a34d6623f5f76e6b40c45393331dc50f.ppt
- Количество слайдов: 24

IHE-Cardiology Technical Committee EP Key Data Elements February 21, 2011 Bryan Jennings - Medical Micrographics Nick Gawrit - Heart. Base 3/17/2018 1
Abstract Define a medical data format for clinical documents containing at a minimum: Ø Patient Conditions Ø EP Lab procedure data Ø Discharge Summary Ø Pointers to other material 2

Problem Statement There is a need to be able to extract these data elements from the medical record in an automated, standards based data model that can operate in a cross-platform environment across multiple electronic health records and cardiac rhythm management devices regardless of vendor. 3

Development of Data Standards Currently, clinical personnel, data managers, clinical investigators, and FDA reviewers must cope with a plethora of data formats and conventions. Some clinical investigators report the presence of many different computer systems for data entry at their sites (for various trials), each of which uses different data conventions. Lack of standardization is not only inefficient, it multiplies the potential for error. … 4
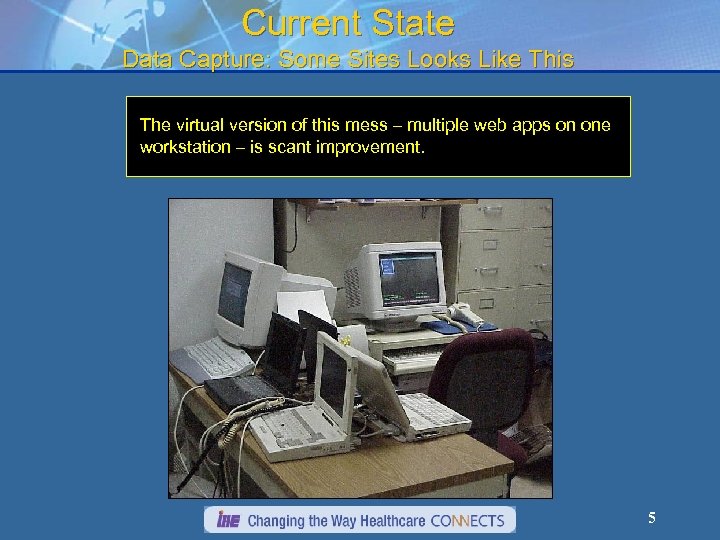
Current State Data Capture: Some Sites Looks Like This The virtual version of this mess – multiple web apps on one workstation – is scant improvement. 5

Standards Used CDA Release 2. 0 ASTM/HL 7 Continuity of Care Document (CCD) HL 7 V 3 Laboratory DMIM HL 7 Care Record Summary LOINC & SNOMED HIPAA Lab Claim Attachment NPRM Document Digital Signature 6

Use Case 1. Health care provider needs to collect data to submit to the ACC NCDR-ICD Registry 2. EMR System would like to display Key Cardiology data in a meaningful way 3. EP Lab System produces data but needs to integrate with other systems to facilitate data sharing 7

Systems that contain data (to name a few) Boston Scientific St. Jude Medical Medtronic Biotronik Siemens Syngo Siemens Axiom Sensis Bard EP Medical Prucka (GE) Witt (Phillips) Lumedx Biosense Mac. Lab (GE) Merge Mc. Kesson Cerner Epic Next. Gen Allscripts 8

Goal: A Common Terminology Boston Scientific Medtronic Epic St. Jude Medical Biotronik Siemens EP Medical GE Prucka Philips Witt 9

Scope The clinical CDA document is: Ø A report of a set of final results (the fulfillment process being completed) will also be shared later as “historical information”. Ø Human-readable, shared between care providers of various specialties and patients (e. g. through a PHR) Ø May contain machine readable coded entries (decision support, registry submissions) 10

A CDA content profile is… A sharable information component that can be exchanged… Ø within an HIE or RHIO Ø via Media or USB Device Ø via Reliable Messages Point to Point Document content using standards Ø CDA Release 2. 0 Ø HL 7 Care Record Summary Ø ASTM/HL 7 Continuity of Care Document More complex documents have a library of reusable parts 11
Where Can Cardiology Use CDA? Clinical Reports (Cath, Echo, EP, etc. ) Ø to go along with our pretty DICOM images Analyses of raw image and waveform data Ø backing up the Clinical Report Documentation of the procedure Ø provide context for the raw data and analyses Input to a clinical and Registry database Ø for patient care over time, or outcomes analysis 12
Value Proposition Supports interchange of PHR Information Ø Patient Demographics Ø Procedure and Device Information Ø Current and Prior Results Ø Medications, Problems, Allergies Ø Diagnoses History Ø Other Information 13
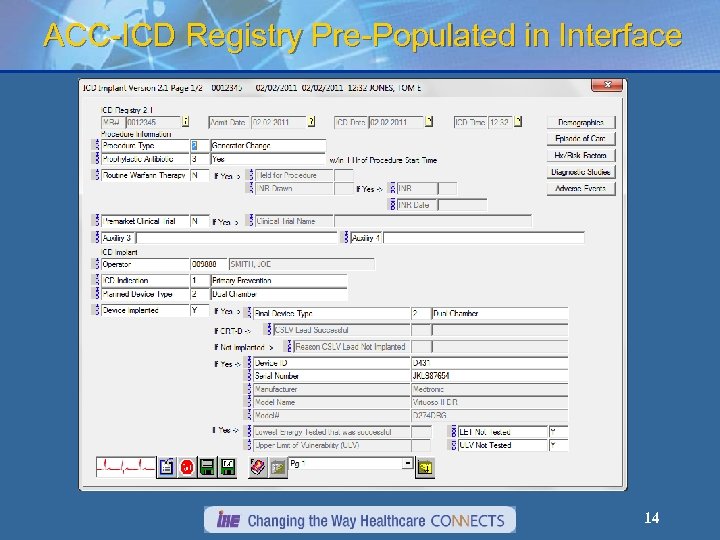
ACC-ICD Registry Pre-Populated in Interface 14
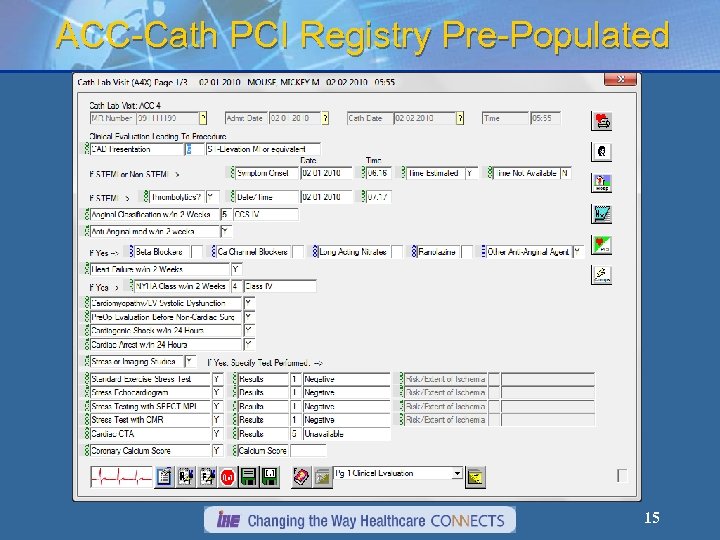
ACC-Cath PCI Registry Pre-Populated 15
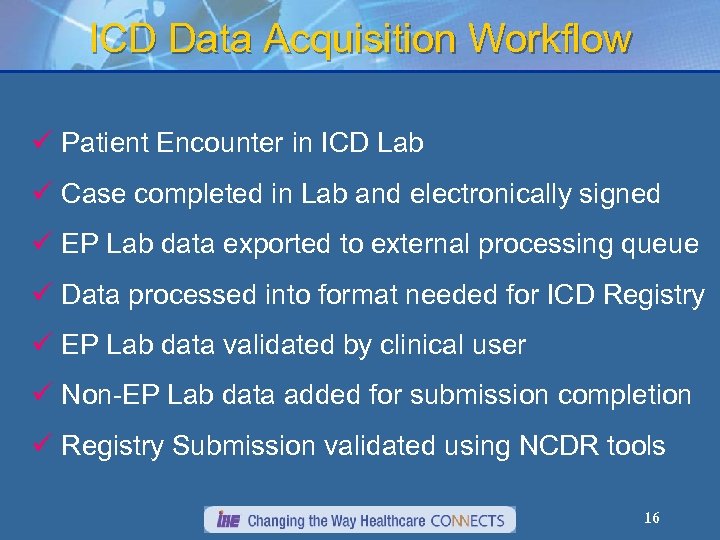
ICD Data Acquisition Workflow ü Patient Encounter in ICD Lab ü Case completed in Lab and electronically signed ü EP Lab data exported to external processing queue ü Data processed into format needed for ICD Registry ü EP Lab data validated by clinical user ü Non-EP Lab data added for submission completion ü Registry Submission validated using NCDR tools 16
Retrieve Forms for Data Capture (RFD) provides a method for gathering data within a user’s current application to meet the requirements of an external system 17
Retrieve Forms for Data Capture A standard way of displaying external data capture forms inside an EHR. Many-to-many integration – any EHR can retrieve forms from many external systems. Low barrier of entry for EHR and external systems. Flexible profile to accommodate both low-tech and sophisticated implementations. 18
Retrieve Forms for Data capture Initial Phase – Done! ü Define standard format forms ü Define standard method for retrieving and submitting forms Content Profile Phase for Cardiology – now! ü Provide domain-specific form requirements ü Enable form population from EHR mapped data 19
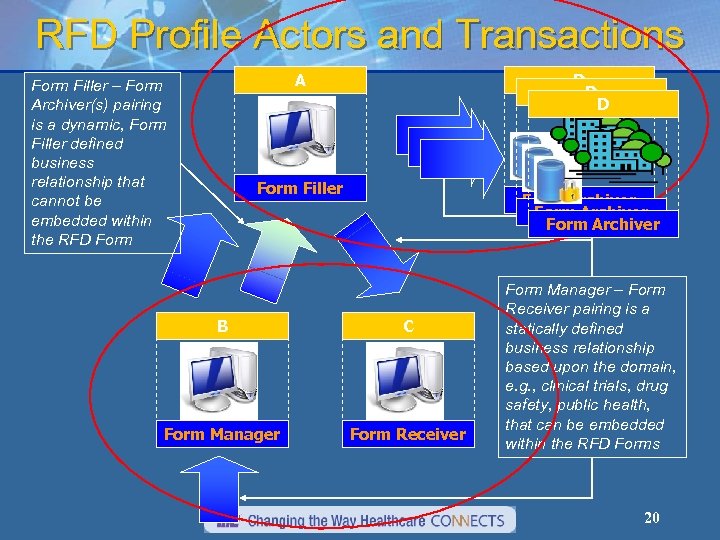
RFD Profile Actors and Transactions D D D A Form Filler – Form Archiver(s) pairing is a dynamic, Form Filler defined business relationship that cannot be embedded within the RFD Form Filler Form Archiver B C Form Manager Form Receiver Form Manager – Form Receiver pairing is a statically defined business relationship based upon the domain, e. g. , clinical trials, drug safety, public health, that can be embedded within the RFD Forms 20
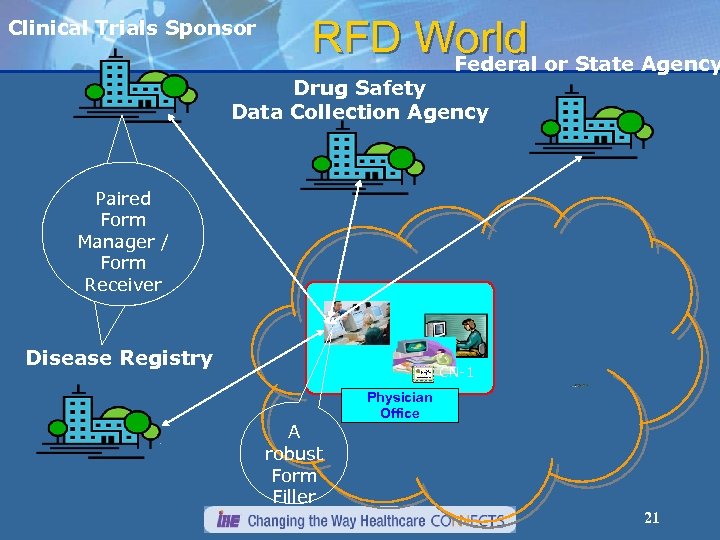
Clinical Trials Sponsor RFD World or State Agency Federal Drug Safety Data Collection Agency Paired Form Manager / Form Receiver Disease Registry CN-1 A robust Form Filler Physician Office 21
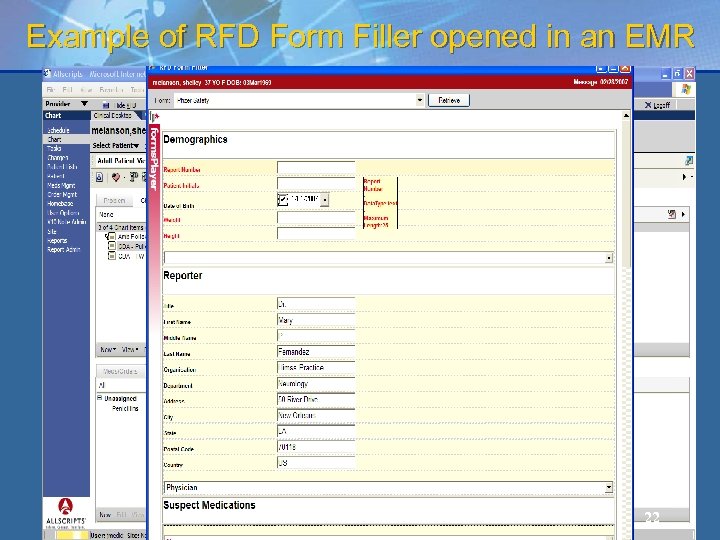
Example of RFD Form Filler opened in an EMR 22
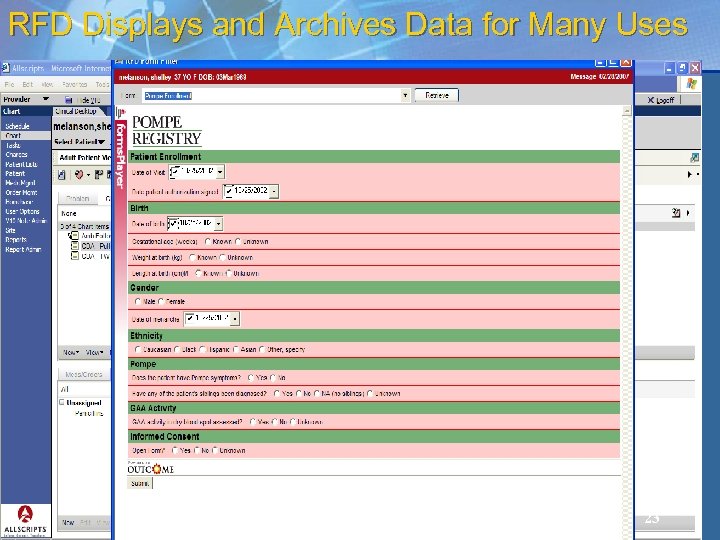
RFD Displays and Archives Data for Many Uses 23

More Information IHE Web Site - http: //www. ihe. net ns? Ø Technical Framework Supplements – Trial io st Implementation ue Q Ø Calls for Participation Ø Technical Frameworks Ø IHE Fact Sheet and FAQ Ø IHE Integration Profiles: Guidelines for Buyers Ø IHE Connectathon Results Ø Vendors’ Product Integration Statements 24
a34d6623f5f76e6b40c45393331dc50f.ppt