295252f128ba8a2236928e5654be7f63.ppt
- Количество слайдов: 1
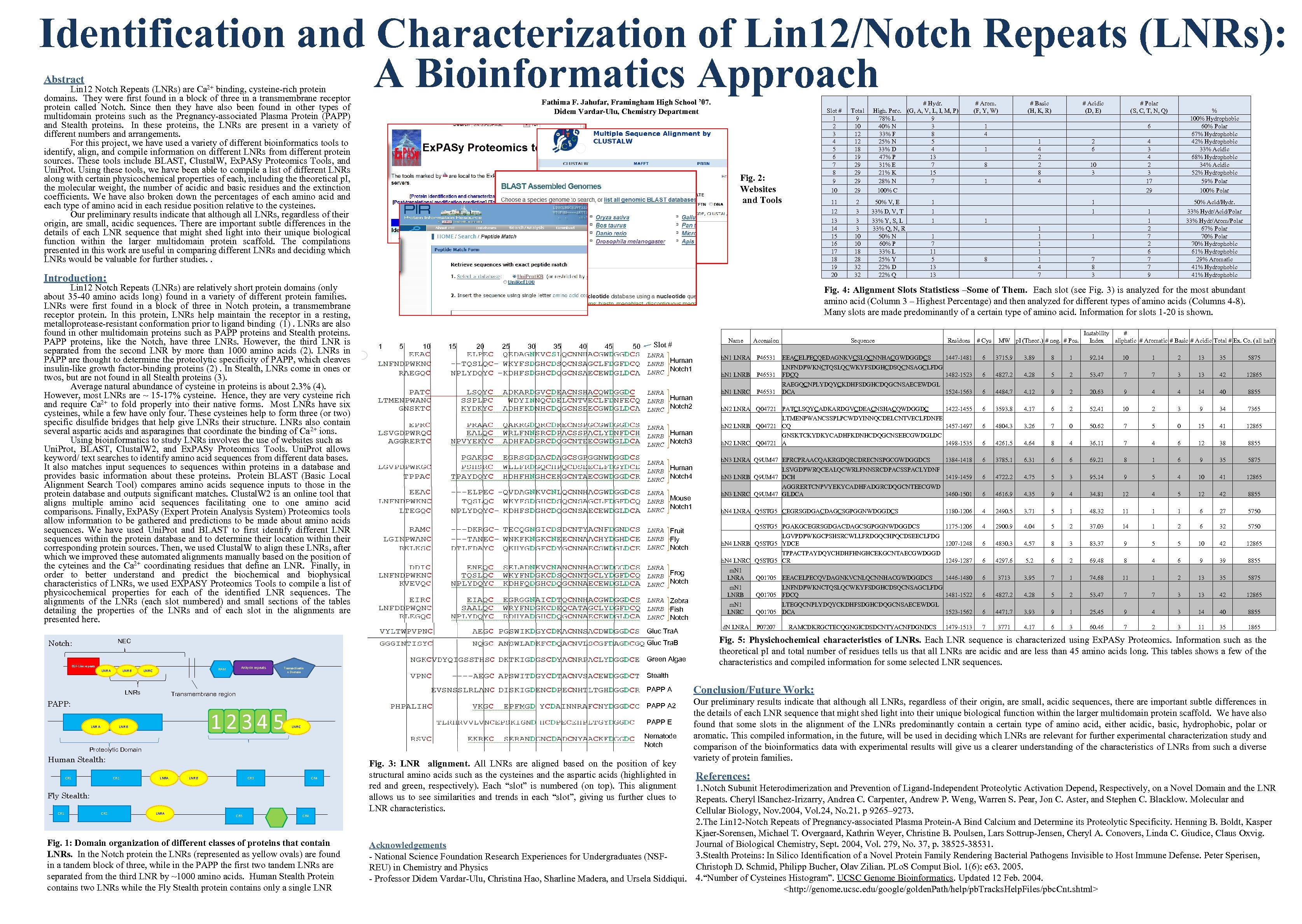 Identification and Characterization of Lin 12/Notch Repeats (LNRs): A Bioinformatics Approach Abstract Lin 12 Notch Repeats (LNRs) are Ca 2+ binding, cysteine-rich protein domains. They were first found in a block of three in a transmembrane receptor protein called Notch. Since then they have also been found in other types of multidomain proteins such as the Pregnancy-associated Plasma Protein (PAPP) and Stealth proteins. In these proteins, the LNRs are present in a variety of different numbers and arrangements. For this project, we have used a variety of different bioinformatics tools to identify, align, and compile information on different LNRs from different protein sources. These tools include BLAST, Clustal. W, Ex. PASy Proteomics Tools, and Uni. Prot. Using these tools, we have been able to compile a list of different LNRs along with certain physicochemical properties of each, including theoretical p. I, the molecular weight, the number of acidic and basic residues and the extinction coefficients. We have also broken down the percentages of each amino acid and each type of amino acid in each residue position relative to the cysteines. Our preliminary results indicate that although all LNRs, regardless of their origin, are small, acidic sequences. There are important subtle differences in the details of each LNR sequence that might shed light into their unique biological function within the larger multidomain protein scaffold. The compilations presented in this work are useful in comparing different LNRs and deciding which LNRs would be valuable for further studies. . Fathima F. Jahufar, Framingham High School ’ 07. Didem Vardar-Ulu, Chemistry Department NEC LNR A LNR B RAM LNRs Transactivatio n Domain 1 5 10 15 20 25 30 35 40 45 50 Slot # LNRA LNRB Human LNRC Notch 1 LNRA LNRB Human LNRC Notch 2 LNRA LNRB Human LNRC Notch 3 LNRA LNRB Human LNRC Notch 4 LNRA LNRB Mouse LNRC Notch 1 LNRA Fruit LNRB Fly LNRC Notch LNRA LNRB Frog LNRC Notch LNRA Zebra LNRB Fish LNRC Notch PAPP A 2 PAPP E LNR C Nematode Notch Proteolytic Domain Human Stealth: CR 1 CR 2 LNRA LNR B CR 3 CR 4 Fly Stealth: CR 1 CR 2 LNRA CR 3 50% V, E 1 1 3 33% D, V, T 1 1 3 3 10 10 18 28 32 32 33% Y, S, L 33% Q, N, R 50% N 60% P 33% L 25% Y 22% D 22% Q 1 1 4 6 1 4 2 2 8 4 1 8 1 2 6 4 3 4 2 3 17 29 10 3 50% Acid/Hydr. 1 1 1 4 7 8 1 7 8 3 33% Hydr/Acid/Polar 1 2 7 2 6 7 7 9 1 1 7 11 5 13 13 % 100% Hydrophobic 60% Polar 67% Hydrophobic 42% Hydrophobic 33% Acidic 68% Hydrophobic 34% Acidic 52% Hydrophobic 59% Polar 100% Polar 33% Hydr/Arom/Polar 67% Polar 70% Hydrophobic 61% Hydrophobic 29% Aromatic 41% Hydrophobic Fig. 4: Alignment Slots Statisticss –Some of Them. Each slot (see Fig. 3) is analyzed for the most abundant amino acid (Column 3 – Highest Percentage) and then analyzed for different types of amino acids (Columns 4 -8). Many slots are made predominantly of a certain type of amino acid. Information for slots 1 -20 is shown. PAPP A 12345 LNR B 2 # Polar (S, C, T, N, Q) Name Accession Residues # Cys h. N 1 LNRA P 46531 EEACELPECQEDAGNKVCSLQCNNHACGWDGGDCS 1447 -1481 6 3715. 9 3. 89 8 1 92. 14 10 1 2 13 35 5875 P 46531 LNFNDPWKNCTQSLQCWKYFSDGHCDSQCNSAGCLFDG 1482 -1523 FDCQ 6 4827. 2 4. 28 5 2 53. 47 7 7 3 13 42 12865 P 46531 RAEGQCNPLYDQYCKDHFSDGHCDQGCNSAECEWDGL 1524 -1563 DCA 6 4484. 7 4. 12 9 2 20. 63 9 4 4 14 40 8855 1422 -1455 6 3593. 8 4. 17 6 2 52. 41 10 2 3 9 34 7365 LTMENPWANCSSPLPCWDYINNQCDELCNTVECLFDNFE h. N 2 LNRB Q 04721 CQ 1457 -1497 6 4804. 3 3. 26 7 0 50. 62 7 5 0 15 41 12865 GNSKTCKYDKYCADHFKDNHCDQGCNSEECGWDGLDC h. N 2 LNRC Q 04721 A 1498 -1535 6 4261. 5 4. 64 8 4 36. 11 7 4 6 12 38 8855 h. N 3 LNRA Q 9 UM 47 EPRCPRAACQAKRGDQRCDRECNSPGCGWDGGDCS 1384 -1418 6 3785. 1 6. 31 6 6 69. 21 8 1 6 9 35 5875 LSVGDPWRQCEALQCWRLFNNSRCDPACSSPACLYDNF h. N 3 LNRB Q 9 UM 47 DCH 1419 -1459 6 4722. 2 4. 75 5 3 95. 14 9 5 4 10 41 12865 AGGRERTCNPVYEKYCADHFADGRCDQGCNTEECGWD h. N 3 LNRC Q 9 UM 47 GLDCA 1460 -1501 6 4616. 9 4. 35 9 4 34. 81 12 4 5 12 42 8855 h. N 4 LNRA Q 5 STG 5 CEGRSGDGACDAGCSGPGGNWDGGDCS 1180 -1206 4 2490. 5 3. 71 5 1 48. 32 11 1 1 6 27 5750 1175 -1206 4 2900. 9 4. 04 5 2 37. 03 14 1 2 6 32 5750 LGVPDPWKGCPSHSRCWLLFRDGQCHPQCDSEECLFDG h. N 4 LNRB Q 5 STG 5 YDCE 1207 -1248 6 4830. 3 4. 57 8 3 83. 37 9 5 5 10 42 12865 TPPACTPAYDQYCHDHFHNGHCEKGCNTAECGWDGGD h. N 4 LNRC Q 5 STG 5 CR 1249 -1287 6 4297. 6 5. 2 69. 48 8 4 6 9 39 8855 h. N 1 LNRB h. N 1 LNRC Sequence Instability # MW p. I (Theor. ) # neg. # Pos. Index aliphatic # Aromatic # Basic # Acidic Total # Ex. Co. (all half) h. N 2 LNRA Q 04721 PATCLSQYCADKARDGVCDEACNSHACQWDGGDC Q 5 STG 5 PGAKGCEGRSGDGACDAGCSGPGGNWDGGDCS m. N 1 LNRA Q 01705 EEACELPECQVDAGNKVCNLQCNNHACGWDGGDCS 1446 -1480 6 3713 3. 95 7 1 74. 68 11 1 2 13 35 5875 m. N 1 LNRB LNFNDPWKNCTQSLQCWKYFSDGHCDSQCNSAGCLFDG Q 01705 FDCQ 1481 -1522 6 4827. 2 4. 28 5 2 53. 47 7 7 3 13 42 12865 m. N 1 LNRC LTEGQCNPLYDQYCKDHFSDGHCDQGCNSAECEWDGL Q 01705 DCA 1523 -1562 6 4471. 7 3. 93 9 1 25. 45 9 4 3 14 40 8855 P 07207 1479 -1513 7 3771 4. 17 6 3 60. 46 7 2 3 11 35 1865 d. N LNRA RAMCDKRGCTECQGNGICDSDCNTYACNFDGNDCS Fig. 5: Physichochemical characteristics of LNRs. Each LNR sequence is characterized using Ex. PASy Proteomics. Information such as theoretical p. I and total number of residues tells us that all LNRs are acidic and are less than 45 amino acids long. This tables shows a few of the characteristics and compiled information for some selected LNR sequences. Stealth Transmembrane region PAPP: LNR A Ankyrin repeats Total 9 10 12 12 18 19 29 29 13 14 15 16 17 18 19 20 Gluc Tra. B LNR C # Acidic (D, E) 12 Green Algae EGF-Like repeats # Basic (H, K, R) 11 Fig. 2: Websites and Tools Gluc Tra. A Notch: # Arom. (F, Y, W) Slot # 1 2 3 4 5 6 7 8 9 10 Introduction: Lin 12 Notch Repeats (LNRs) are relatively short protein domains (only about 35 -40 amino acids long) found in a variety of different protein families. LNRs were first found in a block of three in Notch protein, a transmembrane receptor protein. In this protein, LNRs help maintain the receptor in a resting, metalloprotease-resistant conformation prior to ligand binding (1). LNRs are also found in other multidomain proteins such as PAPP proteins and Stealth proteins. PAPP proteins, like the Notch, have three LNRs. However, the third LNR is separated from the second LNR by more than 1000 amino acids (2). LNRs in PAPP are thought to determine the proteolytic specificity of PAPP, which cleaves insulin-like growth factor-binding proteins (2). In Stealth, LNRs come in ones or twos, but are not found in all Stealth proteins (3). Average natural abundance of cysteine in proteins is about 2. 3% (4). However, most LNRs are ~ 15 -17% cysteine. Hence, they are very cysteine rich and require Ca 2+ to fold properly into their native forms. Most LNRs have six cysteines, while a few have only four. These cysteines help to form three (or two) specific disulfide bridges that help give LNRs their structure. LNRs also contain several aspartic acids and asparagines that coordinate the binding of Ca 2+ ions. Using bioinformatics to study LNRs involves the use of websites such as Uni. Prot, BLAST, Clustal. W 2, and Ex. PASy Proteomics Tools. Uni. Prot allows keyword/ text searches to identify amino acid sequences from different data bases. It also matches input sequences to sequences within proteins in a database and provides basic information about these proteins. Protein BLAST (Basic Local Alignment Search Tool) compares amino acids sequence inputs to those in the protein database and outputs significant matches. Clustal. W 2 is an online tool that aligns multiple amino acid sequences facilitating one to one amino acid comparisons. Finally, Ex. PASy (Expert Protein Analysis System) Proteomics tools allow information to be gathered and predictions to be made about amino acids sequences. We have used Uni. Prot and BLAST to first identify different LNR sequences within the protein database and to determine their location within their corresponding protein sources. Then, we used Clustal. W to align these LNRs, after which we improved these automated alignments manually based on the position of the cyteines and the Ca 2+ coordinating residues that define an LNR. Finally, in order to better understand predict the biochemical and biophysical characteristics of LNRs, we used EXPASY Proteomics Tools to compile a list of physicochemical properties for each of the identified LNR sequences. The alignments of the LNRs (each slot numbered) and small sections of the tables detailing the properties of the LNRs and of each slot in the alignments are presented here. # Hydr. High. Perc. (G, A, V, L, I, M, P) 78% L 9 40% N 3 33% F 8 25% N 5 33% D 4 47% P 13 31% E 7 21% K 15 28% N 7 100% C CR 4 Fig. 1: Domain organization of different classes of proteins that contain LNRs. In the Notch protein the LNRs (represented as yellow ovals) are found in a tandem block of three, while in the PAPP the first two tandem LNRs are separated from the third LNR by ~1000 amino acids. Human Stealth Protein contains two LNRs while the Fly Stealth protein contains only a single LNR Fig. 3: LNR alignment. All LNRs are aligned based on the position of key structural amino acids such as the cysteines and the aspartic acids (highlighted in red and green, respectively). Each “slot” is numbered (on top). This alignment allows us to see similarities and trends in each “slot”, giving us further clues to LNR characteristics. Conclusion/Future Work: Our preliminary results indicate that although all LNRs, regardless of their origin, are small, acidic sequences, there are important subtle differences in the details of each LNR sequence that might shed light into their unique biological function within the larger multidomain protein scaffold. We have also found that some slots in the alignment of the LNRs predominantly contain a certain type of amino acid, either acidic, basic, hydrophobic, polar or aromatic. This compiled information, in the future, will be used in deciding which LNRs are relevant for further experimental characterization study and comparison of the bioinformatics data with experimental results will give us a clearer understanding of the characteristics of LNRs from such a diverse variety of protein families. References: 1. Notch Subunit Heterodimerization and Prevention of Ligand-Independent Proteolytic Activation Depend, Respectively, on a Novel Domain and the LNR Repeats. Cheryl l. Sanchez-Irizarry, Andrea C. Carpenter, Andrew P. Weng, Warren S. Pear, Jon C. Aster, and Stephen C. Blacklow. Molecular and Cellular Biology, Nov. 2004, Vol. 24, No. 21. p 9265– 9273. 2. The Lin 12 -Notch Repeats of Pregnancy-associated Plasma Protein-A Bind Calcium and Determine its Proteolytic Specificity. Henning B. Boldt, Kasper Kjaer-Sorensen, Michael T. Overgaard, Kathrin Weyer, Christine B. Poulsen, Lars Sottrup-Jensen, Cheryl A. Conovers, Linda C. Giudice, Claus Oxvig. Journal of Biological Chemistry, Sept. 2004, Vol. 279, No. 37, p. 38525 -38531. Acknowledgements 3. Stealth Proteins: In Silico Identification of a Novel Protein Family Rendering Bacterial Pathogens Invisible to Host Immune Defense. Peter Sperisen, - National Science Foundation Research Experiences for Undergraduates (NSFChristoph D. Schmid, Philipp Bucher, Olav Zilian. PLo. S Comput Biol. 1(6): e 63. 2005. REU) in Chemistry and Physics - Professor Didem Vardar-Ulu, Christina Hao, Sharline Madera, and Ursela Siddiqui. 4. “Number of Cysteines Histogram”. UCSC Genome Bioinformatics. Updated 12 Feb. 2004.
Identification and Characterization of Lin 12/Notch Repeats (LNRs): A Bioinformatics Approach Abstract Lin 12 Notch Repeats (LNRs) are Ca 2+ binding, cysteine-rich protein domains. They were first found in a block of three in a transmembrane receptor protein called Notch. Since then they have also been found in other types of multidomain proteins such as the Pregnancy-associated Plasma Protein (PAPP) and Stealth proteins. In these proteins, the LNRs are present in a variety of different numbers and arrangements. For this project, we have used a variety of different bioinformatics tools to identify, align, and compile information on different LNRs from different protein sources. These tools include BLAST, Clustal. W, Ex. PASy Proteomics Tools, and Uni. Prot. Using these tools, we have been able to compile a list of different LNRs along with certain physicochemical properties of each, including theoretical p. I, the molecular weight, the number of acidic and basic residues and the extinction coefficients. We have also broken down the percentages of each amino acid and each type of amino acid in each residue position relative to the cysteines. Our preliminary results indicate that although all LNRs, regardless of their origin, are small, acidic sequences. There are important subtle differences in the details of each LNR sequence that might shed light into their unique biological function within the larger multidomain protein scaffold. The compilations presented in this work are useful in comparing different LNRs and deciding which LNRs would be valuable for further studies. . Fathima F. Jahufar, Framingham High School ’ 07. Didem Vardar-Ulu, Chemistry Department NEC LNR A LNR B RAM LNRs Transactivatio n Domain 1 5 10 15 20 25 30 35 40 45 50 Slot # LNRA LNRB Human LNRC Notch 1 LNRA LNRB Human LNRC Notch 2 LNRA LNRB Human LNRC Notch 3 LNRA LNRB Human LNRC Notch 4 LNRA LNRB Mouse LNRC Notch 1 LNRA Fruit LNRB Fly LNRC Notch LNRA LNRB Frog LNRC Notch LNRA Zebra LNRB Fish LNRC Notch PAPP A 2 PAPP E LNR C Nematode Notch Proteolytic Domain Human Stealth: CR 1 CR 2 LNRA LNR B CR 3 CR 4 Fly Stealth: CR 1 CR 2 LNRA CR 3 50% V, E 1 1 3 33% D, V, T 1 1 3 3 10 10 18 28 32 32 33% Y, S, L 33% Q, N, R 50% N 60% P 33% L 25% Y 22% D 22% Q 1 1 4 6 1 4 2 2 8 4 1 8 1 2 6 4 3 4 2 3 17 29 10 3 50% Acid/Hydr. 1 1 1 4 7 8 1 7 8 3 33% Hydr/Acid/Polar 1 2 7 2 6 7 7 9 1 1 7 11 5 13 13 % 100% Hydrophobic 60% Polar 67% Hydrophobic 42% Hydrophobic 33% Acidic 68% Hydrophobic 34% Acidic 52% Hydrophobic 59% Polar 100% Polar 33% Hydr/Arom/Polar 67% Polar 70% Hydrophobic 61% Hydrophobic 29% Aromatic 41% Hydrophobic Fig. 4: Alignment Slots Statisticss –Some of Them. Each slot (see Fig. 3) is analyzed for the most abundant amino acid (Column 3 – Highest Percentage) and then analyzed for different types of amino acids (Columns 4 -8). Many slots are made predominantly of a certain type of amino acid. Information for slots 1 -20 is shown. PAPP A 12345 LNR B 2 # Polar (S, C, T, N, Q) Name Accession Residues # Cys h. N 1 LNRA P 46531 EEACELPECQEDAGNKVCSLQCNNHACGWDGGDCS 1447 -1481 6 3715. 9 3. 89 8 1 92. 14 10 1 2 13 35 5875 P 46531 LNFNDPWKNCTQSLQCWKYFSDGHCDSQCNSAGCLFDG 1482 -1523 FDCQ 6 4827. 2 4. 28 5 2 53. 47 7 7 3 13 42 12865 P 46531 RAEGQCNPLYDQYCKDHFSDGHCDQGCNSAECEWDGL 1524 -1563 DCA 6 4484. 7 4. 12 9 2 20. 63 9 4 4 14 40 8855 1422 -1455 6 3593. 8 4. 17 6 2 52. 41 10 2 3 9 34 7365 LTMENPWANCSSPLPCWDYINNQCDELCNTVECLFDNFE h. N 2 LNRB Q 04721 CQ 1457 -1497 6 4804. 3 3. 26 7 0 50. 62 7 5 0 15 41 12865 GNSKTCKYDKYCADHFKDNHCDQGCNSEECGWDGLDC h. N 2 LNRC Q 04721 A 1498 -1535 6 4261. 5 4. 64 8 4 36. 11 7 4 6 12 38 8855 h. N 3 LNRA Q 9 UM 47 EPRCPRAACQAKRGDQRCDRECNSPGCGWDGGDCS 1384 -1418 6 3785. 1 6. 31 6 6 69. 21 8 1 6 9 35 5875 LSVGDPWRQCEALQCWRLFNNSRCDPACSSPACLYDNF h. N 3 LNRB Q 9 UM 47 DCH 1419 -1459 6 4722. 2 4. 75 5 3 95. 14 9 5 4 10 41 12865 AGGRERTCNPVYEKYCADHFADGRCDQGCNTEECGWD h. N 3 LNRC Q 9 UM 47 GLDCA 1460 -1501 6 4616. 9 4. 35 9 4 34. 81 12 4 5 12 42 8855 h. N 4 LNRA Q 5 STG 5 CEGRSGDGACDAGCSGPGGNWDGGDCS 1180 -1206 4 2490. 5 3. 71 5 1 48. 32 11 1 1 6 27 5750 1175 -1206 4 2900. 9 4. 04 5 2 37. 03 14 1 2 6 32 5750 LGVPDPWKGCPSHSRCWLLFRDGQCHPQCDSEECLFDG h. N 4 LNRB Q 5 STG 5 YDCE 1207 -1248 6 4830. 3 4. 57 8 3 83. 37 9 5 5 10 42 12865 TPPACTPAYDQYCHDHFHNGHCEKGCNTAECGWDGGD h. N 4 LNRC Q 5 STG 5 CR 1249 -1287 6 4297. 6 5. 2 69. 48 8 4 6 9 39 8855 h. N 1 LNRB h. N 1 LNRC Sequence Instability # MW p. I (Theor. ) # neg. # Pos. Index aliphatic # Aromatic # Basic # Acidic Total # Ex. Co. (all half) h. N 2 LNRA Q 04721 PATCLSQYCADKARDGVCDEACNSHACQWDGGDC Q 5 STG 5 PGAKGCEGRSGDGACDAGCSGPGGNWDGGDCS m. N 1 LNRA Q 01705 EEACELPECQVDAGNKVCNLQCNNHACGWDGGDCS 1446 -1480 6 3713 3. 95 7 1 74. 68 11 1 2 13 35 5875 m. N 1 LNRB LNFNDPWKNCTQSLQCWKYFSDGHCDSQCNSAGCLFDG Q 01705 FDCQ 1481 -1522 6 4827. 2 4. 28 5 2 53. 47 7 7 3 13 42 12865 m. N 1 LNRC LTEGQCNPLYDQYCKDHFSDGHCDQGCNSAECEWDGL Q 01705 DCA 1523 -1562 6 4471. 7 3. 93 9 1 25. 45 9 4 3 14 40 8855 P 07207 1479 -1513 7 3771 4. 17 6 3 60. 46 7 2 3 11 35 1865 d. N LNRA RAMCDKRGCTECQGNGICDSDCNTYACNFDGNDCS Fig. 5: Physichochemical characteristics of LNRs. Each LNR sequence is characterized using Ex. PASy Proteomics. Information such as theoretical p. I and total number of residues tells us that all LNRs are acidic and are less than 45 amino acids long. This tables shows a few of the characteristics and compiled information for some selected LNR sequences. Stealth Transmembrane region PAPP: LNR A Ankyrin repeats Total 9 10 12 12 18 19 29 29 13 14 15 16 17 18 19 20 Gluc Tra. B LNR C # Acidic (D, E) 12 Green Algae EGF-Like repeats # Basic (H, K, R) 11 Fig. 2: Websites and Tools Gluc Tra. A Notch: # Arom. (F, Y, W) Slot # 1 2 3 4 5 6 7 8 9 10 Introduction: Lin 12 Notch Repeats (LNRs) are relatively short protein domains (only about 35 -40 amino acids long) found in a variety of different protein families. LNRs were first found in a block of three in Notch protein, a transmembrane receptor protein. In this protein, LNRs help maintain the receptor in a resting, metalloprotease-resistant conformation prior to ligand binding (1). LNRs are also found in other multidomain proteins such as PAPP proteins and Stealth proteins. PAPP proteins, like the Notch, have three LNRs. However, the third LNR is separated from the second LNR by more than 1000 amino acids (2). LNRs in PAPP are thought to determine the proteolytic specificity of PAPP, which cleaves insulin-like growth factor-binding proteins (2). In Stealth, LNRs come in ones or twos, but are not found in all Stealth proteins (3). Average natural abundance of cysteine in proteins is about 2. 3% (4). However, most LNRs are ~ 15 -17% cysteine. Hence, they are very cysteine rich and require Ca 2+ to fold properly into their native forms. Most LNRs have six cysteines, while a few have only four. These cysteines help to form three (or two) specific disulfide bridges that help give LNRs their structure. LNRs also contain several aspartic acids and asparagines that coordinate the binding of Ca 2+ ions. Using bioinformatics to study LNRs involves the use of websites such as Uni. Prot, BLAST, Clustal. W 2, and Ex. PASy Proteomics Tools. Uni. Prot allows keyword/ text searches to identify amino acid sequences from different data bases. It also matches input sequences to sequences within proteins in a database and provides basic information about these proteins. Protein BLAST (Basic Local Alignment Search Tool) compares amino acids sequence inputs to those in the protein database and outputs significant matches. Clustal. W 2 is an online tool that aligns multiple amino acid sequences facilitating one to one amino acid comparisons. Finally, Ex. PASy (Expert Protein Analysis System) Proteomics tools allow information to be gathered and predictions to be made about amino acids sequences. We have used Uni. Prot and BLAST to first identify different LNR sequences within the protein database and to determine their location within their corresponding protein sources. Then, we used Clustal. W to align these LNRs, after which we improved these automated alignments manually based on the position of the cyteines and the Ca 2+ coordinating residues that define an LNR. Finally, in order to better understand predict the biochemical and biophysical characteristics of LNRs, we used EXPASY Proteomics Tools to compile a list of physicochemical properties for each of the identified LNR sequences. The alignments of the LNRs (each slot numbered) and small sections of the tables detailing the properties of the LNRs and of each slot in the alignments are presented here. # Hydr. High. Perc. (G, A, V, L, I, M, P) 78% L 9 40% N 3 33% F 8 25% N 5 33% D 4 47% P 13 31% E 7 21% K 15 28% N 7 100% C CR 4 Fig. 1: Domain organization of different classes of proteins that contain LNRs. In the Notch protein the LNRs (represented as yellow ovals) are found in a tandem block of three, while in the PAPP the first two tandem LNRs are separated from the third LNR by ~1000 amino acids. Human Stealth Protein contains two LNRs while the Fly Stealth protein contains only a single LNR Fig. 3: LNR alignment. All LNRs are aligned based on the position of key structural amino acids such as the cysteines and the aspartic acids (highlighted in red and green, respectively). Each “slot” is numbered (on top). This alignment allows us to see similarities and trends in each “slot”, giving us further clues to LNR characteristics. Conclusion/Future Work: Our preliminary results indicate that although all LNRs, regardless of their origin, are small, acidic sequences, there are important subtle differences in the details of each LNR sequence that might shed light into their unique biological function within the larger multidomain protein scaffold. We have also found that some slots in the alignment of the LNRs predominantly contain a certain type of amino acid, either acidic, basic, hydrophobic, polar or aromatic. This compiled information, in the future, will be used in deciding which LNRs are relevant for further experimental characterization study and comparison of the bioinformatics data with experimental results will give us a clearer understanding of the characteristics of LNRs from such a diverse variety of protein families. References: 1. Notch Subunit Heterodimerization and Prevention of Ligand-Independent Proteolytic Activation Depend, Respectively, on a Novel Domain and the LNR Repeats. Cheryl l. Sanchez-Irizarry, Andrea C. Carpenter, Andrew P. Weng, Warren S. Pear, Jon C. Aster, and Stephen C. Blacklow. Molecular and Cellular Biology, Nov. 2004, Vol. 24, No. 21. p 9265– 9273. 2. The Lin 12 -Notch Repeats of Pregnancy-associated Plasma Protein-A Bind Calcium and Determine its Proteolytic Specificity. Henning B. Boldt, Kasper Kjaer-Sorensen, Michael T. Overgaard, Kathrin Weyer, Christine B. Poulsen, Lars Sottrup-Jensen, Cheryl A. Conovers, Linda C. Giudice, Claus Oxvig. Journal of Biological Chemistry, Sept. 2004, Vol. 279, No. 37, p. 38525 -38531. Acknowledgements 3. Stealth Proteins: In Silico Identification of a Novel Protein Family Rendering Bacterial Pathogens Invisible to Host Immune Defense. Peter Sperisen, - National Science Foundation Research Experiences for Undergraduates (NSFChristoph D. Schmid, Philipp Bucher, Olav Zilian. PLo. S Comput Biol. 1(6): e 63. 2005. REU) in Chemistry and Physics - Professor Didem Vardar-Ulu, Christina Hao, Sharline Madera, and Ursela Siddiqui. 4. “Number of Cysteines Histogram”. UCSC Genome Bioinformatics. Updated 12 Feb. 2004.


