002da2c87595120f8fd086696d792bc1.ppt
- Количество слайдов: 17

IDA FOUNDATION & IDA ARV BV Partners in Affordable Healthcare Connie van Marrewijk IDA Foundation

AGENDA Ø Ø Ø INTRODUCTION IDA FOUNDATION IDA ARV BV SUPPLY OF MEDICINES QA/QC SYSTEM PAPERWORK CONCLUSION

IDA FOUNDATION The world’s leading not-for-profit supplier of medicines and medical supplies to developing countries “To provide high quality essential drugs and medical supplies at the lowest possible price to non-profit health care organisations in developing countries. ” Ø one-stop shop for essential drugs, diagnostics & supplies

ESTABLISHMENT IDA ARV BV Jan 2004 Focus on the specific issues & challenges related to ARV & HIV/AIDS medical supplies procurement Centralisation knowledge in the following areas: Ø patents & registration Ø logistics Ø sourcing Ø one-stop shop for ARV’s, OI’s & Diagnostics

ARV PROJECTS ARV’s are now routinely supplied to projects in over 24 countries Global Fund Cambodia El Salvador Ethiopia Georgia Ghana Haiti Honduras Ivory Coast Nicaragua Lesotho Moldova Tanzania Peru Other Projects Burundi Eritrea East Timor Kenya Guinee-Eq. Uzbekistan Zimbabwe Guinee Sudan Mozambique Úkraine

SUPPLY of ARV’s ARV projects often demand a mixture of generics and branded products GENERIC BRANDED Produced in developing world Produced in Western Europe / US Independent QA & QC QA &QC by manufacturer FDC’s available Combination of different tablets

QAQC at IDA Standardised & Controlled QA/QC system resulting in high quality IDA medicines • To filter out substandard medicines • No experience with counterfeit medicines Note: IDA label copied (2 cases)
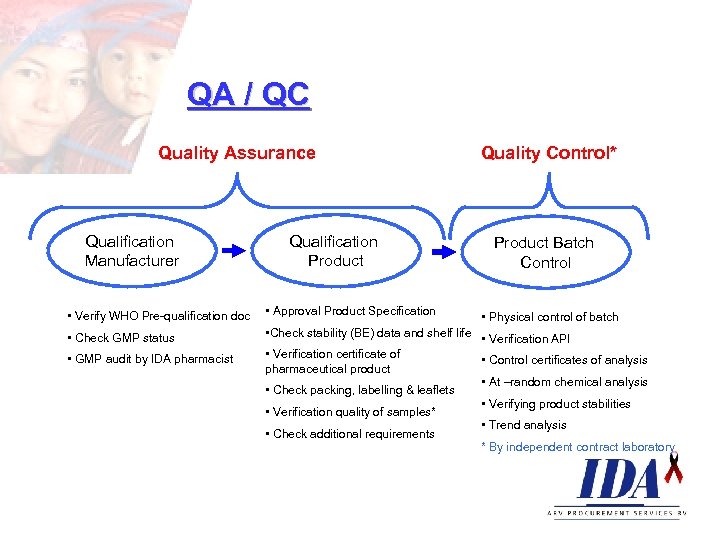
QA / QC Quality Assurance Qualification Manufacturer Qualification Product Quality Control* Product Batch Control • Verify WHO Pre-qualification doc • Approval Product Specification • Physical control of batch • Check stability (BE) data and shelf life • Verification API • Check GMP status • Verification certificate of • GMP audit by IDA pharmacist • Control certificates of analysis pharmaceutical product • At –random chemical analysis • Check packing, labelling & leaflets • Verifying product stabilities • Verification quality of samples* • Trend analysis • Check additional requirements * By independent contract laboratory

QA / Qualification manufacturer GMP o. Manufacturing process o. Equipment and maintenance o. Plant design and environment o. Personnel and training o. Documentation Handling and storage condition o. Special shipment for keep cool items (IDA has 4 classes from *a to ***) o. Controlled temperature in warehouses (IDA and client) o. Good dispensing practice

QA / Qualification product Design of the product o. Drug formulation o. Active ingredient (API) o. Inactive ingredient o. Bio-availability (rate and extend of absorption) o. Assessments of CRO’s o. Stability o. Packaging: Immediate and External o. Labelling & Information leaflet o. Documented evidence o. Validated methods of control & production o. Registration dossier

QC / Product batch control Physical control ØVerification API ØControl certificate of analysis ØAt random chemical analyses ØManufacturer – site combination check ØVerifying product stabilities ØTrend analyses ØDocument number ØAuthorisation signatures ØReferral to Pharmacopeia (eg. UK or US) ØAny added substances ØForm and strength

IDA Product presentation Label §International Nonproprietary Name (INN) §Strength §Dosage form §Route of administration (parenterals) §Storage conditions §Batch mfd exp §Packsize §Other

Product presentation Shipper cartons §No product labels (to prevent theft) §IDA code number, batch number and exp date §Strong quality

GUIDELINES IDA ARV BV compliant with WHO guidelines Antiretrovirals must: Have been accepted by the WHO prequalification project; or a. Have been authorized for consumption by a stringent regulatory authority; or b. Have been authorized by the NDRA in the recipient’s country. Option c. is applicable only until April 2005, pending a Global Fund Board decision

HANDLING PAPERWORK Expertise and experience for required export documentation e. g. : – Packing List – Certificates of Analysis – Airway bill – Required documentation for tax exemptions – Registration requirements in countries Service – Language labelling & leaflets – INCO Terms – Payment conditions – Currency – Pricing requirements (e. g. Clinton Foundation)

CONCLUSION • One stop shop • Standardised 3 -step QA/QC system • Knowledge about registration possibilities • Experience & Expertise PLEASE NOTE THAT IDA FOUNDATION ALSO HAS AN EXTENDED RANGE FOR OPPORTUNISTIC INFECTIONS, TB & MALARIA

BURUNDI CAMBODIA EAST TIMOR EL SALVADOR ERITREA ETHIOPIA GEORGIA GHANA GUINEE-EQ. HAITI HONDURAS IVORY COAST KENYA LESOTHO MOLDOVA MOZAMBIQUE NICARAGUA PERU SUDAN TANZANIA UKRAINE UZBEKISTAN ZIMBABWE
002da2c87595120f8fd086696d792bc1.ppt