db184d00d598355c599dfffdea99d2a1.ppt
- Количество слайдов: 25
 ICON 8 Evaluating Weekly Chemotherapy Scheduling in the First–line Management of Ovarian Cancer Andrew Clamp Senior Lecturer in Medical Oncology The Christie BGCS-NCRI Meeting Westminster 5 th July 2012
ICON 8 Evaluating Weekly Chemotherapy Scheduling in the First–line Management of Ovarian Cancer Andrew Clamp Senior Lecturer in Medical Oncology The Christie BGCS-NCRI Meeting Westminster 5 th July 2012
 Background • Current standard-of-care 3 -weekly carboplatinpaclitaxel Mc. Guire et al NEJM 1996; Piccart et al JNCI 2000; Ozols et al JCO 2003 • No improvement with additional cytotoxics/ maintenance therapy • Increasing role of neoadjuvant chemotherapy with delayed primary surgery Vergote et al NEJM 2010
Background • Current standard-of-care 3 -weekly carboplatinpaclitaxel Mc. Guire et al NEJM 1996; Piccart et al JNCI 2000; Ozols et al JCO 2003 • No improvement with additional cytotoxics/ maintenance therapy • Increasing role of neoadjuvant chemotherapy with delayed primary surgery Vergote et al NEJM 2010
 Weekly Paclitaxel • Dose density – Acceleration of schedule to maximise exposure of tumour cells to PTX in accelerated growth phase • Dose intensity – Achieve higher total dose • Reduced toxicity (myelosuppression) • Anti-angiogenic activity
Weekly Paclitaxel • Dose density – Acceleration of schedule to maximise exposure of tumour cells to PTX in accelerated growth phase • Dose intensity – Achieve higher total dose • Reduced toxicity (myelosuppression) • Anti-angiogenic activity
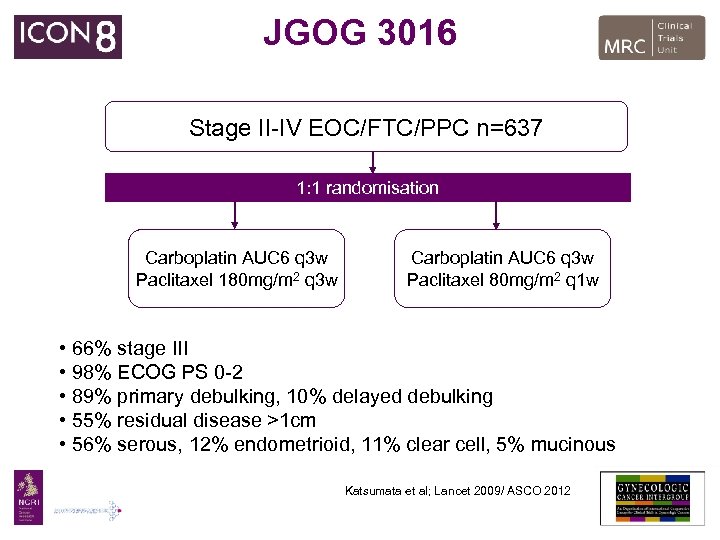 JGOG 3016 Stage II-IV EOC/FTC/PPC n=637 1: 1 randomisation Carboplatin AUC 6 q 3 w Paclitaxel 180 mg/m 2 q 3 w Carboplatin AUC 6 q 3 w Paclitaxel 80 mg/m 2 q 1 w • 66% stage III • 98% ECOG PS 0 -2 • 89% primary debulking, 10% delayed debulking • 55% residual disease >1 cm • 56% serous, 12% endometrioid, 11% clear cell, 5% mucinous Katsumata et al; Lancet 2009/ ASCO 2012
JGOG 3016 Stage II-IV EOC/FTC/PPC n=637 1: 1 randomisation Carboplatin AUC 6 q 3 w Paclitaxel 180 mg/m 2 q 3 w Carboplatin AUC 6 q 3 w Paclitaxel 80 mg/m 2 q 1 w • 66% stage III • 98% ECOG PS 0 -2 • 89% primary debulking, 10% delayed debulking • 55% residual disease >1 cm • 56% serous, 12% endometrioid, 11% clear cell, 5% mucinous Katsumata et al; Lancet 2009/ ASCO 2012
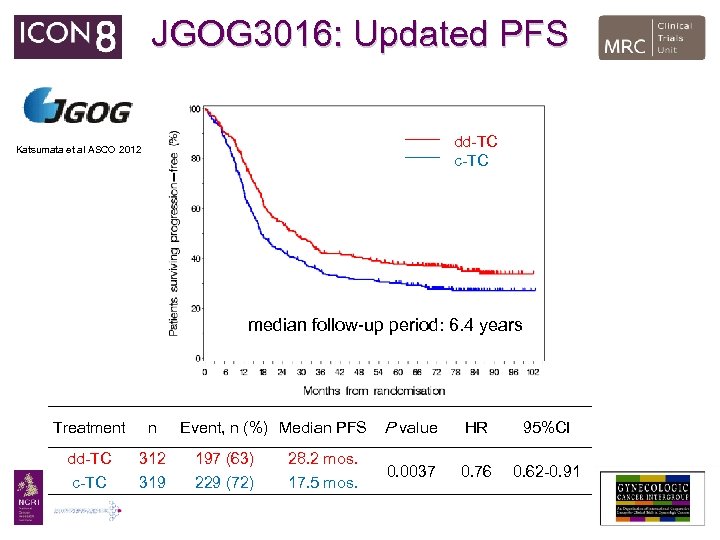 JGOG 3016: Updated PFS dd-TC c-TC Katsumata et al ASCO 2012 median follow-up period: 6. 4 years Treatment n dd-TC c-TC 312 319 Event, n (%) Median PFS 197 (63) 229 (72) 28. 2 mos. 17. 5 mos. P value HR 95%CI 0. 0037 0. 76 0. 62 -0. 91
JGOG 3016: Updated PFS dd-TC c-TC Katsumata et al ASCO 2012 median follow-up period: 6. 4 years Treatment n dd-TC c-TC 312 319 Event, n (%) Median PFS 197 (63) 229 (72) 28. 2 mos. 17. 5 mos. P value HR 95%CI 0. 0037 0. 76 0. 62 -0. 91
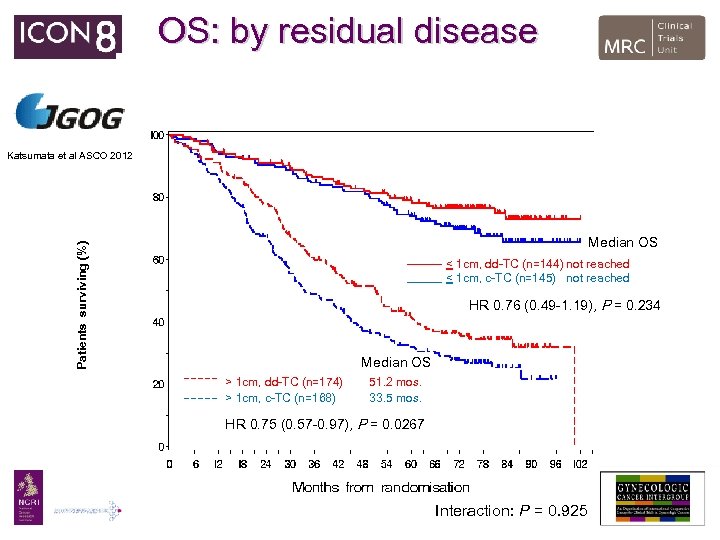 OS: by residual disease Katsumata et al ASCO 2012 Patients surviving (%) Median OS < 1 cm, dd-TC (n=144) not reached < 1 cm, c-TC (n=145) not reached HR 0. 76 (0. 49 -1. 19), P = 0. 234 Median OS > 1 cm, dd-TC (n=174) > 1 cm, c-TC (n=168) 51. 2 mos. 33. 5 mos. HR 0. 75 (0. 57 -0. 97), P = 0. 0267 Interaction: P = 0. 925
OS: by residual disease Katsumata et al ASCO 2012 Patients surviving (%) Median OS < 1 cm, dd-TC (n=144) not reached < 1 cm, c-TC (n=145) not reached HR 0. 76 (0. 49 -1. 19), P = 0. 234 Median OS > 1 cm, dd-TC (n=174) > 1 cm, c-TC (n=168) 51. 2 mos. 33. 5 mos. HR 0. 75 (0. 57 -0. 97), P = 0. 0267 Interaction: P = 0. 925
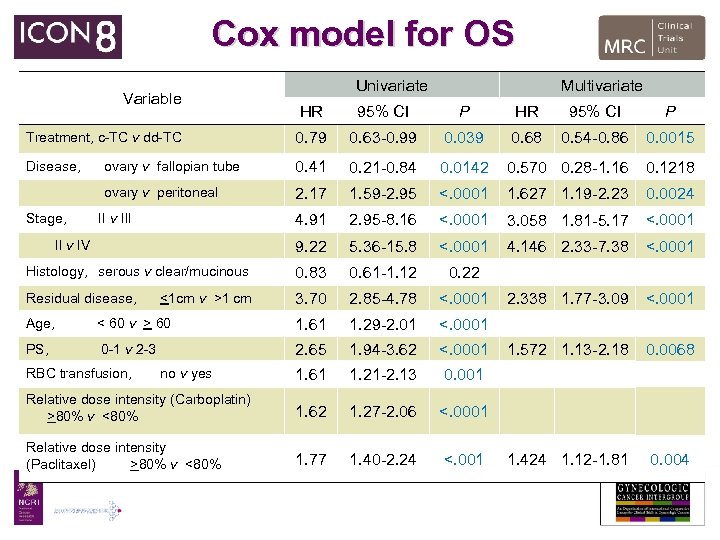 Cox model for OS Variable Univariate Multivariate HR 95% CI P Treatment, c-TC v dd-TC 0. 79 0. 63 -0. 99 0. 039 0. 68 0. 54 -0. 86 0. 0015 Disease, ovary v fallopian tube 0. 41 0. 21 -0. 84 0. 0142 0. 570 0. 28 -1. 16 0. 1218 ovary v peritoneal 2. 17 1. 59 -2. 95 <. 0001 1. 627 1. 19 -2. 23 0. 0024 4. 91 2. 95 -8. 16 <. 0001 3. 058 1. 81 -5. 17 <. 0001 9. 22 5. 36 -15. 8 <. 0001 4. 146 2. 33 -7. 38 <. 0001 Histology, serous v clear/mucinous 0. 83 0. 61 -1. 12 0. 22 Residual disease, 3. 70 2. 85 -4. 78 <. 0001 2. 338 1. 77 -3. 09 <. 0001 1. 572 1. 13 -2. 18 0. 0068 1. 424 1. 12 -1. 81 0. 004 Stage, II v III II v IV <1 cm v >1 cm Age, < 60 v > 60 1. 61 1. 29 -2. 01 <. 0001 PS, 0 -1 v 2 -3 2. 65 1. 94 -3. 62 <. 0001 1. 61 1. 21 -2. 13 0. 001 Relative dose intensity (Carboplatin) >80% v <80% 1. 62 1. 27 -2. 06 <. 0001 Relative dose intensity (Paclitaxel) >80% v <80% 1. 77 1. 40 -2. 24 <. 001 RBC transfusion, no v yes
Cox model for OS Variable Univariate Multivariate HR 95% CI P Treatment, c-TC v dd-TC 0. 79 0. 63 -0. 99 0. 039 0. 68 0. 54 -0. 86 0. 0015 Disease, ovary v fallopian tube 0. 41 0. 21 -0. 84 0. 0142 0. 570 0. 28 -1. 16 0. 1218 ovary v peritoneal 2. 17 1. 59 -2. 95 <. 0001 1. 627 1. 19 -2. 23 0. 0024 4. 91 2. 95 -8. 16 <. 0001 3. 058 1. 81 -5. 17 <. 0001 9. 22 5. 36 -15. 8 <. 0001 4. 146 2. 33 -7. 38 <. 0001 Histology, serous v clear/mucinous 0. 83 0. 61 -1. 12 0. 22 Residual disease, 3. 70 2. 85 -4. 78 <. 0001 2. 338 1. 77 -3. 09 <. 0001 1. 572 1. 13 -2. 18 0. 0068 1. 424 1. 12 -1. 81 0. 004 Stage, II v III II v IV <1 cm v >1 cm Age, < 60 v > 60 1. 61 1. 29 -2. 01 <. 0001 PS, 0 -1 v 2 -3 2. 65 1. 94 -3. 62 <. 0001 1. 61 1. 21 -2. 13 0. 001 Relative dose intensity (Carboplatin) >80% v <80% 1. 62 1. 27 -2. 06 <. 0001 Relative dose intensity (Paclitaxel) >80% v <80% 1. 77 1. 40 -2. 24 <. 001 RBC transfusion, no v yes
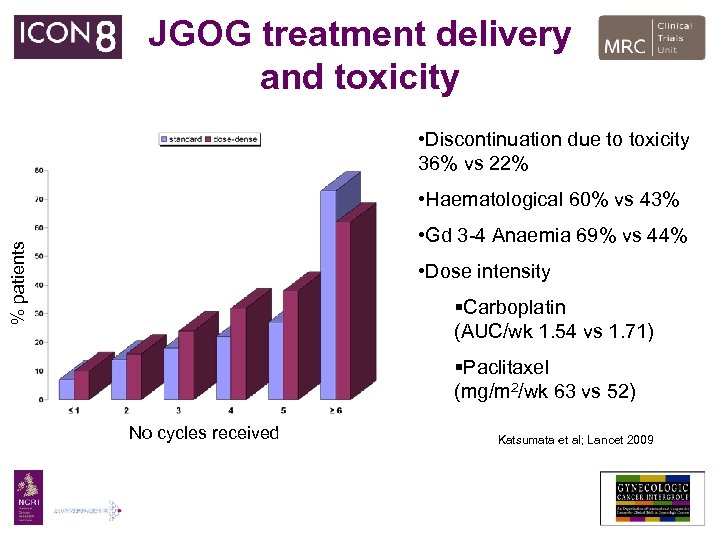 JGOG treatment delivery and toxicity • Discontinuation due to toxicity 36% vs 22% • Haematological 60% vs 43% % patients • Gd 3 -4 Anaemia 69% vs 44% • Dose intensity §Carboplatin (AUC/wk 1. 54 vs 1. 71) §Paclitaxel (mg/m 2/wk 63 vs 52) No cycles received Katsumata et al; Lancet 2009
JGOG treatment delivery and toxicity • Discontinuation due to toxicity 36% vs 22% • Haematological 60% vs 43% % patients • Gd 3 -4 Anaemia 69% vs 44% • Dose intensity §Carboplatin (AUC/wk 1. 54 vs 1. 71) §Paclitaxel (mg/m 2/wk 63 vs 52) No cycles received Katsumata et al; Lancet 2009
 Pharmacogenomics • Delivery of carboplatin- paclitaxel associated with more toxicity in Japanese population – Completion rate 6 cycles >85% in European trials • Lung cancer data – – – Parallel NSCLC phase III trials US/Japan Common CT control arm Improved survival outcomes in Japan Greater haematological toxicity Association of ethnically- distributed PG SNPs (CYP 3 A 4*1 B/ ERCC 2 K 751 Q) with survival and toxicity Gandara et al J Clin Oncol 2009
Pharmacogenomics • Delivery of carboplatin- paclitaxel associated with more toxicity in Japanese population – Completion rate 6 cycles >85% in European trials • Lung cancer data – – – Parallel NSCLC phase III trials US/Japan Common CT control arm Improved survival outcomes in Japan Greater haematological toxicity Association of ethnically- distributed PG SNPs (CYP 3 A 4*1 B/ ERCC 2 K 751 Q) with survival and toxicity Gandara et al J Clin Oncol 2009
 Weekly carboplatin- paclitaxel • reduce myelosuppression • improve tolerability • allow delivery of increased dose intensity • incorporate dose-dense platinum
Weekly carboplatin- paclitaxel • reduce myelosuppression • improve tolerability • allow delivery of increased dose intensity • incorporate dose-dense platinum
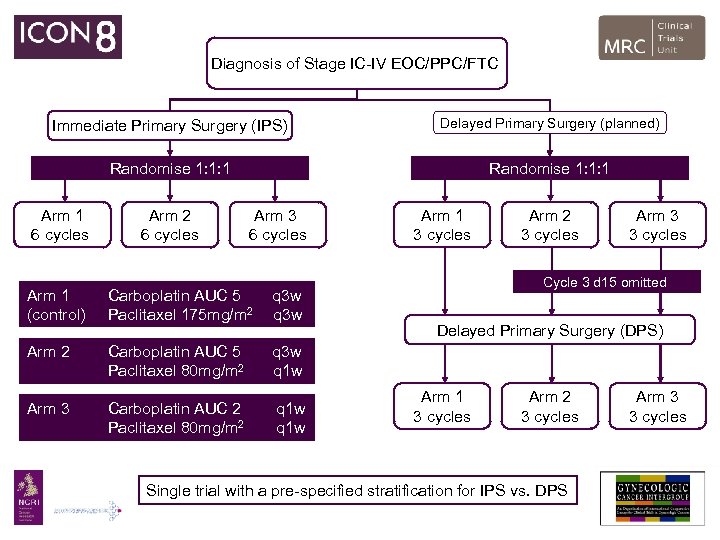 Diagnosis of Stage IC-IV EOC/PPC/FTC Immediate Primary Surgery (IPS) Delayed Primary Surgery (planned) Randomise 1: 1: 1 Arm 1 6 cycles Arm 2 6 cycles Arm 3 6 cycles Arm 1 (control) Carboplatin AUC 5 Paclitaxel 175 mg/m 2 q 3 w Arm 2 Carboplatin AUC 5 Paclitaxel 80 mg/m 2 Carboplatin AUC 2 Paclitaxel 80 mg/m 2 q 1 w Arm 2 3 cycles Arm 3 3 cycles Cycle 3 d 15 omitted q 3 w q 1 w Arm 3 Arm 1 3 cycles Delayed Primary Surgery (DPS) Arm 1 3 cycles Arm 2 3 cycles Single trial with a pre-specified stratification for IPS vs. DPS Arm 3 3 cycles
Diagnosis of Stage IC-IV EOC/PPC/FTC Immediate Primary Surgery (IPS) Delayed Primary Surgery (planned) Randomise 1: 1: 1 Arm 1 6 cycles Arm 2 6 cycles Arm 3 6 cycles Arm 1 (control) Carboplatin AUC 5 Paclitaxel 175 mg/m 2 q 3 w Arm 2 Carboplatin AUC 5 Paclitaxel 80 mg/m 2 Carboplatin AUC 2 Paclitaxel 80 mg/m 2 q 1 w Arm 2 3 cycles Arm 3 3 cycles Cycle 3 d 15 omitted q 3 w q 1 w Arm 3 Arm 1 3 cycles Delayed Primary Surgery (DPS) Arm 1 3 cycles Arm 2 3 cycles Single trial with a pre-specified stratification for IPS vs. DPS Arm 3 3 cycles
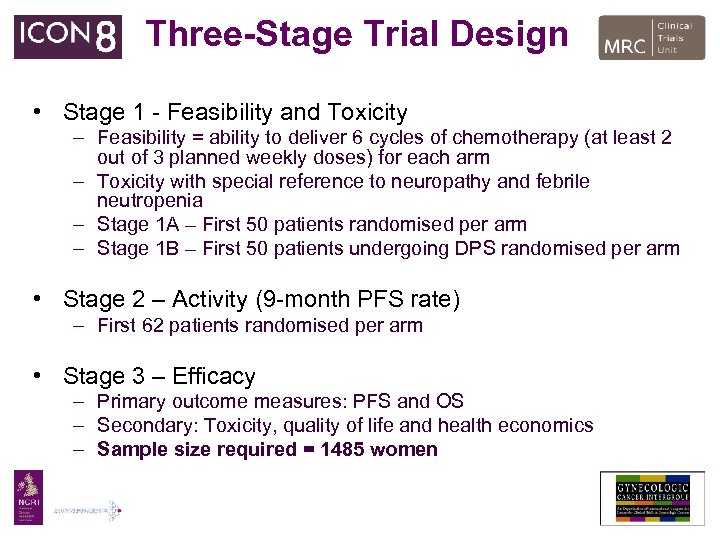 Three-Stage Trial Design • Stage 1 - Feasibility and Toxicity – Feasibility = ability to deliver 6 cycles of chemotherapy (at least 2 out of 3 planned weekly doses) for each arm – Toxicity with special reference to neuropathy and febrile neutropenia – Stage 1 A – First 50 patients randomised per arm – Stage 1 B – First 50 patients undergoing DPS randomised per arm • Stage 2 – Activity (9 -month PFS rate) – First 62 patients randomised per arm • Stage 3 – Efficacy – Primary outcome measures: PFS and OS – Secondary: Toxicity, quality of life and health economics – Sample size required = 1485 women
Three-Stage Trial Design • Stage 1 - Feasibility and Toxicity – Feasibility = ability to deliver 6 cycles of chemotherapy (at least 2 out of 3 planned weekly doses) for each arm – Toxicity with special reference to neuropathy and febrile neutropenia – Stage 1 A – First 50 patients randomised per arm – Stage 1 B – First 50 patients undergoing DPS randomised per arm • Stage 2 – Activity (9 -month PFS rate) – First 62 patients randomised per arm • Stage 3 – Efficacy – Primary outcome measures: PFS and OS – Secondary: Toxicity, quality of life and health economics – Sample size required = 1485 women
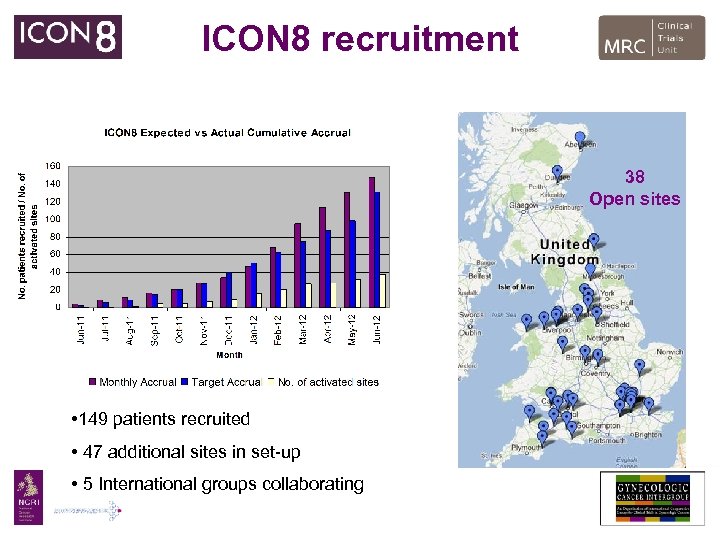 ICON 8 recruitment 38 Open sites • 149 patients recruited • 47 additional sites in set-up • 5 International groups collaborating
ICON 8 recruitment 38 Open sites • 149 patients recruited • 47 additional sites in set-up • 5 International groups collaborating
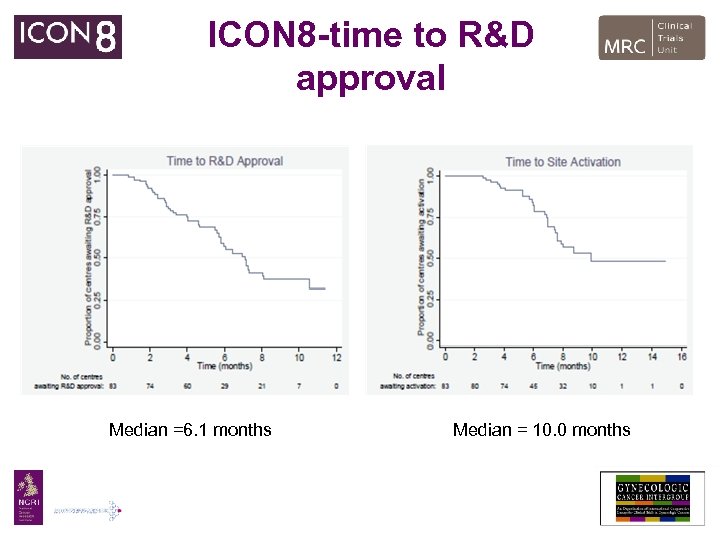 ICON 8 -time to R&D approval Median =6. 1 months Median = 10. 0 months
ICON 8 -time to R&D approval Median =6. 1 months Median = 10. 0 months
 Is ICON 8 still valid in the era of bevacizumab?
Is ICON 8 still valid in the era of bevacizumab?
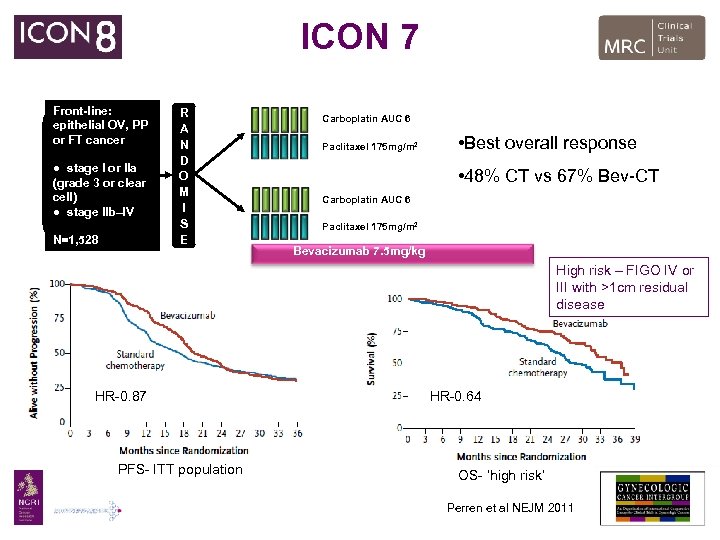 ICON 7 Front-line: epithelial OV, PP or FT cancer ● stage I or IIa (grade 3 or clear cell) ● stage IIb–IV N=1, 528 R A N D O M I S E Carboplatin AUC 6 Paclitaxel 175 mg/m 2 • Best overall response • 48% CT vs 67% Bev-CT Carboplatin AUC 6 1: 1 Paclitaxel 175 mg/m 2 Bevacizumab 7. 5 mg/kg High risk – FIGO IV or III with >1 cm residual disease HR-0. 87 PFS- ITT population HR-0. 64 OS- ‘high risk’ Perren et al NEJM 2011
ICON 7 Front-line: epithelial OV, PP or FT cancer ● stage I or IIa (grade 3 or clear cell) ● stage IIb–IV N=1, 528 R A N D O M I S E Carboplatin AUC 6 Paclitaxel 175 mg/m 2 • Best overall response • 48% CT vs 67% Bev-CT Carboplatin AUC 6 1: 1 Paclitaxel 175 mg/m 2 Bevacizumab 7. 5 mg/kg High risk – FIGO IV or III with >1 cm residual disease HR-0. 87 PFS- ITT population HR-0. 64 OS- ‘high risk’ Perren et al NEJM 2011
 Bevacizumab • Carboplatin-paclitaxel + bevacizumab is becoming a standard of care for “high-risk” ovarian cancer following publication of GOG 218/ICON 7 PFS and interim results – ICON 7 final OS analysis expected 2013 • Bevacizumab is now licensed for the treatment of Stage IIIBIV ovarian cancer in combination with carboplatin/paclitaxel in Europe and is available in England via CDF for “high-risk” disease – Not available for collaborating groups or in Scotland/ Wales
Bevacizumab • Carboplatin-paclitaxel + bevacizumab is becoming a standard of care for “high-risk” ovarian cancer following publication of GOG 218/ICON 7 PFS and interim results – ICON 7 final OS analysis expected 2013 • Bevacizumab is now licensed for the treatment of Stage IIIBIV ovarian cancer in combination with carboplatin/paclitaxel in Europe and is available in England via CDF for “high-risk” disease – Not available for collaborating groups or in Scotland/ Wales
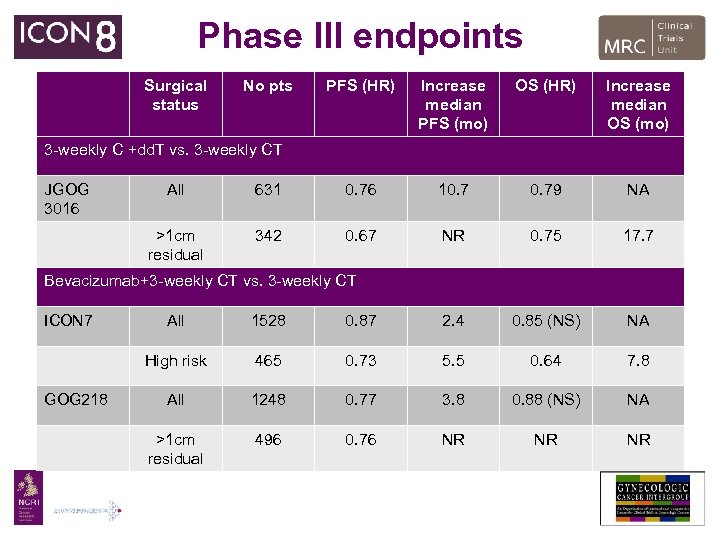 Phase III endpoints Surgical status No pts PFS (HR) Increase median PFS (mo) OS (HR) Increase median OS (mo) 3 -weekly C +dd. T vs. 3 -weekly CT JGOG 3016 All 631 0. 76 10. 79 NA >1 cm residual 342 0. 67 NR 0. 75 17. 7 Bevacizumab+3 -weekly CT vs. 3 -weekly CT ICON 7 1528 0. 87 2. 4 0. 85 (NS) NA High risk GOG 218 All 465 0. 73 5. 5 0. 64 7. 8 All 1248 0. 77 3. 8 0. 88 (NS) NA >1 cm residual 496 0. 76 NR NR NR
Phase III endpoints Surgical status No pts PFS (HR) Increase median PFS (mo) OS (HR) Increase median OS (mo) 3 -weekly C +dd. T vs. 3 -weekly CT JGOG 3016 All 631 0. 76 10. 79 NA >1 cm residual 342 0. 67 NR 0. 75 17. 7 Bevacizumab+3 -weekly CT vs. 3 -weekly CT ICON 7 1528 0. 87 2. 4 0. 85 (NS) NA High risk GOG 218 All 465 0. 73 5. 5 0. 64 7. 8 All 1248 0. 77 3. 8 0. 88 (NS) NA >1 cm residual 496 0. 76 NR NR NR
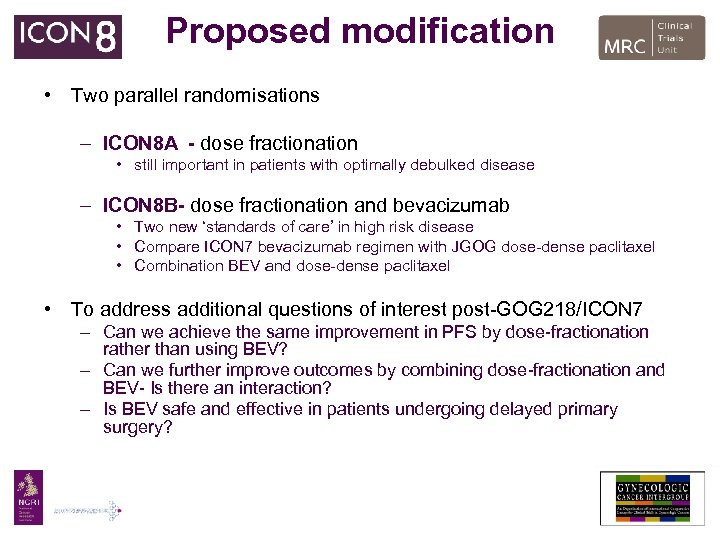 Proposed modification • Two parallel randomisations – ICON 8 A - dose fractionation • still important in patients with optimally debulked disease – ICON 8 B- dose fractionation and bevacizumab • Two new ‘standards of care’ in high risk disease • Compare ICON 7 bevacizumab regimen with JGOG dose-dense paclitaxel • Combination BEV and dose-dense paclitaxel • To address additional questions of interest post-GOG 218/ICON 7 – Can we achieve the same improvement in PFS by dose-fractionation rather than using BEV? – Can we further improve outcomes by combining dose-fractionation and BEV- Is there an interaction? – Is BEV safe and effective in patients undergoing delayed primary surgery?
Proposed modification • Two parallel randomisations – ICON 8 A - dose fractionation • still important in patients with optimally debulked disease – ICON 8 B- dose fractionation and bevacizumab • Two new ‘standards of care’ in high risk disease • Compare ICON 7 bevacizumab regimen with JGOG dose-dense paclitaxel • Combination BEV and dose-dense paclitaxel • To address additional questions of interest post-GOG 218/ICON 7 – Can we achieve the same improvement in PFS by dose-fractionation rather than using BEV? – Can we further improve outcomes by combining dose-fractionation and BEV- Is there an interaction? – Is BEV safe and effective in patients undergoing delayed primary surgery?
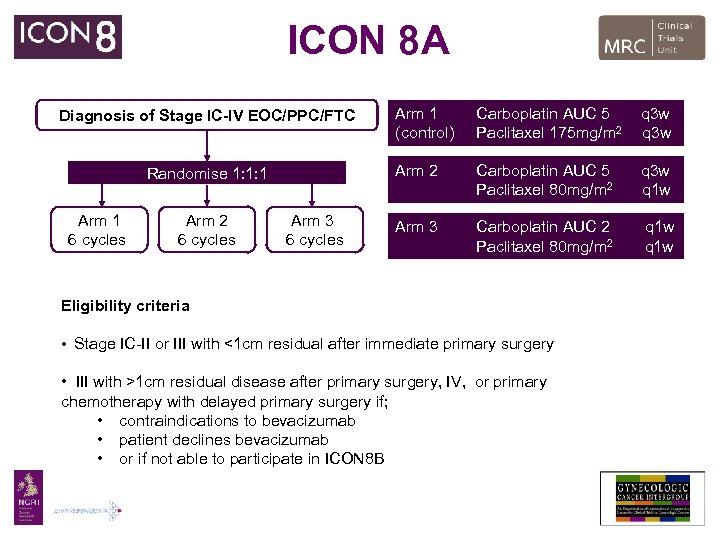 ICON 8 A Randomise 1: 1: 1 Arm 1 6 cycles Arm 2 6 cycles Arm 3 6 cycles Arm 1 (control) Carboplatin AUC 5 Paclitaxel 175 mg/m 2 q 3 w Arm 2 Diagnosis of Stage IC-IV EOC/PPC/FTC Carboplatin AUC 5 Paclitaxel 80 mg/m 2 q 3 w q 1 w Arm 3 Carboplatin AUC 2 Paclitaxel 80 mg/m 2 q 1 w Eligibility criteria • Stage IC-II or III with <1 cm residual after immediate primary surgery • III with >1 cm residual disease after primary surgery, IV, or primary chemotherapy with delayed primary surgery if; • contraindications to bevacizumab • patient declines bevacizumab • or if not able to participate in ICON 8 B
ICON 8 A Randomise 1: 1: 1 Arm 1 6 cycles Arm 2 6 cycles Arm 3 6 cycles Arm 1 (control) Carboplatin AUC 5 Paclitaxel 175 mg/m 2 q 3 w Arm 2 Diagnosis of Stage IC-IV EOC/PPC/FTC Carboplatin AUC 5 Paclitaxel 80 mg/m 2 q 3 w q 1 w Arm 3 Carboplatin AUC 2 Paclitaxel 80 mg/m 2 q 1 w Eligibility criteria • Stage IC-II or III with <1 cm residual after immediate primary surgery • III with >1 cm residual disease after primary surgery, IV, or primary chemotherapy with delayed primary surgery if; • contraindications to bevacizumab • patient declines bevacizumab • or if not able to participate in ICON 8 B
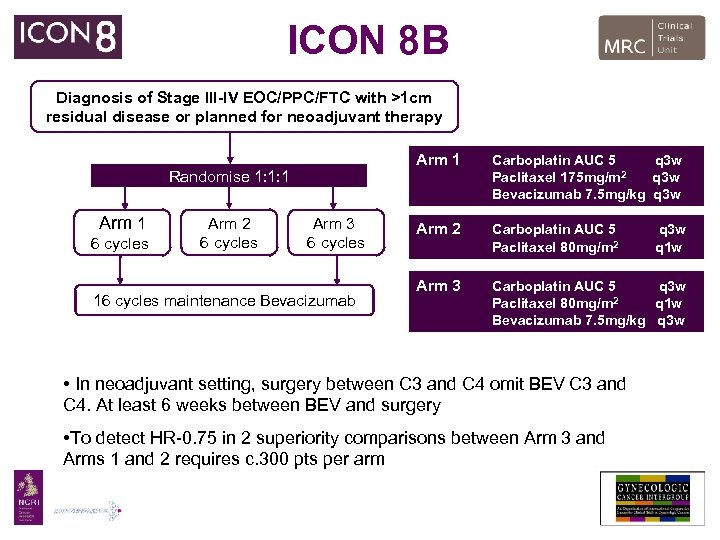 ICON 8 B Diagnosis of Stage III-IV EOC/PPC/FTC with >1 cm residual disease or planned for neoadjuvant therapy Arm 1 Randomise 1: 1: 1 Arm 1 6 cycles Arm 2 6 cycles Arm 3 6 cycles 16 cycles maintenance Bevacizumab Carboplatin AUC 5 q 3 w 2 Paclitaxel 175 mg/m q 3 w Bevacizumab 7. 5 mg/kg q 3 w Arm 2 Carboplatin AUC 5 Paclitaxel 80 mg/m 2 Arm 3 Carboplatin AUC 5 q 3 w 2 Paclitaxel 80 mg/m q 1 w Bevacizumab 7. 5 mg/kg q 3 w • In neoadjuvant setting, surgery between C 3 and C 4 omit BEV C 3 and C 4. At least 6 weeks between BEV and surgery • To detect HR-0. 75 in 2 superiority comparisons between Arm 3 and Arms 1 and 2 requires c. 300 pts per arm q 3 w q 1 w
ICON 8 B Diagnosis of Stage III-IV EOC/PPC/FTC with >1 cm residual disease or planned for neoadjuvant therapy Arm 1 Randomise 1: 1: 1 Arm 1 6 cycles Arm 2 6 cycles Arm 3 6 cycles 16 cycles maintenance Bevacizumab Carboplatin AUC 5 q 3 w 2 Paclitaxel 175 mg/m q 3 w Bevacizumab 7. 5 mg/kg q 3 w Arm 2 Carboplatin AUC 5 Paclitaxel 80 mg/m 2 Arm 3 Carboplatin AUC 5 q 3 w 2 Paclitaxel 80 mg/m q 1 w Bevacizumab 7. 5 mg/kg q 3 w • In neoadjuvant setting, surgery between C 3 and C 4 omit BEV C 3 and C 4. At least 6 weeks between BEV and surgery • To detect HR-0. 75 in 2 superiority comparisons between Arm 3 and Arms 1 and 2 requires c. 300 pts per arm q 3 w q 1 w
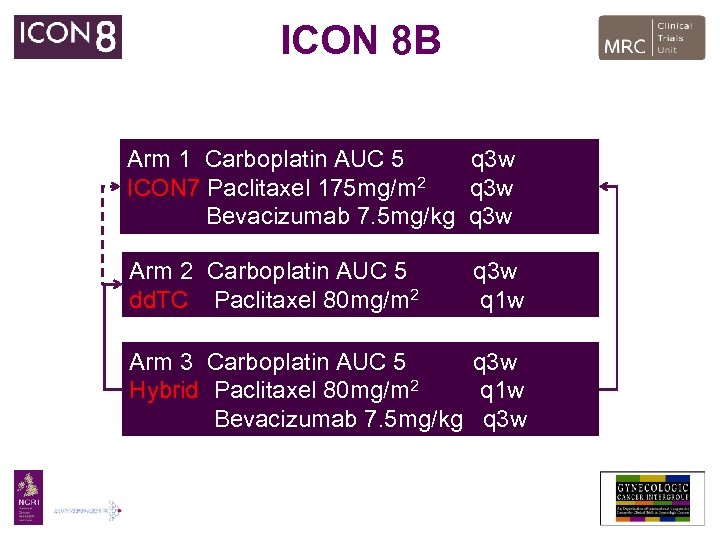 ICON 8 B Arm 1 Carboplatin AUC 5 q 3 w ICON 7 Paclitaxel 175 mg/m 2 q 3 w Bevacizumab 7. 5 mg/kg q 3 w Arm 2 Carboplatin AUC 5 dd. TC Paclitaxel 80 mg/m 2 q 3 w q 1 w Arm 3 Carboplatin AUC 5 q 3 w Hybrid Paclitaxel 80 mg/m 2 q 1 w Bevacizumab 7. 5 mg/kg q 3 w
ICON 8 B Arm 1 Carboplatin AUC 5 q 3 w ICON 7 Paclitaxel 175 mg/m 2 q 3 w Bevacizumab 7. 5 mg/kg q 3 w Arm 2 Carboplatin AUC 5 dd. TC Paclitaxel 80 mg/m 2 q 3 w q 1 w Arm 3 Carboplatin AUC 5 q 3 w Hybrid Paclitaxel 80 mg/m 2 q 1 w Bevacizumab 7. 5 mg/kg q 3 w
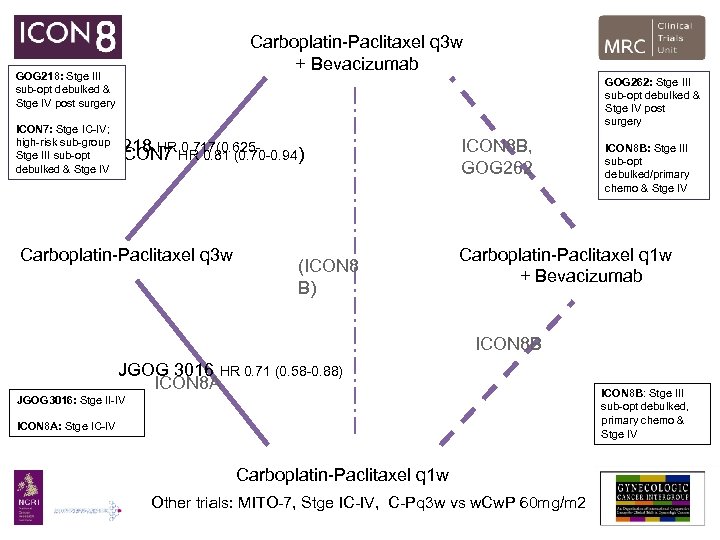 Carboplatin-Paclitaxel q 3 w + Bevacizumab GOG 218: Stge III sub-opt debulked & Stge IV post surgery GOG 262: Stge III sub-opt debulked & Stge IV post surgery ICON 7: Stge IC-IV; high-risk sub-group Stge III sub-opt 0. 824) debulked & Stge IV GOG 218 HR HR 0. 81 (0. 70 -0. 94) ICON 7 0. 717(0. 625 - Carboplatin-Paclitaxel q 3 w (ICON 8 B) ICON 8 B, GOG 262 ICON 8 B: Stge III sub-opt debulked/primary chemo & Stge IV Carboplatin-Paclitaxel q 1 w + Bevacizumab ICON 8 B JGOG 3016 HR 0. 71 (0. 58 -0. 88) ICON 8 A JGOG 3016: Stge II-IV ICON 8 A: Stge IC-IV Carboplatin-Paclitaxel q 1 w Other trials: MITO-7, Stge IC-IV, C-Pq 3 w vs w. Cw. P 60 mg/m 2 ICON 8 B: Stge III sub-opt debulked, primary chemo & Stge IV
Carboplatin-Paclitaxel q 3 w + Bevacizumab GOG 218: Stge III sub-opt debulked & Stge IV post surgery GOG 262: Stge III sub-opt debulked & Stge IV post surgery ICON 7: Stge IC-IV; high-risk sub-group Stge III sub-opt 0. 824) debulked & Stge IV GOG 218 HR HR 0. 81 (0. 70 -0. 94) ICON 7 0. 717(0. 625 - Carboplatin-Paclitaxel q 3 w (ICON 8 B) ICON 8 B, GOG 262 ICON 8 B: Stge III sub-opt debulked/primary chemo & Stge IV Carboplatin-Paclitaxel q 1 w + Bevacizumab ICON 8 B JGOG 3016 HR 0. 71 (0. 58 -0. 88) ICON 8 A JGOG 3016: Stge II-IV ICON 8 A: Stge IC-IV Carboplatin-Paclitaxel q 1 w Other trials: MITO-7, Stge IC-IV, C-Pq 3 w vs w. Cw. P 60 mg/m 2 ICON 8 B: Stge III sub-opt debulked, primary chemo & Stge IV
 Summary • ICON 8 remains open to recruitment and currently meeting target • Bevacizumab will be incorporated if secure funding available • TRICON 8 sample collection (Brenton) awaiting outcome of CTAAC review
Summary • ICON 8 remains open to recruitment and currently meeting target • Bevacizumab will be incorporated if secure funding available • TRICON 8 sample collection (Brenton) awaiting outcome of CTAAC review
 Feedback welcome • Chief Investigators – Andrew Clamp – Jonathan Ledermann • Trials Unit – – – Jane Hook Laura Farrelly Monique Tomiczek Cheryl Courtney Tim Brush Suzanne Freeman andrew. clamp@christie. nhs. uk j. ledermann@ctc. ucl. ac. uk ICON 8@ctu. mrc. ac. uk Trial Physician/CTU Project Lead Project Manager Trial Manager Senior Data Manager Statistician
Feedback welcome • Chief Investigators – Andrew Clamp – Jonathan Ledermann • Trials Unit – – – Jane Hook Laura Farrelly Monique Tomiczek Cheryl Courtney Tim Brush Suzanne Freeman andrew. clamp@christie. nhs. uk j. ledermann@ctc. ucl. ac. uk ICON 8@ctu. mrc. ac. uk Trial Physician/CTU Project Lead Project Manager Trial Manager Senior Data Manager Statistician


