d99ed5ca0c4f09a06675155932e9e4ba.ppt
- Количество слайдов: 24
 ICCS e-Newsletter CSI Jonathan Fromm, MD, Ph. D University of Washington Seattle, WA USA
ICCS e-Newsletter CSI Jonathan Fromm, MD, Ph. D University of Washington Seattle, WA USA
 History and physical examination • The patient is a 24 -year-old woman presenting with shortness of breath (of uncertain duration). She reports episodes of chills but denies fevers, night sweats, and weight loss. • Physical exam is unremarkable. • Past medical history is remarkable for an episode of thrombocytopenia in the setting of sepsis.
History and physical examination • The patient is a 24 -year-old woman presenting with shortness of breath (of uncertain duration). She reports episodes of chills but denies fevers, night sweats, and weight loss. • Physical exam is unremarkable. • Past medical history is remarkable for an episode of thrombocytopenia in the setting of sepsis.
 Radiographic studies • Chest CT demonstrates an anterior mediastinal mass measuring 9. 2 x 6. 8 x 5. 7 cm. • Additional abdomen and pelvis CT was negative for lymphadenopathy or organomegaly.
Radiographic studies • Chest CT demonstrates an anterior mediastinal mass measuring 9. 2 x 6. 8 x 5. 7 cm. • Additional abdomen and pelvis CT was negative for lymphadenopathy or organomegaly.
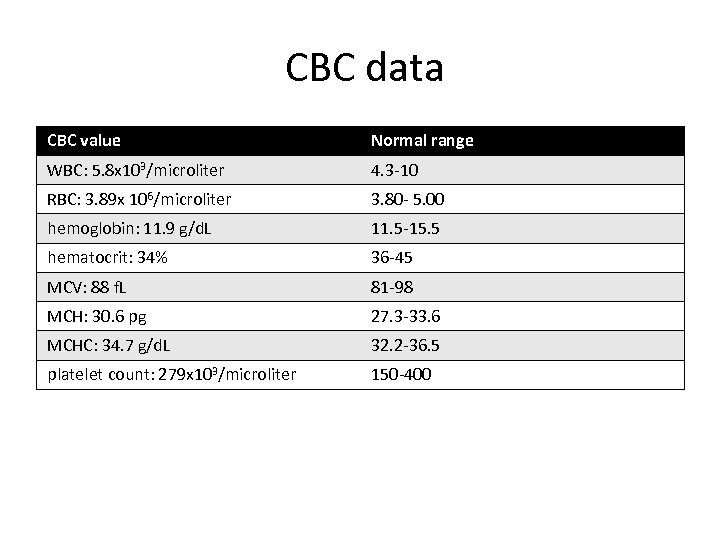 CBC data CBC value Normal range WBC: 5. 8 x 103/microliter 4. 3 -10 RBC: 3. 89 x 106/microliter 3. 80 - 5. 00 hemoglobin: 11. 9 g/d. L 11. 5 -15. 5 hematocrit: 34% 36 -45 MCV: 88 f. L 81 -98 MCH: 30. 6 pg 27. 3 -33. 6 MCHC: 34. 7 g/d. L 32. 2 -36. 5 platelet count: 279 x 103/microliter 150 -400
CBC data CBC value Normal range WBC: 5. 8 x 103/microliter 4. 3 -10 RBC: 3. 89 x 106/microliter 3. 80 - 5. 00 hemoglobin: 11. 9 g/d. L 11. 5 -15. 5 hematocrit: 34% 36 -45 MCV: 88 f. L 81 -98 MCH: 30. 6 pg 27. 3 -33. 6 MCHC: 34. 7 g/d. L 32. 2 -36. 5 platelet count: 279 x 103/microliter 150 -400
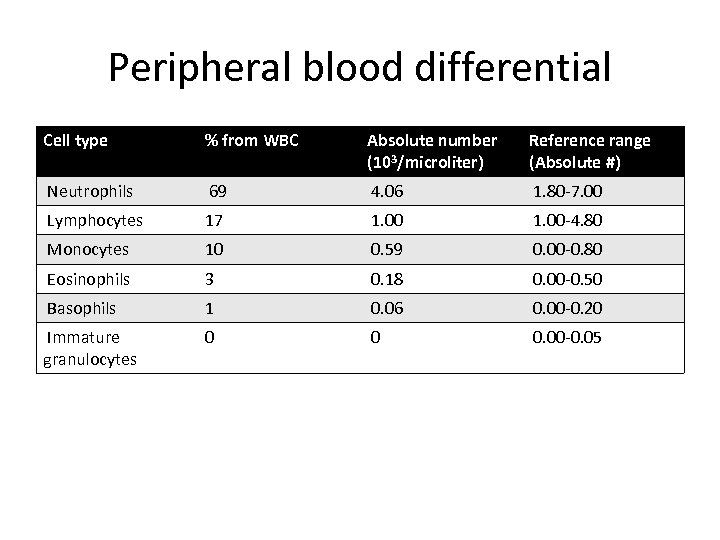 Peripheral blood differential Cell type % from WBC Absolute number (103/microliter) Reference range (Absolute #) Neutrophils 69 4. 06 1. 80 -7. 00 Lymphocytes 17 1. 00 -4. 80 Monocytes 10 0. 59 0. 00 -0. 80 Eosinophils 3 0. 18 0. 00 -0. 50 Basophils 1 0. 06 0. 00 -0. 20 Immature granulocytes 0 0 0. 00 -0. 05
Peripheral blood differential Cell type % from WBC Absolute number (103/microliter) Reference range (Absolute #) Neutrophils 69 4. 06 1. 80 -7. 00 Lymphocytes 17 1. 00 -4. 80 Monocytes 10 0. 59 0. 00 -0. 80 Eosinophils 3 0. 18 0. 00 -0. 50 Basophils 1 0. 06 0. 00 -0. 20 Immature granulocytes 0 0 0. 00 -0. 05
 Work-up and evaluation • The anterior mediastinal mass is biopsied. • Flow cytometric immunophenotyping was performed on this sample. An additional tube is added after reviewing the initial data.
Work-up and evaluation • The anterior mediastinal mass is biopsied. • Flow cytometric immunophenotyping was performed on this sample. An additional tube is added after reviewing the initial data.
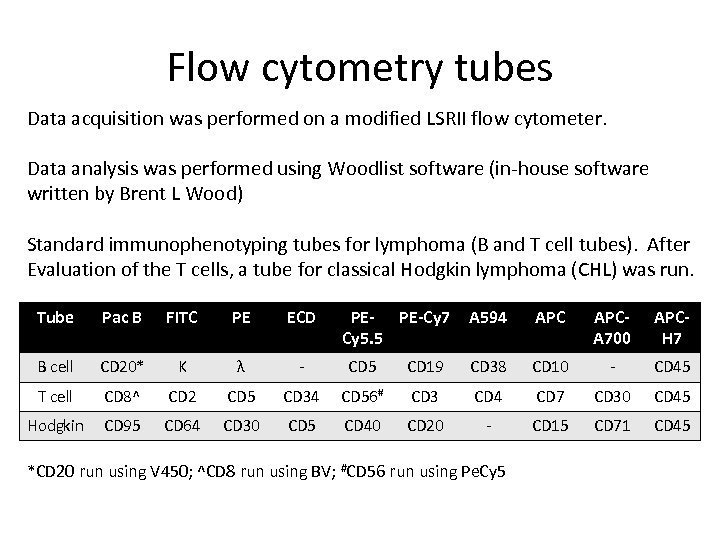 Flow cytometry tubes Data acquisition was performed on a modified LSRII flow cytometer. Data analysis was performed using Woodlist software (in-house software written by Brent L Wood) Standard immunophenotyping tubes for lymphoma (B and T cell tubes). After Evaluation of the T cells, a tube for classical Hodgkin lymphoma (CHL) was run. Tube Pac B FITC PE ECD PE-Cy 7 Cy 5. 5 A 594 APCA 700 APCH 7 B cell CD 20* Κ λ - CD 5 CD 19 CD 38 CD 10 - CD 45 T cell CD 8^ CD 2 CD 5 CD 34 CD 56# CD 3 CD 4 CD 7 CD 30 CD 45 Hodgkin CD 95 CD 64 CD 30 CD 5 CD 40 CD 20 - CD 15 CD 71 CD 45 *CD 20 run using V 450; ^CD 8 run using BV; #CD 56 run using Pe. Cy 5
Flow cytometry tubes Data acquisition was performed on a modified LSRII flow cytometer. Data analysis was performed using Woodlist software (in-house software written by Brent L Wood) Standard immunophenotyping tubes for lymphoma (B and T cell tubes). After Evaluation of the T cells, a tube for classical Hodgkin lymphoma (CHL) was run. Tube Pac B FITC PE ECD PE-Cy 7 Cy 5. 5 A 594 APCA 700 APCH 7 B cell CD 20* Κ λ - CD 5 CD 19 CD 38 CD 10 - CD 45 T cell CD 8^ CD 2 CD 5 CD 34 CD 56# CD 3 CD 4 CD 7 CD 30 CD 45 Hodgkin CD 95 CD 64 CD 30 CD 5 CD 40 CD 20 - CD 15 CD 71 CD 45 *CD 20 run using V 450; ^CD 8 run using BV; #CD 56 run using Pe. Cy 5
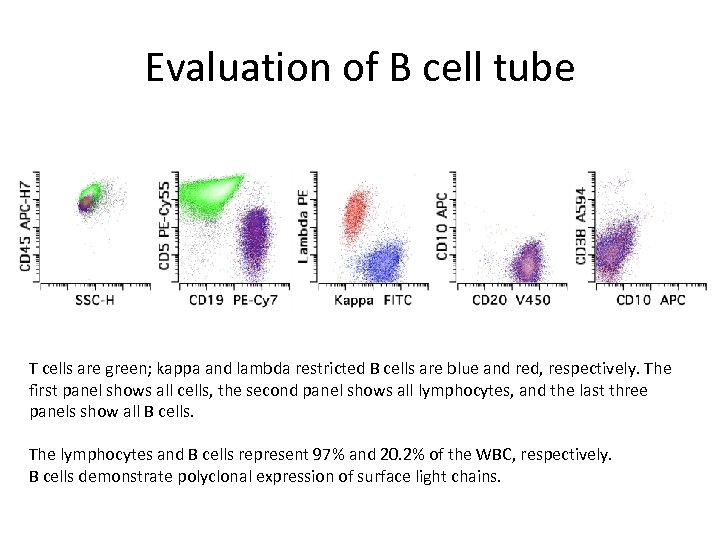 Evaluation of B cell tube T cells are green; kappa and lambda restricted B cells are blue and red, respectively. The first panel shows all cells, the second panel shows all lymphocytes, and the last three panels show all B cells. The lymphocytes and B cells represent 97% and 20. 2% of the WBC, respectively. B cells demonstrate polyclonal expression of surface light chains.
Evaluation of B cell tube T cells are green; kappa and lambda restricted B cells are blue and red, respectively. The first panel shows all cells, the second panel shows all lymphocytes, and the last three panels show all B cells. The lymphocytes and B cells represent 97% and 20. 2% of the WBC, respectively. B cells demonstrate polyclonal expression of surface light chains.
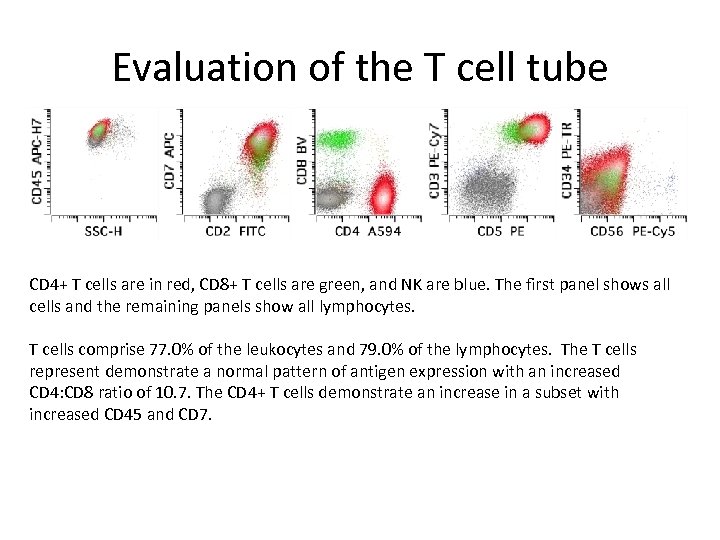 Evaluation of the T cell tube CD 4+ T cells are in red, CD 8+ T cells are green, and NK are blue. The first panel shows all cells and the remaining panels show all lymphocytes. T cells comprise 77. 0% of the leukocytes and 79. 0% of the lymphocytes. The T cells represent demonstrate a normal pattern of antigen expression with an increased CD 4: CD 8 ratio of 10. 7. The CD 4+ T cells demonstrate an increase in a subset with increased CD 45 and CD 7.
Evaluation of the T cell tube CD 4+ T cells are in red, CD 8+ T cells are green, and NK are blue. The first panel shows all cells and the remaining panels show all lymphocytes. T cells comprise 77. 0% of the leukocytes and 79. 0% of the lymphocytes. The T cells represent demonstrate a normal pattern of antigen expression with an increased CD 4: CD 8 ratio of 10. 7. The CD 4+ T cells demonstrate an increase in a subset with increased CD 45 and CD 7.
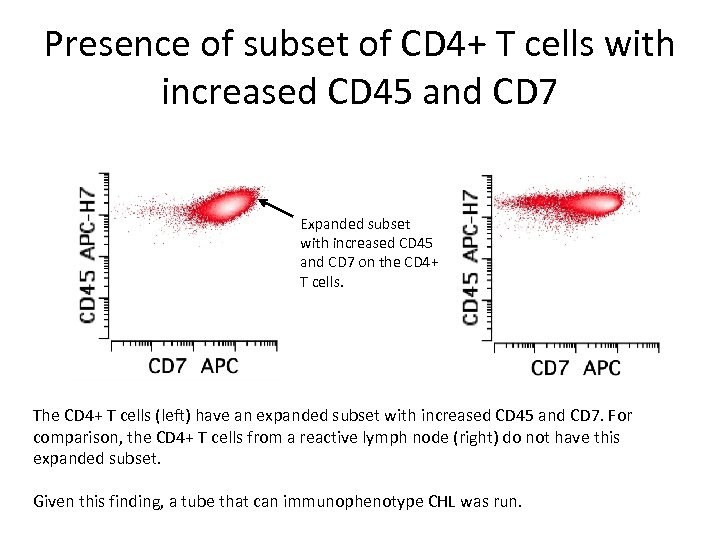 Presence of subset of CD 4+ T cells with increased CD 45 and CD 7 Expanded subset with increased CD 45 and CD 7 on the CD 4+ T cells. The CD 4+ T cells (left) have an expanded subset with increased CD 45 and CD 7. For comparison, the CD 4+ T cells from a reactive lymph node (right) do not have this expanded subset. Given this finding, a tube that can immunophenotype CHL was run.
Presence of subset of CD 4+ T cells with increased CD 45 and CD 7 Expanded subset with increased CD 45 and CD 7 on the CD 4+ T cells. The CD 4+ T cells (left) have an expanded subset with increased CD 45 and CD 7. For comparison, the CD 4+ T cells from a reactive lymph node (right) do not have this expanded subset. Given this finding, a tube that can immunophenotype CHL was run.
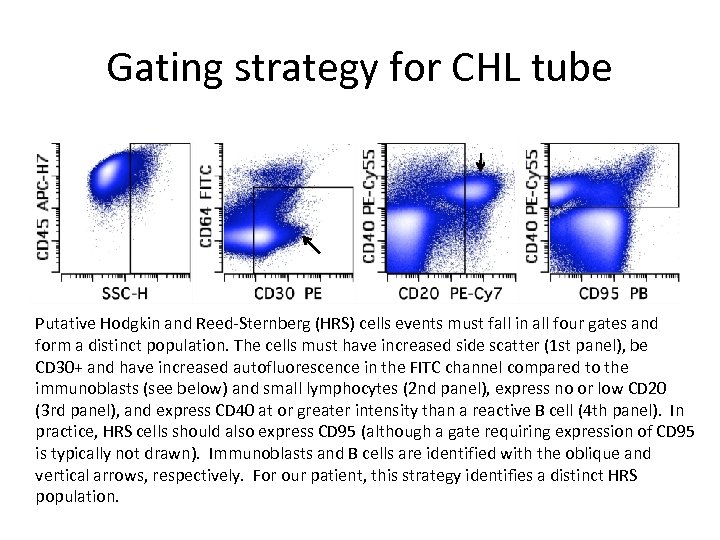 Gating strategy for CHL tube Putative Hodgkin and Reed-Sternberg (HRS) cells events must fall in all four gates and form a distinct population. The cells must have increased side scatter (1 st panel), be CD 30+ and have increased autofluorescence in the FITC channel compared to the immunoblasts (see below) and small lymphocytes (2 nd panel), express no or low CD 20 (3 rd panel), and express CD 40 at or greater intensity than a reactive B cell (4 th panel). In practice, HRS cells should also express CD 95 (although a gate requiring expression of CD 95 is typically not drawn). Immunoblasts and B cells are identified with the oblique and vertical arrows, respectively. For our patient, this strategy identifies a distinct HRS population.
Gating strategy for CHL tube Putative Hodgkin and Reed-Sternberg (HRS) cells events must fall in all four gates and form a distinct population. The cells must have increased side scatter (1 st panel), be CD 30+ and have increased autofluorescence in the FITC channel compared to the immunoblasts (see below) and small lymphocytes (2 nd panel), express no or low CD 20 (3 rd panel), and express CD 40 at or greater intensity than a reactive B cell (4 th panel). In practice, HRS cells should also express CD 95 (although a gate requiring expression of CD 95 is typically not drawn). Immunoblasts and B cells are identified with the oblique and vertical arrows, respectively. For our patient, this strategy identifies a distinct HRS population.
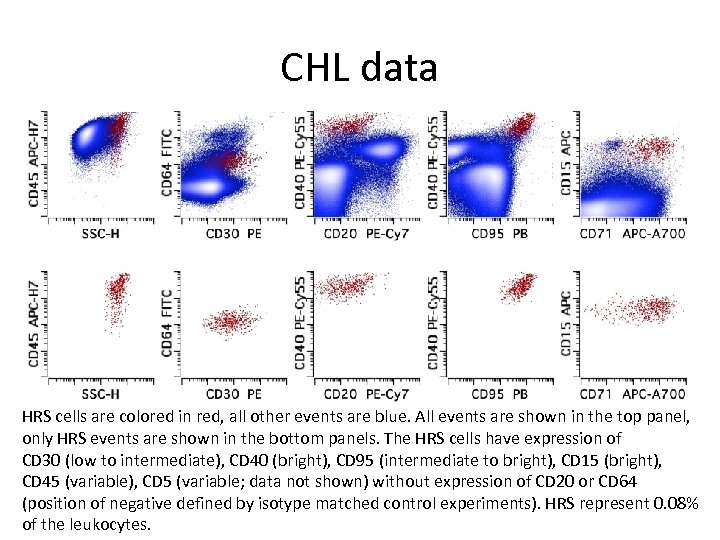 CHL data HRS cells are colored in red, all other events are blue. All events are shown in the top panel, only HRS events are shown in the bottom panels. The HRS cells have expression of CD 30 (low to intermediate), CD 40 (bright), CD 95 (intermediate to bright), CD 15 (bright), CD 45 (variable), CD 5 (variable; data not shown) without expression of CD 20 or CD 64 (position of negative defined by isotype matched control experiments). HRS represent 0. 08% of the leukocytes.
CHL data HRS cells are colored in red, all other events are blue. All events are shown in the top panel, only HRS events are shown in the bottom panels. The HRS cells have expression of CD 30 (low to intermediate), CD 40 (bright), CD 95 (intermediate to bright), CD 15 (bright), CD 45 (variable), CD 5 (variable; data not shown) without expression of CD 20 or CD 64 (position of negative defined by isotype matched control experiments). HRS represent 0. 08% of the leukocytes.
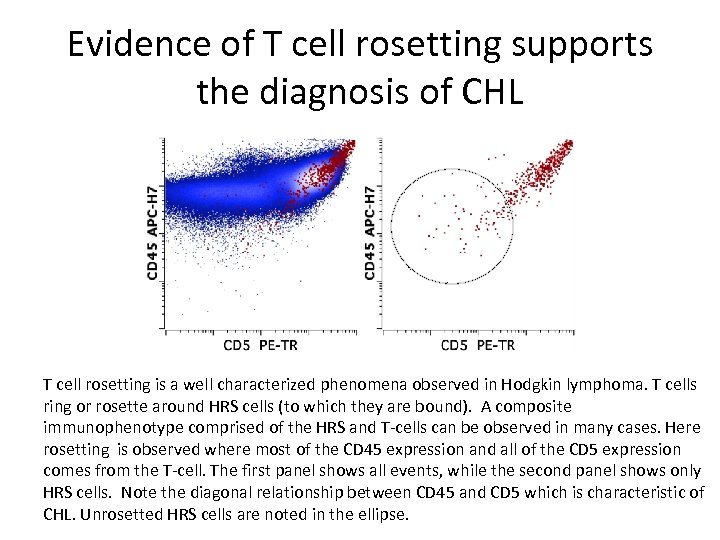 Evidence of T cell rosetting supports the diagnosis of CHL T cell rosetting is a well characterized phenomena observed in Hodgkin lymphoma. T cells ring or rosette around HRS cells (to which they are bound). A composite immunophenotype comprised of the HRS and T-cells can be observed in many cases. Here rosetting is observed where most of the CD 45 expression and all of the CD 5 expression comes from the T-cell. The first panel shows all events, while the second panel shows only HRS cells. Note the diagonal relationship between CD 45 and CD 5 which is characteristic of CHL. Unrosetted HRS cells are noted in the ellipse.
Evidence of T cell rosetting supports the diagnosis of CHL T cell rosetting is a well characterized phenomena observed in Hodgkin lymphoma. T cells ring or rosette around HRS cells (to which they are bound). A composite immunophenotype comprised of the HRS and T-cells can be observed in many cases. Here rosetting is observed where most of the CD 45 expression and all of the CD 5 expression comes from the T-cell. The first panel shows all events, while the second panel shows only HRS cells. Note the diagonal relationship between CD 45 and CD 5 which is characteristic of CHL. Unrosetted HRS cells are noted in the ellipse.
 Diagnosis? Classical Hodgkin lymphoma
Diagnosis? Classical Hodgkin lymphoma
 CHL • CHL is a unique type of B cell lymphoma where the neoplastic HRS cells usually make up less than 1% of the leukocytes. • The neoplastic cells are usually embedded in a reactive infiltrate including lymphocytes, eosinophils, plasma cells, and histiocytes. • Diagnosis historically confirmed by paraffin section immunophenotyping: the HRS cells are CD 30+, CD 15+, Pax-5+, CD 3 -, CD 20 -, CD 45 -.
CHL • CHL is a unique type of B cell lymphoma where the neoplastic HRS cells usually make up less than 1% of the leukocytes. • The neoplastic cells are usually embedded in a reactive infiltrate including lymphocytes, eosinophils, plasma cells, and histiocytes. • Diagnosis historically confirmed by paraffin section immunophenotyping: the HRS cells are CD 30+, CD 15+, Pax-5+, CD 3 -, CD 20 -, CD 45 -.
 CHL • The binding of T cells to HRS is characteristic of Hodgkin lymphoma, forming HRS-T cell rosetting. • This interaction is mediated by CD 54 and CD 58 on the HRS cells binding to LFA-1 and CD 2 on the T cells, resulting in the composite immunophenotype observed by flow cytometry. • The degree of rosetting varies between cases. In our case, many of the events have brighter CD 45 (and CD 5) than the T cells, suggesting more than 1 T cell bound to each HRS cell.
CHL • The binding of T cells to HRS is characteristic of Hodgkin lymphoma, forming HRS-T cell rosetting. • This interaction is mediated by CD 54 and CD 58 on the HRS cells binding to LFA-1 and CD 2 on the T cells, resulting in the composite immunophenotype observed by flow cytometry. • The degree of rosetting varies between cases. In our case, many of the events have brighter CD 45 (and CD 5) than the T cells, suggesting more than 1 T cell bound to each HRS cell.
 Caveats regarding the analysis of CHL cases • HRS cells should demonstrate CD 30, CD 40, and CD 95, have increased forward and side light scatter compared to small lymphocytes, should lack intermediate to bright expression of CD 20, and should define a discrete cluster in multi-dimensional space. • The presence of CD 15 supports a diagnosis when present but is not required.
Caveats regarding the analysis of CHL cases • HRS cells should demonstrate CD 30, CD 40, and CD 95, have increased forward and side light scatter compared to small lymphocytes, should lack intermediate to bright expression of CD 20, and should define a discrete cluster in multi-dimensional space. • The presence of CD 15 supports a diagnosis when present but is not required.
 Caveats regarding the analysis of CHL cases • Other neoplasms commonly in the differential diagnosis of CHL can usually be excluded: – Diffuse large B cell lymphoma-usually have expression of intermediate to bright CD 20, do not have an increase in side scatter to the extent that HRS cells do, do not express CD 15, usually express CD 30 at a lower level than an HRS population (if CD 30 is expressed), and would not show evidence of HRS–T cell rosetting.
Caveats regarding the analysis of CHL cases • Other neoplasms commonly in the differential diagnosis of CHL can usually be excluded: – Diffuse large B cell lymphoma-usually have expression of intermediate to bright CD 20, do not have an increase in side scatter to the extent that HRS cells do, do not express CD 15, usually express CD 30 at a lower level than an HRS population (if CD 30 is expressed), and would not show evidence of HRS–T cell rosetting.
 Caveats regarding the analysis of CHL cases – Nodular lymphocyte predominant Hodgkin lymphoma is a type of Hodgkin lymphoma where the neoplastic L&H cells express CD 20, CD 40, and CD 45, without CD 15 or CD 30 in tissue section. While this neoplastic population cannot be reliably detected by the flow cytometry at present, if identified, the expression of CD 20 without CD 30 would strongly argue against a population being a putative HRS population.
Caveats regarding the analysis of CHL cases – Nodular lymphocyte predominant Hodgkin lymphoma is a type of Hodgkin lymphoma where the neoplastic L&H cells express CD 20, CD 40, and CD 45, without CD 15 or CD 30 in tissue section. While this neoplastic population cannot be reliably detected by the flow cytometry at present, if identified, the expression of CD 20 without CD 30 would strongly argue against a population being a putative HRS population.
 Caveats regarding the analysis of CHL cases – Anaplastic large cell lymphoma (a CD 30 -positive lymphoma of T cell origin) can often have a similar immunophenotype to CHL but CD 40 is not expressed or is expressed at a low level (lower than the reactive small B cells). • Note that other lymphomas (chronic lymphocytic leukemia small lymphocytic lymphoma [CLL/SLL] and peripheral T cell lymphoma) rarely can have HRS cells detected by flow cytometry (and immunohistochemistry). These cases, however, show characteristic abnormal B or T cell populations, strongly suggesting those diagnoses.
Caveats regarding the analysis of CHL cases – Anaplastic large cell lymphoma (a CD 30 -positive lymphoma of T cell origin) can often have a similar immunophenotype to CHL but CD 40 is not expressed or is expressed at a low level (lower than the reactive small B cells). • Note that other lymphomas (chronic lymphocytic leukemia small lymphocytic lymphoma [CLL/SLL] and peripheral T cell lymphoma) rarely can have HRS cells detected by flow cytometry (and immunohistochemistry). These cases, however, show characteristic abnormal B or T cell populations, strongly suggesting those diagnoses.
 T cells in CHL • CD 4+ population with increased expression of CD 45 and CD 7 seen in 82% of CHL, less than 5% of reactive cases. • Can be used to: – Screen for CHL, to prompt evaluation of CHL by flow cytometry. – Supports a diagnosis of CHL.
T cells in CHL • CD 4+ population with increased expression of CD 45 and CD 7 seen in 82% of CHL, less than 5% of reactive cases. • Can be used to: – Screen for CHL, to prompt evaluation of CHL by flow cytometry. – Supports a diagnosis of CHL.
 Summary • Evaluation of T cells can suggest a diagnosis of CHL. • The HRS cells of CHL can be directly detected by flow cytometry. • Rosetting of T cells by HRS cells can be directly detected by flow cytometry. • The flow cytometry assay can be used to distinguish CHL from similar entities that are frequently in the differential diagnosis of CHL.
Summary • Evaluation of T cells can suggest a diagnosis of CHL. • The HRS cells of CHL can be directly detected by flow cytometry. • Rosetting of T cells by HRS cells can be directly detected by flow cytometry. • The flow cytometry assay can be used to distinguish CHL from similar entities that are frequently in the differential diagnosis of CHL.
 References • J. R. Fromm, S. J. Kussick, B. L. Wood, Am. J. Clin. Pathol. 126 (2006) 764– 780. • J. R. Fromm, A. Thomas, B. L. Wood, Am. J. Clin. Pathol. 131 (2009) 322– 332. • J. R. Fromm, A. Thomas, B. L. Wood, Cytometry B Clin. Cytom. 78 (2010) 387– 388. • J. R. Fromm, B. L. Wood, Cytometry B Clin. Cytom. 78 B (2010) 395.
References • J. R. Fromm, S. J. Kussick, B. L. Wood, Am. J. Clin. Pathol. 126 (2006) 764– 780. • J. R. Fromm, A. Thomas, B. L. Wood, Am. J. Clin. Pathol. 131 (2009) 322– 332. • J. R. Fromm, A. Thomas, B. L. Wood, Cytometry B Clin. Cytom. 78 (2010) 387– 388. • J. R. Fromm, B. L. Wood, Cytometry B Clin. Cytom. 78 B (2010) 395.
 References • J. Fromm, D. Wu, United States & Canadian Academy of Pathology 101 st Annual Meeting, Vancouver, BC, Canada, 2012. • R. Schmitz, J. Stanelle, M. L. Hansmann, R. Kuppers, Annu. Rev. Pathol. 4 (2009) 151– 174. • H. Stein, G. Delsol, S. A. Pileri, L. M. Weiss, S. Poppema, E. S. Jaffe, in: S. H. Swerdlow, E. Campos, N. L. Harris, E. S. Jaffe, S. A. Pileri, H. Stein, J. Thiele, J. W. Vardiman (Eds. ), WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, IARC Press, Lyon, 2008, pp. 326– 329.
References • J. Fromm, D. Wu, United States & Canadian Academy of Pathology 101 st Annual Meeting, Vancouver, BC, Canada, 2012. • R. Schmitz, J. Stanelle, M. L. Hansmann, R. Kuppers, Annu. Rev. Pathol. 4 (2009) 151– 174. • H. Stein, G. Delsol, S. A. Pileri, L. M. Weiss, S. Poppema, E. S. Jaffe, in: S. H. Swerdlow, E. Campos, N. L. Harris, E. S. Jaffe, S. A. Pileri, H. Stein, J. Thiele, J. W. Vardiman (Eds. ), WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, IARC Press, Lyon, 2008, pp. 326– 329.


