de07b80c3b376df34f6fab6a10e0b8b5.ppt
- Количество слайдов: 89

Ibritumomab Tiuxetan (Zevalin™) Radioimmunotherapy of Non-Hodgkin’s Lymphoma Oncology Drug Advisory Committee Meeting September 11, 2001 ODAC Presentation 1

Presentation Agenda n Opening remarks n Leslie L. Shelly, Ph. D. - Associate Director, Regulatory Affairs* n Scientific and medical summary of Zevalin n Christine A. White, M. D. - Vice President, Medical Affairs* n Discussion n Christine A. White, M. D. - Vice President, Medical Affairs* n Pratik Multani, M. D. - Director, Medical Affairs* n Bryan Leigh, M. D. - Director, Oncology* *IDEC Pharmaceuticals, San Diego, CA ODAC Presentation 2
Zevalin™ (ibritumomab tiuxetan): Proposed Indication n Treatment of patients with relapsed or refractory, low-grade, follicular, or CD 20+ transformed B-cell non-Hodgkin’s lymphoma (NHL) and treatment of patients with rituximab-refractory follicular NHL ODAC Presentation 3

Non-Hodgkin’s Lymphoma n Most NHL are of B cell origin and express CD 20 n Ranks 5 th in cancer incidence and mortality n Incidence: 54, 900/year n Prevalence: 300, 000 cases n 65% low-grade or follicular n Transformation increases over time n Median age at diagnosis: 60 years n Median survival n Low-grade or follicular: 6. 2 years n From transformation: 7 to 22 months ODAC Presentation 4
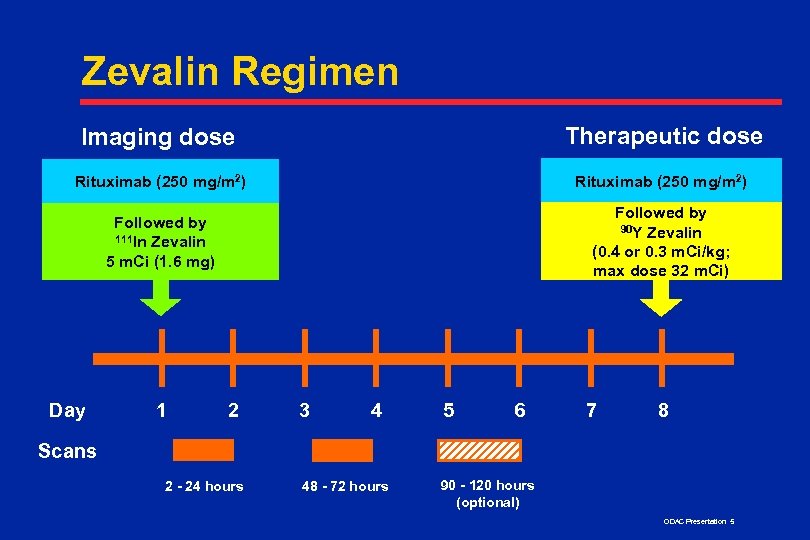
Zevalin Regimen Imaging dose Therapeutic dose Rituximab (250 mg/m 2) Followed by 111 In Zevalin 5 m. Ci (1. 6 mg) Followed by 90 Y Zevalin (0. 4 or 0. 3 m. Ci/kg; max dose 32 m. Ci) Day 1 2 3 4 5 6 7 8 Scans 2 - 24 hours 48 - 72 hours 90 - 120 hours (optional) ODAC Presentation 5
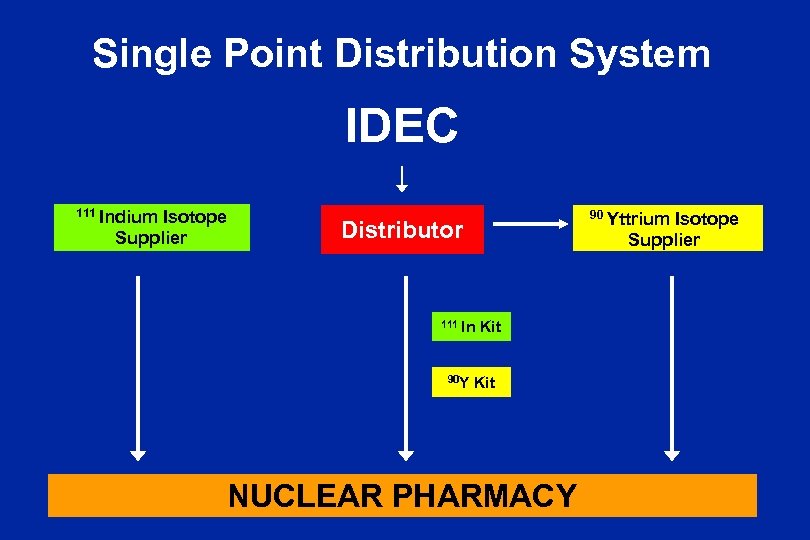
Single Point Distribution System IDEC 111 Indium Isotope Supplier 90 Yttrium Isotope Supplier Distributor 111 In 90 Y Kit NUCLEAR PHARMACY
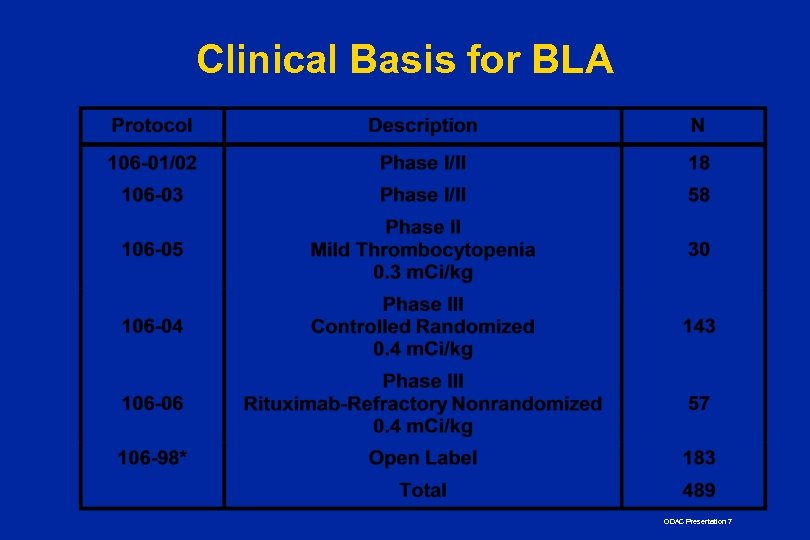
Clinical Basis for BLA ODAC Presentation 7
FDA Agreements n Phase III Randomized Trial (106 -04) n Primary endpoint: ORR n Designed with 80% power to detect a difference in ORR n TTP to be clinically equivalent to control (within 1. 5 months) n Phase III Rituximab-Refractory Trial (106 -06) n Fast Track: June 5, 2000 ODAC Presentation 8
Zevalin™ (ibritumomab tiuxetan): Summary n Clinically significant activity n Acceptable toxicity Zevalin represents a clinically meaningful advance in therapy ODAC Presentation 9

IDEC Investigators n Thomas Witzig, M. D. Mayo Clinic n Sandra Horning, M. D. Stanford University ECOG Lymphoma Chair n Myron Czuczman, M. D. Roswell Park Cancer Center n Gregory A. Wiseman, M. D. Mayo Clinic n Andrew Raubitschek, M. D. City of Hope n Albert Lo. Buglio, M. D. University of Alabama, Birmingham ODAC Presentation 10

IDEC Investigators (cont. ) n Leo Gordon, M. D. n Larry Cripe, M. D. Northwestern University n LEXCOR Indiana University Cancer Center n Radioincorporation Donald Klippenstein, M. D. Roswell Park Cancer Center Michael Zimmer, Ph. D. Northwestern University n Dosimetry Michael Stabin, Ph. D. , C. H. P. Oak Ridge Institute for Science and Education ODAC Presentation 11

Presentation Agenda n Opening remarks n Leslie L. Shelly, Ph. D. - Associate Director, Regulatory Affairs* n Scientific and medical summary of Zevalin n Christine A. White, M. D. - Vice President, Medical Affairs* n Discussion n Christine A. White, M. D. - Vice President, Medical Affairs* n Pratik Multani, M. D. - Director, Medical Affairs* n Bryan Leigh, M. D. - Director, Oncology* *IDEC Pharmaceuticals, San Diego, CA ODAC Presentation 12

Ibritumomab Tiuxetan (Zevalin™) Radioimmunotherapy of Non-Hodgkin’s Lymphoma Christine A. White, M. D. Vice President, Medical Affairs IDEC Pharmaceuticals Corporation Oncology Drug Advisory Committee Meeting September 11, 2001 ODAC Presentation 13
Presentation Outline n Background n Phase I/II conclusions n Imaging and dosimetry n Phase III randomized trial n Phase III rituximab-refractory trial n Efficacy in transformed/nonfollicular low-grade n Integrated safety summary n Conclusions ODAC Presentation 14
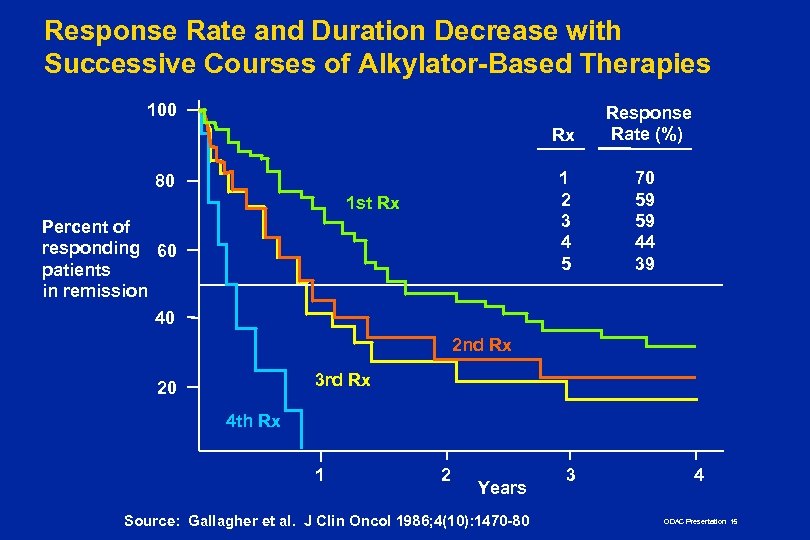
Response Rate and Duration Decrease with Successive Courses of Alkylator-Based Therapies 100 Rx 1 2 3 4 5 80 1 st Rx Percent of responding 60 patients in remission 40 Response Rate (%) 70 59 59 44 39 2 nd Rx 3 rd Rx 20 4 th Rx 1 2 Years Source: Gallagher et al. J Clin Oncol 1986; 4(10): 1470 -80 3 4 ODAC Presentation 15
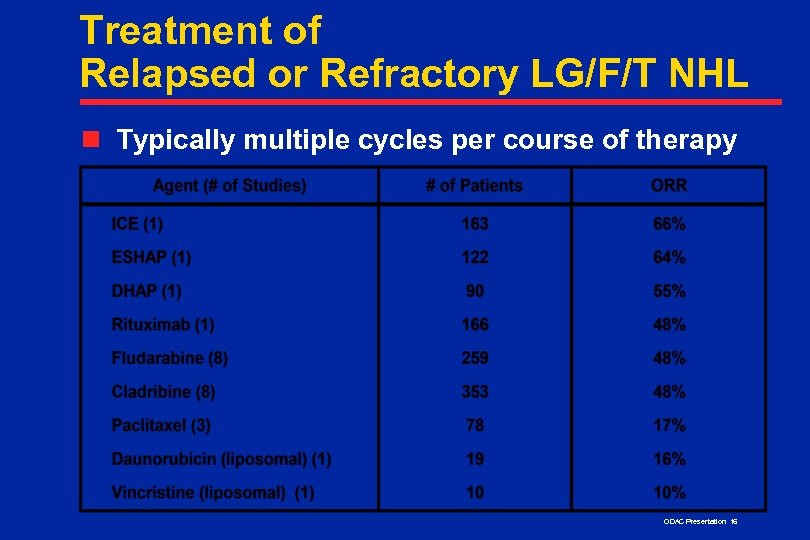
Treatment of Relapsed or Refractory LG/F/T NHL n Typically multiple cycles per course of therapy ODAC Presentation 16
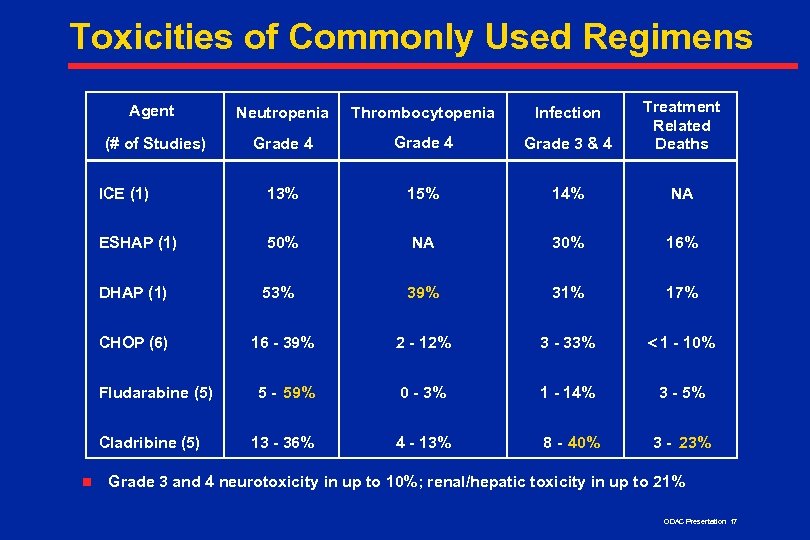
Toxicities of Commonly Used Regimens Agent Neutropenia Thrombocytopenia Infection (# of Studies) Grade 4 Grade 3 & 4 Treatment Related Deaths ICE (1) 13% 15% 14% NA ESHAP (1) 50% NA 30% 16% DHAP (1) 53% 39% 31% 17% CHOP (6) 16 - 39% 2 - 12% 3 - 33% < 1 - 10% 5 - 59% 0 - 3% 1 - 14% 3 - 5% 13 - 36% 4 - 13% 8 - 40% 3 - 23% Fludarabine (5) Cladribine (5) n Grade 3 and 4 neurotoxicity in up to 10%; renal/hepatic toxicity in up to 21% ODAC Presentation 17

Treatment of Relapsed or Refractory LG/F/T NHL n No conventional chemotherapy regimen is curative n No regimen has been shown to be superior with regard to survival n Patients need additional treatment options n In the absence of cure or survival benefit, treatments that induce remission and prolong time off therapy are valuable ODAC Presentation 18

Rationale for Radioimmunotherapy in NHL is inherently sensitive to radiation n RT can be curative in limited stage NHL but can not be applied to advanced stage disease n RIT antibodies target radiation to tumor n RIT can kill both bound and neighboring tumor cells, overcoming the problem of access in bulky or poorly vascularized tumors ODAC Presentation 19

Critical Factors for Successful RIT n Target antigen n Antibody selectivity n Choice of isotope n Stability of antibody-isotope linkage ODAC Presentation 20
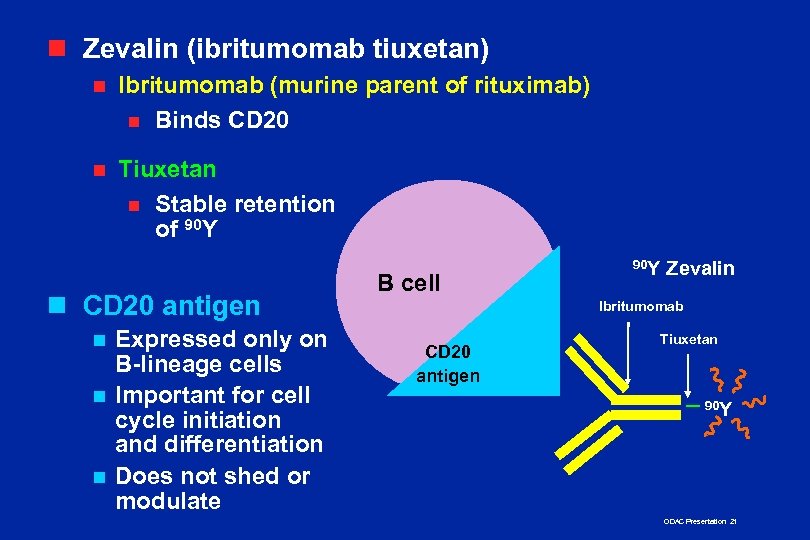
n Zevalin (ibritumomab tiuxetan) n Ibritumomab (murine parent of rituximab) n Binds CD 20 n Tiuxetan n Stable retention of 90 Y n CD 20 antigen Expressed only on B-lineage cells n Important for cell cycle initiation and differentiation n Does not shed or modulate n B cell 90 Y Zevalin Ibritumomab CD 20 antigen Tiuxetan 90 Y ODAC Presentation 21
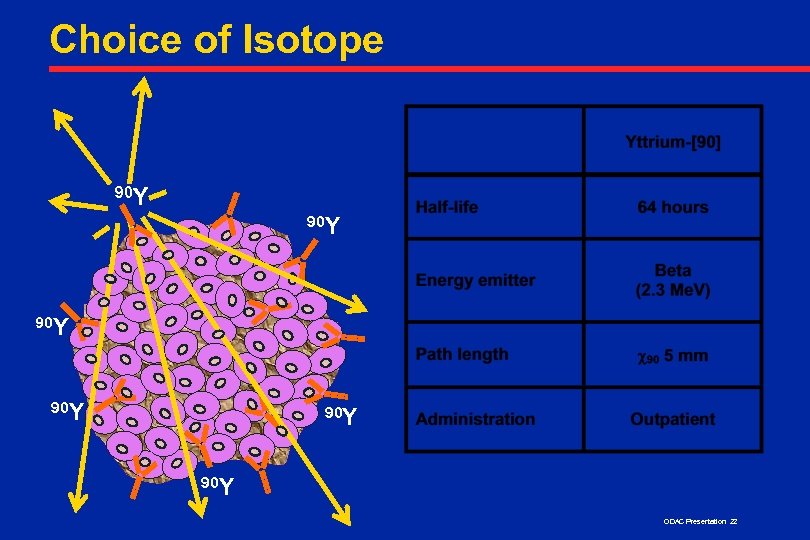
Choice of Isotope 90 Y 90 Y 90 Y ODAC Presentation 22
Presentation Outline n Background n Phase I/II conclusions n Imaging and dosimetry n Phase III randomized trial n Phase III rituximab-refractory trial n Efficacy in transformed/nonfollicular low-grade n Integrated safety summary n Conclusions ODAC Presentation 23
Zevalin Phase I and I/II Conclusions n Unlabeled pretreatment rituximab improves Zevalin biodistribution n MTD: 0. 4 m. Ci/kg (0. 3 m. Ci/kg for patients with mild thrombocytopenia), max 32 m. Ci n Toxicity primarily hematologic and reversible n Correlated with m. Ci/kg dose n Clinical variables (baseline platelets, % marrow NHL involvement) more predictive of toxicity than dosimetry n Clinical parameters adequate for dosing ODAC Presentation 24
Zevalin Phase I/II Study (N = 51) n ORR: 82% in low-grade NHL 42% in intermediate-grade NHL n Median TTP (responders): n 0. 4 m. Ci/kg: 15. 4 months n 0. 4 m. Ci/kg CR pts: 28. 3 - 37. 2+ months ODAC Presentation 25

Presentation Outline n Background n Phase I/II conclusions n Imaging and dosimetry n Phase III randomized trial n Phase III rituximab-refractory trial n Efficacy in transformed/nonfollicular low-grade n Integrated safety summary n Conclusions ODAC Presentation 26
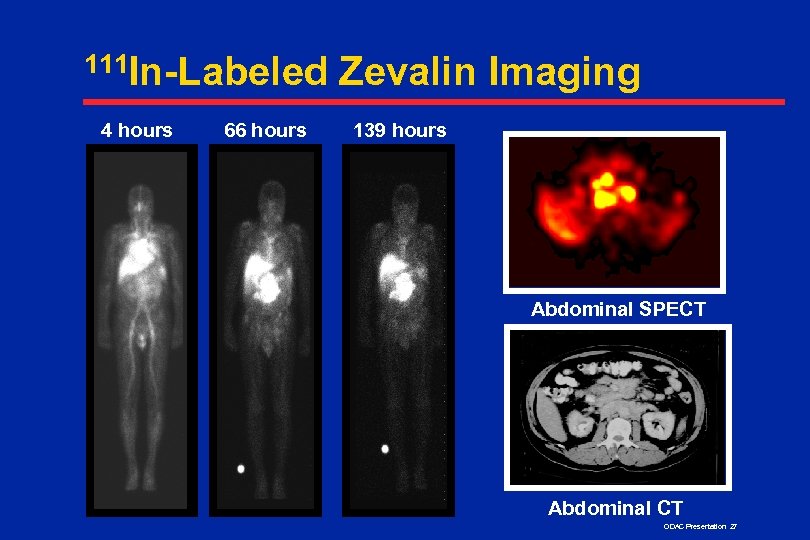
111 In-Labeled 4 hours 66 hours Zevalin Imaging 139 hours Abdominal SPECT Abdominal CT ODAC Presentation 27
Imaging and Dosimetry n Tumors imaged in all LG/F/T patients n Radiation absorbed dose to normal organs acceptable in all patients n Minimal urinary excretion, 7% over 7 days n No correlation between hematologic toxicity and dosimetry or PK parameters ODAC Presentation 28
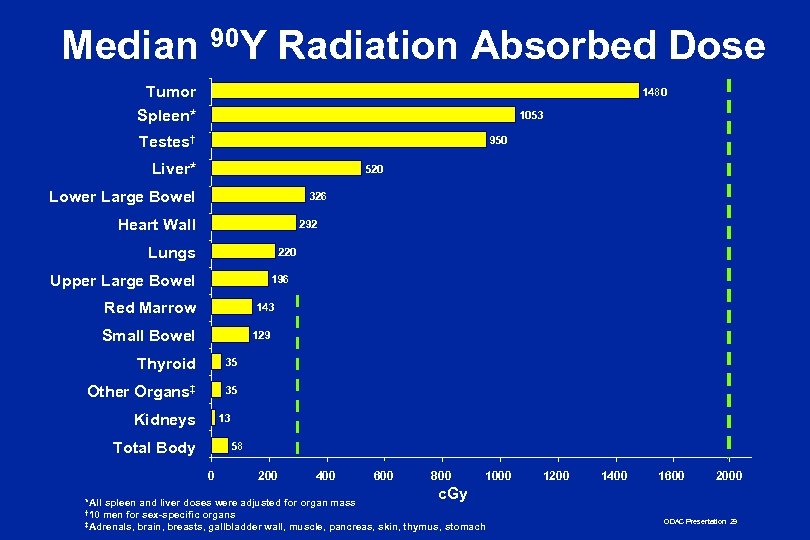
Median 90 Y Radiation Absorbed Dose Tumor Spleen* 1480 1053 Testes† 950 Liver* 520 Lower Large Bowel 326 Heart Wall 292 Lungs 220 Upper Large Bowel 196 Red Marrow 143 Small Bowel 129 Thyroid 35 Other Organs‡ 35 Kidneys 13 Total Body 58 0 200 400 600 800 1000 1200 1400 1600 2000 c. Gy *All spleen and liver doses were adjusted for organ mass † 10 men for sex-specific organs ‡Adrenals, brain, breasts, gallbladder wall, muscle, pancreas, skin, thymus, stomach ODAC Presentation 29
Presentation Outline n Background n Phase I/II conclusions n Imaging and dosimetry n Phase III randomized trial n Phase III rituximab-refractory trial n Efficacy in transformed/nonfollicular low-grade n Integrated safety summary n Conclusions ODAC Presentation 30
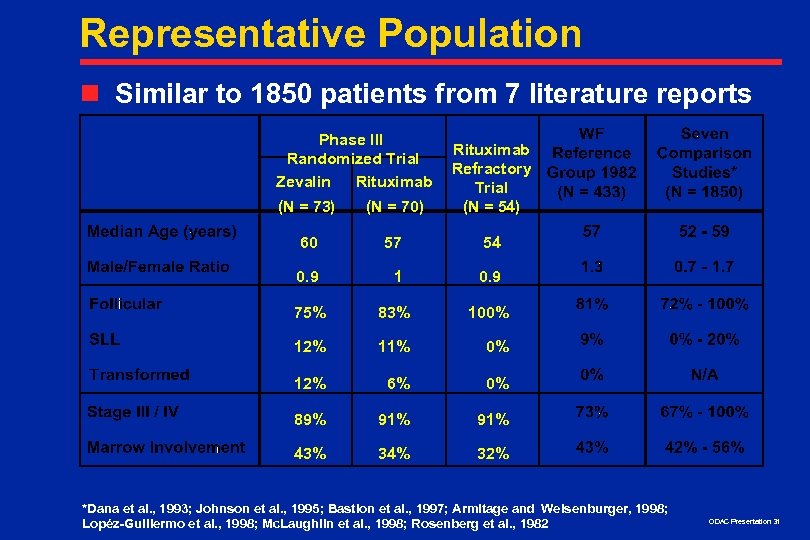
Representative Population n Similar to 1850 patients from 7 literature reports Phase III Randomized Trial Zevalin Rituximab (N = 73) (N = 70) Rituximab Refractory Trial (N = 54) 60 57 54 0. 9 1 0. 9 75% 83% 100% 12% 11% 0% 12% 6% 0% 89% 91% 43% 34% 32% *Dana et al. , 1993; Johnson et al. , 1995; Bastion et al. , 1997; Armitage and Weisenburger, 1998; Lopéz-Guillermo et al. , 1998; Mc. Laughlin et al. , 1998; Rosenberg et al. , 1982 ODAC Presentation 31

Phase III Randomized Controlled Study Mayo Clinic Henry Ford Cancer Center Northwestern Univ Johns Hopkins Univ MD Anderson Kansas Univ UCLA Sutter Cancer Center Roswell Park St. Vincent’s Hospital Harvard/Beth Israel Greenbaum Cancer Center, MD Cleveland Clinic City of Hope Washington Univ Sharp/Sidney Kimmel Cancer Center Jewish Hospital Kenwood, OH NYU Fox Chase Cancer Center Georgetown Univ Mountain States Tumor Institute Western Penn Cancer Institute Univ of Florida New York Hospital Greenville Hospital, SC Kaiser Permanente, CA Univ of Alabama ODAC Presentation 32
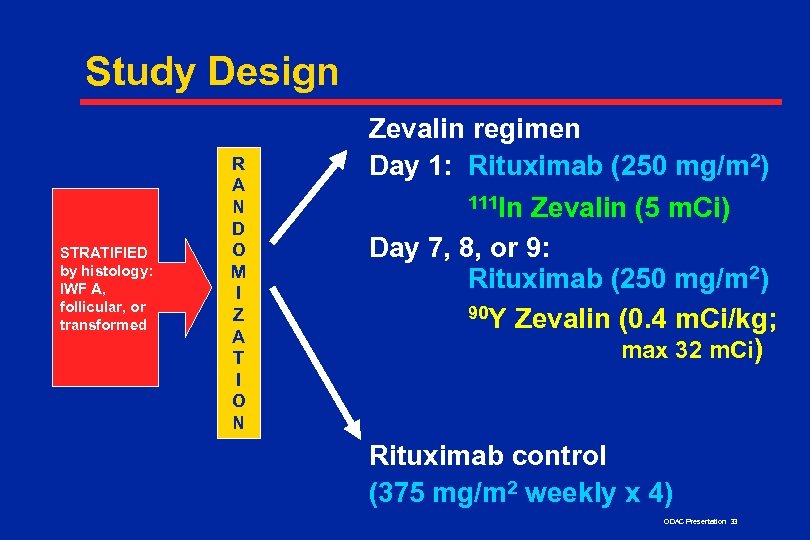
Study Design STRATIFIED by histology: IWF A, follicular, or transformed R A N D O M I Z A T I O N Zevalin regimen Day 1: Rituximab (250 mg/m 2) 111 In Zevalin (5 m. Ci) Day 7, 8, or 9: Rituximab (250 mg/m 2) 90 Y Zevalin (0. 4 m. Ci/kg; max 32 m. Ci) Rituximab control (375 mg/m 2 weekly x 4) ODAC Presentation 33
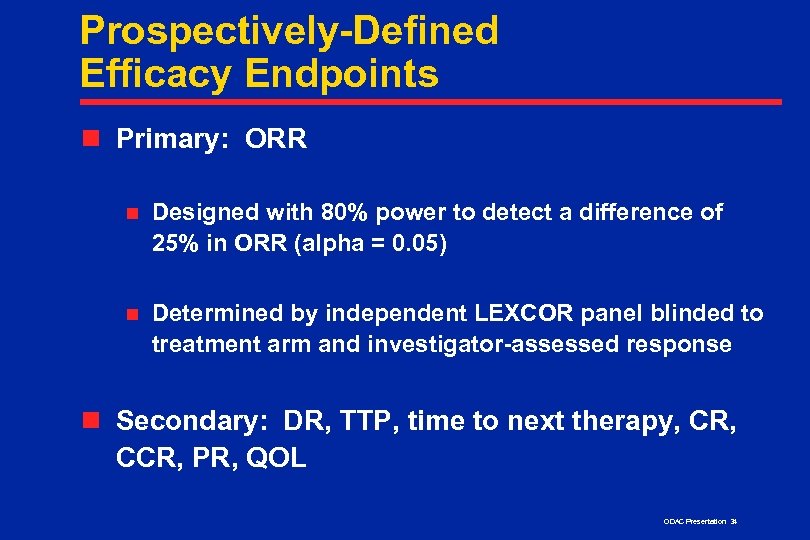
Prospectively-Defined Efficacy Endpoints n Primary: ORR n Designed with 80% power to detect a difference of 25% in ORR (alpha = 0. 05) n Determined by independent LEXCOR panel blinded to treatment arm and investigator-assessed response n Secondary: DR, TTP, time to next therapy, CR, CCR, PR, QOL ODAC Presentation 34
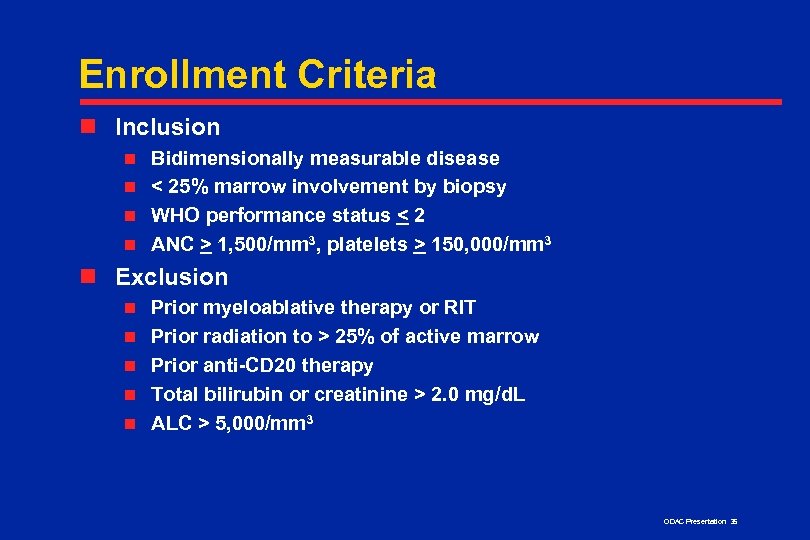
Enrollment Criteria n Inclusion Bidimensionally measurable disease n < 25% marrow involvement by biopsy n WHO performance status < 2 n ANC > 1, 500/mm 3, platelets > 150, 000/mm 3 n n Exclusion n n Prior myeloablative therapy or RIT Prior radiation to > 25% of active marrow Prior anti-CD 20 therapy Total bilirubin or creatinine > 2. 0 mg/d. L ALC > 5, 000/mm 3 ODAC Presentation 35
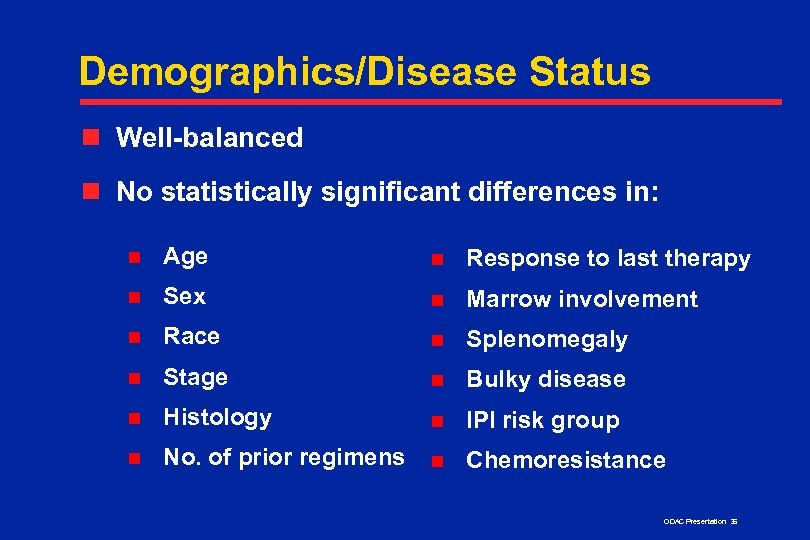
Demographics/Disease Status n Well-balanced n No statistically significant differences in: n Age n Response to last therapy n Sex n Marrow involvement n Race n Splenomegaly n Stage n Bulky disease n Histology n IPI risk group n No. of prior regimens n Chemoresistance ODAC Presentation 36
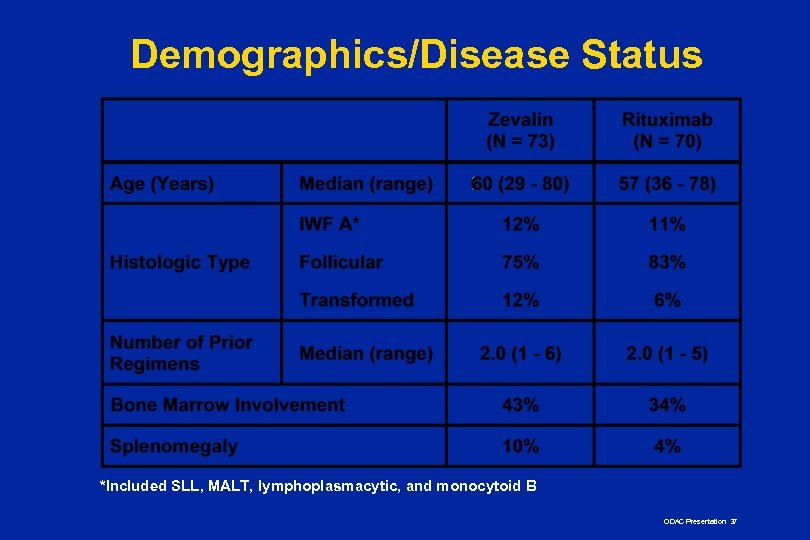
Demographics/Disease Status *Included SLL, MALT, lymphoplasmacytic, and monocytoid B ODAC Presentation 37
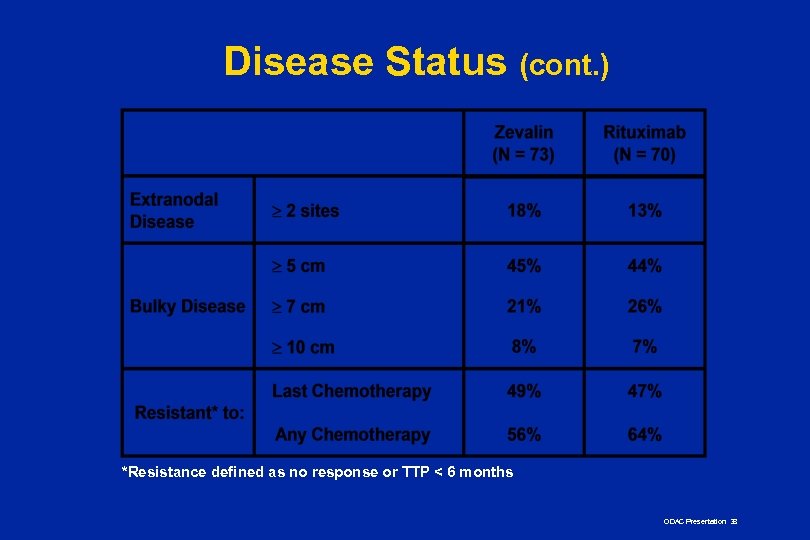
Disease Status (cont. ) *Resistance defined as no response or TTP < 6 months ODAC Presentation 38
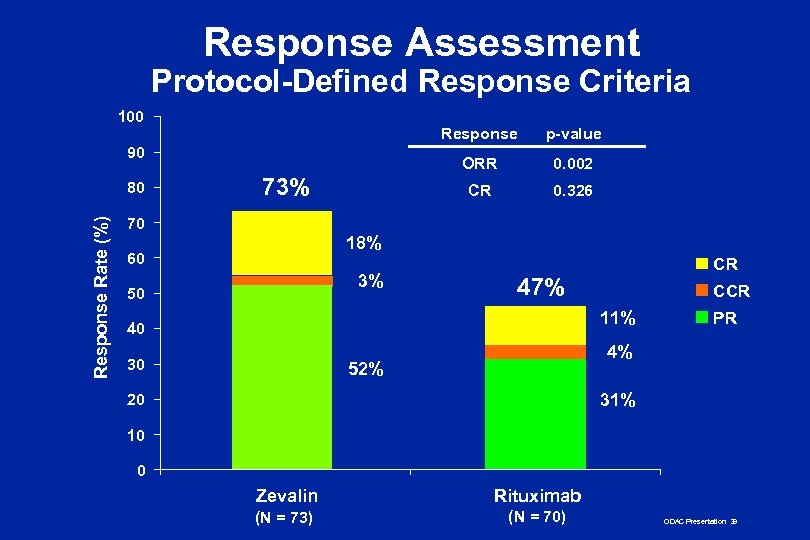
Response Assessment Protocol-Defined Response Criteria 100 Response ORR Response Rate (%) 80 73% 0. 002 CR 90 p-value 0. 326 70 18% 60 3% 50 CR 47% CCR 11% 40 30 PR 4% 52% 31% 20 10 0 Zevalin Rituximab (N = 73) (N = 70) ODAC Presentation 39
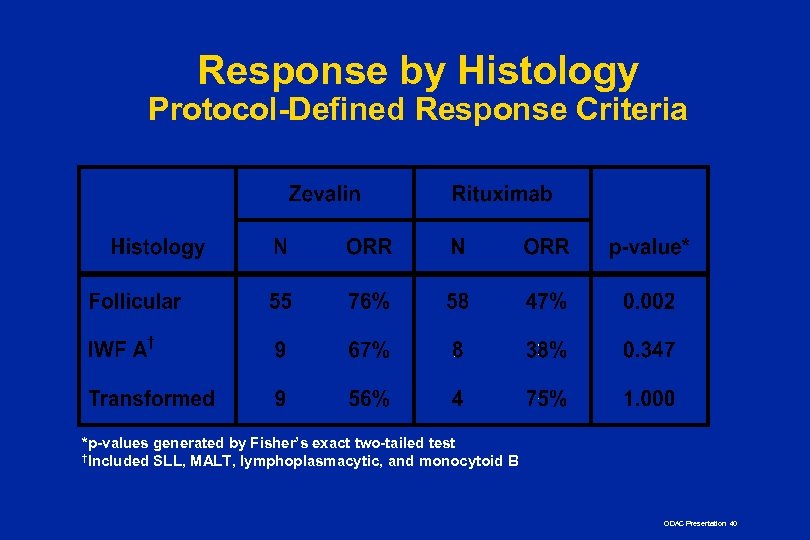
Response by Histology Protocol-Defined Response Criteria *p-values generated by Fisher’s exact two-tailed test †Included SLL, MALT, lymphoplasmacytic, and monocytoid B ODAC Presentation 40
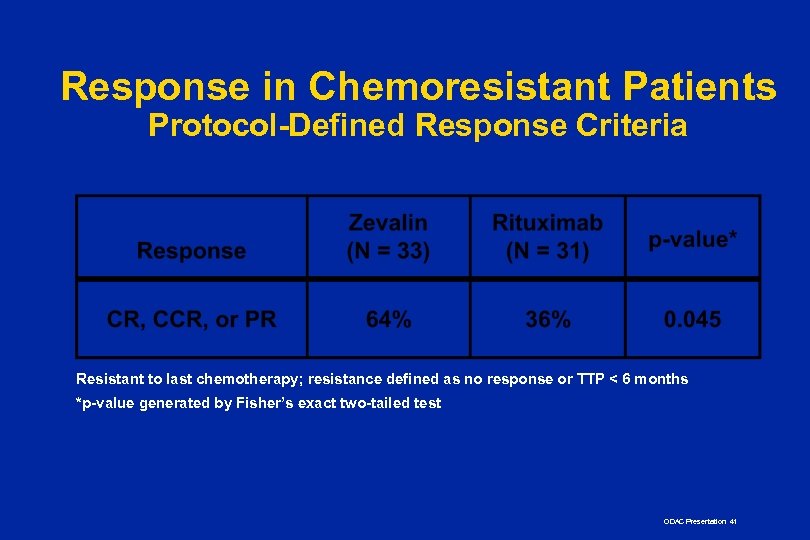
Response in Chemoresistant Patients Protocol-Defined Response Criteria Resistant to last chemotherapy; resistance defined as no response or TTP < 6 months *p-value generated by Fisher’s exact two-tailed test ODAC Presentation 41
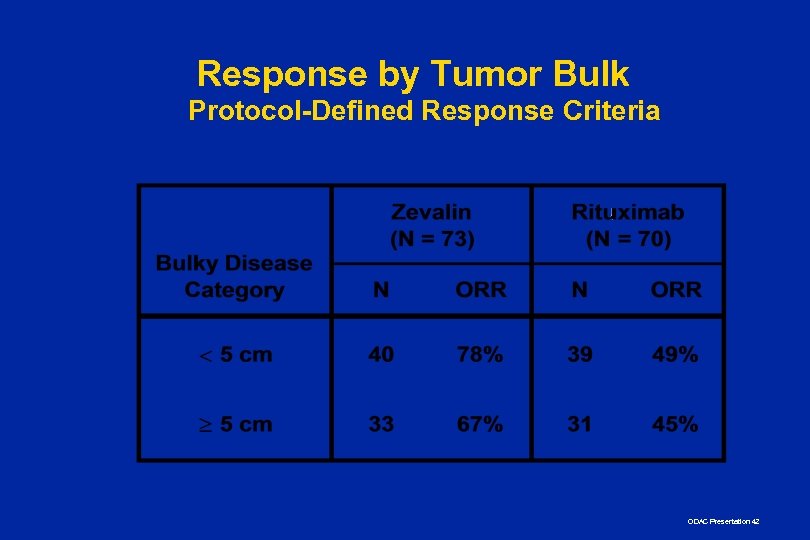
Response by Tumor Bulk Protocol-Defined Response Criteria ODAC Presentation 42
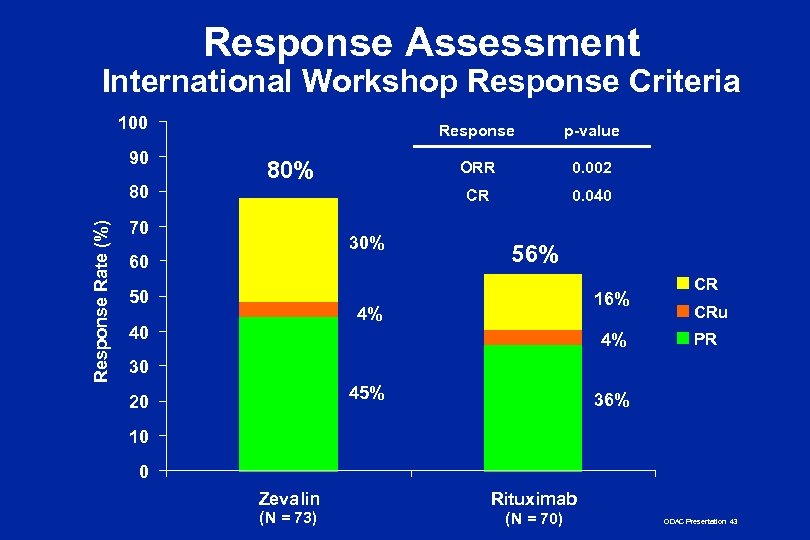
Response Assessment International Workshop Response Criteria 100 90 Response Rate (%) 80 Response ORR 70 30% 60 50 0. 002 CR 80% p-value 0. 040 56% 16% 4% 40 4% CR CRu PR 30 45% 20 36% 10 0 Zevalin (N = 73) Rituximab (N = 70) ODAC Presentation 43
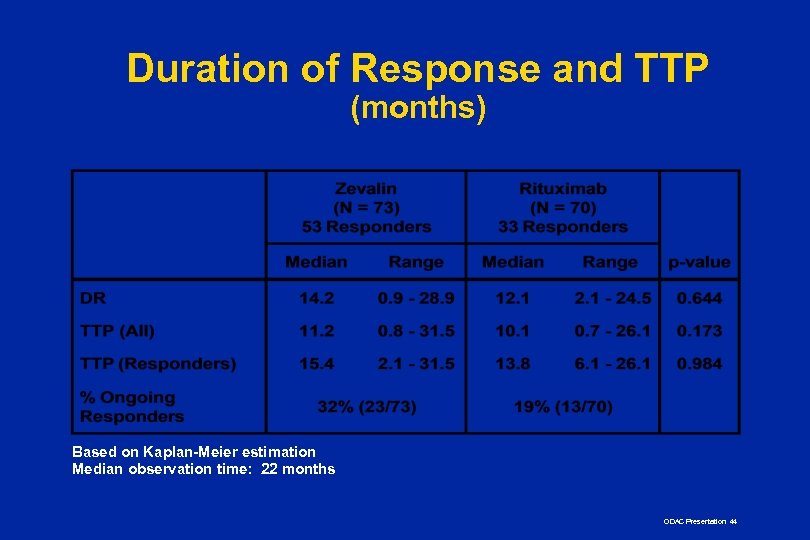
Duration of Response and TTP (months) Based on Kaplan-Meier estimation Median observation time: 22 months ODAC Presentation 44
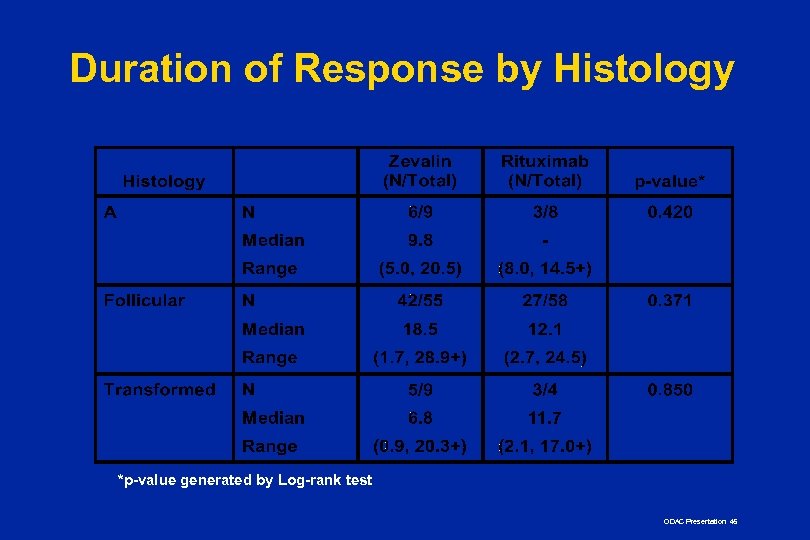
Duration of Response by Histology *p-value generated by Log-rank test ODAC Presentation 45
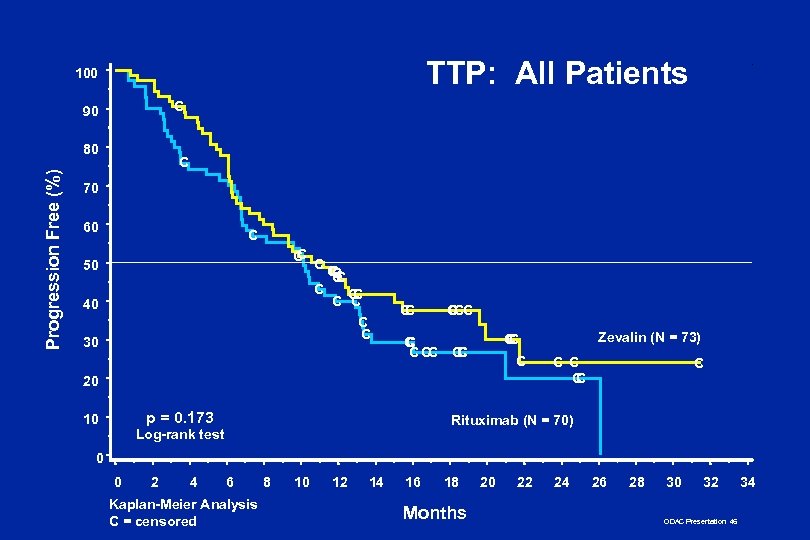
TTP: All Patients 100 C 90 Progression Free (%) 80 C 70 60 C C C 50 40 30 CC CC C C C CC C C C 20 p = 0. 173 10 Zevalin (N = 73) C C C C Rituximab (N = 70) Log-rank test 0 0 2 4 6 Kaplan-Meier Analysis C = censored 8 10 12 14 16 18 Months 20 22 24 26 28 30 32 ODAC Presentation 46 34
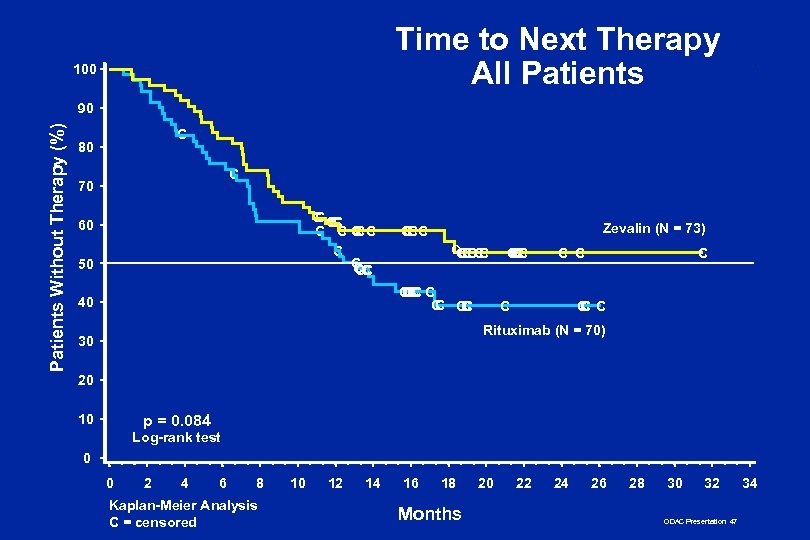
Time to Next Therapy All Patients 100 Patients Without Therapy (%) 90 C 80 C 70 CC CC C CC CC C 60 50 Zevalin (N = 73) CCC C C CC CC C C 40 CC C C Rituximab (N = 70) 30 20 10 p = 0. 084 Log-rank test 0 0 2 4 6 8 Kaplan-Meier Analysis C = censored 10 12 14 16 18 Months 20 22 24 26 28 30 32 ODAC Presentation 47 34
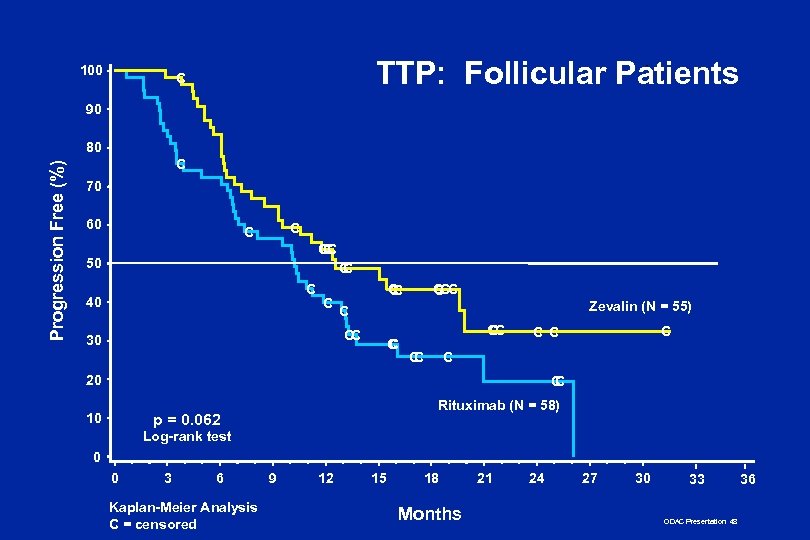
100 TTP: Follicular Patients C 90 Progression Free (%) 80 C 70 60 C C CC C 50 C C C 40 C C C Zevalin (N = 55) C CC 30 C CC C 20 C C 10 Rituximab (N = 58) p = 0. 062 Log-rank test 0 0 3 6 Kaplan-Meier Analysis C = censored 9 12 15 18 Months 21 24 27 30 33 ODAC Presentation 48 36
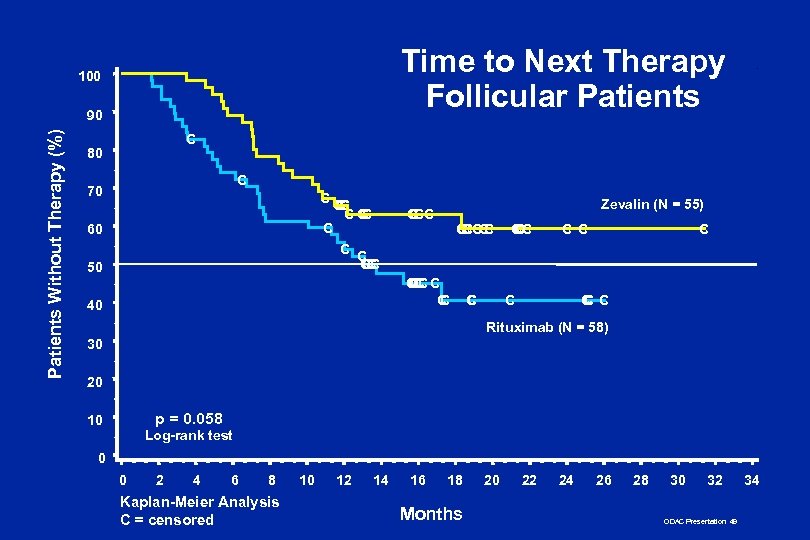
Time to Next Therapy Follicular Patients 100 Patients Without Therapy (%) 90 C 80 C 70 CC C C C 60 C 50 Zevalin (N = 55) CCC C C CC CC CC C C C C 40 C C C Rituximab (N = 58) 30 20 p = 0. 058 10 Log-rank test 0 0 2 4 6 8 Kaplan-Meier Analysis C = censored 10 12 14 16 18 Months 20 22 24 26 28 30 32 ODAC Presentation 49 34
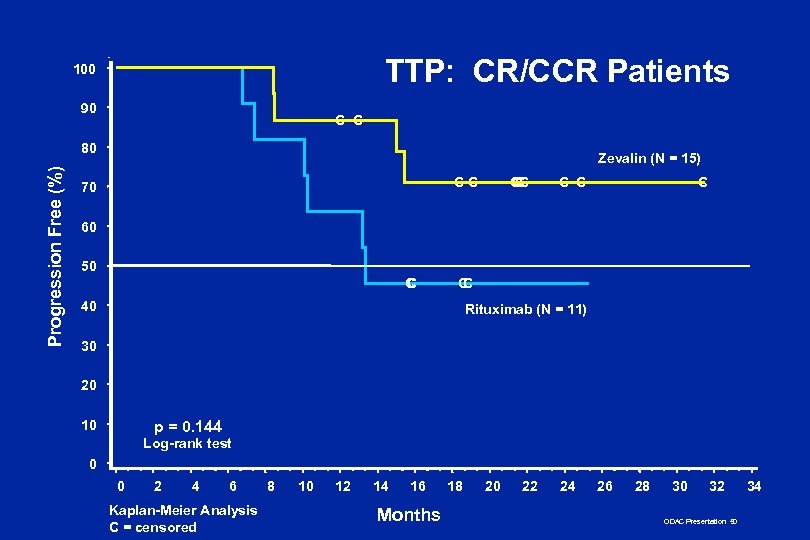
TTP: CR/CCR Patients 100 90 C C Progression Free (%) 80 Zevalin (N = 15) CC 70 CC C C 60 50 C C C 40 Rituximab (N = 11) 30 20 10 p = 0. 144 Log-rank test 0 0 2 4 6 Kaplan-Meier Analysis C = censored 8 10 12 14 16 Months 18 20 22 24 26 28 30 32 ODAC Presentation 50 34
Phase III Randomized Summary n Efficacy objectives met n Primary n n Significantly higher ORR as determined by independent, blinded LEXCOR panel Secondary n Overall TTP comparable n Trend toward longer TTP in follicular and CR/CCR patients n Trend toward longer time to next therapy in all patients n Median TTP in responders: 15. 4 months ODAC Presentation 51
Presentation Outline n Background n Phase I/II conclusions n Imaging and dosimetry n Phase III randomized trial n Phase III rituximab-refractory trial n Efficacy in transformed/nonfollicular low-grade n Integrated safety summary n Conclusions ODAC Presentation 52
Phase III Nonrandomized Controlled Study in Rituximab-Refractory Follicular NHL Mayo Clinic Western Penn Cancer Institute Johns Hopkins Univ Harvard/Beth Israel Northwestern Univ Washington Univ UCLA Henry Ford Cancer Center Indiana Univ Sutter Cancer Center, CA Roswell Park Univ of Miami Sharp/Sidney Kimmel Cancer Center Radiation Therapy Assoc. , FL Univ of Florida Kaiser Permanente, CA Kansas Univ ODAC Presentation 53
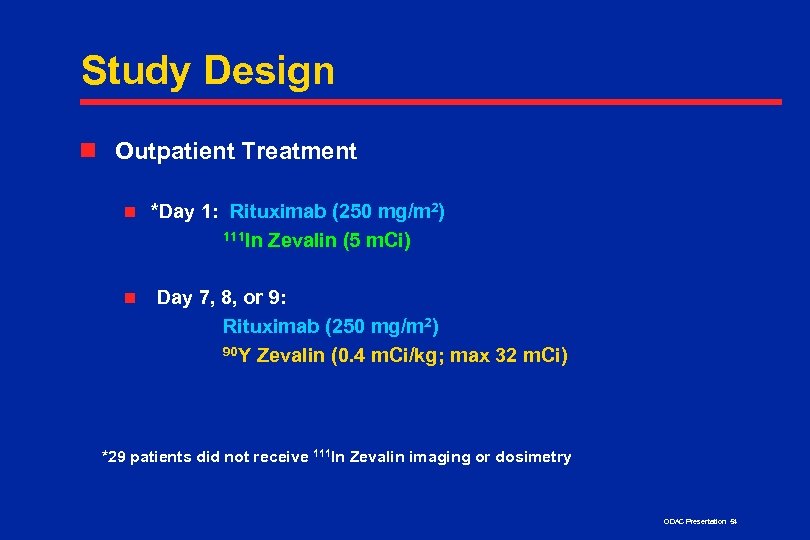
Study Design n Outpatient Treatment n n *Day 1: Rituximab (250 mg/m 2) 111 In Zevalin (5 m. Ci) Day 7, 8, or 9: Rituximab (250 mg/m 2) 90 Y Zevalin (0. 4 m. Ci/kg; max 32 m. Ci) *29 patients did not receive 111 In Zevalin imaging or dosimetry ODAC Presentation 54
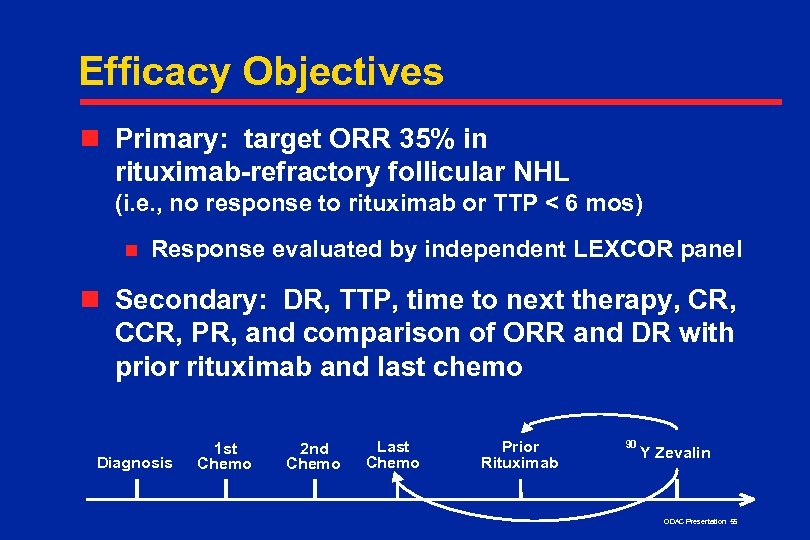
Efficacy Objectives n Primary: target ORR 35% in rituximab-refractory follicular NHL (i. e. , no response to rituximab or TTP < 6 mos) n Response evaluated by independent LEXCOR panel n Secondary: DR, TTP, time to next therapy, CR, CCR, PR, and comparison of ORR and DR with prior rituximab and last chemo Diagnosis 1 st Chemo 2 nd Chemo Last Chemo Prior Rituximab 90 Y Zevalin ODAC Presentation 55
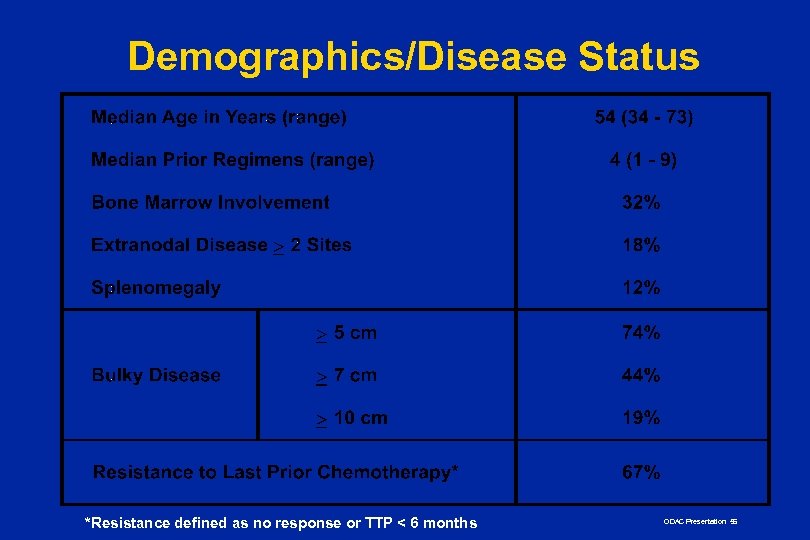
Demographics/Disease Status *Resistance defined as no response or TTP < 6 months ODAC Presentation 56
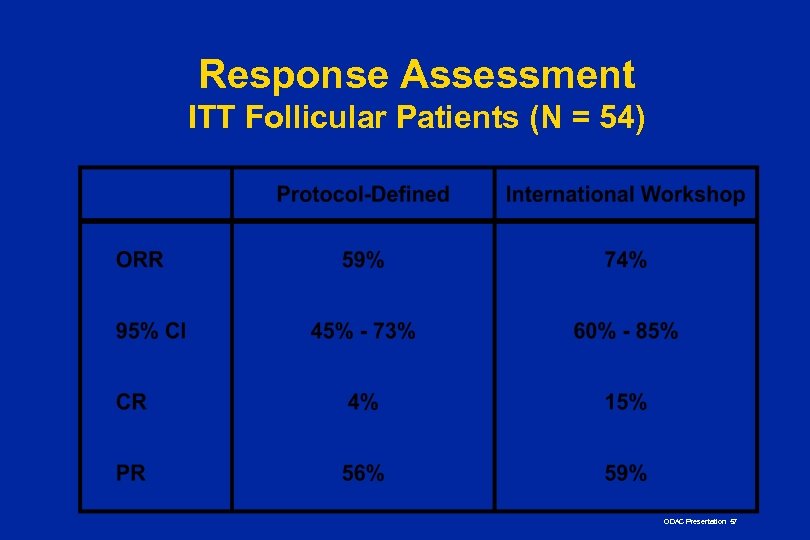
Response Assessment ITT Follicular Patients (N = 54) ODAC Presentation 57
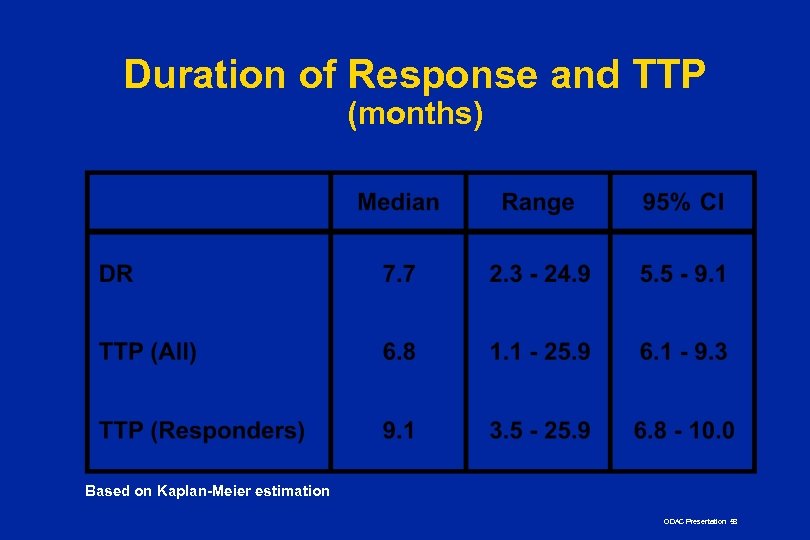
Duration of Response and TTP (months) Based on Kaplan-Meier estimation ODAC Presentation 58

Zevalin vs Prior Therapy Analysis n Favors if: n Responded to one therapy but not the other n Responded to both but DR at least 3 months longer n Neutral if: n Did not respond to eitherapy, or n Responded to both therapies but DR within 3 months ODAC Presentation 59
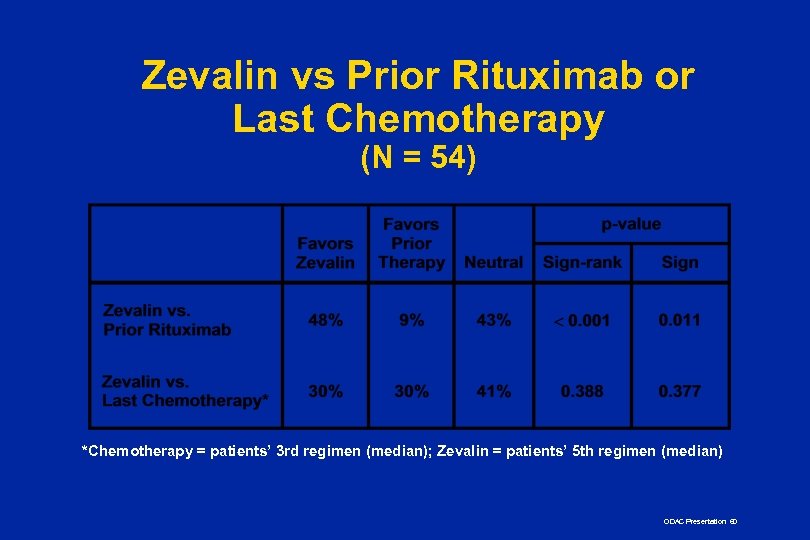
Zevalin vs Prior Rituximab or Last Chemotherapy (N = 54) *Chemotherapy = patients’ 3 rd regimen (median); Zevalin = patients’ 5 th regimen (median) ODAC Presentation 60
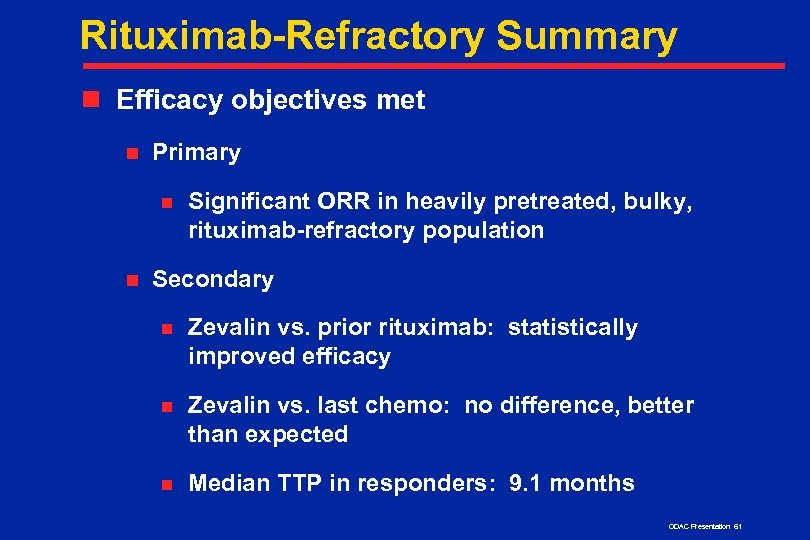
Rituximab-Refractory Summary n Efficacy objectives met n Primary n n Significant ORR in heavily pretreated, bulky, rituximab-refractory population Secondary n Zevalin vs. prior rituximab: statistically improved efficacy n Zevalin vs. last chemo: no difference, better than expected n Median TTP in responders: 9. 1 months ODAC Presentation 61
Presentation Outline n Background n Phase I/II conclusions n Imaging and dosimetry n Phase III randomized trial n Phase III rituximab-refractory trial n Efficacy in transformed/nonfollicular low-grade n Integrated safety summary n Conclusions ODAC Presentation 62
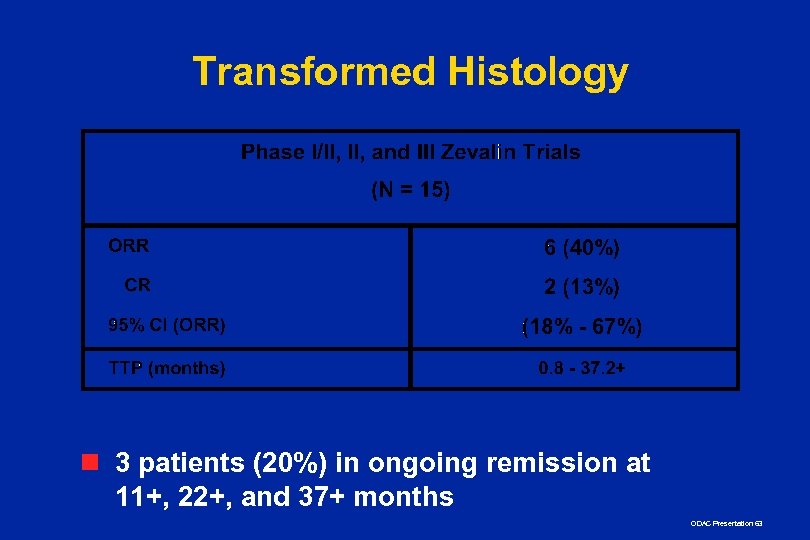
Transformed Histology n 3 patients (20%) in ongoing remission at 11+, 22+, and 37+ months ODAC Presentation 63
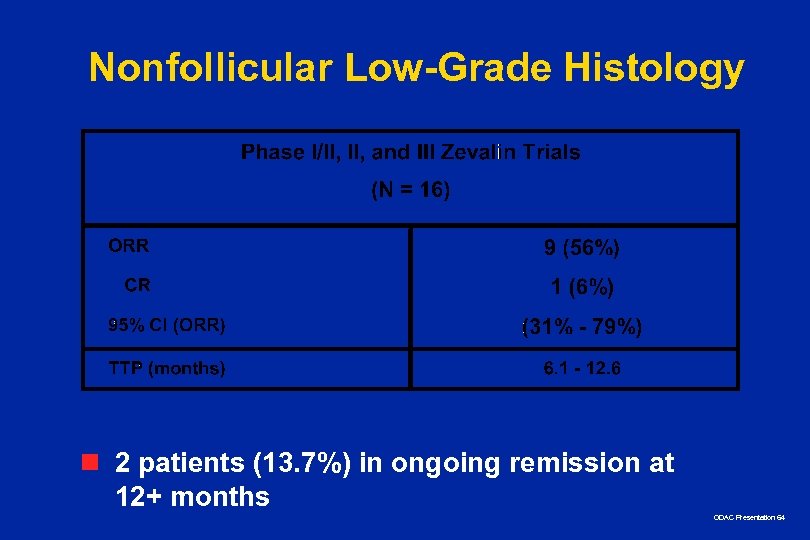
Nonfollicular Low-Grade Histology n 2 patients (13. 7%) in ongoing remission at 12+ months ODAC Presentation 64
Presentation Outline n Background n Phase I/II conclusions n Imaging and dosimetry n Phase III randomized trial n Phase III rituximab-refractory trial n Efficacy in transformed/nonfollicular low-grade n Integrated safety summary n Conclusions ODAC Presentation 65

Integrated Safety n AEs primarily hematologic n Nonhematologic AEs primarily Grade 1 or 2 n Not associated with hair loss, severe mucositis, persistent nausea and vomiting common with chemotherapy n Low incidence of serious infection n Low incidence of HAMA/HACA ODAC Presentation 66
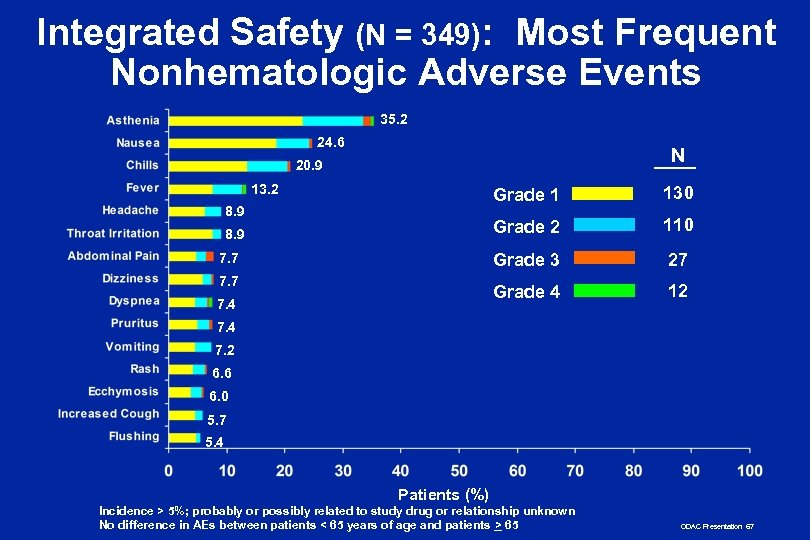
Integrated Safety (N = 349): Most Frequent Nonhematologic Adverse Events 35. 2 24. 6 N 20. 9 13. 2 Grade 1 Grade 2 7. 7 7. 4 27 Grade 4 8. 9 110 Grade 3 8. 9 130 12 7. 4 7. 2 6. 6 6. 0 5. 7 5. 4 Patients (%) Incidence > 5%; probably or possibly related to study drug or relationship unknown No difference in AEs between patients < 65 years of age and patients > 65 ODAC Presentation 67
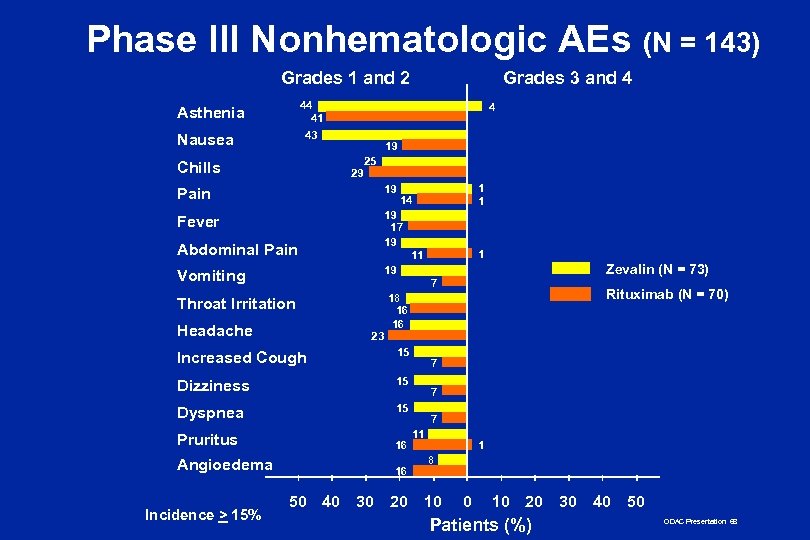
Phase III Nonhematologic AEs (N = 143) Grades 1 and 2 Grades 3 and 4 44 41 Asthenia 4 43 Nausea 19 25 Chills 29 1 1 19 Pain 14 19 17 19 11 19 Fever Abdominal Pain Vomiting 1 Throat Irritation Headache 23 15 Dizziness 15 Dyspnea 15 Pruritus 16 Angioedema 16 50 40 Rituximab (N = 70) 18 16 16 Increased Cough Incidence > 15% Zevalin (N = 73) 7 30 20 7 7 7 11 1 8 10 0 10 20 Patients (%) 30 40 50 ODAC Presentation 68
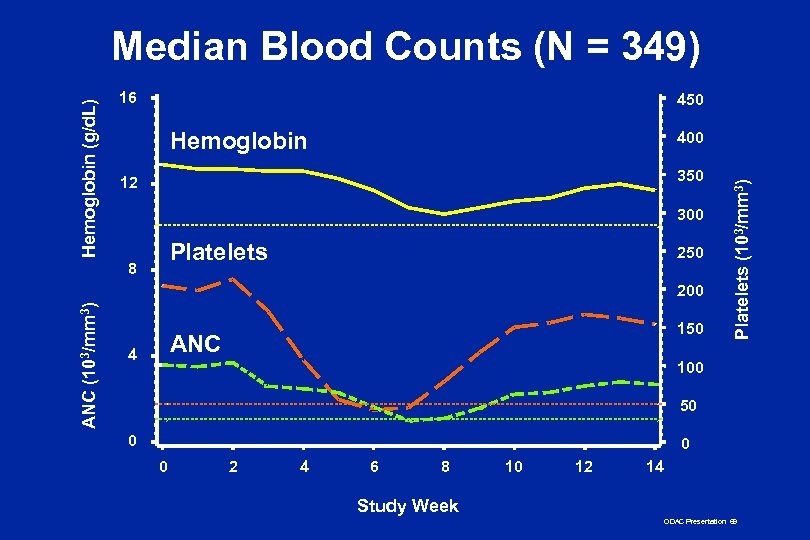
16 450 Hemoglobin 400 350 12 300 Platelets 8 250 ANC (103/mm 3) 200 150 ANC 4 Platelets (103/mm 3) Hemoglobin (g/d. L) Median Blood Counts (N = 349) 100 50 0 2 4 6 8 10 12 14 Study Week ODAC Presentation 69
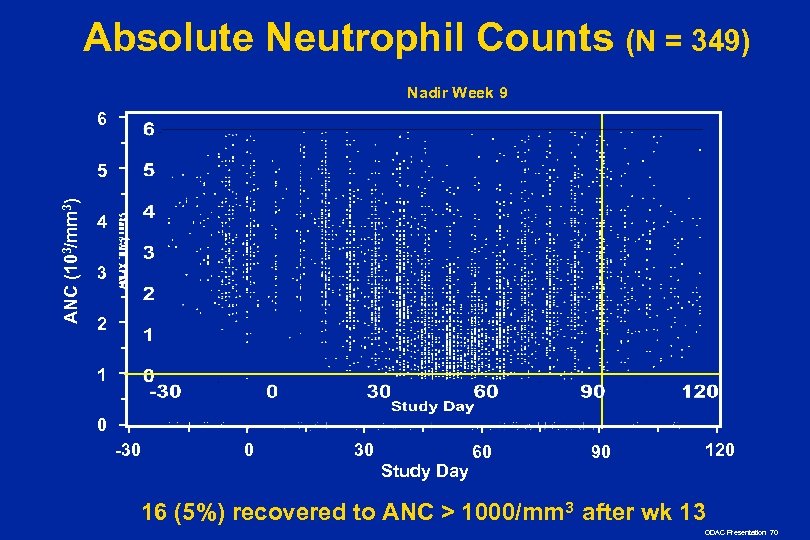
Absolute Neutrophil Counts (N = 349) Nadir Week 9 6 ANC (103/mm 3) 5 4 3 2 1 0 -30 0 30 Study Day 60 90 120 16 (5%) recovered to ANC > 1000/mm 3 after wk 13 ODAC Presentation 70
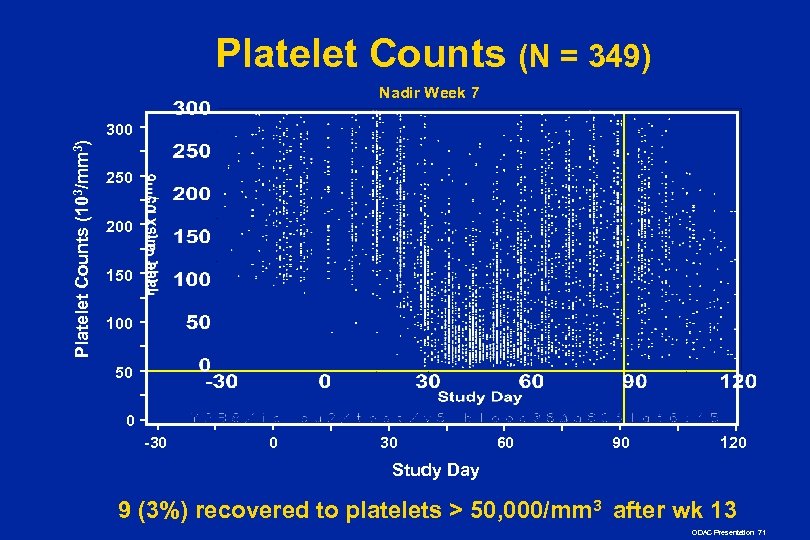
Platelet Counts (N = 349) Nadir Week 7 Platelet Counts (103/mm 3) 300 250 200 150 100 50 0 -30 0 30 60 90 120 Study Day 9 (3%) recovered to platelets > 50, 000/mm 3 after wk 13 ODAC Presentation 71
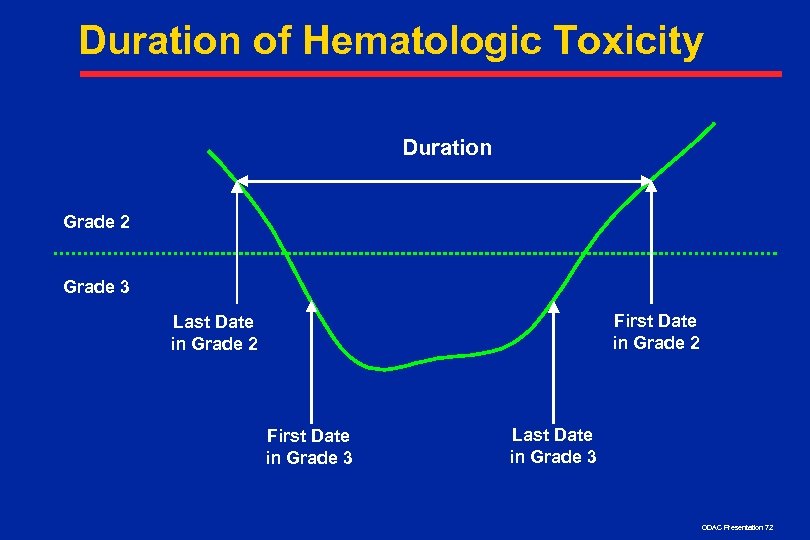
Duration of Hematologic Toxicity Duration Grade 2 Grade 3 First Date in Grade 2 Last Date in Grade 2 First Date in Grade 3 Last Date in Grade 3 ODAC Presentation 72
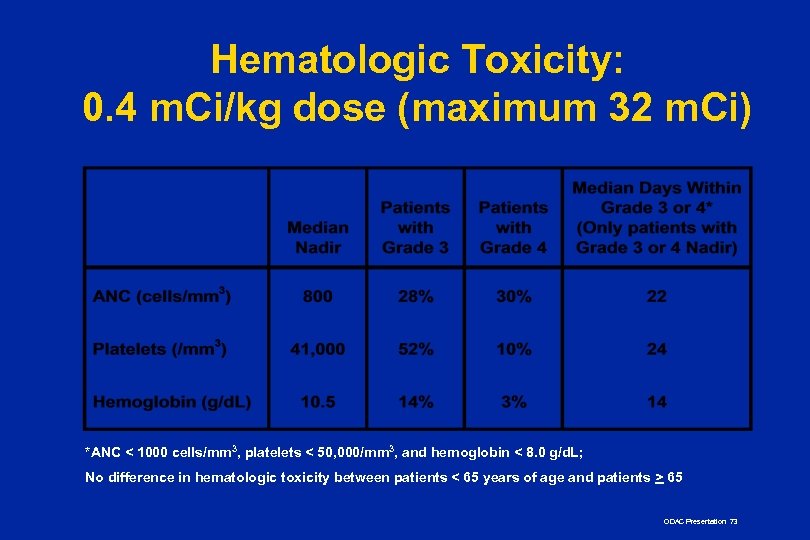
Hematologic Toxicity: 0. 4 m. Ci/kg dose (maximum 32 m. Ci) *ANC < 1000 cells/mm 3, platelets < 50, 000/mm 3, and hemoglobin < 8. 0 g/d. L; No difference in hematologic toxicity between patients < 65 years of age and patients > 65 ODAC Presentation 73
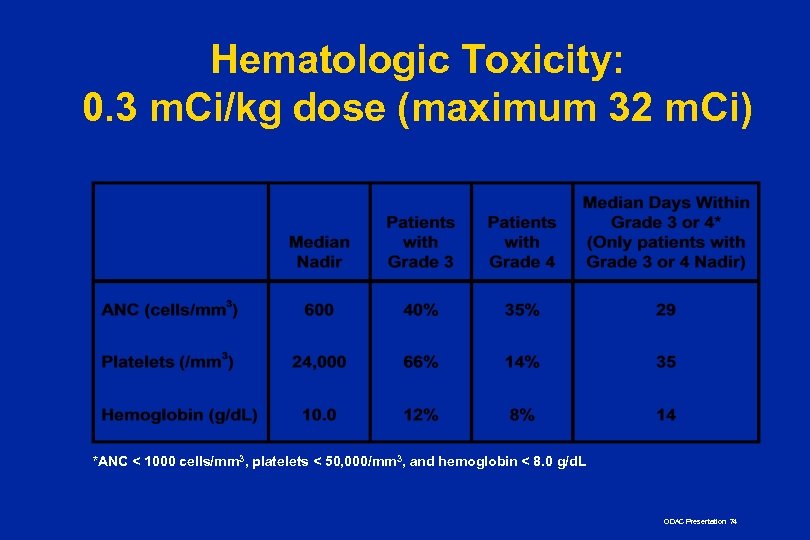
Hematologic Toxicity: 0. 3 m. Ci/kg dose (maximum 32 m. Ci) *ANC < 1000 cells/mm 3, platelets < 50, 000/mm 3, and hemoglobin < 8. 0 g/d. L ODAC Presentation 74
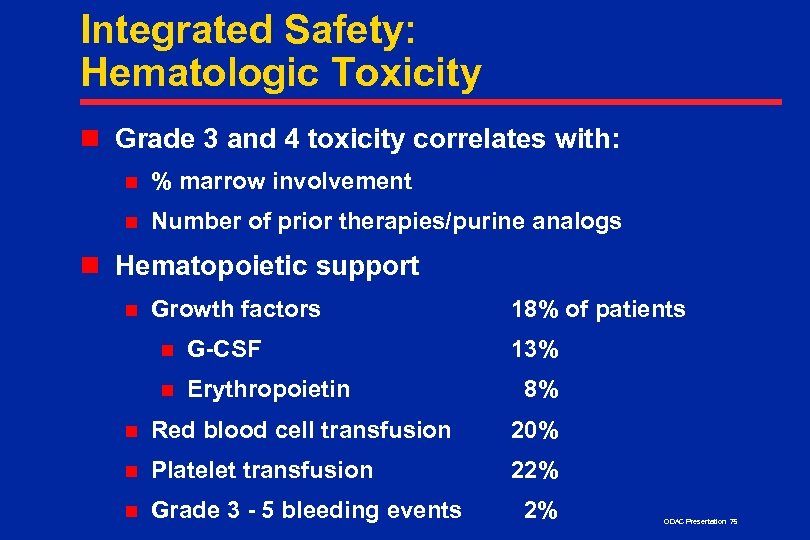
Integrated Safety: Hematologic Toxicity n Grade 3 and 4 toxicity correlates with: n % marrow involvement n Number of prior therapies/purine analogs n Hematopoietic support n Growth factors n G-CSF n Erythropoietin 18% of patients 13% 8% n Red blood cell transfusion 20% n Platelet transfusion 22% n Grade 3 - 5 bleeding events 2% ODAC Presentation 75
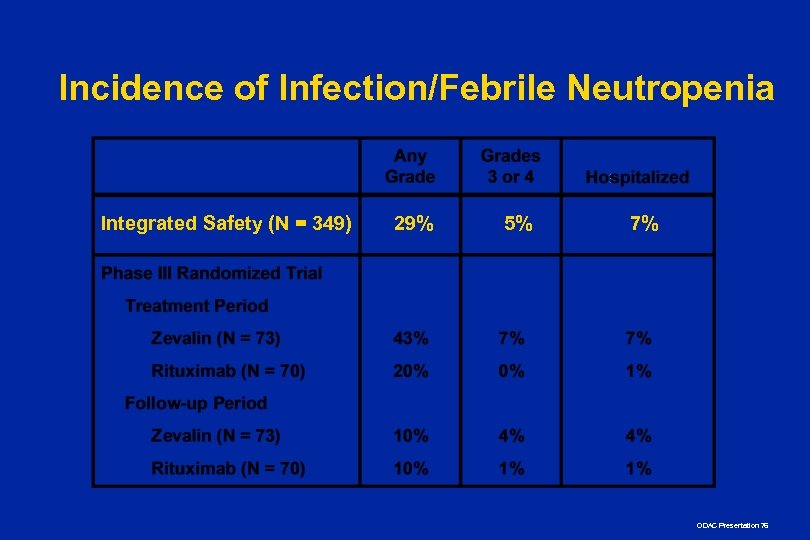
Incidence of Infection/Febrile Neutropenia Integrated Safety (N = 349) 29% 5% 7% ODAC Presentation 76
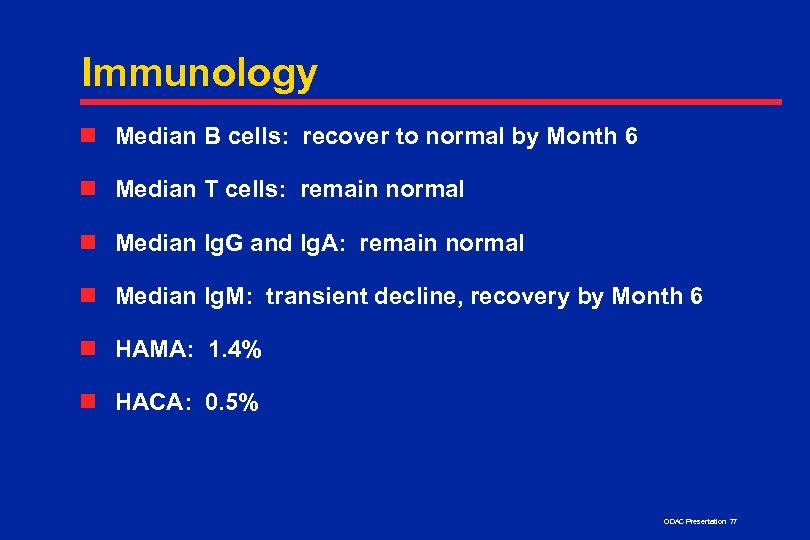
Immunology n Median B cells: recover to normal by Month 6 n Median T cells: remain normal n Median Ig. G and Ig. A: remain normal n Median Ig. M: transient decline, recovery by Month 6 n HAMA: 1. 4% n HACA: 0. 5% ODAC Presentation 77
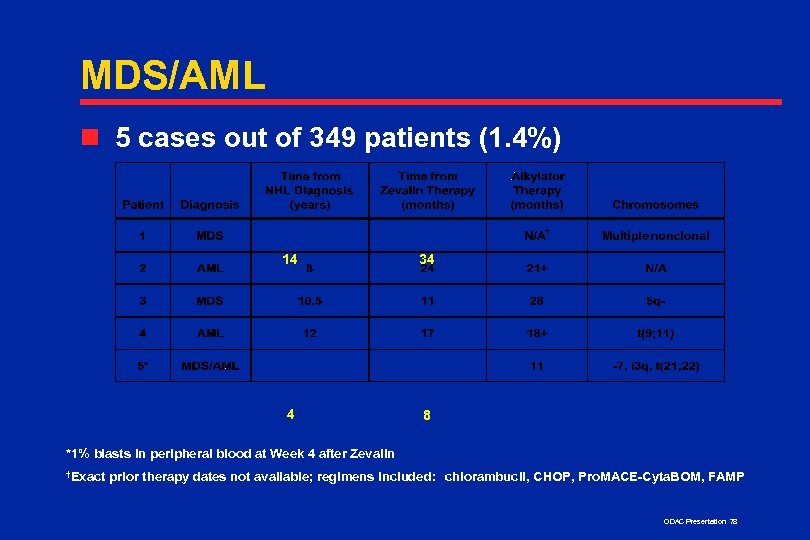
MDS/AML n 5 cases out of 349 patients (1. 4%) 14 34 4 8 *1% blasts in peripheral blood at Week 4 after Zevalin †Exact prior therapy dates not available; regimens included: chlorambucil, CHOP, Pro. MACE-Cyta. BOM, FAMP ODAC Presentation 78
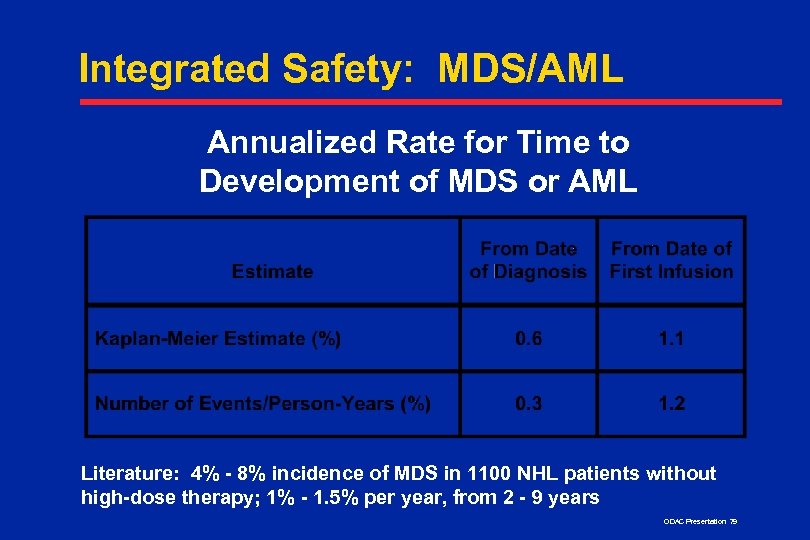
Integrated Safety: MDS/AML Annualized Rate for Time to Development of MDS or AML Literature: 4% - 8% incidence of MDS in 1100 NHL patients without high-dose therapy; 1% - 1. 5% per year, from 2 - 9 years ODAC Presentation 79

Mortality (N = 349) n 70 deaths to date n 56 secondary to NHL n 2 subsequent chemo induced neutropenic sepsis n 5 MDS/AML n 2 intracranial hemorrhage n 5 due to unrelated or pre-existing illness ODAC Presentation 80
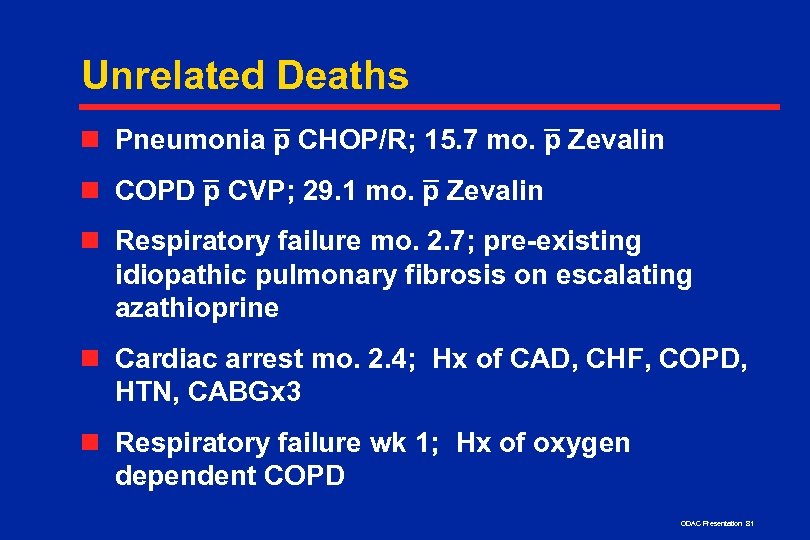
Unrelated Deaths – n Pneumonia p CHOP/R; 15. 7 mo. – Zevalin p – n COPD – CVP; 29. 1 mo. p Zevalin p n Respiratory failure mo. 2. 7; pre-existing idiopathic pulmonary fibrosis on escalating azathioprine n Cardiac arrest mo. 2. 4; Hx of CAD, CHF, COPD, HTN, CABGx 3 n Respiratory failure wk 1; Hx of oxygen dependent COPD ODAC Presentation 81
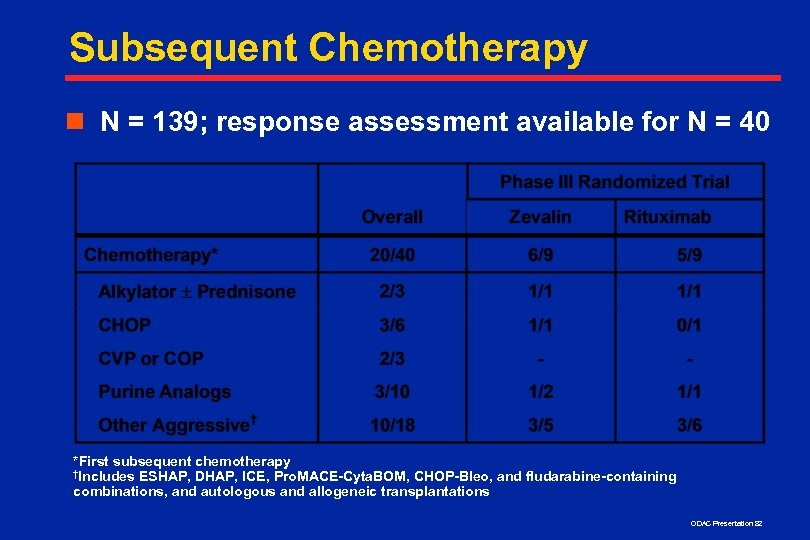
Subsequent Chemotherapy n N = 139; response assessment available for N = 40 *First subsequent chemotherapy †Includes ESHAP, DHAP, ICE, Pro. MACE-Cyta. BOM, CHOP-Bleo, and fludarabine-containing combinations, and autologous and allogeneic transplantations ODAC Presentation 82
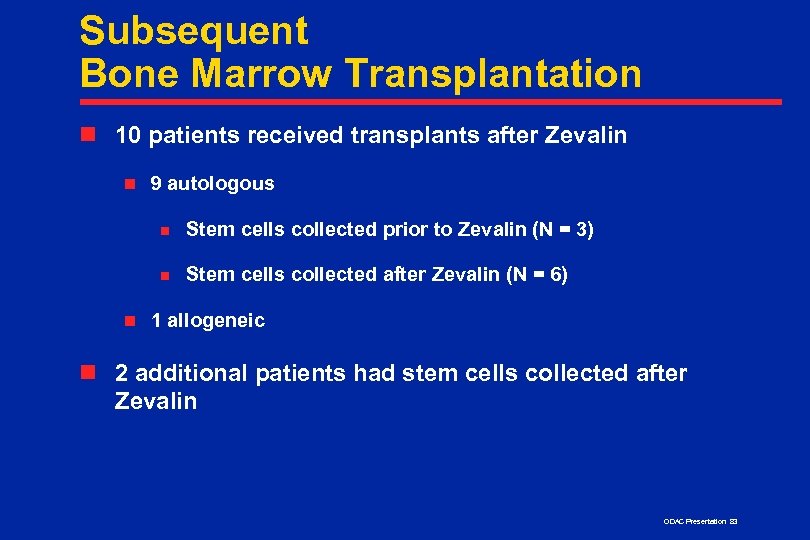
Subsequent Bone Marrow Transplantation n 10 patients received transplants after Zevalin n 9 autologous n n n Stem cells collected prior to Zevalin (N = 3) Stem cells collected after Zevalin (N = 6) 1 allogeneic n 2 additional patients had stem cells collected after Zevalin ODAC Presentation 83
Presentation Outline n Background n Phase I/II conclusions n Imaging and dosimetry n Phase III randomized trial n Phase III rituximab-refractory trial n Efficacy in transformed/nonfollicular low-grade n Integrated safety summary n Conclusions ODAC Presentation 84
Summary n Zevalin represents a new class of targeted therapy n Well-tolerated, outpatient therapy completed in 8 days n AEs primarily hematologic n Severity related to baseline platelet count and % bone marrow involvement n Nonhematologic AEs predominantly Grade 1 and 2 n Low incidence of severe infection n < 1% treatment related mortality n Incidence of HAMA/HACA: < 2% n Rare cases of MDS; within expected rate ODAC Presentation 85
Summary (cont. ) n Clinical benefit established n ORR statistically higher than rituximab control in randomized Phase III study n Trend towards longer TTP in follicular and CR/CCR patients; longer TTNT in all patients n Significant activity in heavily pretreated, bulky, rituximabrefractory population n Median TTP in responders: 15. 4 months in Phase I/II and randomized Phase III studies ODAC Presentation 86

Conclusion Zevalin therapy represents a clinically meaningful advance for patients with relapsed or refractory, low-grade, follicular, or CD 20+ transformed B-cell NHL and rituximab-refractory NHL ODAC Presentation 87

Presentation Agenda n Opening remarks n Leslie L. Shelly, Ph. D. - Associate Director, Regulatory Affairs* n Scientific and medical summary of Zevalin n Christine A. White, M. D. - Vice President, Medical Affairs* n Discussion n Christine A. White, M. D. - Vice President, Medical Affairs* n Pratik Multani, M. D. - Director, Medical Affairs* n Bryan Leigh, M. D. - Director, Oncology* *IDEC Pharmaceuticals, San Diego, CA ODAC Presentation 88

de07b80c3b376df34f6fab6a10e0b8b5.ppt