37b7bc5592159ec6c6b41b51e8d4df69.ppt
- Количество слайдов: 47

I do not object to people looking at their watches when I'm speaking. But I strongly object when they start shaking them to make sure they are still going. William Norman Birkett 1

Update on Regulatory Requirements for Combination Products Bradley Merrill Thompson, MBA, JD, RAC Epstein Becker & Green PC June 2, 2009 Cambridge Healthtech Institute 2

Topics 1. Overview a. What are combination products? b. What is the Combination Product Coalition? 2. Where are Combination Products Going? 3. Where is Combination Product Regulation Going? 4. Where are the Challenges and Opportunities? I feel like Zsa Gabor’s fifth husband. I know what I'm supposed to do but I don't know if I can make it interesting. Al Gore 3

What is a Combination Product? t. Statute -- 503(g)(1) ►Products that constitute a combination of a drug, device, or biologic t. Combination products are diverse: ►Drug-device ►Device-biologic ►Drug-device-biologic 4
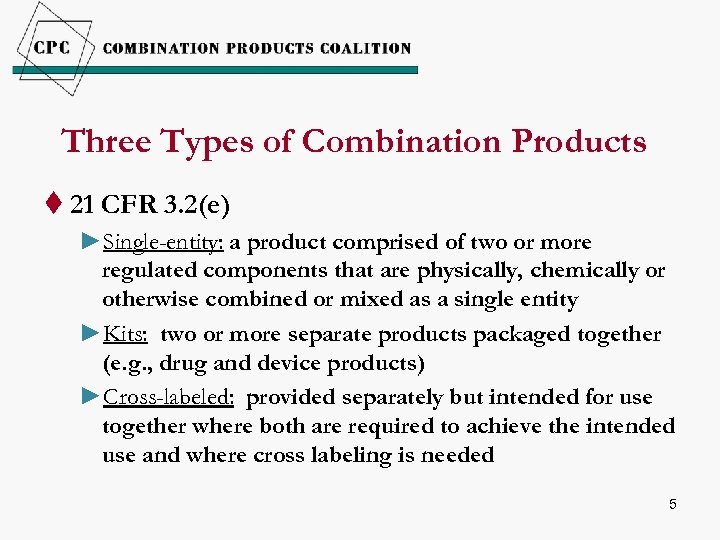
Three Types of Combination Products t 21 CFR 3. 2(e) ►Single-entity: a product comprised of two or more regulated components that are physically, chemically or otherwise combined or mixed as a single entity ►Kits: two or more separate products packaged together (e. g. , drug and device products) ►Cross-labeled: provided separately but intended for use together where both are required to achieve the intended use and where cross labeling is needed 5

Not. Combination Products t Most concomitant use of drugs, devices and biologics t Drug-drug, device-device, or biologic-biologic combinations ►Example: Products with two biologics, even if shared CDER and CBER role t General devices intended for use with a class of or otherwise unspecified drug/biologic products ►Example: Unfilled syringe or diagnostic test without specifying a particular drug 6
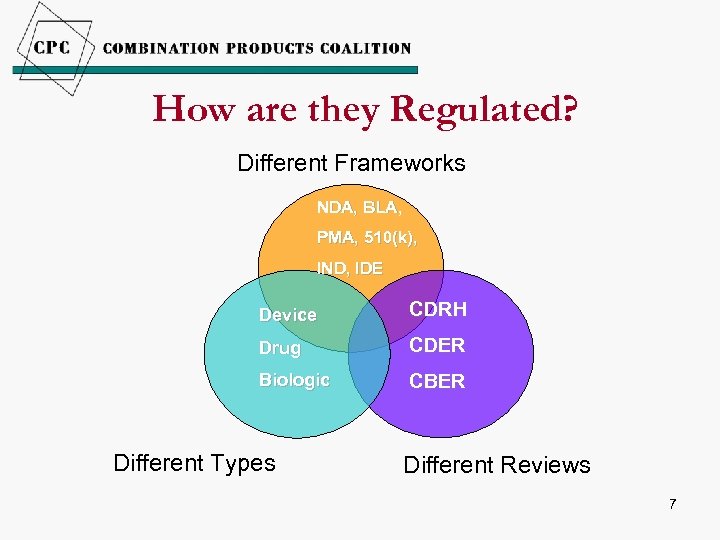
How are they Regulated? Different Frameworks NDA, BLA, PMA, 510(k), IND, IDE Device CDRH Drug CDER Biologic CBER Different Types Different Reviews 7

CPC: Purpose t To clarify and streamline the regulatory paradigm for combination products t While protecting the public health I have always wanted to be somebody. I guess I should have been more specific. -Lily Tomlin 8

Membership t Up to 20 drug, device and biologics companies have engaged in CPC activities. Some members include: ► ► ► t t Abbott Baxter Becton Dickinson Genentech Pfizer Roche Diagnostics Most active participants are regulatory affairs professionals for member companies. Diversity of industry representation is encouraged. 9
Activities t Started in 2003 with developing consensus policy positions t Advocating policy positions and working collaboratively with FDA ► Providing comments to FDA on proposed rules and guidances t Partnered with RAPS to host January 2005 policy summit attended by about 150 people. ►Topics included cross labeling, kit labeling and the labeling of single entity products. ►The summit resulted in a consensus white paper that was submitted to FDA. 10

Activities t Will partner with RAPS to host policy summit on GMPs when proposed rule is published, during comment period. t Working on comments re injector guidance (more later) t Shepherding a clinical trials proposed guidance t Legislative work 11

2007 Survey t Goals ►Evaluate current industry concerns and priorities ►Communicate these to FDA ►Inform CPC policy agenda t Why? ►In 2007, the OCP underwent several leadership and personnel changes; new permanent director effective Jan. 7, 2008 ►Also wanted to take a step back and reflect on CPC activities 12

Survey Scope & Methodology t Focused questions on: ►Demographics ►Satisfaction with existing guidance (FDA and non-FDA) ►Topics on which more or better FDA guidance is needed t Disseminated widely among industry t Asked companies to complete only one survey, but to collaborate with their colleagues 13
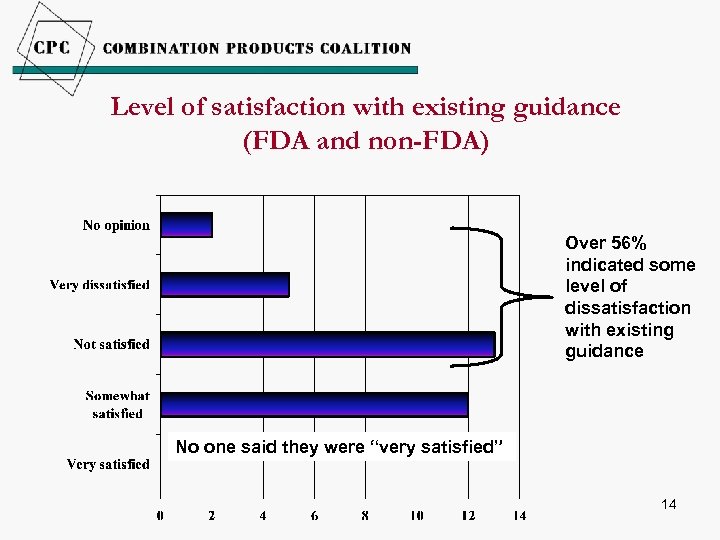
Level of satisfaction with existing guidance (FDA and non-FDA) Over 56% indicated some level of dissatisfaction with existing guidance No one said they were “very satisfied” 14
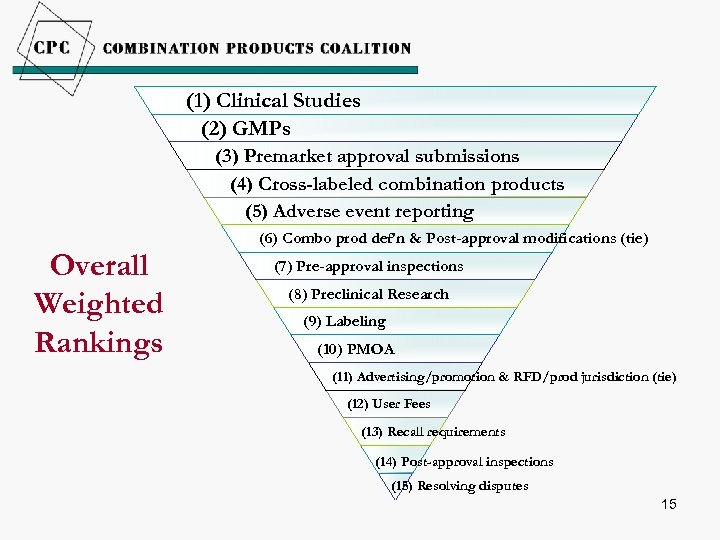
(1) Clinical Studies (2) GMPs (3) Premarket approval submissions (4) Cross-labeled combination products (5) Adverse event reporting Overall Weighted Rankings (6) Combo prod def’n & Post-approval modifications (tie) (7) Pre-approval inspections (8) Preclinical Research (9) Labeling (10) PMOA (11) Advertising/promotion & RFD/prod jurisdiction (tie) (12) User Fees (13) Recall requirements (14) Post-approval inspections (15) Resolving disputes 15

Current CPC Key Priorities t. Draft guidance on injectors t. Quality systems/GMPs t. Combo product clinical trials t. Modification of approved combination products t. Adverse incident reporting t. Clarification of OCP role Priorities are organic and change as new developments occur and progress is made. 16

Ways to Get Involved t Companies interested in CPC should visit: www. combinationproducts. com ►Membership structure ►Policy Positions t Active Linked. In group (you don’t need to be a member to join) t Free wiki experiment for drafting injector comment, link www. combinationproduct. com I've often wondered how some people in positions of this kind. . . manage without having had any acting experience. Ronald Reagan 17

Topics 1. Overview 2. Where are Combination Products Going? a. FDA experience b. Trends in submissions 3. Where is Combination Product Regulation Going? 4. Where are the Challenges and Opportunities? Politics gives guys so much power that they tend to behave badly around women. And I hope I never get into that. 18 Bill Clinton
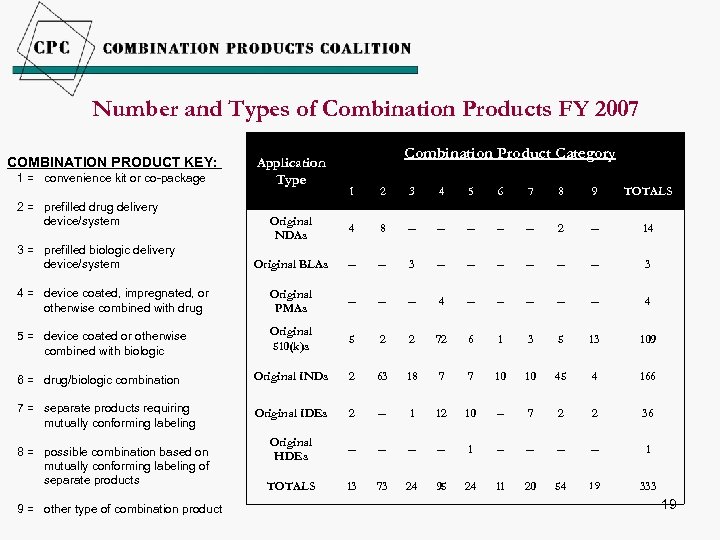
Number and Types of Combination Products FY 2007 COMBINATION PRODUCT KEY: 1 = convenience kit or co-package Application Type Combination Product Category 1 2 3 4 5 6 7 8 9 TOTALS Original NDAs 4 8 -- -- -- 2 -- 14 Original BLAs -- -- 3 -- -- -- 3 4 = device coated, impregnated, or otherwise combined with drug Original PMAs -- -- -- 4 5 = device coated or otherwise combined with biologic Original 510(k)s 5 2 2 72 6 1 3 5 13 109 6 = drug/biologic combination Original INDs 2 63 18 7 7 10 10 45 4 166 7 = separate products requiring mutually conforming labeling Original IDEs 2 -- 1 12 10 -- 7 2 2 36 Original HDEs -- -- 1 TOTALS 13 73 24 95 24 11 20 54 19 333 2 = prefilled drug delivery device/system 3 = prefilled biologic delivery device/system 8 = possible combination based on mutually conforming labeling of separate products 9 = other type of combination product 19
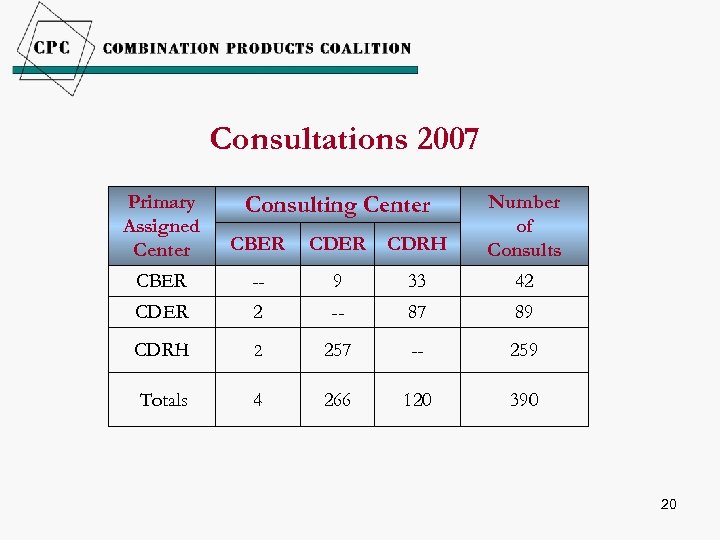
Consultations 2007 Primary Assigned Center CBER CDRH Number of Consults CBER -- 9 33 42 CDER 2 -- 87 89 CDRH 2 257 -- 259 Totals 4 266 120 390 Consulting Center 20

Number of Submissions Combination Product Application Trend 21

Topics 1. 2. 3. 4. Overview Where are Combination Products Going? Where is Combination Product Regulation Going? a. Congress b. FDA c. Internationally Where are the Challenges and Opportunities? One way to make sure crime doesn't pay would be to let the government run it. Ronald Reagan 22

Congress t Where has Congress been recently? t Where is Congress going? The voters have spoken—the bastards. Richard M. Nixon 23

Historical Development t Safe Medical Devices Act (1990) ► Added § 503(g) ► Required determination of “primary mode of action” (i. e. , drug, device, or biologic) ► Gave primary jurisdiction to the center with premarket review authority for that type of product 24

Historical Development t Food and Drug Administration Modernization Act of 1997 (“FDAMA”) ►Added § 563, Request For Designation ►Allowed sponsor to request designation as drug, biologic, device, or combination product, and/or reviewing center 25

Historical Development t Medical Device User Fee and Modernization Act of 2002 (“MDUFMA”) ► Established Office of Combination Products in order to assure: n Prompt designations and review assignments n Timely and effective premarket review n Consistent and appropriate postmarket regulation 26

Where is Congress Going? t FDA Commissioner confirmation hearings and budget discussions ►The need for combination product policy development specifically discussed t Future issues ►Some talk of unified regulation for combination products, but not serious yet ►Other talk of unified adverse reporting system t Congress trails technology, instead of leading ►That’s not a bad thing, unless they fall too far behind 27

1. 2. 3. 4. 5. 6. 7. Where is FDA Going? Office of Combination Products Clinical Trials Injector Draft Guidance GMPs Adverse Events Cross Labeling Submissions Actual Trial Question How far apart were the vehicles at the time of collision? 28

Office of Combination Products t Relatively new leadership ► Thinh Nguyen replaced Dr. Joanne Less who replaced Mark Kramer n Formerly Director Premarket Approval Section at ODE/CDRH ► Patricia Y. Love, MD, MBA - Associate Director ► Barr Weiner, from Chief Counsel’s Office ► Leigh Hayes, JD - Product Assignment Officer t Statutory Duties ► Assignment of combination products ► Ensure timely and effective premarket review ► Consistent and appropriate postmarket regulation ► Dispute resolution (timeliness vs. substance) ► Review/update guidance, agreement, practices ► Reports to Congress ► Resource to sponsors and review staff » P. L. 107 -250 – enacted 10 -26 -02 29

CPC Draft Clinical Trial Guidance t Feb. 27, 2009, the CPC filed draft guidance, FAQs on Pre-Clinical and Clinical Research on Combination Products t Developed in response to industry’s desire for guidance in this area t Topics addressed include: ►Pre-clinical safety studies ►IND and IDE submissions ►Clinical study design ►Labeling, GMP, and safety reporting issues ►Issues pertaining to specific technologies 30

Draft Injector Guidance t FDA released draft injector guidance on April 27, 2009 - Technical Considerations for Pen, Jet, and Relate Injectors Intended for Use with Drugs and Biological P t Comments due July 27, 2009 t CPC soliciting open/public comments via Wiki t Very broad scope ►Injector – “jet injectors, pen injectors, piston syringes, needle-free injectors, mechanically operated injectors, and injectors with computerized or electronic elements” ►Combination products with injector part and standalone, general use injectors 31

Draft Injector Guidance t Major concerns ►Could significantly increase burden for certain injectors (maybe 3 X for example stand-alone ), device injectors and simpler types of injectors ►Potential inconsistencies with existing device guidance (e. g. , piston syringe guidance) ►Laundry list of data requirements, rather than focused, least burdensome guidance 32

Draft Injector Guidance t Major concerns (con’t) ►Omits discussion of fundamental policy issues, for example: n. How the type/composition of injector influences regulatory requirements n. Types of submissions, e. g. , when an injector requires a separate clearance or approval n. Post-market modifications to injectors n. Any specific expectations for GMP or adverse event reporting 33

Draft Injector Guidance t More specific concerns ►Definitions n Scope of “injector” – currently the definition seems all encompassing n Other terms (e. g. , “product class”, “product line”) ►Clinical studies n Implies that there should be a clinical study for all types of injectors n Needs to clarify when FDA believes clinical data are needed ►Very prescriptive data requirements 34

t t GMPs Proposed Rule expected anytime Likely themes ► ► Combination product manufacturers must meet the requirements of both sets of applicable GMP regulations. Manufacturers may choose an “umbrella” system under which to operate, but this system must meet the requirements of bothsets of applicable GMP regulations. Manufacturers must implement certain specific provisions in order to achieve compliance with both sets of regulations (e. g. , design controls, purchasing controls, and CAPA for devices). May be a regulatory obligation to comply with certain GMP requirements even before constituent parts are physically combined, merged, or joined. Manufacturers cannot delegate ultimate responsibility for GMP compliance. 35

GMP Comment Meeting with FDA and RAPS t Will be organized quickly during comment period t Will focus on pre-written case studies t Will be in person and virtual 36

Adverse Events t Proposed Rule expected anytime. t Likely content might propose: ►mechanisms by which the postmarket safety reporting requirements ordinarily associated with the marketing application used to approve or clear a combination product may be supplemented, as appropriate, to take into account the combination nature of the product, or ►a reporting scheme in which the same types of postmarket safety reports would be submitted for a combination product, regardless of the type of marketing application used for its approval or clearance t Look at September 2005 Concept Paper 37

Cross Labeling t May 10, 2005 Public Meeting ►Transcript and presentations accessible on OCP website t New straw man proposal likely ►New public meeting planned to discuss proposal My mother never saw the irony in calling me a son-of-a-bitch. Jack Nicholson 38

What is Cross Labeling? A drug, device, or biological product packaged separately that according to its investigational plan or proposed labeling is intended for use only with an approved individually specified drug, device, or biological product where both are required to achieve the intended use, indication or effect and where upon approval of the proposed product the labeling of the approved product would need to be changed, e. g. to reflect a change in intended use, dosage form, strength, route of administration, or significant change in dose…. 21 CFR 3. 2(e)(3) Winter related injuries occur more often in the winter. -newswoman for WHIZ-TV, Zanesville, Ohio 39

Submissions t Questions: ►Initial submissions—number of them ►Supplements for product modifications t Guidance ►September 2005 Concept Paper for initial submissions ►Close to guidance on product modifications, unless goes to rulemaking 40

Initial Submissions t Agency goal seems to be to prescribe the number and type to be filed t CPC has argued for greater freedom to determine the approval pathway, within the confines of the law. ►We explain that a lot of factors, many of which the agency won’t know, affect the optimal approval route t Not clear where the agency is going 41

Submissions for Product Modifications t Agency has a draft guidance in hand ►However, still grappling with fundamental questions such as guidance or rulemaking ►Addresses pathway/type of submission issue, rather than type of evidence or data required t CPC has drafted its own guidance ►Will shift to developing questions and case studies 42

International Trends t Other jurisdictions are lagging behind FDA in the development of new guidance and approaches ►In Europe, specific regs not yet in place t Europe's approach is similarly based on primary mode of action, although it is determined differently t Medical Device Directive lays out pathway for combination products that operate as devices ►If the drug and device are a single integral product that is intended exclusively for use in a given combination, gets regulated as a drug. ►On the other hand, if a device incorporates a drug as an integral part and the drug acts on the body in an ancillary manner, the product is regulated as a device. ►In the case of a tie, it’s a drug t There is a consultation procedure (MEDDEV 2. 1/3 rev. 2 (2001)) t Little energy is being directed at harmonization (2008 Initiative)43

Topics 1. Overview 2. Where are Combination Products Going? 3. Where is Combination Product Regulation Going? 4. Where are the Challenges and Opportunities? What orators lack in depth, they make up for in length. Charles-Louis De Secondat Montesquieu 44

Practical Challenges t Combination products: ► Increasingly state-of-the-art, innovative technologies that challenge existing regulatory and scientific knowledge ► Require regulators to apply very different regulatory paradigms to one – often unique – product ► Force FDA’s nearly autonomous centers to work together t The OCP is still somewhat new, with limited resources t Different industries have different perspectives and priorities – leaving OCP to weigh the options and make choices t Most existing trade association structures mirror FDA’s product-based centers 45

Practical Opportunities t The OCP will actively seek input on its initiatives. For example: ►Injectors ►GMP ►Adverse Events t Because the OCP is so thinly staffed, industry has an opportunity to help fill the gaps with: ► Regulatory, scientific and practical knowledge ► Research ► Idea generation ► Feedback 46

Questions? Arguing with a lawyer is like mud wrestling with a pig: after a while you realize that the pig actually enjoys it. 47
37b7bc5592159ec6c6b41b51e8d4df69.ppt