d5aecefee021f1592ba2ec8260919f53.ppt
- Количество слайдов: 101
 HYPERTENSION IN THE INPATIENT SETTING Mechanisms and Pharmacologic Management
HYPERTENSION IN THE INPATIENT SETTING Mechanisms and Pharmacologic Management
 Dedicated to the memory of LEON I. GOLDBERG, MD, PHD A pioneer in the research of dopamine receptor pharmacology and physiology
Dedicated to the memory of LEON I. GOLDBERG, MD, PHD A pioneer in the research of dopamine receptor pharmacology and physiology
 Learning Objectives Outline the prevalence, pathology, and pathophysiology of hypertension in the inpatient setting. Identify treatment goals and treatment options for the severely hypertensive patient. Discuss the pharmacologic profile and potential benefits of fenoldopam in the treatment of hypertension.
Learning Objectives Outline the prevalence, pathology, and pathophysiology of hypertension in the inpatient setting. Identify treatment goals and treatment options for the severely hypertensive patient. Discuss the pharmacologic profile and potential benefits of fenoldopam in the treatment of hypertension.
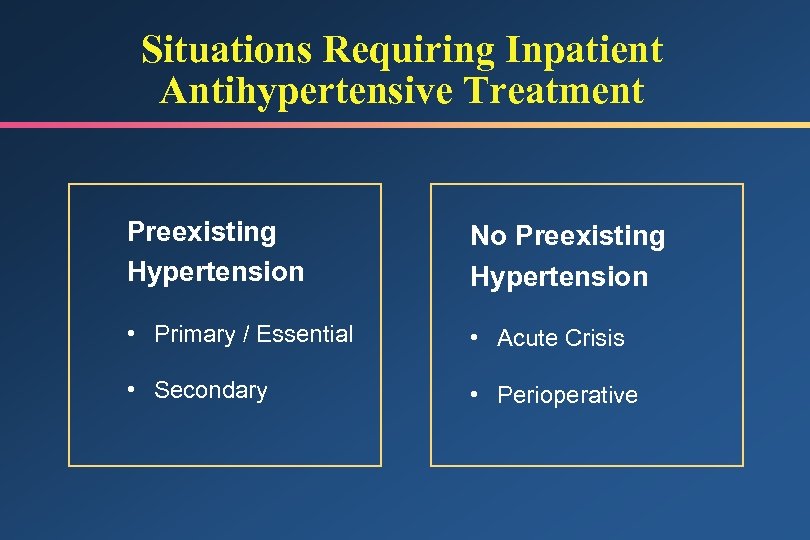 Situations Requiring Inpatient Antihypertensive Treatment Preexisting Hypertension No Preexisting Hypertension • Primary / Essential • Acute Crisis • Secondary • Perioperative
Situations Requiring Inpatient Antihypertensive Treatment Preexisting Hypertension No Preexisting Hypertension • Primary / Essential • Acute Crisis • Secondary • Perioperative
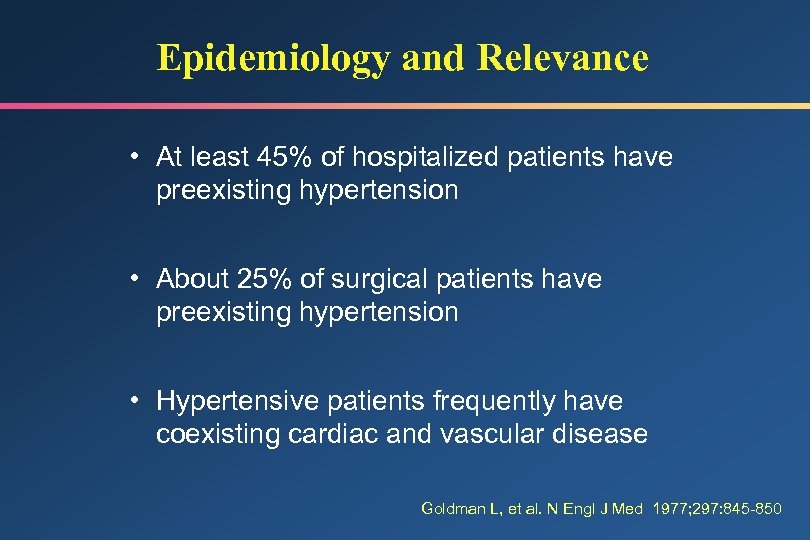 Epidemiology and Relevance • At least 45% of hospitalized patients have preexisting hypertension • About 25% of surgical patients have preexisting hypertension • Hypertensive patients frequently have coexisting cardiac and vascular disease Goldman L, et al. N Engl J Med 1977; 297: 845 -850
Epidemiology and Relevance • At least 45% of hospitalized patients have preexisting hypertension • About 25% of surgical patients have preexisting hypertension • Hypertensive patients frequently have coexisting cardiac and vascular disease Goldman L, et al. N Engl J Med 1977; 297: 845 -850
 Parenteral Treatment of Hypertension May be Required in. . . • EM • MICU • SICU • OR • PACU • Obstetrics Suite
Parenteral Treatment of Hypertension May be Required in. . . • EM • MICU • SICU • OR • PACU • Obstetrics Suite
 Parenteral Treatment of Hypertension May be Required for Medical Emergencies • Uncontrolled or Malignant Hypertension • Drug-Induced Hypertension – cocaine, amphetamines – drug withdrawal – drug-drug interactions • Endocrine Disorders
Parenteral Treatment of Hypertension May be Required for Medical Emergencies • Uncontrolled or Malignant Hypertension • Drug-Induced Hypertension – cocaine, amphetamines – drug withdrawal – drug-drug interactions • Endocrine Disorders
 Parenteral Treatment of Hypertension May Be Required During/After Perioperative Period • Cardiac Surgery • Major Vascular Surgery – carotid endarterectomy – aortic surgery • Neurosurgery • Head and Neck Surgery • Renal Transplantation • Major Trauma - Burns or Head Injury
Parenteral Treatment of Hypertension May Be Required During/After Perioperative Period • Cardiac Surgery • Major Vascular Surgery – carotid endarterectomy – aortic surgery • Neurosurgery • Head and Neck Surgery • Renal Transplantation • Major Trauma - Burns or Head Injury
 Factors in the Development of Acute Hypertension ER/CC OR PACU Myocardial Ischemia Vascular clamping (afterload) Pain Hypercarbia/ Hypoxemia Reduced organ perfusion -Renal -Cerebral Hyperdynamic Myocardium Malignant Hyperthermia Diastolic Dysfunction Anxiety Distended Bladder Hypervolemia Vasoconstriction
Factors in the Development of Acute Hypertension ER/CC OR PACU Myocardial Ischemia Vascular clamping (afterload) Pain Hypercarbia/ Hypoxemia Reduced organ perfusion -Renal -Cerebral Hyperdynamic Myocardium Malignant Hyperthermia Diastolic Dysfunction Anxiety Distended Bladder Hypervolemia Vasoconstriction
 Adverse Consequences of Uncontrolled Hypertension • Postsurgical – Hemorrhage – Suture line disruption – Aortic dissection • End Organ Injury – Myocardial ischemia – Stroke – Renal failure • Pulmonary Edema
Adverse Consequences of Uncontrolled Hypertension • Postsurgical – Hemorrhage – Suture line disruption – Aortic dissection • End Organ Injury – Myocardial ischemia – Stroke – Renal failure • Pulmonary Edema
 Sympathetic Nervous System Regulation of Blood Pressure CNS Adrenal Gland Baroreceptor Reflexes Adrenergic Tone Catecholamines Veins Capacitance Arteries Resistance Afterload Preload Heart Cardiac Output Volume/Pressure Renin/Angiotensin Blood Pressure Kidney
Sympathetic Nervous System Regulation of Blood Pressure CNS Adrenal Gland Baroreceptor Reflexes Adrenergic Tone Catecholamines Veins Capacitance Arteries Resistance Afterload Preload Heart Cardiac Output Volume/Pressure Renin/Angiotensin Blood Pressure Kidney
 Renin-Angiotensin-Aldosterone Regulation of Blood Pressure Renin Substrate Angiotensin II Renin Aldosterone Kidney Sodium & Water Reabsorption Adrenal Cortex Blood Pressure Vasoconstriction
Renin-Angiotensin-Aldosterone Regulation of Blood Pressure Renin Substrate Angiotensin II Renin Aldosterone Kidney Sodium & Water Reabsorption Adrenal Cortex Blood Pressure Vasoconstriction
 Preoperative Hypertension “Effective intraoperative management may be more important than preoperative hypertensive control in terms of decreasing clinically significant blood pressure lability and cardiovascular complications in patients who have mild to moderate hypertension. ” Goldman L, Caldera DL. Anesthesiology 1979; 50: 285 -292
Preoperative Hypertension “Effective intraoperative management may be more important than preoperative hypertensive control in terms of decreasing clinically significant blood pressure lability and cardiovascular complications in patients who have mild to moderate hypertension. ” Goldman L, Caldera DL. Anesthesiology 1979; 50: 285 -292
 Inpatient Hypertension: Therapeutic Considerations Therapy – Treat the underlying cause – Provide adequate anesthesia/analgesia – Administer antihypertensive medications
Inpatient Hypertension: Therapeutic Considerations Therapy – Treat the underlying cause – Provide adequate anesthesia/analgesia – Administer antihypertensive medications
 Hypertension in the United States · 50 million adults have high blood pressure · 25% are unaware of this condition · 72. 6% are not well controlled at goal of <140/90 · Majority have additional CV risk factors JNC VI. Arch Intern Med 1997; 157: 2413 -2448
Hypertension in the United States · 50 million adults have high blood pressure · 25% are unaware of this condition · 72. 6% are not well controlled at goal of <140/90 · Majority have additional CV risk factors JNC VI. Arch Intern Med 1997; 157: 2413 -2448
 Classification of Blood Pressure* Hypertensive+ Stage 1 140 -159 Or 90 -99 Stage 2 160 -179 Or 100 -109 Stage 3** 180 Or 110 *When SBP and DBP fall into different categories, use higher classification. +Based on average of at least two readings or at least two visits. **Assess for presence of risk factors and target organ disease. JNC VI. Arch Intern Med 1997; 157: 2413 -2448
Classification of Blood Pressure* Hypertensive+ Stage 1 140 -159 Or 90 -99 Stage 2 160 -179 Or 100 -109 Stage 3** 180 Or 110 *When SBP and DBP fall into different categories, use higher classification. +Based on average of at least two readings or at least two visits. **Assess for presence of risk factors and target organ disease. JNC VI. Arch Intern Med 1997; 157: 2413 -2448
 Classification of Severe Hypertension Uncomplicated Stage 3 HTN Hypertensive Crises · urgencies · emergencies JNC VI. Arch Intern Med 1997; 157: 2413 -2448
Classification of Severe Hypertension Uncomplicated Stage 3 HTN Hypertensive Crises · urgencies · emergencies JNC VI. Arch Intern Med 1997; 157: 2413 -2448
 Hypertensive Urgencies: Defined by Effects or Setting Hypertension with Progressive target organ damage
Hypertensive Urgencies: Defined by Effects or Setting Hypertension with Progressive target organ damage
 Hypertensive Emergencies: Defined by Effects Severe HTN with acute end organ damage: Central nervous system Myocardial ischemia or heart failure Renal damage Active hemorrhage Eclampsia Microangiopathic hemolytic anemia Aortic dissection
Hypertensive Emergencies: Defined by Effects Severe HTN with acute end organ damage: Central nervous system Myocardial ischemia or heart failure Renal damage Active hemorrhage Eclampsia Microangiopathic hemolytic anemia Aortic dissection
 Hypertensive Emergencies Are More Than Blood Pressure Measurement • Hypertensive emergencies generally occur with DBP 140 mm Hg, but can be much lower • Baseline level of hypertension and rate of rise are also important • There is much overlap between groups and categories, i. e. , cannot be defined by BP alone Kincaid-Smith P. Aust N Z J Med 1981; 11(Suppl 1): 64 -68
Hypertensive Emergencies Are More Than Blood Pressure Measurement • Hypertensive emergencies generally occur with DBP 140 mm Hg, but can be much lower • Baseline level of hypertension and rate of rise are also important • There is much overlap between groups and categories, i. e. , cannot be defined by BP alone Kincaid-Smith P. Aust N Z J Med 1981; 11(Suppl 1): 64 -68
 Hypertensive Emergencies: Common Etiologies • Medication noncompliance / withdrawal • Accelerated hypertension in a patient with preexisting hypertension • Renovascular hypertension • Acute glomerulonephritis
Hypertensive Emergencies: Common Etiologies • Medication noncompliance / withdrawal • Accelerated hypertension in a patient with preexisting hypertension • Renovascular hypertension • Acute glomerulonephritis
 Hypertensive Emergencies: Other Etiologies Sympathomimetic drug poisonings Eclampsia Pheochromocytoma MAO inhibitor interactions
Hypertensive Emergencies: Other Etiologies Sympathomimetic drug poisonings Eclampsia Pheochromocytoma MAO inhibitor interactions
 Treatment Guidelines* Hypertensive Emergencies Initiate treatment immediately Hypertensive Urgencies Reduce BP within a few hours Non-urgent Stage 3 Hypertension Reduce BP within one week *JNC VI. Arch Intern Med 1997; 157: 2413 -2448
Treatment Guidelines* Hypertensive Emergencies Initiate treatment immediately Hypertensive Urgencies Reduce BP within a few hours Non-urgent Stage 3 Hypertension Reduce BP within one week *JNC VI. Arch Intern Med 1997; 157: 2413 -2448
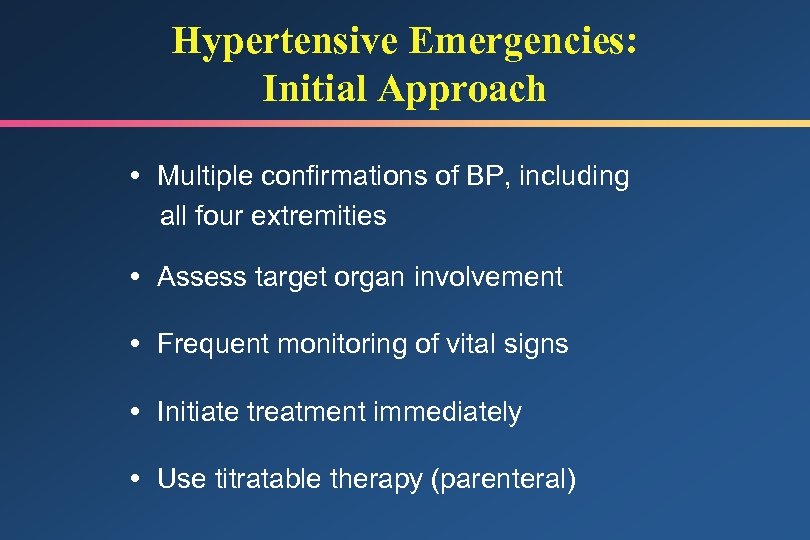 Hypertensive Emergencies: Initial Approach Multiple confirmations of BP, including all four extremities Assess target organ involvement Frequent monitoring of vital signs Initiate treatment immediately Use titratable therapy (parenteral)
Hypertensive Emergencies: Initial Approach Multiple confirmations of BP, including all four extremities Assess target organ involvement Frequent monitoring of vital signs Initiate treatment immediately Use titratable therapy (parenteral)
 Endpoints of Antihypertensive Therapy Reduce MAP by 20 -25% or Reduce MAP to 110 -120 mm. Hg (whichever is higher) Achieve target BP within 2 -4 hours
Endpoints of Antihypertensive Therapy Reduce MAP by 20 -25% or Reduce MAP to 110 -120 mm. Hg (whichever is higher) Achieve target BP within 2 -4 hours
 Hypertensive Emergencies: Control the BP for Patients with. . . • Aortic dissection • Active arterial hemorrhage • Acute myocardial infarction • Intracranial hemorrhage
Hypertensive Emergencies: Control the BP for Patients with. . . • Aortic dissection • Active arterial hemorrhage • Acute myocardial infarction • Intracranial hemorrhage
 IV Therapeutics • • • Alpha Blockers ACE Inhibitors Beta Blockers Calcium Channel Blockers Diuretics Dopamine-1 Agonists Ganglionic Blockers Nitrovasodilators Other Vasodilators
IV Therapeutics • • • Alpha Blockers ACE Inhibitors Beta Blockers Calcium Channel Blockers Diuretics Dopamine-1 Agonists Ganglionic Blockers Nitrovasodilators Other Vasodilators
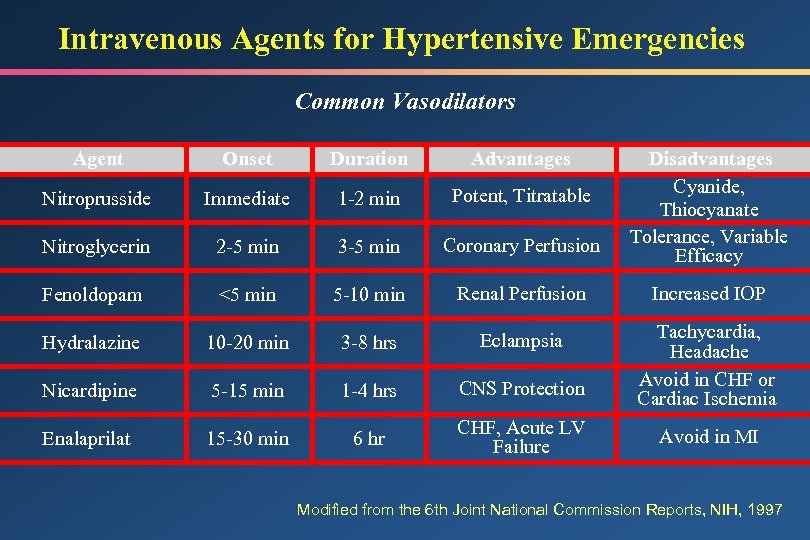 Intravenous Agents for Hypertensive Emergencies Common Vasodilators Agent Onset Duration Advantages Disadvantages Cyanide, Thiocyanate Tolerance, Variable Efficacy Nitroprusside Immediate 1 -2 min Potent, Titratable Nitroglycerin 2 -5 min 3 -5 min Coronary Perfusion Fenoldopam <5 min 5 -10 min Renal Perfusion Increased IOP Hydralazine 10 -20 min 3 -8 hrs Eclampsia Nicardipine 5 -15 min 1 -4 hrs CNS Protection Tachycardia, Headache Avoid in CHF or Cardiac Ischemia Enalaprilat 15 -30 min 6 hr CHF, Acute LV Failure Avoid in MI Modified from the 6 th Joint National Commission Reports, NIH, 1997
Intravenous Agents for Hypertensive Emergencies Common Vasodilators Agent Onset Duration Advantages Disadvantages Cyanide, Thiocyanate Tolerance, Variable Efficacy Nitroprusside Immediate 1 -2 min Potent, Titratable Nitroglycerin 2 -5 min 3 -5 min Coronary Perfusion Fenoldopam <5 min 5 -10 min Renal Perfusion Increased IOP Hydralazine 10 -20 min 3 -8 hrs Eclampsia Nicardipine 5 -15 min 1 -4 hrs CNS Protection Tachycardia, Headache Avoid in CHF or Cardiac Ischemia Enalaprilat 15 -30 min 6 hr CHF, Acute LV Failure Avoid in MI Modified from the 6 th Joint National Commission Reports, NIH, 1997
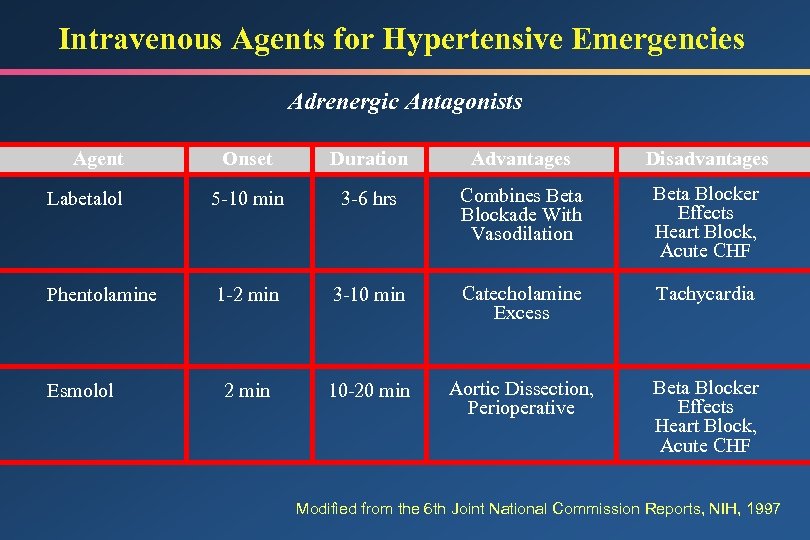 Intravenous Agents for Hypertensive Emergencies Adrenergic Antagonists Onset Duration Advantages Disadvantages Labetalol 5 -10 min 3 -6 hrs Combines Beta Blockade With Vasodilation Beta Blocker Effects Heart Block, Acute CHF Phentolamine 1 -2 min 3 -10 min Catecholamine Excess Tachycardia 2 min 10 -20 min Aortic Dissection, Perioperative Beta Blocker Effects Heart Block, Acute CHF Agent Esmolol Modified from the 6 th Joint National Commission Reports, NIH, 1997
Intravenous Agents for Hypertensive Emergencies Adrenergic Antagonists Onset Duration Advantages Disadvantages Labetalol 5 -10 min 3 -6 hrs Combines Beta Blockade With Vasodilation Beta Blocker Effects Heart Block, Acute CHF Phentolamine 1 -2 min 3 -10 min Catecholamine Excess Tachycardia 2 min 10 -20 min Aortic Dissection, Perioperative Beta Blocker Effects Heart Block, Acute CHF Agent Esmolol Modified from the 6 th Joint National Commission Reports, NIH, 1997
 Acute Hypertensive Situations Ideal Therapeutic Agent Parenteral administration Rapid onset and offset (minutes) Easy titratability Reliable efficacy Safe across patient populations Ease of use Cost effectiveness
Acute Hypertensive Situations Ideal Therapeutic Agent Parenteral administration Rapid onset and offset (minutes) Easy titratability Reliable efficacy Safe across patient populations Ease of use Cost effectiveness
 Sodium Nitroprusside Profile Advantages • Immediate onset • Short duration of action • Potent Limitations • Light sensitive • Arterial catheter usually recommended • ICU-level care usually required
Sodium Nitroprusside Profile Advantages • Immediate onset • Short duration of action • Potent Limitations • Light sensitive • Arterial catheter usually recommended • ICU-level care usually required
 Sodium Nitroprusside Adverse Effects • Excessive Hypotension • Tachyphylaxis (hyperdynamic response) • Redistribution of Flow • Intrapulmonary Shunt • Coronary Steal • Reduced Renal Blood Flow • Platelet Dysfunction • Toxicity • Cyanide • Thiocyanate
Sodium Nitroprusside Adverse Effects • Excessive Hypotension • Tachyphylaxis (hyperdynamic response) • Redistribution of Flow • Intrapulmonary Shunt • Coronary Steal • Reduced Renal Blood Flow • Platelet Dysfunction • Toxicity • Cyanide • Thiocyanate
 Metabolism of Sodium Nitroprusside Oxyhemoglobin Nitroprusside Nonenzymatic Nitroprusside Radical CN Thiosulfate Hepatic - Methemoglobin Cyanmethemoglobin Cytochrome Oxidases Rhodanase - Inactive Cytochromes Thiocyanate (SCN ) Renal Excretion TOXICITY Tinker JH, Michenfelder JD. Anesthesiology 1976; 45: 340 -354
Metabolism of Sodium Nitroprusside Oxyhemoglobin Nitroprusside Nonenzymatic Nitroprusside Radical CN Thiosulfate Hepatic - Methemoglobin Cyanmethemoglobin Cytochrome Oxidases Rhodanase - Inactive Cytochromes Thiocyanate (SCN ) Renal Excretion TOXICITY Tinker JH, Michenfelder JD. Anesthesiology 1976; 45: 340 -354
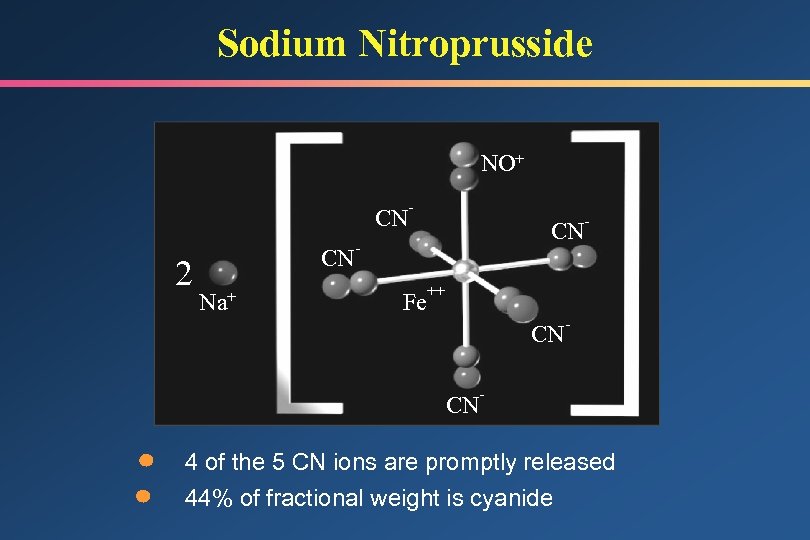 Sodium Nitroprusside NO+ CN 2 CN Na+ - CN - Fe - ++ CN CN - - 4 of the 5 CN ions are promptly released 44% of fractional weight is cyanide
Sodium Nitroprusside NO+ CN 2 CN Na+ - CN - Fe - ++ CN CN - - 4 of the 5 CN ions are promptly released 44% of fractional weight is cyanide
 Signs Of Cyanide Toxicity • Increased mixed venous saturation • Increased metabolic acidosis • Loss of consciousness and abnormal breathing patterns • Death may be very rapid
Signs Of Cyanide Toxicity • Increased mixed venous saturation • Increased metabolic acidosis • Loss of consciousness and abnormal breathing patterns • Death may be very rapid
 Additional Costs Often Associated With Nitroprusside Infusions • Arterial blood gas measurements • Lactate concentrations • Cyanide / thiocyanate monitoring • Invasive blood pressure monitoring
Additional Costs Often Associated With Nitroprusside Infusions • Arterial blood gas measurements • Lactate concentrations • Cyanide / thiocyanate monitoring • Invasive blood pressure monitoring
 Nitroglycerin • Coronary vasodilator • Direct venodilator (variable arterial effects) • Requires special tubing for administration • Side effects: headaches and tachycardia • Variable efficacy and tachyphylaxis • Methemoglobinemia
Nitroglycerin • Coronary vasodilator • Direct venodilator (variable arterial effects) • Requires special tubing for administration • Side effects: headaches and tachycardia • Variable efficacy and tachyphylaxis • Methemoglobinemia
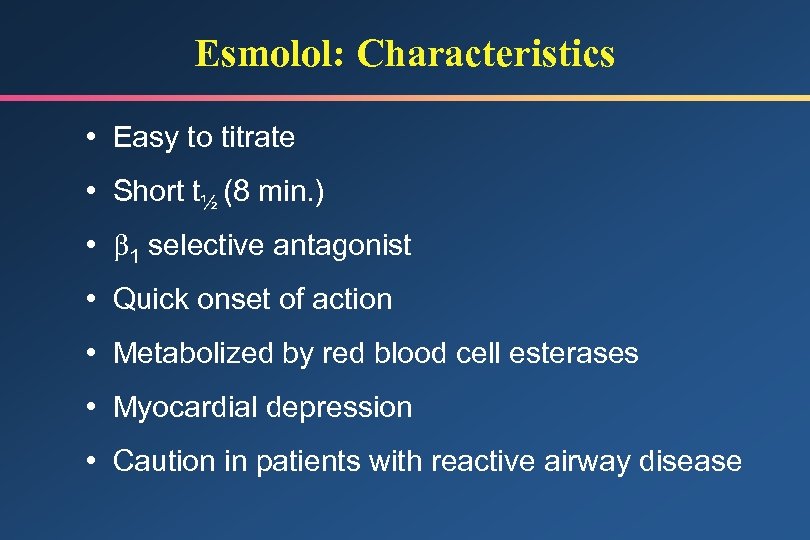 Esmolol: Characteristics • Easy to titrate • Short t½ (8 min. ) • 1 selective antagonist • Quick onset of action • Metabolized by red blood cell esterases • Myocardial depression • Caution in patients with reactive airway disease
Esmolol: Characteristics • Easy to titrate • Short t½ (8 min. ) • 1 selective antagonist • Quick onset of action • Metabolized by red blood cell esterases • Myocardial depression • Caution in patients with reactive airway disease
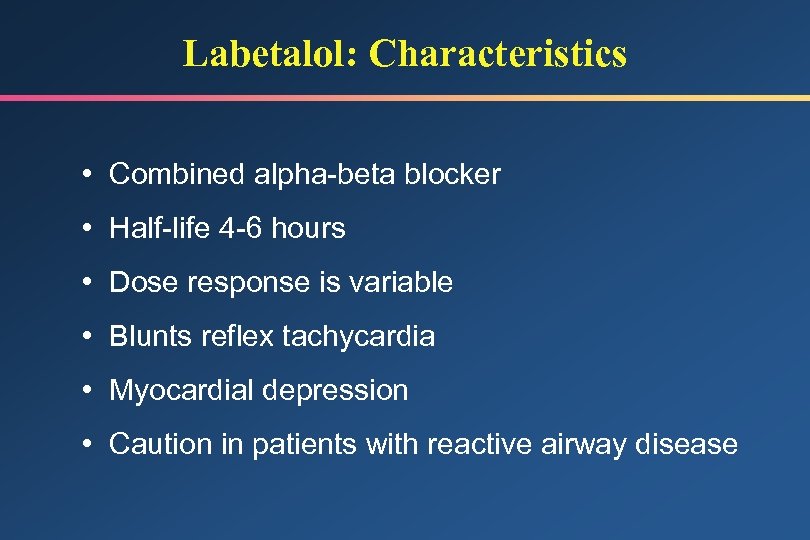 Labetalol: Characteristics • Combined alpha-beta blocker • Half-life 4 -6 hours • Dose response is variable • Blunts reflex tachycardia • Myocardial depression • Caution in patients with reactive airway disease
Labetalol: Characteristics • Combined alpha-beta blocker • Half-life 4 -6 hours • Dose response is variable • Blunts reflex tachycardia • Myocardial depression • Caution in patients with reactive airway disease
 Nifedipine Capsules: Characteristics · Provides non-oral route for NPO patients · Requires breaking capsule, sublingual administration · Absorption variable - Abrupt hypotension may occur - May exacerbate myocardial ischemia
Nifedipine Capsules: Characteristics · Provides non-oral route for NPO patients · Requires breaking capsule, sublingual administration · Absorption variable - Abrupt hypotension may occur - May exacerbate myocardial ischemia
 Nicardipine: Characteristics • Dihydropyridine • Water soluble and light stable (allows for IV infusion) • Slow onset and offset • Arterial catheter not mandatory • May accumulate • Variable duration of hypertensive effect
Nicardipine: Characteristics • Dihydropyridine • Water soluble and light stable (allows for IV infusion) • Slow onset and offset • Arterial catheter not mandatory • May accumulate • Variable duration of hypertensive effect
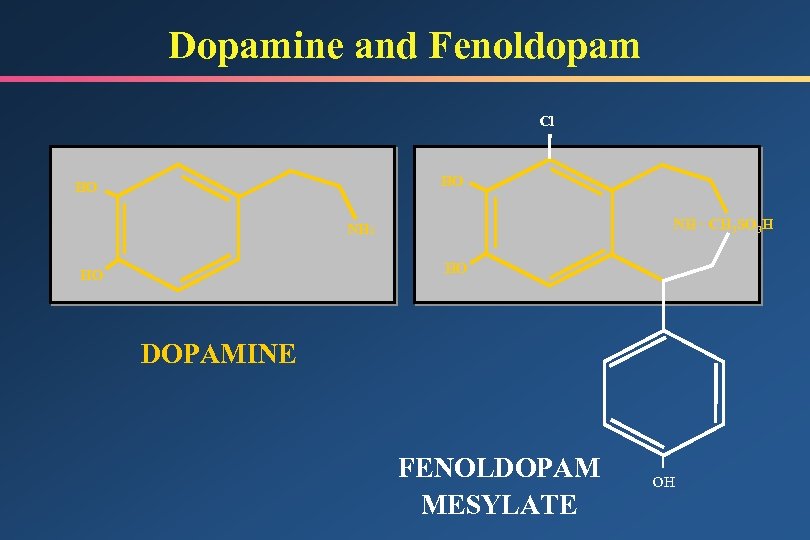 Dopamine and Fenoldopam Cl HO HO NH · CH 3 SO 3 H NH 2 HO HO DOPAMINE FENOLDOPAM MESYLATE OH
Dopamine and Fenoldopam Cl HO HO NH · CH 3 SO 3 H NH 2 HO HO DOPAMINE FENOLDOPAM MESYLATE OH
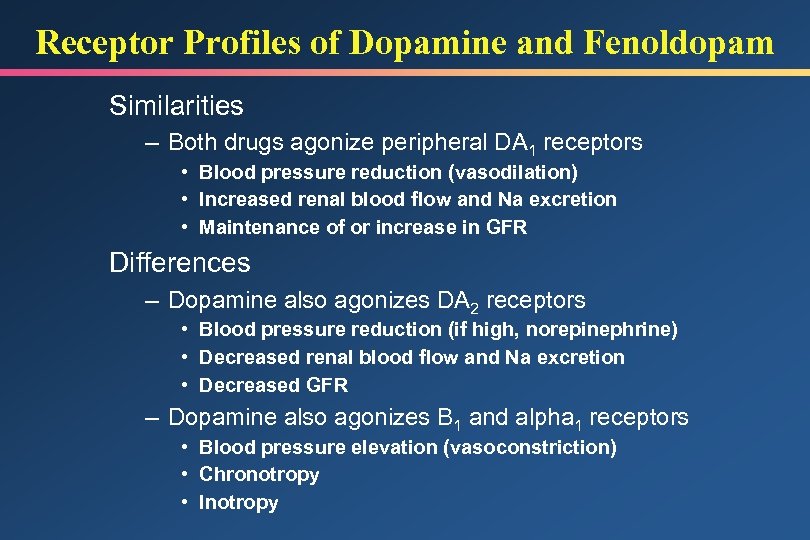 Receptor Profiles of Dopamine and Fenoldopam Similarities – Both drugs agonize peripheral DA 1 receptors • Blood pressure reduction (vasodilation) • Increased renal blood flow and Na excretion • Maintenance of or increase in GFR Differences – Dopamine also agonizes DA 2 receptors • Blood pressure reduction (if high, norepinephrine) • Decreased renal blood flow and Na excretion • Decreased GFR – Dopamine also agonizes B 1 and alpha 1 receptors • Blood pressure elevation (vasoconstriction) • Chronotropy • Inotropy
Receptor Profiles of Dopamine and Fenoldopam Similarities – Both drugs agonize peripheral DA 1 receptors • Blood pressure reduction (vasodilation) • Increased renal blood flow and Na excretion • Maintenance of or increase in GFR Differences – Dopamine also agonizes DA 2 receptors • Blood pressure reduction (if high, norepinephrine) • Decreased renal blood flow and Na excretion • Decreased GFR – Dopamine also agonizes B 1 and alpha 1 receptors • Blood pressure elevation (vasoconstriction) • Chronotropy • Inotropy
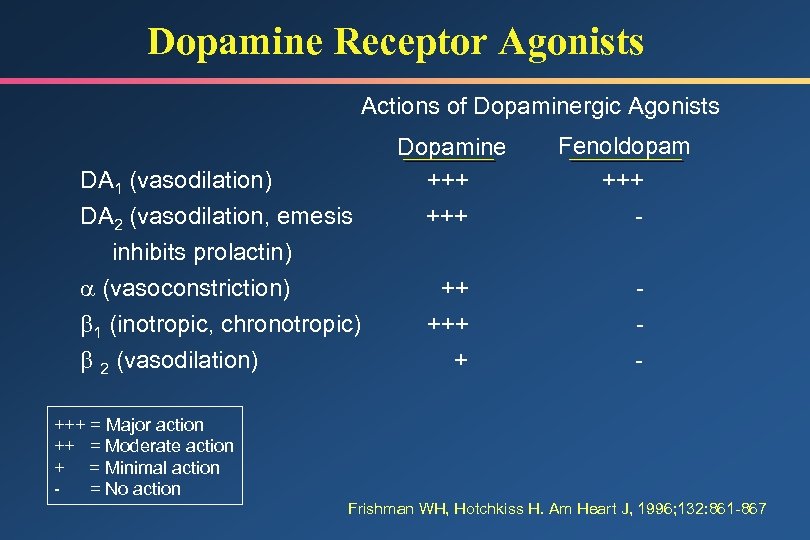 Dopamine Receptor Agonists Actions of Dopaminergic Agonists DA 1 (vasodilation) DA 2 (vasodilation, emesis Dopamine +++ inhibits prolactin) (vasoconstriction) 1 (inotropic, chronotropic) 2 (vasodilation) ++ + Fenoldopam +++ - +++ = Major action ++ = Moderate action + = Minimal action = No action Frishman WH, Hotchkiss H. Am Heart J, 1996; 132: 861 -867
Dopamine Receptor Agonists Actions of Dopaminergic Agonists DA 1 (vasodilation) DA 2 (vasodilation, emesis Dopamine +++ inhibits prolactin) (vasoconstriction) 1 (inotropic, chronotropic) 2 (vasodilation) ++ + Fenoldopam +++ - +++ = Major action ++ = Moderate action + = Minimal action = No action Frishman WH, Hotchkiss H. Am Heart J, 1996; 132: 861 -867
 Peripheral Dopamine Receptor Subtypes Location DA 1 • Postsynaptic smooth muscle • Proximal tubule • Cortical collecting duct DA 2 • Presynaptic • Glomerulus • Renal nerves • Adrenal cortex Secondary Messenger G-protein linked increased adenylate cyclase Inhibition of adenylate cyclase decreased NE release Systemic Effects Peripheral vasodilation • Increased RBF Renal Effects* • Increased GFR or no change • Natriuresis (inhibition of NA/K ATPase via protein kinase C and NA/H exchanger via adenyl cyclase) • Diuresis • Decreased RBF • Decreased GFR • Decreased Na and H 20 excretion • Decreased aldosterone * Carey RM, et al. Am J Hypertens, 1990; 3(6 Pt 2): 59 S-63 S
Peripheral Dopamine Receptor Subtypes Location DA 1 • Postsynaptic smooth muscle • Proximal tubule • Cortical collecting duct DA 2 • Presynaptic • Glomerulus • Renal nerves • Adrenal cortex Secondary Messenger G-protein linked increased adenylate cyclase Inhibition of adenylate cyclase decreased NE release Systemic Effects Peripheral vasodilation • Increased RBF Renal Effects* • Increased GFR or no change • Natriuresis (inhibition of NA/K ATPase via protein kinase C and NA/H exchanger via adenyl cyclase) • Diuresis • Decreased RBF • Decreased GFR • Decreased Na and H 20 excretion • Decreased aldosterone * Carey RM, et al. Am J Hypertens, 1990; 3(6 Pt 2): 59 S-63 S
 Dopamine: Lack of Pharmacological Specificity • BP effects variable, dose-dependent • 1: increased heart rate, tachyarrhythmias • 1: vasoconstriction • Minute ventilation decreases • Possible respiratory depression
Dopamine: Lack of Pharmacological Specificity • BP effects variable, dose-dependent • 1: increased heart rate, tachyarrhythmias • 1: vasoconstriction • Minute ventilation decreases • Possible respiratory depression
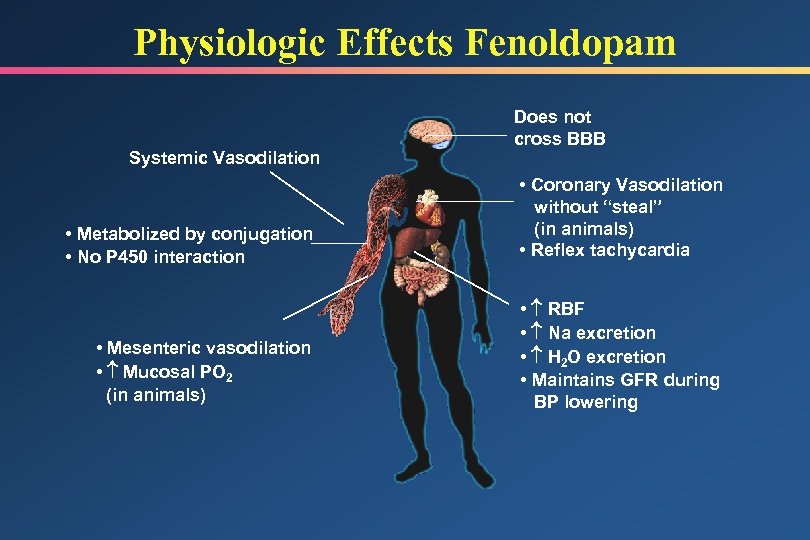 Physiologic Effects Fenoldopam Systemic Vasodilation Does not cross BBB • Metabolized by conjugation • No P 450 interaction • Coronary Vasodilation without “steal” (in animals) • Reflex tachycardia • Mesenteric vasodilation • Mucosal PO 2 (in animals) • RBF • Na excretion • H 2 O excretion • Maintains GFR during BP lowering
Physiologic Effects Fenoldopam Systemic Vasodilation Does not cross BBB • Metabolized by conjugation • No P 450 interaction • Coronary Vasodilation without “steal” (in animals) • Reflex tachycardia • Mesenteric vasodilation • Mucosal PO 2 (in animals) • RBF • Na excretion • H 2 O excretion • Maintains GFR during BP lowering
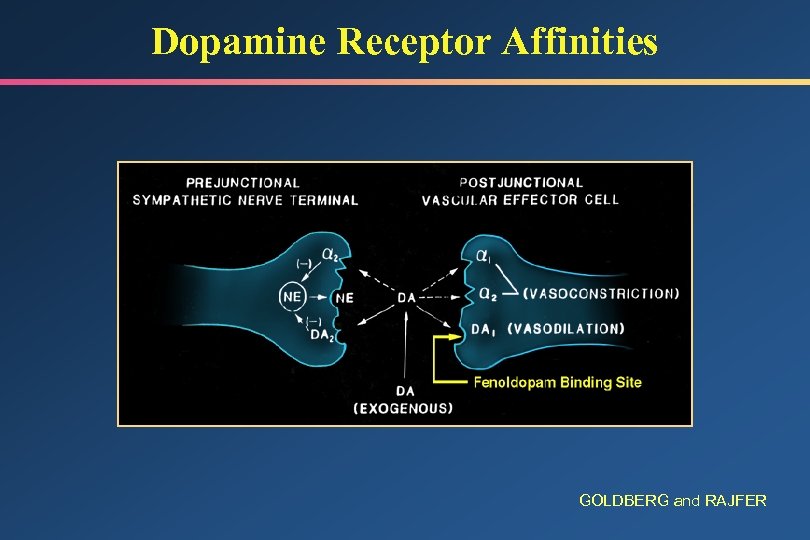 Dopamine Receptor Affinities GOLDBERG and RAJFER
Dopamine Receptor Affinities GOLDBERG and RAJFER
 Fenoldopam Receptor Activity • Selective peripheral dopamine-1 (DA 1) receptor agonism – Systemic vasodilation – Regional vasodilation (especially renal) – Renal proximal and distal tubular effects • No binding to DA 2 or beta-adrenergic receptors • No alpha-adrenergic agonism, but is an alpha 1 antagonist • Does not cross blood brain barrier
Fenoldopam Receptor Activity • Selective peripheral dopamine-1 (DA 1) receptor agonism – Systemic vasodilation – Regional vasodilation (especially renal) – Renal proximal and distal tubular effects • No binding to DA 2 or beta-adrenergic receptors • No alpha-adrenergic agonism, but is an alpha 1 antagonist • Does not cross blood brain barrier
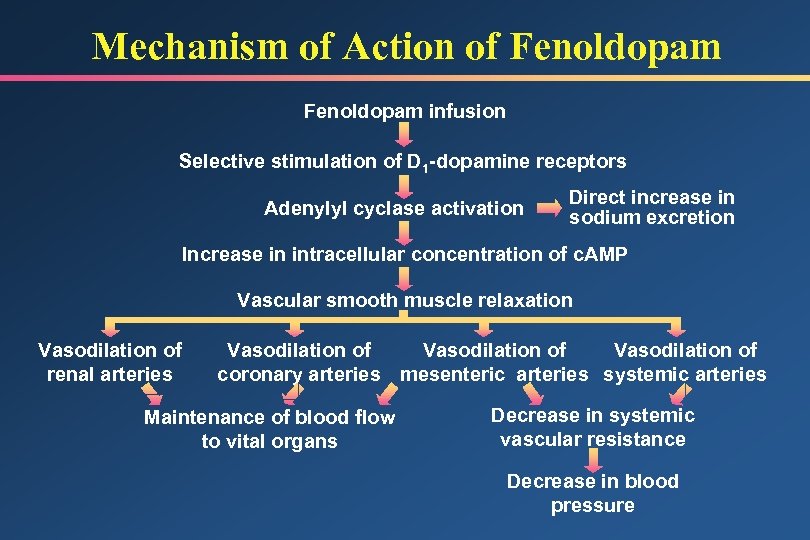 Mechanism of Action of Fenoldopam infusion Selective stimulation of D 1 -dopamine receptors Adenylyl cyclase activation Direct increase in sodium excretion Increase in intracellular concentration of c. AMP Vascular smooth muscle relaxation Vasodilation of renal arteries Vasodilation of coronary arteries mesenteric arteries systemic arteries Maintenance of blood flow to vital organs Decrease in systemic vascular resistance Decrease in blood pressure
Mechanism of Action of Fenoldopam infusion Selective stimulation of D 1 -dopamine receptors Adenylyl cyclase activation Direct increase in sodium excretion Increase in intracellular concentration of c. AMP Vascular smooth muscle relaxation Vasodilation of renal arteries Vasodilation of coronary arteries mesenteric arteries systemic arteries Maintenance of blood flow to vital organs Decrease in systemic vascular resistance Decrease in blood pressure
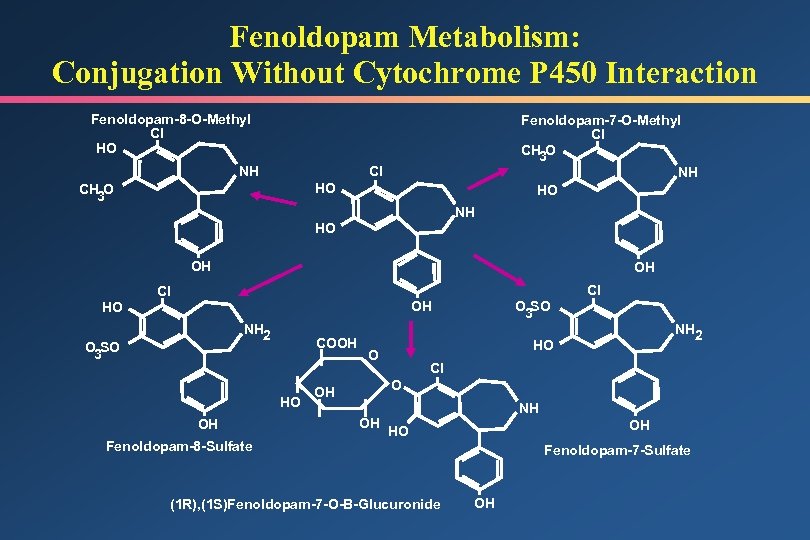 Fenoldopam Metabolism: Conjugation Without Cytochrome P 450 Interaction Fenoldopam-8 -O-Methyl Cl HO NH Fenoldopam-7 -O-Methyl Cl CH 3 O Cl NH HO CH 3 O HO NH HO OH Cl O 3 SO OH NH 2 O 3 SO COOH HO OH Fenoldopam-8 -Sulfate Cl NH 2 HO O Cl O OH OH NH OH HO (1 R), (1 S)Fenoldopam-7 -O-B-Glucuronide Fenoldopam-7 -Sulfate OH
Fenoldopam Metabolism: Conjugation Without Cytochrome P 450 Interaction Fenoldopam-8 -O-Methyl Cl HO NH Fenoldopam-7 -O-Methyl Cl CH 3 O Cl NH HO CH 3 O HO NH HO OH Cl O 3 SO OH NH 2 O 3 SO COOH HO OH Fenoldopam-8 -Sulfate Cl NH 2 HO O Cl O OH OH NH OH HO (1 R), (1 S)Fenoldopam-7 -O-B-Glucuronide Fenoldopam-7 -Sulfate OH
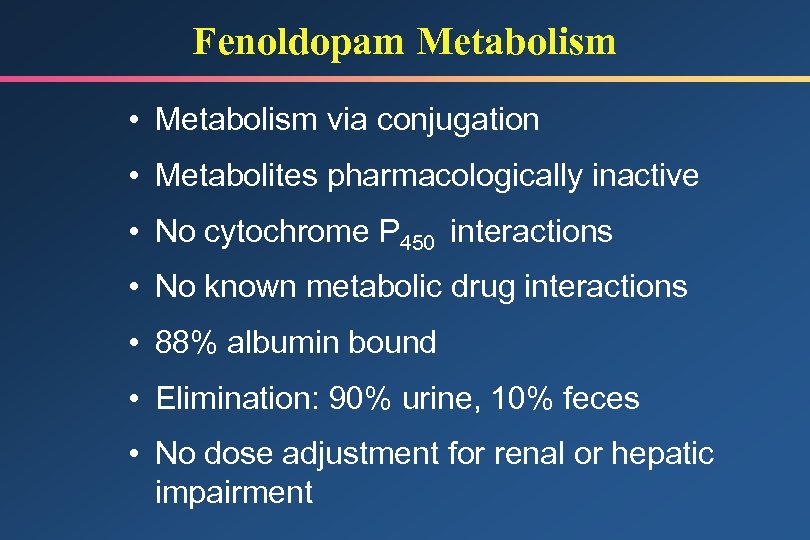 Fenoldopam Metabolism • Metabolism via conjugation • Metabolites pharmacologically inactive • No cytochrome P 450 interactions • No known metabolic drug interactions • 88% albumin bound • Elimination: 90% urine, 10% feces • No dose adjustment for renal or hepatic impairment
Fenoldopam Metabolism • Metabolism via conjugation • Metabolites pharmacologically inactive • No cytochrome P 450 interactions • No known metabolic drug interactions • 88% albumin bound • Elimination: 90% urine, 10% feces • No dose adjustment for renal or hepatic impairment
 Plasma Fenoldopam (ng/ml) Pharmacokinetics 40 40 Onset 30 20 10 10 0 Dose 0. 00 g/kg/min Dose 0. 04 g/kg/min Dose 0. 1 g/kg/min Dose 0. 4 g/kg/min Dose 0. 8 g/kg/min 30 20 Offset 0 0 1 2 3 4 Time (hr) 5 6 48 49 50 51 52 53 54 Time (hr) Neurex: data on file
Plasma Fenoldopam (ng/ml) Pharmacokinetics 40 40 Onset 30 20 10 10 0 Dose 0. 00 g/kg/min Dose 0. 04 g/kg/min Dose 0. 1 g/kg/min Dose 0. 4 g/kg/min Dose 0. 8 g/kg/min 30 20 Offset 0 0 1 2 3 4 Time (hr) 5 6 48 49 50 51 52 53 54 Time (hr) Neurex: data on file
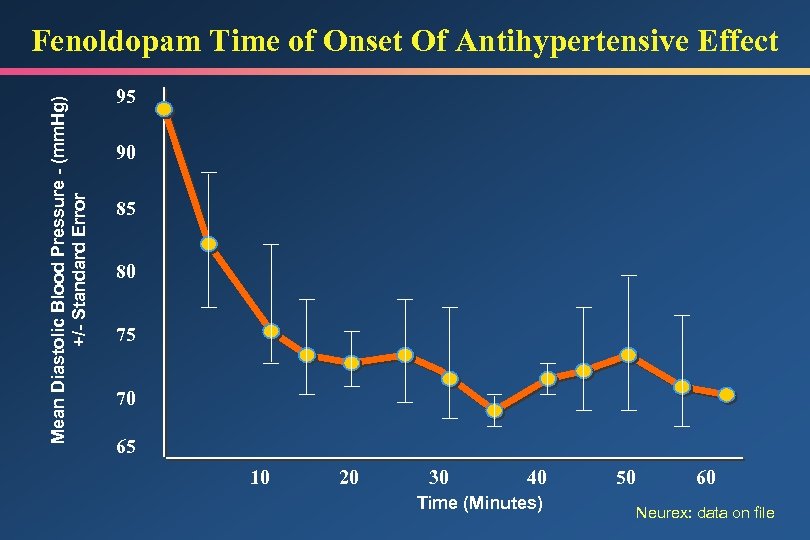 Mean Diastolic Blood Pressure - (mm. Hg) +/- Standard Error Fenoldopam Time of Onset Of Antihypertensive Effect 95 90 85 80 75 70 65 10 20 30 40 Time (Minutes) 50 60 Neurex: data on file
Mean Diastolic Blood Pressure - (mm. Hg) +/- Standard Error Fenoldopam Time of Onset Of Antihypertensive Effect 95 90 85 80 75 70 65 10 20 30 40 Time (Minutes) 50 60 Neurex: data on file
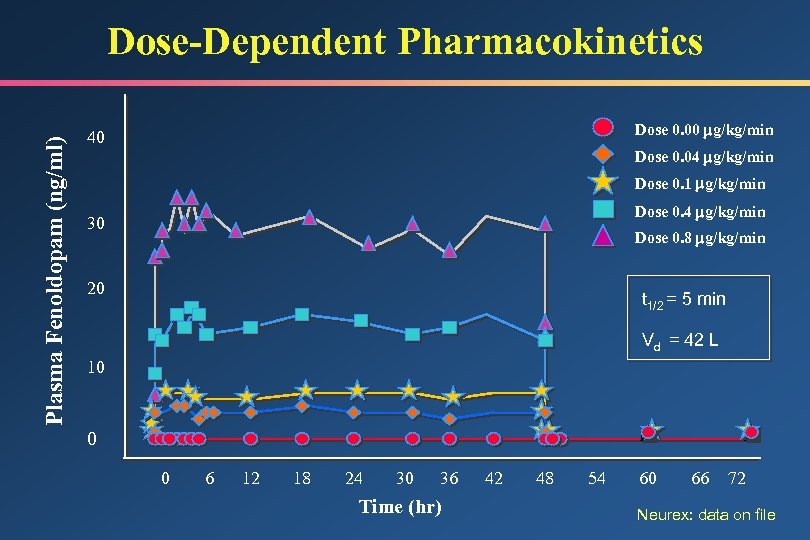 Plasma Fenoldopam (ng/ml) Dose-Dependent Pharmacokinetics Dose 0. 00 g/kg/min 40 Dose 0. 04 g/kg/min Dose 0. 1 g/kg/min Dose 0. 4 g/kg/min 30 Dose 0. 8 g/kg/min 20 t 1/2 = 5 min Vd = 42 L 10 0 0 6 12 18 24 30 36 Time (hr) 42 48 54 60 66 72 Neurex: data on file
Plasma Fenoldopam (ng/ml) Dose-Dependent Pharmacokinetics Dose 0. 00 g/kg/min 40 Dose 0. 04 g/kg/min Dose 0. 1 g/kg/min Dose 0. 4 g/kg/min 30 Dose 0. 8 g/kg/min 20 t 1/2 = 5 min Vd = 42 L 10 0 0 6 12 18 24 30 36 Time (hr) 42 48 54 60 66 72 Neurex: data on file
 Fenoldopam: Pharmacokinetics t½ (~ 5 min) Small volume of distribution Rapid attainment of steady state (~ 30 min) Plasma concentrations proportional to dose No alteration in pharmacokinetics over 48 hr infusion Rapid elimination upon discontinuation
Fenoldopam: Pharmacokinetics t½ (~ 5 min) Small volume of distribution Rapid attainment of steady state (~ 30 min) Plasma concentrations proportional to dose No alteration in pharmacokinetics over 48 hr infusion Rapid elimination upon discontinuation
 Fenoldopam: Pharmacodynamics Predictable hemodynamic effect Rapid onset of effect Predictable dose response for lowering BP No rebound hypertension
Fenoldopam: Pharmacodynamics Predictable hemodynamic effect Rapid onset of effect Predictable dose response for lowering BP No rebound hypertension
 Fenoldopam: Potential Benefits Rapid, predictable, dose-dependent blood pressure decrease (without overshoot) Short t½, rapid attainment of steady state titration Linear pharmacokinetics No cytochrome P 450 interactions Dose-response curves well defined No dosing adjustment for pre-existing renal or hepatic impairment Increases renal blood flow and maintains GFR Ease of use
Fenoldopam: Potential Benefits Rapid, predictable, dose-dependent blood pressure decrease (without overshoot) Short t½, rapid attainment of steady state titration Linear pharmacokinetics No cytochrome P 450 interactions Dose-response curves well defined No dosing adjustment for pre-existing renal or hepatic impairment Increases renal blood flow and maintains GFR Ease of use
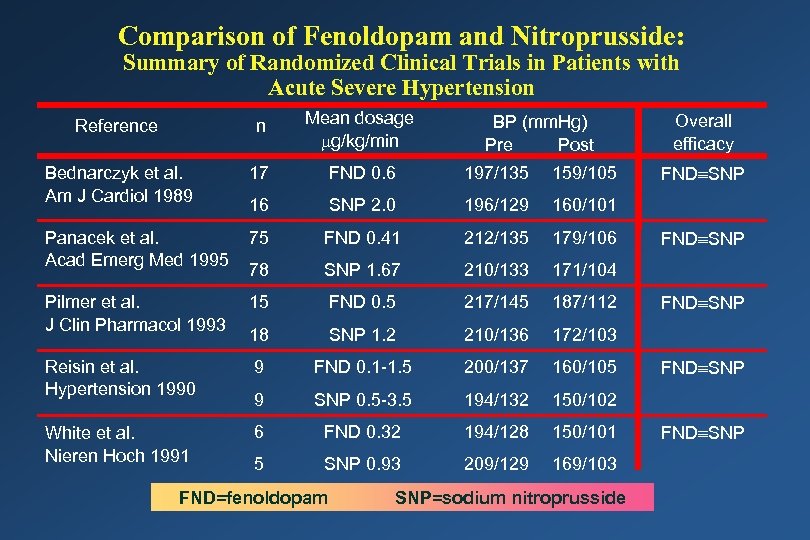 Comparison of Fenoldopam and Nitroprusside: Summary of Randomized Clinical Trials in Patients with Acute Severe Hypertension Reference n Mean dosage g/kg/min Bednarczyk et al. Am J Cardiol 1989 17 FND 0. 6 197/135 159/105 16 SNP 2. 0 196/129 160/101 Panacek et al. Acad Emerg Med 1995 75 FND 0. 41 212/135 179/106 78 SNP 1. 67 210/133 171/104 Pilmer et al. J Clin Pharmacol 1993 15 FND 0. 5 217/145 187/112 18 SNP 1. 2 210/136 172/103 Reisin et al. Hypertension 1990 9 FND 0. 1 -1. 5 200/137 160/105 9 SNP 0. 5 -3. 5 194/132 150/102 White et al. Nieren Hoch 1991 6 FND 0. 32 194/128 150/101 5 SNP 0. 93 209/129 169/103 FND=fenoldopam BP (mm. Hg) Pre Post SNP=sodium nitroprusside Overall efficacy FND SNP FND SNP
Comparison of Fenoldopam and Nitroprusside: Summary of Randomized Clinical Trials in Patients with Acute Severe Hypertension Reference n Mean dosage g/kg/min Bednarczyk et al. Am J Cardiol 1989 17 FND 0. 6 197/135 159/105 16 SNP 2. 0 196/129 160/101 Panacek et al. Acad Emerg Med 1995 75 FND 0. 41 212/135 179/106 78 SNP 1. 67 210/133 171/104 Pilmer et al. J Clin Pharmacol 1993 15 FND 0. 5 217/145 187/112 18 SNP 1. 2 210/136 172/103 Reisin et al. Hypertension 1990 9 FND 0. 1 -1. 5 200/137 160/105 9 SNP 0. 5 -3. 5 194/132 150/102 White et al. Nieren Hoch 1991 6 FND 0. 32 194/128 150/101 5 SNP 0. 93 209/129 169/103 FND=fenoldopam BP (mm. Hg) Pre Post SNP=sodium nitroprusside Overall efficacy FND SNP FND SNP
 Randomized Prospective Trial Fenoldopam vs. Sodium Nitroprusside in Treatment of Acute Severe Hypertension Prospective, randomized, open-label, multicenter clinical trial 183 patients enrolled with balanced demographics (153 completed) FND efficacy equal to SNP Similar adverse event profile Panacek EA, et al. Acad Emerg Med 1995; 2: 959 -965
Randomized Prospective Trial Fenoldopam vs. Sodium Nitroprusside in Treatment of Acute Severe Hypertension Prospective, randomized, open-label, multicenter clinical trial 183 patients enrolled with balanced demographics (153 completed) FND efficacy equal to SNP Similar adverse event profile Panacek EA, et al. Acad Emerg Med 1995; 2: 959 -965
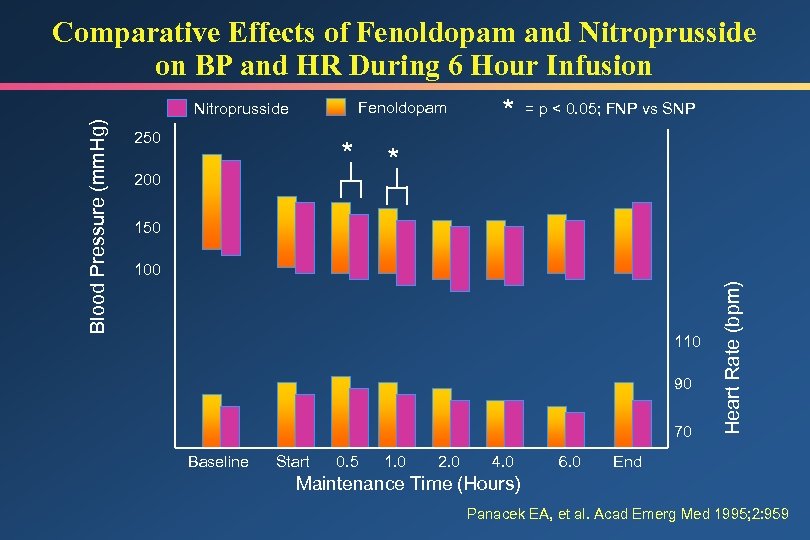 Comparative Effects of Fenoldopam and Nitroprusside on BP and HR During 6 Hour Infusion Fenoldopam 250 * 200 * = p < 0. 05; FNP vs SNP * 150 100 110 90 70 Baseline Start 0. 5 1. 0 2. 0 4. 0 6. 0 Heart Rate (bpm) Blood Pressure (mm. Hg) Nitroprusside End Maintenance Time (Hours) Panacek EA, et al. Acad Emerg Med 1995; 2: 959
Comparative Effects of Fenoldopam and Nitroprusside on BP and HR During 6 Hour Infusion Fenoldopam 250 * 200 * = p < 0. 05; FNP vs SNP * 150 100 110 90 70 Baseline Start 0. 5 1. 0 2. 0 4. 0 6. 0 Heart Rate (bpm) Blood Pressure (mm. Hg) Nitroprusside End Maintenance Time (Hours) Panacek EA, et al. Acad Emerg Med 1995; 2: 959
 Comparative Effects of Fenoldopam and Nitroprusside on BP and HR after 12 Hours of Infusion Regimen 9 8 SBP 229 ± 8 225 ± 10 DBP 148 ± 6 134 ± 2 HR 94 ± 5 86 ± 4 SBP Change (± SEM) Nitroprusside n Baseline (± SEM) Fenoldopam -54 ± 10 -45 ± 10 DBP -45 ± 5 -32 ± 6 HR -7 ± 5 -6 ± 4 Panacek EA, et al. Acad Emerg Med 1995; 2: 959 -965
Comparative Effects of Fenoldopam and Nitroprusside on BP and HR after 12 Hours of Infusion Regimen 9 8 SBP 229 ± 8 225 ± 10 DBP 148 ± 6 134 ± 2 HR 94 ± 5 86 ± 4 SBP Change (± SEM) Nitroprusside n Baseline (± SEM) Fenoldopam -54 ± 10 -45 ± 10 DBP -45 ± 5 -32 ± 6 HR -7 ± 5 -6 ± 4 Panacek EA, et al. Acad Emerg Med 1995; 2: 959 -965
 Hypertensive Emergency Trial Study Design • Determine pharmacokinetic/pharmacodynamic parameters • Patients with end organ damage and DBP 120 mm. Hg • Double-blind, constant infusion, 4 rates – 0. 01, 0. 03, 0. 1, 0. 3 g/kg/min • 24 -hour infusion, transition to PO after 18 hours • No target BP specified • Reduction in DBP at 4 hours primary endpoint • Statistical comparison vs. 0. 01 dose group Ellis D, et al. Crit Care Med 1998; 26(Suppl): A 23 (abstract)
Hypertensive Emergency Trial Study Design • Determine pharmacokinetic/pharmacodynamic parameters • Patients with end organ damage and DBP 120 mm. Hg • Double-blind, constant infusion, 4 rates – 0. 01, 0. 03, 0. 1, 0. 3 g/kg/min • 24 -hour infusion, transition to PO after 18 hours • No target BP specified • Reduction in DBP at 4 hours primary endpoint • Statistical comparison vs. 0. 01 dose group Ellis D, et al. Crit Care Med 1998; 26(Suppl): A 23 (abstract)
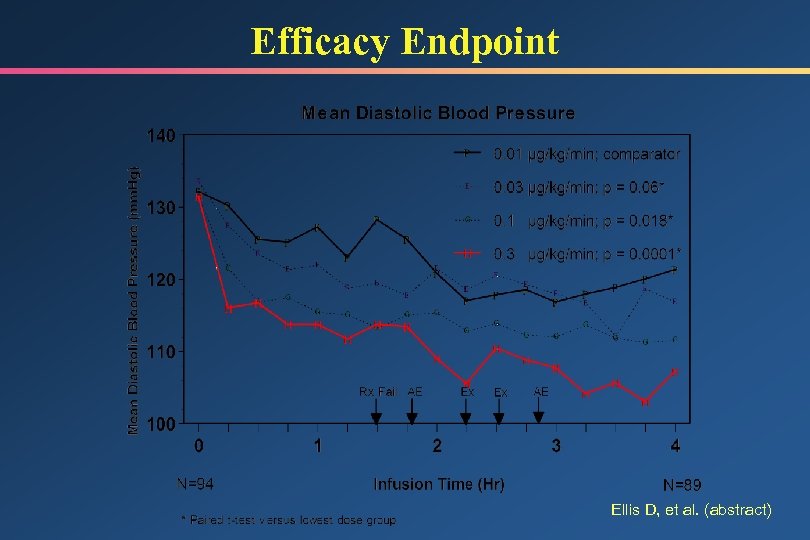 Efficacy Endpoint Ellis D, et al. (abstract)
Efficacy Endpoint Ellis D, et al. (abstract)
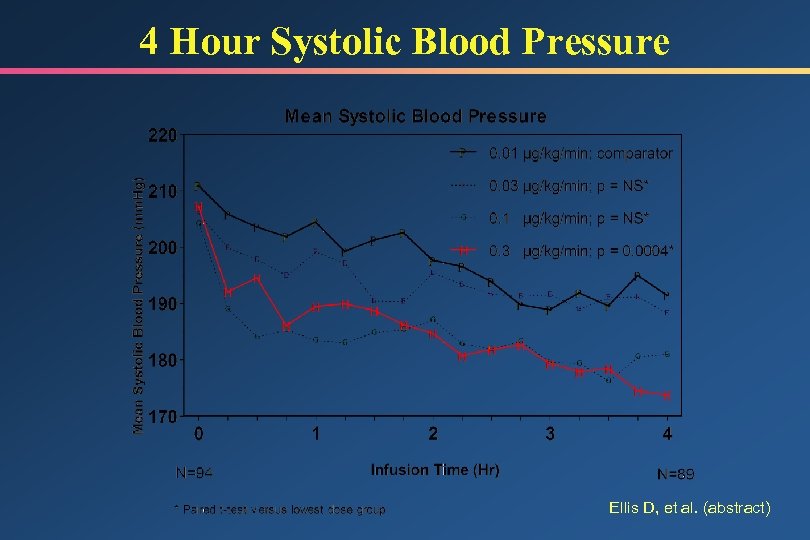 4 Hour Systolic Blood Pressure Ellis D, et al. (abstract)
4 Hour Systolic Blood Pressure Ellis D, et al. (abstract)
 4 Hour Heart Rate Ellis D, et al. (abstract)
4 Hour Heart Rate Ellis D, et al. (abstract)
 Objective End Organ Damage Malignant Hypertension Trial Encephalopathy (Confusion, TIA) Retinal (III-IV, hemorrhage) Renal Insufficiency (Cr >2. 4) Myocardial Ischemia (ECG, chest pain) Papilledema CHF (Pulmonary) Hematuria (edema, CXR, rales) 0 5 10 15 20 25 30 35 Number of Patients Ellis D, et al. (abstract)
Objective End Organ Damage Malignant Hypertension Trial Encephalopathy (Confusion, TIA) Retinal (III-IV, hemorrhage) Renal Insufficiency (Cr >2. 4) Myocardial Ischemia (ECG, chest pain) Papilledema CHF (Pulmonary) Hematuria (edema, CXR, rales) 0 5 10 15 20 25 30 35 Number of Patients Ellis D, et al. (abstract)
 Transition to Oral Medications No evidence of rebound effects Rapid disappearance of drug Administration before or after discontinuation of infusion Wide variety of drugs used Generally successful transfer to oral drugs
Transition to Oral Medications No evidence of rebound effects Rapid disappearance of drug Administration before or after discontinuation of infusion Wide variety of drugs used Generally successful transfer to oral drugs
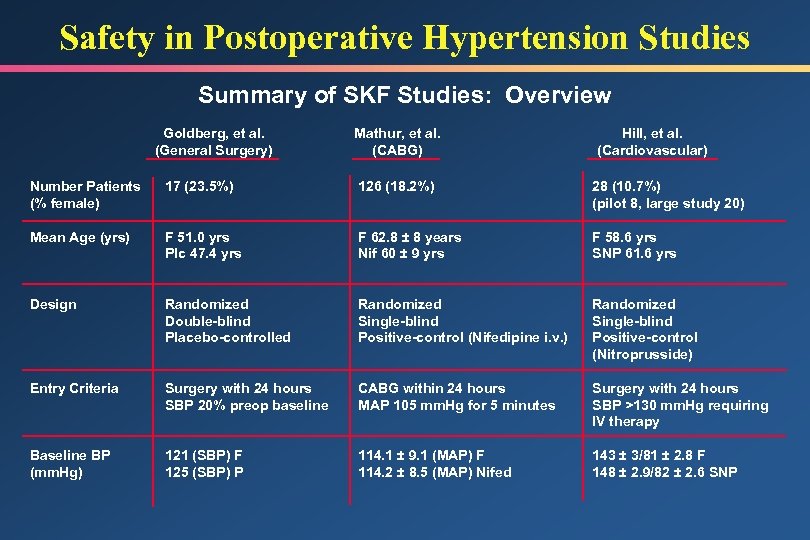 Safety in Postoperative Hypertension Studies Summary of SKF Studies: Overview Goldberg, et al. (General Surgery) Mathur, et al. (CABG) Hill, et al. (Cardiovascular) Number Patients (% female) 17 (23. 5%) 126 (18. 2%) 28 (10. 7%) (pilot 8, large study 20) Mean Age (yrs) F 51. 0 yrs Plc 47. 4 yrs F 62. 8 ± 8 years Nif 60 ± 9 yrs F 58. 6 yrs SNP 61. 6 yrs Design Randomized Double-blind Placebo-controlled Randomized Single-blind Positive-control (Nifedipine i. v. ) Randomized Single-blind Positive-control (Nitroprusside) Entry Criteria Surgery with 24 hours SBP 20% preop baseline CABG within 24 hours MAP 105 mm. Hg for 5 minutes Surgery with 24 hours SBP >130 mm. Hg requiring IV therapy Baseline BP (mm. Hg) 121 (SBP) F 125 (SBP) P 114. 1 ± 9. 1 (MAP) F 114. 2 ± 8. 5 (MAP) Nifed 143 ± 3/81 ± 2. 8 F 148 ± 2. 9/82 ± 2. 6 SNP
Safety in Postoperative Hypertension Studies Summary of SKF Studies: Overview Goldberg, et al. (General Surgery) Mathur, et al. (CABG) Hill, et al. (Cardiovascular) Number Patients (% female) 17 (23. 5%) 126 (18. 2%) 28 (10. 7%) (pilot 8, large study 20) Mean Age (yrs) F 51. 0 yrs Plc 47. 4 yrs F 62. 8 ± 8 years Nif 60 ± 9 yrs F 58. 6 yrs SNP 61. 6 yrs Design Randomized Double-blind Placebo-controlled Randomized Single-blind Positive-control (Nifedipine i. v. ) Randomized Single-blind Positive-control (Nitroprusside) Entry Criteria Surgery with 24 hours SBP 20% preop baseline CABG within 24 hours MAP 105 mm. Hg for 5 minutes Surgery with 24 hours SBP >130 mm. Hg requiring IV therapy Baseline BP (mm. Hg) 121 (SBP) F 125 (SBP) P 114. 1 ± 9. 1 (MAP) F 114. 2 ± 8. 5 (MAP) Nifed 143 ± 3/81 ± 2. 8 F 148 ± 2. 9/82 ± 2. 6 SNP
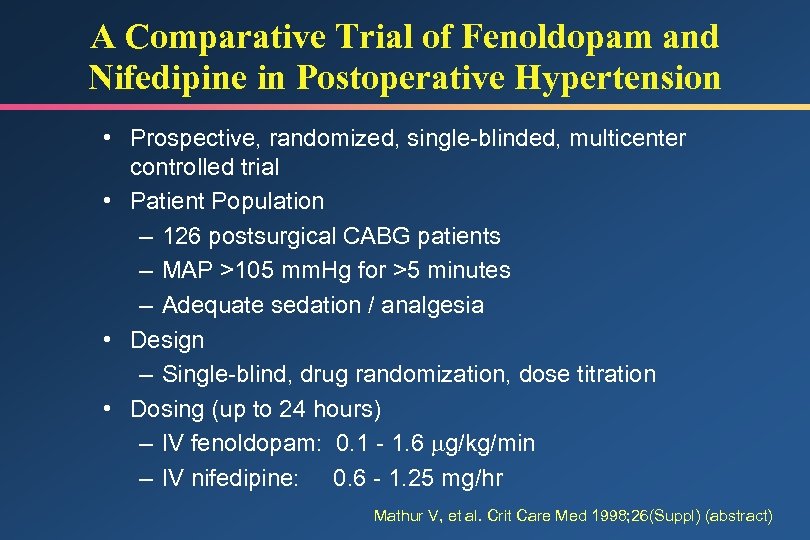 A Comparative Trial of Fenoldopam and Nifedipine in Postoperative Hypertension • Prospective, randomized, single-blinded, multicenter controlled trial • Patient Population – 126 postsurgical CABG patients – MAP >105 mm. Hg for >5 minutes – Adequate sedation / analgesia • Design – Single-blind, drug randomization, dose titration • Dosing (up to 24 hours) – IV fenoldopam: 0. 1 - 1. 6 g/kg/min – IV nifedipine: 0. 6 - 1. 25 mg/hr Mathur V, et al. Crit Care Med 1998; 26(Suppl) (abstract)
A Comparative Trial of Fenoldopam and Nifedipine in Postoperative Hypertension • Prospective, randomized, single-blinded, multicenter controlled trial • Patient Population – 126 postsurgical CABG patients – MAP >105 mm. Hg for >5 minutes – Adequate sedation / analgesia • Design – Single-blind, drug randomization, dose titration • Dosing (up to 24 hours) – IV fenoldopam: 0. 1 - 1. 6 g/kg/min – IV nifedipine: 0. 6 - 1. 25 mg/hr Mathur V, et al. Crit Care Med 1998; 26(Suppl) (abstract)
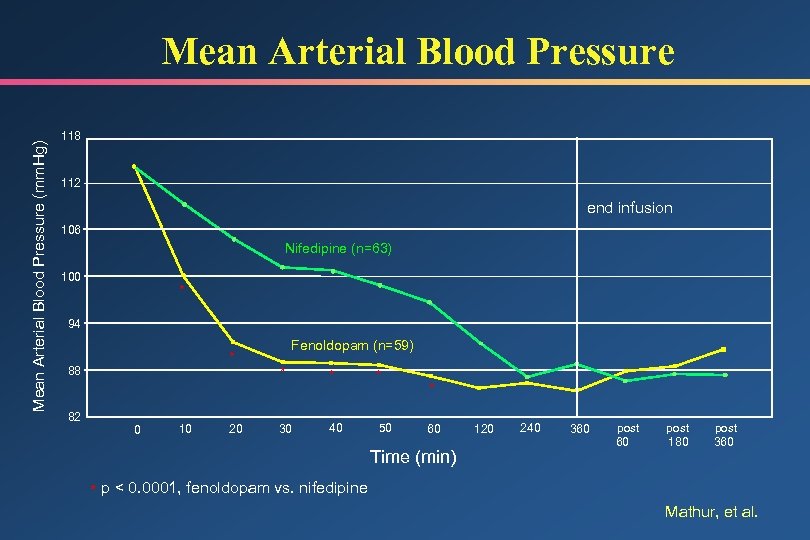 Mean Arterial Blood Pressure (mm. Hg) Mean Arterial Blood Pressure 118 112 end infusion 106 Nifedipine (n=63) 100 * 94 * 88 82 0 10 20 Fenoldopam (n=59) * 30 * * 40 50 * 60 Time (min) 120 240 360 post 180 post 360 * p < 0. 0001, fenoldopam vs. nifedipine Mathur, et al.
Mean Arterial Blood Pressure (mm. Hg) Mean Arterial Blood Pressure 118 112 end infusion 106 Nifedipine (n=63) 100 * 94 * 88 82 0 10 20 Fenoldopam (n=59) * 30 * * 40 50 * 60 Time (min) 120 240 360 post 180 post 360 * p < 0. 0001, fenoldopam vs. nifedipine Mathur, et al.
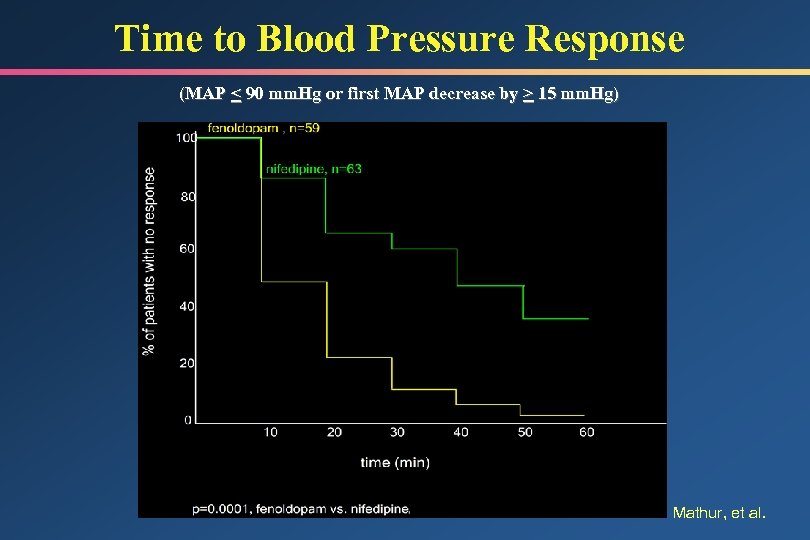 Time to Blood Pressure Response (MAP < 90 mm. Hg or first MAP decrease by > 15 mm. Hg) Mathur, et al.
Time to Blood Pressure Response (MAP < 90 mm. Hg or first MAP decrease by > 15 mm. Hg) Mathur, et al.
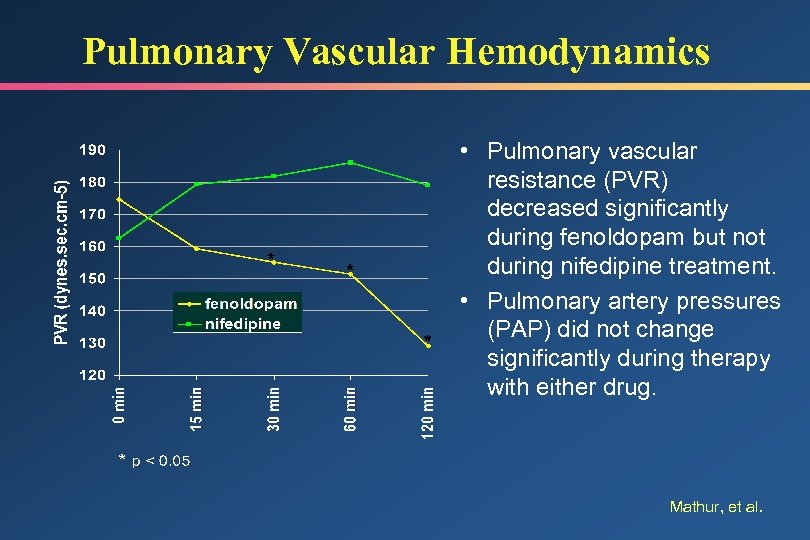 Pulmonary Vascular Hemodynamics • Pulmonary vascular resistance (PVR) decreased significantly during fenoldopam but not during nifedipine treatment. • Pulmonary artery pressures (PAP) did not change significantly during therapy with either drug. Mathur, et al.
Pulmonary Vascular Hemodynamics • Pulmonary vascular resistance (PVR) decreased significantly during fenoldopam but not during nifedipine treatment. • Pulmonary artery pressures (PAP) did not change significantly during therapy with either drug. Mathur, et al.
 Filling Pressures and Cardiac Output Mathur, et al.
Filling Pressures and Cardiac Output Mathur, et al.
 Heart Rate 110 fenoldopam Heart Rate (bpm) 100 nifedipine 90 80 70 p = NS, fenoldopam vs. nifedipine 60 0 10 20 30 Time (min) 40 50 60 Mathur, et al.
Heart Rate 110 fenoldopam Heart Rate (bpm) 100 nifedipine 90 80 70 p = NS, fenoldopam vs. nifedipine 60 0 10 20 30 Time (min) 40 50 60 Mathur, et al.
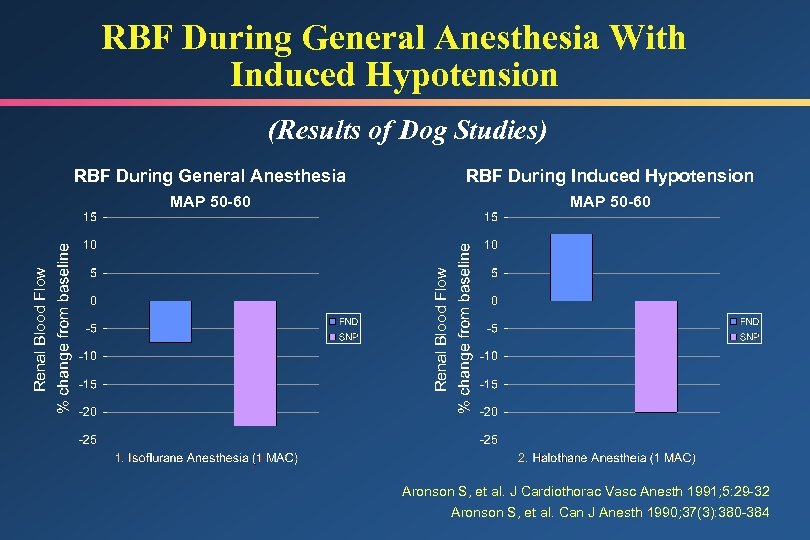 RBF During General Anesthesia With Induced Hypotension (Results of Dog Studies) RBF During Induced Hypotension MAP 50 -60 Renal Blood Flow RBF During General Anesthesia 1. 2. Aronson S, et al. J Cardiothorac Vasc Anesth 1991; 5: 29 -32 Aronson S, et al. Can J Anesth 1990; 37(3): 380 -384
RBF During General Anesthesia With Induced Hypotension (Results of Dog Studies) RBF During Induced Hypotension MAP 50 -60 Renal Blood Flow RBF During General Anesthesia 1. 2. Aronson S, et al. J Cardiothorac Vasc Anesth 1991; 5: 29 -32 Aronson S, et al. Can J Anesth 1990; 37(3): 380 -384
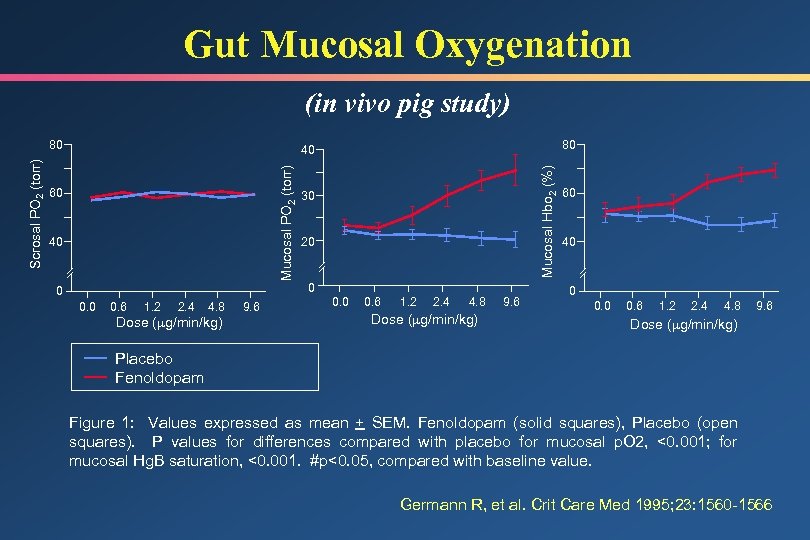 Gut Mucosal Oxygenation (in vivo pig study) 80 60 40 0 0. 6 1. 2 2. 4 4. 8 Dose ( g/min/kg) 9. 6 Mucosal Hbo 2 (%) 40 Mucosal PO 2 (torr) Scrosal PO 2 (torr) 80 30 20 0 0. 6 1. 2 2. 4 4. 8 Dose ( g/min/kg) 9. 6 60 40 0 0. 6 1. 2 2. 4 4. 8 9. 6 Dose ( g/min/kg) Placebo Fenoldopam Figure 1: Values expressed as mean + SEM. Fenoldopam (solid squares), Placebo (open squares). P values for differences compared with placebo for mucosal p. O 2, <0. 001; for mucosal Hg. B saturation, <0. 001. #p<0. 05, compared with baseline value. Germann R, et al. Crit Care Med 1995; 23: 1560 -1566
Gut Mucosal Oxygenation (in vivo pig study) 80 60 40 0 0. 6 1. 2 2. 4 4. 8 Dose ( g/min/kg) 9. 6 Mucosal Hbo 2 (%) 40 Mucosal PO 2 (torr) Scrosal PO 2 (torr) 80 30 20 0 0. 6 1. 2 2. 4 4. 8 Dose ( g/min/kg) 9. 6 60 40 0 0. 6 1. 2 2. 4 4. 8 9. 6 Dose ( g/min/kg) Placebo Fenoldopam Figure 1: Values expressed as mean + SEM. Fenoldopam (solid squares), Placebo (open squares). P values for differences compared with placebo for mucosal p. O 2, <0. 001; for mucosal Hg. B saturation, <0. 001. #p<0. 05, compared with baseline value. Germann R, et al. Crit Care Med 1995; 23: 1560 -1566
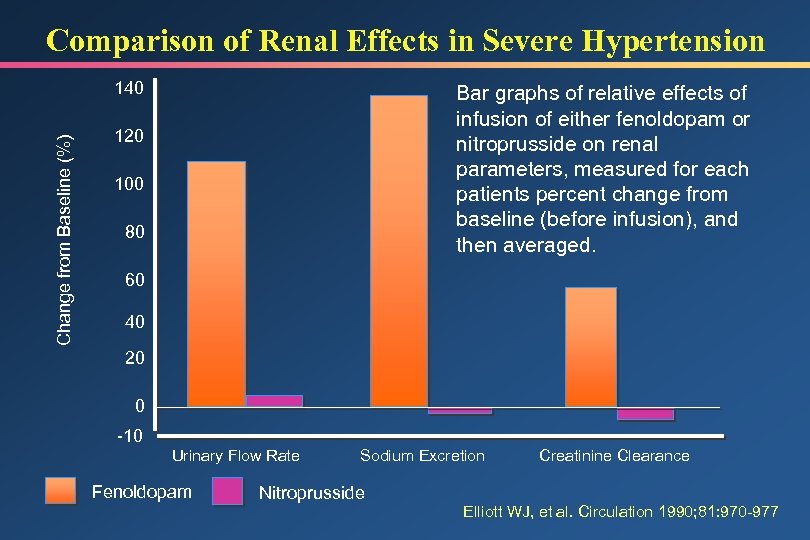 Comparison of Renal Effects in Severe Hypertension Change from Baseline (%) 140 Bar graphs of relative effects of infusion of either fenoldopam or nitroprusside on renal parameters, measured for each patients percent change from baseline (before infusion), and then averaged. 120 100 80 60 40 20 0 -10 Urinary Flow Rate Fenoldopam Sodium Excretion Nitroprusside Creatinine Clearance Elliott WJ, et al. Circulation 1990; 81: 970 -977
Comparison of Renal Effects in Severe Hypertension Change from Baseline (%) 140 Bar graphs of relative effects of infusion of either fenoldopam or nitroprusside on renal parameters, measured for each patients percent change from baseline (before infusion), and then averaged. 120 100 80 60 40 20 0 -10 Urinary Flow Rate Fenoldopam Sodium Excretion Nitroprusside Creatinine Clearance Elliott WJ, et al. Circulation 1990; 81: 970 -977
 Fenoldopam: Renal Function in Hypertensives Results V (m. L/min) 25 Baseline Experimental Recovery 20 15 10 5 0 30 90 150 210 270 30 90 150 210 Urine volume (UV) and urinary sodium (UNa. V) before, during and after infusion 270 750 UNa. V ( Eq/min) Placebo Fenoldopam 625 500 375 250 125 0 Murphy MB, et al. Circulation 1987; 76: 1312 -1318
Fenoldopam: Renal Function in Hypertensives Results V (m. L/min) 25 Baseline Experimental Recovery 20 15 10 5 0 30 90 150 210 270 30 90 150 210 Urine volume (UV) and urinary sodium (UNa. V) before, during and after infusion 270 750 UNa. V ( Eq/min) Placebo Fenoldopam 625 500 375 250 125 0 Murphy MB, et al. Circulation 1987; 76: 1312 -1318
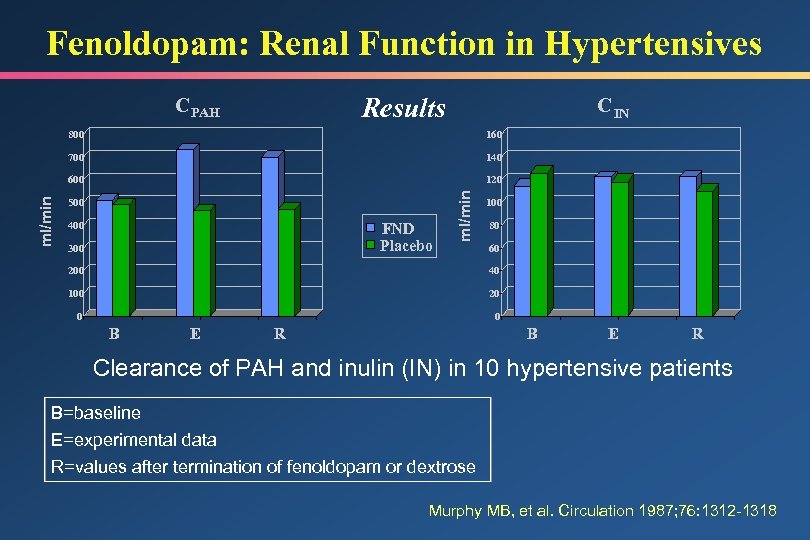 Fenoldopam: Renal Function in Hypertensives Results C PAH C IN 140 600 120 500 FND Placebo 400 300 ml/min 160 700 ml/min 800 100 80 60 200 40 100 20 0 0 B E R Clearance of PAH and inulin (IN) in 10 hypertensive patients B=baseline E=experimental data R=values after termination of fenoldopam or dextrose Murphy MB, et al. Circulation 1987; 76: 1312 -1318
Fenoldopam: Renal Function in Hypertensives Results C PAH C IN 140 600 120 500 FND Placebo 400 300 ml/min 160 700 ml/min 800 100 80 60 200 40 100 20 0 0 B E R Clearance of PAH and inulin (IN) in 10 hypertensive patients B=baseline E=experimental data R=values after termination of fenoldopam or dextrose Murphy MB, et al. Circulation 1987; 76: 1312 -1318
 Reversal of Hemodynamic Effects of Cyclosporine In Cs. A-treated renal transplant patients • Renal plasma increased significantly • Fe. Na in urine volume tended to increase • GFR and free water clearance were unchanged • BP fell (mean of 18/6 mm. Hg) • No change in Cs. A levels while on fenoldopam Jorkasky DK, et al. Am J Kidney Dis 1992; 19: 567 -572
Reversal of Hemodynamic Effects of Cyclosporine In Cs. A-treated renal transplant patients • Renal plasma increased significantly • Fe. Na in urine volume tended to increase • GFR and free water clearance were unchanged • BP fell (mean of 18/6 mm. Hg) • No change in Cs. A levels while on fenoldopam Jorkasky DK, et al. Am J Kidney Dis 1992; 19: 567 -572
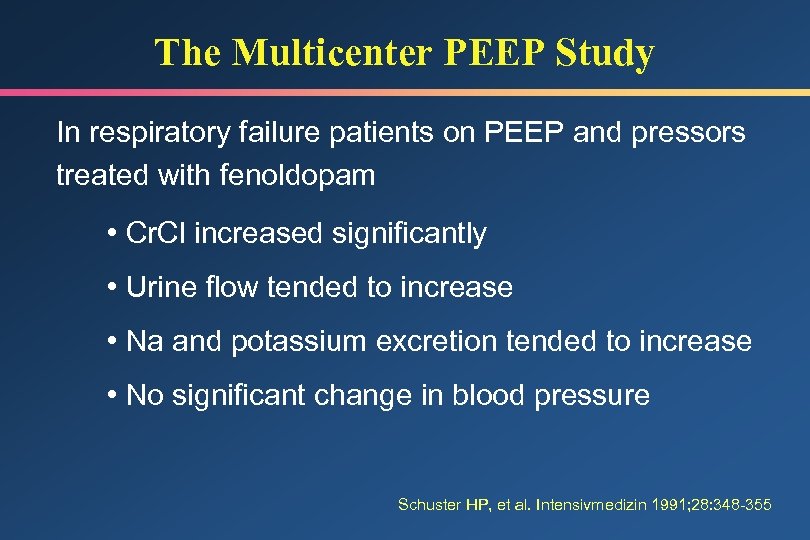 The Multicenter PEEP Study In respiratory failure patients on PEEP and pressors treated with fenoldopam • Cr. Cl increased significantly • Urine flow tended to increase • Na and potassium excretion tended to increase • No significant change in blood pressure Schuster HP, et al. Intensivmedizin 1991; 28: 348 -355
The Multicenter PEEP Study In respiratory failure patients on PEEP and pressors treated with fenoldopam • Cr. Cl increased significantly • Urine flow tended to increase • Na and potassium excretion tended to increase • No significant change in blood pressure Schuster HP, et al. Intensivmedizin 1991; 28: 348 -355
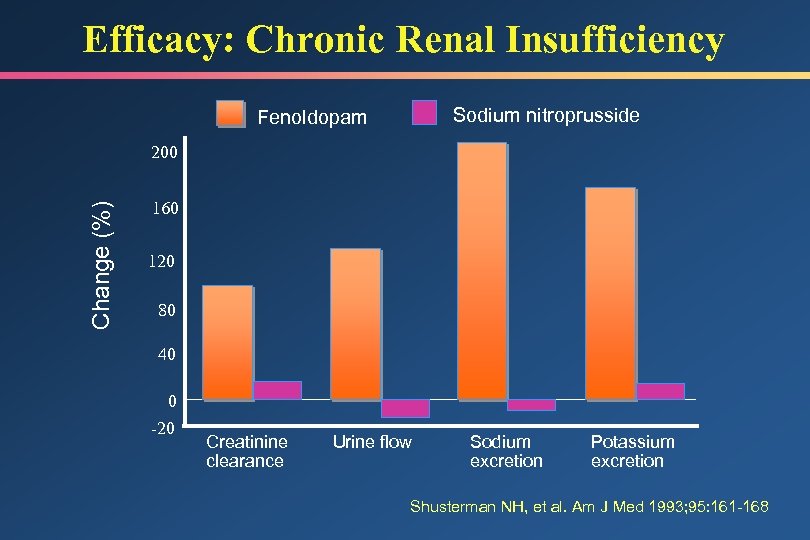 Efficacy: Chronic Renal Insufficiency Sodium nitroprusside Fenoldopam Change (%) 200 160 120 80 40 0 -20 Creatinine clearance Urine flow Sodium excretion Potassium excretion Shusterman NH, et al. Am J Med 1993; 95: 161 -168
Efficacy: Chronic Renal Insufficiency Sodium nitroprusside Fenoldopam Change (%) 200 160 120 80 40 0 -20 Creatinine clearance Urine flow Sodium excretion Potassium excretion Shusterman NH, et al. Am J Med 1993; 95: 161 -168
 Fenoldopam: Renal Plasma Flow * * * Fenoldopam 20 18 16 14 12 Placebo 10 8 6 4 2 0 Fenoldopam Concentration (ng/m. L) Renal Plasma Flow (m. L/min/1. 73 m 2) Dose Response of RBF in normotensives Infusion Dose (mcg/kg/min) *p<0. 05 compared with placebo with both diets combined Neurex: data on file
Fenoldopam: Renal Plasma Flow * * * Fenoldopam 20 18 16 14 12 Placebo 10 8 6 4 2 0 Fenoldopam Concentration (ng/m. L) Renal Plasma Flow (m. L/min/1. 73 m 2) Dose Response of RBF in normotensives Infusion Dose (mcg/kg/min) *p<0. 05 compared with placebo with both diets combined Neurex: data on file
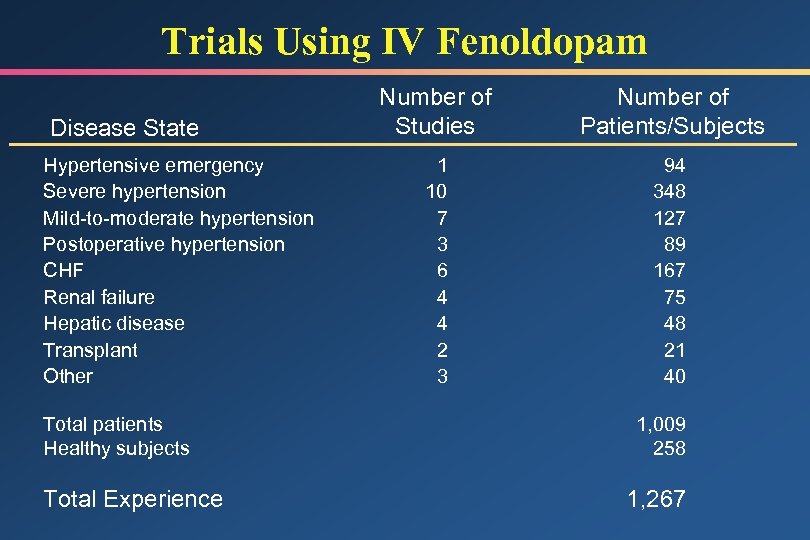 Trials Using IV Fenoldopam Disease State Hypertensive emergency Severe hypertension Mild-to-moderate hypertension Postoperative hypertension CHF Renal failure Hepatic disease Transplant Other Total patients Healthy subjects Total Experience Number of Studies Number of Patients/Subjects 1 10 7 3 6 4 4 2 3 94 348 127 89 167 75 48 21 40 1, 009 258 1, 267
Trials Using IV Fenoldopam Disease State Hypertensive emergency Severe hypertension Mild-to-moderate hypertension Postoperative hypertension CHF Renal failure Hepatic disease Transplant Other Total patients Healthy subjects Total Experience Number of Studies Number of Patients/Subjects 1 10 7 3 6 4 4 2 3 94 348 127 89 167 75 48 21 40 1, 009 258 1, 267
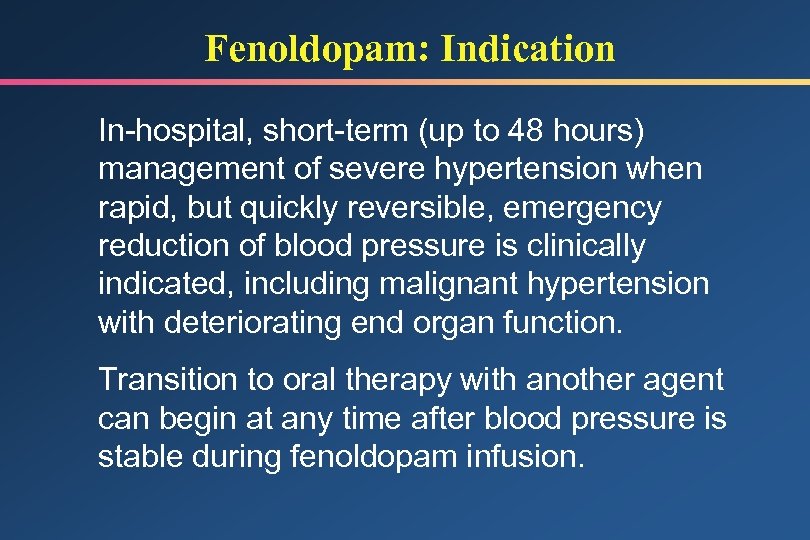 Fenoldopam: Indication In-hospital, short-term (up to 48 hours) management of severe hypertension when rapid, but quickly reversible, emergency reduction of blood pressure is clinically indicated, including malignant hypertension with deteriorating end organ function. Transition to oral therapy with another agent can begin at any time after blood pressure is stable during fenoldopam infusion.
Fenoldopam: Indication In-hospital, short-term (up to 48 hours) management of severe hypertension when rapid, but quickly reversible, emergency reduction of blood pressure is clinically indicated, including malignant hypertension with deteriorating end organ function. Transition to oral therapy with another agent can begin at any time after blood pressure is stable during fenoldopam infusion.
 Fenoldopam: Contraindications None Known
Fenoldopam: Contraindications None Known
 Fenoldopam: Warnings Contains sodium metabisulfate, a sulfite that may cause allergic-type reactions, including anaphylactic symptoms and life-threatening or less severe asthmatic episodes, in certain susceptible people. Overall prevalence of sulfite sensitivity in general population is unknown but probably low. Sulfite sensitivity is seen more frequently in asthmatic than in nonasthmatic people.
Fenoldopam: Warnings Contains sodium metabisulfate, a sulfite that may cause allergic-type reactions, including anaphylactic symptoms and life-threatening or less severe asthmatic episodes, in certain susceptible people. Overall prevalence of sulfite sensitivity in general population is unknown but probably low. Sulfite sensitivity is seen more frequently in asthmatic than in nonasthmatic people.
 Fenolodopam: Precautions • • Intraocular pressure that changes within diurnal variation Tachycardia Hypotension Hypokalemia Pregnancy category B Nursing mothers Data suggests no carcinogenesis, mutagenesis, or impairment of fertility • Safety and effectiveness in children has not been established
Fenolodopam: Precautions • • Intraocular pressure that changes within diurnal variation Tachycardia Hypotension Hypokalemia Pregnancy category B Nursing mothers Data suggests no carcinogenesis, mutagenesis, or impairment of fertility • Safety and effectiveness in children has not been established
 Fenoldopam: Precautions • No formal interaction studies; intravenous fenoldopam has been administered safely with drugs such as digitalis and sublingual nitroglycerin. • Limited experience with concomitant antihypertensive agents: beta blockers, alpha blockers, calcium channel blockers, ACE inhibitors, and diuretics (both thiazidelike and loop). • Use of beta-blockers in conjunction with fenoldopam not studied in hypertensive patients: concomitant use should be avoided. • Caution should be exercised: unexpected hypotension cou result from beta-blocker inhibition of reflex response to fenoldopam.
Fenoldopam: Precautions • No formal interaction studies; intravenous fenoldopam has been administered safely with drugs such as digitalis and sublingual nitroglycerin. • Limited experience with concomitant antihypertensive agents: beta blockers, alpha blockers, calcium channel blockers, ACE inhibitors, and diuretics (both thiazidelike and loop). • Use of beta-blockers in conjunction with fenoldopam not studied in hypertensive patients: concomitant use should be avoided. • Caution should be exercised: unexpected hypotension cou result from beta-blocker inhibition of reflex response to fenoldopam.
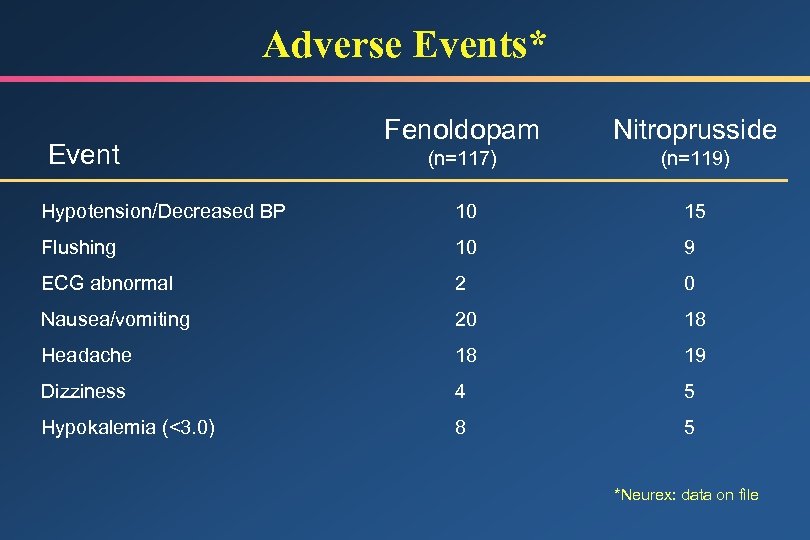 Adverse Events* Fenoldopam Nitroprusside (n=117) (n=119) Hypotension/Decreased BP 10 15 Flushing 10 9 ECG abnormal 2 0 Nausea/vomiting 20 18 Headache 18 19 Dizziness 4 5 Hypokalemia (<3. 0) 8 5 Event *Neurex: data on file
Adverse Events* Fenoldopam Nitroprusside (n=117) (n=119) Hypotension/Decreased BP 10 15 Flushing 10 9 ECG abnormal 2 0 Nausea/vomiting 20 18 Headache 18 19 Dizziness 4 5 Hypokalemia (<3. 0) 8 5 Event *Neurex: data on file
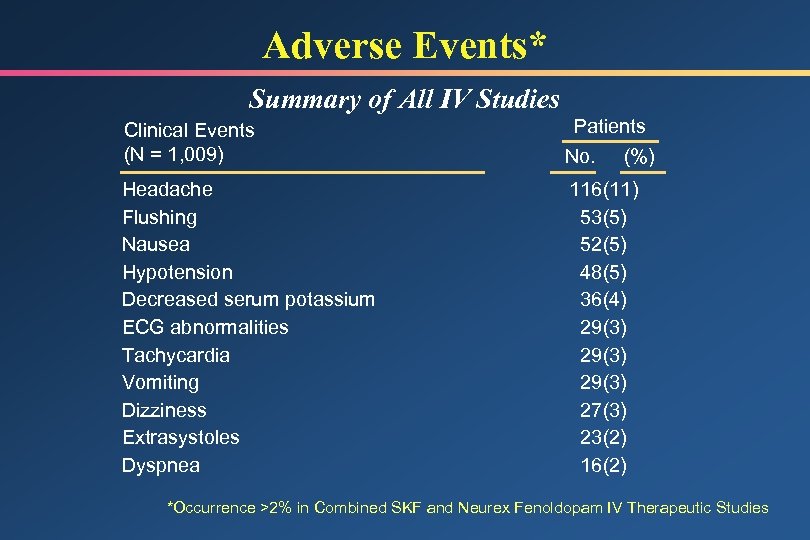 Adverse Events* Summary of All IV Studies Clinical Events (N = 1, 009) Patients No. (%) Headache Flushing Nausea Hypotension Decreased serum potassium ECG abnormalities Tachycardia Vomiting Dizziness Extrasystoles Dyspnea 116(11) 53(5) 52(5) 48(5) 36(4) 29(3) 27(3) 23(2) 16(2) *Occurrence >2% in Combined SKF and Neurex Fenoldopam IV Therapeutic Studies
Adverse Events* Summary of All IV Studies Clinical Events (N = 1, 009) Patients No. (%) Headache Flushing Nausea Hypotension Decreased serum potassium ECG abnormalities Tachycardia Vomiting Dizziness Extrasystoles Dyspnea 116(11) 53(5) 52(5) 48(5) 36(4) 29(3) 27(3) 23(2) 16(2) *Occurrence >2% in Combined SKF and Neurex Fenoldopam IV Therapeutic Studies
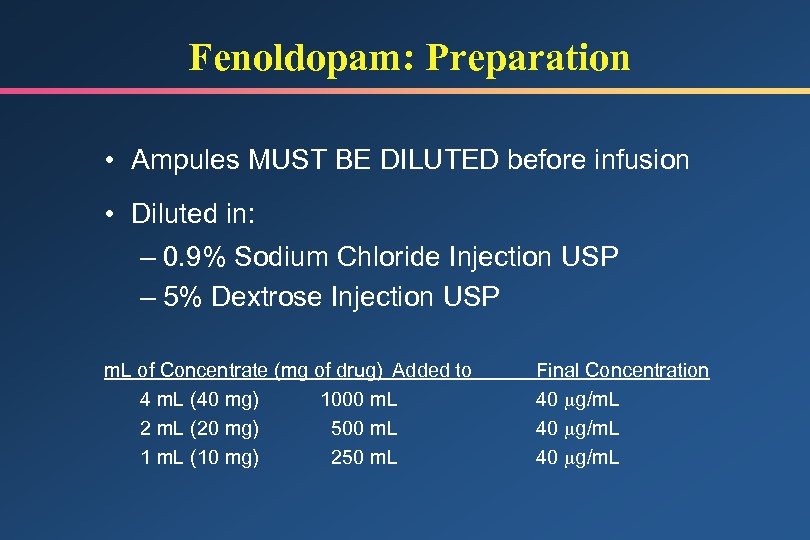 Fenoldopam: Preparation • Ampules MUST BE DILUTED before infusion • Diluted in: – 0. 9% Sodium Chloride Injection USP – 5% Dextrose Injection USP m. L of Concentrate (mg of drug) Added to 4 m. L (40 mg) 1000 m. L 2 m. L (20 mg) 500 m. L 1 m. L (10 mg) 250 m. L Final Concentration 40 g/m. L
Fenoldopam: Preparation • Ampules MUST BE DILUTED before infusion • Diluted in: – 0. 9% Sodium Chloride Injection USP – 5% Dextrose Injection USP m. L of Concentrate (mg of drug) Added to 4 m. L (40 mg) 1000 m. L 2 m. L (20 mg) 500 m. L 1 m. L (10 mg) 250 m. L Final Concentration 40 g/m. L
 Fenoldopam: Dosage and Administration Dosing Recommendations • Usual starting dose = 0. 1 g/kg/min – Rapid titratable blood pressure control – Minimal increase in heart rate • Higher starting dose recommended – For more rapid onset of blood pressure control – For greater magnitude of effect
Fenoldopam: Dosage and Administration Dosing Recommendations • Usual starting dose = 0. 1 g/kg/min – Rapid titratable blood pressure control – Minimal increase in heart rate • Higher starting dose recommended – For more rapid onset of blood pressure control – For greater magnitude of effect
 Fenoldopam: Dosage and Administration • Fenoldopam should be administered by continuous intravenous infusion • A bolus dose should not be used • Initial dose should be titrated upward or downward, no more frequently than every 15 minutes • Recommended increments for titration are 0. 05 to 0. 1 g/kg/min • Use of infusion pump or syringe pump recommended • Intraarterial hemodynamic monitoring at discretion of treating physician
Fenoldopam: Dosage and Administration • Fenoldopam should be administered by continuous intravenous infusion • A bolus dose should not be used • Initial dose should be titrated upward or downward, no more frequently than every 15 minutes • Recommended increments for titration are 0. 05 to 0. 1 g/kg/min • Use of infusion pump or syringe pump recommended • Intraarterial hemodynamic monitoring at discretion of treating physician
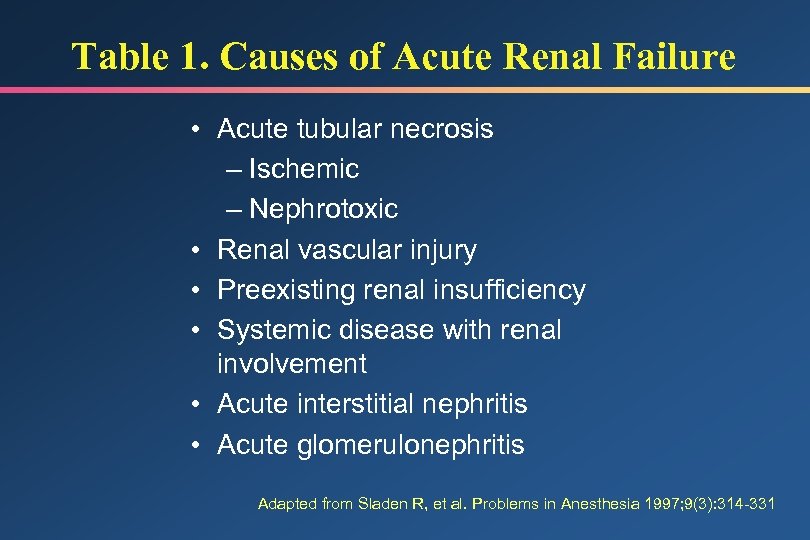 Table 1. Causes of Acute Renal Failure • Acute tubular necrosis – Ischemic – Nephrotoxic • Renal vascular injury • Preexisting renal insufficiency • Systemic disease with renal involvement • Acute interstitial nephritis • Acute glomerulonephritis Adapted from Sladen R, et al. Problems in Anesthesia 1997; 9(3): 314 -331
Table 1. Causes of Acute Renal Failure • Acute tubular necrosis – Ischemic – Nephrotoxic • Renal vascular injury • Preexisting renal insufficiency • Systemic disease with renal involvement • Acute interstitial nephritis • Acute glomerulonephritis Adapted from Sladen R, et al. Problems in Anesthesia 1997; 9(3): 314 -331
 Table 2. High-Risk Procedures and Events • Cardiac surgery • Vascular surgery • Biliary tract and hepatic surgery • Urogenital surgery • Complicated obstetrics • Major trauma Adapted from Sladen R, et al. Problems in Anesthesia 1997; 9(3): 314 -331
Table 2. High-Risk Procedures and Events • Cardiac surgery • Vascular surgery • Biliary tract and hepatic surgery • Urogenital surgery • Complicated obstetrics • Major trauma Adapted from Sladen R, et al. Problems in Anesthesia 1997; 9(3): 314 -331
 Incidence of Acute Renal Failure: Perioperative Risk Factors Requiring Dialysis CR CL <60 No Yes Prior Heart Surgery IABP No Yes Valve No NYHA IV Yes 0. 4% No 0. 9% NYHA IV Yes 2. 1% Yes 9. 5% Cardiomegaly Yes 1. 3% No No No Yes 2. 8% No PVD Yes No NYHA IV 2. 3% 1. 1% Yes 5. 0% Valve No 2. 1% Yes 6. 1% Chertow GM, et al. Circulation 1997; 95: 878 -884
Incidence of Acute Renal Failure: Perioperative Risk Factors Requiring Dialysis CR CL <60 No Yes Prior Heart Surgery IABP No Yes Valve No NYHA IV Yes 0. 4% No 0. 9% NYHA IV Yes 2. 1% Yes 9. 5% Cardiomegaly Yes 1. 3% No No No Yes 2. 8% No PVD Yes No NYHA IV 2. 3% 1. 1% Yes 5. 0% Valve No 2. 1% Yes 6. 1% Chertow GM, et al. Circulation 1997; 95: 878 -884
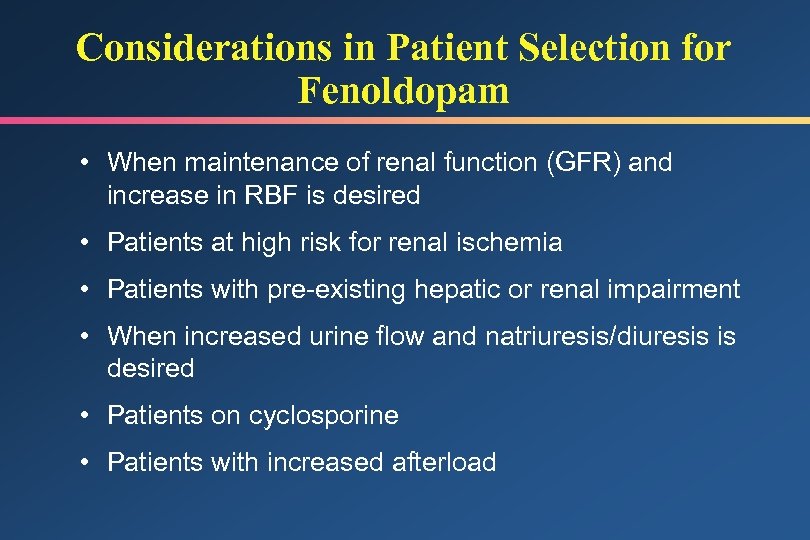 Considerations in Patient Selection for Fenoldopam • When maintenance of renal function (GFR) and increase in RBF is desired • Patients at high risk for renal ischemia • Patients with pre-existing hepatic or renal impairment • When increased urine flow and natriuresis/diuresis is desired • Patients on cyclosporine • Patients with increased afterload
Considerations in Patient Selection for Fenoldopam • When maintenance of renal function (GFR) and increase in RBF is desired • Patients at high risk for renal ischemia • Patients with pre-existing hepatic or renal impairment • When increased urine flow and natriuresis/diuresis is desired • Patients on cyclosporine • Patients with increased afterload
 Considerations when Choosing IV Therapies • Cost of drug • Cost of intensive care setting (ICU vs. floor) • Cost of monitoring (A-line vs. cuff) • Cost of medical personnel • Cost of monitoring for side effects (lactate levels) • Cost of treating side effects (colloid/crystalloid for hypotension)
Considerations when Choosing IV Therapies • Cost of drug • Cost of intensive care setting (ICU vs. floor) • Cost of monitoring (A-line vs. cuff) • Cost of medical personnel • Cost of monitoring for side effects (lactate levels) • Cost of treating side effects (colloid/crystalloid for hypotension)
 The Cost of Renal Failure Variable Length of Stay in Critical Care Unit Length of Stay in Hospital Ward Unadjusted All patients 1. No renal dysfunction 2. Renal dysfunction 3. Renal failure Adjusted for Preoperative Factors days 2. 0 4. 8 11. 6 3. 1 6. 5 14. 9 5. 9 10. 0 12. 4 7. 5 11. 7 13. 9 Mangano CM, et al, Ann Intern Med 1998; (3): 194 -203
The Cost of Renal Failure Variable Length of Stay in Critical Care Unit Length of Stay in Hospital Ward Unadjusted All patients 1. No renal dysfunction 2. Renal dysfunction 3. Renal failure Adjusted for Preoperative Factors days 2. 0 4. 8 11. 6 3. 1 6. 5 14. 9 5. 9 10. 0 12. 4 7. 5 11. 7 13. 9 Mangano CM, et al, Ann Intern Med 1998; (3): 194 -203


