ecb928af6d639e90ef1bbafb10656ba3.ppt
- Количество слайдов: 22
 Hydrogen and Fuel Cells 1
Hydrogen and Fuel Cells 1
 Hydrogen: The Reality - Hydrogen is the lightest of all gases - Its physical properties are incompatible with the requirements of the energy market (Low energy density) CV = 13 MJ/ m 3 & = 0. 019 kg/m 3 at STP Production, packaging, storage, transfer and delivery of the gas. - All key components of a hydrogen economy - So energy intensive that alternatives should be considered.
Hydrogen: The Reality - Hydrogen is the lightest of all gases - Its physical properties are incompatible with the requirements of the energy market (Low energy density) CV = 13 MJ/ m 3 & = 0. 019 kg/m 3 at STP Production, packaging, storage, transfer and delivery of the gas. - All key components of a hydrogen economy - So energy intensive that alternatives should be considered.
 Relative Energy Consumption • A hydrogen economy will involve transport by road • H 2 or methane stored at 200 bar, delivered in a 40 ton tanker • These tanks can be emptied to only 42 bar to accommodate the 40 bar pressure systems of the receiver (such pressure cascades are standard practice) • Thus, pressurised gas carriers deliver only 80% of their freight, while 20% of the load remains in the tanks and returned to the gas plant.
Relative Energy Consumption • A hydrogen economy will involve transport by road • H 2 or methane stored at 200 bar, delivered in a 40 ton tanker • These tanks can be emptied to only 42 bar to accommodate the 40 bar pressure systems of the receiver (such pressure cascades are standard practice) • Thus, pressurised gas carriers deliver only 80% of their freight, while 20% of the load remains in the tanks and returned to the gas plant.
 Relative Energy Consumption • At 200 bar pressure: 3. 2 tons of methane, but only 320 kg of H 2 can be delivered by a 40 ton tanker. • A direct consequence of the low density of H 2 + the weight of the 200 bar PRESSURE VESSEL and many safety installations. • Allowing for future %wt improvements in GH 2 storage to provide 500 kg, over 39 tons of dead weight have to be moved on the road to deliver 400 kg of H 2.
Relative Energy Consumption • At 200 bar pressure: 3. 2 tons of methane, but only 320 kg of H 2 can be delivered by a 40 ton tanker. • A direct consequence of the low density of H 2 + the weight of the 200 bar PRESSURE VESSEL and many safety installations. • Allowing for future %wt improvements in GH 2 storage to provide 500 kg, over 39 tons of dead weight have to be moved on the road to deliver 400 kg of H 2.
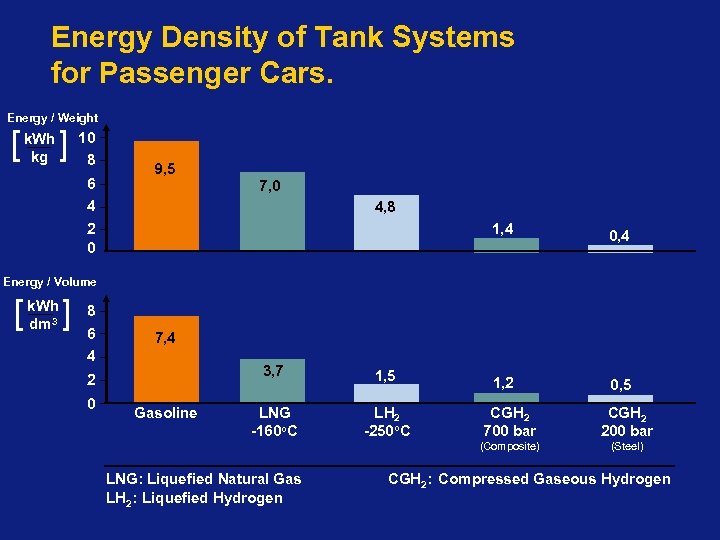 Energy Density of Tank Systems for Passenger Cars. Energy / Weight [ k. Wh ] 10 kg 8 6 4 9, 5 7, 0 4, 8 2 0 1, 4 0, 4 1, 2 0, 5 Energy / Volume [ k. Wh dm 3 ] 8 6 7, 4 4 3, 7 2 0 1, 5 LNG -160 o. C LH 2 -250 o. C LNG: Liquefied Natural Gas LH 2: Liquefied Hydrogen CGH 2 700 bar CGH 2 200 bar (Composite) Gasoline (Steel) CGH 2: Compressed Gaseous Hydrogen
Energy Density of Tank Systems for Passenger Cars. Energy / Weight [ k. Wh ] 10 kg 8 6 4 9, 5 7, 0 4, 8 2 0 1, 4 0, 4 1, 2 0, 5 Energy / Volume [ k. Wh dm 3 ] 8 6 7, 4 4 3, 7 2 0 1, 5 LNG -160 o. C LH 2 -250 o. C LNG: Liquefied Natural Gas LH 2: Liquefied Hydrogen CGH 2 700 bar CGH 2 200 bar (Composite) Gasoline (Steel) CGH 2: Compressed Gaseous Hydrogen
 What are the issues for hydrogen in fuel cells? • • • Fuel cells – what, how, why and when? ‘Markets’ for fuel cells Hydrogen for stationary fuel cells Hydrogen for transport fuel cells The International Perspective The future? © Imperial College London
What are the issues for hydrogen in fuel cells? • • • Fuel cells – what, how, why and when? ‘Markets’ for fuel cells Hydrogen for stationary fuel cells Hydrogen for transport fuel cells The International Perspective The future? © Imperial College London
 Fuel Cells • Fuel cell is an electrical cell, which unlike a battery can be fed with a continous supply of fuel so that electrical power production can be sustained indefinitely. • Several different fuel cell types, all work on the same principle: converting hydrogen directly into electrical energy and heat through the electrochemical reaction of hydrogen and oxygen:
Fuel Cells • Fuel cell is an electrical cell, which unlike a battery can be fed with a continous supply of fuel so that electrical power production can be sustained indefinitely. • Several different fuel cell types, all work on the same principle: converting hydrogen directly into electrical energy and heat through the electrochemical reaction of hydrogen and oxygen:
 Fuel Cell Operation • A fuel cell consists of an electrolyte sandwiched between two thin electrodes (a porous anode and cathode). • Hydrogen, is fed to the anode where a catalyst separates dissociates into charged electrons, e-, and positively charged ions (protons), H+. • Electrons at anode side of cell can’t pass through electrolyte to positively charged cathode; must travel to it via an electrical circuit (electrical current). • Protons move through the electrolyte to the cathode and combine with oxygen and electrons, producing water and heat.
Fuel Cell Operation • A fuel cell consists of an electrolyte sandwiched between two thin electrodes (a porous anode and cathode). • Hydrogen, is fed to the anode where a catalyst separates dissociates into charged electrons, e-, and positively charged ions (protons), H+. • Electrons at anode side of cell can’t pass through electrolyte to positively charged cathode; must travel to it via an electrical circuit (electrical current). • Protons move through the electrolyte to the cathode and combine with oxygen and electrons, producing water and heat.
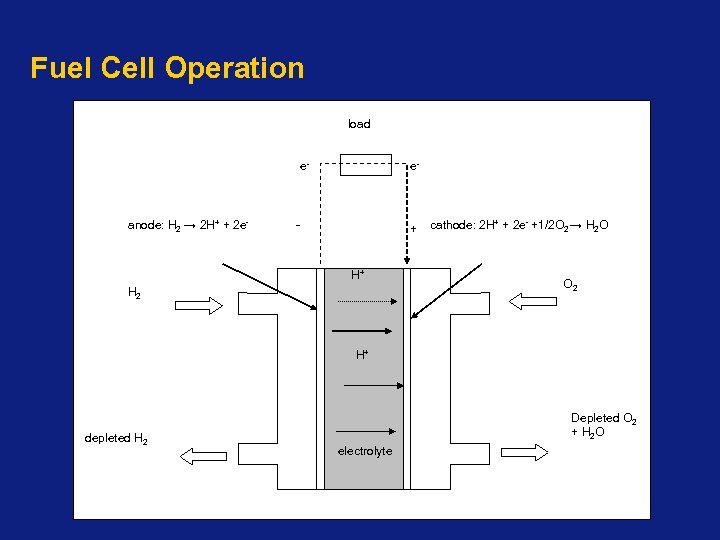 Fuel Cell Operation load e- anode: H 2 → 2 H+ + 2 e- e- - + H+ H 2 cathode: 2 H+ + 2 e- +1/2 O 2 → H 2 O O 2 H+ depleted H 2 Depleted O 2 + H 2 O electrolyte
Fuel Cell Operation load e- anode: H 2 → 2 H+ + 2 e- e- - + H+ H 2 cathode: 2 H+ + 2 e- +1/2 O 2 → H 2 O O 2 H+ depleted H 2 Depleted O 2 + H 2 O electrolyte
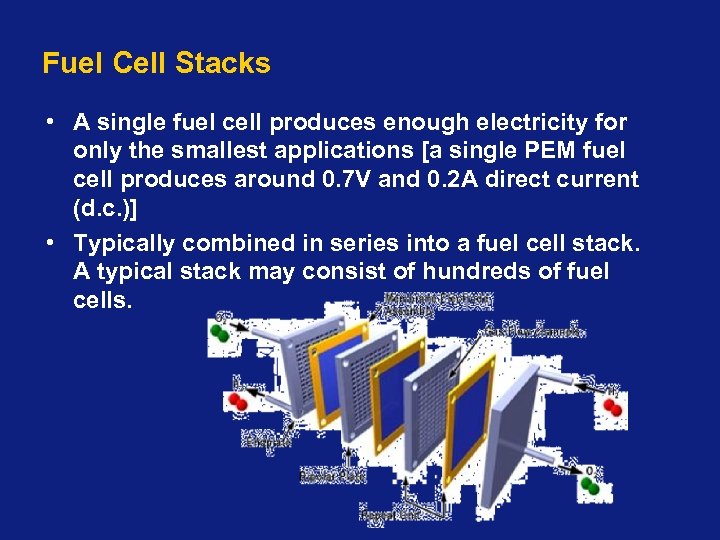 Fuel Cell Stacks • A single fuel cell produces enough electricity for only the smallest applications [a single PEM fuel cell produces around 0. 7 V and 0. 2 A direct current (d. c. )] • Typically combined in series into a fuel cell stack. A typical stack may consist of hundreds of fuel cells.
Fuel Cell Stacks • A single fuel cell produces enough electricity for only the smallest applications [a single PEM fuel cell produces around 0. 7 V and 0. 2 A direct current (d. c. )] • Typically combined in series into a fuel cell stack. A typical stack may consist of hundreds of fuel cells.
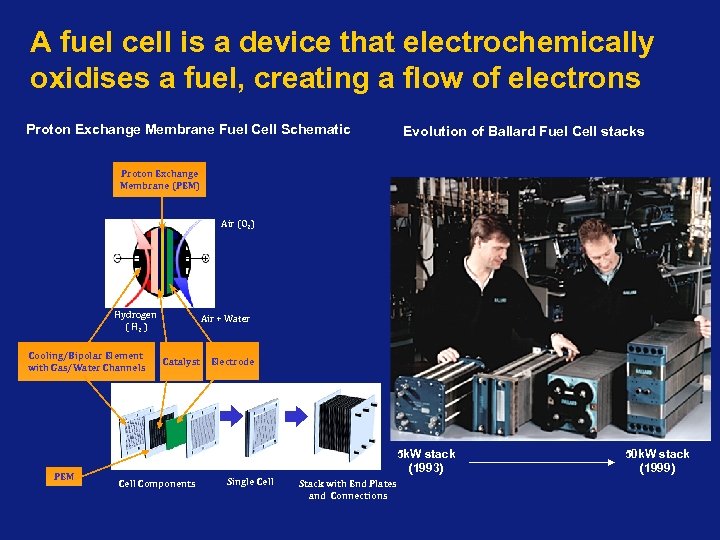 A fuel cell is a device that electrochemically oxidises a fuel, creating a flow of electrons Proton Exchange Membrane Fuel Cell Schematic Evolution of Ballard Fuel Cell stacks Proton Exchange Membrane (PEM) Air (O 2) Hydrogen ( H 2 ) Cooling/Bipolar Element with Gas/Water Channels PEM Air + Water Catalyst Electrode 5 k. W stack (1993) Cell Components Single Cell Stack with End Plates and Connections 50 k. W stack (1999)
A fuel cell is a device that electrochemically oxidises a fuel, creating a flow of electrons Proton Exchange Membrane Fuel Cell Schematic Evolution of Ballard Fuel Cell stacks Proton Exchange Membrane (PEM) Air (O 2) Hydrogen ( H 2 ) Cooling/Bipolar Element with Gas/Water Channels PEM Air + Water Catalyst Electrode 5 k. W stack (1993) Cell Components Single Cell Stack with End Plates and Connections 50 k. W stack (1999)
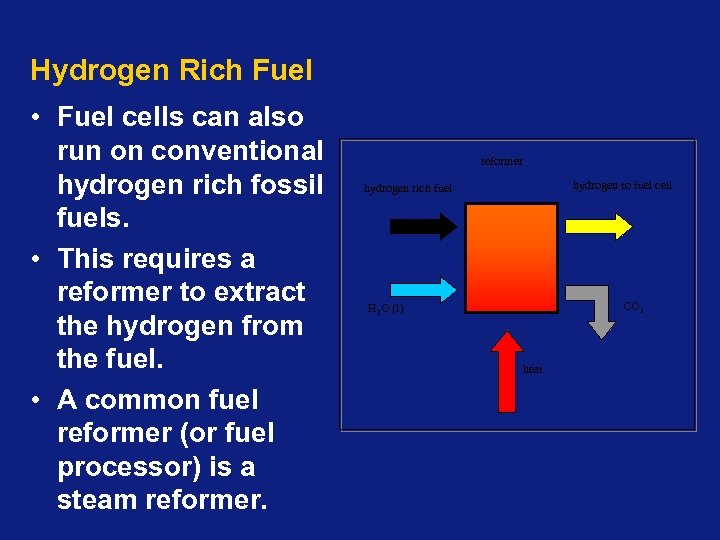 Hydrogen Rich Fuel • Fuel cells can also run on conventional hydrogen rich fossil fuels. • This requires a reformer to extract the hydrogen from the fuel. • A common fuel reformer (or fuel processor) is a steam reformer hydrogen to fuel cell hydrogen rich fuel CO 2 H 2 O (l) heat
Hydrogen Rich Fuel • Fuel cells can also run on conventional hydrogen rich fossil fuels. • This requires a reformer to extract the hydrogen from the fuel. • A common fuel reformer (or fuel processor) is a steam reformer hydrogen to fuel cell hydrogen rich fuel CO 2 H 2 O (l) heat
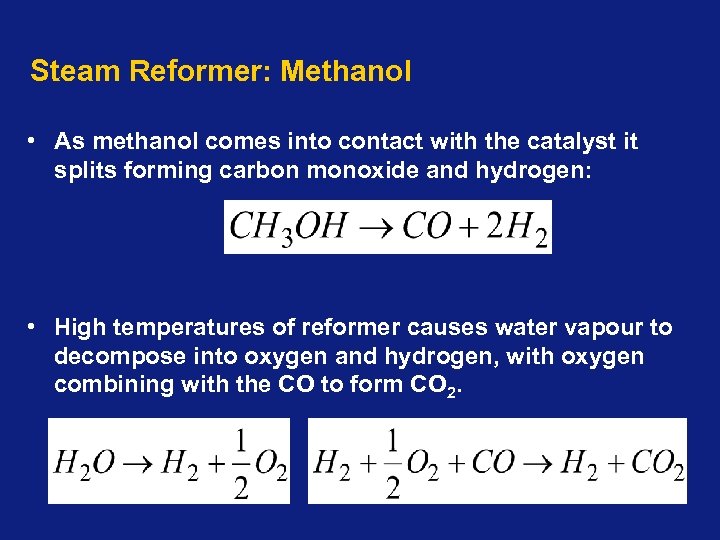 Steam Reformer: Methanol • As methanol comes into contact with the catalyst it splits forming carbon monoxide and hydrogen: • High temperatures of reformer causes water vapour to decompose into oxygen and hydrogen, with oxygen combining with the CO to form CO 2.
Steam Reformer: Methanol • As methanol comes into contact with the catalyst it splits forming carbon monoxide and hydrogen: • High temperatures of reformer causes water vapour to decompose into oxygen and hydrogen, with oxygen combining with the CO to form CO 2.
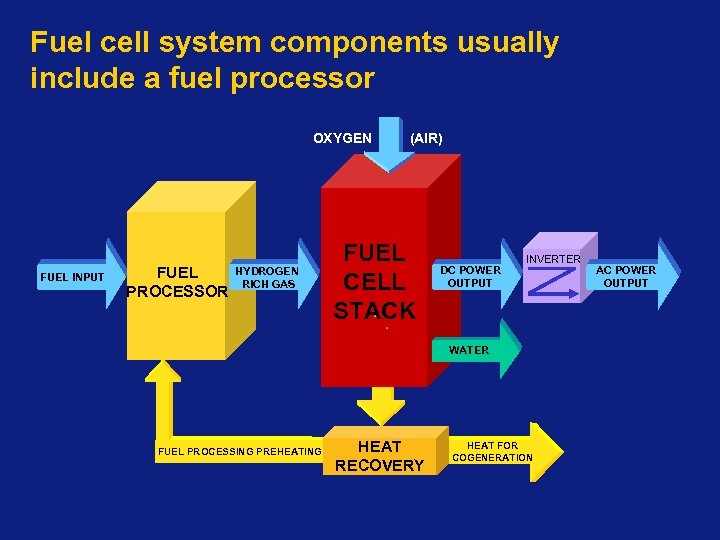 Fuel cell system components usually include a fuel processor OXYGEN FUEL INPUT FUEL PROCESSOR HYDROGEN RICH GAS (AIR) FUEL CELL STACK DC POWER OUTPUT INVERTER WATER FUEL PROCESSING PREHEATING HEAT RECOVERY HEAT FOR COGENERATION AC POWER OUTPUT
Fuel cell system components usually include a fuel processor OXYGEN FUEL INPUT FUEL PROCESSOR HYDROGEN RICH GAS (AIR) FUEL CELL STACK DC POWER OUTPUT INVERTER WATER FUEL PROCESSING PREHEATING HEAT RECOVERY HEAT FOR COGENERATION AC POWER OUTPUT
 Though significant barriers exist, fuel cells are emerging in applications • Other technologies already establish and perform the functions we want. • Energy markets are frequently conservative (slow to change). • Fuel cell costs are high, performance low (like many new technologies) • But fuel cells are becoming available: – PAFC systems are already installed in many areas – PEM systems are becoming available – The first FCVs are leased to customers – Hundreds of fuel cells are in test and demonstration worldwide
Though significant barriers exist, fuel cells are emerging in applications • Other technologies already establish and perform the functions we want. • Energy markets are frequently conservative (slow to change). • Fuel cell costs are high, performance low (like many new technologies) • But fuel cells are becoming available: – PAFC systems are already installed in many areas – PEM systems are becoming available – The first FCVs are leased to customers – Hundreds of fuel cells are in test and demonstration worldwide
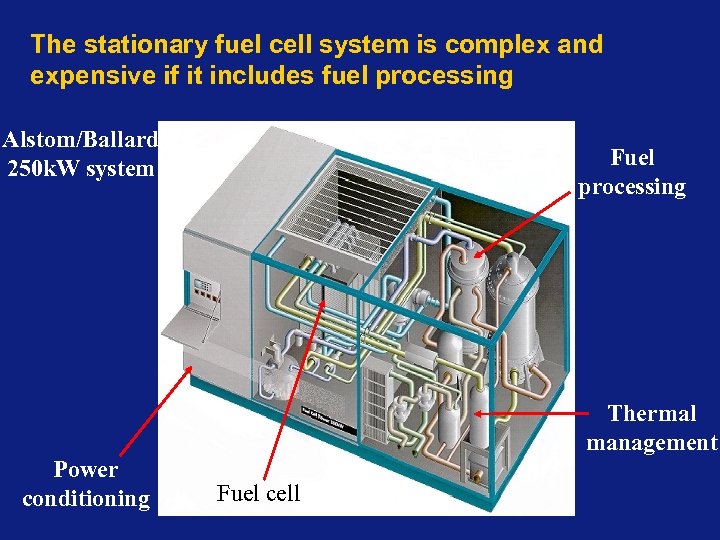 The stationary fuel cell system is complex and expensive if it includes fuel processing Alstom/Ballard 250 k. W system Fuel processing Thermal management Power conditioning Fuel cell
The stationary fuel cell system is complex and expensive if it includes fuel processing Alstom/Ballard 250 k. W system Fuel processing Thermal management Power conditioning Fuel cell
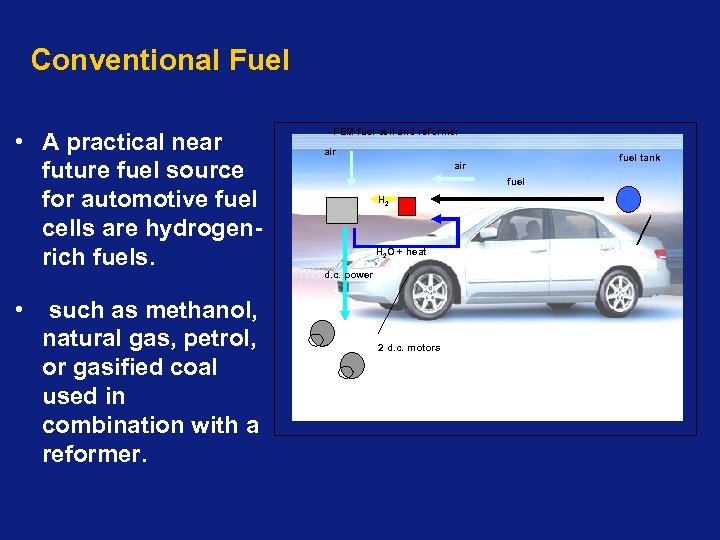 Conventional Fuel • A practical near future fuel source for automotive fuel cells are hydrogenrich fuels. • such as methanol, natural gas, petrol, or gasified coal used in combination with a reformer. PEM fuel cell and reformer air fuel tank air fuel H 2 O + heat d. c. power 2 d. c. motors
Conventional Fuel • A practical near future fuel source for automotive fuel cells are hydrogenrich fuels. • such as methanol, natural gas, petrol, or gasified coal used in combination with a reformer. PEM fuel cell and reformer air fuel tank air fuel H 2 O + heat d. c. power 2 d. c. motors
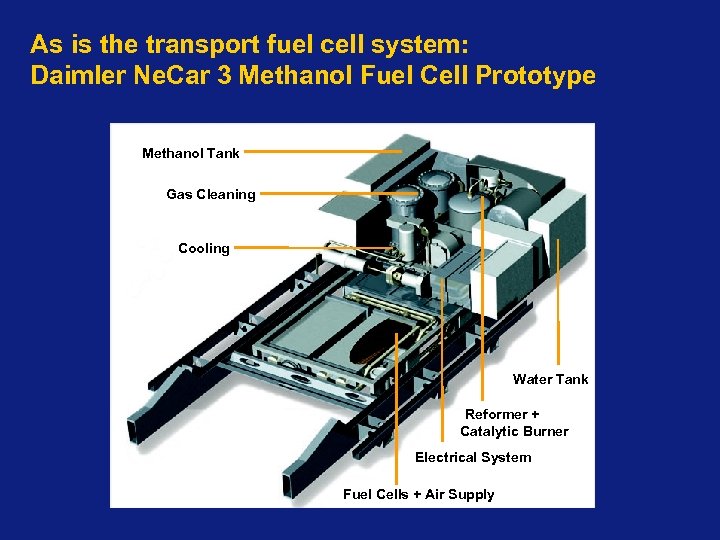 As is the transport fuel cell system: Daimler Ne. Car 3 Methanol Fuel Cell Prototype Methanol Tank Gas Cleaning Cooling Water Tank Reformer + Catalytic Burner Electrical System Fuel Cells + Air Supply
As is the transport fuel cell system: Daimler Ne. Car 3 Methanol Fuel Cell Prototype Methanol Tank Gas Cleaning Cooling Water Tank Reformer + Catalytic Burner Electrical System Fuel Cells + Air Supply
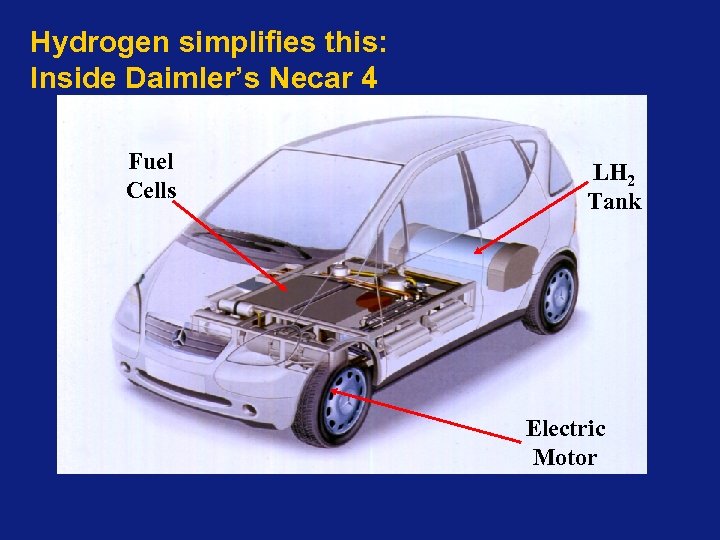 Hydrogen simplifies this: Inside Daimler’s Necar 4 Fuel Cells LH 2 Tank Electric Motor
Hydrogen simplifies this: Inside Daimler’s Necar 4 Fuel Cells LH 2 Tank Electric Motor
 The fuel cell ‘fleet’ is now mostly hydrogen fuelled Daimler. Chrysler Ford Iris. Bus Toyota MAN GM/Opel
The fuel cell ‘fleet’ is now mostly hydrogen fuelled Daimler. Chrysler Ford Iris. Bus Toyota MAN GM/Opel
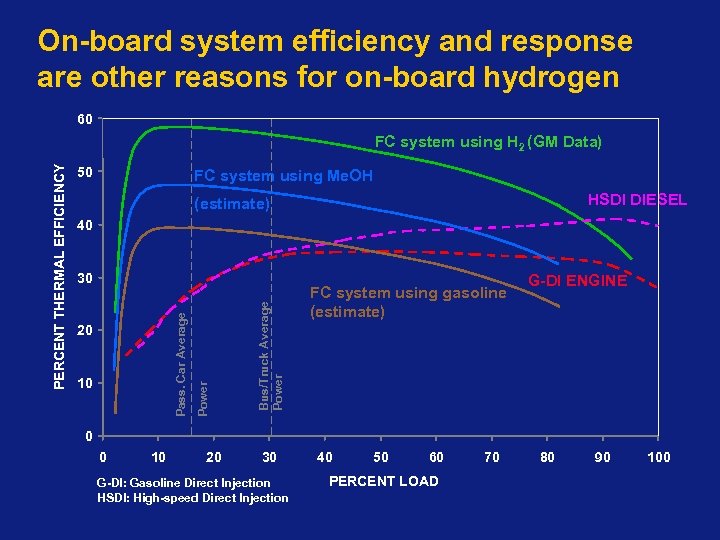 On-board system efficiency and response are other reasons for on-board hydrogen 60 50 FC system using Me. OH HSDI DIESEL (estimate) 40 10 Bus/Truck Average Power 20 Power 30 Pass. Car Average PERCENT THERMAL EFFICIENCY FC system using H 2 (GM Data) FC system using gasoline (estimate) G-DI ENGINE 0 0 10 20 30 G-DI: Gasoline Direct Injection HSDI: High-speed Direct Injection 40 50 60 PERCENT LOAD 70 80 90 100
On-board system efficiency and response are other reasons for on-board hydrogen 60 50 FC system using Me. OH HSDI DIESEL (estimate) 40 10 Bus/Truck Average Power 20 Power 30 Pass. Car Average PERCENT THERMAL EFFICIENCY FC system using H 2 (GM Data) FC system using gasoline (estimate) G-DI ENGINE 0 0 10 20 30 G-DI: Gasoline Direct Injection HSDI: High-speed Direct Injection 40 50 60 PERCENT LOAD 70 80 90 100
 What about the future? • In the very long term, electricity and hydrogen are likely to become complementary energy vectors of choice. • Hydrogen and the fuel cell are complementary, and each enables the other. • The transition to the ‘long term’ is unclear, but the ubiquitous interest in fuel cells and hydrogen suggests it may be underway.
What about the future? • In the very long term, electricity and hydrogen are likely to become complementary energy vectors of choice. • Hydrogen and the fuel cell are complementary, and each enables the other. • The transition to the ‘long term’ is unclear, but the ubiquitous interest in fuel cells and hydrogen suggests it may be underway.


