3fefcca731b1fb454e41546a381d4c6c.ppt
- Количество слайдов: 22
 Hydrogen A Fuel for Today and Tomorrow
Hydrogen A Fuel for Today and Tomorrow
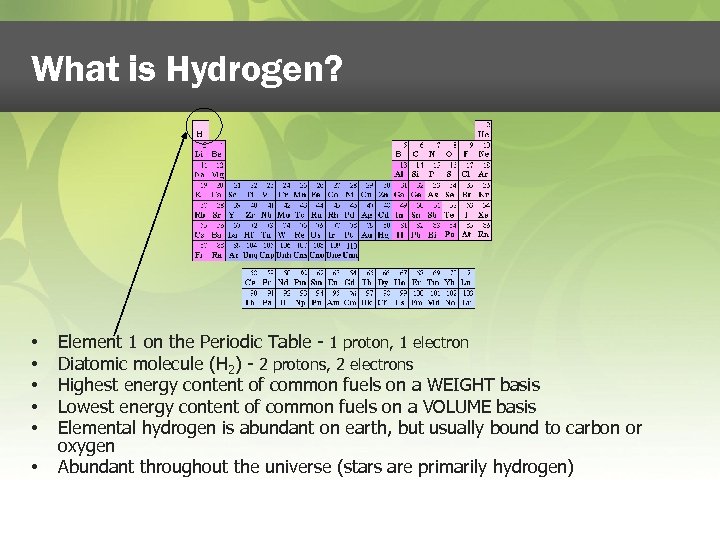 What is Hydrogen? • • • Element 1 on the Periodic Table - 1 proton, 1 electron Diatomic molecule (H 2) - 2 protons, 2 electrons Highest energy content of common fuels on a WEIGHT basis Lowest energy content of common fuels on a VOLUME basis Elemental hydrogen is abundant on earth, but usually bound to carbon or oxygen Abundant throughout the universe (stars are primarily hydrogen)
What is Hydrogen? • • • Element 1 on the Periodic Table - 1 proton, 1 electron Diatomic molecule (H 2) - 2 protons, 2 electrons Highest energy content of common fuels on a WEIGHT basis Lowest energy content of common fuels on a VOLUME basis Elemental hydrogen is abundant on earth, but usually bound to carbon or oxygen Abundant throughout the universe (stars are primarily hydrogen)
 • Energy carriers move energy in a usable form from one place to another. • Electricity is an energy carrier • So are gasoline and hydrogen • Hydrogen allows us to store energy from many sources and bring it to where we need it. Hydrogen is an Energy Carrier
• Energy carriers move energy in a usable form from one place to another. • Electricity is an energy carrier • So are gasoline and hydrogen • Hydrogen allows us to store energy from many sources and bring it to where we need it. Hydrogen is an Energy Carrier
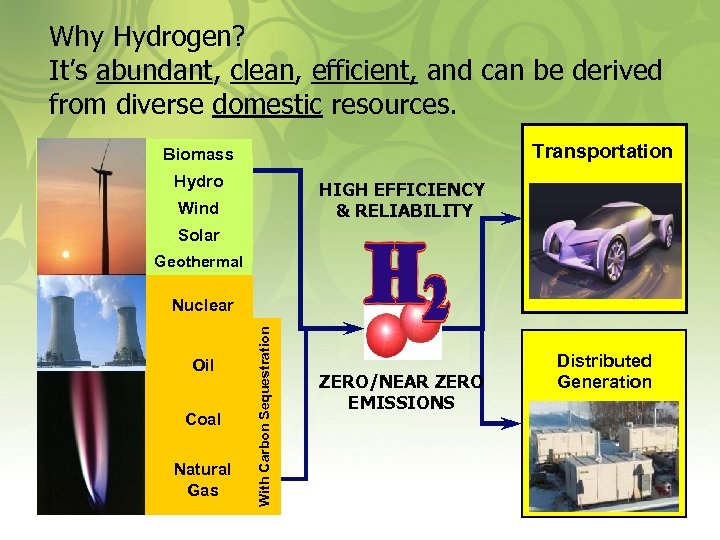 Why Hydrogen? It’s abundant, clean, efficient, and can be derived from diverse domestic resources. Transportation Biomass Hydro HIGH EFFICIENCY & RELIABILITY Wind Solar . Geothermal Oil Coal Natural Gas With Carbon Sequestration Nuclear ZERO/NEAR ZERO EMISSIONS Distributed Generation
Why Hydrogen? It’s abundant, clean, efficient, and can be derived from diverse domestic resources. Transportation Biomass Hydro HIGH EFFICIENCY & RELIABILITY Wind Solar . Geothermal Oil Coal Natural Gas With Carbon Sequestration Nuclear ZERO/NEAR ZERO EMISSIONS Distributed Generation
 Where is Hydrogen Found? • Hydrogen as a gas in NOT abundant in underground reservoirs. • Hydrogen bonds easily to other elements and is rarely found on its own. • While hydrogen can be stripped from underground deposits of natural gas (methane) there are no underground deposits of pure hydrogen.
Where is Hydrogen Found? • Hydrogen as a gas in NOT abundant in underground reservoirs. • Hydrogen bonds easily to other elements and is rarely found on its own. • While hydrogen can be stripped from underground deposits of natural gas (methane) there are no underground deposits of pure hydrogen.
 • Hydrogen can be produced from water; from carbon-containing materials (usually reacting with water); as a byproduct of chemical processes • Regional variations in traditional energy resources are no longer an issue • Every region has some indigenous fossil or renewable resource that can be used to make hydrogen Flexibility of Source
• Hydrogen can be produced from water; from carbon-containing materials (usually reacting with water); as a byproduct of chemical processes • Regional variations in traditional energy resources are no longer an issue • Every region has some indigenous fossil or renewable resource that can be used to make hydrogen Flexibility of Source
 Commercial Product Today Steam Methane Reforming (SMR) 48% of world production Nearly 95% of the U. S. hydrogen production Strong economy-of-scale Heat integration within and outside of SMR Overall energy efficiency is affected by the ability to make use of the steam by-product
Commercial Product Today Steam Methane Reforming (SMR) 48% of world production Nearly 95% of the U. S. hydrogen production Strong economy-of-scale Heat integration within and outside of SMR Overall energy efficiency is affected by the ability to make use of the steam by-product
 Current Hydrogen Fuel Use in the U. S. • 70 fueling stations – 23 in California – 9 in New York – 4 in Michigan – 1 -2 in AZ, CO, CT, HI, IL, MA, MO, NV, ND, OH, PA, SC, TX, VT, VA, WV • 421 Hydrogen Vehicles, a 34% increase since 2008 • Honda FCX sedan and the Mercedes-Benz B-Class F-Cell are the only fuel cell cars available to the public on a limited release agreement (mostly in S. California) Data from Transportation Energy Data Book, Dept. of Energy, 2010 3/19/2018 Footer Goes Here 8
Current Hydrogen Fuel Use in the U. S. • 70 fueling stations – 23 in California – 9 in New York – 4 in Michigan – 1 -2 in AZ, CO, CT, HI, IL, MA, MO, NV, ND, OH, PA, SC, TX, VT, VA, WV • 421 Hydrogen Vehicles, a 34% increase since 2008 • Honda FCX sedan and the Mercedes-Benz B-Class F-Cell are the only fuel cell cars available to the public on a limited release agreement (mostly in S. California) Data from Transportation Energy Data Book, Dept. of Energy, 2010 3/19/2018 Footer Goes Here 8
 Commercial Production Today Petroleum Refining 30% of world production Used within the refinery Coal Gasification 18% of world production Byproduct of steel industry Coke off-gas Primarily found in Europe and Asia Electrolysis 5% of world production High-purity for on-site generation and use Cost is a strong function of electricity cost
Commercial Production Today Petroleum Refining 30% of world production Used within the refinery Coal Gasification 18% of world production Byproduct of steel industry Coke off-gas Primarily found in Europe and Asia Electrolysis 5% of world production High-purity for on-site generation and use Cost is a strong function of electricity cost
 Other Ways to Liberate Hydrogen From Water • Steam Electrolysis • Split water with heat, pressure, and electricity • Thermochemical • Split water with chemicals and heat • Photoelectrochemical • Split water using sunlight directly, or using chemicals and heat • Biological • Split water using organisms
Other Ways to Liberate Hydrogen From Water • Steam Electrolysis • Split water with heat, pressure, and electricity • Thermochemical • Split water with chemicals and heat • Photoelectrochemical • Split water using sunlight directly, or using chemicals and heat • Biological • Split water using organisms
 • Storage of hydrogen on board a vehicle is a tough technical challenge • Installation of a hydrogen delivery and dispensing infrastructure is expensive • It’s not just the transportation sector that is affected by hydrogen and fuel cells –stationary and portable applications also affected. Challenges of Hydrogen
• Storage of hydrogen on board a vehicle is a tough technical challenge • Installation of a hydrogen delivery and dispensing infrastructure is expensive • It’s not just the transportation sector that is affected by hydrogen and fuel cells –stationary and portable applications also affected. Challenges of Hydrogen
 Hydrogen Storage and Transportation • Hydrogen can be cooled and stored as a o liquid. It must be cooled to -253 • It can also be stored as a gas. It must be compressed to be stored efficiently.
Hydrogen Storage and Transportation • Hydrogen can be cooled and stored as a o liquid. It must be cooled to -253 • It can also be stored as a gas. It must be compressed to be stored efficiently.
 Hydrogen Storage • High-pressure storage tanks. Hydrogen gas can be compressed and stored in storage tanks at high pressure, but these tanks must be very strong. • Liquid hydrogen. Hydrogen can be stored as a liquid. In this form, more hydrogen can be stored per volume, but it must be kept at very cold temperature (about -253° C).
Hydrogen Storage • High-pressure storage tanks. Hydrogen gas can be compressed and stored in storage tanks at high pressure, but these tanks must be very strong. • Liquid hydrogen. Hydrogen can be stored as a liquid. In this form, more hydrogen can be stored per volume, but it must be kept at very cold temperature (about -253° C).
 Hydrogen Storage • Metal hydrides. Hydrogen combines chemically with some metals, which can store it more efficiently than high-pressure storage tanks. • Carbon nanotubes are microscopic tubes of carbon, two nanometers (billionths of a meter) across, which store hydrogen in their microscopic pores.
Hydrogen Storage • Metal hydrides. Hydrogen combines chemically with some metals, which can store it more efficiently than high-pressure storage tanks. • Carbon nanotubes are microscopic tubes of carbon, two nanometers (billionths of a meter) across, which store hydrogen in their microscopic pores.
 Hydrogen Storage Hydrogen storage takes place… – On-board a vehicle – At production sites, in transit, and at refueling stations Hydrogen can be stored in its pure form, or can be reformed on board a vehicle from other fuels
Hydrogen Storage Hydrogen storage takes place… – On-board a vehicle – At production sites, in transit, and at refueling stations Hydrogen can be stored in its pure form, or can be reformed on board a vehicle from other fuels
 Hydrogen Fuel Cells for Transportation • PEM fuel cells are favored because they operate at low temperature (~80°C) • less waste heat…but also limits CHP applications compared to other fuel cell types • Quick startup, lower thermal stresses • Efficient at low loads (typical operating region for vehicles)
Hydrogen Fuel Cells for Transportation • PEM fuel cells are favored because they operate at low temperature (~80°C) • less waste heat…but also limits CHP applications compared to other fuel cell types • Quick startup, lower thermal stresses • Efficient at low loads (typical operating region for vehicles)
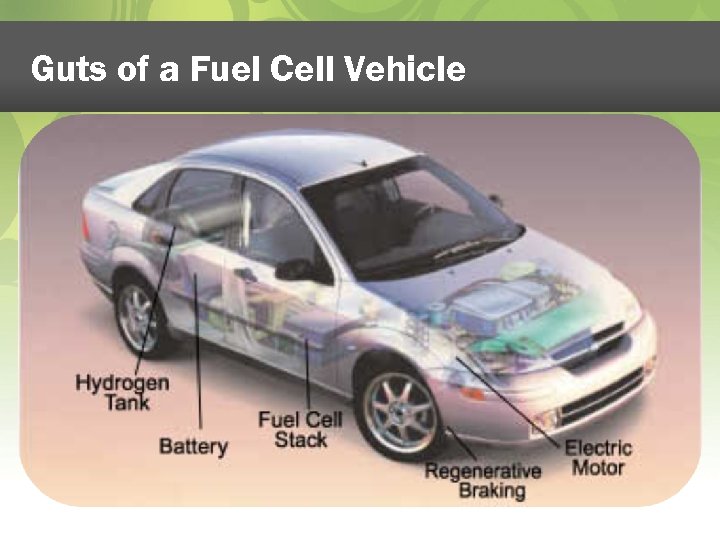 Guts of a Fuel Cell Vehicle
Guts of a Fuel Cell Vehicle
 Fuel Cell Life While fuel cells do wear out over time, A PEM fuel cell in a vehicle should have a 4, 000 hour service life, while stationary applications should last 40, 000 hours.
Fuel Cell Life While fuel cells do wear out over time, A PEM fuel cell in a vehicle should have a 4, 000 hour service life, while stationary applications should last 40, 000 hours.
 Hydrogen Safety Hydrogen Gasoline Three Second seconds Fuel leak simulation Hydrogen on left Gasoline on right Equivalent energy release One minute
Hydrogen Safety Hydrogen Gasoline Three Second seconds Fuel leak simulation Hydrogen on left Gasoline on right Equivalent energy release One minute
 Flexibility Of Use Transportation Desired range can be achieved with on-board hydrogen storage (unlike Battery Electric Vehicle) Can be used in internal combustion engines Trains, automobiles, buses, and ships Buildings Combined heat, power, and fuel Reliable energy services for critical applications Grid independence Industrial Sector Already plays an important role as a chemical Opportunities for additional revenue streams
Flexibility Of Use Transportation Desired range can be achieved with on-board hydrogen storage (unlike Battery Electric Vehicle) Can be used in internal combustion engines Trains, automobiles, buses, and ships Buildings Combined heat, power, and fuel Reliable energy services for critical applications Grid independence Industrial Sector Already plays an important role as a chemical Opportunities for additional revenue streams
 So– why hydrogen? • Energy security • Diverse domestic sources • Flexibility of system • Economic security • International leadership in technical • development and deployment • Price stability • Environmental security • Potential to meet GHG targets • Urban air quality improvements • Reduction in air pollutants
So– why hydrogen? • Energy security • Diverse domestic sources • Flexibility of system • Economic security • International leadership in technical • development and deployment • Price stability • Environmental security • Potential to meet GHG targets • Urban air quality improvements • Reduction in air pollutants
 The NEED Project acknowledges… Catherine E. Grégoire Padró Los Alamos National Laboratory Dr. Rajat K. Sen, Patty Kappaz Sentech, Inc.
The NEED Project acknowledges… Catherine E. Grégoire Padró Los Alamos National Laboratory Dr. Rajat K. Sen, Patty Kappaz Sentech, Inc.


