efb07a0b169dceaf537909baa283e671.ppt
- Количество слайдов: 14

Human Subjects in Clinical Trials Prepared for Involve Conference – People in research Presented by Jane Fiona Cumming September 2006 Invigoration through innovation

Agenda • Context • What was put in place • How members of the public have been involved • Lessons learnt • Issues for discussion Invigoration through innovation
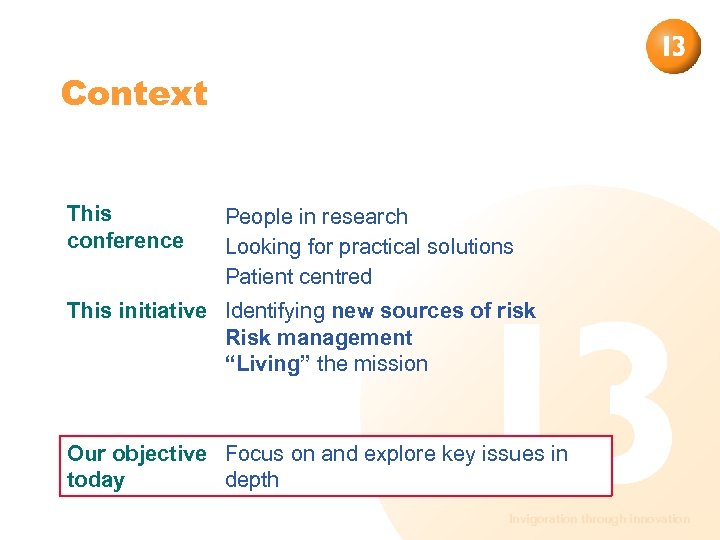
Context This conference People in research Looking for practical solutions Patient centred This initiative Identifying new sources of risk Risk management “Living” the mission Our objective Focus on and explore key issues in today depth Invigoration through innovation

The press Mirror 1 April 2006 THE ELEPHANT MAN EXCLUSIVE: HIS STORY By Victoria Ward TERRIFIED Mohamed Abdelhady lay on his hospital bed awaiting his drug trial hideously aware other volunteers were already writhing in agony. Unknown to him in minutes he would suffer excruciating pain, his head would balloon to three times its size and he would become the original Elephant Man in the biggest health trial blunder in living memory. Yet for now he was helpless just waiting his turn. Invigoration through innovation

2004 - Risk – was informed consent an emerging issue? A number of catastrophes as well, trials going wrong • Needed to be explored Reputation of the ‘industry’ • Externally, consent and understanding issues emerging in the health sector as a whole (Alder Hey, Bristol Royal Infirmary) Share prices and Corporate governance • Focuses on risk – this lack of understanding of informed consent raised internally as a possible risk • Share prices – and ratings - of pharma companies being affected by behaviour in trials Reputation of the company • Journalist scrutiny • Employee concern/motivation Invigoration through innovation
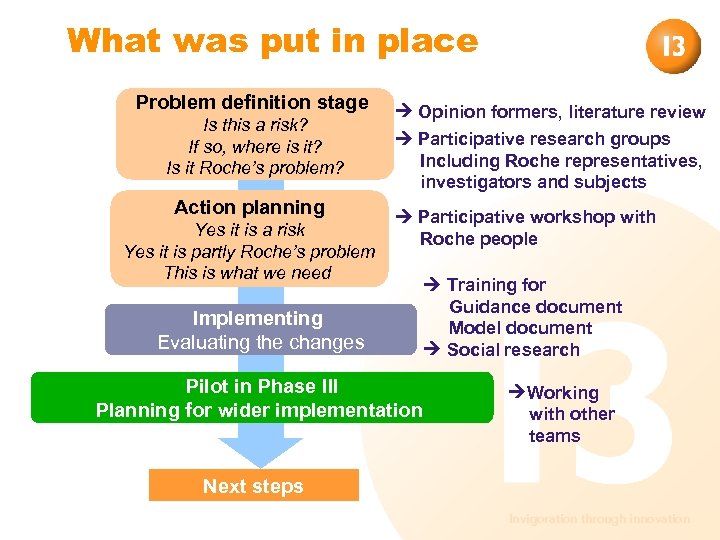
What was put in place Problem definition stage Is this a risk? If so, where is it? Is it Roche’s problem? Action planning Yes it is a risk Yes it is partly Roche’s problem This is what we need Opinion formers, literature review Participative research groups Including Roche representatives, investigators and subjects Participative workshop with Roche people Implementing Evaluating the changes Pilot in Phase III Planning for wider implementation Training for Guidance document Model document Social research Working with other teams Next steps Invigoration through innovation
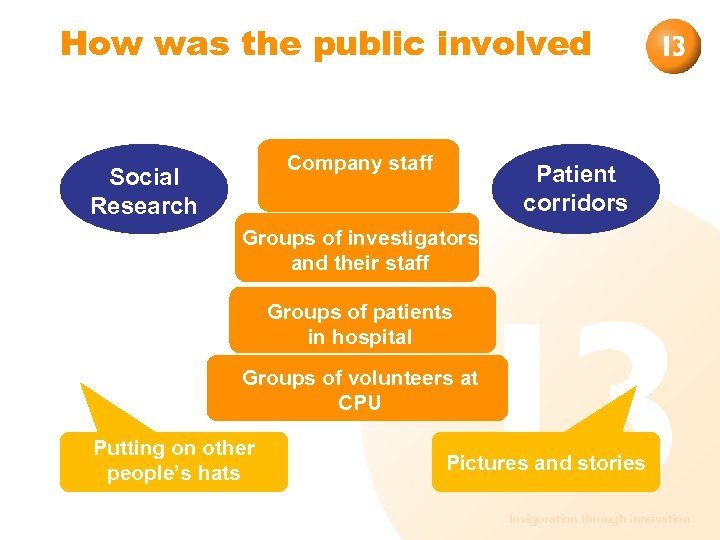
How was the public involved Company staff Social Research Patient corridors Groups of investigators and their staff Groups of patients in hospital Groups of volunteers at CPU Putting on other people’s hats Pictures and stories Invigoration through innovation

What was learnt - Risks and opportunities Risks Opportunities • Lack of standardisation of approach across Investigator sites • • • Focus on the subject (Userfriendly) • Design of ICF & Subject Lack of evidence and transparency as Information documents to what sites do • Appending aids e. g. FAQs Inconsistent data • Subject Understanding Early withdrawal of subjects • Time to read, Time to ask Litigation (subject) questions • Documentation and checking Rejection of trial (regulator / ethics committees) • Focus on the process • Unfavourable publicity • Ethical • Minimum standards followed consistently Invigoration through innovation

Lessons learnt - implementation Objectives: • To increase the transparency of the informed consent procedure: within Roche, to the investigators and to the subjects. • To build evidence within Roche. Implementation: • Process for Roche staff • Guidance document • Model consent form • Training Invigoration through innovation
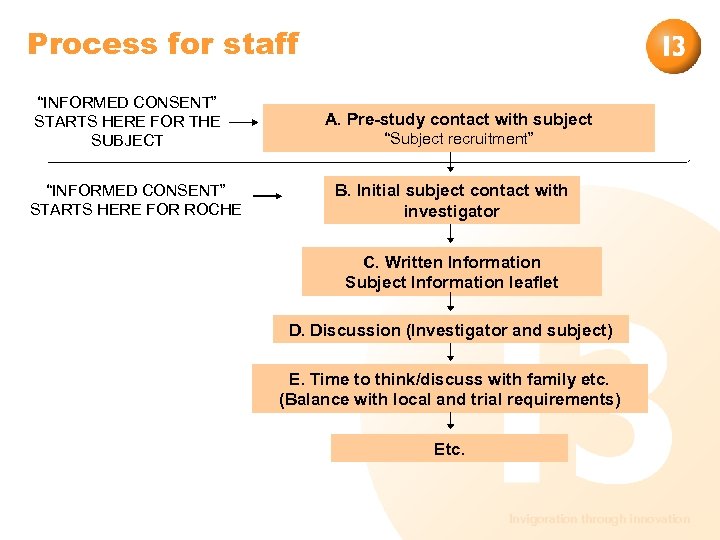
Process for staff “INFORMED CONSENT” STARTS HERE FOR THE SUBJECT “INFORMED CONSENT” STARTS HERE FOR ROCHE A. Pre-study contact with subject “Subject recruitment” B. Initial subject contact with investigator C. Written Information Subject Information leaflet D. Discussion (Investigator and subject) E. Time to think/discuss with family etc. (Balance with local and trial requirements) Etc. Invigoration through innovation
Guidance document Developed guidance document: We already have guidelines and SOPs – surely following those is enough? Put ourselves in the subject’s shoes…? What will this approach deliver to me / Roche? Better subject understanding can deliver: - Reputation for new and innovative ways of doing things - Lower risk of litigation - More reliable study data - Lower risk of subject withdrawal - Reputation for being an ethical company committed to good clinical practice Invigoration through innovation
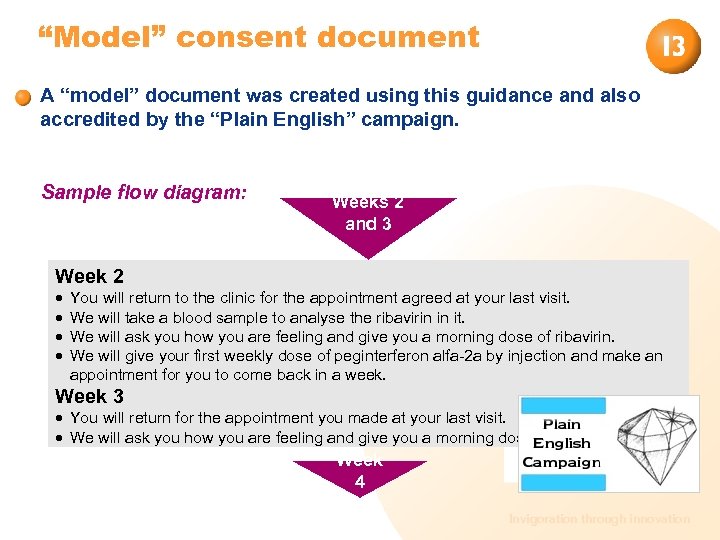
“Model” consent document A “model” document was created using this guidance and also accredited by the “Plain English” campaign. Sample flow diagram: Weeks 2 and 3 Week 2 You will return to the clinic for the appointment agreed at your last visit. We will take a blood sample to analyse the ribavirin in it. We will ask you how you are feeling and give you a morning dose of ribavirin. We will give your first weekly dose of peginterferon alfa-2 a by injection and make an appointment for you to come back in a week. Week 3 You will return for the appointment you made at your last visit. We will ask you how you are feeling and give you a morning dose of ribavirin. Week 4 Invigoration through innovation

Issues for discussion Assumption: that the full informed consent procedure is carried out consistently by sites. The findings in total demonstrate that the focus before the initiative was on “procedure”: i. e. ensuring IC is in line with current ICH GCP guidelines, ensuring that consent is granted before the trial, advising subjects on their right to withdraw etc. However this needs to be widened to include the least mentioned part of the IC procedures i. e. Ensuring subject understanding and subject safety and well being. (Greater Subject Focus). The risk for Roche was/is: • Where is the evidence of interaction and checking understanding? • Why is subject safety and well being not a salient aspect of IC? The evaluation to date demonstrates a useful process has been developed to address this. Invigoration through innovation

For further information contact Jane Fiona Cumming Article 13 Bradley House 26 St Alban’s Lane London NW 11 7 QE www. article 13. com email: janefionac@Article 13. com 0208 731 7700 September 2006 Invigoration through innovation
efb07a0b169dceaf537909baa283e671.ppt