a476b97188ff6c6a66b69377b059baf9.ppt
- Количество слайдов: 91

Human Physiology: Endomembrane system BY DR BOOMINATHAN Ph. D. M. Sc. , (Med. Bio, JIPMER), M. Sc. , (FGSWI, Israel), Ph. D (NUS, SINGAPORE) PONDICHERRY UNIVERSITY III Lecture 9/August/2012 Source: Collected from different sources on the internet-http: //koning. ecsu. ctstateu. edu/cell. html

Learning objectives 1. The structure and function of endoplasmic reticulum(ER); 2. The structure and function of Golgi complex; 3. The structure and function of lysosomes.
Introduction The Compartmentalization in Eukaryotic Cells n Membranes divide the cytoplasm of eukaryotic cells into distinct compartments. Three categories in eukaryotic cells: 1. the endomembrane system: endoplasmic reticu- lum, Golgi complex, Lysosomes. 2. the cytosol. 3. mitochondria, peroxisomes and the nucleus. Membrane-bound structures (organelles) are found in all eukaryotic cells.
Introduce Endomembrane System : The structural and functional relationship organelles include endoplasmic reticulum , Golgi complex, lysosome, secretory vesicles. n Membrane-bound structures (organelles) are found in all eukaryotic cells. n
Endomembrane System endoplasmic reticulum(ER) Golgi complex lysosome secretory vesicles
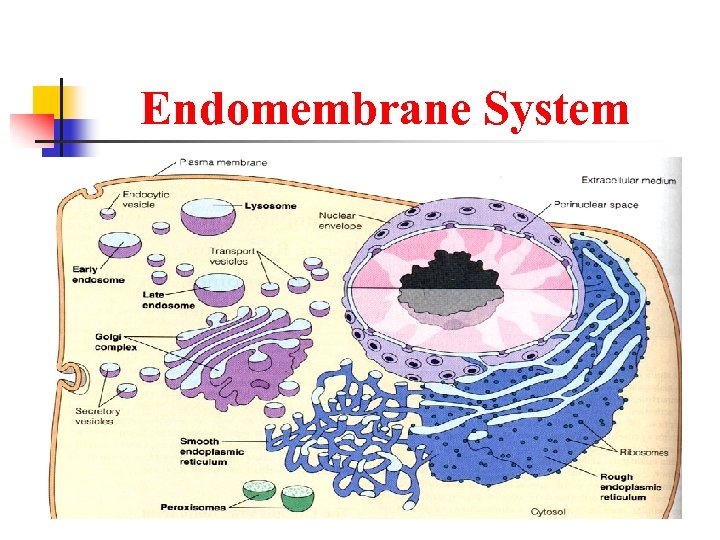
Endomembrane System
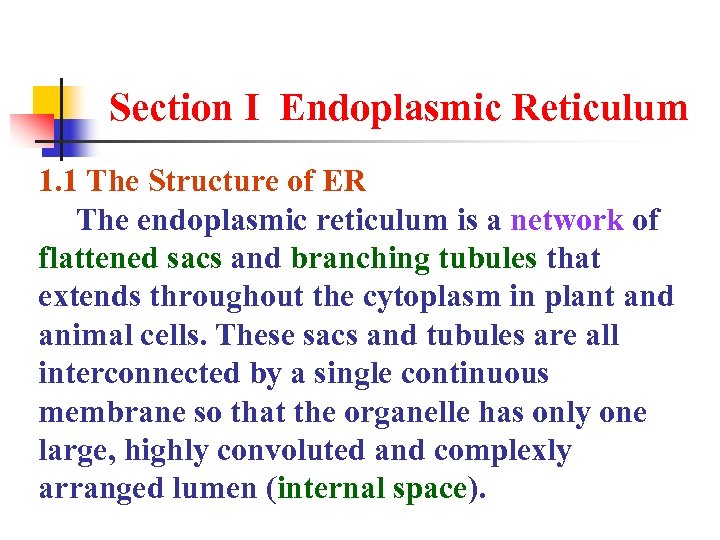
Section I Endoplasmic Reticulum 1. 1 The Structure of ER The endoplasmic reticulum is a network of flattened sacs and branching tubules that extends throughout the cytoplasm in plant and animal cells. These sacs and tubules are all interconnected by a single continuous membrane so that the organelle has only one large, highly convoluted and complexly arranged lumen (internal space).
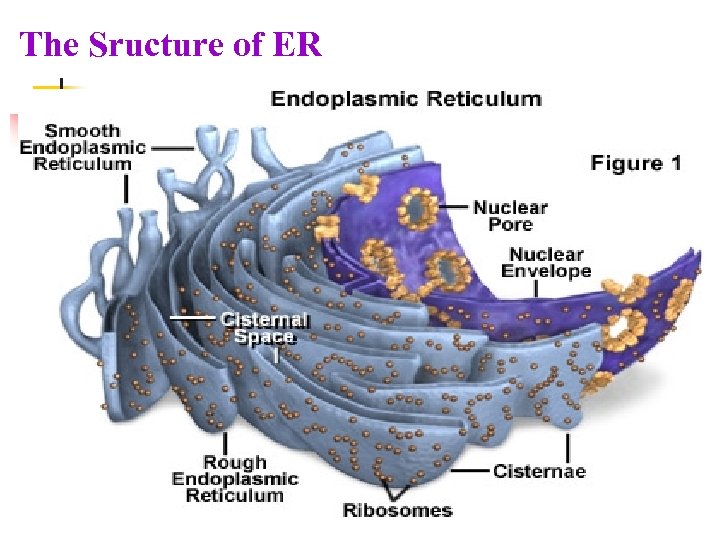
The Sructure of ER

1. 2 The types of ER The ER comes in two forms. Rough endoplasmic reticulum(RER) Smooth endoplasmic reticulum(SER)
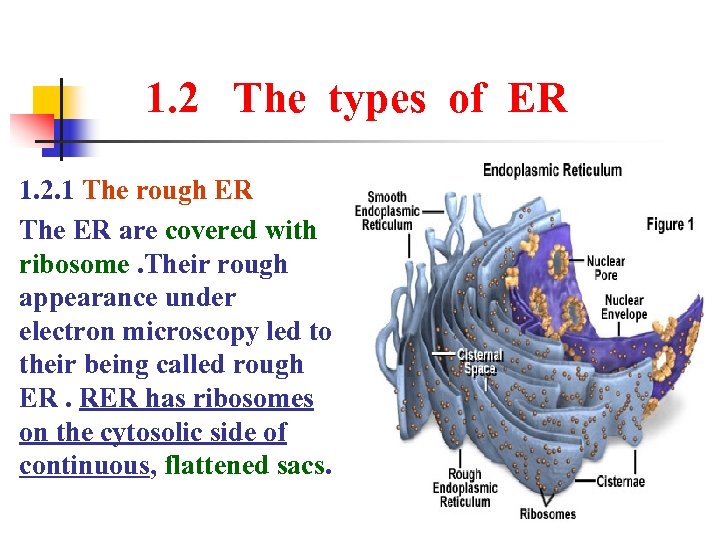
1. 2 The types of ER 1. 2. 1 The rough ER The ER are covered with ribosome. Their rough appearance under electron microscopy led to their being called rough ER. RER has ribosomes on the cytosolic side of continuous, flattened sacs.
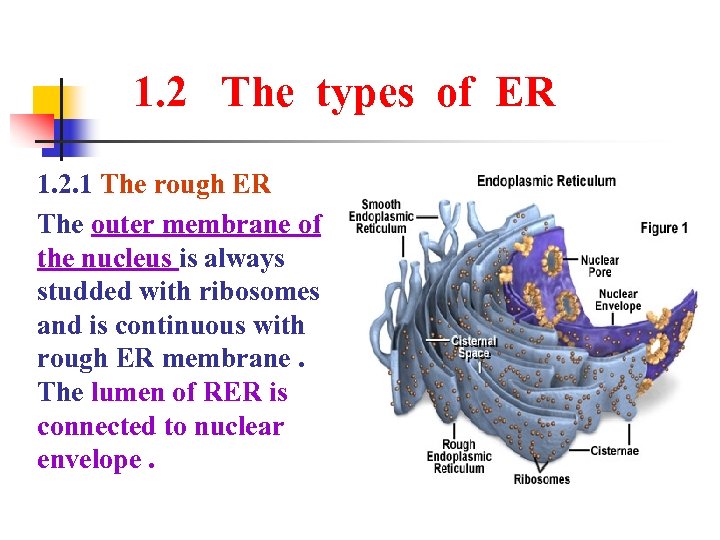
1. 2 The types of ER 1. 2. 1 The rough ER The outer membrane of the nucleus is always studded with ribosomes and is continuous with rough ER membrane. The lumen of RER is connected to nuclear envelope.
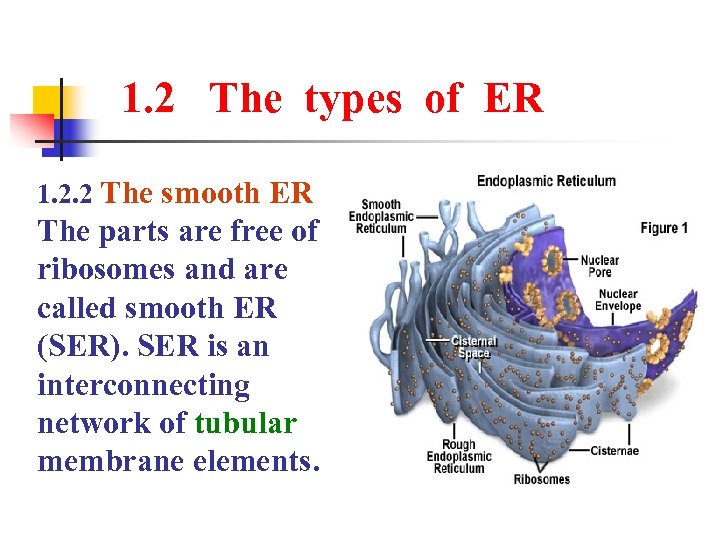
1. 2 The types of ER 1. 2. 2 The smooth ER The parts are free of ribosomes and are called smooth ER (SER). SER is an interconnecting network of tubular membrane elements.

1. 2 The types of ER Rough and smooth ER differ not only in structure, but also in function.
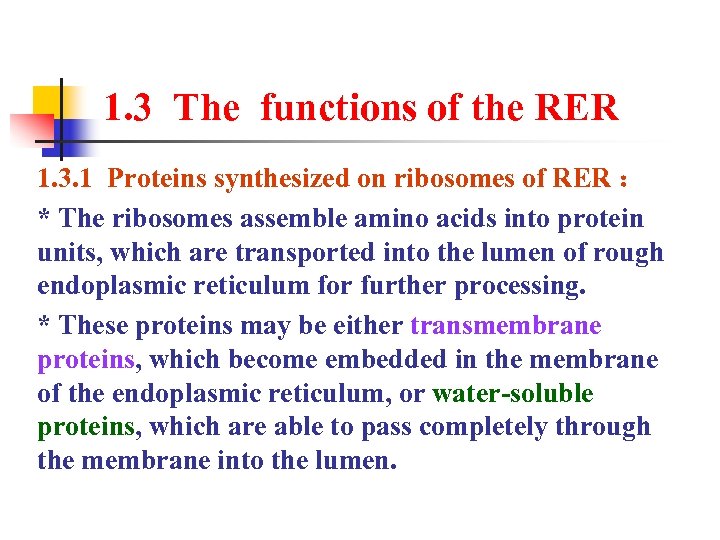
1. 3 The functions of the RER 1. 3. 1 Proteins synthesized on ribosomes of RER : * The ribosomes assemble amino acids into protein units, which are transported into the lumen of rough endoplasmic reticulum for further processing. * These proteins may be either transmembrane proteins, which become embedded in the membrane of the endoplasmic reticulum, or water-soluble proteins, which are able to pass completely through the membrane into the lumen.

1. 3 The functions of the RER Those proteins that reach the inside of the endoplasmic reticulum are folded into the correct three-dimensional conformation. Chemicals, such as carbohydrates or sugars, are added, then the ER either transports the completed proteins to areas of the cell where they are needed, or they are sent to the Golgi apparatus for further processing and modification.
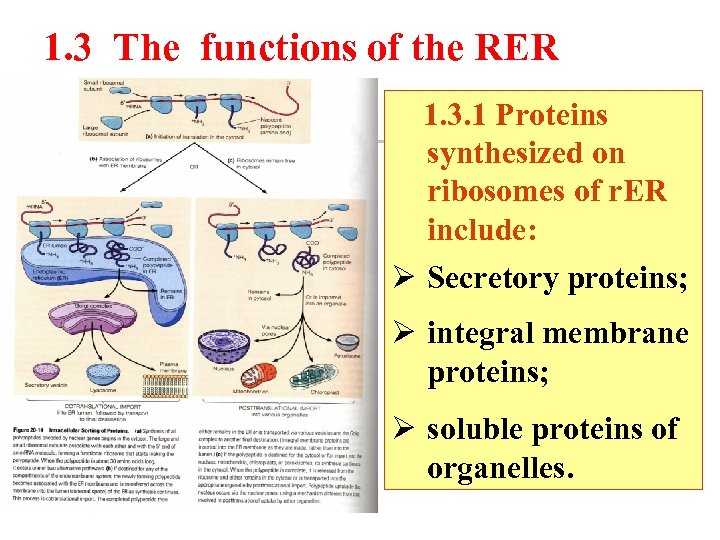
1. 3 The functions of the RER 1. 3. 1 Proteins synthesized on ribosomes of r. ER include: Ø Secretory proteins; Ø integral membrane proteins; Ø soluble proteins of organelles.

1. 3 The functions of the RER ? How do the ribosomes (a membranebound ribosome ) attach to the outer surface of r. ER , and then the newly synthesized protein pass completely through the membrane into the lumen of r. ER?

1. 3 The functions of the RER The Signal Hypothesis would explain completely the mechanism that the ribosomes attach to the outer surface of r. ER , and then the newly protein pass completely through the membrane into the lumen of r. ER?
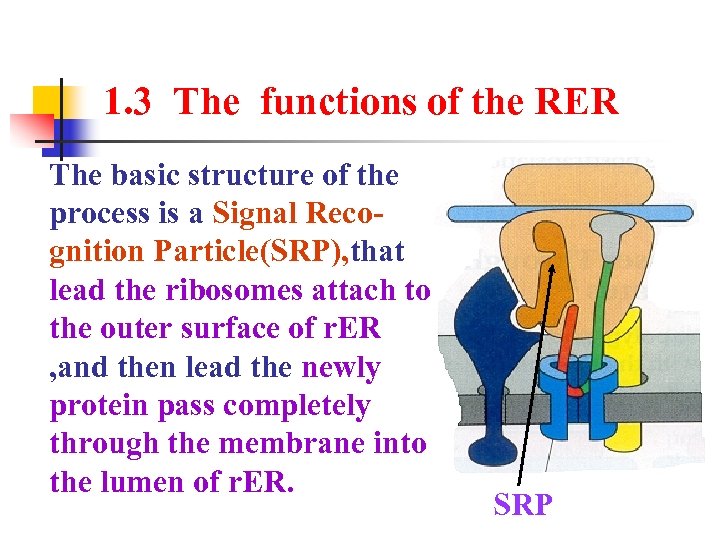
1. 3 The functions of the RER The basic structure of the process is a Signal Recognition Particle(SRP), that lead the ribosomes attach to the outer surface of r. ER , and then lead the newly protein pass completely through the membrane into the lumen of r. ER. SRP
1. 3 The functions of the RER • Signal-recognition particle(SRP) 1. synthesis: Six different polypeptides complexed with a 300 -nucleotide molecule of RNA.

1. 3 The functions of the RER 2. SRP have three main active sites: * One that recognizes and binds to ER signal sequence; * One that interacts with the ribosome to block further translation; * One that binds to the ER membrane (docking The site to protein: receptor protein) recognize and bind to ER signal sequence The site to block further translation The site to recognize receptor protein

1. 3 The functions of the RER • ER signal sequence: 1. synthesis : Typically 15 -30 amino acids. 2. consist of three domains: a positively charged N-terminal region; a central hydrophobic region; a polar region adjoining the site where cleavage from the mature protein will take place. A signal sequence on nascent seretory proteins targets them to the ER and is then cleaved off. SRP receptor (a binding protein or docking protein: receptor protein)
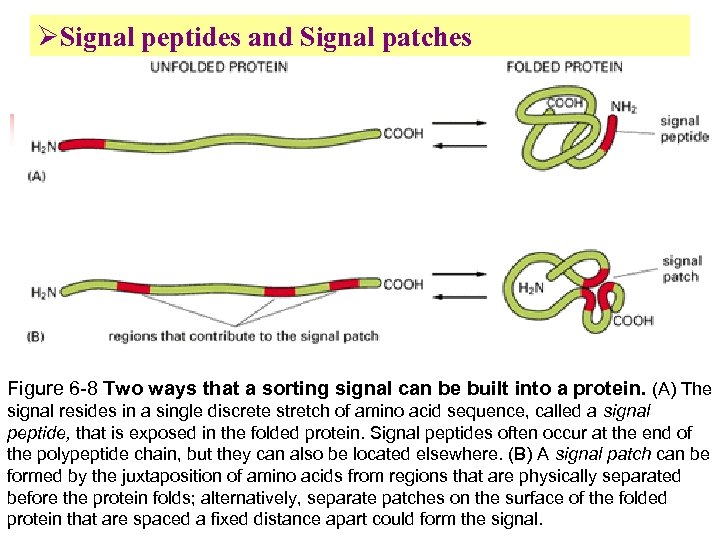
ØSignal peptides and Signal patches Figure 6 -8 Two ways that a sorting signal can be built into a protein. (A) The signal resides in a single discrete stretch of amino acid sequence, called a signal peptide, that is exposed in the folded protein. Signal peptides often occur at the end of the polypeptide chain, but they can also be located elsewhere. (B) A signal patch can be formed by the juxtaposition of amino acids from regions that are physically separated before the protein folds; alternatively, separate patches on the surface of the folded protein that are spaced a fixed distance apart could form the signal.
1. 3 The functions of the RER The basic structure of the mechanism of Signal Hypothesis : 1. Signal Recognition Particle(SRP) 2. ER signal sequence 3. SRP receptor 1 3 SRP
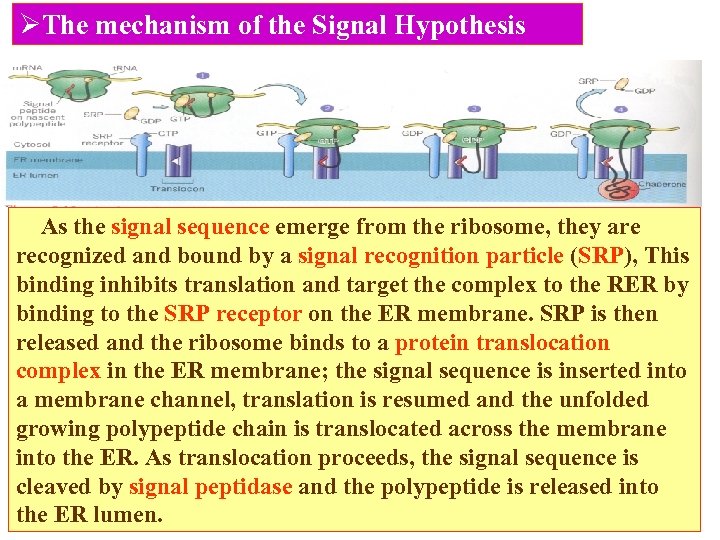
ØThe mechanism of the Signal Hypothesis As the signal sequence emerge from the ribosome, they are recognized and bound by a signal recognition particle (SRP), This binding inhibits translation and target the complex to the RER by binding to the SRP receptor on the ER membrane. SRP is then released and the ribosome binds to a protein translocation complex in the ER membrane; the signal sequence is inserted into a membrane channel, translation is resumed and the unfolded growing polypeptide chain is translocated across the membrane into the ER. As translocation proceeds, the signal sequence is cleaved by signal peptidase and the polypeptide is released into the ER lumen.
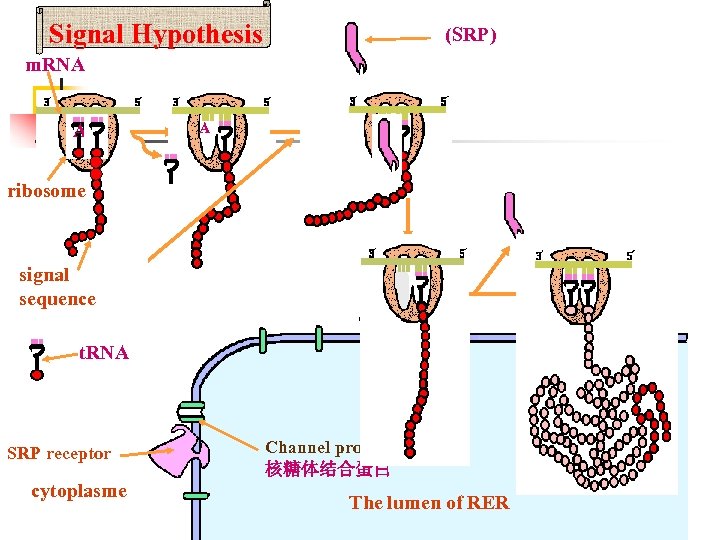
Signal Hypothesis (SRP) m. RNA AP A ribosome signal sequence t. RNA SRP receptor cytoplasme Channel protein 核糖体结合蛋白 The lumen of RER
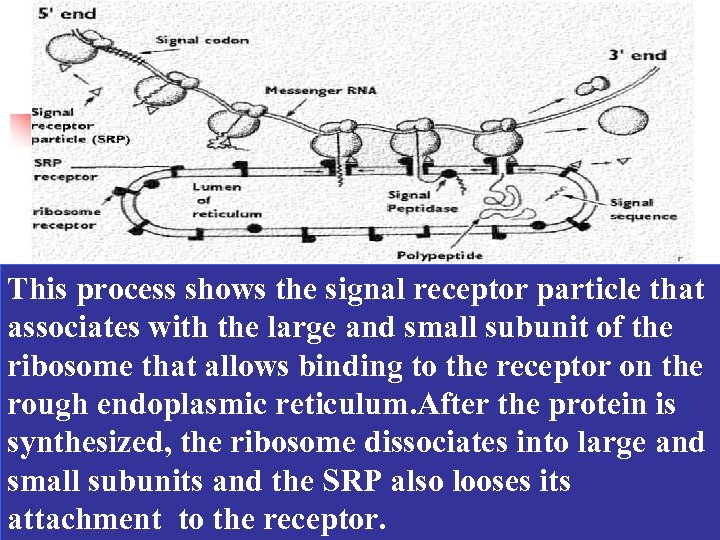
This process shows the signal receptor particle that associates with the large and small subunit of the ribosome that allows binding to the receptor on the rough endoplasmic reticulum. After the protein is synthesized, the ribosome dissociates into large and small subunits and the SRP also looses its attachment to the receptor.
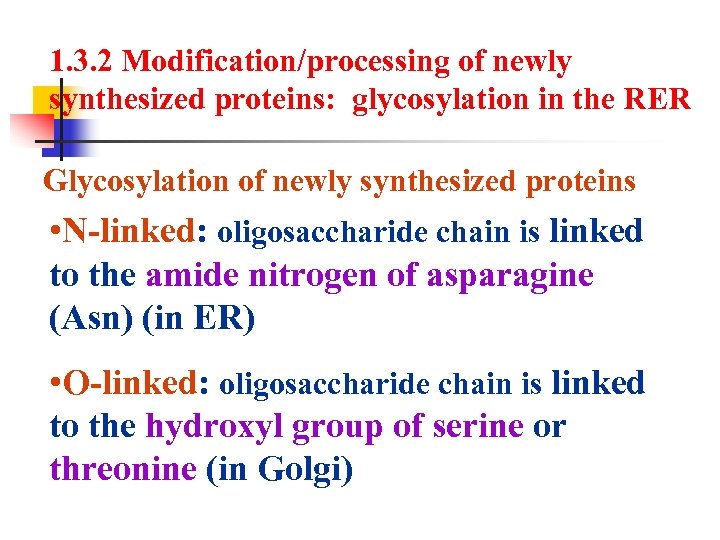
1. 3. 2 Modification/processing of newly synthesized proteins: glycosylation in the RER Glycosylation of newly synthesized proteins • N-linked: oligosaccharide chain is linked to the amide nitrogen of asparagine (Asn) (in ER) • O-linked: oligosaccharide chain is linked to the hydroxyl group of serine or threonine (in Golgi)
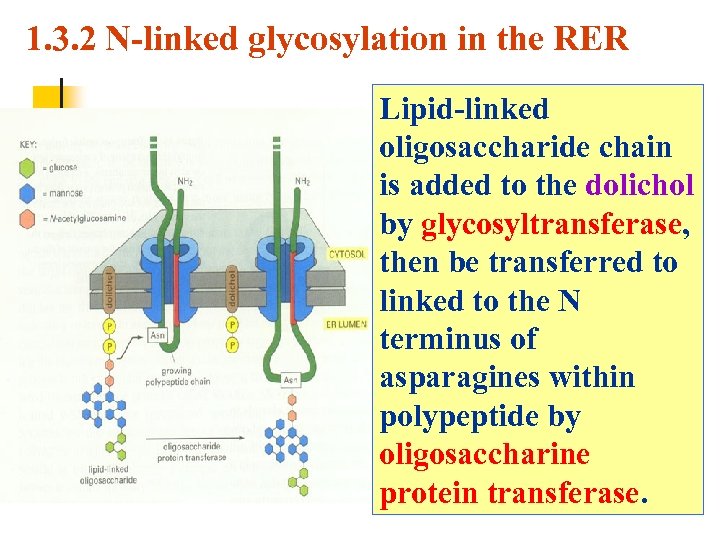
1. 3. 2 N-linked glycosylation in the RER Lipid-linked oligosaccharide chain is added to the dolichol by glycosyltransferase, then be transferred to linked to the N terminus of asparagines within polypeptide by oligosaccharine protein transferase.
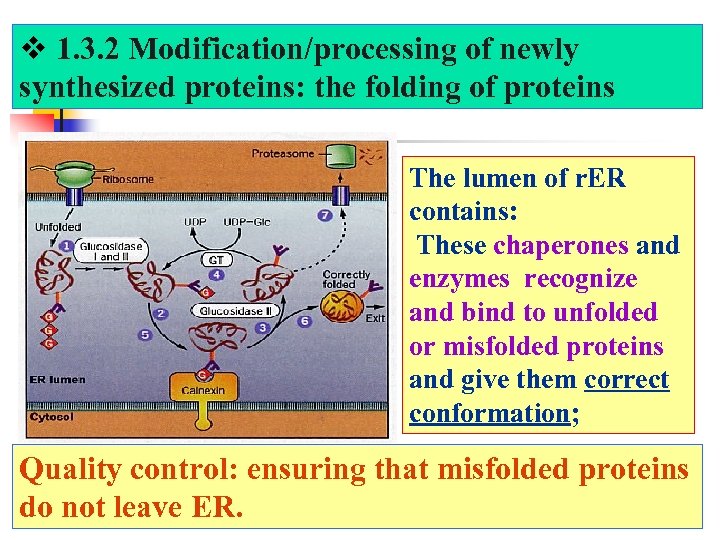
1. 3. 2 Modification/processing of newly synthesized proteins: the folding of proteins The lumen of r. ER contains: These chaperones and enzymes recognize and bind to unfolded or misfolded proteins and give them correct conformation; Quality control: ensuring that misfolded proteins do not leave ER.
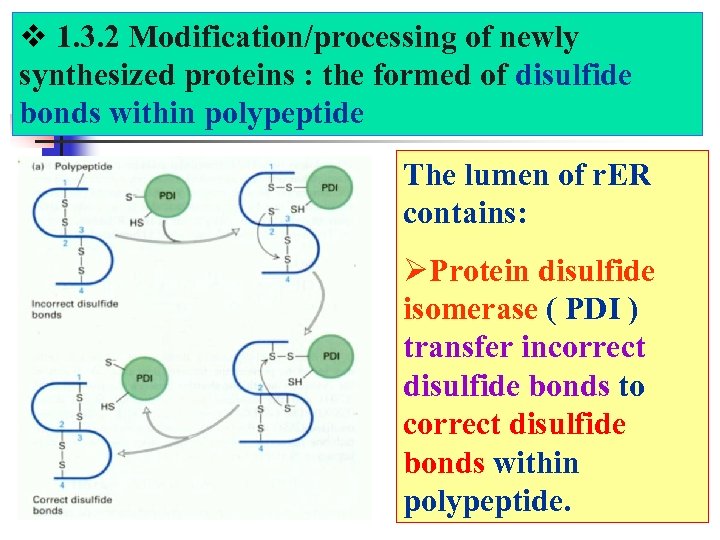
1. 3. 2 Modification/processing of newly synthesized proteins : the formed of disulfide bonds within polypeptide The lumen of r. ER contains: ØProtein disulfide isomerase ( PDI ) transfer incorrect disulfide bonds to correct disulfide bonds within polypeptide.
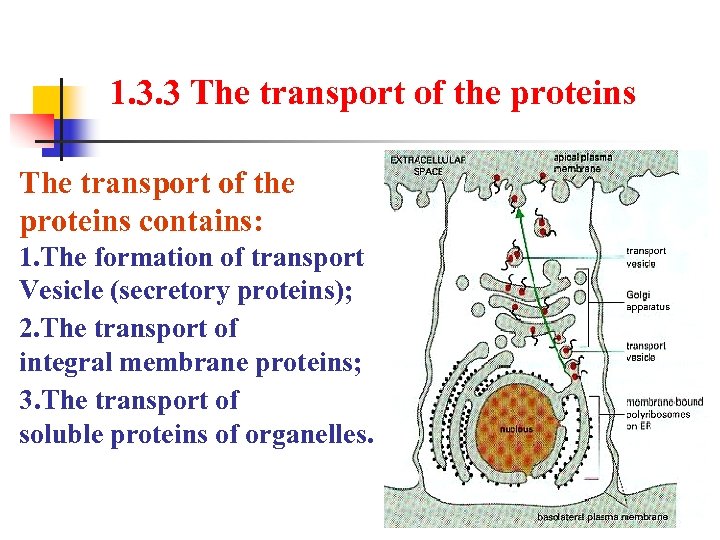
1. 3. 3 The transport of the proteins contains: 1. The formation of transport Vesicle (secretory proteins); 2. The transport of integral membrane proteins; 3. The transport of soluble proteins of organelles.
1. 3 The functions of the RER 1. Proteins synthesized on ribosomes of RER. 2. Modification and processing of newly synthesized proteins ; A. glycosylation in the RER. B. the folding of proteins. C. the formation of disulfide bonds within polypeptide. 3. The transport of the proteins.
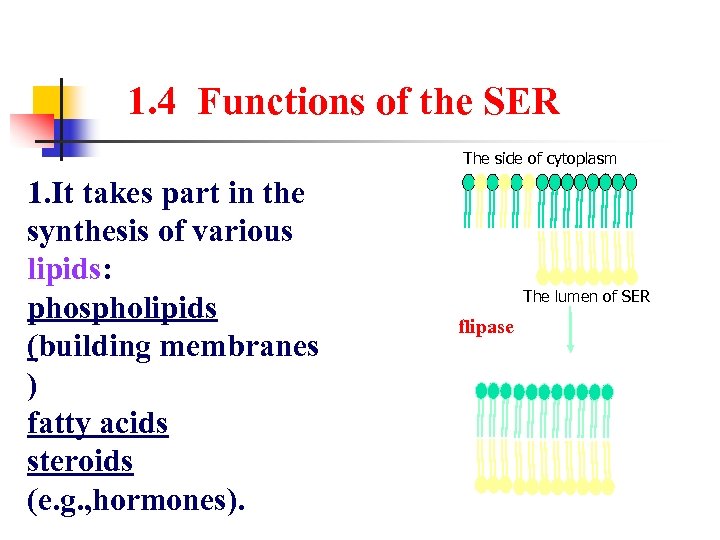
1. 4 Functions of the SER The side of cytoplasm 1. It takes part in the synthesis of various lipids: phospholipids (building membranes ) fatty acids steroids (e. g. , hormones). The lumen of SER flipase
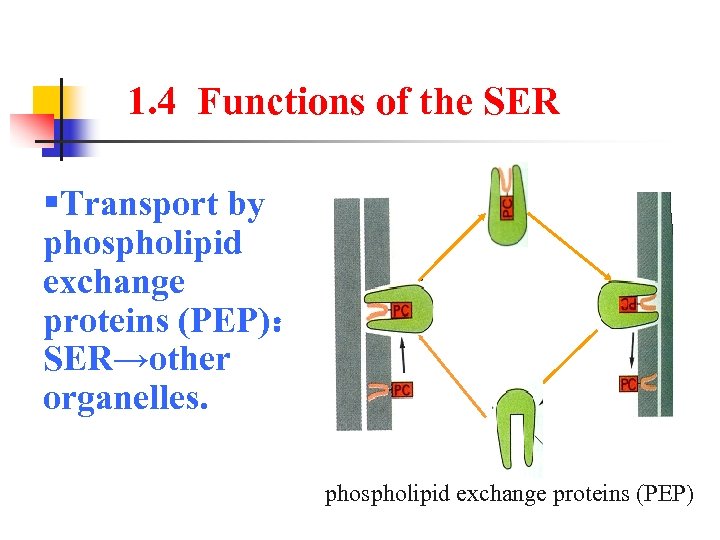
1. 4 Functions of the SER §Transport by phospholipid exchange proteins (PEP): SER→other organelles. phospholipid exchange proteins (PEP)

1. 4 Functions of the SER 2. Detoxification of organic compounds in liver cells. It contains enzymes needed to detoxify drugs. 3. Metabolism of heparin. 4. The SER serve as a storage place for calcium.
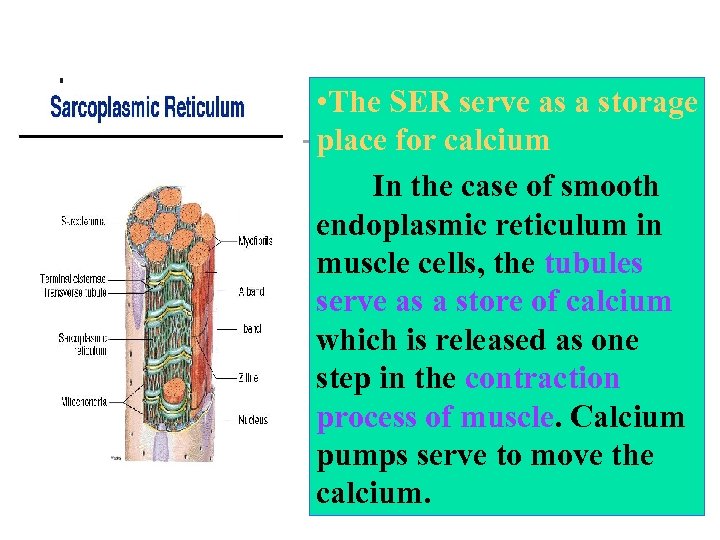
• The SER serve as a storage place for calcium In the case of smooth endoplasmic reticulum in muscle cells, the tubules serve as a store of calcium which is released as one step in the contraction process of muscle. Calcium pumps serve to move the calcium.
1. 4 Functions of the SER 1. Synthesis of lipid; 2. Detoxification of organic compounds in liver cells; 3. Metabolism of heparin; 4. The SER serve as a storage place for calcium.
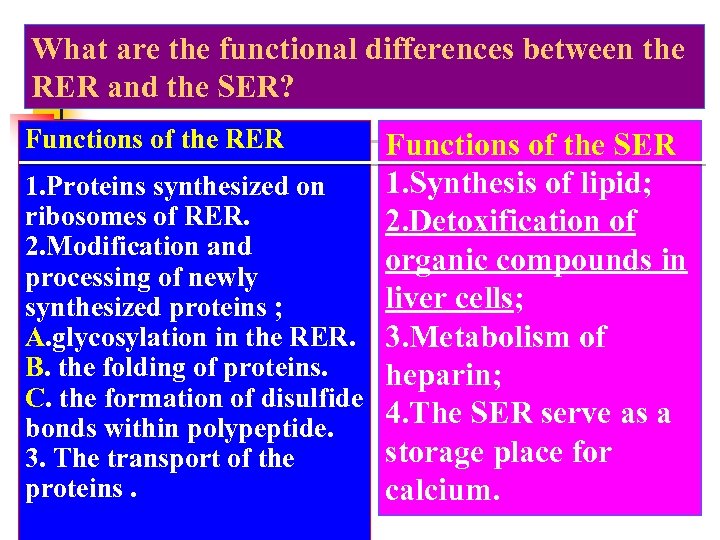
What are the functional differences between the RER and the SER? Functions of the RER Functions of the SER 1. Synthesis of lipid; 1. Proteins synthesized on ribosomes of RER. 2. Detoxification of 2. Modification and organic compounds in processing of newly liver cells; synthesized proteins ; A. glycosylation in the RER. 3. Metabolism of B. the folding of proteins. heparin; C. the formation of disulfide 4. The SER serve as a bonds within polypeptide. storage place for 3. The transport of the proteins. calcium.
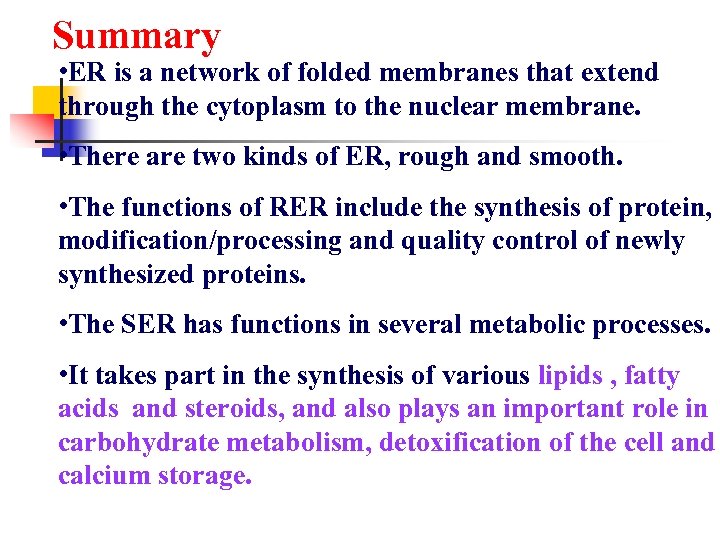
Summary • ER is a network of folded membranes that extend through the cytoplasm to the nuclear membrane. • There are two kinds of ER, rough and smooth. • The functions of RER include the synthesis of protein, modification/processing and quality control of newly synthesized proteins. • The SER has functions in several metabolic processes. • It takes part in the synthesis of various lipids , fatty acids and steroids, and also plays an important role in carbohydrate metabolism, detoxification of the cell and calcium storage.

Self-Quiz : Choosed-question 1. Where does protein synthesis take place in eucaryotic cells ? A. granum B. nucleus C. endoplasmic reticulum D. golgi apparatus
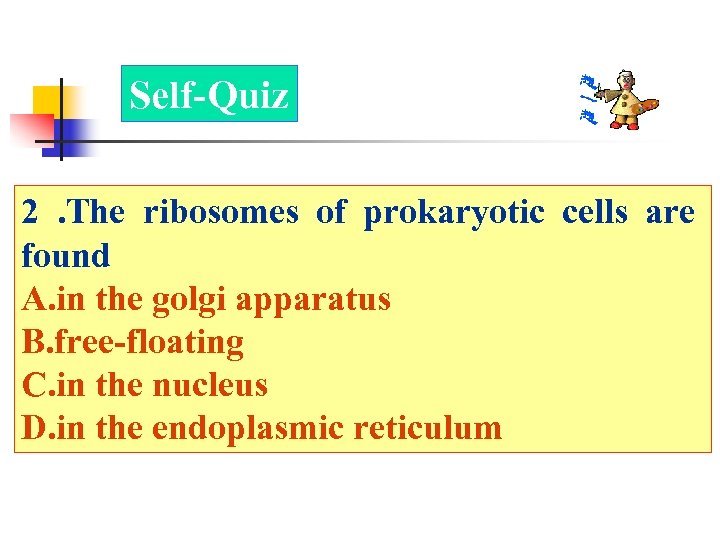
Self-Quiz 2 . The ribosomes of prokaryotic cells are found A. in the golgi apparatus B. free-floating C. in the nucleus D. in the endoplasmic reticulum
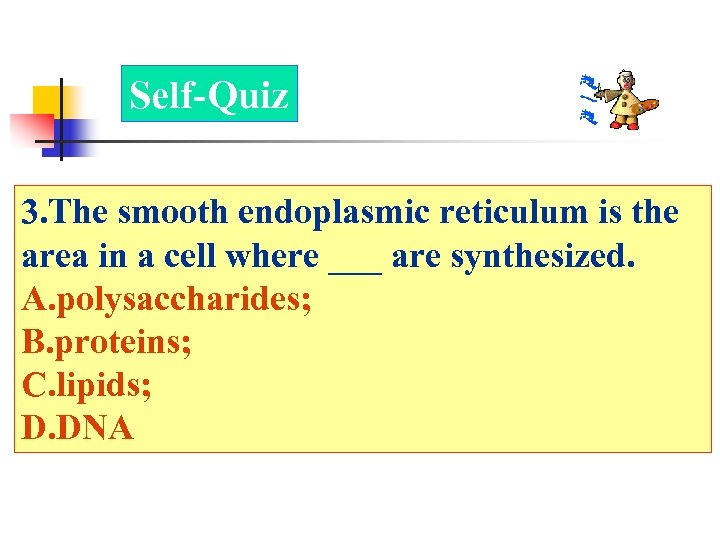
Self-Quiz 3. The smooth endoplasmic reticulum is the area in a cell where ___ are synthesized. A. polysaccharides; B. proteins; C. lipids; D. DNA
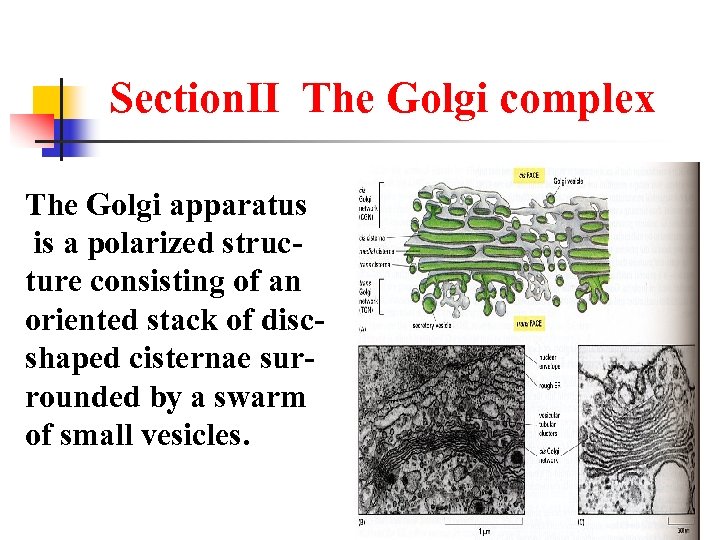
SectionⅡ The Golgi complex The Golgi apparatus is a polarized structure consisting of an oriented stack of discshaped cisternae surrounded by a swarm of small vesicles.
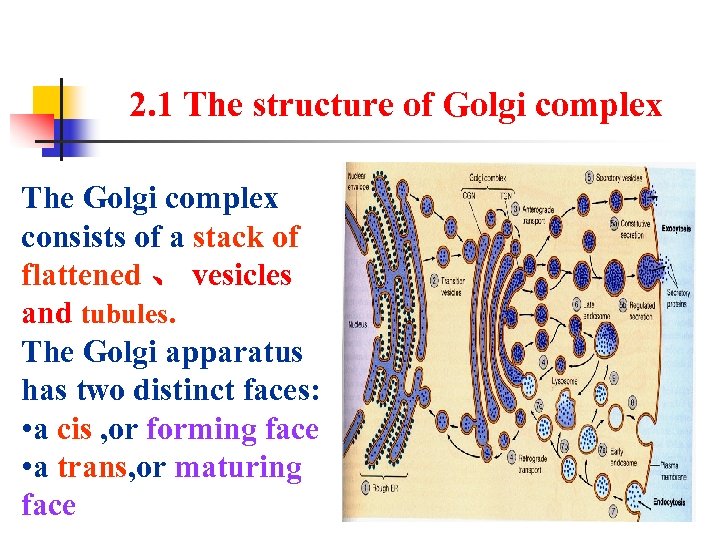
2. 1 The structure of Golgi complex The Golgi complex consists of a stack of flattened 、 vesicles and tubules. The Golgi apparatus has two distinct faces: • a cis , or forming face • a trans, or maturing face
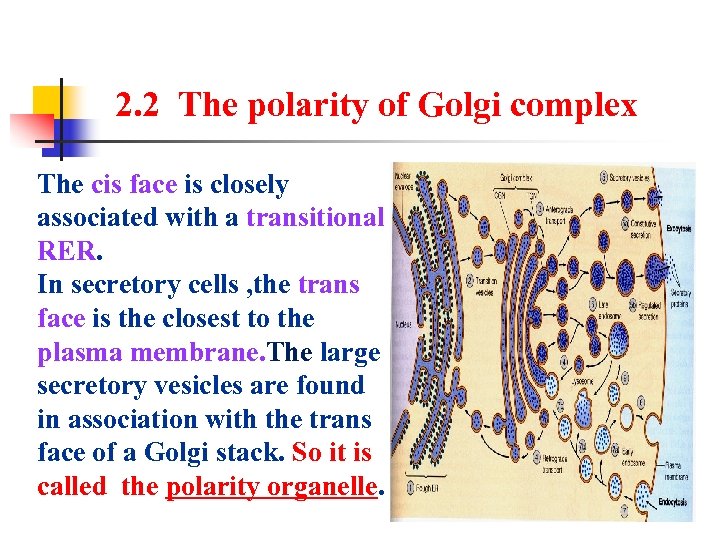
2. 2 The polarity of Golgi complex The cis face is closely associated with a transitional RER. In secretory cells , the trans face is the closest to the plasma membrane. The large secretory vesicles are found in association with the trans face of a Golgi stack. So it is called the polarity organelle.
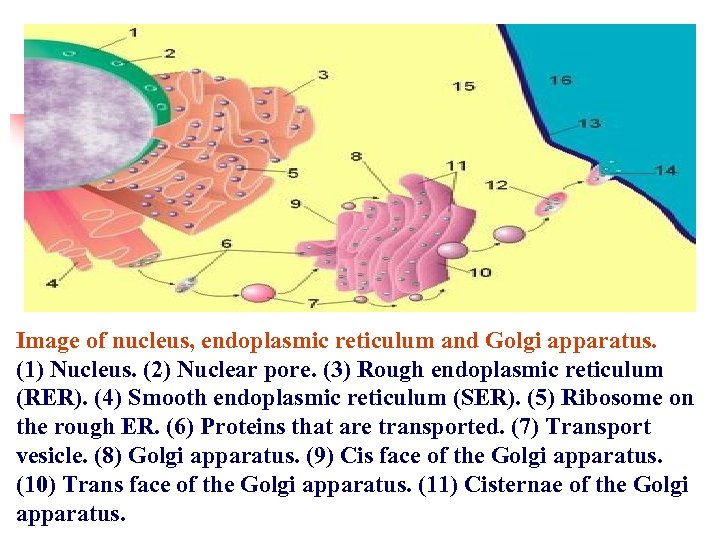
Image of nucleus, endoplasmic reticulum and Golgi apparatus. (1) Nucleus. (2) Nuclear pore. (3) Rough endoplasmic reticulum (RER). (4) Smooth endoplasmic reticulum (SER). (5) Ribosome on the rough ER. (6) Proteins that are transported. (7) Transport vesicle. (8) Golgi apparatus. (9) Cis face of the Golgi apparatus. (10) Trans face of the Golgi apparatus. (11) Cisternae of the Golgi apparatus.
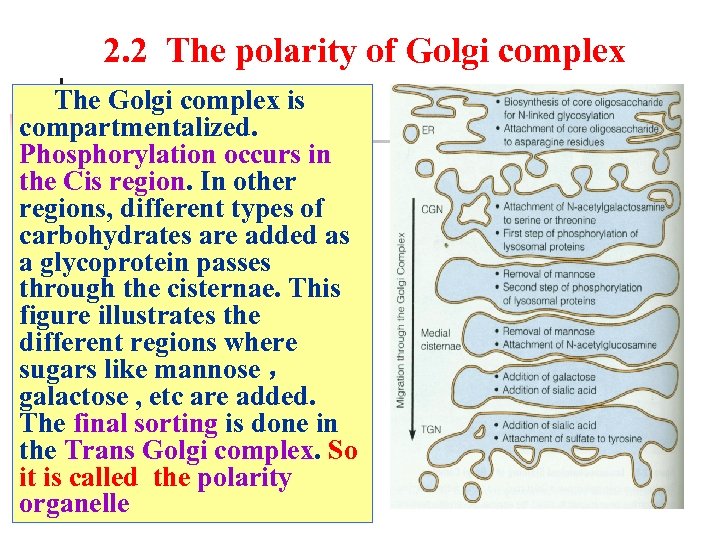
2. 2 The polarity of Golgi complex The Golgi complex is compartmentalized. Phosphorylation occurs in the Cis region. In other regions, different types of carbohydrates are added as a glycoprotein passes through the cisternae. This figure illustrates the different regions where sugars like mannose , galactose , etc are added. The final sorting is done in the Trans Golgi complex. So it is called the polarity organelle
2. 3 The functions of Golgi complex 2. 3. 1 Glycosylation in the Golgi complex plays a key role in the assembly of the carbohydrate component of glycoproteins and glycolipids. O-linked oligosaccharides takes place in Golgi complex. Oligosaccharide chain is linked to the hydroxyl group of serine or threonine.
2. 3. 1 Glycosylation in the Golgi complex The important role of Glycosylation : 1. One might suspect that they function to aid folding and the transport process; for example, carbohydrate as a marker during protein folding in ER and the use of carbohydrate-binding lectins in guilding ER-to. Golgi transport. 2. Limit the approach of other macromolecules to the protein surface, more resist to digestion by proteases. 3. Regulatory roles in signaling through the cellsurface receptor notch, to allow these cells to respond selectively to activating stimuli.
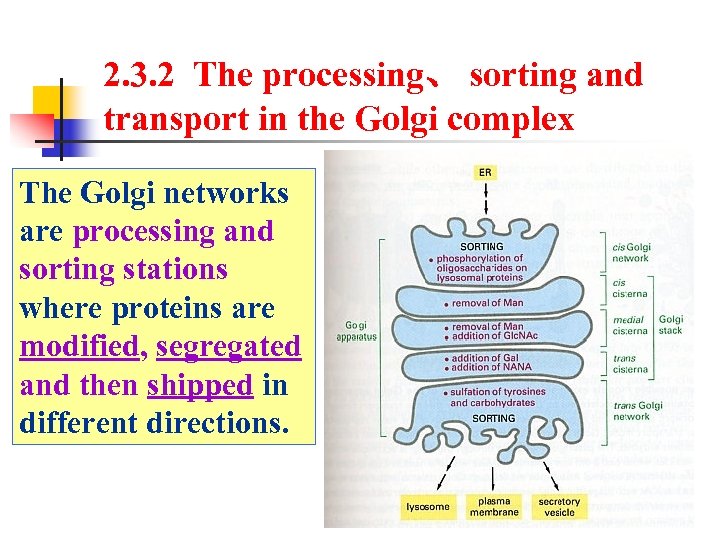
2. 3. 2 The processing、 sorting and transport in the Golgi complex The Golgi networks are processing and sorting stations where proteins are modified, segregated and then shipped in different directions.
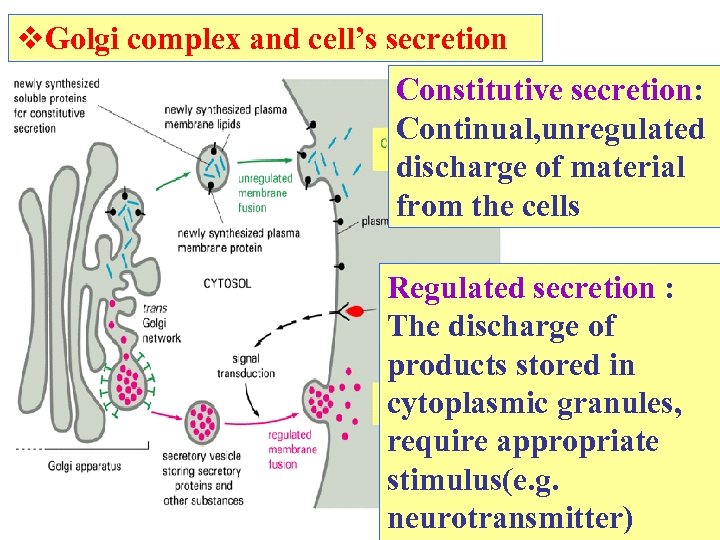
Golgi complex and cell’s secretion Constitutive secretion: Continual, unregulated discharge of material from the cells Regulated secretion : The discharge of products stored in cytoplasmic granules, require appropriate stimulus(e. g. neurotransmitter)
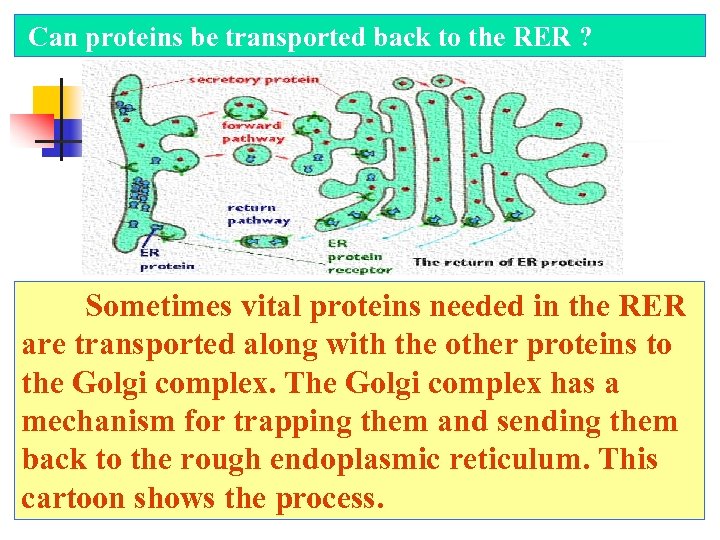
Can proteins be transported back to the RER ? Sometimes vital proteins needed in the RER are transported along with the other proteins to the Golgi complex. The Golgi complex has a mechanism for trapping them and sending them back to the rough endoplasmic reticulum. This cartoon shows the process.
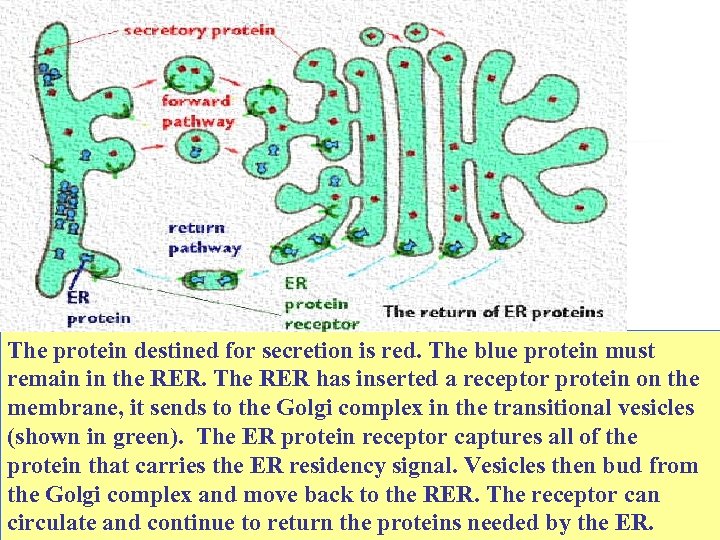
The protein destined for secretion is red. The blue protein must remain in the RER. The RER has inserted a receptor protein on the membrane, it sends to the Golgi complex in the transitional vesicles (shown in green). The ER protein receptor captures all of the protein that carries the ER residency signal. Vesicles then bud from the Golgi complex and move back to the RER. The receptor can circulate and continue to return the proteins needed by the ER.

2. 3 The functions of Golgi complex 2. 3. 1 Glycosylation in the Golgi complex 2. 3. 2 The processing、 sorting and tran-sport in the Golgi complex
Summary The Golgi complex consists of a stack of flattened、 vesicles and tubules. The Golgi apparatus has two distinct faces: a cis face and a trans face. It is called the polarity organelle. The Golgi complex receives newly synthesized proteins and lipids from the ER and then processing、sorting and distributes them to the plasma membrane 、lysosomes and secretory vesicles. Golgi is the site of O-linked glycosylation.

Self-Quiz 1. Which cell component consists of a stack of smooth membrane elements, through which newly synthesized proteins travel by vesicles budding off and fusing while they are being chemically modified and targeted for export or other destinations? A. cytoplasm B. cell membrane C. Golgi body D. SER E. RER
Self-Quiz 2. What is the function of the golgi apparatus? A. It produces DNA molecules B. It propels the cell C. It produces ribosomes D. It secretes cell products
Self-Quiz 3. What is the correct sequence of membrane compartments through which a secretory protein moves from synthesis to release from the cell? A. SER → Golgi → RER → cell membrane B. cell membrane → Golgi → RER → SER C. RER → Golgi → cell membrane → SER D. Golgi → RER → SER → cell membrane E. RER → Golgi → cell membrane
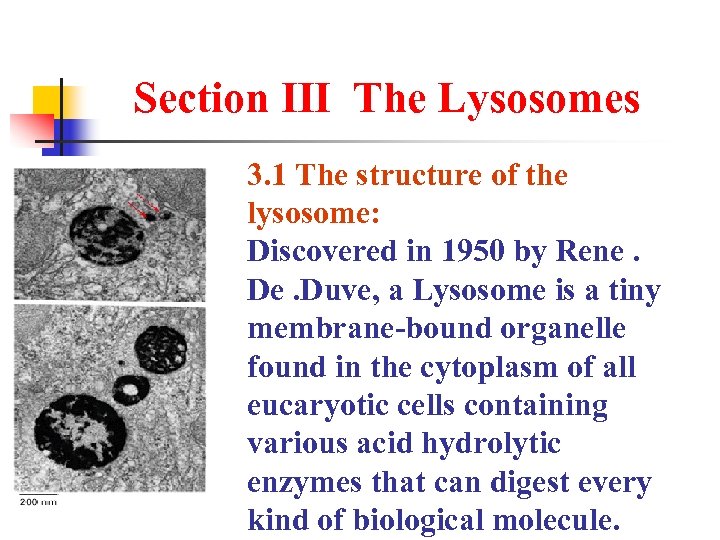
Section Ⅲ The Lysosomes 3. 1 The structure of the lysosome: Discovered in 1950 by Rene. De. Duve, a Lysosome is a tiny membrane-bound organelle found in the cytoplasm of all eucaryotic cells containing various acid hydrolytic enzymes that can digest every kind of biological molecule.
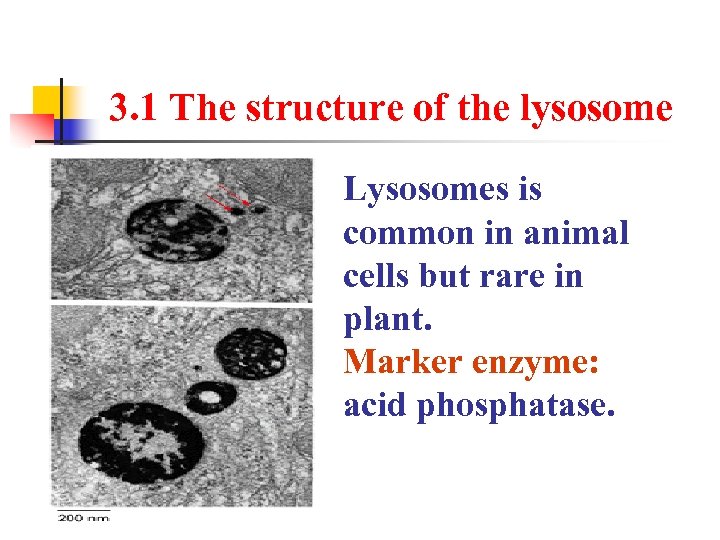
3. 1 The structure of the lysosome Lysosomes is common in animal cells but rare in plant. Marker enzyme: acid phosphatase.
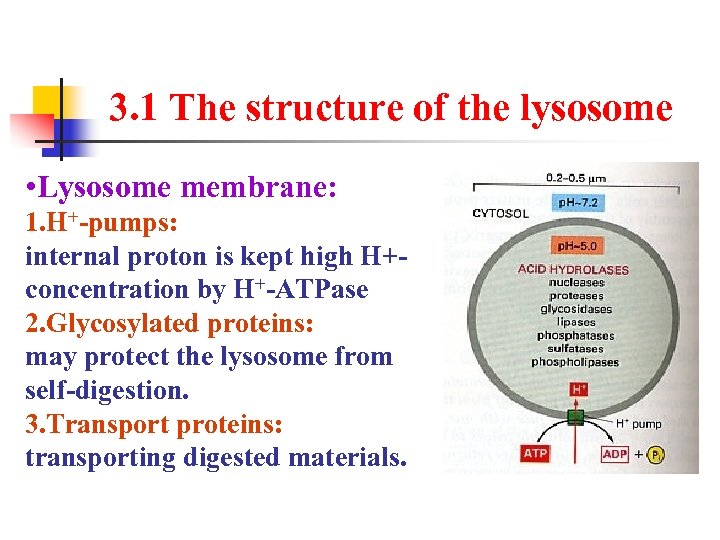
3. 1 The structure of the lysosome • Lysosome membrane: 1. H+-pumps: internal proton is kept high H+- concentration by H+-ATPase 2. Glycosylated proteins: may protect the lysosome from self-digestion. 3. Transport proteins: transporting digested materials.
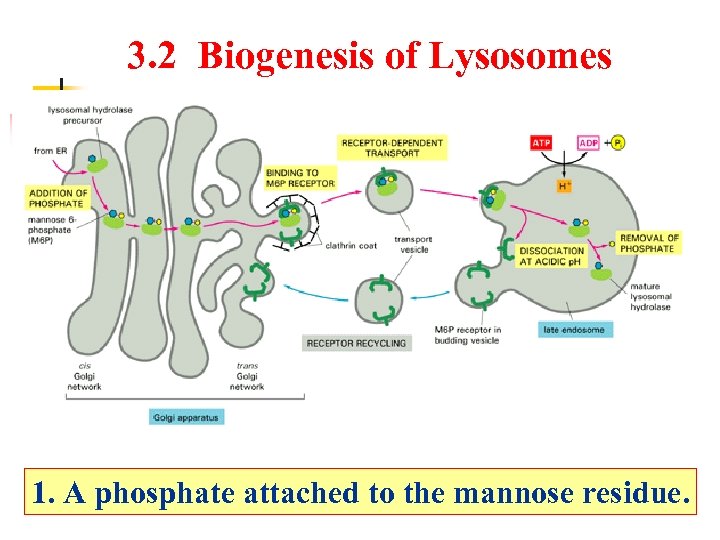
3. 2 Biogenesis of Lysosomes 1. A phosphate attached to the mannose residue.
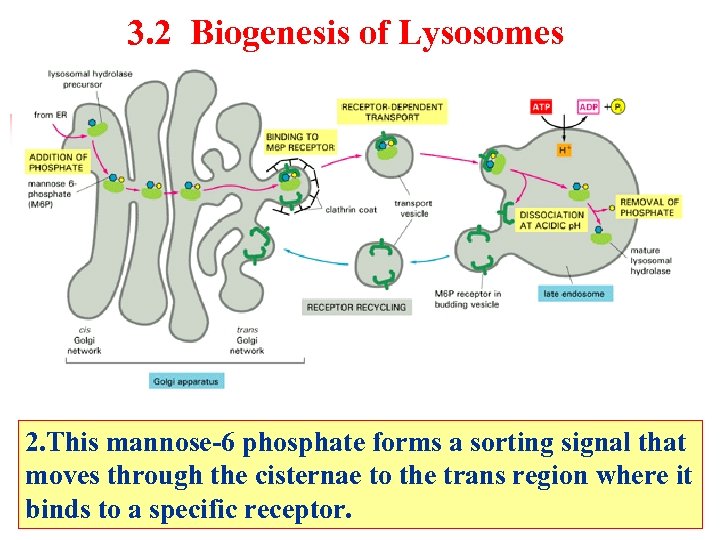
3. 2 Biogenesis of Lysosomes 2. This mannose-6 phosphate forms a sorting signal that moves through the cisternae to the trans region where it binds to a specific receptor.
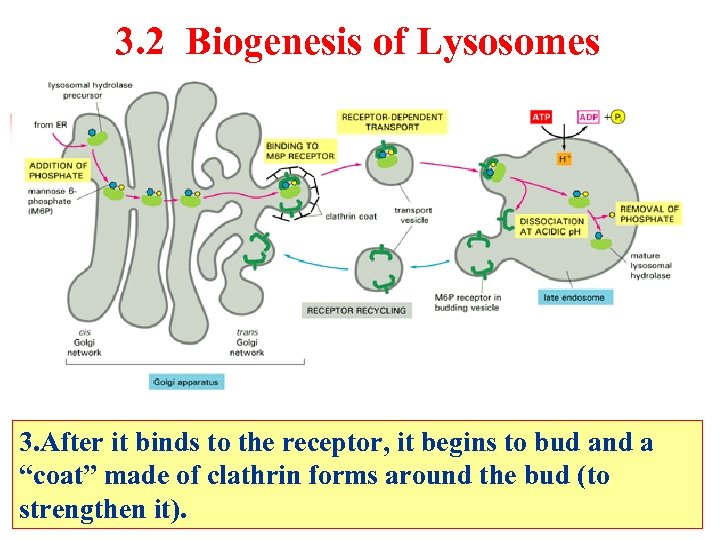
3. 2 Biogenesis of Lysosomes 3. After it binds to the receptor, it begins to bud and a “coat” made of clathrin forms around the bud (to strengthen it).
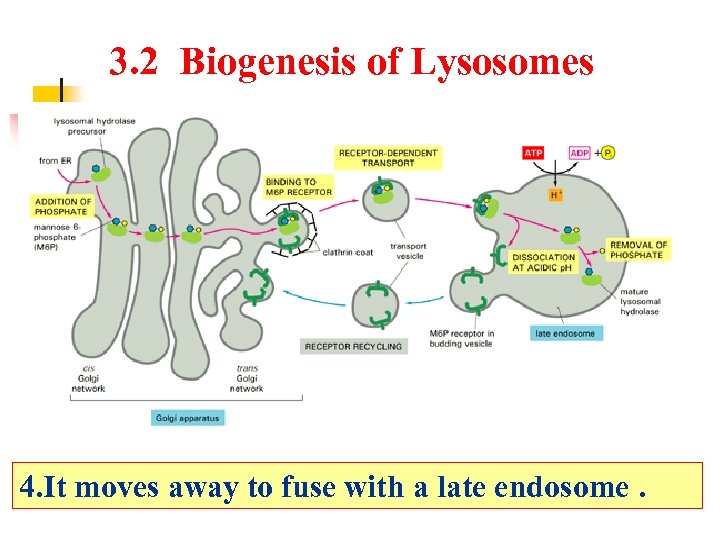
3. 2 Biogenesis of Lysosomes 4. It moves away to fuse with a late endosome.
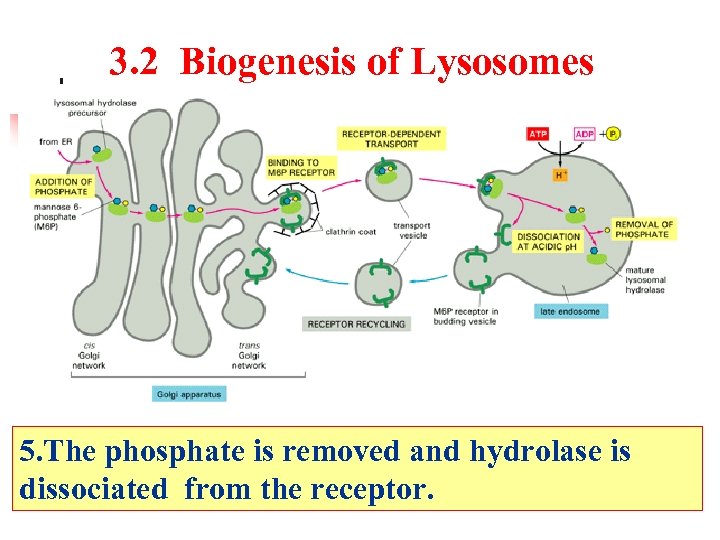
3. 2 Biogenesis of Lysosomes 5. The phosphate is removed and hydrolase is dissociated from the receptor.
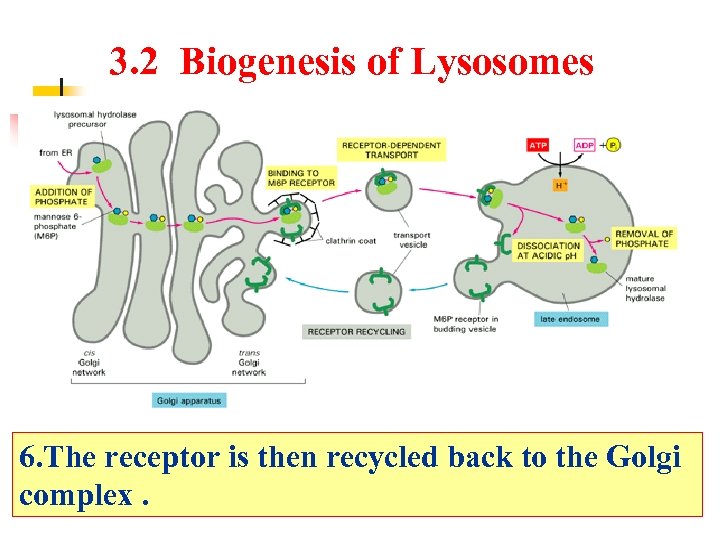
3. 2 Biogenesis of Lysosomes 6. The receptor is then recycled back to the Golgi complex.
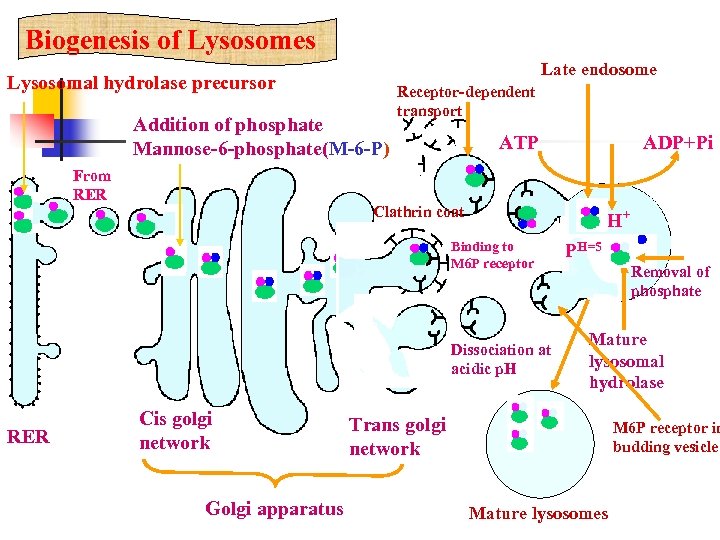
Biogenesis of Lysosomes Late endosome Lysosomal hydrolase precursor Addition of phosphate Mannose-6 -phosphate(M-6 -P) From RER Receptor-dependent transport ATP Clathrin coat Binding to M 6 P receptor Dissociation at acidic p. H RER ADP+Pi Cis golgi network Golgi apparatus P H+ 内吞体 H=5 Removal of phosphate Mature lysosomal hydrolase Trans golgi network M 6 P receptor in budding vesicle Mature lysosomes
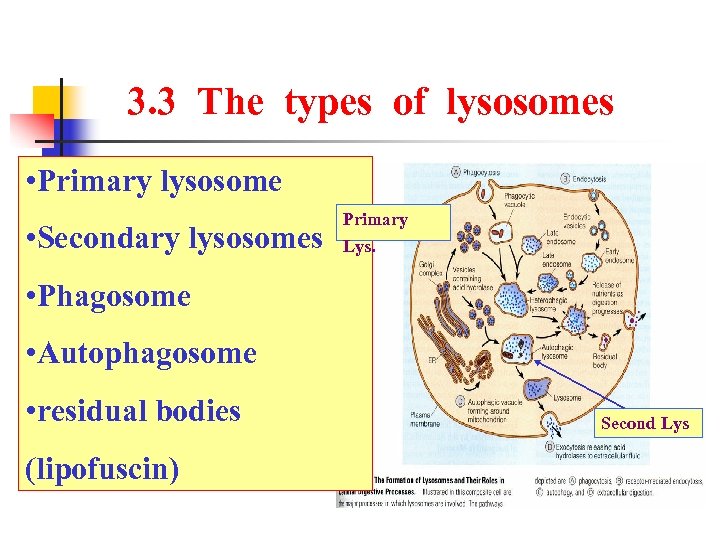
3. 3 The types of lysosomes • Primary lysosome • Secondary lysosomes Primary Lys. • Phagosome • Autophagosome • residual bodies (lipofuscin) Second Lys
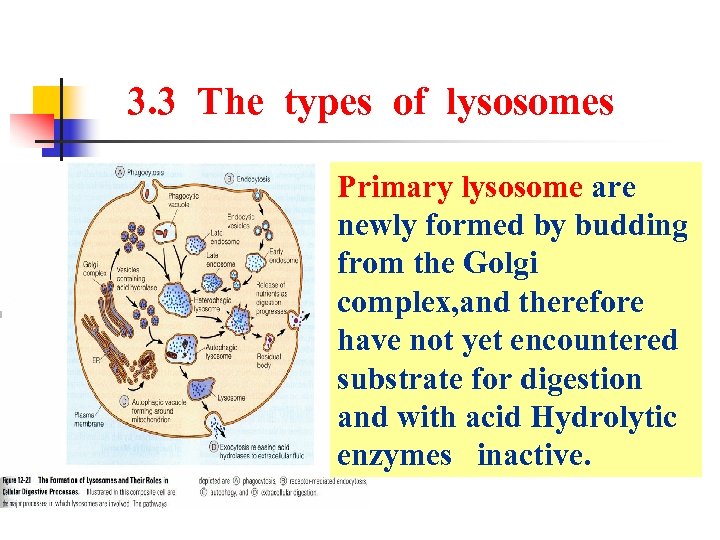
3. 3 The types of lysosomes Primary lysosome are newly formed by budding from the Golgi complex, and therefore have not yet encountered substrate for digestion and with acid Hydrolytic enzymes inactive.
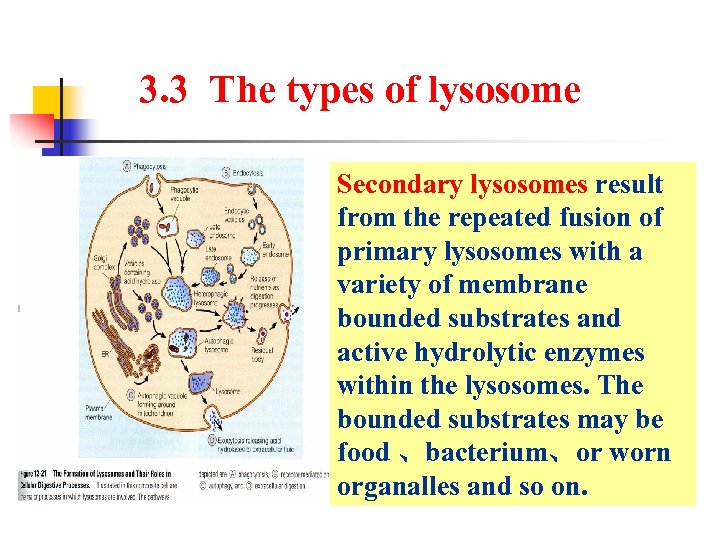
3. 3 The types of lysosome Secondary lysosomes result from the repeated fusion of primary lysosomes with a variety of membrane bounded substrates and active hydrolytic enzymes within the lysosomes. The bounded substrates may be food 、bacterium、or worn organalles and so on.
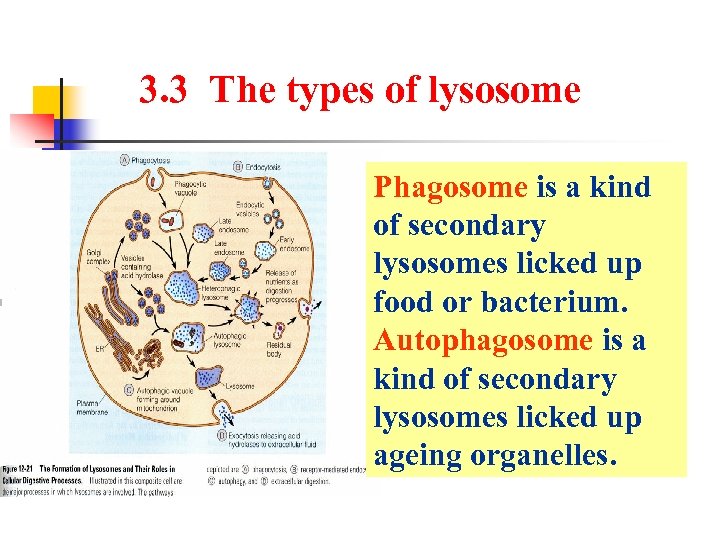
3. 3 The types of lysosome Phagosome is a kind of secondary lysosomes licked up food or bacterium. Autophagosome is a kind of secondary lysosomes licked up ageing organelles.
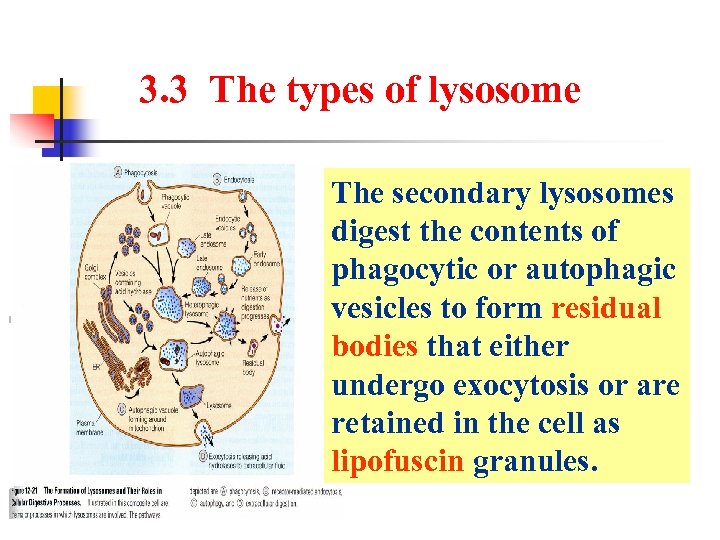
3. 3 The types of lysosome The secondary lysosomes digest the contents of phagocytic or autophagic vesicles to form residual bodies that either undergo exocytosis or are retained in the cell as lipofuscin granules.
3. 4 The functions of Lysosomes are involved in five major cell functions: D. The Functions of Lysosomes 1. Heterophagy; 2. autophagy; 3. The extracellular digest; 4. Autocytolysis;
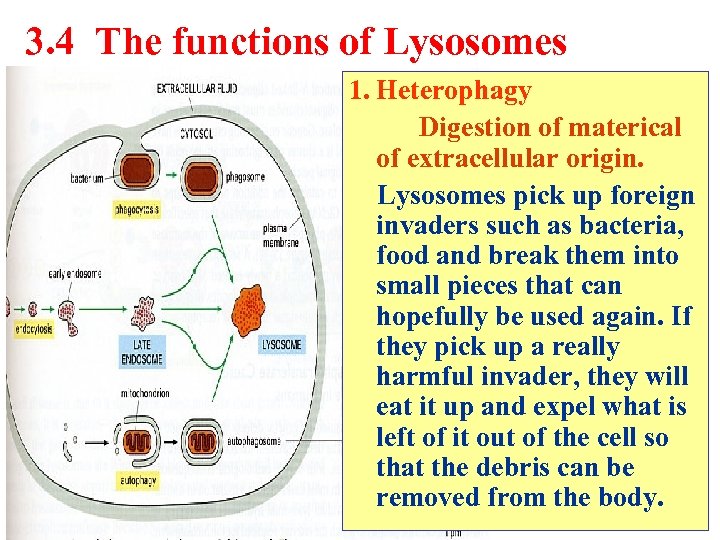
3. 4 The functions of Lysosomes 1. Heterophagy Digestion of materical of extracellular origin. Lysosomes pick up foreign invaders such as bacteria, food and break them into small pieces that can hopefully be used again. If they pick up a really harmful invader, they will eat it up and expel what is left of it out of the cell so that the debris can be removed from the body.

3. 4 The functions of Lysosomes 2. Autophagy Digestion of materical of intracellular origin. Lysosomes also play a key role in destroying old organelles within the cell and thus allow them to be replaced with fresher, more effective ones. This process is known as autophagy and is accomplished in two stages.
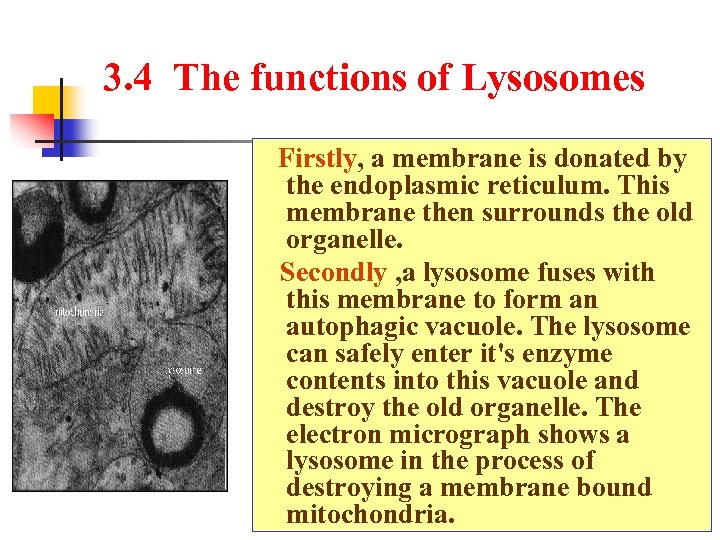
3. 4 The functions of Lysosomes Firstly, a membrane is donated by the endoplasmic reticulum. This membrane then surrounds the old organelle. Secondly , a lysosome fuses with this membrane to form an autophagic vacuole. The lysosome can safely enter it's enzyme contents into this vacuole and destroy the old organelle. The electron micrograph shows a lysosome in the process of destroying a membrane bound mitochondria.
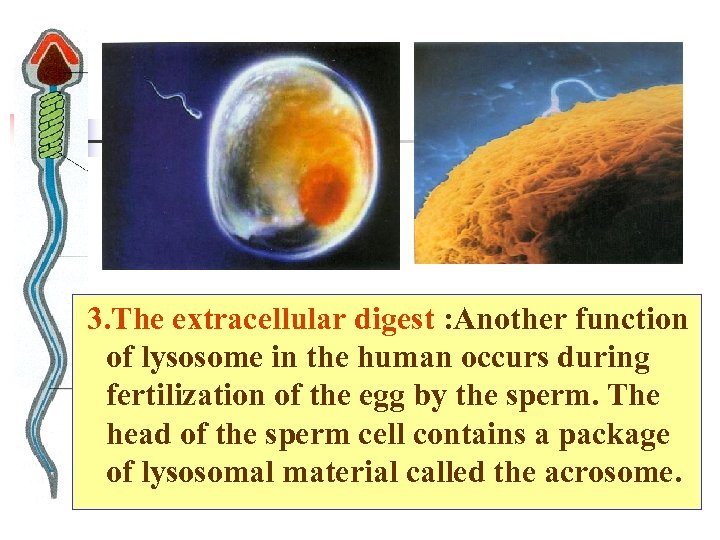
3. The extracellular digest : Another function of lysosome in the human occurs during fertilization of the egg by the sperm. The head of the sperm cell contains a package of lysosomal material called the acrosome.
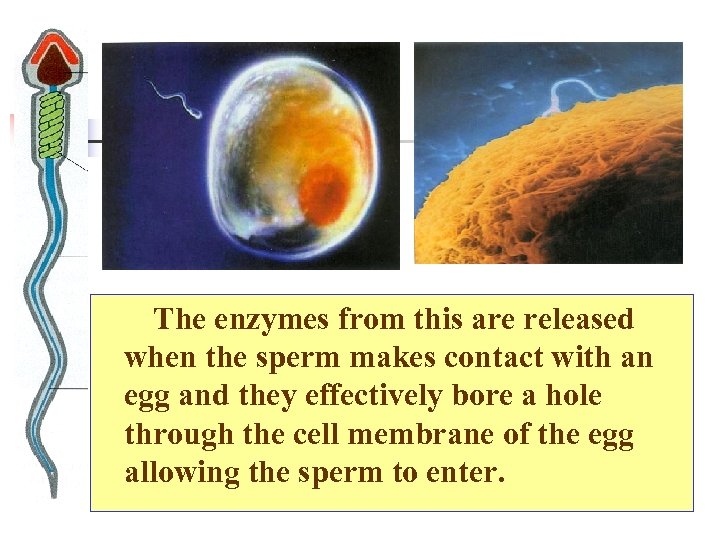
The enzymes from this are released when the sperm makes contact with an egg and they effectively bore a hole through the cell membrane of the egg allowing the sperm to enter.
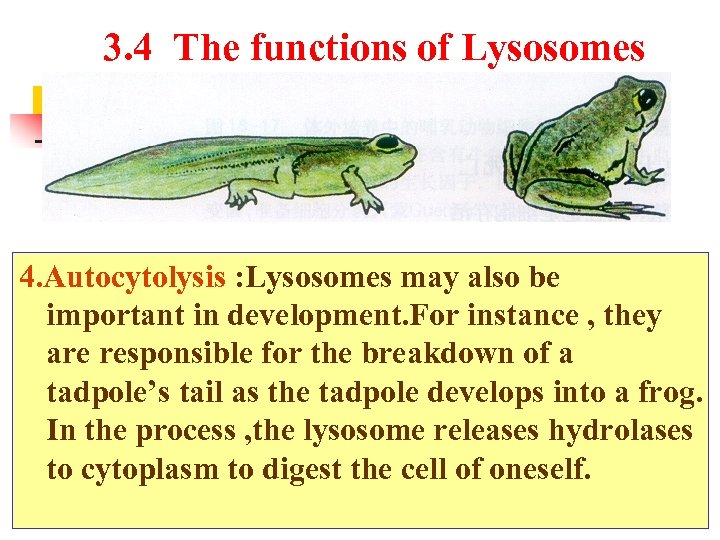
3. 4 The functions of Lysosomes 4. Autocytolysis : Lysosomes may also be important in development. For instance , they are responsible for the breakdown of a tadpole’s tail as the tadpole develops into a frog. In the process , the lysosome releases hydrolases to cytoplasm to digest the cell of oneself.
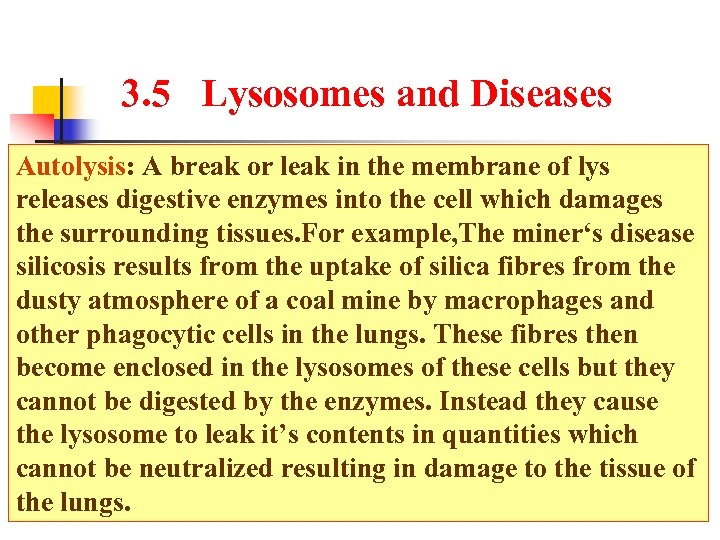
3. 5 Lysosomes and Diseases Autolysis: A break or leak in the membrane of lys releases digestive enzymes into the cell which damages the surrounding tissues. For example, The miner‘s disease silicosis results from the uptake of silica fibres from the dusty atmosphere of a coal mine by macrophages and other phagocytic cells in the lungs. These fibres then become enclosed in the lysosomes of these cells but they cannot be digested by the enzymes. Instead they cause the lysosome to leak it’s contents in quantities which cannot be neutralized resulting in damage to the tissue of the lungs.

5. Lysosomes and Diseases Autolysis: The same process occurs in asbestos workers resulting in the disease asbestosis. Both conditions can be severely debilitating or even fatal. It is also thought that as we age lysosomes become “leaky” and can cause damage to our own tissues. Rheumatoid Arthritis is thought to occur partly due to damage caused to cartilage cells in the joints by enzymes leaked from lysosomes.

5. Lysosomes and Diseases Lysosomal storage diseases are due to the absence of one or more lysosomal enzymes, and resulting in accumulation of material in lysosomes as large inclusions. 1、Acid Maltase Deficiency (Lysosomal Glycogen Storage Disease) which leads to the accumulation of glycogen in muscle tissue.

5. Lysosomes and Diseases 2、Tay-Sachs Disease is due to a deficiency in one of the enzymes which breaks down certain types of fat (lipid) called hexosaminidase A. As a result of this deficiency, huge amounts of lipids are deposited in neuronal (nerve) tissue and leads to severe brain damage and nervous degeneration. The disease is progressive and terminal resulting in early death around 3 years of age.

5. Lysosomes and Diseases 3、Gaucher‘s Disease is due to the deficiency of the lysosomal enzyme glucocerebrosidase. The disease results in liver and spleen enlargement and erosion of the long bones such as the femur. If the disease manifests itself in infancy there is also brain damage causing learning disability.
Summary Lysosome is A membrane-bound organelle in the cytoplasm of most cells containing various acid hydrolases. Lysosomes are involved in four major cell functions: Phagocytosis; Autophagy; Extracellular digest; Autocytolysis. Primary lys fuse with either phagocytic or autophagic vesicles, forming residual bodies that either undergo exocytosis or are retained in the cell as lipofuscin granules.
Choosed-question 1. The cell organelle that contains acid hydrolases is the A. endoplasmic reticulum B. lysosome C. golgi D. ribosome

Choosed-question 2. Lysosomes are found in …… A. ribosomes B. animal cells C. enzymes D. bacteria
Fill-blank questions 1. Endomembrane System include ER, _______, lysosome, secretory vesicles. 2. There are two types of glycosylation : N-linked: linked to the amide nitrogen of asparagine (Asn). These process take place in _______; O-linked: linked to the hydroxyl group serine or threonine via Gal. Nac. These process take place in RER and _____. 5. Primary lysosome are newly formed by budding from _____.
The short-answer questions 1. what are the functions of the lysosome? 2. what are the differences between the primary lysosome and secondary lysosome?
a476b97188ff6c6a66b69377b059baf9.ppt