625096abd44e8e13250e2c488c1cf0dc.ppt
- Количество слайдов: 52
 Human Gonadotropins: A Regulatory Perspective Shelley R. Slaughter, M. D. , Ph. D. Reproductive Medical Officer Team Leader Division of Reproductive and Urologic Drug Products Food and Drug Administration
Human Gonadotropins: A Regulatory Perspective Shelley R. Slaughter, M. D. , Ph. D. Reproductive Medical Officer Team Leader Division of Reproductive and Urologic Drug Products Food and Drug Administration
 Introduction • Review the physiology and role of gonadotropin therapy in female infertility • Overview of the regulatory history of selected approved gonadotropin drug products • Discussion: population, design, efficacy endpoints, analysis and safety endpoints for trials of these drug products 2
Introduction • Review the physiology and role of gonadotropin therapy in female infertility • Overview of the regulatory history of selected approved gonadotropin drug products • Discussion: population, design, efficacy endpoints, analysis and safety endpoints for trials of these drug products 2
 Human Gonadotropins Endocrinology • Link between hypothalamic-pituitary axis and the ovary • Required at threshold levels for follicular development 3
Human Gonadotropins Endocrinology • Link between hypothalamic-pituitary axis and the ovary • Required at threshold levels for follicular development 3
 Human Gonadotropins • Control of gonadotropin release occurs through pulsatile hypothalamic production of gonadotropin releasing hormone (Gn. RH) – Pulses vary over the course of the menstrual cycle. – The timing and amplitude of pulses determine gonadotropin release from the pituitary. 4
Human Gonadotropins • Control of gonadotropin release occurs through pulsatile hypothalamic production of gonadotropin releasing hormone (Gn. RH) – Pulses vary over the course of the menstrual cycle. – The timing and amplitude of pulses determine gonadotropin release from the pituitary. 4
 Types of Gonadotropins • In females, the reproductive axis is responsive to two main gonadotropin types: – Follicle Stimulating Hormone (FSH) – Luteinizing Hormone (LH) 5
Types of Gonadotropins • In females, the reproductive axis is responsive to two main gonadotropin types: – Follicle Stimulating Hormone (FSH) – Luteinizing Hormone (LH) 5
 Follicle Stimulating Hormone (FSH) • • Half-life of 180 - 240 minutes Stimulates the growth of an ovarian follicle Increases the production of estrogen Stimulates production of luteinizing hormone (LH) receptors and other factors (inhibin, activin) in preparation for ovulation 6
Follicle Stimulating Hormone (FSH) • • Half-life of 180 - 240 minutes Stimulates the growth of an ovarian follicle Increases the production of estrogen Stimulates production of luteinizing hormone (LH) receptors and other factors (inhibin, activin) in preparation for ovulation 6
 Luteinizing Hormone (LH) • Half-life is 38 -60 minutes • LH role in folliculogenesis is unclear • Induces follicular maturation and the sequence of events leading to ovulation • Responsible for steroid production by theca cells 7
Luteinizing Hormone (LH) • Half-life is 38 -60 minutes • LH role in folliculogenesis is unclear • Induces follicular maturation and the sequence of events leading to ovulation • Responsible for steroid production by theca cells 7
 Exogenous Gonadotropin Therapy
Exogenous Gonadotropin Therapy
 Exogenous Gonadotropin Therapy • The goal: 9
Exogenous Gonadotropin Therapy • The goal: 9
 Exogenous Gonadotropin Therapy • Patient Types – – Substitution - hypogonadal women Stimulation – women with hypothalamic dysfunction Regulation - oligo-anovulatory women Hyperstimulation therapy – women undergoing Assisted Reproductive Technology procedures 10
Exogenous Gonadotropin Therapy • Patient Types – – Substitution - hypogonadal women Stimulation – women with hypothalamic dysfunction Regulation - oligo-anovulatory women Hyperstimulation therapy – women undergoing Assisted Reproductive Technology procedures 10
 Exogenous Gonadotropin Therapy • Objective: simulate a normal menstrual cycle • Action: override the hypothalamic-pituitary axis and direct: – the onset and duration of follicular development – the timing and number of follicles that reach maturity – the production of gonadal steroids 11
Exogenous Gonadotropin Therapy • Objective: simulate a normal menstrual cycle • Action: override the hypothalamic-pituitary axis and direct: – the onset and duration of follicular development – the timing and number of follicles that reach maturity – the production of gonadal steroids 11
 A Typical U. S. Gonadotropin Treatment Protocol • • Baseline serum estradiol (E 2) level Baseline ultrasound scan Administer daily for 7 - 10 days Repeat E 2 level and ultrasound approximately every 2 to 3 days until follicular maturity is achieved • Administer human chorionic gonadotropin (h. CG) 12
A Typical U. S. Gonadotropin Treatment Protocol • • Baseline serum estradiol (E 2) level Baseline ultrasound scan Administer daily for 7 - 10 days Repeat E 2 level and ultrasound approximately every 2 to 3 days until follicular maturity is achieved • Administer human chorionic gonadotropin (h. CG) 12
 Gonadotropin Drugs Development History • 1926 -1927: Discovery of pituitary hormones • 1955: Clinical use of urinary hormone assays (steroids and gonadotropins) 13
Gonadotropin Drugs Development History • 1926 -1927: Discovery of pituitary hormones • 1955: Clinical use of urinary hormone assays (steroids and gonadotropins) 13
 Gonadotropin Drug Development (continued) • 1959: Extraction of gonadotropins from human pituitary and urine. • 1979: Initial use of ultrasound to determine human ovarian follicle size • 1985: Use of human pituitary extracts was abandoned after literature reports of patients contracting Jacob-Creutzfeldt disease 14
Gonadotropin Drug Development (continued) • 1959: Extraction of gonadotropins from human pituitary and urine. • 1979: Initial use of ultrasound to determine human ovarian follicle size • 1985: Use of human pituitary extracts was abandoned after literature reports of patients contracting Jacob-Creutzfeldt disease 14
 Types of Gonadotropin Therapy Marketed • Urinary derived human gonadotropins - menotropins, urofolitropin, chorionic gonadotropin • Recombinant human gonadotropins - follitropin alfa and follitropin beta, chorionic gonadotropin alfa 15
Types of Gonadotropin Therapy Marketed • Urinary derived human gonadotropins - menotropins, urofolitropin, chorionic gonadotropin • Recombinant human gonadotropins - follitropin alfa and follitropin beta, chorionic gonadotropin alfa 15
 Urinary-derived Human Gonadotropins • Urine is pooled from postmenopausal women • Urine pool is processed to concentrate gonadotropins • Gonadotropins are purified by either antibody affinity column or conventional chromatography 16
Urinary-derived Human Gonadotropins • Urine is pooled from postmenopausal women • Urine pool is processed to concentrate gonadotropins • Gonadotropins are purified by either antibody affinity column or conventional chromatography 16
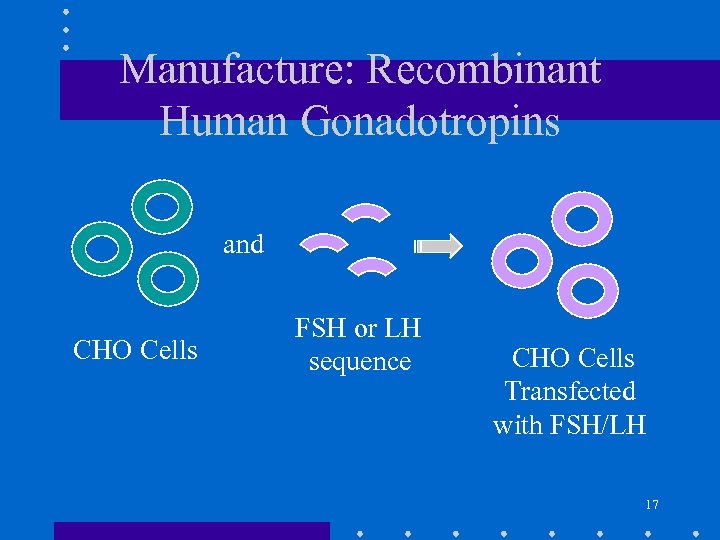 Manufacture: Recombinant Human Gonadotropins and CHO Cells FSH or LH sequence CHO Cells Transfected with FSH/LH 17
Manufacture: Recombinant Human Gonadotropins and CHO Cells FSH or LH sequence CHO Cells Transfected with FSH/LH 17
 Manufacture: Recombinant Human Gonadotropins • DNA constructs containing coding sequences of either alfa or beta subunit of FSH or LH are prepared. • Chinese hamster ovary (CHO) cells are co-transfected with two DNA constructs • Stable CHO cell lines containing integrated FSH or LH sequence are selected. • Master and Working Cell Banks are prepared for production in bioreactors • FSH or LH in the cell culture harvests are purified by chromatography 18
Manufacture: Recombinant Human Gonadotropins • DNA constructs containing coding sequences of either alfa or beta subunit of FSH or LH are prepared. • Chinese hamster ovary (CHO) cells are co-transfected with two DNA constructs • Stable CHO cell lines containing integrated FSH or LH sequence are selected. • Master and Working Cell Banks are prepared for production in bioreactors • FSH or LH in the cell culture harvests are purified by chromatography 18
 Pergonal® • Generic Name: menotropins for injection, USP • Active Ingredients: Follicle Stimulating Hormone (FSH) and Luteinizing Hormone (LH) • Derived: Post-menopausal human urine 19
Pergonal® • Generic Name: menotropins for injection, USP • Active Ingredients: Follicle Stimulating Hormone (FSH) and Luteinizing Hormone (LH) • Derived: Post-menopausal human urine 19
 Pergonal® • In women: – “Pergonal and h. CG given in a sequential manner are indicated for: • Induction of ovulation in anovulatory women (Approved – June 23, 1970) • Development of multiple follicles in ovulatory patients participating in an IVF program” (Approved - March 1, 1988) 20
Pergonal® • In women: – “Pergonal and h. CG given in a sequential manner are indicated for: • Induction of ovulation in anovulatory women (Approved – June 23, 1970) • Development of multiple follicles in ovulatory patients participating in an IVF program” (Approved - March 1, 1988) 20
 Pergonal® • Efficacy and safety data : – Retrospective IVF data representing the clinical experience with 192 patients at the Jones Institute (1981 – 1984) – IVF data from Australia and New Zealand (1979 -1984) – Published Literature 21
Pergonal® • Efficacy and safety data : – Retrospective IVF data representing the clinical experience with 192 patients at the Jones Institute (1981 – 1984) – IVF data from Australia and New Zealand (1979 -1984) – Published Literature 21
 Pergonal® • Primary Efficacy Endpoints: – Mean number of oocytes retrieved at time of laparoscopy – 3. 82 22
Pergonal® • Primary Efficacy Endpoints: – Mean number of oocytes retrieved at time of laparoscopy – 3. 82 22
 Pergonal® • Safety Endpoints: – Rate of ovarian hyperstimulation syndrome– 1. 3% • (In the retrospective analysis of the Jones Institute data, no severe ovarian hyperstimulation or other adverse reactions were noted in 192 IVF subjects) – Multiple pregnancy rate – 20 % 23
Pergonal® • Safety Endpoints: – Rate of ovarian hyperstimulation syndrome– 1. 3% • (In the retrospective analysis of the Jones Institute data, no severe ovarian hyperstimulation or other adverse reactions were noted in 192 IVF subjects) – Multiple pregnancy rate – 20 % 23
 Metrodin® • • Generic name: urofollitropin for injection Active ingredient: FSH Derived: Post-menopausal human urine Approved: September 18, 1986 24
Metrodin® • • Generic name: urofollitropin for injection Active ingredient: FSH Derived: Post-menopausal human urine Approved: September 18, 1986 24
 Metrodin® • Indication: – “Metrodin and human chorionic gonadotropin (h. CG) given in a sequential manner are indicated for: • the induction of ovulation in patients with polycystic ovarian disease who have an elevated FSH/LH ratio and who have failed to respond to adequate clomiphene citrate therapy” 25
Metrodin® • Indication: – “Metrodin and human chorionic gonadotropin (h. CG) given in a sequential manner are indicated for: • the induction of ovulation in patients with polycystic ovarian disease who have an elevated FSH/LH ratio and who have failed to respond to adequate clomiphene citrate therapy” 25
 Metrodin® • Efficacy and safety data : – Literature review of retrospective data from five open-label, non-comparative, clinical studies of ovulation induction (n=80 patients) 26
Metrodin® • Efficacy and safety data : – Literature review of retrospective data from five open-label, non-comparative, clinical studies of ovulation induction (n=80 patients) 26
 Metrodin® • Efficacy: – Observational reports of ovulation and pregnancy 27
Metrodin® • Efficacy: – Observational reports of ovulation and pregnancy 27
 Metrodin® • Safety Endpoints : – Ovarian hyperstimulation syndrome rate – 6% – Multiple birth rate – 17% 28
Metrodin® • Safety Endpoints : – Ovarian hyperstimulation syndrome rate – 6% – Multiple birth rate – 17% 28
 Gonal-f® • Generic Name: follitropin alfa for injection • Active Ingredient: Follicle Stimulating Hormone (FSH) • Derived: Chinese hamster ovary (CHO) cells (Recombinant) • Approved: September 29, 1997 29
Gonal-f® • Generic Name: follitropin alfa for injection • Active Ingredient: Follicle Stimulating Hormone (FSH) • Derived: Chinese hamster ovary (CHO) cells (Recombinant) • Approved: September 29, 1997 29
 Gonal-f® • Indications: – “Induction of ovulation and pregnancy in the anovulatory infertile patient in whom the cause of infertility is not functional and not due to primary ovarian failure” – “Development of multiple follicles in the ovulatory patient participating in an Assisted Reproductive Technology program” 30
Gonal-f® • Indications: – “Induction of ovulation and pregnancy in the anovulatory infertile patient in whom the cause of infertility is not functional and not due to primary ovarian failure” – “Development of multiple follicles in the ovulatory patient participating in an Assisted Reproductive Technology program” 30
 Gonal-f® • Efficacy and safety data from four controlled studies : – IVF • Study 5503 - multicenter (Europe), randomized, open-label, active comparator, parallel group, equivalence trial of Gonal-f® vs. Metrodin® for multiple follicular development in IVF • Study 5533 – multicenter (U. S. ), randomized, openlabel, active comparator, parallel group, equivalence trial of Gonal-f® vs. Metrodin® for superovulation in IVF 31
Gonal-f® • Efficacy and safety data from four controlled studies : – IVF • Study 5503 - multicenter (Europe), randomized, open-label, active comparator, parallel group, equivalence trial of Gonal-f® vs. Metrodin® for multiple follicular development in IVF • Study 5533 – multicenter (U. S. ), randomized, openlabel, active comparator, parallel group, equivalence trial of Gonal-f® vs. Metrodin® for superovulation in IVF 31
 Gonal-f® • Efficacy and safety data from four controlled studies: – Ovulation Induction • Study 5642 - multicenter (Europe, Israel), randomized, open-label, active comparator, parallel group, equivalence trial of Gonal-f® vs. Metrodin® for ovulation induction in WHO Type II anovulation • Study 5727 - multicenter (U. S. ), randomized, openlabel, active comparator, parallel group, equivalence trial of Gonal-f® vs. Metrodin® for ovulation induction in WHO Type II anovulation 32
Gonal-f® • Efficacy and safety data from four controlled studies: – Ovulation Induction • Study 5642 - multicenter (Europe, Israel), randomized, open-label, active comparator, parallel group, equivalence trial of Gonal-f® vs. Metrodin® for ovulation induction in WHO Type II anovulation • Study 5727 - multicenter (U. S. ), randomized, openlabel, active comparator, parallel group, equivalence trial of Gonal-f® vs. Metrodin® for ovulation induction in WHO Type II anovulation 32
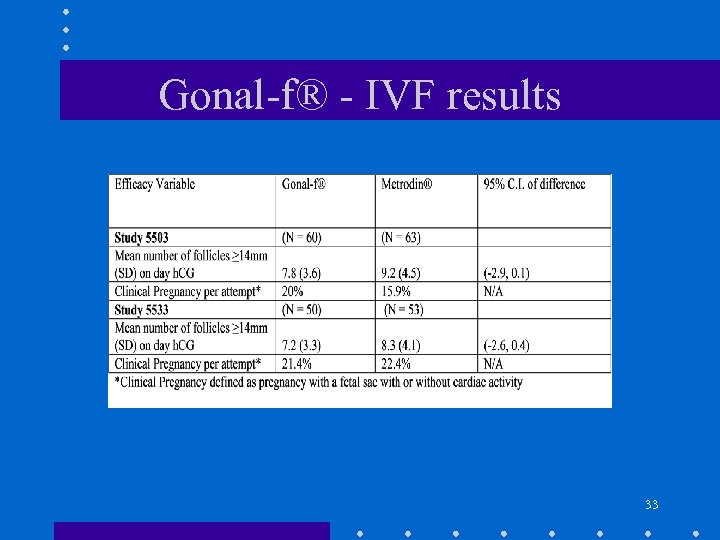 Gonal-f® - IVF results 33
Gonal-f® - IVF results 33
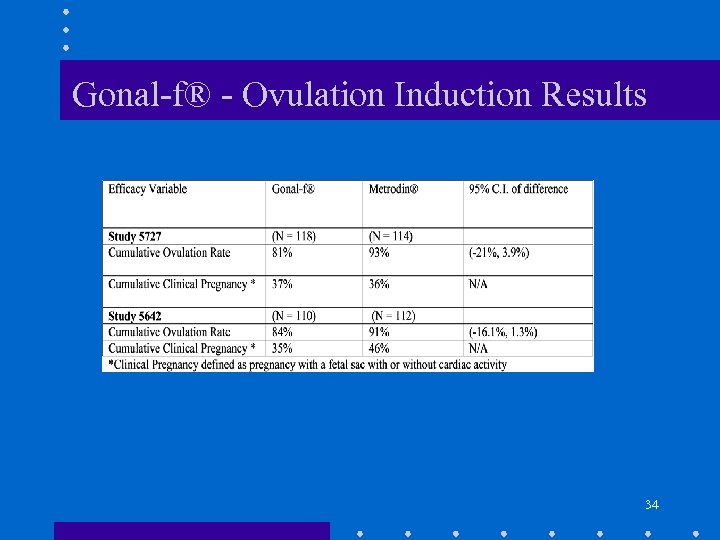 Gonal-f® - Ovulation Induction Results 34
Gonal-f® - Ovulation Induction Results 34
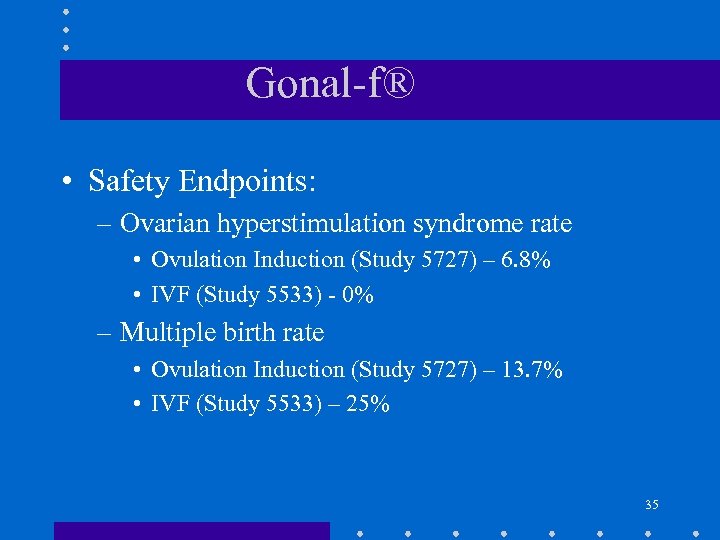 Gonal-f® • Safety Endpoints: – Ovarian hyperstimulation syndrome rate • Ovulation Induction (Study 5727) – 6. 8% • IVF (Study 5533) - 0% – Multiple birth rate • Ovulation Induction (Study 5727) – 13. 7% • IVF (Study 5533) – 25% 35
Gonal-f® • Safety Endpoints: – Ovarian hyperstimulation syndrome rate • Ovulation Induction (Study 5727) – 6. 8% • IVF (Study 5533) - 0% – Multiple birth rate • Ovulation Induction (Study 5727) – 13. 7% • IVF (Study 5533) – 25% 35
 Follistim® • Generic name: follitropin beta for injection • Active Ingredient: Follicle Stimulating Hormone • Derived: Chinese Hamster Ovary Cells (Recombinant) • Approved: September 29, 1997 36
Follistim® • Generic name: follitropin beta for injection • Active Ingredient: Follicle Stimulating Hormone • Derived: Chinese Hamster Ovary Cells (Recombinant) • Approved: September 29, 1997 36
 Follistim® • Indications: – “Induction of ovulation and pregnancy in anovulatory infertile patients in whom the cause of infertility is functional and not due to ovarian failure” – “Development of multiple follicles in ovulatory patients participating in an Assisted Reproductive Technology program” 37
Follistim® • Indications: – “Induction of ovulation and pregnancy in anovulatory infertile patients in whom the cause of infertility is functional and not due to ovarian failure” – “Development of multiple follicles in ovulatory patients participating in an Assisted Reproductive Technology program” 37
 Follistim® • IVF – Study 37604 - single center (Netherlands), randomized, assessor blind, active comparator, equivalence trial of Follistim® vs. Humegon® for infertile women treated with IVF – Study 37608 - multicenter (Europe), randomized, assessor-blind, active comparator, equivalence trial of Follistim® vs. Metrodin® for infertile women treated with IVF 38
Follistim® • IVF – Study 37604 - single center (Netherlands), randomized, assessor blind, active comparator, equivalence trial of Follistim® vs. Humegon® for infertile women treated with IVF – Study 37608 - multicenter (Europe), randomized, assessor-blind, active comparator, equivalence trial of Follistim® vs. Metrodin® for infertile women treated with IVF 38
 Follistim® • IVF – Study 37611 - multicenter (France), randomized, assessor-blind, active comparator, equivalence trial of Follistim® vs. Metrodin® for infertile women treated with IVF – Study 37613 - multicenter (non-U. S. ), randomized, open-label, active comparator, equivalence trial designed to compare the safety and efficacy of two routes of administration of Follistim® subcutaneously and intramuscular for infertile women treated with IVF 39
Follistim® • IVF – Study 37611 - multicenter (France), randomized, assessor-blind, active comparator, equivalence trial of Follistim® vs. Metrodin® for infertile women treated with IVF – Study 37613 - multicenter (non-U. S. ), randomized, open-label, active comparator, equivalence trial designed to compare the safety and efficacy of two routes of administration of Follistim® subcutaneously and intramuscular for infertile women treated with IVF 39
 Follistim® • Ovulation induction: – Study 37609 - multicenter (European), randomized, assessor-blind, active comparator, equivalence trial of Follistim® vs. Metrodin® for induction of ovulation in chronic anovulation who failed to ovulate and/or conceive during clomiphene citrate treatment (WHO Type II). 40
Follistim® • Ovulation induction: – Study 37609 - multicenter (European), randomized, assessor-blind, active comparator, equivalence trial of Follistim® vs. Metrodin® for induction of ovulation in chronic anovulation who failed to ovulate and/or conceive during clomiphene citrate treatment (WHO Type II). 40
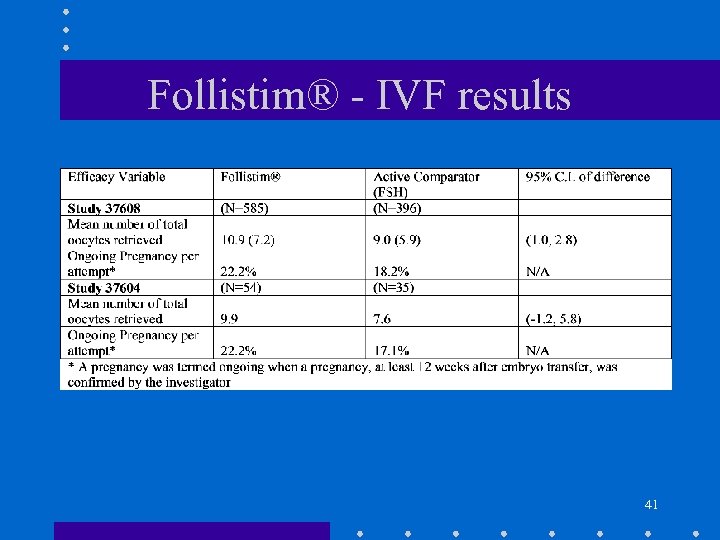 Follistim® - IVF results 41
Follistim® - IVF results 41
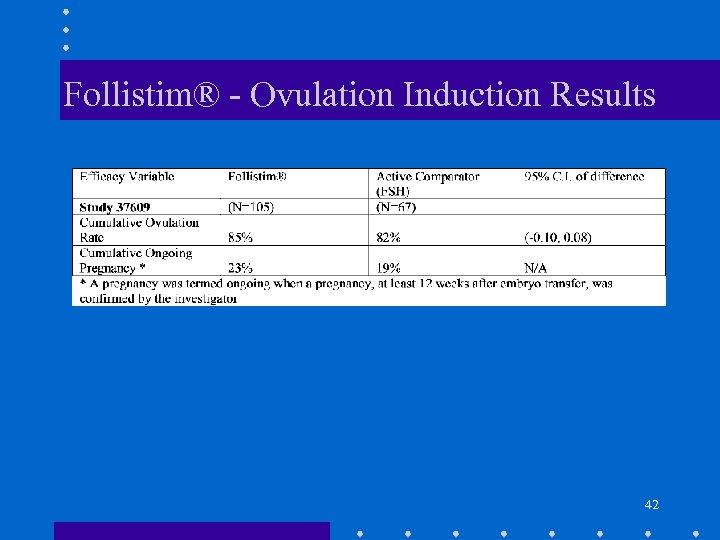 Follistim® - Ovulation Induction Results 42
Follistim® - Ovulation Induction Results 42
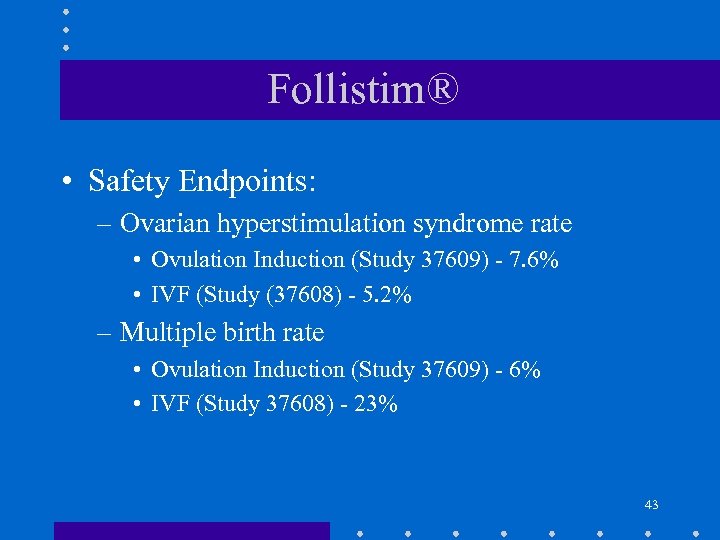 Follistim® • Safety Endpoints: – Ovarian hyperstimulation syndrome rate • Ovulation Induction (Study 37609) - 7. 6% • IVF (Study (37608) - 5. 2% – Multiple birth rate • Ovulation Induction (Study 37609) - 6% • IVF (Study 37608) - 23% 43
Follistim® • Safety Endpoints: – Ovarian hyperstimulation syndrome rate • Ovulation Induction (Study 37609) - 7. 6% • IVF (Study (37608) - 5. 2% – Multiple birth rate • Ovulation Induction (Study 37609) - 6% • IVF (Study 37608) - 23% 43
 For Discussion
For Discussion
 Study Population – Ovulation Induction • The following populations are enrolled: – WHO Group I (hypogonadotropic hypogonadism) – WHO Group II (chronic anovulation) – Does the committee have any advice on these? 45
Study Population – Ovulation Induction • The following populations are enrolled: – WHO Group I (hypogonadotropic hypogonadism) – WHO Group II (chronic anovulation) – Does the committee have any advice on these? 45
 Study Population - ART • The following populations are enrolled: – Normal ovulatory (defined by serum progesterone levels) women – WHO Group I (hypogonadotropic hypogonadism) – WHO Group II (chronic anovulation) – Does the committee have any advice on these? • How do we take into account differences in the procedures? • IVF • ICSI • Donor Oocyte 46
Study Population - ART • The following populations are enrolled: – Normal ovulatory (defined by serum progesterone levels) women – WHO Group I (hypogonadotropic hypogonadism) – WHO Group II (chronic anovulation) – Does the committee have any advice on these? • How do we take into account differences in the procedures? • IVF • ICSI • Donor Oocyte 46
 Study Design • What study designs should be used? – Blinding • double or assessor blind – Comparators • active or placebo 47
Study Design • What study designs should be used? – Blinding • double or assessor blind – Comparators • active or placebo 47
 Primary Efficacy Endpoint • Discuss the advantages and disadvantages of the following as primary or secondary endpoints: – Live birth rate – Ongoing viable pregnancy (presence of a fetal heartbeat) rate – Gestational sac development rate – Rate of Positive ß-h. CG – Ovulation rate [as defined by serum progesterone level(s)] – Follicular development rate (as defined by two or three criteria) 48
Primary Efficacy Endpoint • Discuss the advantages and disadvantages of the following as primary or secondary endpoints: – Live birth rate – Ongoing viable pregnancy (presence of a fetal heartbeat) rate – Gestational sac development rate – Rate of Positive ß-h. CG – Ovulation rate [as defined by serum progesterone level(s)] – Follicular development rate (as defined by two or three criteria) 48
 Primary Efficacy Endpoint • How should the primary endpoint(s) be analyzed? – For Ovulation Induction • Intent-to-Treat Population • Per protocol population – For ART • Per treatment initiation? • Per retrieval? • Per embryo transfer? 49
Primary Efficacy Endpoint • How should the primary endpoint(s) be analyzed? – For Ovulation Induction • Intent-to-Treat Population • Per protocol population – For ART • Per treatment initiation? • Per retrieval? • Per embryo transfer? 49
 Study Analysis • How should success be defined? – Superiority to comparator (placebo; active control) – Equivalence to active comparator – Non-inferiority to active comparator 50
Study Analysis • How should success be defined? – Superiority to comparator (placebo; active control) – Equivalence to active comparator – Non-inferiority to active comparator 50
 Safety Endpoint Questions • Discuss the advantages and disadvantages of evaluating the following safety endpoint(s): – – Rate of ovarian hyperstimulation syndrome Rate of miscarriages Rate of multiple pregnancies Rate of ectopic pregnancies 51
Safety Endpoint Questions • Discuss the advantages and disadvantages of evaluating the following safety endpoint(s): – – Rate of ovarian hyperstimulation syndrome Rate of miscarriages Rate of multiple pregnancies Rate of ectopic pregnancies 51
 Acknowledgements • • Audrey Gassman, M. D. Ridgely Bennett, M. D. Barbara Wesley, M. D. , M. P. H. Phill Price, M. D. Dornette Spell-Lesane, C. N. P. Donna Griebel, M. D. Dan Shames, M. D. Florence Houn, M. D. 52
Acknowledgements • • Audrey Gassman, M. D. Ridgely Bennett, M. D. Barbara Wesley, M. D. , M. P. H. Phill Price, M. D. Dornette Spell-Lesane, C. N. P. Donna Griebel, M. D. Dan Shames, M. D. Florence Houn, M. D. 52


