a40708661e26ee448f4111f757fa8a77.ppt
- Количество слайдов: 88

Human Error, Root Cause Analysis and Conducting Objective and Effective Investigations Presented by: Karen Ginsbury. For IFF Denmark November 2012 PCI Pharmaceutical Consulting Israel Ltd 1

Workshop Objective • To understand why operators make mistakes and how to identify the real problem rather than “human error – retrain” corrective actions PCI Pharmaceutical Consulting Israel Ltd 2



What is an investigation? • You are not investigating if when looking for EVIDENCE you start out BELIEVING you know what happened • EVIDENCE provides proof • Proof is convincing objective evidence that compels the mind to accept a truth or fact

MHRA – April 2011 – March 2012 • 303 inspections: 26 criticals; 644 majors • Top 10:

EU GMPs Part 1 Finished Product

Chapter 1 - PQS • Under Quality Assurance: 8

US GMP Trends – Risk Management

Suicide or Murder? “I’ll never find the killer syndrome” PCI Pharmaceutical Consulting Israel Ltd 10

Sudoku – “it’s impossible” How many of you completed the puzzle How many of you have done a Sudoku in the past? Here is a fiendish puzzle See if you can finish it by the end of the day. You can ask anyone else or do it in a group AFTER you have spent 30 minutes trying to do it yourself (NOT during the class – take it home if necessary) PCI Pharmaceutical Consulting Israel Ltd 11
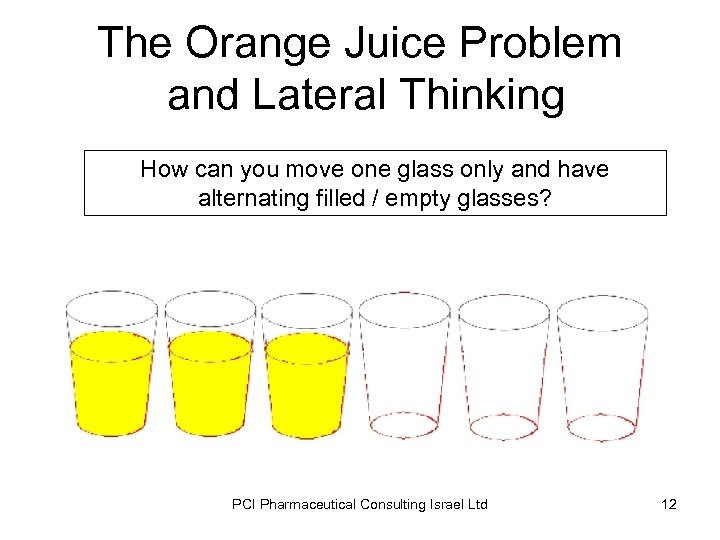
The Orange Juice Problem and Lateral Thinking How can you move one glass only and have alternating filled / empty glasses? PCI Pharmaceutical Consulting Israel Ltd 12

Impossible? You tell me. Logical / Rationale Thinking • There are three boxes, one contains only apples, one contains only oranges, and one contains both apples and oranges. The boxes have been incorrectly labeled such that no label identifies the actual contents of the box it labels. Opening just one box, and without looking in the box, you take out one piece of fruit. By looking at the fruit, how can you immediately label all of the boxes correctly? PCI Pharmaceutical Consulting Israel Ltd 13

Course Objective – Take Home • Understand how to: – Effectively and objectively investigate: Deviations, Non-conformities, Unexpected, Unwanted, Undesirable, OOS, Out of trend – events and document the investigation • Concisely • precisely PCI Pharmaceutical Consulting Israel Ltd Toolki t 14
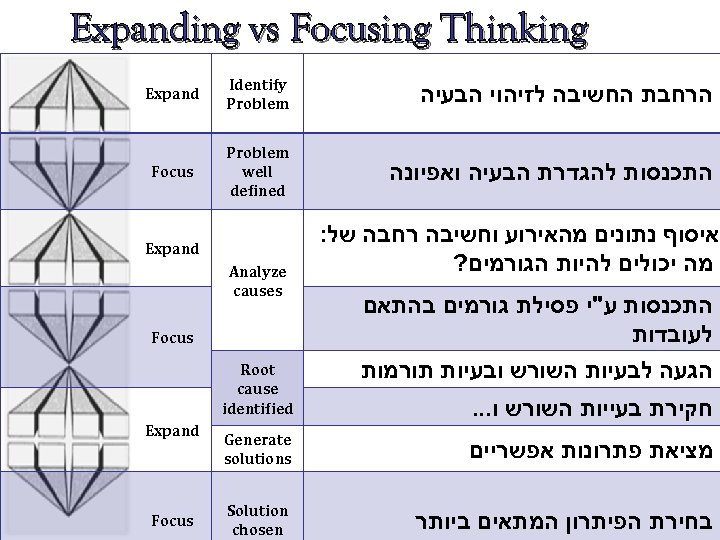
Expanding vs Focusing Thinking הרחבת החשיבה לזיהוי הבעיה Identify Problem Expand התכנסות להגדרת הבעיה ואפיונה Problem well defined Focus איסוף נתונים מהאירוע וחשיבה רחבה של: מה יכולים להיות הגורמים? התכנסות ע"י פסילת גורמים בהתאם לעובדות Expand Analyze causes Focus הגעה לבעיות השורש ובעיות תורמות Root cause identified מציאת פתרונות אפשריים Generate solutions בחירת הפיתרון המתאים ביותר Solution chosen חקירת בעייות השורש ו. . . Expand Focus
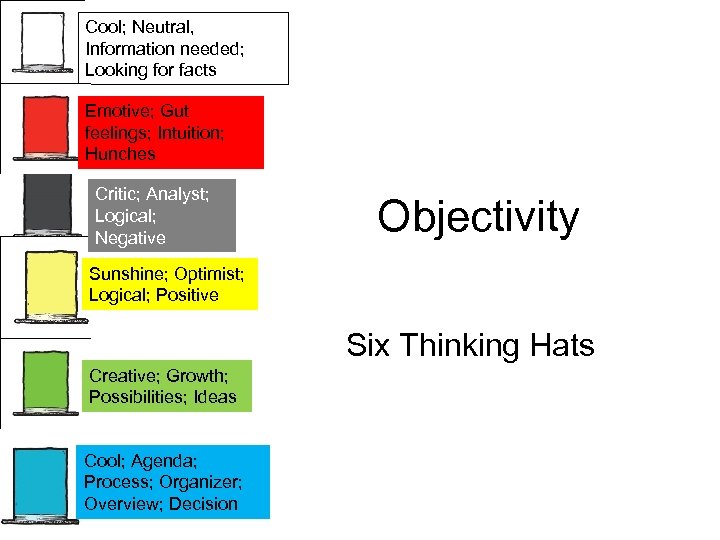
Cool; Neutral, Information needed; Looking for facts Emotive; Gut feelings; Intuition; Hunches Critic; Analyst; Logical; Negative Objectivity Sunshine; Optimist; Logical; Positive Six Thinking Hats Creative; Growth; Possibilities; Ideas Cool; Agenda; Process; Organizer; Overview; Decision
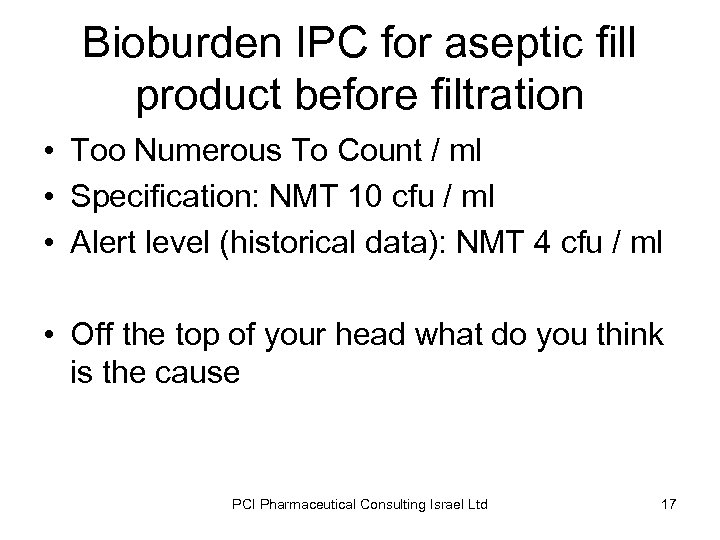
Bioburden IPC for aseptic fill product before filtration • Too Numerous To Count / ml • Specification: NMT 10 cfu / ml • Alert level (historical data): NMT 4 cfu / ml • Off the top of your head what do you think is the cause PCI Pharmaceutical Consulting Israel Ltd 17

Karen’s questions 1. 2. 3. 4. 5. 6. 7. TNTC / ml = too numerous to count a very different result to those normally obtained if the alert is 4 cfu / ml Any other samples TNTC for this or other products in the past months: - answered in the investigation checklist BUT not analyzed and no conclusions in report (Has anything changed in the sampling plan? Is the same lot of containers being used? What about different starting materials? ) Who took / tested the sample? Was EM performed? Where was the test performed? What was tested immediately before this product? What disinfectant is used for the BSC? Do the cleaning records show that the BSC was indeed disinfected? Who did that? When were they last observed doing it? What was the ID and where is the colony morphology of the plate documented? Was there a single colony type? Is there a picture of the plate? How was ID performed? Is the organism in the library etc.
The Investigation • • • Test Procedure Two 15 ml portions were filtered and washed with PW and PWTL and filters were transferred to TSA and SDA Another 1 ml portion was filtered and washed with PW only and then the filter transferred to TSA incubated 30 – 35; 2 days. SDA 20 – 25; 5 days For exact sample testing see method ID 43253 ver 02, attachment 2 This is the most important part – it is the analyst interview… Did the 15 ml sample show an identical pattern to the 1 ml plate? Same contamination – single strain or more than one microbe? After the same incubation time? Why is the procedure different for the 1 ml sample (PW only and not PWTL) and only TSA? PCI Pharmaceutical Consulting Israel Ltd 19

More Details TSA plates: tested for GPT – well they would be valid – they showed TNTC – so are only interested in STERILITY – was this part of the control testing? What does “vastly” mean… MUST give figures: how many plates were received and how many used in tests? Did the tests show zero counts or low counts where zero is usually obtained? PCI Pharmaceutical Consulting Israel Ltd 20

Experienced Operator PCI Pharmaceutical Consulting Israel Ltd 21
More Details • Historical results show no or very low microbial bioburden counts for this product • Contamination is Micrococcus Luteus Historical results from when to when? Was Micrococcus luteus the ONLY bacterium seen on the TNTC plate Is there a photo of the plate Is there a morphological description of the contamination documented? Next piece of information says BET <0. 005 EU/ml – so what: M. luteus isn’t an endotoxin producer! Can give the information but draw the appropriate conclusion… PCI Pharmaceutical Consulting Israel Ltd 22

A Few Minutes of GMPs • The GMP regulations: – EU and US on handling deviations • Q 10 – Pharmaceutical Quality System and CAPA management PCI Pharmaceutical Consulting Israel Ltd 23

The GMP Regulations European Directive • 1. 3 (vi) …any significant deviations are fully recorded and investigated • 5. 15 Any deviation from instructions or procedures should be avoided as far as possible • 5. 39 Any significant deviation from the expected yield should be recorded and investigated PCI Pharmaceutical Consulting Israel Ltd 24

21 cfr 211. 100 Written procedures; deviations (b) Written procedures and process control procedures shall be followed in the execution of the various production and process control functions…… Any deviations from the written procedures shall be recorded and justified PCI Pharmaceutical Consulting Israel Ltd 25

Q 10: CAPA • A pharmaceutical company should have a system for implementing corrective actions and preventive actions resulting from investigation of product quality feedback and trends from process performance and product quality monitoring PCI Pharmaceutical Consulting Israel Ltd 26

Q 10: CAPA • A structured approach to the investigation process should be used with the objective of determining root cause • The level of effort and formality of the investigation should be commensurate with the level of risk • CAPA methodology should result in product and process improvements and enhanced product and process understanding PCI Pharmaceutical Consulting Israel Ltd 27
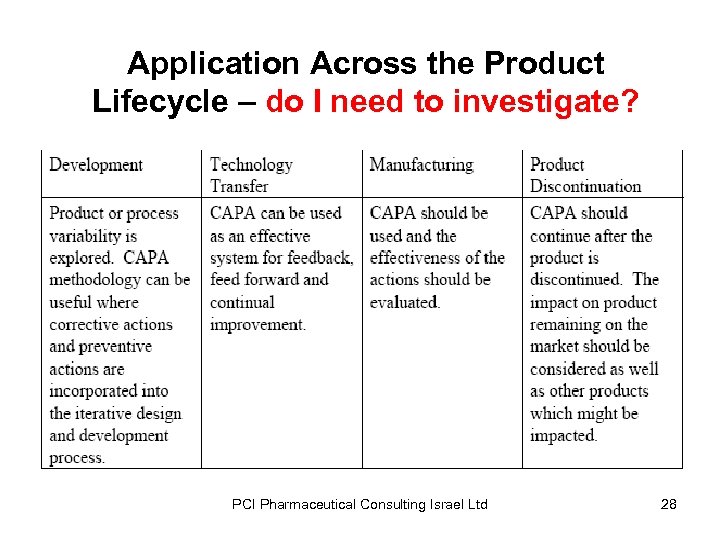
Application Across the Product Lifecycle – do I need to investigate? PCI Pharmaceutical Consulting Israel Ltd 28

Investigations • Always covered in inspections • The display window of the firm – Reporting culture – Level of understanding – Level of efforts invested – Level of documentation skills – A sole effort of QA? – Do they lead to continuous improvement?

RED FLAGS • Last year we had less deviations than complaints…. • In a deviation categorized as MAJOR, QA decided not to conduct an investigation • Same effort invested in trivial and major deviations • Majority of “root cause” defined as human error and retraining is chosen as the sole corrective action • Deviations are not closed in a timely manner and the statistics looks bad

RED FLAGS • Environmental monitoring deviations starts and ends with ID of the microorganism • Recurrent deviations with “copy paste” investigations • Immediate cause for deviations (e. g. electrical blackout) is defined as root cause • Conclusion are drawn based on R&D scientist’s statement ”the product is known to be stable at this temperature” with no supporting scientific evidence

RED FLAGS • Failure in systems (e. g. , HVAC) not correlated with production activities in the affected area • Written documentation does not allow understanding of what happened and lengthy verbal explanations are required • Shallow investigations if at all following calibration failures • Investigation only at the level of the impact on the product

RED FLAGS • Trends revealed in PQRs or System/Equipment QR not investigated • Internal e-mail correspondence presented as an investigation • Majority of CAPA activities as response to failures; preventive actions based on proactive activities such as risk assessments or internal audits are rare

Circumstantial Evidence “…is a very tricky thing “ answered Holmes thoughtfully “it may seem to point very straight to one thing but if you shift your own point of view a little, you may find it pointing in an equally uncompromising manner to something entirely different”

“Obvious” facts “ there is nothing more deceptive than an obvious fact, ” he answered laughing “ besides, we may chance to hit upon some other obvious facts which may have been by no means obvious to Mr Lestrade. ” “ I shall approach this case from the point of view that what this young man says is true, and see where that hypothesis leads us. ”

…and finally…look for the flaw Challenge the evidence • “you have read the evidence. You have formed some conclusion? Do you not see some loophole, some flaw? Do you not think that he is innocent?

Capturing the Event Description of the Deviation • Four case studies will be distributed – work with your colleagues and come up with a list of questions regarding additional information you need in order to understand the nature of the deviation PCI Pharmaceutical Consulting Israel Ltd 37

#1: PCI Pharmaceutical Consulting Israel Ltd 38

#2 PCI Pharmaceutical Consulting Israel Ltd 39

#3 PCI Pharmaceutical Consulting Israel Ltd 40

#4 PCI Pharmaceutical Consulting Israel Ltd 41
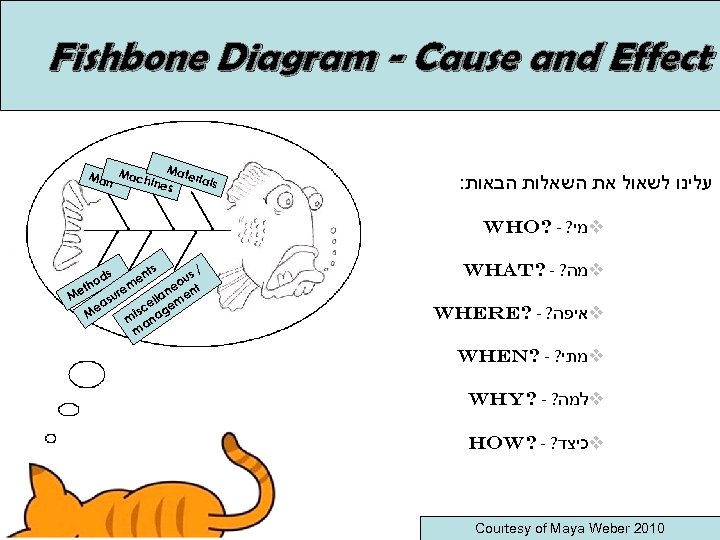
Fishbone Diagram - Cause and Effect Mat eria Man Machi ls nes : עלינו לשאול את השאלות הבאות Who? - ? מי v ts s/ ou t ne n r Me lla me su e ea isc ge M m ana m s d tho en m e What? - ? מה v where? - ? איפה v when? - ? מתי v why? - ? למה v how? - ? כיצד v Courtesy of Maya Weber 2010

Possible Causes • You are going to Brainstorm – but because we are not good at doing that – let’s warm up first PCI Pharmaceutical Consulting Israel Ltd 43

Problem statement: There is a mouse in the cheese • A large block of very good Swiss cheese on a kitchen table a few feet away from an open screen door. The weather outside is warm. A man comes to the table for some wine and cheese and sees a mouse in the cheese • Comments, questions, hypotheses regarding contributing causes and most probable cause(s) please work on your own write it down and think laterally there are no wrong answers at this stage 44

Comments, Hypotheses PCI Pharmaceutical Consulting Israel Ltd 45

Mouse in Cheese • Here is a solution to the problem: • Throw out the cheese with the mouse and open up a new packet of cheese • Have I solved the problem – Yes / No? • Defend your answer 46

Mouse in Cheese – 6 M’s Fishbone • • Man Group #1 Machines Materials Group #2 Methods Measurement Group #3 Miscellaneous / Mother Nature / Management please work in groups – be a bit crazy write it down – at least three for each category - we need ideas there are no wrong answers at this stage PCI Pharmaceutical Consulting Israel Ltd 47

A Tool • Root Cause Analysis is a tool for identifying prevention strategies. It is a process that is part of the effort to build a culture of doing things right, continuous improvement and move beyond the culture of blame PCI Pharmaceutical Consulting Israel Ltd 48
Root Cause Methodology • NOT intended to identify a single cause • Look for: – Contributing cause(s) – Most probable cause(s)
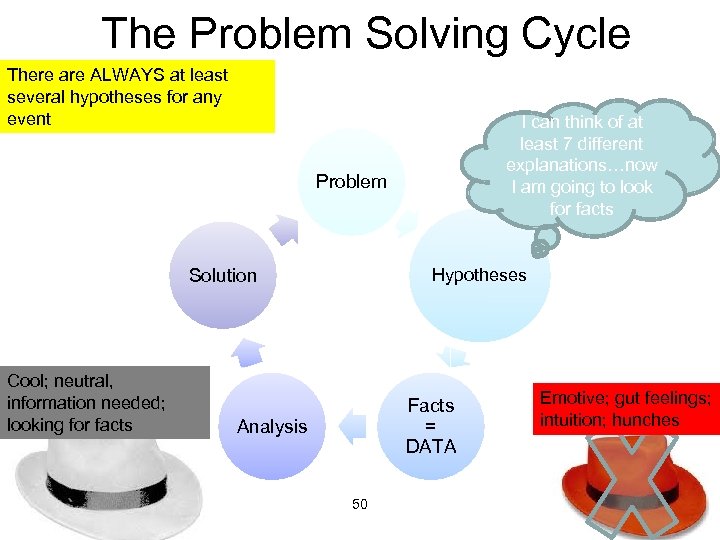
The Problem Solving Cycle There are ALWAYS at least several hypotheses for any event I can think of at least 7 different explanations…now I am going to look for facts Problem Solution Cool; neutral, information needed; looking for facts Hypotheses Facts = DATA Analysis 50 Emotive; gut feelings; intuition; hunches
Expand Thinking Using – Process Flow chart : from test method and SOPs – Fishbone / Ishikawa: 6 m’s – MANY possible causes • Relates cause and effect • Sorts ideas – Why- why diagram 51
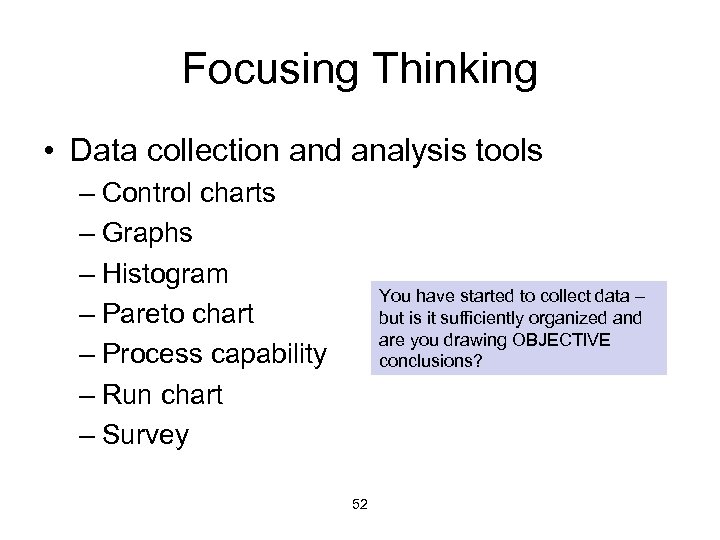
Focusing Thinking • Data collection and analysis tools – Control charts – Graphs – Histogram – Pareto chart – Process capability – Run chart – Survey You have started to collect data – but is it sufficiently organized and are you drawing OBJECTIVE conclusions? 52
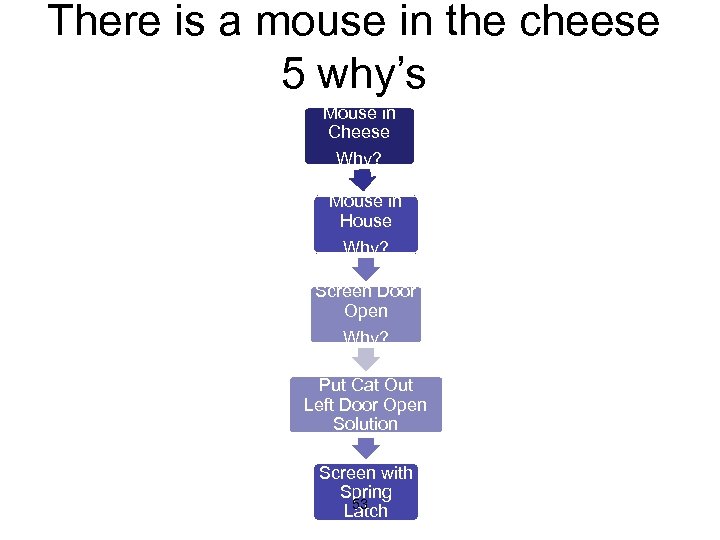
There is a mouse in the cheese 5 why’s Mouse in Cheese Why? Mouse in House Why? Screen Door Open Why? Put Cat Out Left Door Open Solution Screen with Spring 53 Latch

There is a mouse in the cheese • Alternative solutions: – "Be sure to close screen door“ – "Put a note on door asking, 'Did you latch me? • Comments please 54

Mouse in the Cheese • Should the cheese be left on the table? • Why was it left on the table? • If it is left on the table shouldn’t it be covered? • Why wasn’t it covered? • Did the mouse definitely come from outside? • Should there be a pest control program

Juran’s Universal Sequence for Making Quality Improvements • Study the symptoms of the defects and failures • Theorize as to the causes of these symptoms • Test theories until the cause(s) is known • Stimulate remedial action by the appropriate department Use Project Teams – A Project is a Problem Scheduled for Solution = CAPA PCI Pharmaceutical Consulting Israel Ltd 56

Root Cause Analysis • The goal of a Root Cause Analysis is to find out · what happened · why did it happen · What is the risk to product – through understanding cause(s) of the event rather than just symptoms · what do you do to prevent it from happening again. PCI Pharmaceutical Consulting Israel Ltd 57

Let’s take the case studies and Hypothesize • What could be the reasons for the event PCI Pharmaceutical Consulting Israel Ltd 58

Case #1 PCI Pharmaceutical Consulting Israel Ltd 59

Case #2 PCI Pharmaceutical Consulting Israel Ltd 60

Case #3 PCI Pharmaceutical Consulting Israel Ltd 61

Case #4 PCI Pharmaceutical Consulting Israel Ltd 62

Now select some most probable and contributing causes for case study #1 PCI Pharmaceutical Consulting Israel Ltd 63

Now do 5 why’s for the most probable causes PCI Pharmaceutical Consulting Israel Ltd 64

Now suggest some corrective actions PCI Pharmaceutical Consulting Israel Ltd 65

Completing the Cycle A whole is that which has beginning, middle and end, Aristotle, Rhetoric To complete a deviation / change report: – all relevant details documented – complete investigation recorded – final product disposition verified as implemented – corrective actions / tests verified as successfully performed PCI Pharmaceutical Consulting Israel Ltd 66

Deviation Documentation • Let’s Write a Deviation Report as we think it should look for one of the cases we studied today PCI Pharmaceutical Consulting Israel Ltd 67

MHRA – UK Regulator • Paul Hargreaves emphasised that the MHRA, and other regulatory agencies, expect companies to actively reduce the frequency and severity of human error • Regulatory agencies expect companies to actively prevent errors from happening, not to react after the event

MHRA – UK Regulator • The root cause of a vast majority of quality problems is not ‘human error’. Such a conclusion is usually symptomatic of a poor and inadequate investigation. Most informed regulatory auditors will refuse to accept ‘retraining’ as a suitable corrective action

MHRA – UK Regulator Error Reduction: Practical Tools and Techniques • Achieving a genuinely open and blame free working environment • Taking a positive approach to mistakes and error • Driving out complexity • The importance of User Centred Design (UCD) • The crucial importance of SYSTEM SAFEGUARDS • Avoid overly complex quality management systems which can cause errors
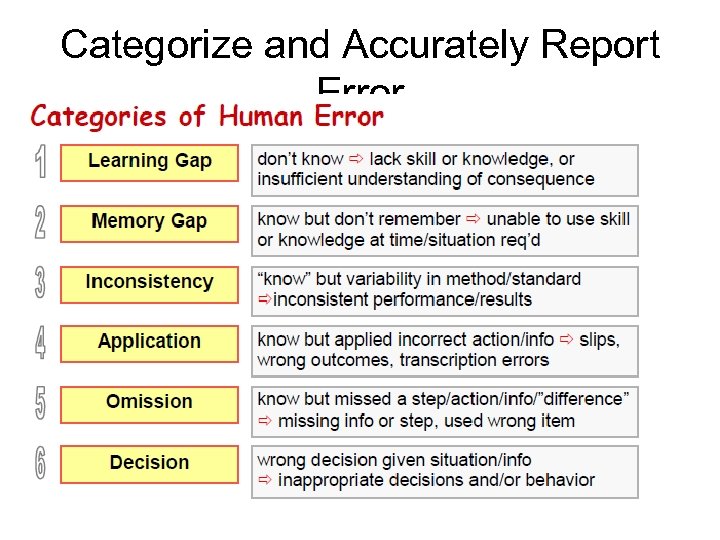
Categorize and Accurately Report Error

Learning Gap • Don’t know what to do: – Missing skill – Missing knowledge – Failure to understand the consequences – Lack of risk communication

Memory Gap • “know” but don’t remember at the time of performing an action or • Unable to apply the skill at the time of action or • Unable to apply the skill in a particular situation

Omission Errors • Failure to document a step in a procedure – Generally arises because of poor documents – user friendly documents encourage accurate recording • Skip a step in a procedure e. g. because of an interruption or lack of concentration (or perform same step twice) • Use wrong reagent / wrong type of water…

Inconsistent Performance • “Know” and “Understand” but variability in method / standard leads to inconsistent results e. g. – – Poor instrument maintenance Unclean instruments / contaminated incubator Poor reagent control e. g. contaminated LAL reagent Unclear method leads to inconsistent performance between different or even the same analyst – Analyst’s mood / tired / angry / bored / stressed / overworked: too many tests per analyst…

Application • “know” but apply technique wrongly or use the information incorrectly: – Transcription errors – Touch tip to sample and then use for a new sample – Scratch face before opening reagent – Failure to disinfect BSC

Decision Errors • Wrong decision made – In a particular situation or – Given a series of facts / information • E. g. put plates back in incubator rather than report breakage • “analyst error” when results may be true

MHRA – UK Regulator Actively REDUCE and REMOVE Risk Increasing Factors • Can conduct audits to identify RIFs – Structural (poor lighting, material and people flows etc) – Organisational (poor communication, leadership and complex, hierarchical bureaucracies) – Process related (poor documentation / process design) – Poor work place ergonomics – Individual (fatigue, stress, competence, skill related)

Actively remove RISK INCREASING FACTORS(RIF) • After an event – conduct an audit for RIFs • Observe several analysts performing the operation and ask “why? ” • Why did the plates drop: – was the incubator overcrowded? – Was there no trolley nearby? – Was there no workspace available? – Was the operator rushed / stressed?

Juran – inadvertent error • Inadvertent errors come from workers’ inability to maintain attention • The errors are unintentional, unwitting, and unpredictable (they exhibit randomness) • The bulk of remedies lie in fool-proofing the system

Juran – Technique Errors • Technique errors arise because the worker lacks some essential technique, skill, or knowledge needed to prevent the error from happening • They are typically unintentional, specific, consistent, and unavoidable • Remedies often require the discovery (using the diagnostic techniques) of differences of technique which represent the beneficial “knack” that produces superior results • and training others or changing the process to embody the better method

Inadvertent and Technique Errors • Usually workers cannot find the reasons for defects themselves, and therefore they will keep on doing what they have been doing • They will keep on producing defects • This will go on until they get the help they need from management

And regarding operator error: Juran on operator error: Management has the responsibility to provide a: 1. Means for knowing what he is supposed to do 2. Means for knowing whether he is doing what he is supposed to do 3. Means for changing what he is doing if it does not conform with what he is supposed to do PCI Pharmaceutical Consulting Israel Ltd 83

In Conclusion • Expand your thinking: #1 question: three or more possible explanations for every deviation event – write down at least three each time and be sure to include “I / my department messed up” • Collect data and analyze to convert into information • Apply the information as knowledge to draw a scientifically defendable conclusion

Thank You for your Attention Any questions Find us at pcikaren@netvision. net. il PCI Pharmaceutical Consulting Israel Ltd 85

Some answers from earlier on… BUT NOT ALL THE ANSWERS KEEP AN OPEN MIND PCI Pharmaceutical Consulting Israel Ltd 86

Documenting the Deviation • What was missing in the report: – When (did it occur) – Who (discovered it) – How and When (did they discover it) – Whom (did they notify) – When (did they make the notification) – What (immediate action was taken) – What was the scale of the deviation e. g. whole batch affected, portion of the batch segregated; how far out of specification (give the spec) PCI Pharmaceutical Consulting Israel Ltd 87

Which are “Contributing” vs “Most Probable”? For the cheese • cheese left on the table – why? E. g. refrigerator broken / man lazy CONTRIBUTING • If it is left on the table shouldn’t it be covered? Why wasn’t it covered? No cover available / man lazy CONTRIBUTING • Did the mouse definitely come from outside? Should there be a pest control program – depends – if the mouse is the kid’s then that could be the most probable cause – if not then contributing cause • Screen door open? – most probable cause Automatic latch / get rid of cat? • What will happen if you get rid of cat? ? ? Be careful not to be too quick to chose a corrective action plan without examining potential consequences and collecting more data to support your hypothesis
a40708661e26ee448f4111f757fa8a77.ppt