2f6b5dae803da4cdebd2852e83fa7c03.ppt
- Количество слайдов: 44
 HPV Campaign Update 5 th Welsh Immunisation Conference Wednesday 12 March 2008 Venue Cymru, Llandudno Conference Centre
HPV Campaign Update 5 th Welsh Immunisation Conference Wednesday 12 March 2008 Venue Cymru, Llandudno Conference Centre
 Overview § Background § Vaccines § (implementation update) § Catch-up campaign § Consent § Training resources
Overview § Background § Vaccines § (implementation update) § Catch-up campaign § Consent § Training resources
 HPV infection § Human papillomavirus (HPV) is a small DNA virus § It infects the deeper layers of the skin and internal lining of organs such as the vagina and mouth § HPV infection is often asymptomatic § HPV infections normally resolve spontaneously 90% do so within two years § Persistent HPV infection causes the cell changes that eventually lead to cancer § There are more than 100 types, of which c. 40 infect the genital area
HPV infection § Human papillomavirus (HPV) is a small DNA virus § It infects the deeper layers of the skin and internal lining of organs such as the vagina and mouth § HPV infection is often asymptomatic § HPV infections normally resolve spontaneously 90% do so within two years § Persistent HPV infection causes the cell changes that eventually lead to cancer § There are more than 100 types, of which c. 40 infect the genital area
 HPV transmission § HPV is spread by direct physical contact § Any genital contact is important, not just sexual intercourse § Hand to genital contact may cause some infections § Anyone who is sexually active is at risk § The risk of acquiring HPV increases with the number of sexual partners Computerised image of the human papillomavirus Courtesy of Dept of Pathology, University of Cambridge
HPV transmission § HPV is spread by direct physical contact § Any genital contact is important, not just sexual intercourse § Hand to genital contact may cause some infections § Anyone who is sexually active is at risk § The risk of acquiring HPV increases with the number of sexual partners Computerised image of the human papillomavirus Courtesy of Dept of Pathology, University of Cambridge
 Current approaches to preventing HPV infection § Condoms reduce the risk of acquiring HPV § HPV can be transmitted by contact of areas not covered by condoms § Cervical screening does not prevent HPV infection or pre-cancerous changes § Cervical screening remains important as: § vaccination will take several years to reduce cervical cancer § vaccination does not protect against all HPV types § and unvaccinated women will not be protected
Current approaches to preventing HPV infection § Condoms reduce the risk of acquiring HPV § HPV can be transmitted by contact of areas not covered by condoms § Cervical screening does not prevent HPV infection or pre-cancerous changes § Cervical screening remains important as: § vaccination will take several years to reduce cervical cancer § vaccination does not protect against all HPV types § and unvaccinated women will not be protected
 Epidemiology of HPV infection § HPV infection is common § 75% of women infected by the age of 50 § HPV infection increases from age 14 § Women are most likely to be infected in their late teens and early twenties § One UK study showed 15% of 20 - to 24 -year-old women were recently infected with HPV 16 or 18
Epidemiology of HPV infection § HPV infection is common § 75% of women infected by the age of 50 § HPV infection increases from age 14 § Women are most likely to be infected in their late teens and early twenties § One UK study showed 15% of 20 - to 24 -year-old women were recently infected with HPV 16 or 18
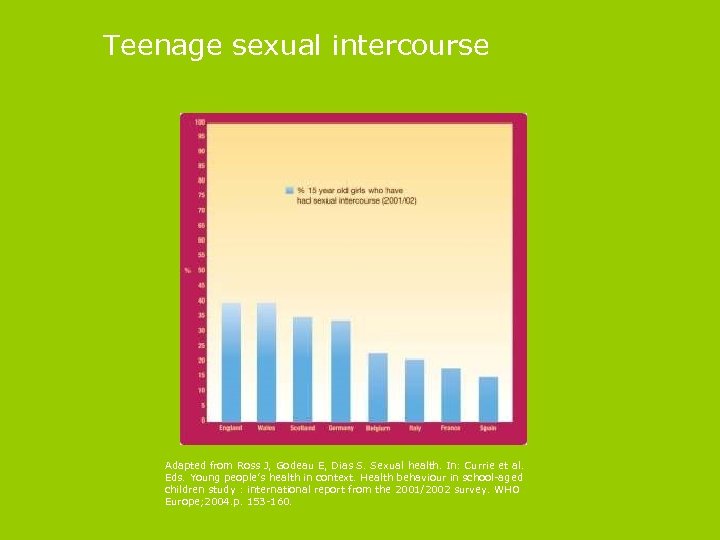 Teenage sexual intercourse Adapted from Ross J, Godeau E, Dias S. Sexual health. In: Currie et al. Eds. Young people’s health in context. Health behaviour in school-aged children study : international report from the 2001/2002 survey. WHO Europe; 2004. p. 153 -160.
Teenage sexual intercourse Adapted from Ross J, Godeau E, Dias S. Sexual health. In: Currie et al. Eds. Young people’s health in context. Health behaviour in school-aged children study : international report from the 2001/2002 survey. WHO Europe; 2004. p. 153 -160.
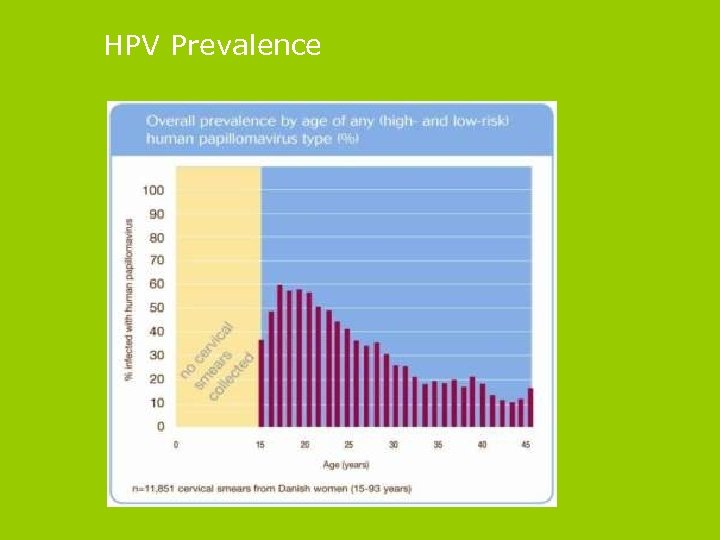 HPV Prevalence
HPV Prevalence
 Categories of genital HPV infection § Genital HPV types are categorised as either: § High-risk (HR) (oncogenic) types - which cause cervical intraepithelial neoplasia and invasive cancer § Low-risk (LR) types - which cause genital warts and some low grade pre-cancerous lesions § Over 99% of cases of cervical cancer are caused by HPV infection § Two high-risk types, HPV 16 and 18, cause over 70% of cervical cancers § 90% Genital warts caused by types 6 and 11 § Other HPV types can also cause cervical cancer
Categories of genital HPV infection § Genital HPV types are categorised as either: § High-risk (HR) (oncogenic) types - which cause cervical intraepithelial neoplasia and invasive cancer § Low-risk (LR) types - which cause genital warts and some low grade pre-cancerous lesions § Over 99% of cases of cervical cancer are caused by HPV infection § Two high-risk types, HPV 16 and 18, cause over 70% of cervical cancers § 90% Genital warts caused by types 6 and 11 § Other HPV types can also cause cervical cancer
 Genital warts § Genital warts are the commonest sexually transmitted viral infection in the UK § In one study, 4% of adults aged 18 to 44 reported having been diagnosed with genital warts § Genital warts can be recurrent and difficult to treat § Genital warts, although not life threatening, can cause significant distress § HPV types 6 and 11 cause 90% of cases (Gardasil) § Prevention of cervical cancer is, however, the primary purpose of the HPV vaccination programme
Genital warts § Genital warts are the commonest sexually transmitted viral infection in the UK § In one study, 4% of adults aged 18 to 44 reported having been diagnosed with genital warts § Genital warts can be recurrent and difficult to treat § Genital warts, although not life threatening, can cause significant distress § HPV types 6 and 11 cause 90% of cases (Gardasil) § Prevention of cervical cancer is, however, the primary purpose of the HPV vaccination programme
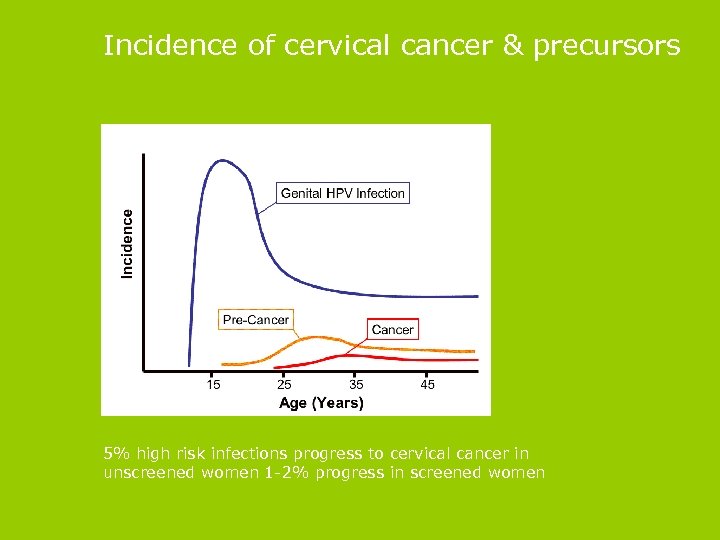 Incidence of cervical cancer & precursors 5% high risk infections progress to cervical cancer in unscreened women 1 -2% progress in screened women
Incidence of cervical cancer & precursors 5% high risk infections progress to cervical cancer in unscreened women 1 -2% progress in screened women
 Epidemiology of cervical cancer § It is the second most common cancer of women worldwide § 471, 000 new cases per year § 273, 000 deaths per year § Most cases occur in women in their late 30 s or in their 70 s/80 s (latter group were not screened when younger) § In developed countries, most cases are prevented by cervical screening
Epidemiology of cervical cancer § It is the second most common cancer of women worldwide § 471, 000 new cases per year § 273, 000 deaths per year § Most cases occur in women in their late 30 s or in their 70 s/80 s (latter group were not screened when younger) § In developed countries, most cases are prevented by cervical screening
 UK facts & figures: incidence & Mortality § 2, 726 new cases (2004) § Second most common cancer in < 35 yr olds § UK lifetime risk 1 in 116 § UK Incidence 8. 0/100, 000 women § (England 7. 8 cf Wales 9. 3) § 1, 061 deaths (2005) § 3 women die daily in UK
UK facts & figures: incidence & Mortality § 2, 726 new cases (2004) § Second most common cancer in < 35 yr olds § UK lifetime risk 1 in 116 § UK Incidence 8. 0/100, 000 women § (England 7. 8 cf Wales 9. 3) § 1, 061 deaths (2005) § 3 women die daily in UK
 Case history ED 23 years, para 1 - daughter 4 yrs § 7/05 abdominal pain § 6 cm cervical tumour involving upper 1/3 vagina § MRI: Bilateral iliac lymphadenopathy & parametrial involvement § Stage IIb, Squamous cell carcinoma § Chemoradiation § 6/06 Pain, lymphoedema of legs, recurrence § 8/06 Hospice § Died 12/06
Case history ED 23 years, para 1 - daughter 4 yrs § 7/05 abdominal pain § 6 cm cervical tumour involving upper 1/3 vagina § MRI: Bilateral iliac lymphadenopathy & parametrial involvement § Stage IIb, Squamous cell carcinoma § Chemoradiation § 6/06 Pain, lymphoedema of legs, recurrence § 8/06 Hospice § Died 12/06
 Precursor disease cervical intraepithelial neoplasia § 24, 105 new cases CIN 3 (UK 2003) § 19, 527 new cases CIN 3 (UK 2002) § 91% in women <45 years § Peak incidence 25 -29 yrs
Precursor disease cervical intraepithelial neoplasia § 24, 105 new cases CIN 3 (UK 2003) § 19, 527 new cases CIN 3 (UK 2002) § 91% in women <45 years § Peak incidence 25 -29 yrs
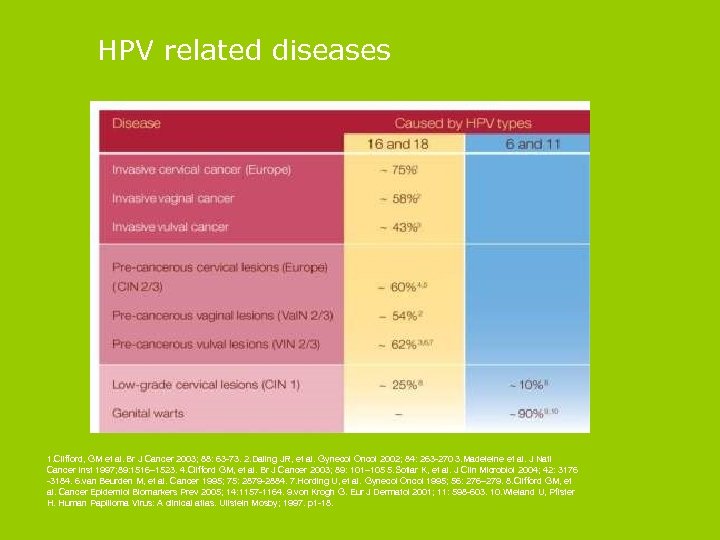 HPV related diseases 1. Clifford, GM et al. Br J Cancer 2003; 88: 63 -73. 2. Daling JR, et al. Gynecol Oncol 2002; 84: 263 -270 3. Madeleine et al. J Natl Cancer Inst 1997; 89: 1516– 1523. 4. Clifford GM, et al. Br J Cancer 2003; 89: 101– 105 5. Sotlar K, et al. J Clin Microbiol 2004; 42: 3176 -3184. 6. van Beurden M, et al. Cancer 1995; 75: 2879 -2884. 7. Hording U, et al. Gynecol Oncol 1995; 56: 276– 279. 8. Clifford GM, et al. Cancer Epidemiol Biomarkers Prev 2005; 14: 1157 -1164. 9. von Krogh G. Eur J Dermatol 2001; 11: 598 -603. 10. Wieland U, Pfister H. Human Papilloma Virus: A clinical atlas. Ullstein Mosby; 1997. p 1 -18.
HPV related diseases 1. Clifford, GM et al. Br J Cancer 2003; 88: 63 -73. 2. Daling JR, et al. Gynecol Oncol 2002; 84: 263 -270 3. Madeleine et al. J Natl Cancer Inst 1997; 89: 1516– 1523. 4. Clifford GM, et al. Br J Cancer 2003; 89: 101– 105 5. Sotlar K, et al. J Clin Microbiol 2004; 42: 3176 -3184. 6. van Beurden M, et al. Cancer 1995; 75: 2879 -2884. 7. Hording U, et al. Gynecol Oncol 1995; 56: 276– 279. 8. Clifford GM, et al. Cancer Epidemiol Biomarkers Prev 2005; 14: 1157 -1164. 9. von Krogh G. Eur J Dermatol 2001; 11: 598 -603. 10. Wieland U, Pfister H. Human Papilloma Virus: A clinical atlas. Ullstein Mosby; 1997. p 1 -18.
 HPV carcinogenesis § Persistent HPV infection with eventual HPV DNA integration into host cell DNA § HPV oncogenes interfere with host cell tumour suppressor proteins: § E 6 inactivates p 53 § E 7 inactivates Rb protein § Resultant cell cycle progression, multiplication of genetic mistakes & genetic instability
HPV carcinogenesis § Persistent HPV infection with eventual HPV DNA integration into host cell DNA § HPV oncogenes interfere with host cell tumour suppressor proteins: § E 6 inactivates p 53 § E 7 inactivates Rb protein § Resultant cell cycle progression, multiplication of genetic mistakes & genetic instability
 History of HPV research 1982 HPV 16/18 identified in cervical cancer 1986 Genital HR HPVs associated with cervical ca. 1989 E 6/E 7 expression in cervical cancer 1990 E 6/E 7 results in transformation 1992 HR HPV common STI in young people 1994 Persistent HR HPV necessary for CIN 3/cervical cancer 1999 99. 7% cervical cancer HR HPV+
History of HPV research 1982 HPV 16/18 identified in cervical cancer 1986 Genital HR HPVs associated with cervical ca. 1989 E 6/E 7 expression in cervical cancer 1990 E 6/E 7 results in transformation 1992 HR HPV common STI in young people 1994 Persistent HR HPV necessary for CIN 3/cervical cancer 1999 99. 7% cervical cancer HR HPV+
 Recent history of HPV research: infection to vaccine 1991 HPV 16 L 1&L 2 VLPs 1992 HPV 16 L 1 alone VLPs 1994 VLPs protect against viral challenge with neutralising abs 1995 Ig Ab following VLP vaccination passive transfer & protection 2002 Proof of principle HPV 16 VLP study - Koutsky
Recent history of HPV research: infection to vaccine 1991 HPV 16 L 1&L 2 VLPs 1992 HPV 16 L 1 alone VLPs 1994 VLPs protect against viral challenge with neutralising abs 1995 Ig Ab following VLP vaccination passive transfer & protection 2002 Proof of principle HPV 16 VLP study - Koutsky
 The nature of HPV vaccines § Proteins, like those that coat the HPV virus, are made in either yeast or baculovirus (insect virus) cells using recombinant DNA technology § These purified antigenic proteins assemble into small spheres called virus-like particles (VLPs) § VLPs cannot cause infection or cancer § HPV vaccination causes the body to mount an immune response against the antigens in the VLPs § Immunised individuals mount a rapid immune response when subsequently exposed to HPV
The nature of HPV vaccines § Proteins, like those that coat the HPV virus, are made in either yeast or baculovirus (insect virus) cells using recombinant DNA technology § These purified antigenic proteins assemble into small spheres called virus-like particles (VLPs) § VLPs cannot cause infection or cancer § HPV vaccination causes the body to mount an immune response against the antigens in the VLPs § Immunised individuals mount a rapid immune response when subsequently exposed to HPV
 The available HPV vaccines § There are two licensed HPV vaccines: § Gardasil. TM (quadrivalent), which protects against HPV types 16/18 and 6/11, and § Cervarix. TM (bivalent), which protects against types 16/18 only. § HR 16 and 18 covers only 70% Cervical ca. § Prevent initial infection with HPV § No head to head comparisons § £ 240 for 3 dose regimen § Current procurement process
The available HPV vaccines § There are two licensed HPV vaccines: § Gardasil. TM (quadrivalent), which protects against HPV types 16/18 and 6/11, and § Cervarix. TM (bivalent), which protects against types 16/18 only. § HR 16 and 18 covers only 70% Cervical ca. § Prevent initial infection with HPV § No head to head comparisons § £ 240 for 3 dose regimen § Current procurement process
 Vaccine effectiveness § Large numbers of patients in good clinical trials § Studies use CIN 2/3 as a surrogate marker § cervical cancer takes many years to develop § ethics § In uninfected women, vaccination is shown to be 99 -100% effective in preventing pre-cancerous lesions caused by types 16 and 18
Vaccine effectiveness § Large numbers of patients in good clinical trials § Studies use CIN 2/3 as a surrogate marker § cervical cancer takes many years to develop § ethics § In uninfected women, vaccination is shown to be 99 -100% effective in preventing pre-cancerous lesions caused by types 16 and 18
 Phase 2/3 Trial Data § Quadrivalent vaccine prevents genital warts and some low grade disease § Evidence of cross-protective antibodies against other HR HPV types - studies are in progress to confirm their clinical impact § No clear evidence that vaccination is effective against an HPV type that has already caused infection § Effectiveness in practice? § Acceptability, feasibility & uptake of 3 IM dose regime
Phase 2/3 Trial Data § Quadrivalent vaccine prevents genital warts and some low grade disease § Evidence of cross-protective antibodies against other HR HPV types - studies are in progress to confirm their clinical impact § No clear evidence that vaccination is effective against an HPV type that has already caused infection § Effectiveness in practice? § Acceptability, feasibility & uptake of 3 IM dose regime
 Vaccine safety § Rigorous safety testing is a requirement for licensing. § Studies have shown HPV vaccine to be safe and welltolerated. § Vaccine safety, as a routine, is monitored long term. § 13 million doses have been given to tens of thousands of women worldwide. § The fainting episodes reported in Australia are often seen in adolescents with any injection procedure. § The reports of death in USA, and more recently in Europe, have been investigated and were not linked to vaccination.
Vaccine safety § Rigorous safety testing is a requirement for licensing. § Studies have shown HPV vaccine to be safe and welltolerated. § Vaccine safety, as a routine, is monitored long term. § 13 million doses have been given to tens of thousands of women worldwide. § The fainting episodes reported in Australia are often seen in adolescents with any injection procedure. § The reports of death in USA, and more recently in Europe, have been investigated and were not linked to vaccination.
 Vaccine side effects § Mild to moderate swelling, redness and pain at site of injection is common. § Other less commonly reported side effects are: § slight temperature § sickness, dizziness and diarrhoea, and § muscle aches. § Anaphylaxis following vaccination is possible but extremely rare.
Vaccine side effects § Mild to moderate swelling, redness and pain at site of injection is common. § Other less commonly reported side effects are: § slight temperature § sickness, dizziness and diarrhoea, and § muscle aches. § Anaphylaxis following vaccination is possible but extremely rare.
 Vaccine contraindications and precautions § Contraindications to HPV vaccination are: § a confirmed anaphylactic reaction to a previous dose, or § a confirmed anaphylactic reaction to any component of the vaccine § Yeast allergy is not a contraindication to HPV vaccination § Those who are immunocompromised can be safely vaccinated, although vaccination may be less effective
Vaccine contraindications and precautions § Contraindications to HPV vaccination are: § a confirmed anaphylactic reaction to a previous dose, or § a confirmed anaphylactic reaction to any component of the vaccine § Yeast allergy is not a contraindication to HPV vaccination § Those who are immunocompromised can be safely vaccinated, although vaccination may be less effective
 Vaccine schedule and administration § A course of three doses of vaccine is recommended for protection § The three doses of vaccine should be given over a sixmonth period § The vaccination course is best completed within 12 months § HPV vaccination will normally be given by the usual route, in the upper arm by IM injection § As the incidence of HPV infection starts to rise from age 14, vaccination should occur before this age
Vaccine schedule and administration § A course of three doses of vaccine is recommended for protection § The three doses of vaccine should be given over a sixmonth period § The vaccination course is best completed within 12 months § HPV vaccination will normally be given by the usual route, in the upper arm by IM injection § As the incidence of HPV infection starts to rise from age 14, vaccination should occur before this age
 Implementation Issues § Acceptability and uptake? § Booster requirements? § Cross protection? § Efficacy in older women? § Effective in men? § Long term efficacy of screening v. vaccination strategies?
Implementation Issues § Acceptability and uptake? § Booster requirements? § Cross protection? § Efficacy in older women? § Effective in men? § Long term efficacy of screening v. vaccination strategies?
 Research on attitudes to HPV vaccination § Parents and girls knew little about HPV infection as a cause of cervical cancer § Parents were more accepting of immunisation at secondary school age than in primary schools § Parents expressed concerns about vaccine safety and consent issues § Teenage girls mostly concerned about having injections § The primary message should be that HPV vaccination protects against cervical cancer § A stepped approach to communication is planned, with further resources signposted for those who want more detailed information
Research on attitudes to HPV vaccination § Parents and girls knew little about HPV infection as a cause of cervical cancer § Parents were more accepting of immunisation at secondary school age than in primary schools § Parents expressed concerns about vaccine safety and consent issues § Teenage girls mostly concerned about having injections § The primary message should be that HPV vaccination protects against cervical cancer § A stepped approach to communication is planned, with further resources signposted for those who want more detailed information
 Vaccine Acceptability in UK § Controversy in USA § Marlow, Waller, Wardle 2007: § Mothers of girls 8 -14 yrs § 75% acceptance § 19% unsure § 6% non acceptance § Factors affecting acceptance/non acceptance
Vaccine Acceptability in UK § Controversy in USA § Marlow, Waller, Wardle 2007: § Mothers of girls 8 -14 yrs § 75% acceptance § 19% unsure § 6% non acceptance § Factors affecting acceptance/non acceptance
 Factors affecting acceptability in UK § Acceptance § White v non-white § Positive attitudes to HPV vaccination § Socially normative beliefs about HPV vaccination § Non acceptance § Religious § Concerns about effect on sexual behaviour § Concerns about need to discuss HPV with children § Concerns about safety
Factors affecting acceptability in UK § Acceptance § White v non-white § Positive attitudes to HPV vaccination § Socially normative beliefs about HPV vaccination § Non acceptance § Religious § Concerns about effect on sexual behaviour § Concerns about need to discuss HPV with children § Concerns about safety
 Duration of immunity § The immune response to HPV vaccination lasts at least six years (the current maximum length of post-vaccination follow-up). § For at least five years post-vaccination, antibody levels have been shown to be higher from vaccination than from natural infection. § At present there is no evidence for waning immunity, but important long-term follow-up studies are taking place to establish whether boosting will be necessary.
Duration of immunity § The immune response to HPV vaccination lasts at least six years (the current maximum length of post-vaccination follow-up). § For at least five years post-vaccination, antibody levels have been shown to be higher from vaccination than from natural infection. § At present there is no evidence for waning immunity, but important long-term follow-up studies are taking place to establish whether boosting will be necessary.
 Cross Protection v Type Replacement § Neutralising antibodies type specific § Cross protection against other HPV types? § Could type replacement occur? § Polyvalent vaccines? § 5 -6 HPV types for 80 -90% coverage
Cross Protection v Type Replacement § Neutralising antibodies type specific § Cross protection against other HPV types? § Could type replacement occur? § Polyvalent vaccines? § 5 -6 HPV types for 80 -90% coverage
 Vaccination of boys and young women 18 and over § The benefits of HPV vaccination are less for boys and JCVI has advised that vaccination of boys is not cost-effective. § The vaccination of girls will reduce HPV infections in boys by a herd immunity effect. § JCVI has also advised that vaccination for all women aged 18 to 25 years is not cost effective. § Some older girls and young women could benefit however, and this is under consideration. § Young women not eligible for vaccination will still be covered by the cervical screening programme.
Vaccination of boys and young women 18 and over § The benefits of HPV vaccination are less for boys and JCVI has advised that vaccination of boys is not cost-effective. § The vaccination of girls will reduce HPV infections in boys by a herd immunity effect. § JCVI has also advised that vaccination for all women aged 18 to 25 years is not cost effective. § Some older girls and young women could benefit however, and this is under consideration. § Young women not eligible for vaccination will still be covered by the cervical screening programme.
 Developed countries § Screening reduces cervical cancer by 75% (75 -80% coverage) § Vaccines protect against 70 -75% (100% coverage) § Over reliance on vaccine and failure to continue with screening could increase cervical cancer!
Developed countries § Screening reduces cervical cancer by 75% (75 -80% coverage) § Vaccines protect against 70 -75% (100% coverage) § Over reliance on vaccine and failure to continue with screening could increase cervical cancer!
 Combination HPV vaccination & screening potential health gain § Reduction of abnormal cytology & pre-invasive disease (CIN 2/3) § Reduction in colposcopy workload § Reduction in incidence, morbidity & mortality of cervical cancer § Reduction in morbidity of screening § Other benefits § Reduction in other anogenital neoplasia § Reduction in head and neck cancers
Combination HPV vaccination & screening potential health gain § Reduction of abnormal cytology & pre-invasive disease (CIN 2/3) § Reduction in colposcopy workload § Reduction in incidence, morbidity & mortality of cervical cancer § Reduction in morbidity of screening § Other benefits § Reduction in other anogenital neoplasia § Reduction in head and neck cancers
 Predicted effect of HPV vaccines on vulval disease § VIN 3 - 70% reduction in 20 years § Vulval cancer - 40% reduction in 30 years § Gential warts - 80 -90% reduction in 10 years (with quadrivalent vaccine)
Predicted effect of HPV vaccines on vulval disease § VIN 3 - 70% reduction in 20 years § Vulval cancer - 40% reduction in 30 years § Gential warts - 80 -90% reduction in 10 years (with quadrivalent vaccine)
 Some key implementation issues for PCTs § JCVI has recommended a schools-based programme but PCTs will decide which delivery model to use. § It is likely that: § vaccine coverage would be higher in schools § costs would be lower than a GP-delivered model, and § using schools would be more acceptable to parents/pupils. § Offering vaccination in the summer term is problematic, so an early start in the school year will be necessary. § Specific educational input from school health services is likely to be needed.
Some key implementation issues for PCTs § JCVI has recommended a schools-based programme but PCTs will decide which delivery model to use. § It is likely that: § vaccine coverage would be higher in schools § costs would be lower than a GP-delivered model, and § using schools would be more acceptable to parents/pupils. § Offering vaccination in the summer term is problematic, so an early start in the school year will be necessary. § Specific educational input from school health services is likely to be needed.
 Practical lessons from a Hepatitis B pilot study A study in Glasgow* in 2001/2002 examined a three-dose hepatitis B regime provided in secondary schools and found: § schools, parents and pupils were supportive § consent was obtained for 92% of pupils § only 0. 2% of parents actively refused consent § 91% of pupils had at least one vaccination, and 80% had all three § deprivation was the most significant predictor of uptake § community ‘mop-up’ clinics proved unpopular with parents, and § education of healthcare workers, as well as working closely with school staff, was essential. * Bramley JC et al. Universal Hepatitis B vaccination of UK adolescents : a feasibility and acceptability study. Comm Dis and Public Health 2002; 5(4): 318 -20.
Practical lessons from a Hepatitis B pilot study A study in Glasgow* in 2001/2002 examined a three-dose hepatitis B regime provided in secondary schools and found: § schools, parents and pupils were supportive § consent was obtained for 92% of pupils § only 0. 2% of parents actively refused consent § 91% of pupils had at least one vaccination, and 80% had all three § deprivation was the most significant predictor of uptake § community ‘mop-up’ clinics proved unpopular with parents, and § education of healthcare workers, as well as working closely with school staff, was essential. * Bramley JC et al. Universal Hepatitis B vaccination of UK adolescents : a feasibility and acceptability study. Comm Dis and Public Health 2002; 5(4): 318 -20.
 Key messages for vaccinee’s § HPV infection is very common § Occasionally causes abnormal smears and cervical cancer § Vaccines protect against 75% cervical cancer § But not against all cancer causing types § Still need cervical screening § Vaccines don’t protect against other STI’s § Safe sex still important
Key messages for vaccinee’s § HPV infection is very common § Occasionally causes abnormal smears and cervical cancer § Vaccines protect against 75% cervical cancer § But not against all cancer causing types § Still need cervical screening § Vaccines don’t protect against other STI’s § Safe sex still important
 Proposed HPV vaccination programme § Girls aged 12 -13 years (school year 8) will be immunised routinely, starting from September 2008. § A catch-up programme, over two years, will start in autumn 2009: § girls aged 16 to 18 (years 12 and 13) will be offered immunisation in 2009/2010 § girls aged 15 to 17 (years 11 and 12) will be offered immunisation in 2010/2011.
Proposed HPV vaccination programme § Girls aged 12 -13 years (school year 8) will be immunised routinely, starting from September 2008. § A catch-up programme, over two years, will start in autumn 2009: § girls aged 16 to 18 (years 12 and 13) will be offered immunisation in 2009/2010 § girls aged 15 to 17 (years 11 and 12) will be offered immunisation in 2010/2011.
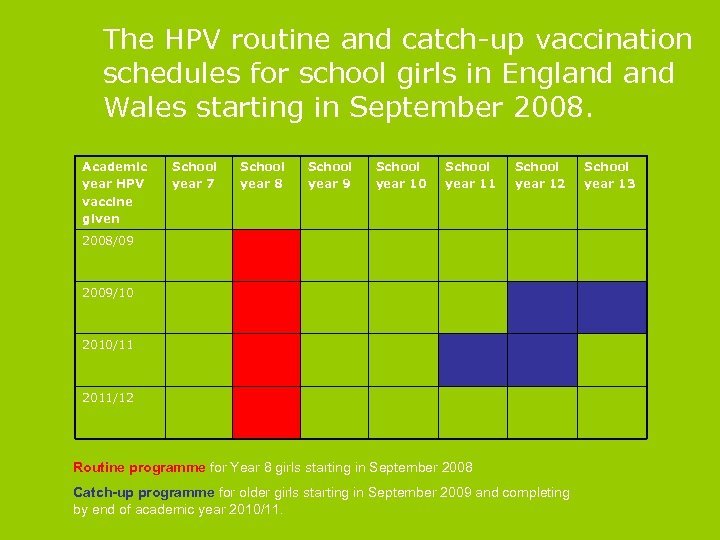 The HPV routine and catch-up vaccination schedules for school girls in England Wales starting in September 2008. Academic year HPV vaccine given School year 7 School year 8 School year 9 School year 10 School year 11 School year 12 2008/09 2009/10 2010/11 2011/12 Routine programme for Year 8 girls starting in September 2008 Catch-up programme for older girls starting in September 2009 and completing by end of academic year 2010/11. School year 13
The HPV routine and catch-up vaccination schedules for school girls in England Wales starting in September 2008. Academic year HPV vaccine given School year 7 School year 8 School year 9 School year 10 School year 11 School year 12 2008/09 2009/10 2010/11 2011/12 Routine programme for Year 8 girls starting in September 2008 Catch-up programme for older girls starting in September 2009 and completing by end of academic year 2010/11. School year 13
 Consent?
Consent?
 Further information § www. immunisation. nhs. uk website: § the DH press release and Q&A of 26 Oct 2007, which outlines DH policy on HPV vaccination; § a report of the first national HPV vaccination conference for Immunisation Coordinators, held by DH on 11 Oct 2007; and § training slide set. § It is planned that an information pack will be available in spring, but DH will make guidance and information materials available earlier, as they are finalised. § Drug companies
Further information § www. immunisation. nhs. uk website: § the DH press release and Q&A of 26 Oct 2007, which outlines DH policy on HPV vaccination; § a report of the first national HPV vaccination conference for Immunisation Coordinators, held by DH on 11 Oct 2007; and § training slide set. § It is planned that an information pack will be available in spring, but DH will make guidance and information materials available earlier, as they are finalised. § Drug companies


