59afbb94b040ab0983b527a08484ef76.ppt
- Количество слайдов: 95

HPLC – Basic Principles and Instrumentation 1

High Performance Liquid Chromatography (HPLC) 2
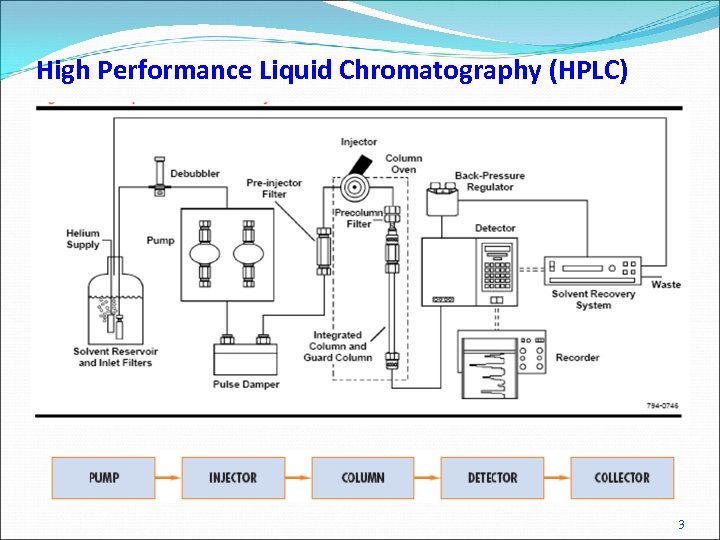
High Performance Liquid Chromatography (HPLC) 3
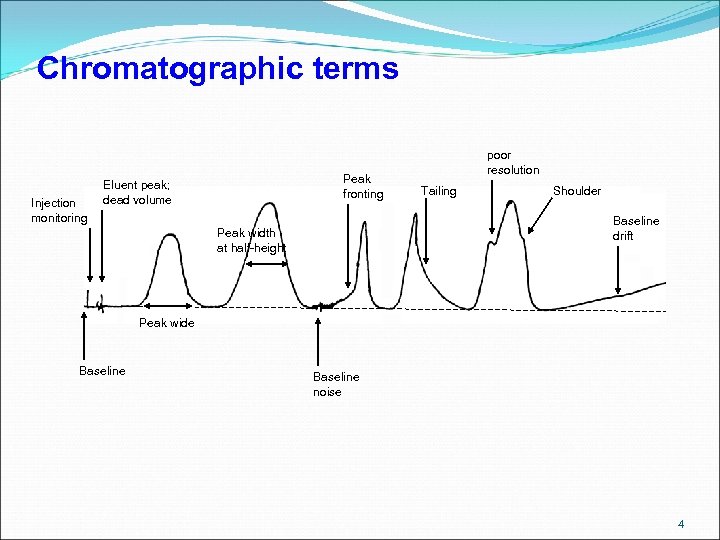
Chromatographic terms Injection monitoring Peak fronting Eluent peak; dead volume poor resolution Tailing Shoulder Baseline drift Peak width at half-height Peak wide Baseline noise 4
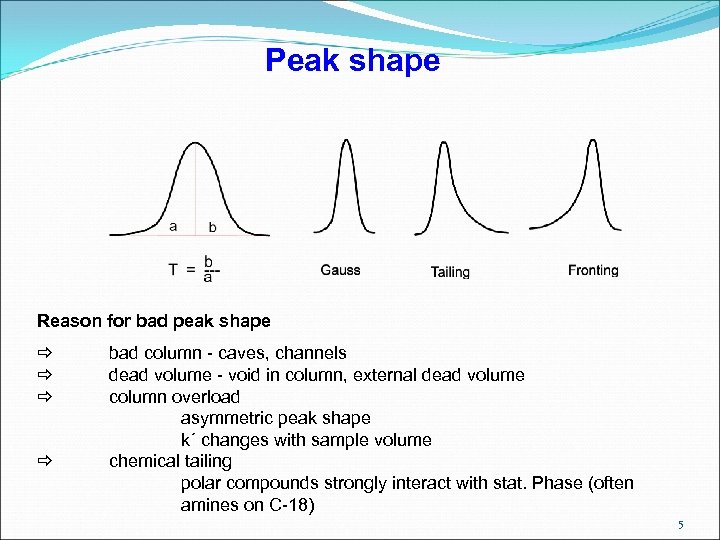
Peak shape Reason for bad peak shape bad column - caves, channels dead volume - void in column, external dead volume column overload asymmetric peak shape k´ changes with sample volume chemical tailing polar compounds strongly interact with stat. Phase (often amines on C-18) 5
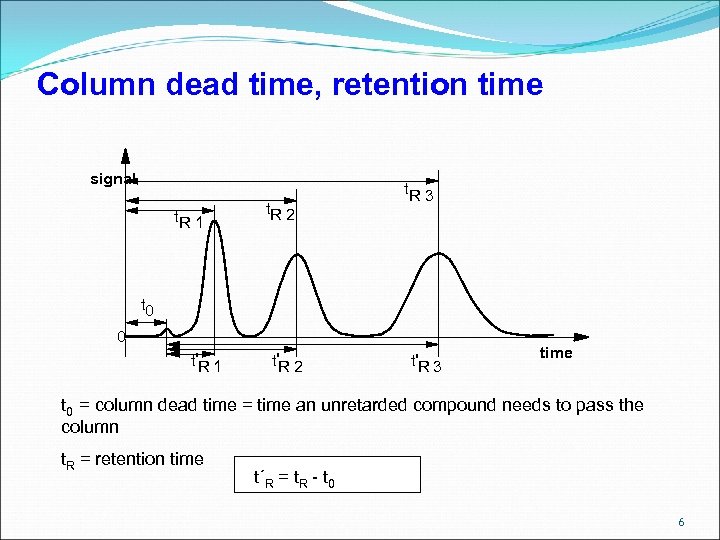
Column dead time, retention time signal t. R 1 t. R 2 t. R 3 t 0 0 t'R 1 t'R 2 t'R 3 time t 0 = column dead time = time an unretarded compound needs to pass the column t. R = retention time t´R = t. R - t 0 6
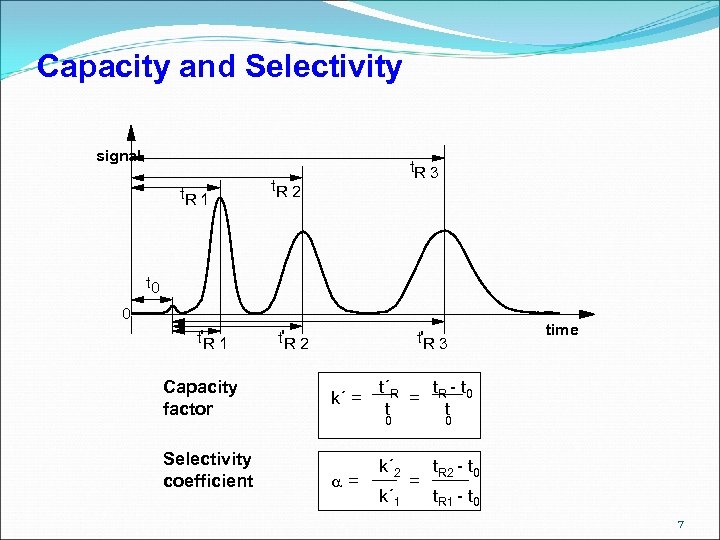
Capacity and Selectivity signal t. R 1 t. R 3 t. R 2 t 0 0 t'R 1 Capacity factor Selectivity coefficient t'R 2 t'R 3 k´ = t´R t - t = R 0 t t 0 = time k´ 2 k´ 1 0 = t. R 2 - t 0 t. R 1 - t 0 7
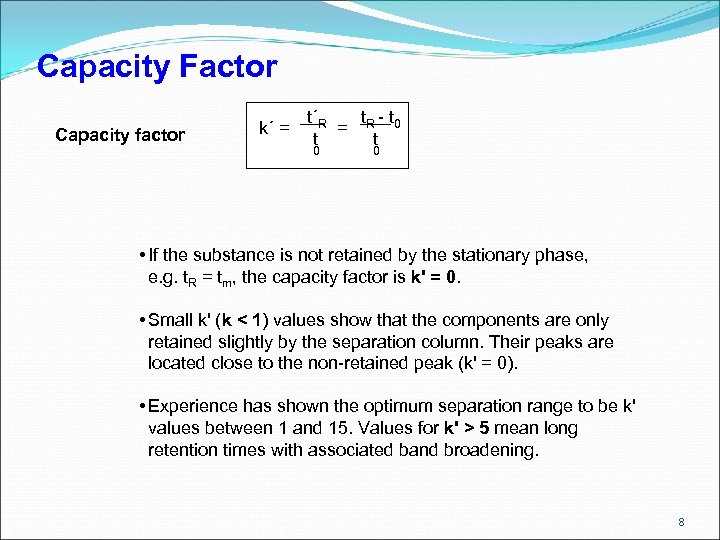
Capacity Factor Capacity factor k´ = t´R t - t = R 0 t t 0 0 • If the substance is not retained by the stationary phase, e. g. t. R = tm, the capacity factor is k' = 0. • Small k' (k < 1) values show that the components are only retained slightly by the separation column. Their peaks are located close to the non-retained peak (k' = 0). • Experience has shown the optimum separation range to be k' values between 1 and 15. Values for k' > 5 mean long retention times with associated band broadening. 8
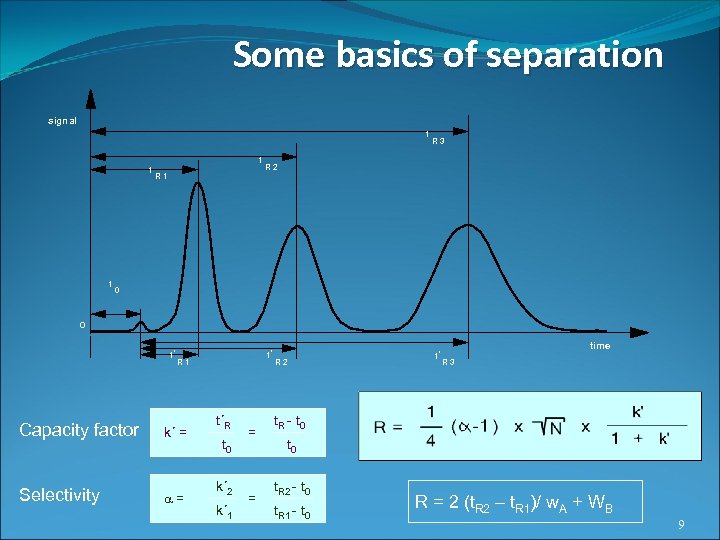
Some basics of separation signal t t t t R 3 R 2 R 1 0 0 t' t' R 1 Capacity factor k´ = Selectivity = t´R t 0 k´ 2 k´ 1 = = R 2 t' time R 3 t. R - t 0 t. R 2 - t 0 t. R 1 - t 0 R = 2 (t. R 2 – t. R 1)/ w. A + WB 9
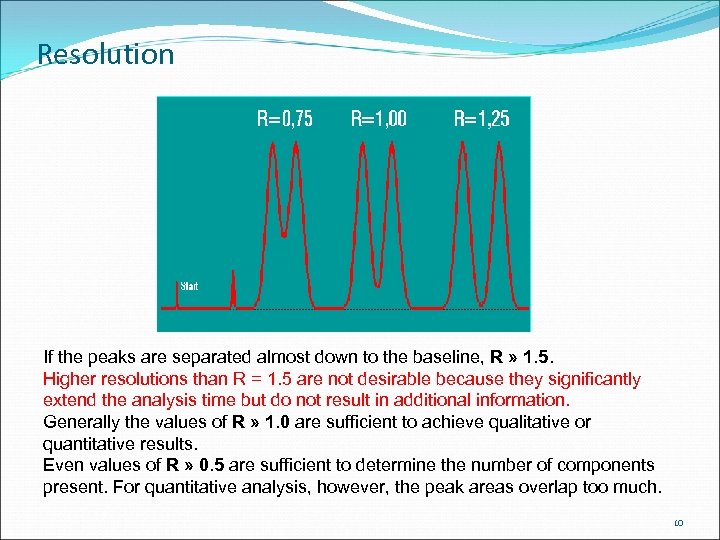
Resolution If the peaks are separated almost down to the baseline, R » 1. 5. Higher resolutions than R = 1. 5 are not desirable because they significantly extend the analysis time but do not result in additional information. Generally the values of R » 1. 0 are sufficient to achieve qualitative or quantitative results. Even values of R » 0. 5 are sufficient to determine the number of components present. For quantitative analysis, however, the peak areas overlap too much. 10
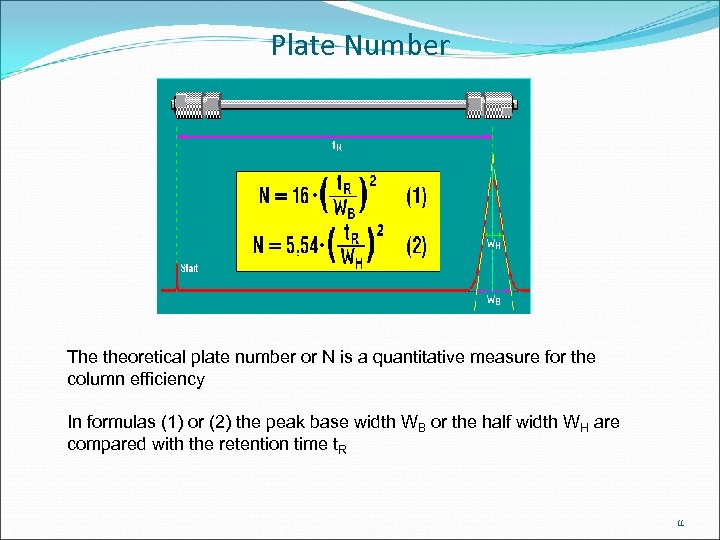
Plate Number The theoretical plate number or N is a quantitative measure for the column efficiency In formulas (1) or (2) the peak base width WB or the half width WH are compared with the retention time t. R 11
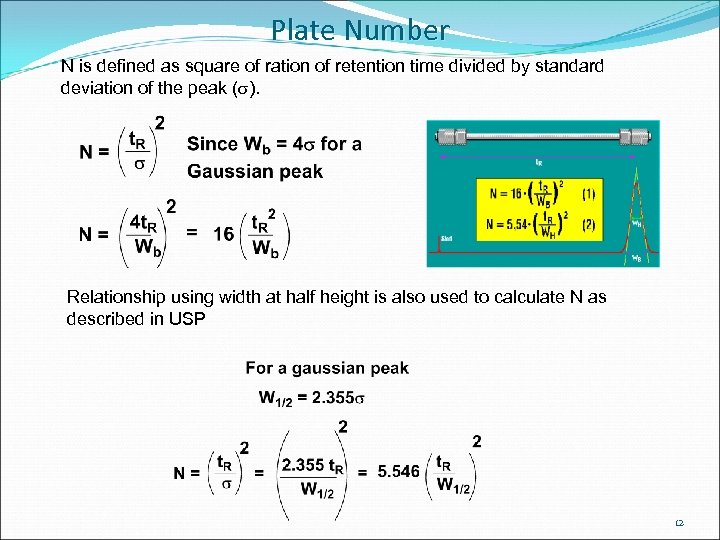
Plate Number N is defined as square of ration of retention time divided by standard deviation of the peak (s). Relationship using width at half height is also used to calculate N as described in USP 12
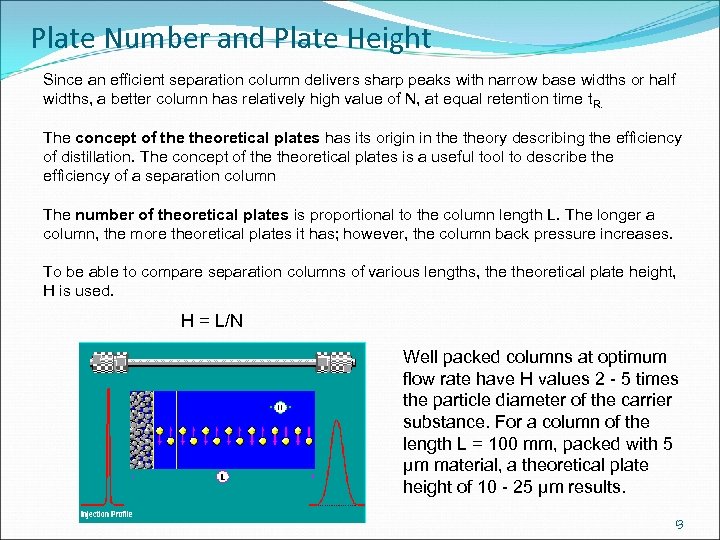
Plate Number and Plate Height Since an efficient separation column delivers sharp peaks with narrow base widths or half widths, a better column has relatively high value of N, at equal retention time t. R. The concept of theoretical plates has its origin in theory describing the efficiency of distillation. The concept of theoretical plates is a useful tool to describe the efficiency of a separation column The number of theoretical plates is proportional to the column length L. The longer a column, the more theoretical plates it has; however, the column back pressure increases. To be able to compare separation columns of various lengths, theoretical plate height, H is used. H = L/N Well packed columns at optimum flow rate have H values 2 - 5 times the particle diameter of the carrier substance. For a column of the length L = 100 mm, packed with 5 µm material, a theoretical plate height of 10 - 25 µm results. 13
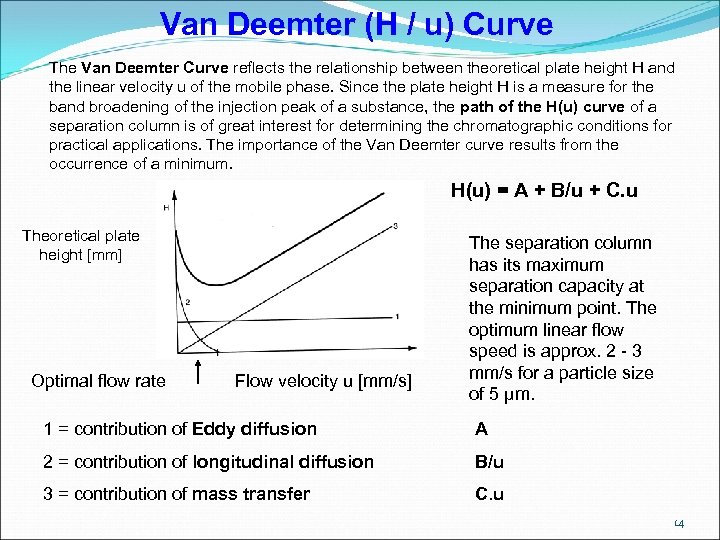
Van Deemter (H / u) Curve The Van Deemter Curve reflects the relationship between theoretical plate height H and the linear velocity u of the mobile phase. Since the plate height H is a measure for the band broadening of the injection peak of a substance, the path of the H(u) curve of a separation column is of great interest for determining the chromatographic conditions for practical applications. The importance of the Van Deemter curve results from the occurrence of a minimum. H(u) = A + B/u + C. u Theoretical plate height [mm] Optimal flow rate Flow velocity u [mm/s] The separation column has its maximum separation capacity at the minimum point. The optimum linear flow speed is approx. 2 - 3 mm/s for a particle size of 5 µm. 1 = contribution of Eddy diffusion A 2 = contribution of longitudinal diffusion B/u 3 = contribution of mass transfer C. u 14
Reasons for band broadening Instrument effects longitudinal diffusion turbulences and mixing at edges, borders, in dead volumes Column effects 15
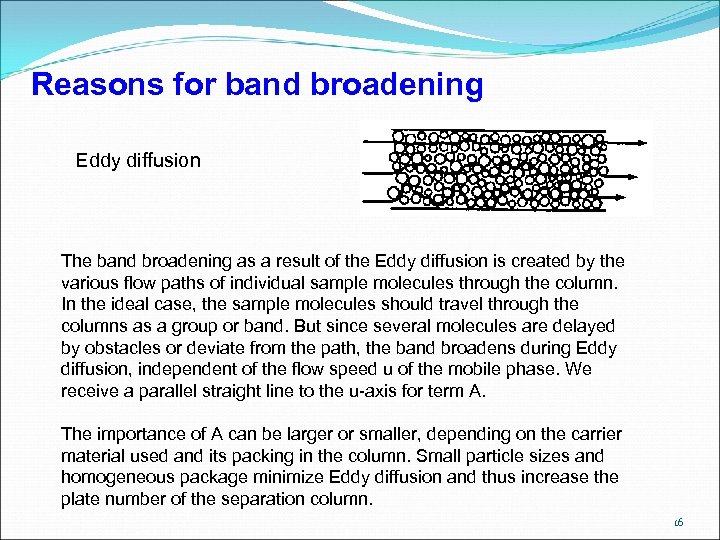
Reasons for band broadening Eddy diffusion The band broadening as a result of the Eddy diffusion is created by the various flow paths of individual sample molecules through the column. In the ideal case, the sample molecules should travel through the columns as a group or band. But since several molecules are delayed by obstacles or deviate from the path, the band broadens during Eddy diffusion, independent of the flow speed u of the mobile phase. We receive a parallel straight line to the u-axis for term A. The importance of A can be larger or smaller, depending on the carrier material used and its packing in the column. Small particle sizes and homogeneous package minimize Eddy diffusion and thus increase the plate number of the separation column. 16
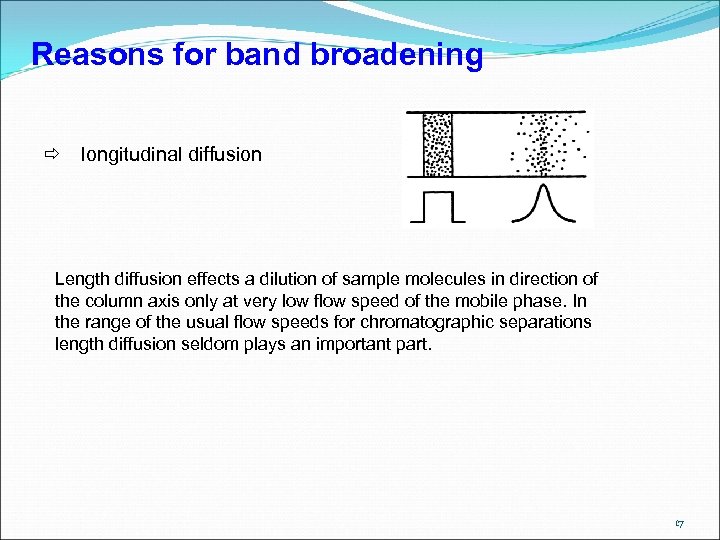
Reasons for band broadening longitudinal diffusion Length diffusion effects a dilution of sample molecules in direction of the column axis only at very low flow speed of the mobile phase. In the range of the usual flow speeds for chromatographic separations length diffusion seldom plays an important part. 17
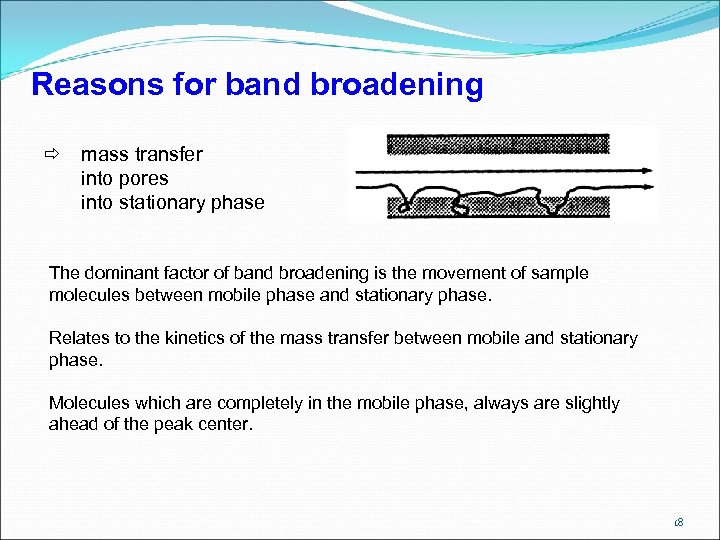
Reasons for band broadening mass transfer into pores into stationary phase The dominant factor of band broadening is the movement of sample molecules between mobile phase and stationary phase. Relates to the kinetics of the mass transfer between mobile and stationary phase. Molecules which are completely in the mobile phase, always are slightly ahead of the peak center. 18

Instrumentation 19
Pumps • There a number of different types of pumps that can provide the necessary pressures and flow-rates required by the modern liquid chromatograph. • In the early years of the LC renaissance, there were two types of pump in common use; they were the • Pneumatic pump, where the necessary high pressures were achieved by pneumatic amplification • syringe pump, which was simply a large, strongly constructed syringe with a plunger that was driven by a motor. • Today the majority of modern chromatographs are fitted with reciprocating pumps fitted with either pistons or diaphragms. 20
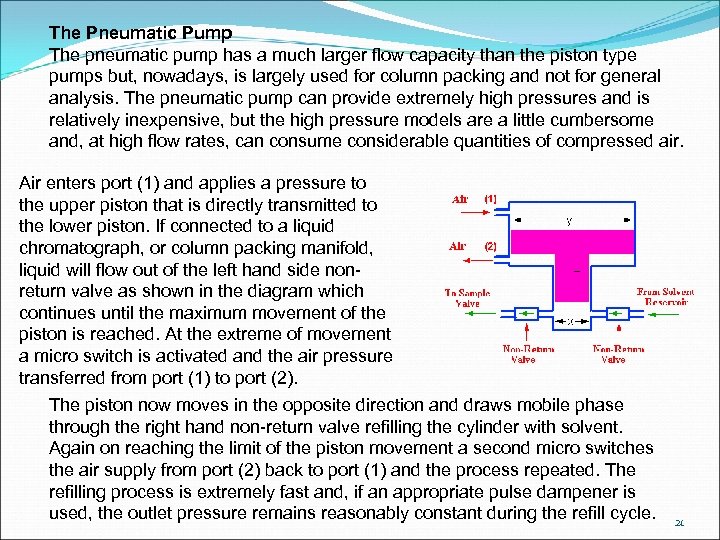
The Pneumatic Pump The pneumatic pump has a much larger flow capacity than the piston type pumps but, nowadays, is largely used for column packing and not for general analysis. The pneumatic pump can provide extremely high pressures and is relatively inexpensive, but the high pressure models are a little cumbersome and, at high flow rates, can consume considerable quantities of compressed air. Air enters port (1) and applies a pressure to the upper piston that is directly transmitted to the lower piston. If connected to a liquid chromatograph, or column packing manifold, liquid will flow out of the left hand side nonreturn valve as shown in the diagram which continues until the maximum movement of the piston is reached. At the extreme of movement a micro switch is activated and the air pressure transferred from port (1) to port (2). The piston now moves in the opposite direction and draws mobile phase through the right hand non-return valve refilling the cylinder with solvent. Again on reaching the limit of the piston movement a second micro switches the air supply from port (2) back to port (1) and the process repeated. The refilling process is extremely fast and, if an appropriate pulse dampener is used, the outlet pressure remains reasonably constant during the refill cycle. 21
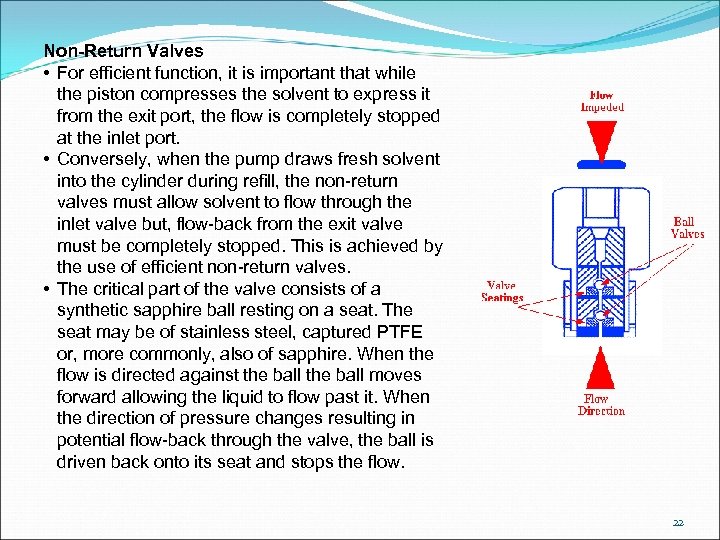
Non-Return Valves • For efficient function, it is important that while the piston compresses the solvent to express it from the exit port, the flow is completely stopped at the inlet port. • Conversely, when the pump draws fresh solvent into the cylinder during refill, the non-return valves must allow solvent to flow through the inlet valve but, flow-back from the exit valve must be completely stopped. This is achieved by the use of efficient non-return valves. • The critical part of the valve consists of a synthetic sapphire ball resting on a seat. The seat may be of stainless steel, captured PTFE or, more commonly, also of sapphire. When the flow is directed against the ball moves forward allowing the liquid to flow past it. When the direction of pressure changes resulting in potential flow-back through the valve, the ball is driven back onto its seat and stops the flow. 22
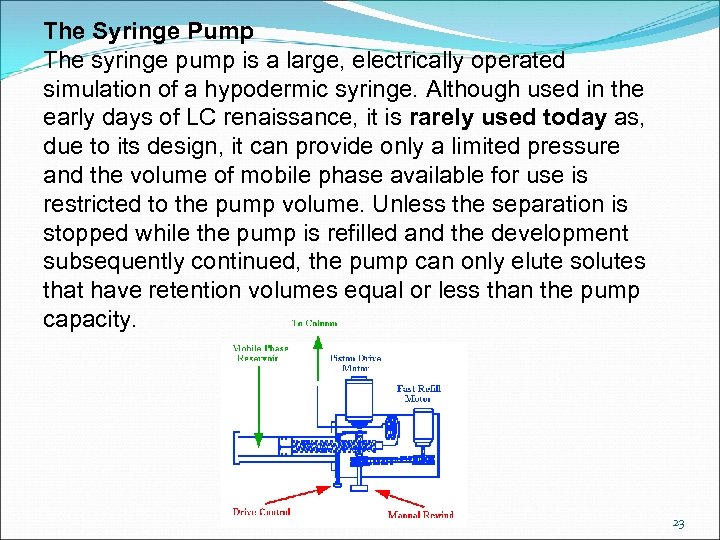
The Syringe Pump The syringe pump is a large, electrically operated simulation of a hypodermic syringe. Although used in the early days of LC renaissance, it is rarely used today as, due to its design, it can provide only a limited pressure and the volume of mobile phase available for use is restricted to the pump volume. Unless the separation is stopped while the pump is refilled and the development subsequently continued, the pump can only elute solutes that have retention volumes equal or less than the pump capacity. 23
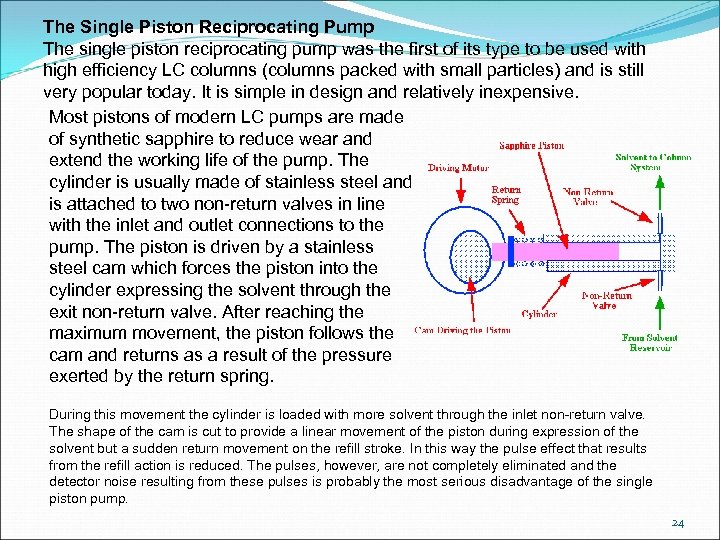
The Single Piston Reciprocating Pump The single piston reciprocating pump was the first of its type to be used with high efficiency LC columns (columns packed with small particles) and is still very popular today. It is simple in design and relatively inexpensive. Most pistons of modern LC pumps are made of synthetic sapphire to reduce wear and extend the working life of the pump. The cylinder is usually made of stainless steel and is attached to two non-return valves in line with the inlet and outlet connections to the pump. The piston is driven by a stainless steel cam which forces the piston into the cylinder expressing the solvent through the exit non-return valve. After reaching the maximum movement, the piston follows the cam and returns as a result of the pressure exerted by the return spring. During this movement the cylinder is loaded with more solvent through the inlet non-return valve. The shape of the cam is cut to provide a linear movement of the piston during expression of the solvent but a sudden return movement on the refill stroke. In this way the pulse effect that results from the refill action is reduced. The pulses, however, are not completely eliminated and the detector noise resulting from these pulses is probably the most serious disadvantage of the single piston pump. 24
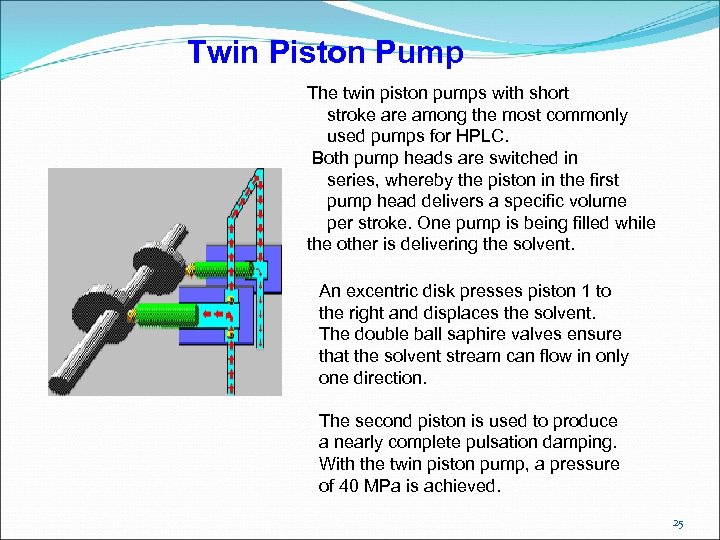
Twin Piston Pump The twin piston pumps with short stroke are among the most commonly used pumps for HPLC. Both pump heads are switched in series, whereby the piston in the first pump head delivers a specific volume per stroke. One pump is being filled while the other is delivering the solvent. An excentric disk presses piston 1 to the right and displaces the solvent. The double ball saphire valves ensure that the solvent stream can flow in only one direction. The second piston is used to produce a nearly complete pulsation damping. With the twin piston pump, a pressure of 40 MPa is achieved. 25
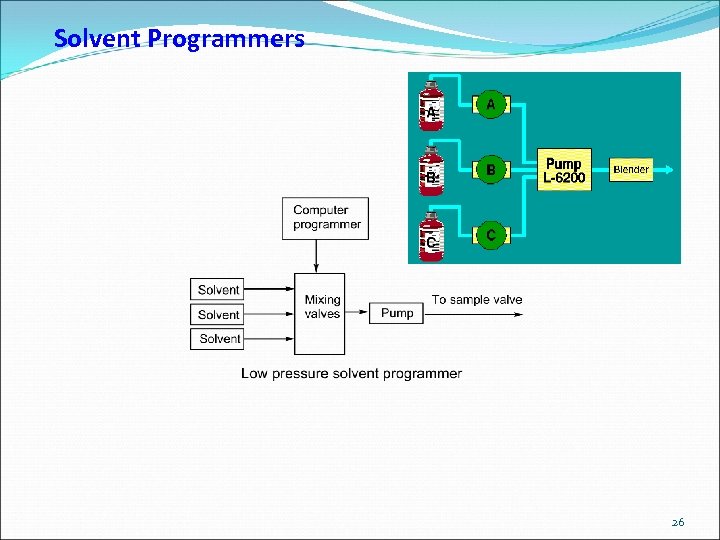
Solvent Programmers 26
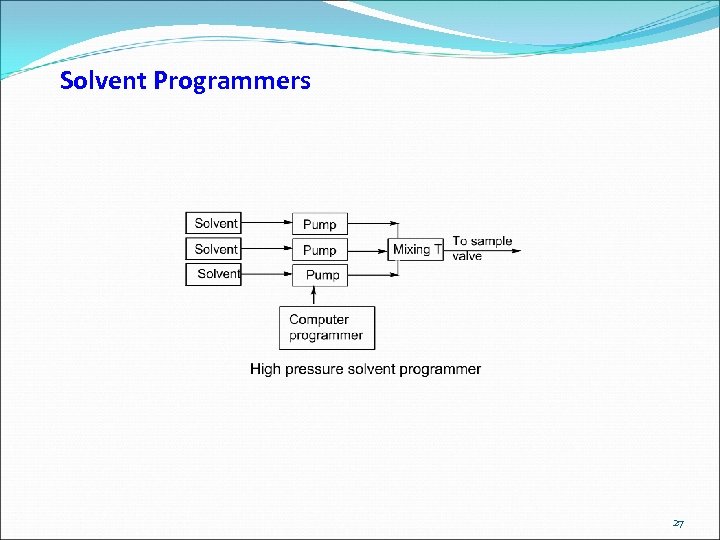
Solvent Programmers 27
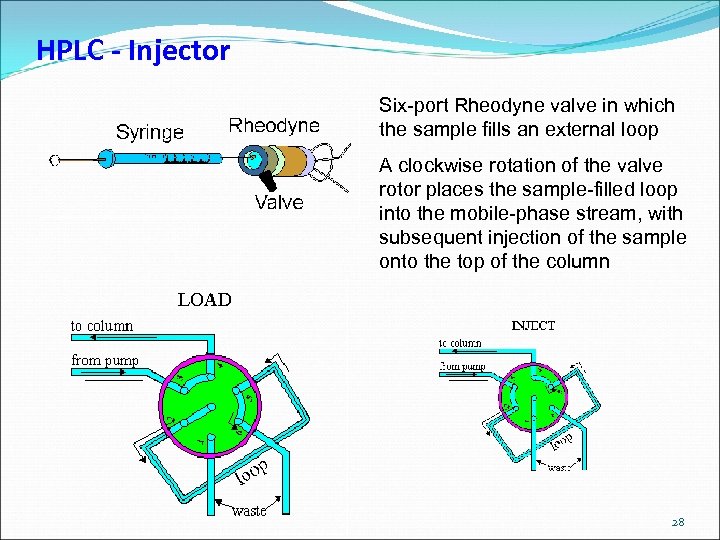
HPLC - Injector Six-port Rheodyne valve in which the sample fills an external loop A clockwise rotation of the valve rotor places the sample-filled loop into the mobile-phase stream, with subsequent injection of the sample onto the top of the column 28
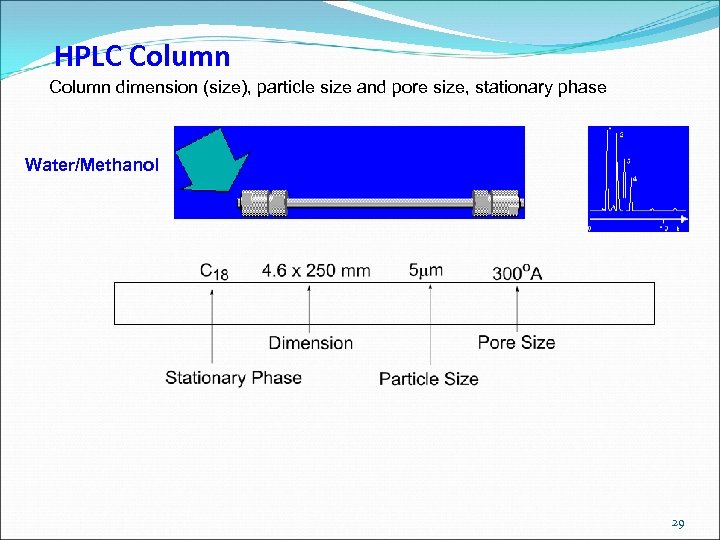
HPLC Column dimension (size), particle size and pore size, stationary phase Water/Methanol 29
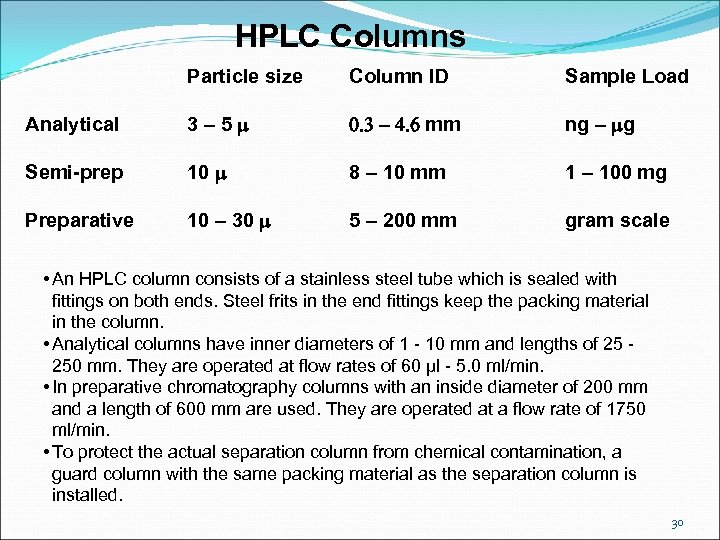
HPLC Columns Particle size Column ID Sample Load Analytical 3 – 5 m 0. 3 - 4. 6 mm ng – mg Semi-prep 10 m 8 – 10 mm 1 – 100 mg Preparative 10 – 30 m 5 – 200 mm gram scale • An HPLC column consists of a stainless steel tube which is sealed with fittings on both ends. Steel frits in the end fittings keep the packing material in the column. • Analytical columns have inner diameters of 1 - 10 mm and lengths of 25 - 250 mm. They are operated at flow rates of 60 µl - 5. 0 ml/min. • In preparative chromatography columns with an inside diameter of 200 mm and a length of 600 mm are used. They are operated at a flow rate of 1750 ml/min. • To protect the actual separation column from chemical contamination, a guard column with the same packing material as the separation column is installed. 30
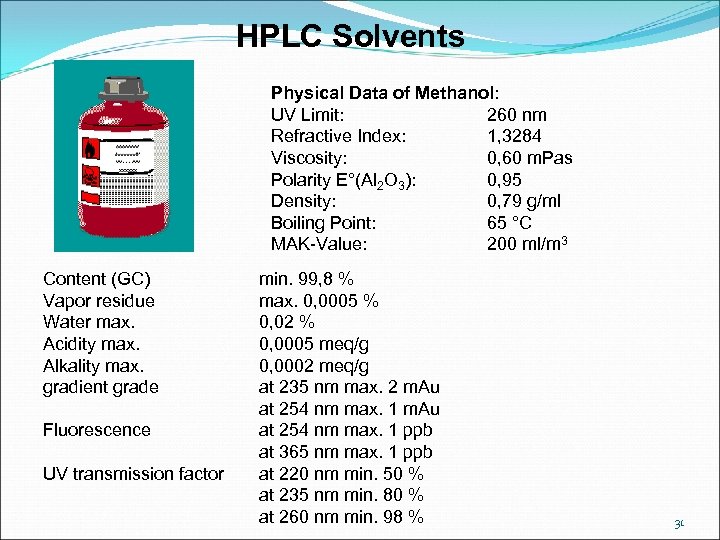
HPLC Solvents Physical Data of Methanol: UV Limit: 260 nm Refractive Index: 1, 3284 Viscosity: 0, 60 m. Pas Polarity E°(Al 2 O 3): 0, 95 Density: 0, 79 g/ml Boiling Point: 65 °C MAK-Value: 200 ml/m 3 Content (GC) Vapor residue Water max. Acidity max. Alkality max. gradient grade min. 99, 8 % max. 0, 0005 % 0, 02 % 0, 0005 meq/g 0, 0002 meq/g at 235 nm max. 2 m. Au at 254 nm max. 1 m. Au Fluorescence at 254 nm max. 1 ppb at 365 nm max. 1 ppb UV transmission factor at 220 nm min. 50 % at 235 nm min. 80 % at 260 nm min. 98 % 31
HPLC Solvents UV transmission and refraction index are important characteristics for the detection of the substances. When using a UV detector, the mobile phase in the absorption range of the sample molecules must be as transparent as possible to UV light. The UV limit specifies the wavelength minimum which can be used for detection with any particular solvent. Methanol, for example, can be used as a mobile phase component only with wavelength 260 nm or higher when detecting compounds. When using refractive detectors (RI detectors) the difference between the refractive indices from the sample substance and mobile phase should be as large as possible. Viscosity is a physical measurement that qualifies the flow characteristic of a solvent. It is measured in m. Pas = millipascal seconds. The viscosity influences the column backpressure, i. e. the column backpressure increases with increasing viscosity of the eluent. A low viscosity mobile phase always improves the separation capacity of a column compared with a high viscosity mobile phase, because the mass transfer processes occur faster and thus theoretical plate number of the column increases. 32

HPLC Solvents The Eluotropic Series Table shows that solvents with low boiling points usually also have low viscosity. In addition to the solvent bioling point, the vapor pressure also plays an important role. A low boiling point is always coupled with high vapor pressure. This behavior can lead to gas bubble formation when the solvent is as pirated into the pump during use. This results in pulsations, base line fluctuations, peak broadening, shoulder formation and pump shutdown. The solvent(s) should be inert compared to the sample material, i. e. it should not react with the sample mixture. When selecting solvents the aspects Operating Safety and Environmental Protection must also be considered. Many solvents are combustible, easily flammable and often toxic. Therefore, environmentally safe disposal at the end of the LC equipment is especially important. 33
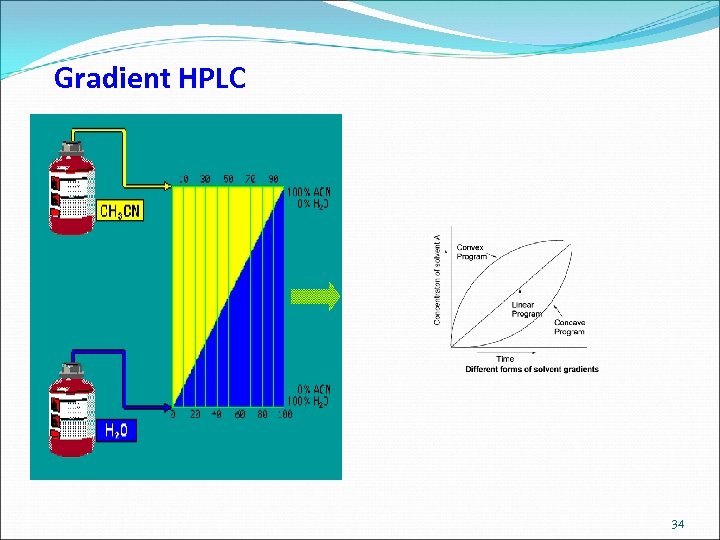
Gradient HPLC 34

HPLC Detectors 35
Detectors • Ultraviolet (UV) Fixed wavelength detector Variable wavelength detector Diode Array • Fluorescence • Electrical Conductivity • Refractive Index • Electrochemical • Light scattering 36
UV Detectors • The UV detector is by far the most popular and useful LC detector that is available to the analyst at this time. • This is particularly true if multi-wavelength technology is included in this class of detectors. • Although the UV detector has some definite limitations (particularly for the detection of non polar solutes that do not possess a UV chromaphores) it has the best combination of sensitivity, linearity, versatility and reliability of all the LC detectors so far developed. • Most compounds absorb UV light in the range of 200 -350Å including all substances having one or more double bonds (p electrons) and all substances that have unshared (non bonded) electrons; e. g. all olefins, all aromatics and all substances containing >CO, >CS, -N=O and groups. • The relationship between the intensity of UV light transmitted through the detector cell and solute concentration is given by "Beers' Law IT = I 0 e-klc where, (Io) is the intensity of the light entering the cell; (IT) is the intensity of the transmitted light; (L) is the path length of the cell; (c) is the concentration of the solute; (k) is the molar extinction coefficient of the solute for the specific wavelength of the UV light. 37
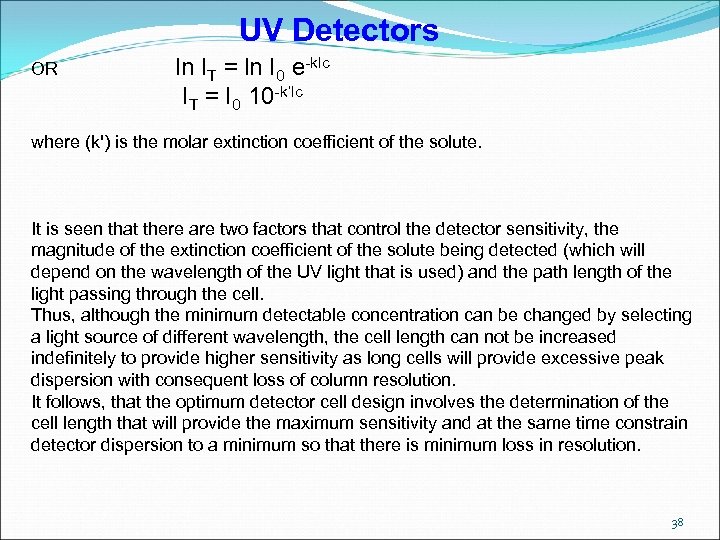
UV Detectors OR ln IT = ln I 0 e-klc IT = I 0 10 -k’lc where (k') is the molar extinction coefficient of the solute. It is seen that there are two factors that control the detector sensitivity, the magnitude of the extinction coefficient of the solute being detected (which will depend on the wavelength of the UV light that is used) and the path length of the light passing through the cell. Thus, although the minimum detectable concentration can be changed by selecting a light source of different wavelength, the cell length can not be increased indefinitely to provide higher sensitivity as long cells will provide excessive peak dispersion with consequent loss of column resolution. It follows, that the optimum detector cell design involves the determination of the cell length that will provide the maximum sensitivity and at the same time constrain detector dispersion to a minimum so that there is minimum loss in resolution. 38
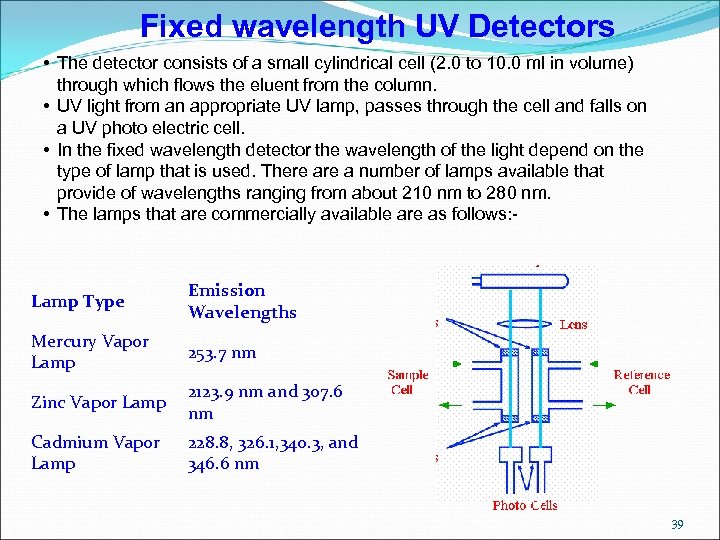
Fixed wavelength UV Detectors • The detector consists of a small cylindrical cell (2. 0 to 10. 0 ml in volume) through which flows the eluent from the column. • UV light from an appropriate UV lamp, passes through the cell and falls on a UV photo electric cell. • In the fixed wavelength detector the wavelength of the light depend on the type of lamp that is used. There a number of lamps available that provide of wavelengths ranging from about 210 nm to 280 nm. • The lamps that are commercially available are as follows: - Lamp Type Emission Wavelengths Mercury Vapor Lamp 253. 7 nm Zinc Vapor Lamp 2123. 9 nm and 307. 6 nm Cadmium Vapor Lamp 228. 8, 326. 1, 340. 3, and 346. 6 nm 39
Mercury Vapor Lamp § The mercury vapor lamp is the most popular as it has an emission wavelength that allows the detector to sense a wide range of solute types. § The detector usually contains both a sample and reference cell and the output from the reference cell is compared to that from the sample cell. The difference is fed to a non linear amplifier that converts the signal to one that is linearly related to concentration of solute in the sample cell. § The fixed wavelength detector is the least expensive and, as the majority of the light is emitted at a specific wavelength(s) it has a high intensity, and thus, a higher intrinsic sensitivity than the multi-wavelength UV detectors. § However, the multi-wavelength detector can often compensate for the lower sensitivity by choosing a wavelength that has the highest extinction coefficient for the solutes of interest. Average specifications for commercially available fixed wavelength UV detectors are as follows: Sensitivity (toluene) 5 x 10 -8 g/ml Linear Dynamic Range 5 x 10 -8 to 5 x 10 -4 g/ml Response Index 0. 98 - 1. 02 40
Multi-Wavelength UV Detector • Multi-Wavelength UV detectors utilize a single (perhaps more accurately a narrow range) of wavelengths to detect the solute. Most multi wavelength UV detectors can also provide a UV spectrum of the eluted solute if appropriately arranged. • There are two types of multi-wavelength detectors • dispersion detector that monitors the eluent at one wavelength only and the • diode array detector that monitors the eluted solute over a range of wavelengths simultaneously. • The former passes the light from a broad emission light source through a monochromator, selects a specific wavelength and allows it to pass through the detecting cell. The second, also uses a broad emission light source, but all the light is allowed to pass through the sensing cell and subsequently the light is dispersed by means of a holographic grating and the dispersed light allowed to fall on an array of diodes. 41
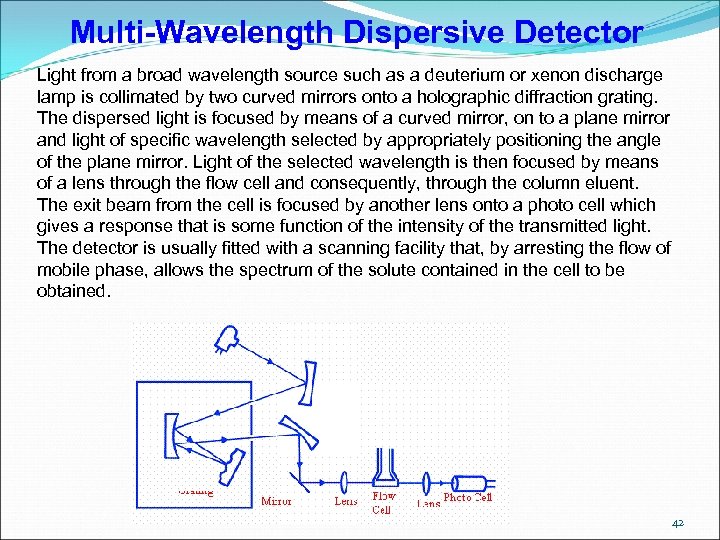
Multi-Wavelength Dispersive Detector Light from a broad wavelength source such as a deuterium or xenon discharge lamp is collimated by two curved mirrors onto a holographic diffraction grating. The dispersed light is focused by means of a curved mirror, on to a plane mirror and light of specific wavelength selected by appropriately positioning the angle of the plane mirror. Light of the selected wavelength is then focused by means of a lens through the flow cell and consequently, through the column eluent. The exit beam from the cell is focused by another lens onto a photo cell which gives a response that is some function of the intensity of the transmitted light. The detector is usually fitted with a scanning facility that, by arresting the flow of mobile phase, allows the spectrum of the solute contained in the cell to be obtained. 42
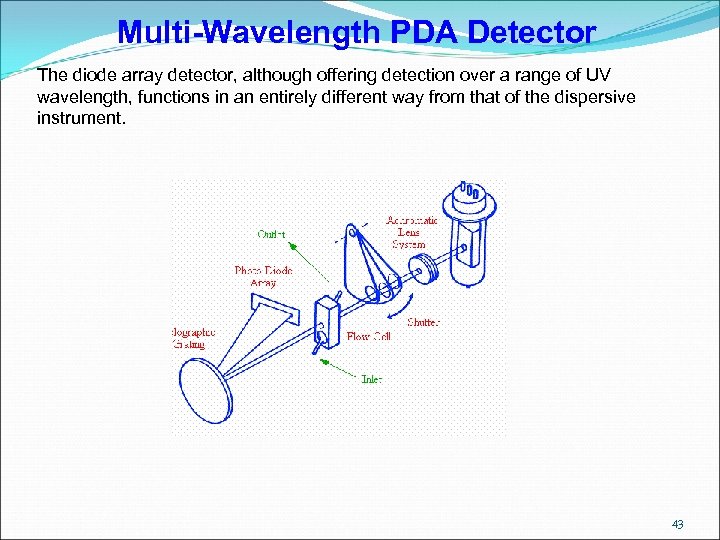
Multi-Wavelength PDA Detector The diode array detector, although offering detection over a range of UV wavelength, functions in an entirely different way from that of the dispersive instrument. 43
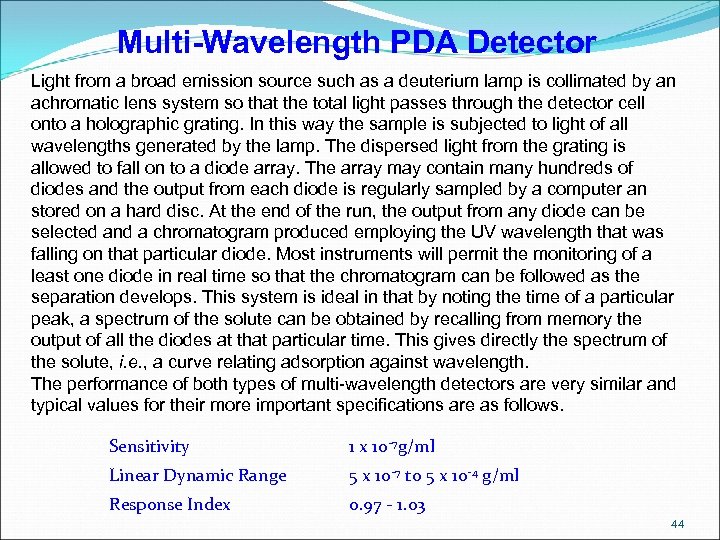
Multi-Wavelength PDA Detector Light from a broad emission source such as a deuterium lamp is collimated by an achromatic lens system so that the total light passes through the detector cell onto a holographic grating. In this way the sample is subjected to light of all wavelengths generated by the lamp. The dispersed light from the grating is allowed to fall on to a diode array. The array may contain many hundreds of diodes and the output from each diode is regularly sampled by a computer an stored on a hard disc. At the end of the run, the output from any diode can be selected and a chromatogram produced employing the UV wavelength that was falling on that particular diode. Most instruments will permit the monitoring of a least one diode in real time so that the chromatogram can be followed as the separation develops. This system is ideal in that by noting the time of a particular peak, a spectrum of the solute can be obtained by recalling from memory the output of all the diodes at that particular time. This gives directly the spectrum of the solute, i. e. , a curve relating adsorption against wavelength. The performance of both types of multi-wavelength detectors are very similar and typical values for their more important specifications are as follows. Sensitivity 1 x 10 -7 g/ml Linear Dynamic Range 5 x 10 -7 to 5 x 10 -4 g/ml Response Index 0. 97 - 1. 03 44
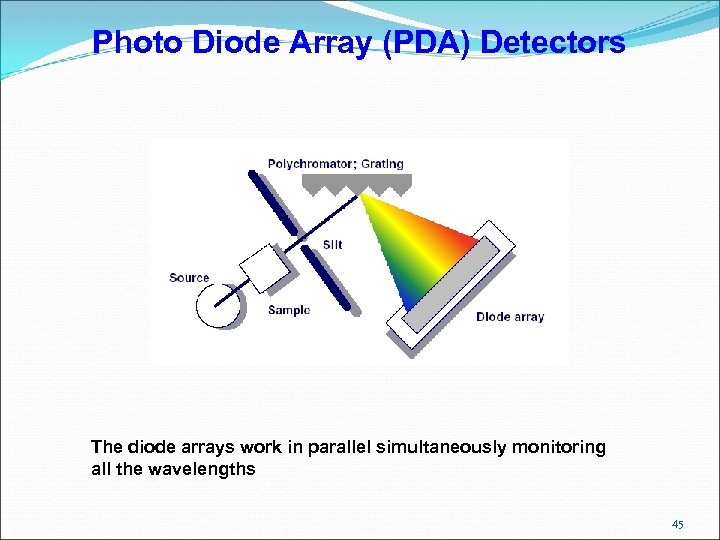
Photo Diode Array (PDA) Detectors The diode arrays work in parallel simultaneously monitoring all the wavelengths 45
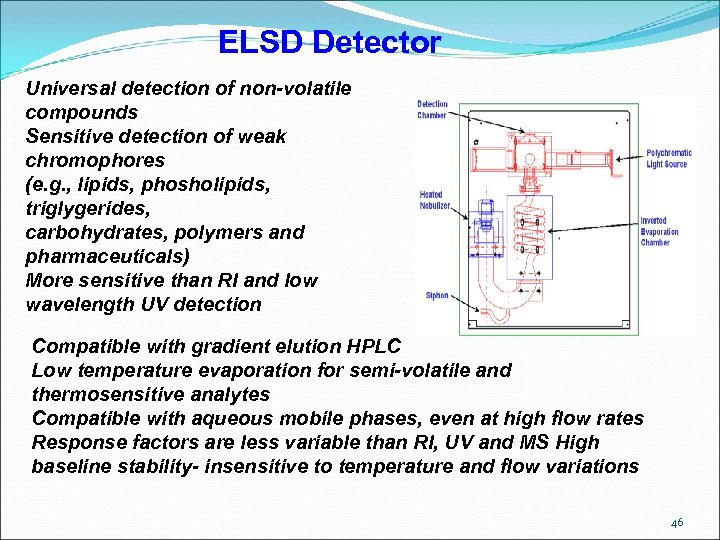
ELSD Detector Universal detection of non-volatile compounds Sensitive detection of weak chromophores (e. g. , lipids, phosholipids, triglygerides, carbohydrates, polymers and pharmaceuticals) More sensitive than RI and low wavelength UV detection Compatible with gradient elution HPLC Low temperature evaporation for semi-volatile and thermosensitive analytes Compatible with aqueous mobile phases, even at high flow rates Response factors are less variable than RI, UV and MS High baseline stability- insensitive to temperature and flow variations 46
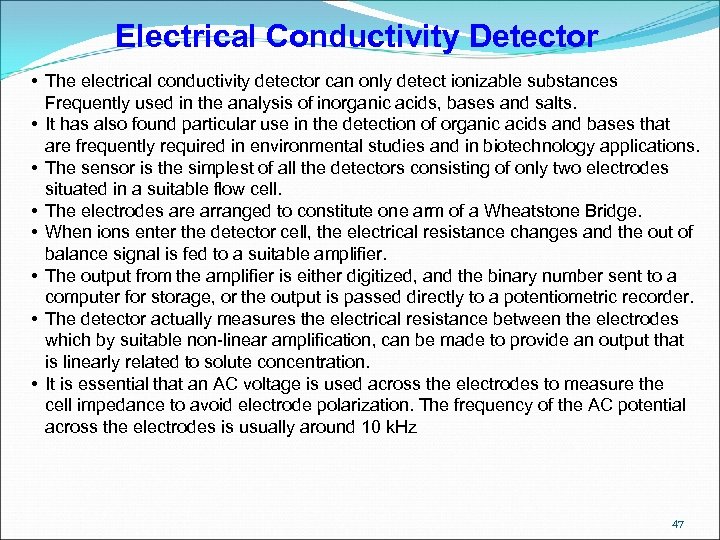
Electrical Conductivity Detector • The electrical conductivity detector can only detect ionizable substances Frequently used in the analysis of inorganic acids, bases and salts. • It has also found particular use in the detection of organic acids and bases that are frequently required in environmental studies and in biotechnology applications. • The sensor is the simplest of all the detectors consisting of only two electrodes situated in a suitable flow cell. • The electrodes are arranged to constitute one arm of a Wheatstone Bridge. • When ions enter the detector cell, the electrical resistance changes and the out of balance signal is fed to a suitable amplifier. • The output from the amplifier is either digitized, and the binary number sent to a computer for storage, or the output is passed directly to a potentiometric recorder. • The detector actually measures the electrical resistance between the electrodes which by suitable non-linear amplification, can be made to provide an output that is linearly related to solute concentration. • It is essential that an AC voltage is used across the electrodes to measure the cell impedance to avoid electrode polarization. The frequency of the AC potential across the electrodes is usually around 10 k. Hz 47
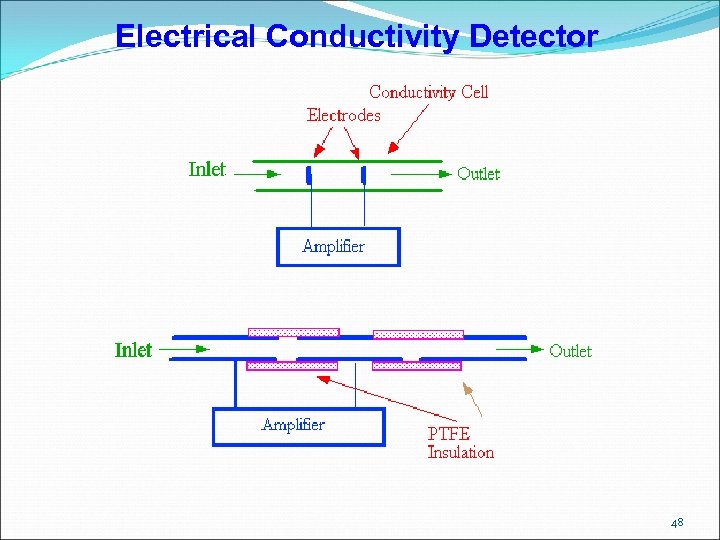
Electrical Conductivity Detector 48
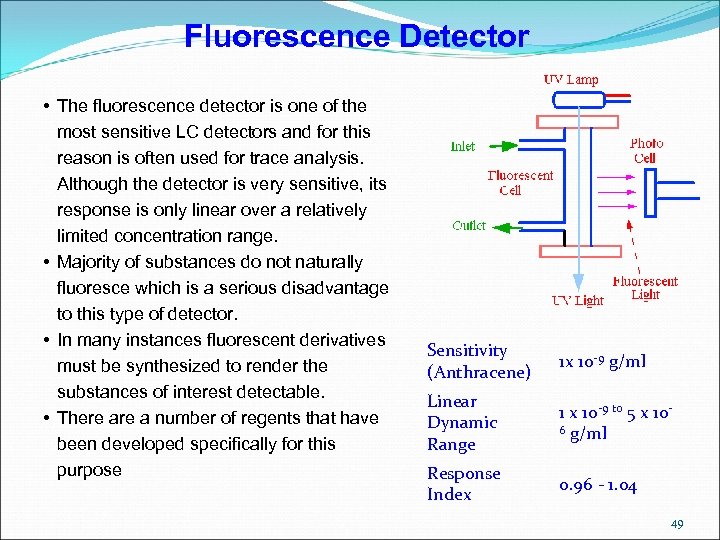
Fluorescence Detector • The fluorescence detector is one of the most sensitive LC detectors and for this reason is often used for trace analysis. Although the detector is very sensitive, its response is only linear over a relatively limited concentration range. • Majority of substances do not naturally fluoresce which is a serious disadvantage to this type of detector. • In many instances fluorescent derivatives must be synthesized to render the substances of interest detectable. • There a number of regents that have been developed specifically for this purpose Sensitivity (Anthracene) 1 x 10 -9 g/ml Linear Dynamic Range 1 x 10 -9 to 5 x 106 g/ml Response Index 0. 96 - 1. 04 49
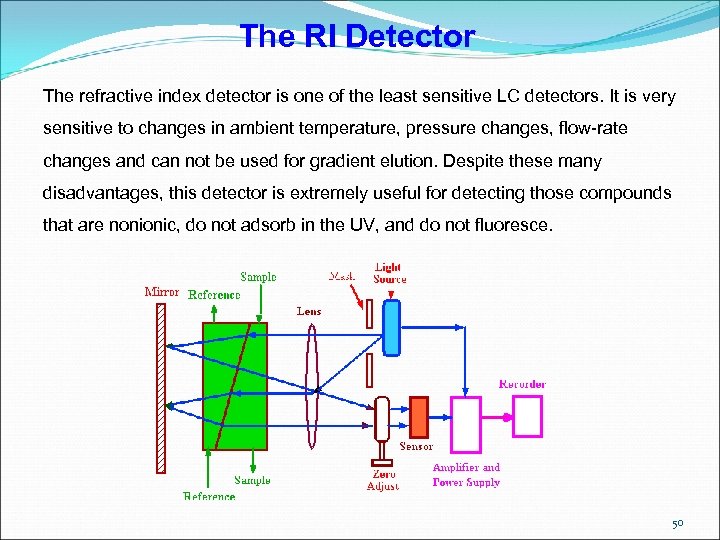
The RI Detector The refractive index detector is one of the least sensitive LC detectors. It is very sensitive to changes in ambient temperature, pressure changes, flow-rate changes and can not be used for gradient elution. Despite these many disadvantages, this detector is extremely useful for detecting those compounds that are nonionic, do not adsorb in the UV, and do not fluoresce. 50
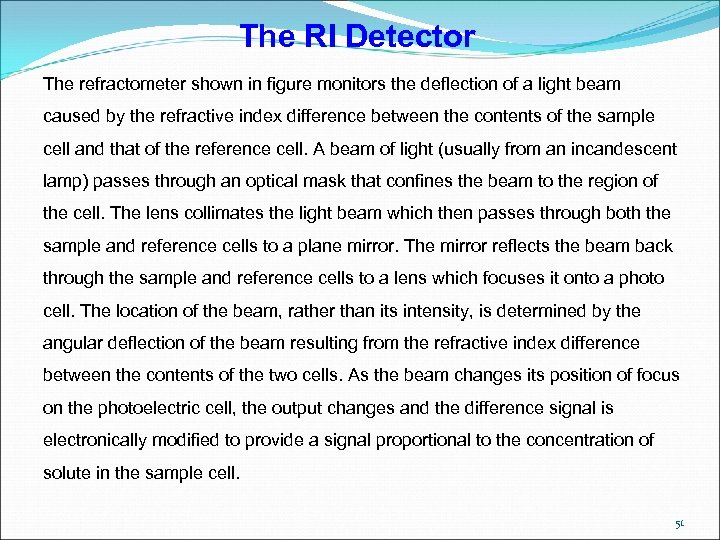
The RI Detector The refractometer shown in figure monitors the deflection of a light beam caused by the refractive index difference between the contents of the sample cell and that of the reference cell. A beam of light (usually from an incandescent lamp) passes through an optical mask that confines the beam to the region of the cell. The lens collimates the light beam which then passes through both the sample and reference cells to a plane mirror. The mirror reflects the beam back through the sample and reference cells to a lens which focuses it onto a photo cell. The location of the beam, rather than its intensity, is determined by the angular deflection of the beam resulting from the refractive index difference between the contents of the two cells. As the beam changes its position of focus on the photoelectric cell, the output changes and the difference signal is electronically modified to provide a signal proportional to the concentration of solute in the sample cell. 51
The RI Detector The refractive index detector is often a 'choice of last resort' and is selected for those applications where, for one reason or another, all other detectors are inappropriate or impractical. However, the detector has one particular and unique area of application and that is in the separation and analysis of polymers. For those polymers that contain more than ten monomer units, the refractive index is directly proportional to the concentration of the polymer and is practically independent of the molecular weight. A quantitative analysis of a polymer mixture can, therefore, be obtained by the simple normalization of the peak areas in the chromatogram (there being no need for the use of individual response factors). Some typical specifications for the refractive index detector are as follows: Sensitivity (benzene) 1 x 10 -6 g/ml Linear Dynamic Range 1 x 10 -6 to 1 x 10 -4 g/ml Response Index 0. 97 - 1. 03 A typical application of the RI detector is for carbohydrate analysis. Carbohydrates do not adsorb in the UV, do not ionize and although fluorescent derivatives can be made, the procedure is tedious and time consuming. 52
• • • Sample Preparation Clean-up • to get homogenous sample free from interfering particles • A sample that will not damage the column Concentration - To concentrate or dilute the analyte Solvent transfer - Miscible with mobile phase Fractionation of polarity classes Derivatization – to get the analyte suitable for detection (UV, fluorescence etc) • To change polarity of the sample Sample preparation should Give quantitative recovery Involve minimum number of steps 53
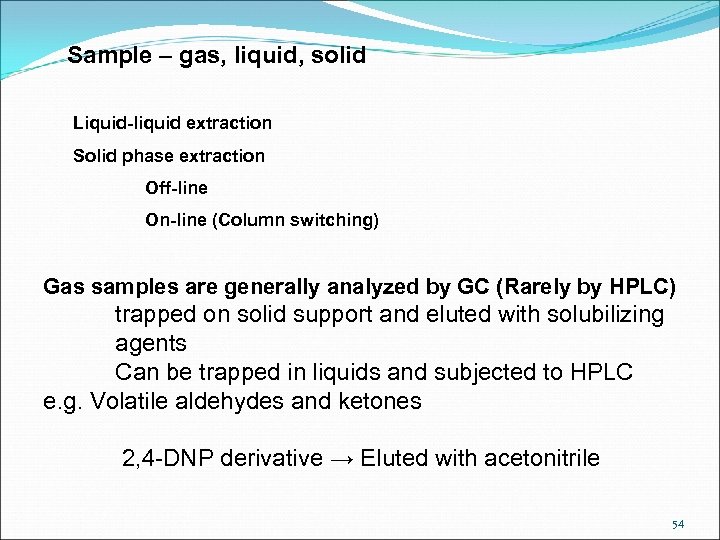
Sample – gas, liquid, solid Liquid-liquid extraction Solid phase extraction Off-line On-line (Column switching) Gas samples are generally analyzed by GC (Rarely by HPLC) trapped on solid support and eluted with solubilizing agents Can be trapped in liquids and subjected to HPLC e. g. Volatile aldehydes and ketones 2, 4 -DNP derivative → Eluted with acetonitrile 54
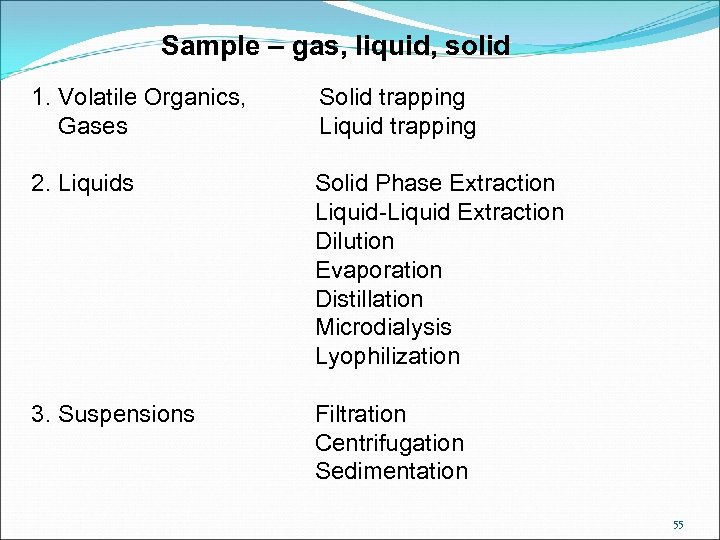
Sample – gas, liquid, solid 1. Volatile Organics, Gases Solid trapping Liquid trapping 2. Liquids Solid Phase Extraction Liquid-Liquid Extraction Dilution Evaporation Distillation Microdialysis Lyophilization 3. Suspensions Filtration Centrifugation Sedimentation 55
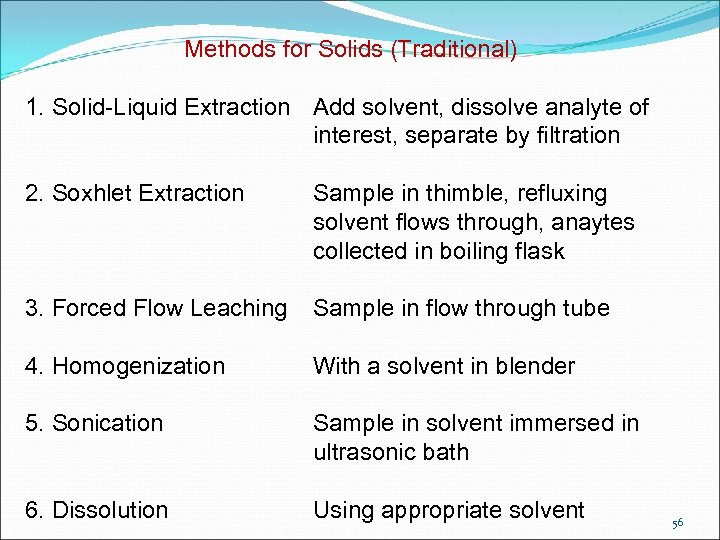
Methods for Solids (Traditional) 1. Solid-Liquid Extraction Add solvent, dissolve analyte of interest, separate by filtration 2. Soxhlet Extraction Sample in thimble, refluxing solvent flows through, anaytes collected in boiling flask 3. Forced Flow Leaching Sample in flow through tube 4. Homogenization With a solvent in blender 5. Sonication Sample in solvent immersed in ultrasonic bath 6. Dissolution Using appropriate solvent 56
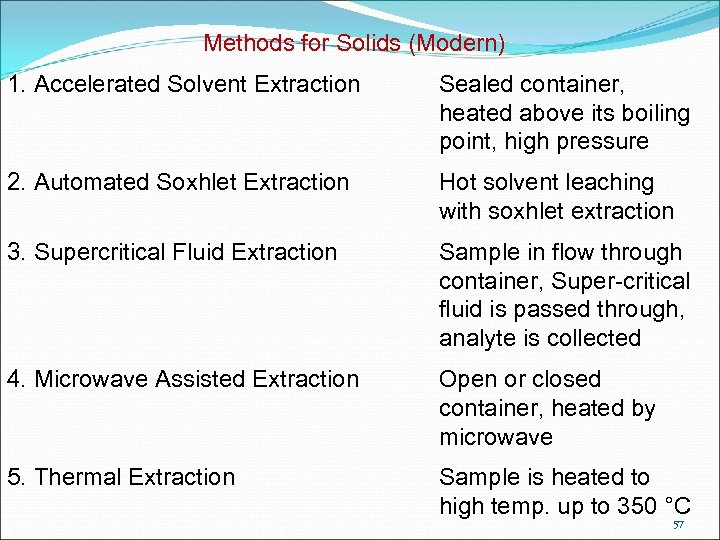
Methods for Solids (Modern) 1. Accelerated Solvent Extraction Sealed container, heated above its boiling point, high pressure 2. Automated Soxhlet Extraction Hot solvent leaching with soxhlet extraction 3. Supercritical Fluid Extraction Sample in flow through container, Super-critical fluid is passed through, analyte is collected 4. Microwave Assisted Extraction Open or closed container, heated by microwave 5. Thermal Extraction Sample is heated to high temp. up to 350 °C 57
Preliminary Processing of Solid and Semi-Solid samples 1. Reducing sample size Finely divided samples are more homogenous Dissolve faster Easier to extract due to larger surface area Several Methods: Blending, Chopping, Crushing, Cutting, Grinding, Macerating, Milling etc. For thermally labile or volatile components – Minimize heating Elastic samples should be cooled to make them brittle 2. Drying the sample Drying necessary to get constant weight for reliable analysis 58
Preliminary Processing of Liquid Samples Filtration Paper or Cellulose Removal of larger particles (> 40 µm) Membrane Filters (Nylon, Polypropylene) Removal of smaller particles (<10 µm) Functionalized Membranes (Ion exchange/Affinity) Removal of particulate matrix SPE cartridges (Silica) -do- SPE Disks (PTFE, PVE) -do- Filters range 0. 25 to 2 m porosity most common, Disposable filters can be attached to syringe. 59
Sample Pre-treatment for Liquid Samples Most common methods are: 1. Liquid-Liquid Extraction 2. Liquid-Solid Extraction LLE – Partitioning between two immiscible liquid (aqueous and organic) Organic solvent should have: 1. Low solubility in water (<10%) 2. Volatile (For easy recovery) 3. Polarity and H-bonding so as to enhance recovery in organic phase 4. Highly pure to minimize sample contamination 60
Problems encountered in LLE 1. Emulsion formation: No sharp boundary, recovery affected Ø Ø Ø Remedy Add salt to aqueous phase Heating or cooling Filtering though glass wool Add different solvent Centrifuge 2. Analyte strongly sorbed to particulates – Wash 3. Analytes bound to large molecules – Detergent, organic solvent, water 4. Mutual solubility of two phases 61
Solid Phase Extraction (SPE) Most important technique for sample pre-treatment before HPLC (Like chromatography) Advantages Ø Complete extraction Ø Efficient separation of interferences Ø Reduced solvent Ø Removal of particulate Ø Easy collection Disadvantages Ø Variability of cartridges Ø Irreversible adsorption 62
Cartridges Disks Coated Fibers Sample – Liquid Solvent – K = 0 or K >> 1 (Either unretained or strongly retained) When K >> 1, cartridge is washed to remove interferences When K = 0, interferences are strongly retained Goals of Solid Phase Extraction Clean up: Removal of interferences and column killers (Fats, oils, grease) Concentration of analyte: e. g. ppb pesticides from water Solvent transfer: Of aqueous solution to unpolar phase Of unpolar solution to aqueous phase Fractionating of polarity Classes Desalting Sample storage and transport 63
SPE Method Development – 4 Steps 1. Conditioning e. g. using RP-SPE, analyte to be retained Cartridge is conditioned by passing few bed volumes of solvent (e. g. Methanol or Acetonitrile for RP) Removes impurities Sorbent solvation (Dried RP packings show less retention) 2. Sample Application Remove excess conditioning solvent Do not allow cartridge to dry out Sample in weak solvent added to cartridge (For RP-SPE, water or buffer or 10% organic) Size: Match to volume of sample (0. 5 to 10 m. L for 35 mg to 2 g) Flow Rate: 2 to 4 m. L/min 64
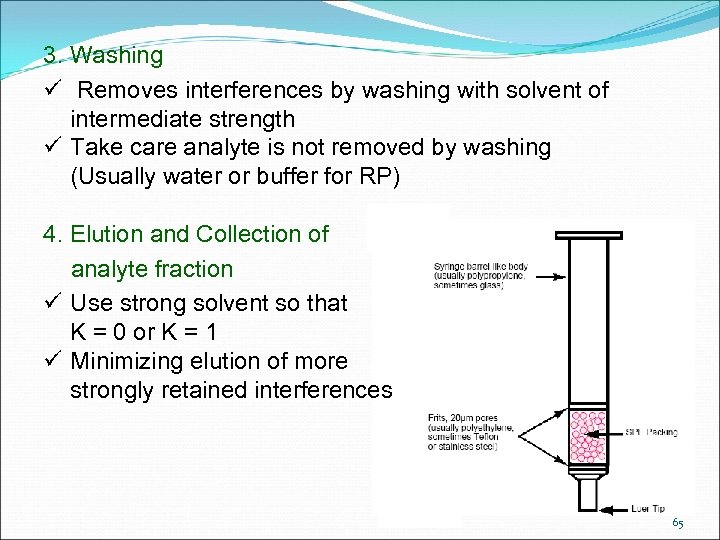
3. Washing Removes interferences by washing with solvent of intermediate strength Take care analyte is not removed by washing (Usually water or buffer for RP) 4. Elution and Collection of analyte fraction Use strong solvent so that K = 0 or K = 1 Minimizing elution of more strongly retained interferences 65
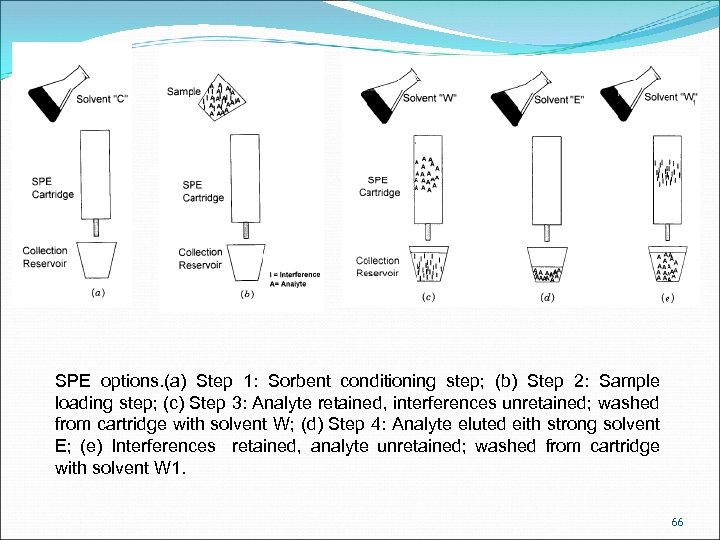
SPE options. (a) Step 1: Sorbent conditioning step; (b) Step 2: Sample loading step; (c) Step 3: Analyte retained, interferences unretained; washed from cartridge with solvent W; (d) Step 4: Analyte eluted eith strong solvent E; (e) Interferences retained, analyte unretained; washed from cartridge with solvent W 1. 66
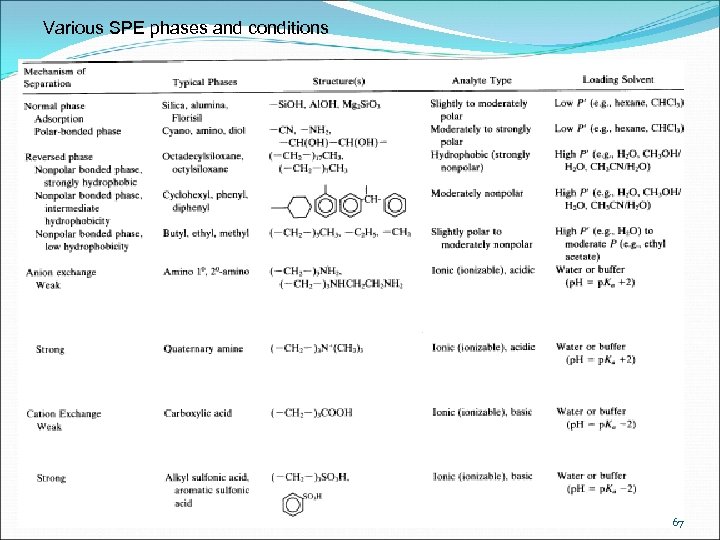
Various SPE phases and conditions 67
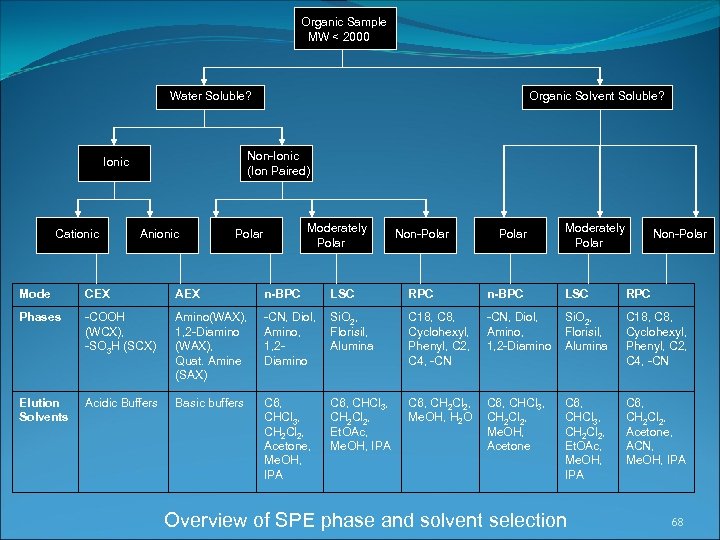
Organic Sample MW < 2000 Water Soluble? Non-Ionic (Ion Paired) Ionic Cationic Organic Solvent Soluble? Anionic Moderately Polar Mode CEX AEX n-BPC Phases -COOH (WCX), -SO 3 H (SCX) Amino(WAX), 1, 2 -Diamino (WAX), Quat. Amine (SAX) Elution Solvents Acidic Buffers Basic buffers LSC Non-Polar Moderately Polar Non-Polar RPC n-BPC LSC RPC -CN, Diol, Si. O 2, Amino, Florisil, 1, 2 Alumina Diamino C 18, C 8, Cyclohexyl, Phenyl, C 2, C 4, -CN, Diol, Amino, 1, 2 -Diamino Si. O 2, Florisil, Alumina C 18, C 8, Cyclohexyl, Phenyl, C 2, C 4, -CN C 6, CHCl 3, CH 2 Cl 2, Acetone, Me. OH, IPA C 6, CH 2 Cl 2, Me. OH, H 2 O C 6, CHCl 3, CH 2 Cl 2, Me. OH, Acetone C 6, CHCl 3, CH 2 Cl 2, Et. OAc, Me. OH, IPA C 6, CH 2 Cl 2, Acetone, ACN, Me. OH, IPA C 6, CHCl 3, CH 2 Cl 2, Et. OAc, Me. OH, IPA Overview of SPE phase and solvent selection 68

Important things to know before SPE 1. Nature of Sample (Polarity, Solubility, Acidic or Basic nature) 2. Composition – Any functional groups 3. What is goal of SPE Removal of interferences Increased detection sensitivity Removal of column killers Ø Collect analyte in the smallest possible volume and obtain analyte fraction in a form that can be injected directly into HPLC. Ø If acid or base, solvent strength in washing and elution may be adjusted by p. H. 69
Advantages of SPE over LLE On-site enrichment of samples Simple and fast handling No emulsions Safe Low costs Reduced solvent consumption Flexible choice of mobile and stationery phases More complete extraction of analyte More efficient separation on interferences Easy collection of analyte fraction Removal of particulate 70
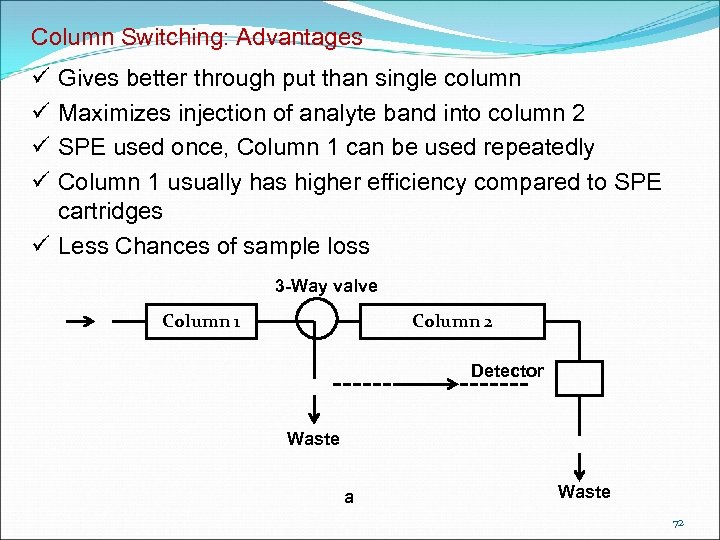
Column Switching (Multidimensional Column Chromatography or Coupled Column Chromatography) A portion of chromatogram from an initial column is passed selectively to second column for further separation Aims at: Removal of column killers Removal of Late eluters Removal of Interferences Trace Enrichment 71
Column Switching: Advantages Gives better through put than single column Maximizes injection of analyte band into column 2 SPE used once, Column 1 can be used repeatedly Column 1 usually has higher efficiency compared to SPE cartridges Less Chances of sample loss 3 -Way valve Column 1 Column 2 Detector Waste a Waste 72
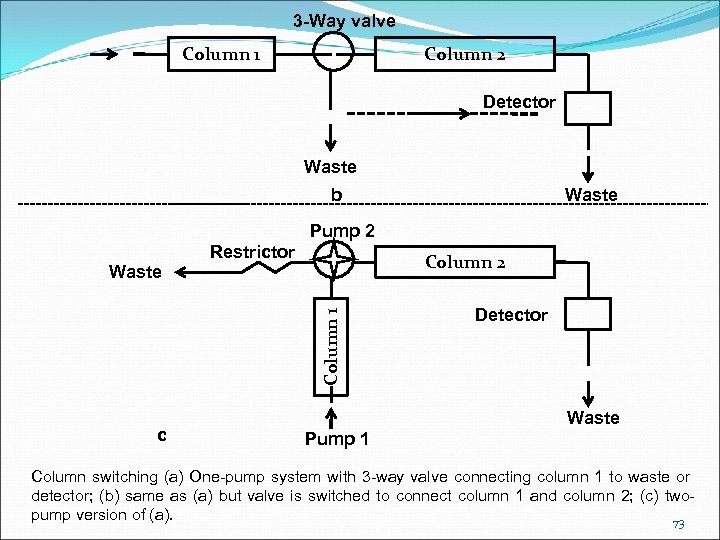
3 -Way valve Column 1 Column 2 Detector Waste b Waste Pump 2 Column 1 Waste Restrictor c Detector Waste Pump 1 Column switching (a) One-pump system with 3 -way valve connecting column 1 to waste or detector; (b) same as (a) but valve is switched to connect column 1 and column 2; (c) twopump version of (a). 73
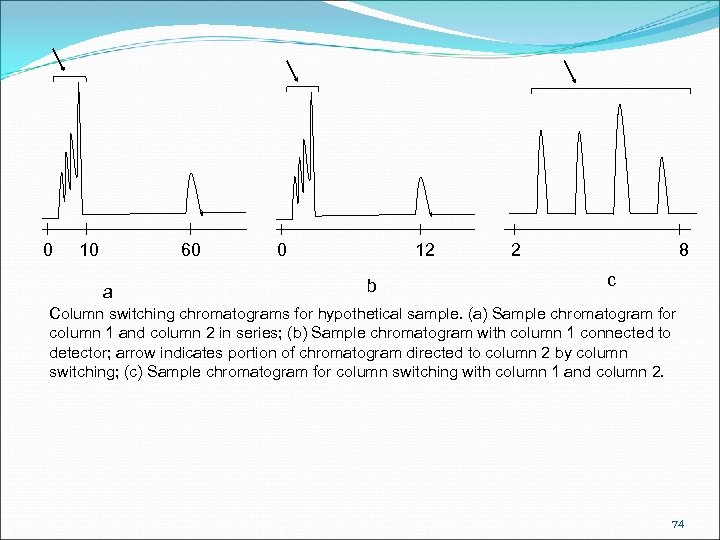
0 10 60 a 0 12 b 2 8 c Column switching chromatograms for hypothetical sample. (a) Sample chromatogram for column 1 and column 2 in series; (b) Sample chromatogram with column 1 connected to detector; arrow indicates portion of chromatogram directed to column 2 by column switching; (c) Sample chromatogram for column switching with column 1 and column 2. 74
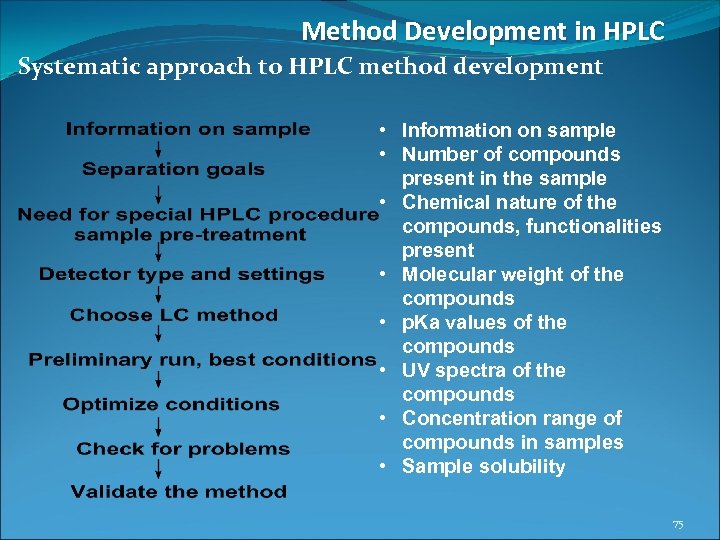
Method Development in HPLC Systematic approach to HPLC method development • Information on sample • Number of compounds present in the sample • Chemical nature of the compounds, functionalities present • Molecular weight of the compounds • p. Ka values of the compounds • UV spectra of the compounds • Concentration range of compounds in samples • Sample solubility 75
What are the separation goals • Is the primary goal qualitative analysis, quantitative analysis, isolation of pure compound • Is it necessary to resolve all compounds or a specific compound is of interest • For how many sample matrices the method has to be designed • Number of samples to be analyzed at a particular time Sample pretreatment • Solids, Liquids, Gases Detectors • What detector type 76
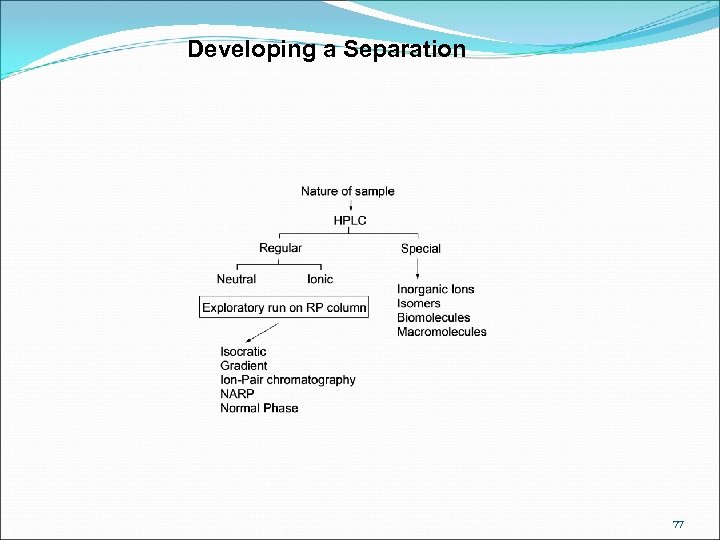
Developing a Separation 77
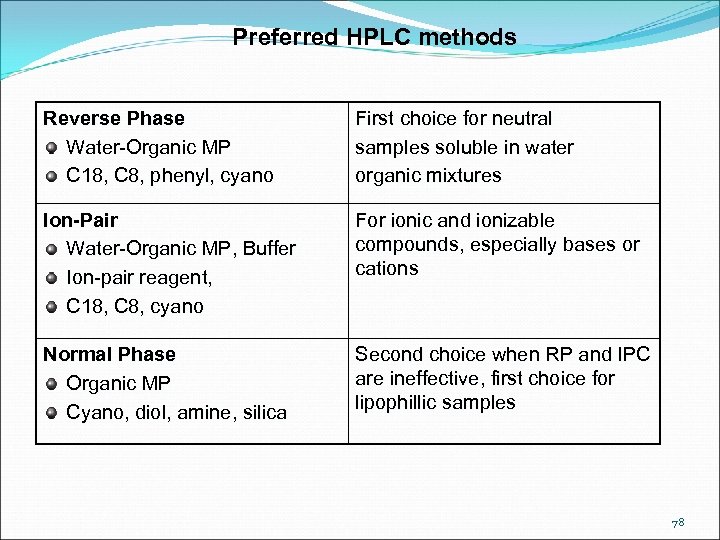
Preferred HPLC methods Reverse Phase Water-Organic MP C 18, C 8, phenyl, cyano First choice for neutral samples soluble in water organic mixtures Ion-Pair Water-Organic MP, Buffer Ion-pair reagent, C 18, C 8, cyano For ionic and ionizable compounds, especially bases or cations Normal Phase Organic MP Cyano, diol, amine, silica Second choice when RP and IPC are ineffective, first choice for lipophillic samples 78
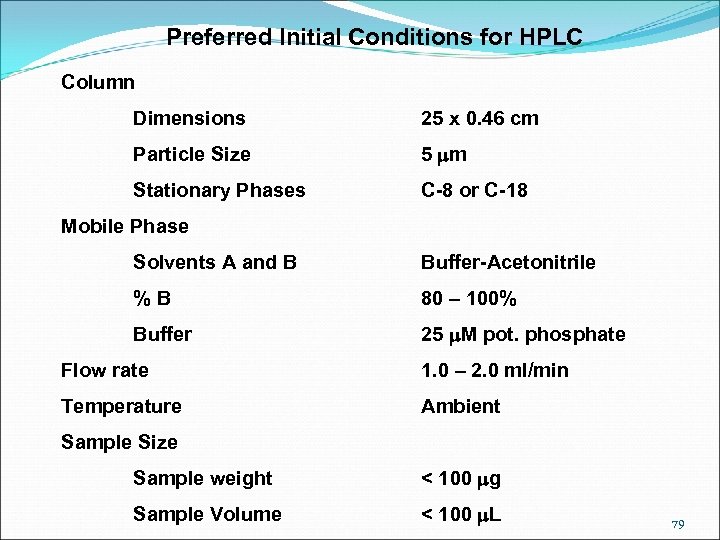
Preferred Initial Conditions for HPLC Column Dimensions 25 x 0. 46 cm Particle Size 5 mm Stationary Phases C-8 or C-18 Mobile Phase Solvents A and B Buffer-Acetonitrile % B 80 – 100% Buffer 25 m. M pot. phosphate Flow rate 1. 0 – 2. 0 ml/min Temperature Ambient Sample Size Sample weight < 100 mg Sample Volume < 100 m. L 79
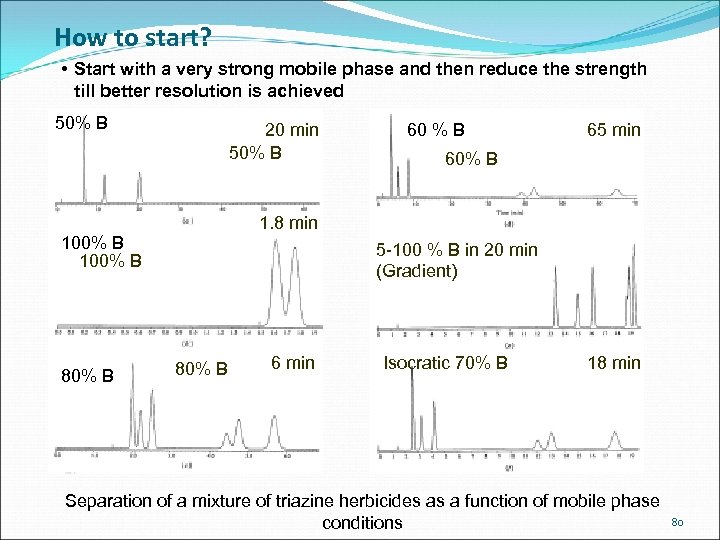
How to start? • Start with a very strong mobile phase and then reduce the strength till better resolution is achieved 50% B 20 min 50% B 65 min 60% B 1. 8 min 100% B 80% B 60 % B 5 -100 % B in 20 min (Gradient) 80% B 6 min Isocratic 70% B 18 min Separation of a mixture of triazine herbicides as a function of mobile phase conditions 80
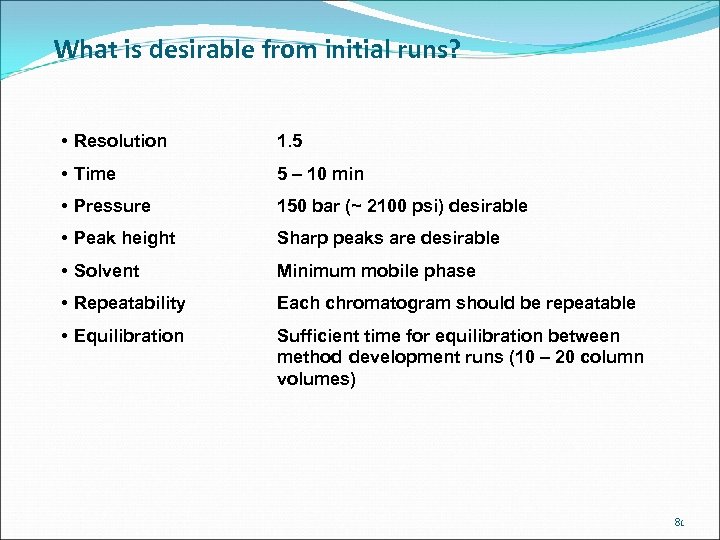
What is desirable from initial runs? • Resolution 1. 5 • Time 5 – 10 min • Pressure 150 bar (~ 2100 psi) desirable • Peak height Sharp peaks are desirable • Solvent Minimum mobile phase • Repeatability Each chromatogram should be repeatable • Equilibration Sufficient time for equilibration between method development runs (10 – 20 column volumes) 81

Trouble shooting in HPLC 82
See problem locate problem correct problem prevent it from happening again 83
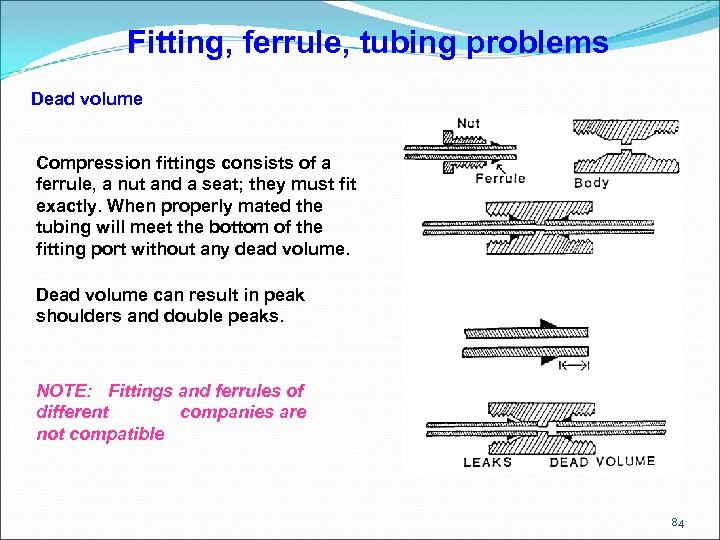
Fitting, ferrule, tubing problems Dead volume Compression fittings consists of a ferrule, a nut and a seat; they must fit exactly. When properly mated the tubing will meet the bottom of the fitting port without any dead volume. Dead volume can result in peak shoulders and double peaks. NOTE: Fittings and ferrules of different companies are not compatible 84
Capillaries The inner diameter of capillaries should be reduced from pump to detector. (e. g. pump - injector 0. 5 mm i. D. ; injector - column and column - detector 0. 2 mm i. D. ) Especially using narrow bore columns (3 mm or 2 mm i. D. ) or using gradients the inner diameter and the length of capillaries is of great importance. A large I. D. of capillaries can result in wide peaks. Very long tubings can result in bad peak resolution, especially when changing to narrow bore columns. NOTE: Tubing related extra column effects will be minimized by using short lengths of small-bore tubing. 85
Solvents Preparing eluents Ø use only chemicals and solvents of highest purity Ø filter all solvents and buffers (0. 45 µm filter) Ø use HPLC-grade sovents (they have been previously filtered) Ø acetate buffers are better than phosphate buffers but look at UV absorbance Ø preparing of buffers from acid and base is often better than preparing of salt using aqueous solvents Ø sodium azide (0. 04%) avoids bacterial grow 86
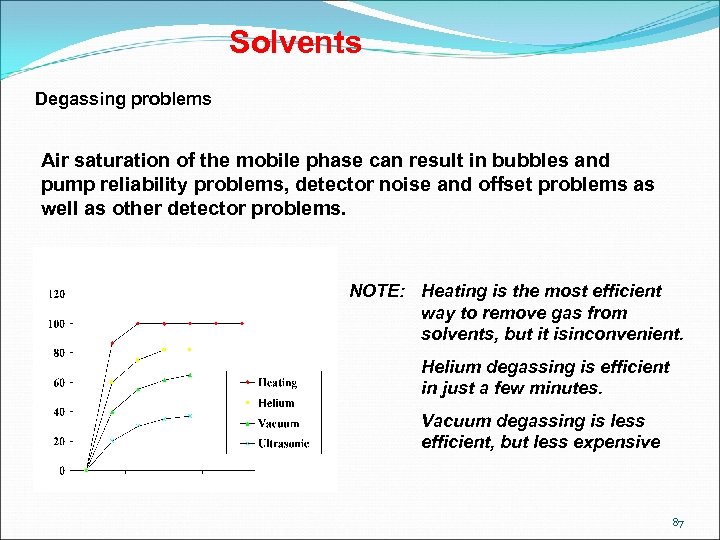
Solvents Degassing problems Air saturation of the mobile phase can result in bubbles and pump reliability problems, detector noise and offset problems as well as other detector problems. NOTE: Heating is the most efficient way to remove gas from solvents, but it isinconvenient. Helium degassing is efficient in just a few minutes. Vacuum degassing is less efficient, but less expensive 87
Solvents Solvent purity and mixing problems Solvent impurities can result in ghost peaks especially using gradients Mixing problems can result in baseline drift, bad reproducibility of retention times, peak shoulders and ghost peaks. Use HPLC quality only! Do not use old or unfiltered buffers! Phosphate buffers can precipitate with organic solvents due to their concentration >20 m. M and <50 m. M 88
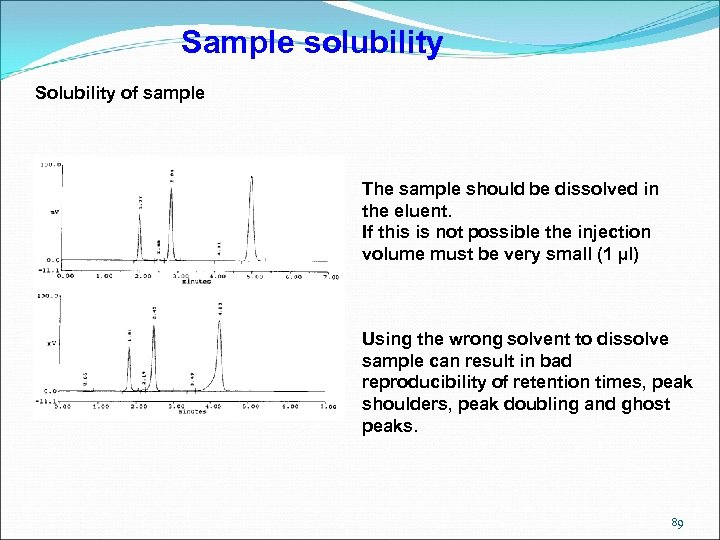
Sample solubility Solubility of sample The sample should be dissolved in the eluent. If this is not possible the injection volume must be very small (1 µl) Using the wrong solvent to dissolve sample can result in bad reproducibility of retention times, peak shoulders, peak doubling and ghost peaks. 89

Solvents Solvent filter problems Symptom ghost peaks (from contaminated frit) retention times are changing (blocked or partly blocked frit) Cleaning process: 1. Water 2. Diluted Nitric acid 3. Water 4. Isopropanol 5. Water (in ultrasonic bath) 90
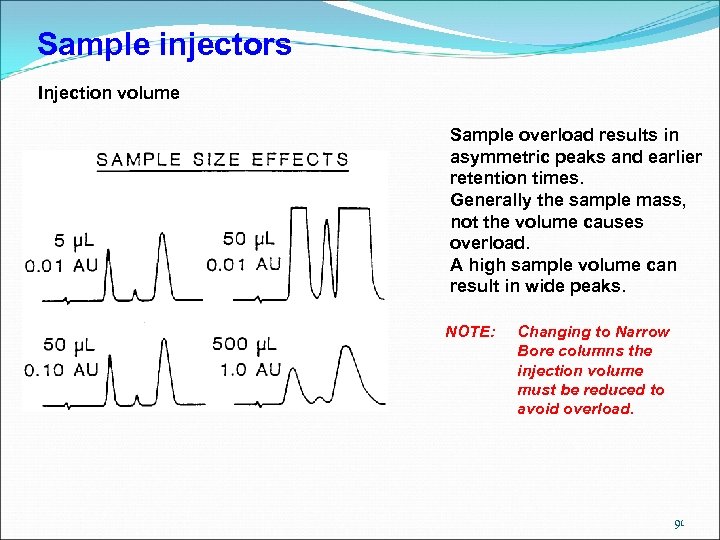
Sample injectors Injection volume Sample overload results in asymmetric peaks and earlier retention times. Generally the sample mass, not the volume causes overload. A high sample volume can result in wide peaks. NOTE: Changing to Narrow Bore columns the injection volume must be reduced to avoid overload. 91
Column Requirements to get reproducible results good reproducibility from batch to batch good reproducibility from column to column good peak symmetry good efficiency and selectivity Reasons for column problems column life time high backpressure reproducibility efficiency 92
Column Prevent problems Ø use pre- or guard columns (silica is soluble in polar/ionic mobile phases) Ø the p. H stability of most RP phases is limited from 2 - 7. 5 p. H Ø avoid radical changes of the elution conditions Ø the mobile phase must be filtered and degassed Ø if polar or ionic buffers are used as eluent store the column in acetonitrile Ø rising pressure could be a signal to change the precolumn 93
Column Regeneration of columns connect the column directly to the pump flush 20 column volume of each cleaning solvent through the column Regeneration of polar phases (Si, NH 2, CN, DIOL): heptane chloroform ethylacetate aceton ethanol water Regeneration of RP phases: water acetonitrile chloroform (or isopropanol) acetonitrile water 0. 05 M H 2 SO 4 can by used to clean soiled columns NOTE: In borderline case it is better to change the column. is much more expensive to risk wrong results and time spending troubleshooting. It 94

THANK YOU 95
59afbb94b040ab0983b527a08484ef76.ppt