7a58fd5c2182d370ee3e61692237e9c8.ppt
- Количество слайдов: 61
HP 1: A coupled numerical code for variably saturated water flow, solute transport and biogeochemical reactions in soils and sediments D. Mallants, D. Jacques, J. Šimůnek, and M. Th. van Genuchten 1

Outline § HP 1: HYDRUS 1 D-PHREEQC § Possibilities of the code § Benchmarking Ø PCE-dissolution Ø Migration of decay chain of adsorbing contaminants during precipitation/evaporation § Illustration of ‘coupled’ effects Ø TNT degradation under steady state flow Ø Cd leaching in an acid podzol: lysimeter experiments Ø Long-term transient flow and transport of major cations and heavy metals in a soil profile 2 Ø U-transport in agricultural field soils

§ HP 1: HYDRUS 1 D-PHREEQC § Possibilities of the code § Benchmarking Ø PCE-dissolution Ø Migration of decay chain of adsorbing contaminants during precipitation/evapotranspiration § Illustration of ‘coupled’ effects Ø TNT degradation under steady state flow Ø Cd leaching in an acid podzol: lysimeter experiments Ø Long-term transient flow and transport of major cations and heavy metals in a soil profile 3 Ø U-transport in agricultural field soils
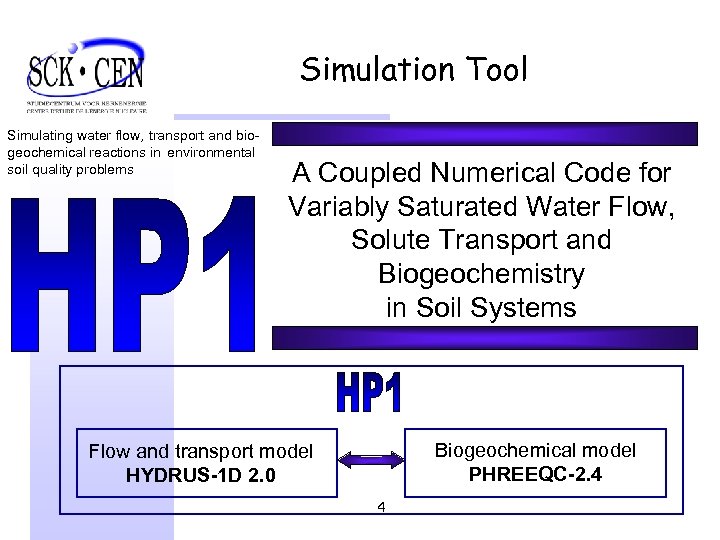
Simulation Tool Simulating water flow, transport and biogeochemical reactions in environmental soil quality problems A Coupled Numerical Code for Variably Saturated Water Flow, Solute Transport and Biogeochemistry in Soil Systems Biogeochemical model PHREEQC-2. 4 Flow and transport model HYDRUS-1 D 2. 0 4
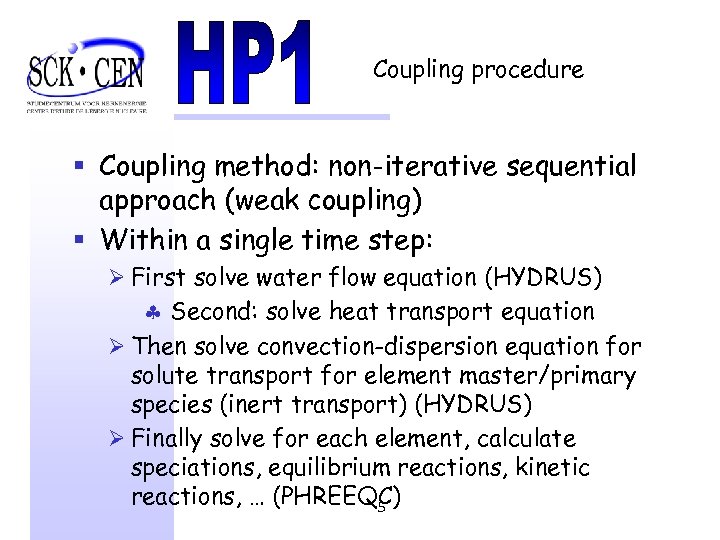
Coupling procedure § Coupling method: non-iterative sequential approach (weak coupling) § Within a single time step: Ø First solve water flow equation (HYDRUS) § Second: solve heat transport equation Ø Then solve convection-dispersion equation for solute transport for element master/primary species (inert transport) (HYDRUS) Ø Finally solve for each element, calculate speciations, equilibrium reactions, kinetic reactions, … (PHREEQC) 5

§ HP 1: HYDRUS 1 D-PHREEQC § Possibilities of the code § Benchmarking Ø PCE-dissolution Ø Migration of decay chain of adsorbing contaminants during precipitation/evapotranspiration § Illustration of ‘coupled’ effects Ø TNT degradation under steady state flow Ø Cd leaching in an acid podzol: lysimeter experiments Ø Long term transient flow and transport of major cations and heavy metals in a soil profile 6 Ø U-transport in agricultural field soils
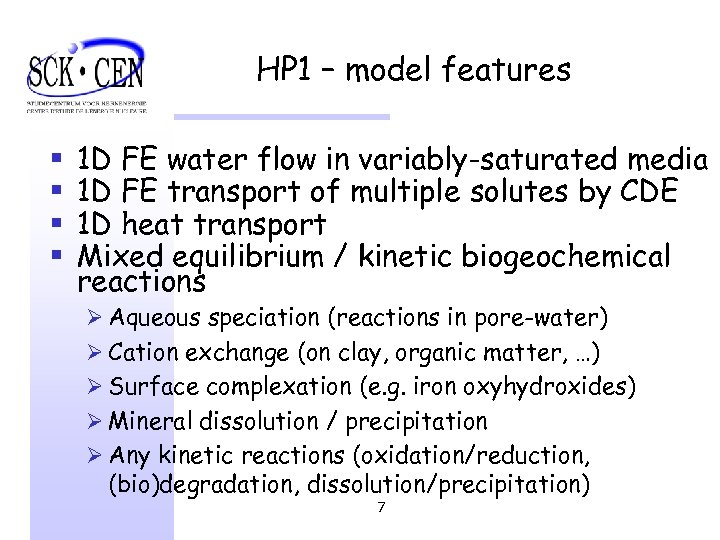
HP 1 – model features § § 1 D FE water flow in variably-saturated media 1 D FE transport of multiple solutes by CDE 1 D heat transport Mixed equilibrium / kinetic biogeochemical reactions Ø Aqueous speciation (reactions in pore-water) Ø Cation exchange (on clay, organic matter, …) Ø Surface complexation (e. g. iron oxyhydroxides) Ø Mineral dissolution / precipitation Ø Any kinetic reactions (oxidation/reduction, (bio)degradation, dissolution/precipitation) 7
HP 1 examples § § § Transport of heavy metals (Zn 2+, Pb 2+, and Cd 2+) subject to multiple cation exchange Transport with mineral dissolution of amorphous Si. O 2 and gibbsite (Al(OH)3) Heavy metal transport in a medium with a p. Hdependent cation exchange complex Infiltration of a hyperalkaline solution in a clay sample (kinetic precipitation-dissolution of kaolinite, illite, quartz, calcite, dolomite, gypsum, …) Long-term transient flow and transport of major cations (Na+, K+, Ca 2+, and Mg 2+) and heavy metals (Cd 2+, Zn 2+, and Pb 2+) in a soil profile. Kinetic biodegradation of TNT (multiple degradation pathways) 8

Typical application and processes involved § Cycling of radionuclides/metals in soilplant systems Ø Heterogeneous physical/chemical properties Ø Water flow under rainfall - evapotranspiration conditions Ø Root growth and water uptake Ø Microbiological growth Ø Degradation of organic matter with radionuclide/metal release Ø Transport/adsorption/decay Ø Uptake of radionuclides/metals by plants 9

§ HP 1: HYDRUS 1 D-PHREEQC § Possibilities of the code § Benchmarking Ø PCE-dissolution under steady-state flow conditions Ø Migration of decay chain of adsorbing contaminants during precipitation/evapotranspiration § Illustration of ‘coupled’ effects Ø TNT degradation under steady state flow Ø Cd leaching in an acid podzol: lysimeter experiments Ø Long-term transient flow and transport of major cations and heavy metals in a soil profile 10 Ø U-transport in agricultural field soils
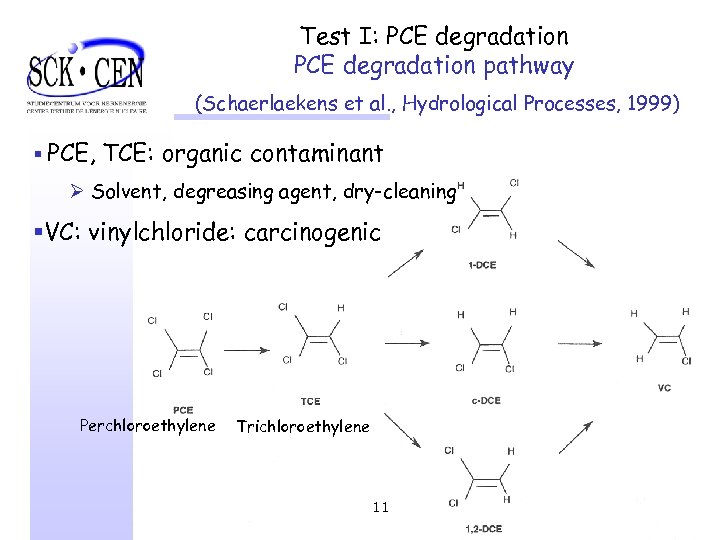
Test I: PCE degradation pathway (Schaerlaekens et al. , Hydrological Processes, 1999) § PCE, TCE: organic contaminant Ø Solvent, degreasing agent, dry-cleaning §VC: vinylchloride: carcinogenic Perchloroethylene Trichloroethylene 11
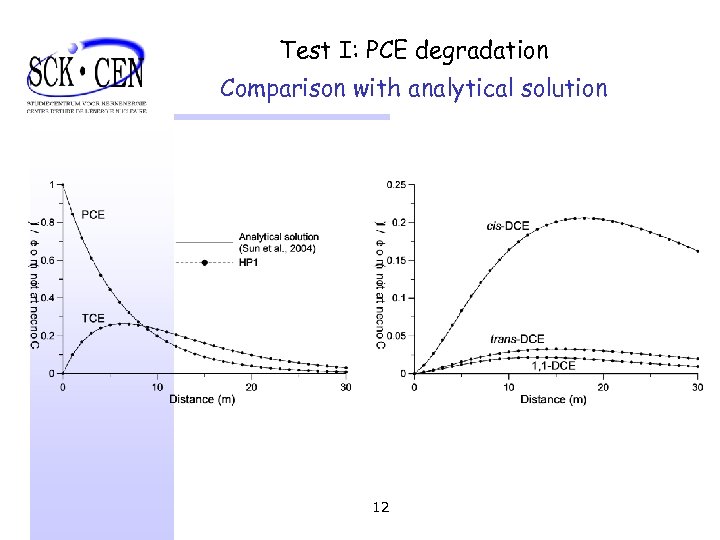
Test I: PCE degradation Comparison with analytical solution 12
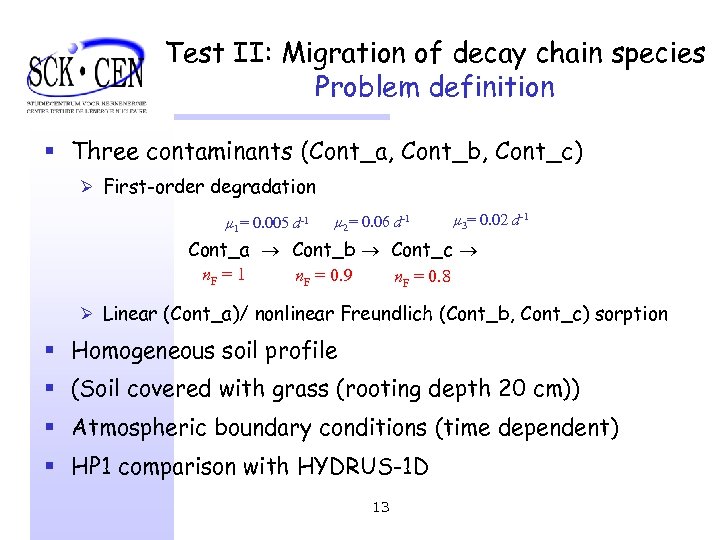
Test II: Migration of decay chain species Problem definition § Three contaminants (Cont_a, Cont_b, Cont_c) Ø First-order degradation µ 1= 0. 005 d-1 µ 2= 0. 06 d-1 µ 3= 0. 02 d-1 Cont_a Cont_b Cont_c n. F = 1 n. F = 0. 9 n. F = 0. 8 Ø Linear (Cont_a)/ nonlinear Freundlich (Cont_b, Cont_c) sorption § Homogeneous soil profile § (Soil covered with grass (rooting depth 20 cm)) § Atmospheric boundary conditions (time dependent) § HP 1 comparison with HYDRUS-1 D 13
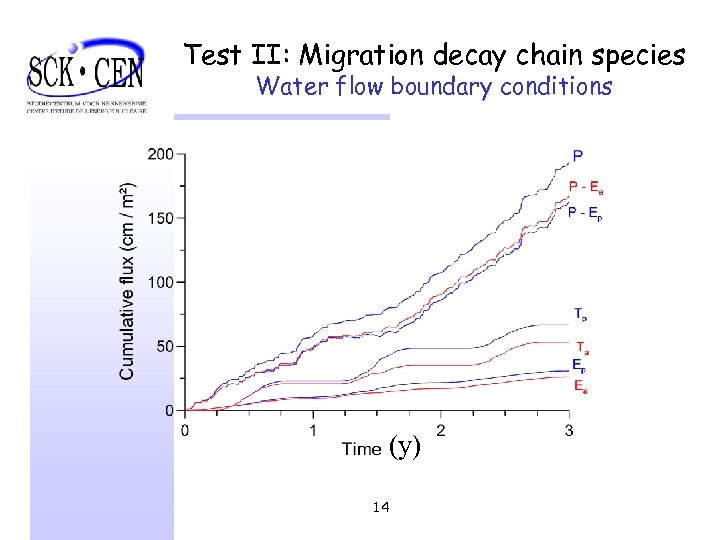
Test II: Migration decay chain species Water flow boundary conditions (y) 14
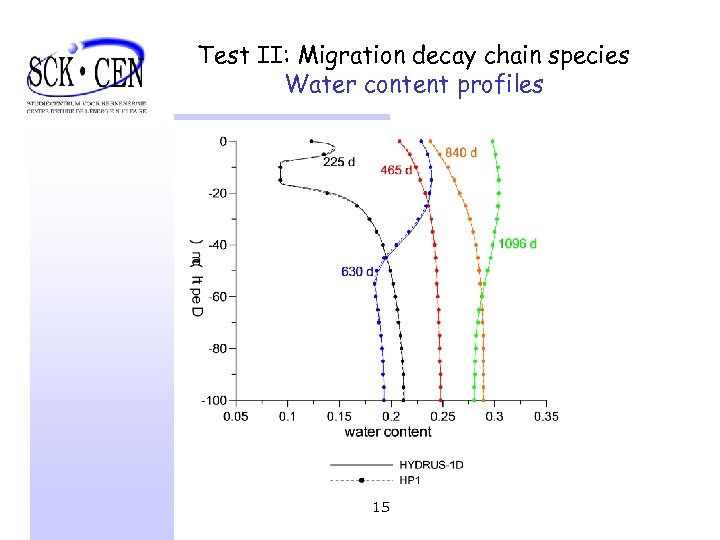
Test II: Migration decay chain species Water content profiles 15
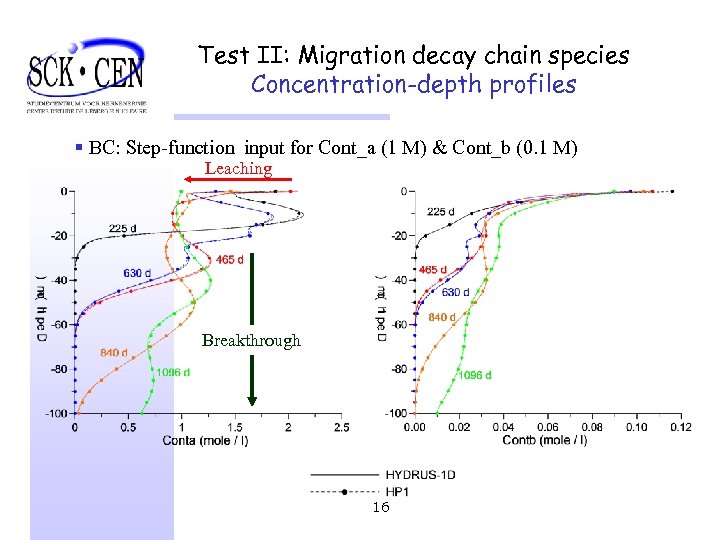
Test II: Migration decay chain species Concentration-depth profiles § BC: Step-function input for Cont_a (1 M) & Cont_b (0. 1 M) Leaching Breakthrough 16
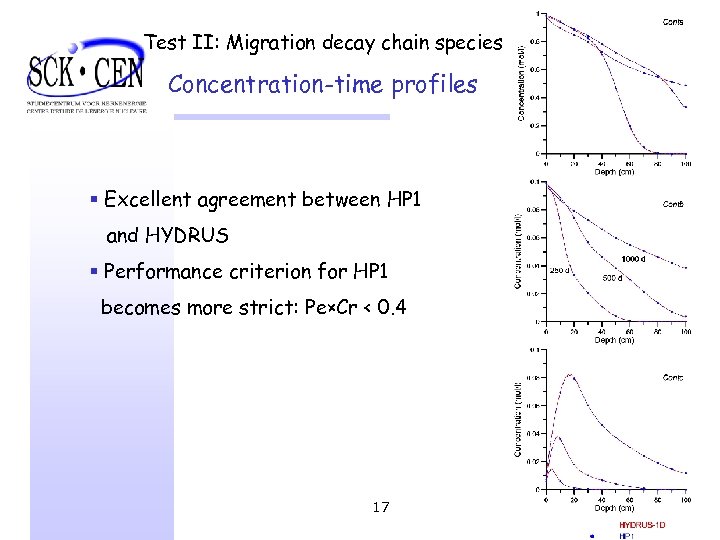
Test II: Migration decay chain species Concentration-time profiles § Excellent agreement between HP 1 and HYDRUS § Performance criterion for HP 1 becomes more strict: Pe×Cr < 0. 4 17
§ HP 1: HYDRUS 1 D-PHREEQC § Possibilities of the code § Benchmarking Ø PCE-dissolution under steady-state flow conditions Ø Migration of decay chain of adsorbing contaminants during precipitation/evapotranspiration § Illustration of ‘coupled’ effects § TNT degradation under steady-state flow Ø Cd leaching in an acid podzol: lysimeter experiments Ø Long-term transient flow and transport of major cations and heavy metals in a soil profile 18 Ø U-transport in agricultural field soils
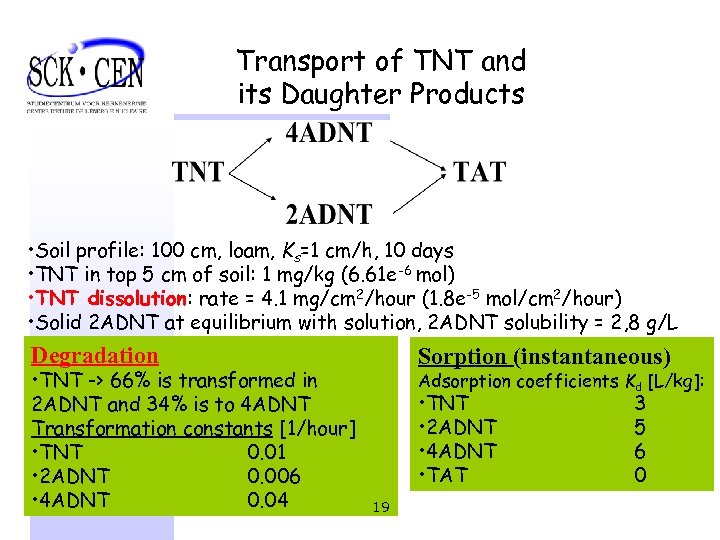
Transport of TNT and its Daughter Products • Soil profile: 100 cm, loam, Ks=1 cm/h, 10 days • TNT in top 5 cm of soil: 1 mg/kg (6. 61 e-6 mol) • TNT dissolution: rate = 4. 1 mg/cm 2/hour (1. 8 e-5 mol/cm 2/hour) • Solid 2 ADNT at equilibrium with solution, 2 ADNT solubility = 2, 8 g/L Degradation • TNT -> 66% is transformed in 2 ADNT and 34% is to 4 ADNT Transformation constants [1/hour] • TNT 0. 01 • 2 ADNT 0. 006 • 4 ADNT 0. 04 Sorption (instantaneous) Adsorption coefficients Kd [L/kg]: • TNT • 2 ADNT • 4 ADNT • TAT 19 3 5 6 0
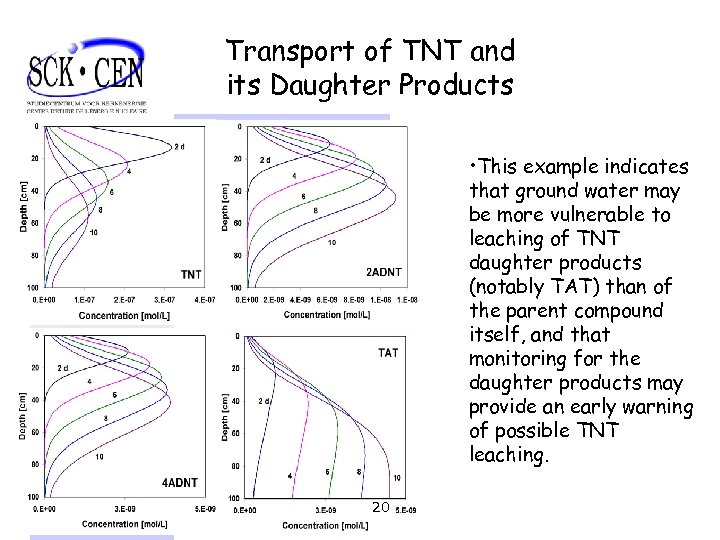
Transport of TNT and its Daughter Products • This example indicates that ground water may be more vulnerable to leaching of TNT daughter products (notably TAT) than of the parent compound itself, and that monitoring for the daughter products may provide an early warning of possible TNT leaching. 20

Cd leaching in acid podzol Introduction § Nothern region of Belgium: historical contamination of soils with Cd, Pb, Cu, Zn by atmospheric deposition originated from the non-ferro industry (historical contamination, beginning 20 th century) § Risk of flooding with water containing increased salt concentrations 21
Cd leaching in acid podzol Objectives § To describe the leaching of major cations, Zn and Cd from a lysimeter after application of an increased salt concentration (tracter test) § To assess the effect of increased salt concentrations (Ca. Cl 2) on Cd leaching using a new coupled reactive transport model HP 1 22
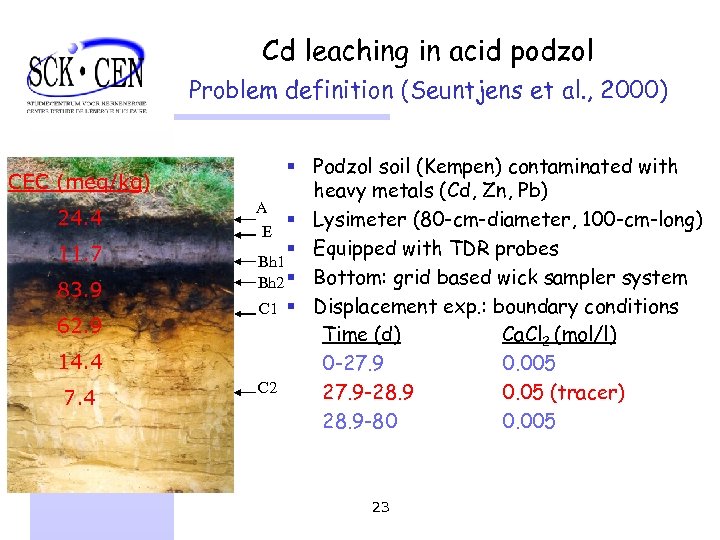
Cd leaching in acid podzol Problem definition (Seuntjens et al. , 2000) CEC (meq/kg) 24. 4 11. 7 83. 9 62. 9 14. 4 7. 4 § Podzol soil (Kempen) contaminated with heavy metals (Cd, Zn, Pb) A § Lysimeter (80 -cm-diameter, 100 -cm-long) E § Equipped with TDR probes Bh 1 Bh 2 § Bottom: grid based wick sampler system C 1 § Displacement exp. : boundary conditions Time (d) Ca. Cl 2 (mol/l) 0 -27. 9 0. 005 C 2 27. 9 -28. 9 0. 05 (tracer) 28. 9 -80 0. 005 23

Cd leaching in acid podzol Leaching experiment set-up TDR probes Cable tester 24 Leachate collectors
Cd leaching in acid podzol Leaching experiment modelling (1) § Components in solution: H, Ca, Na, K, Mg, Al, Cl, Br, Cd, Zn § Speciation reactions in soil solution Ø Complexation reactions of Zn, Cd with OH-, Cl-: § Cd(OH)+, Cd(OH)2, Cd(OH)3 -, Cd(OH)32§ Cd(Cl)+, Cd(Cl)2, Cd(Cl)3 -, Cd(Cl)32 - 25
Cd leaching in acid podzol Leaching experiment modelling (2) § Ion exchange reactions (solid phase interaction) Ø Half reactions (X-: exchange complex): H+ + X- = HX Ca 2+ + 2 X- = Ca. X 2 Ø H, Ca, Na, K, Mg, Cd, Zn Ø Equilibrium constants are adapted to fit the measurements (site-specific Log_K values) § Equilibrium with gibbsite (Al(OH)3) 26
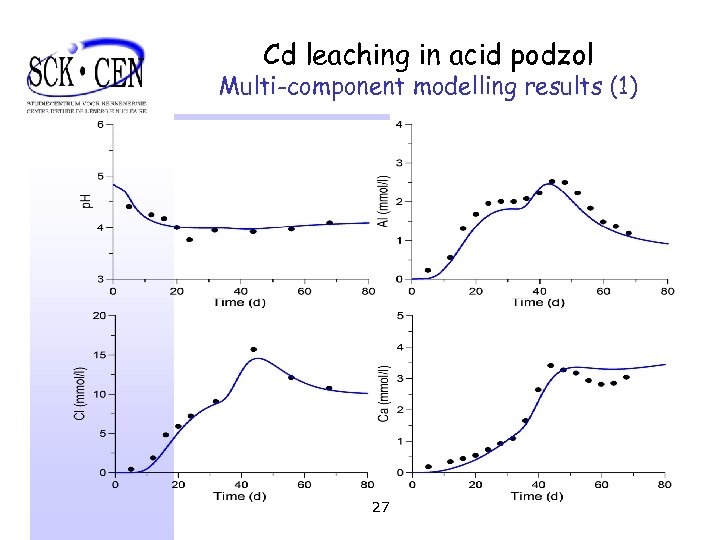
Cd leaching in acid podzol Multi-component modelling results (1) 27
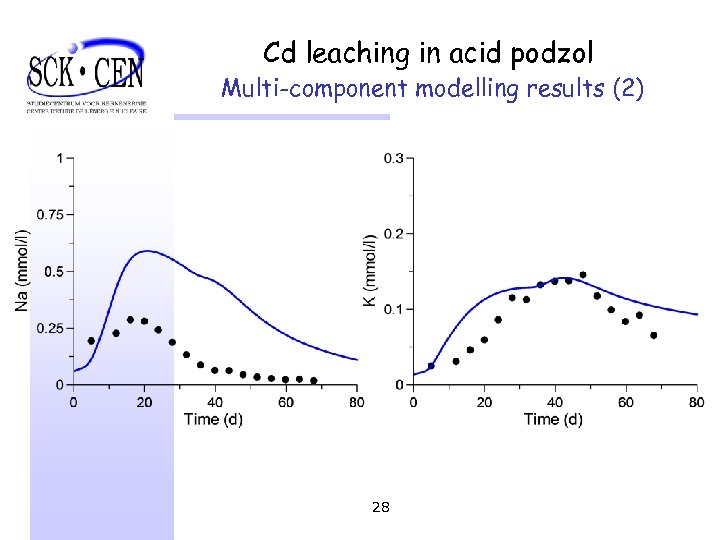
Cd leaching in acid podzol Multi-component modelling results (2) 28
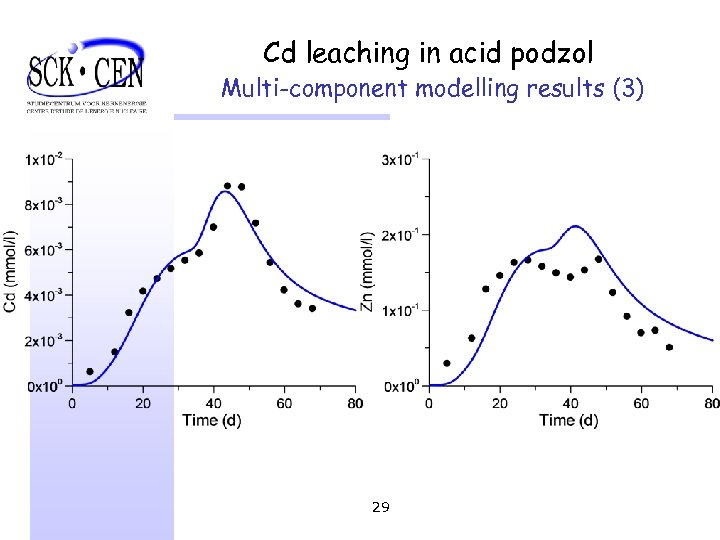
Cd leaching in acid podzol Multi-component modelling results (3) 29
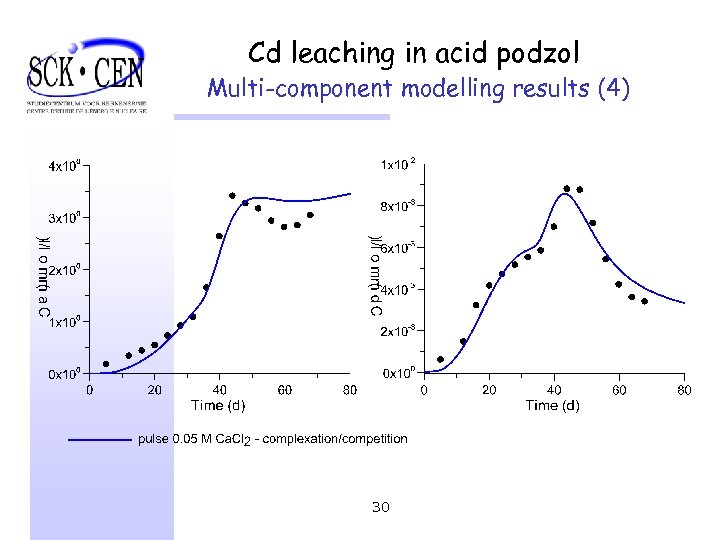
Cd leaching in acid podzol Multi-component modelling results (4) 30
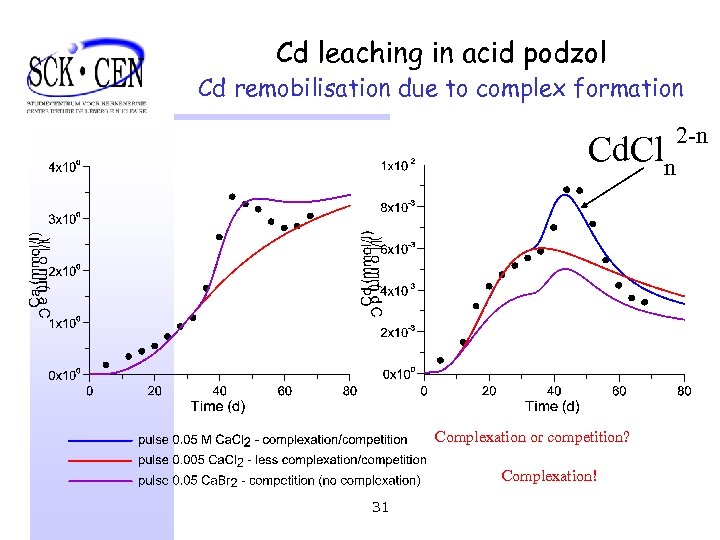
Cd leaching in acid podzol Cd remobilisation due to complex formation Cd. Cln Complexation or competition? Complexation! 31 2 -n
Cd leaching in acid podzol Conclusion § Increased Cd mobilization due to Ø exchange Ca-Cd Ø complexation with Cl- (most important) § Geochemical speciation models required (instead of e. g. Kd approach) § HP 1: allows for transient flow conditions 32

§ HP 1: HYDRUS 1 D-PHREEQC § Possibilities of the code § Benchmarking Ø PCE-dissolution Ø Migration of decay chain of adsorbing contaminants during precipitation/evapotranspiration § Illustration of ‘coupled’ effects Ø TNT degradation under steady state flow Ø Cd leaching in an acid podzol: lysimeter experiments Ø Long term transient flow and transport of major cations and heavy metals in a soil profile 33 Ø U-transport in agricultural field soils
Geochemical transport under transient variably-saturated flow § Cycling of metals in soil-plant systems Ø Heterogeneous physical/chemical properties Ø Water flow under rainfall - evaporation conditions Ø Root growth and water uptake Ø Metal transport/adsorption/speciation Ø Uptake of metals by plants Ø Degradation of organic matter with metal release 34
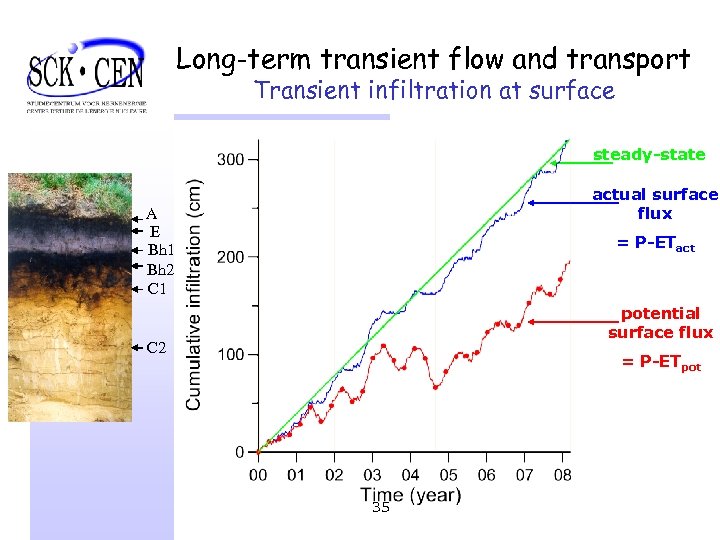
Long-term transient flow and transport Transient infiltration at surface steady-state actual surface flux A E Bh 1 Bh 2 C 1 = P-ETact potential surface flux C 2 = P-ETpot 35
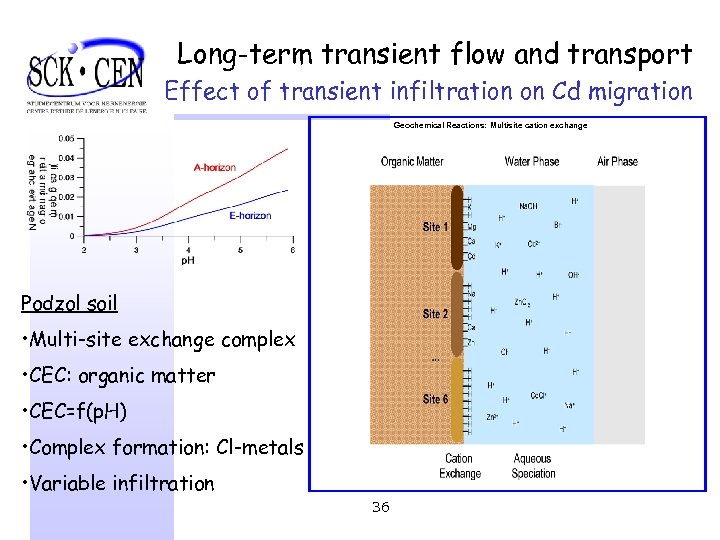
Long-term transient flow and transport Effect of transient infiltration on Cd migration Geochemical Reactions: Multisite cation exchange Podzol soil • Multi-site exchange complex • CEC: organic matter • CEC=f(p. H) • Complex formation: Cl-metals • Variable infiltration 36
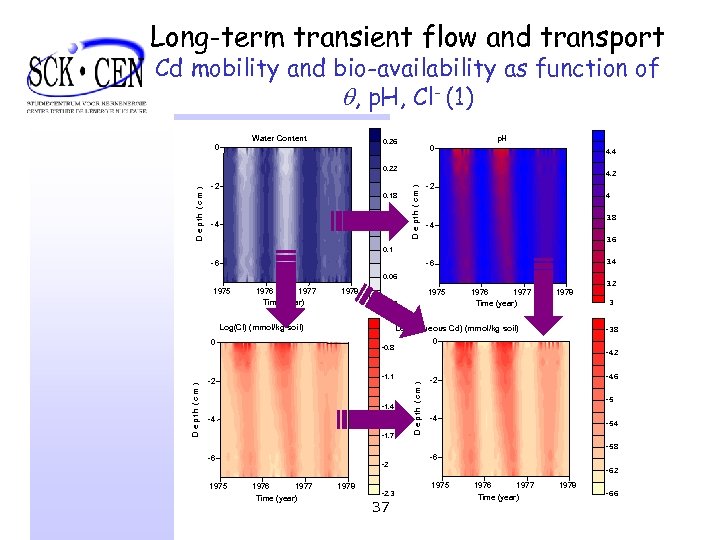
Long-term transient flow and transport Cd mobility and bio-availability as function of , p. H, Cl- (1) 0 Water Content p. H 0. 26 0 4. 4 -2 0. 18 0. 14 -4 4. 2 D e pth ( c m ) D e p th ( c m ) 0. 22 -2 4 3. 8 -4 3. 6 0. 1 -6 3. 4 -6 0. 06 1977 Time (year) 1978 1975 0. 02 Log(Cl) (mmol/kg soil) 0 D e p t h (c m ) 1976 1977 Time (year) 1978 -4. 2 -1. 1 -1. 4 -4 -1. 7 -3. 8 0 -0. 8 -2 3. 2 3 Log(Aqueous Cd) (mmol/kg soil) D e p th (c m ) 1975 -4. 6 -2 -5 -4 -5. 8 -6 1975 -2 1976 1977 Time (year) 1978 -6 -6. 2 1975 -2. 3 37 1976 1977 Time (year) 1978 -6. 6
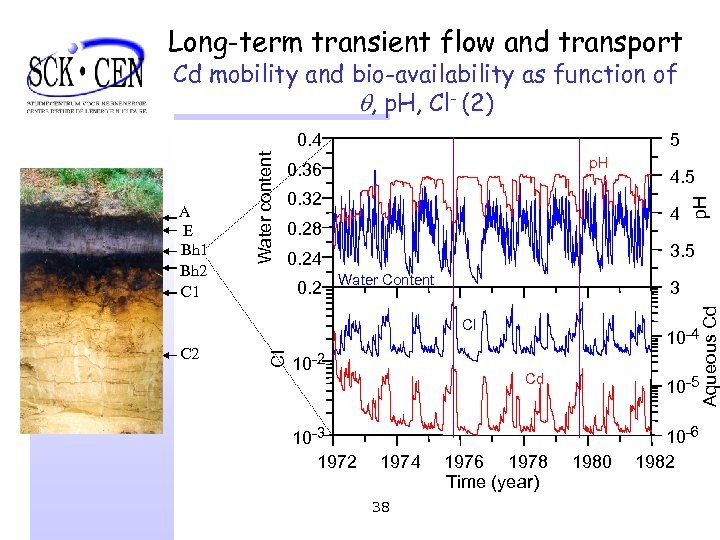
Long-term transient flow and transport Cd mobility and bio-availability as function of , p. H, Cl- (2) 5 0. 32 4 0. 28 3. 5 0. 24 0. 2 Water Content 3 Cl Cl C 2 4. 5 p. H 0. 36 10 -2 10 -3 1972 10 -4 Cd 1974 38 1976 1978 Time (year) 10 -5 1980 10 -6 1982 Aqueous Cd A E Bh 1 Bh 2 C 1 Water content 0. 4
Long-term transient flow and transport Conclusions § Temporal variability of physical soil variables (θ) results in temporal variability in geochemical variables (p. H, Cl-, …) § Applied to heavy metal mobility and bioavailability: Ø Water content variations linearly related to p. H and inversely to Cl- variations Ø p. H inversely related to dissolved metal concentration (multi-site cation exchange f(p. H)) Ø Cl- concentration linearly related to dissolved metal concentration (complex formation) 39

§ HP 1: HYDRUS 1 D-PHREEQC § Possibilities of the code § Benchmarking Ø PCE-dissolution under steady-state flow conditions Ø Migration of decay chain of adsorbing contaminants during precipitation/evapotranspiration § Illustration of ‘coupled’ effects Ø TNT degradation under steady state flow Ø Cd leaching in an acid podzol Ø Long term transient flow and transport of major cations and heavy metals in a soil profile 40 Ø U-transport in agricultural field soils

Introduction / objectives (1) § Motivation: assessment of post-closure safety for surface repository § Inherent uncertainties, especially for the long-term § Use of multiple lines of reasoning § Complementary safety indicators for evaluating and confirming safety: e. g. , RN fluxes, U-concentration § Objective: estimate long-term U-leaching from agricultural soils, compare with U-fluxes from planned surface repository 41
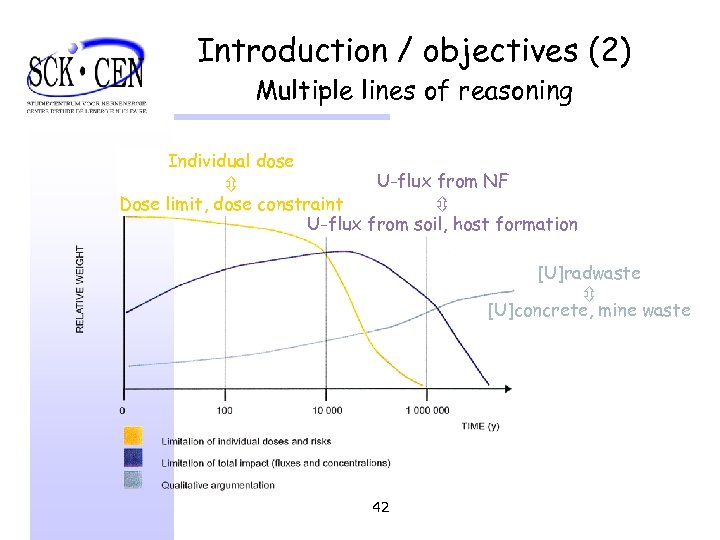
Introduction / objectives (2) Multiple lines of reasoning Individual dose U-flux from NF Dose limit, dose constraint U-flux from soil, host formation [U]radwaste [U]concrete, mine waste 42

• Introduction • A new biogeochemical transport code: HP 1 • Problem statement: soil, geochemical reactions, BC/IC • Simulation results • U-fluxes from soil vs. surface repository • Conclusions 43
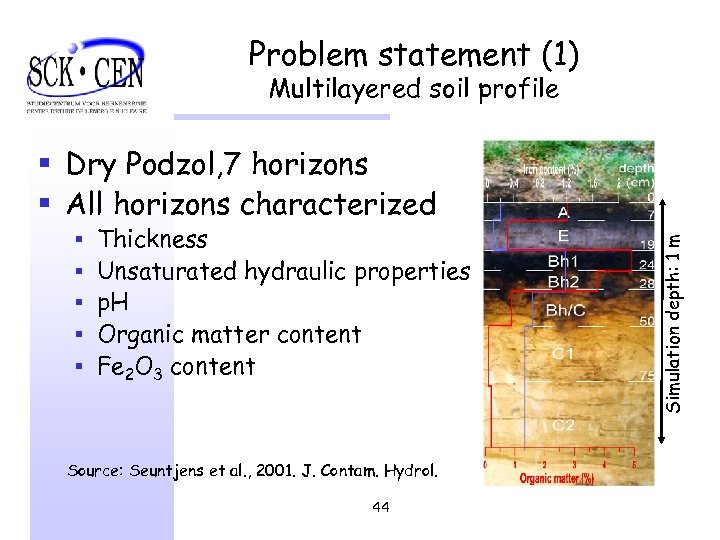
Problem statement (1) Multilayered soil profile § § § Thickness Unsaturated hydraulic properties p. H Organic matter content Fe 2 O 3 content Source: Seuntjens et al. , 2001. J. Contam. Hydrol. 44 Simulation depth: 1 m § Dry Podzol, 7 horizons § All horizons characterized
Problem statement (2) Geochemical equilibrium reactions § Aqueous speciation reactions § Chemical components: C, Ca, Cl, F, H, K, Mg, N(5), Na, O(0), O(-2), P, S(6), U(6) § Multi-site cation exchange reactions § Related to amount of organic matter § Increases with increasing p. H § Surface complexation reactions § Specific binding to charged surfaces ( Fe. OH) § Related to amount of Fe-oxides 45
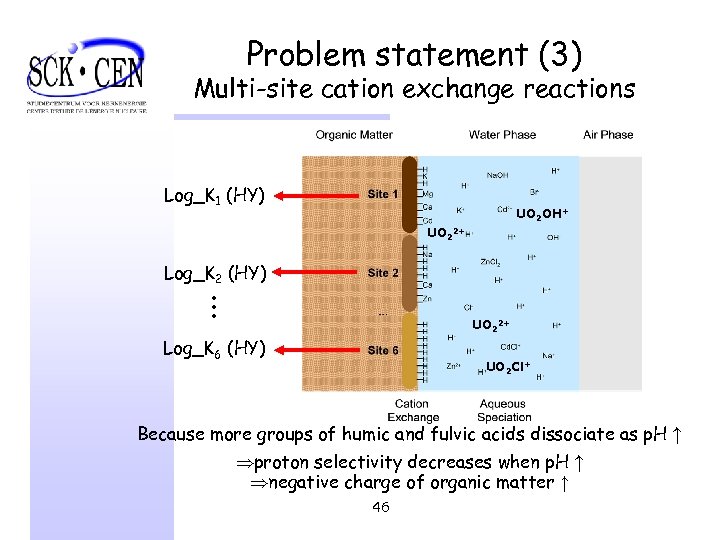
Problem statement (3) Multi-site cation exchange reactions Log_K 1 (HY) UO 2 OH+ UO 22+ Log_K 2 (HY) . . . UO 22+ Log_K 6 (HY) UO 2 Cl+ Because more groups of humic and fulvic acids dissociate as p. H ↑ Þproton selectivity decreases when p. H ↑ Þnegative charge of organic matter ↑ 46
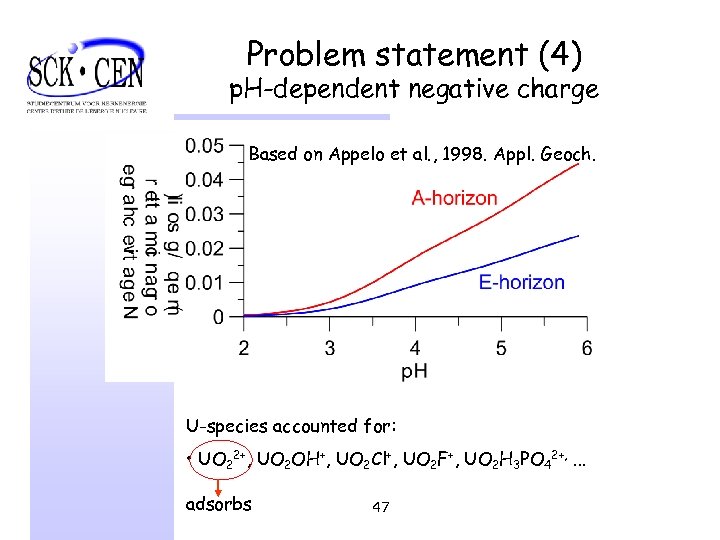
Problem statement (4) p. H-dependent negative charge Based on Appelo et al. , 1998. Appl. Geoch. U-species accounted for: • UO 22+, UO 2 OH+, UO 2 Cl+, UO 2 F+, UO 2 H 3 PO 42+, . . . adsorbs 47
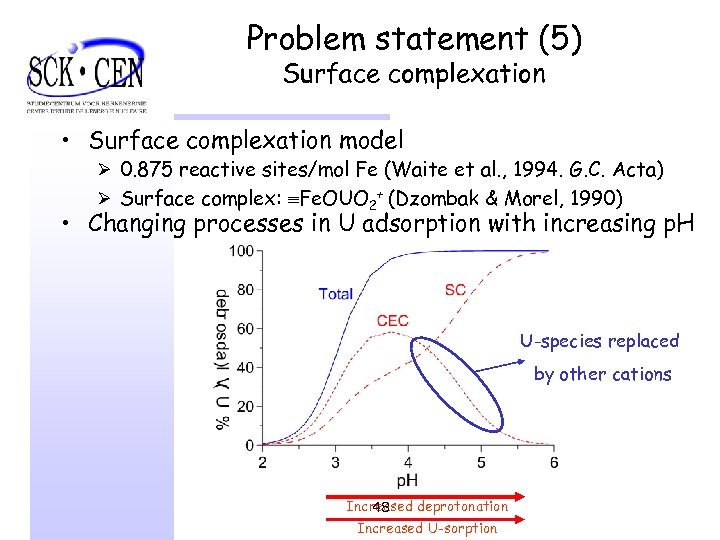
Problem statement (5) Surface complexation • Surface complexation model Ø 0. 875 reactive sites/mol Fe (Waite et al. , 1994. G. C. Acta) Ø Surface complex: Fe. OUO 2+ (Dzombak & Morel, 1990) • Changing processes in U adsorption with increasing p. H U-species replaced by other cations Increased deprotonation 48 Increased U-sorption
Problem statement (6) Initial and Boundary conditions § Initial condition § No U initially present in soil profile (<> few 10 Bq/kg) § Boundary condition § 200 -year time series of synthetic meteorological data to calculate preciptiation and potential evaporation § Composition rain water from measurements § P-fertilizer (Ca(H 2 PO 4)2): ~3000 Bq 238 U/kg § Applied each year on May 1 (1 g P/m 2) § 1. 6 10 -1 mol Ca(H 2 PO 4)2 /m² in 1 cm of rain § =>3. 8 10 -6 mol U /m 2 in 1 cm of rain (~105 Bq/ha) 49

• Introduction • A new biogeochemical transport code: HP 1 • Problem statement: soil, geochemical reactions, BC/IC • Simulation results • U-fluxes from soil vs. surface repository • Conclusions 50
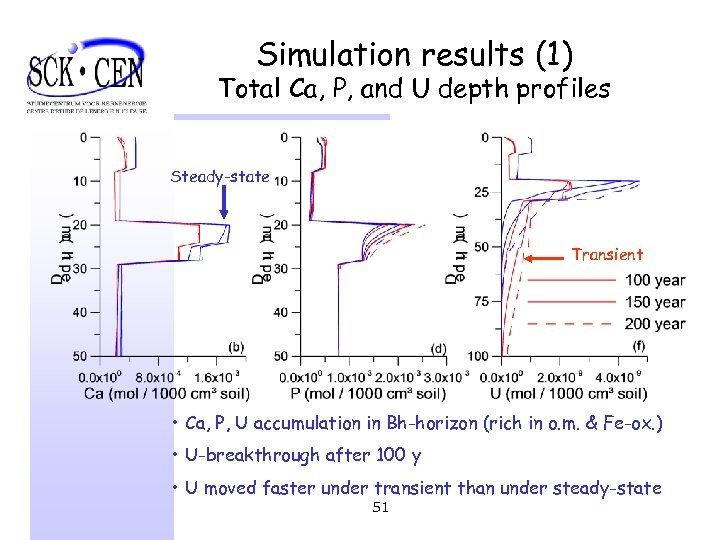
Simulation results (1) Total Ca, P, and U depth profiles Steady-state Transient • Ca, P, U accumulation in Bh-horizon (rich in o. m. & Fe-ox. ) • U-breakthrough after 100 y • U moved faster under transient than under steady-state 51
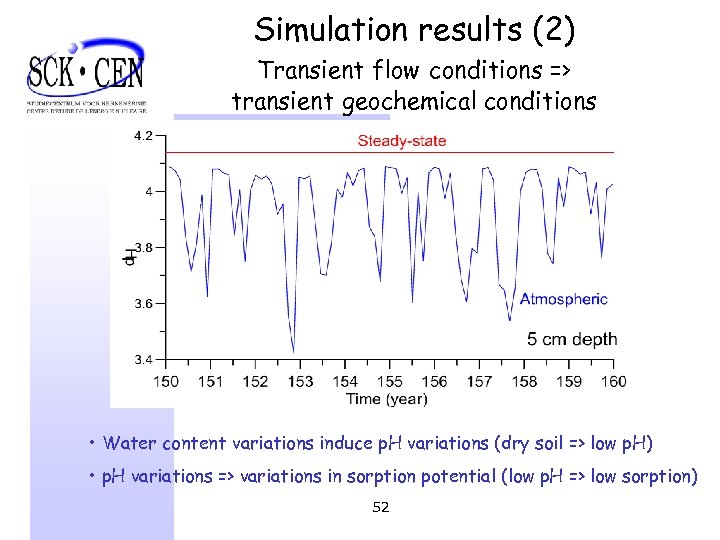
Simulation results (2) Transient flow conditions => transient geochemical conditions • Water content variations induce p. H variations (dry soil => low p. H) • p. H variations => variations in sorption potential (low p. H => low sorption) 52
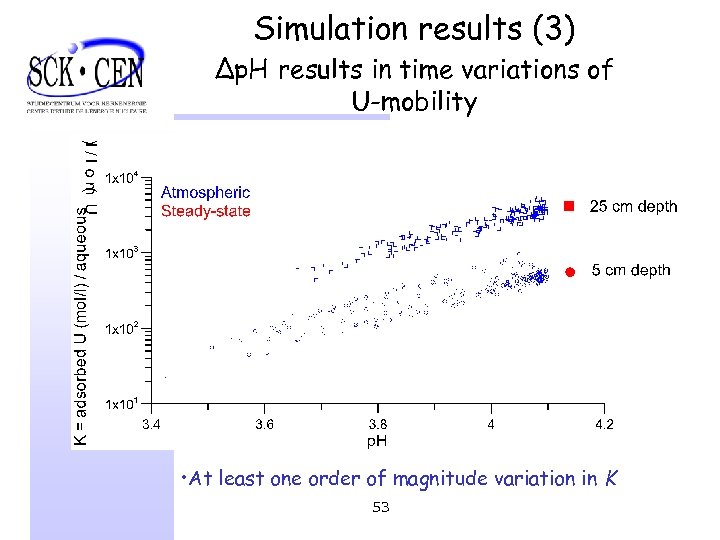
Simulation results (3) ∆p. H results in time variations of U-mobility • At least one order of magnitude variation in K 53
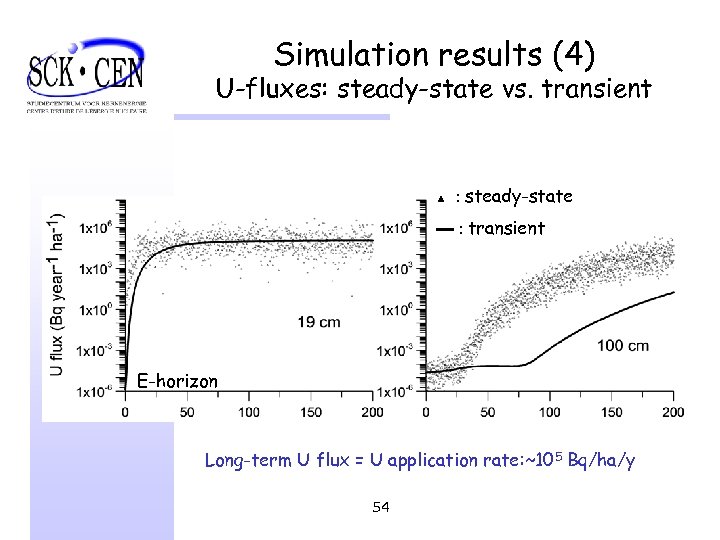
Simulation results (4) U-fluxes: steady-state vs. transient ▲ : steady-state ▬ : transient E-horizon Long-term U flux = U application rate: ~105 Bq/ha/y 54

• Introduction • A new biogeochemical transport code: HP 1 • Problem statement: soil, geochemical reactions, BC/IC • Simulation results • U-fluxes from soil vs. surface repository • Conclusions 55
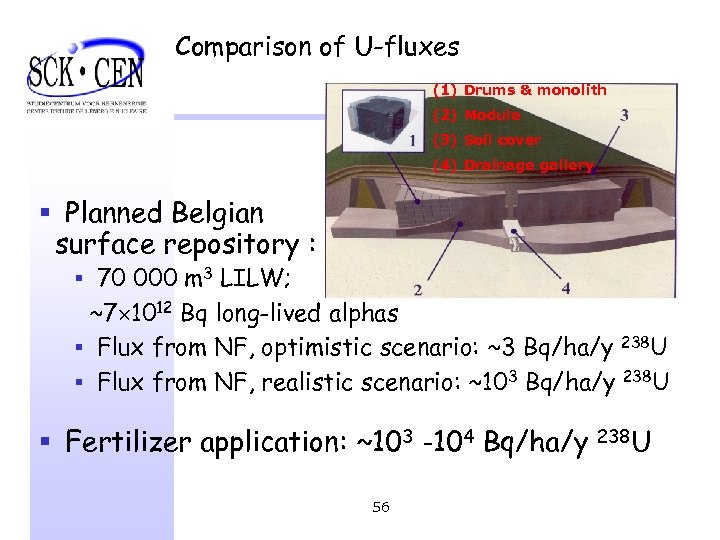
Comparison of U-fluxes (1) Drums & monolith (2) Module (3) Soil cover (4) Drainage gallery § Planned Belgian surface repository : § 70 000 m 3 LILW; ~7 1012 Bq long-lived alphas § Flux from NF, optimistic scenario: ~3 Bq/ha/y 238 U § Flux from NF, realistic scenario: ~103 Bq/ha/y 238 U § Fertilizer application: ~103 -104 Bq/ha/y 238 U 56
• Introduction • A new biogeochemical transport code: HP 1 • Problem statement: soil, geochemical reactions, BC/IC • Simulation results • U-fluxes from soil vs. surface repository • Conclusions 57
Conclusions (1) § New biogeochemical transport code HP 1 provides useful insight into complex U-migration processes § U migration under atmospheric boundary conditions faster than under steady-state flow conditions § Due to changing flow and geochemical conditions (∆ p. H =>∆ sorption) § Atmospheric boundary conditions important when assessing U-flux to groundwater 58
Conclusions (2) § Calculated U-fluxes from soil same order of magnitude as U-flux from surface repository § Limitations of the study § § § No interactions U-nitrate CO 2 transport not accounted for More typical agricultural soils Include plant uptake Need verification experiments. . . 59
Use of Geochemical Transport Models § Process Coupling and Interactions Tools for investigating the impacts of multiple coupled biogeochemical reactions in the presence of complex flow fields and spatial heterogeneity. Enable extrapolation to environmentally relevant temporal and spatial scales. § Interpretation of Laboratory and Field Data Provide a useful framework for interpreting experimental results. Serve as a tool for understanding qualitative and quantitative trends and relationships present in the data. § Sensitivity Analysis Permit the systematic evaluation of the impact of model parameters (both reactive and hydrogeological), initial conditions, and boundary conditions upon the model output. § Integration and Synthesis Tool for integrating all of the knowledge obtained from simulation, sensitivity analyses, and laboratory and field 60 experimentation.

Find out more about HP 1! www. sckcen. be/hp 1 61
7a58fd5c2182d370ee3e61692237e9c8.ppt