dea8b4733c8cd0463f58de26aa217210.ppt
- Количество слайдов: 38
 How to use data to get “The Right Answer” Donna Spiegelman Departments of Epidemiology and Biostatistics Harvard School of Public Health stdls@channing. harvard. edu
How to use data to get “The Right Answer” Donna Spiegelman Departments of Epidemiology and Biostatistics Harvard School of Public Health stdls@channing. harvard. edu
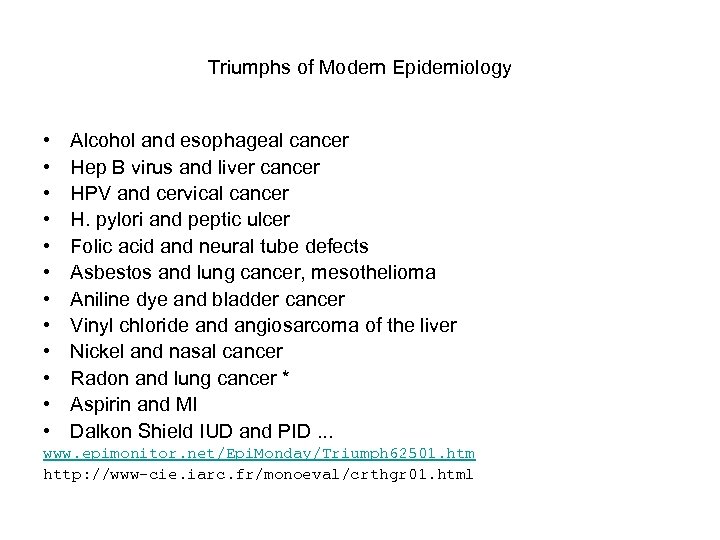 Triumphs of Modern Epidemiology • • • Alcohol and esophageal cancer Hep B virus and liver cancer HPV and cervical cancer H. pylori and peptic ulcer Folic acid and neural tube defects Asbestos and lung cancer, mesothelioma Aniline dye and bladder cancer Vinyl chloride and angiosarcoma of the liver Nickel and nasal cancer Radon and lung cancer * Aspirin and MI Dalkon Shield IUD and PID. . . www. epimonitor. net/Epi. Monday/Triumph 62501. htm http: //www-cie. iarc. fr/monoeval/crthgr 01. html
Triumphs of Modern Epidemiology • • • Alcohol and esophageal cancer Hep B virus and liver cancer HPV and cervical cancer H. pylori and peptic ulcer Folic acid and neural tube defects Asbestos and lung cancer, mesothelioma Aniline dye and bladder cancer Vinyl chloride and angiosarcoma of the liver Nickel and nasal cancer Radon and lung cancer * Aspirin and MI Dalkon Shield IUD and PID. . . www. epimonitor. net/Epi. Monday/Triumph 62501. htm http: //www-cie. iarc. fr/monoeval/crthgr 01. html
 The Start of Junk Science? ? Definition of junk science: “It is a hodgepodge of biased data, spurious inference, and logical legerdemain, patched together by researchers whose enthusiasm for discovery and diagnosis far outstrips their dredging, wishful thinking, truculent dogmatism, and, now and again, outright fraud. ”
The Start of Junk Science? ? Definition of junk science: “It is a hodgepodge of biased data, spurious inference, and logical legerdemain, patched together by researchers whose enthusiasm for discovery and diagnosis far outstrips their dredging, wishful thinking, truculent dogmatism, and, now and again, outright fraud. ”
 The Junkyard Dogs “Unfortunately, and increasingly today, one can find examples of junk science that compromise the integrity of the field of science and, at the same time, create a scare environment where unnecessary regulations on industry in particular, are rammed through without respect to rhyme, reason, effect or cause. ” —Michael A. Miles, former CEO of the Phillip Morris tobacco company
The Junkyard Dogs “Unfortunately, and increasingly today, one can find examples of junk science that compromise the integrity of the field of science and, at the same time, create a scare environment where unnecessary regulations on industry in particular, are rammed through without respect to rhyme, reason, effect or cause. ” —Michael A. Miles, former CEO of the Phillip Morris tobacco company

 More controversial: “weak effects” • Air pollution and all-cause mortality, CVD mortality • Low dose exposure to radon and lung cancer • Low dose exposure to lead and neurotoxicity in children • Passive cigarette smoking and lung cancer • Alcohol and breast cancer • Oral contraceptives and breast cancer
More controversial: “weak effects” • Air pollution and all-cause mortality, CVD mortality • Low dose exposure to radon and lung cancer • Low dose exposure to lead and neurotoxicity in children • Passive cigarette smoking and lung cancer • Alcohol and breast cancer • Oral contraceptives and breast cancer
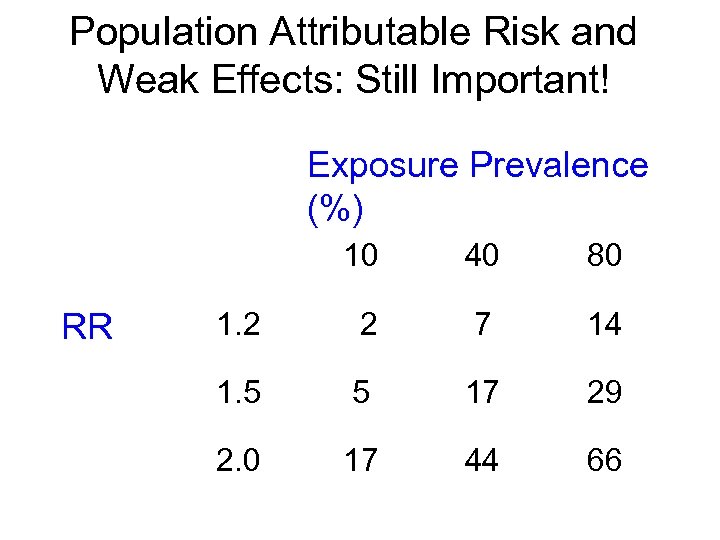 Population Attributable Risk and Weak Effects: Still Important! Exposure Prevalence (%) 10 RR 40 80 1. 2 2 7 14 1. 5 5 17 29 2. 0 17 44 66
Population Attributable Risk and Weak Effects: Still Important! Exposure Prevalence (%) 10 RR 40 80 1. 2 2 7 14 1. 5 5 17 29 2. 0 17 44 66
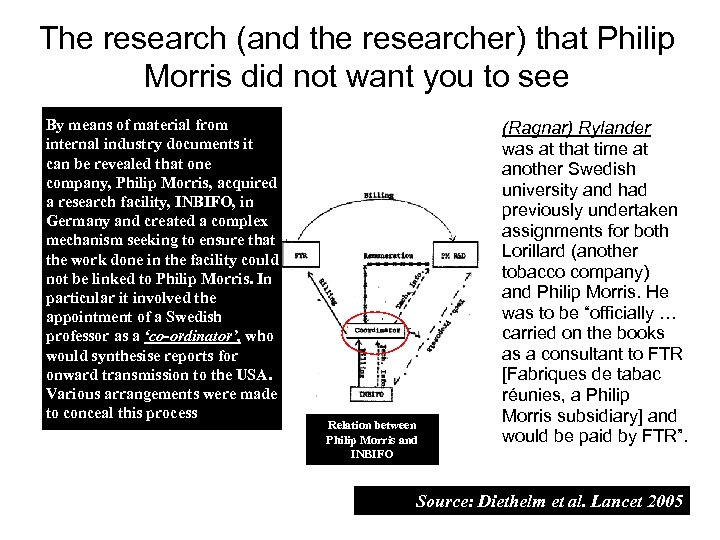 The research (and the researcher) that Philip Morris did not want you to see By means of material from internal industry documents it can be revealed that one company, Philip Morris, acquired a research facility, INBIFO, in Germany and created a complex mechanism seeking to ensure that the work done in the facility could not be linked to Philip Morris. In particular it involved the appointment of a Swedish professor as a ‘co-ordinator’, who would synthesise reports for onward transmission to the USA. Various arrangements were made to conceal this process Relation between Philip Morris and INBIFO (Ragnar) Rylander was at that time at another Swedish university and had previously undertaken assignments for both Lorillard (another tobacco company) and Philip Morris. He was to be “officially … carried on the books as a consultant to FTR [Fabriques de tabac réunies, a Philip Morris subsidiary] and would be paid by FTR”. Source: Diethelm et al. Lancet 2005
The research (and the researcher) that Philip Morris did not want you to see By means of material from internal industry documents it can be revealed that one company, Philip Morris, acquired a research facility, INBIFO, in Germany and created a complex mechanism seeking to ensure that the work done in the facility could not be linked to Philip Morris. In particular it involved the appointment of a Swedish professor as a ‘co-ordinator’, who would synthesise reports for onward transmission to the USA. Various arrangements were made to conceal this process Relation between Philip Morris and INBIFO (Ragnar) Rylander was at that time at another Swedish university and had previously undertaken assignments for both Lorillard (another tobacco company) and Philip Morris. He was to be “officially … carried on the books as a consultant to FTR [Fabriques de tabac réunies, a Philip Morris subsidiary] and would be paid by FTR”. Source: Diethelm et al. Lancet 2005
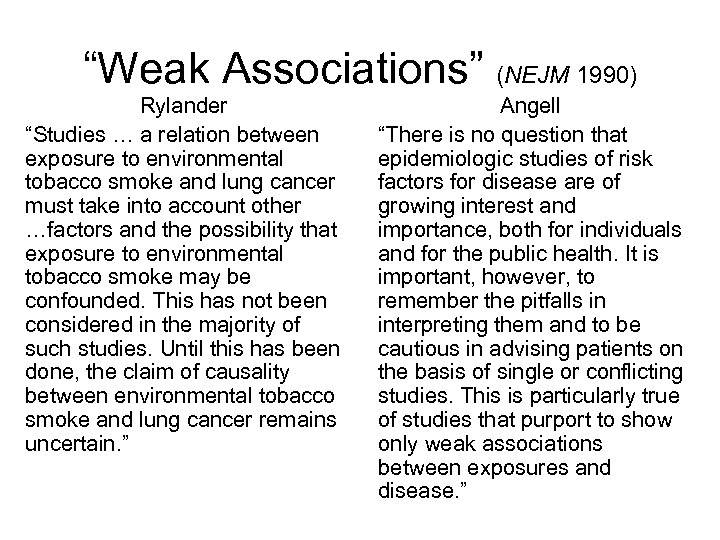 “Weak Associations” (NEJM 1990) Rylander “Studies … a relation between exposure to environmental tobacco smoke and lung cancer must take into account other …factors and the possibility that exposure to environmental tobacco smoke may be confounded. This has not been considered in the majority of such studies. Until this has been done, the claim of causality between environmental tobacco smoke and lung cancer remains uncertain. ” Angell “There is no question that epidemiologic studies of risk factors for disease are of growing interest and importance, both for individuals and for the public health. It is important, however, to remember the pitfalls in interpreting them and to be cautious in advising patients on the basis of single or conflicting studies. This is particularly true of studies that purport to show only weak associations between exposures and disease. ”
“Weak Associations” (NEJM 1990) Rylander “Studies … a relation between exposure to environmental tobacco smoke and lung cancer must take into account other …factors and the possibility that exposure to environmental tobacco smoke may be confounded. This has not been considered in the majority of such studies. Until this has been done, the claim of causality between environmental tobacco smoke and lung cancer remains uncertain. ” Angell “There is no question that epidemiologic studies of risk factors for disease are of growing interest and importance, both for individuals and for the public health. It is important, however, to remember the pitfalls in interpreting them and to be cautious in advising patients on the basis of single or conflicting studies. This is particularly true of studies that purport to show only weak associations between exposures and disease. ”
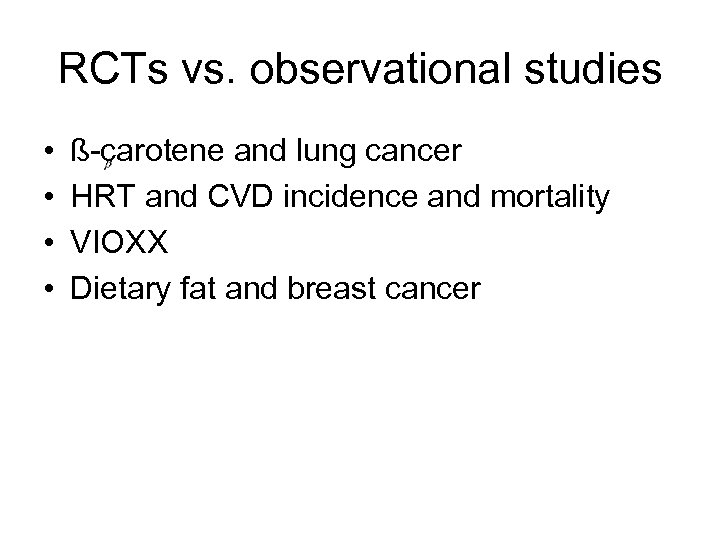 RCTs vs. observational studies • • ß-carotene and lung cancer HRT and CVD incidence and mortality VIOXX Dietary fat and breast cancer
RCTs vs. observational studies • • ß-carotene and lung cancer HRT and CVD incidence and mortality VIOXX Dietary fat and breast cancer
 - Standard designs & analysis sometimes not adequately controlling for - confounding - information bias - selection bias Wrong answer? - Agreed: We can be doing a better job - Not agreed: HOW
- Standard designs & analysis sometimes not adequately controlling for - confounding - information bias - selection bias Wrong answer? - Agreed: We can be doing a better job - Not agreed: HOW
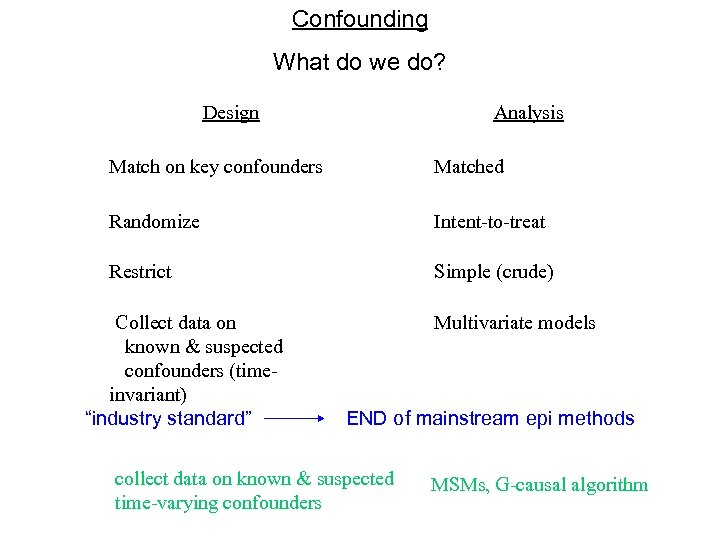 Confounding What do we do? Design Analysis Match on key confounders Matched Randomize Intent-to-treat Restrict Simple (crude) Collect data on known & suspected confounders (timeinvariant) “industry standard” Multivariate models END of mainstream epi methods collect data on known & suspected time-varying confounders MSMs, G-causal algorithm
Confounding What do we do? Design Analysis Match on key confounders Matched Randomize Intent-to-treat Restrict Simple (crude) Collect data on known & suspected confounders (timeinvariant) “industry standard” Multivariate models END of mainstream epi methods collect data on known & suspected time-varying confounders MSMs, G-causal algorithm
 Confounding – outstanding problems • unmeasured confounding • known or suspected confounders • unknown confounders Fact: ~ 47% of US breast cancer incidence explained by known risk factors (Madigan et al. , JNCI, 1987: 1681 -1695) r 2 in most epi regressions (blood pressure, serum hormones) 20%-40% (Pediatric Task Force on BP Control in Children, Pediatrics, 2004; Hankinson, personal communication) Undiscovered genes? Unimagined environmental factors? Complex non-linear interactions?
Confounding – outstanding problems • unmeasured confounding • known or suspected confounders • unknown confounders Fact: ~ 47% of US breast cancer incidence explained by known risk factors (Madigan et al. , JNCI, 1987: 1681 -1695) r 2 in most epi regressions (blood pressure, serum hormones) 20%-40% (Pediatric Task Force on BP Control in Children, Pediatrics, 2004; Hankinson, personal communication) Undiscovered genes? Unimagined environmental factors? Complex non-linear interactions?
 Solution to confounding by unknown risk factors: randomization VERY limited applicability Outstanding questions: a few strong risk factors or many weak ones? many rare ones or a few common ones? modeling of scenarios: do biases cancel? NEW IDEAS NEEDED
Solution to confounding by unknown risk factors: randomization VERY limited applicability Outstanding questions: a few strong risk factors or many weak ones? many rare ones or a few common ones? modeling of scenarios: do biases cancel? NEW IDEAS NEEDED
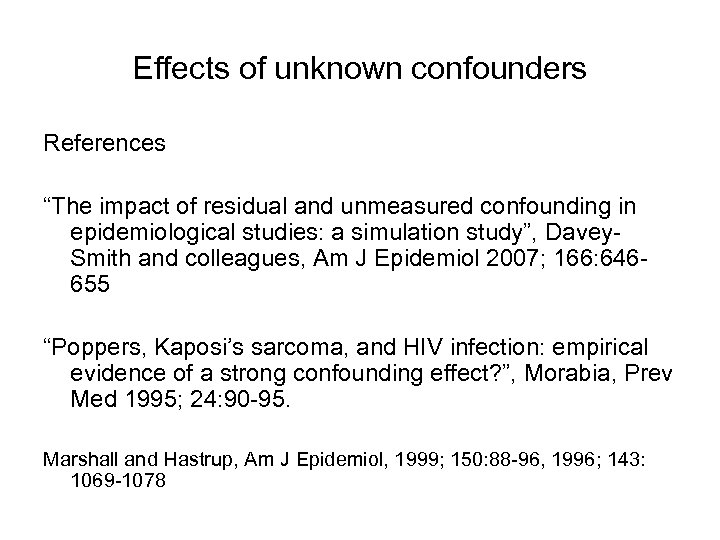 Effects of unknown confounders References “The impact of residual and unmeasured confounding in epidemiological studies: a simulation study”, Davey. Smith and colleagues, Am J Epidemiol 2007; 166: 646655 “Poppers, Kaposi’s sarcoma, and HIV infection: empirical evidence of a strong confounding effect? ”, Morabia, Prev Med 1995; 24: 90 -95. Marshall and Hastrup, Am J Epidemiol, 1999; 150: 88 -96, 1996; 143: 1069 -1078
Effects of unknown confounders References “The impact of residual and unmeasured confounding in epidemiological studies: a simulation study”, Davey. Smith and colleagues, Am J Epidemiol 2007; 166: 646655 “Poppers, Kaposi’s sarcoma, and HIV infection: empirical evidence of a strong confounding effect? ”, Morabia, Prev Med 1995; 24: 90 -95. Marshall and Hastrup, Am J Epidemiol, 1999; 150: 88 -96, 1996; 143: 1069 -1078
 Unmeasured confounding by known or suspected risk factors: We can use the data to get ‘the right answer’/’improve the validity of new studies’! Design: two-stage (Reilly & Salim, http: //meb. ki. se/~marrei/software/) Stage 1 (Di, Ei, C 1 i), i = 1, . . . , n Stage 2 (Di, Ei, C 1 i, C 2 i), i = 1, . . . , n 2 or (Ei, C 1 i, C 2 i), i = 1, . . . , n 2 So that (Di, Ei, C 1 i, . ), i = n 2+ 1, . . . , n 1 + n 2 n 1 >> n 2 Analysis: MLE of 2 -stage likelihood Cain & Breslow, AJE, 1988 References: Reilly M, 1996; Weinberg & Wacholder, 1990; Zhao & Lipsitz, 1992; Robins et al. , 1994; + many others
Unmeasured confounding by known or suspected risk factors: We can use the data to get ‘the right answer’/’improve the validity of new studies’! Design: two-stage (Reilly & Salim, http: //meb. ki. se/~marrei/software/) Stage 1 (Di, Ei, C 1 i), i = 1, . . . , n Stage 2 (Di, Ei, C 1 i, C 2 i), i = 1, . . . , n 2 or (Ei, C 1 i, C 2 i), i = 1, . . . , n 2 So that (Di, Ei, C 1 i, . ), i = n 2+ 1, . . . , n 1 + n 2 n 1 >> n 2 Analysis: MLE of 2 -stage likelihood Cain & Breslow, AJE, 1988 References: Reilly M, 1996; Weinberg & Wacholder, 1990; Zhao & Lipsitz, 1992; Robins et al. , 1994; + many others
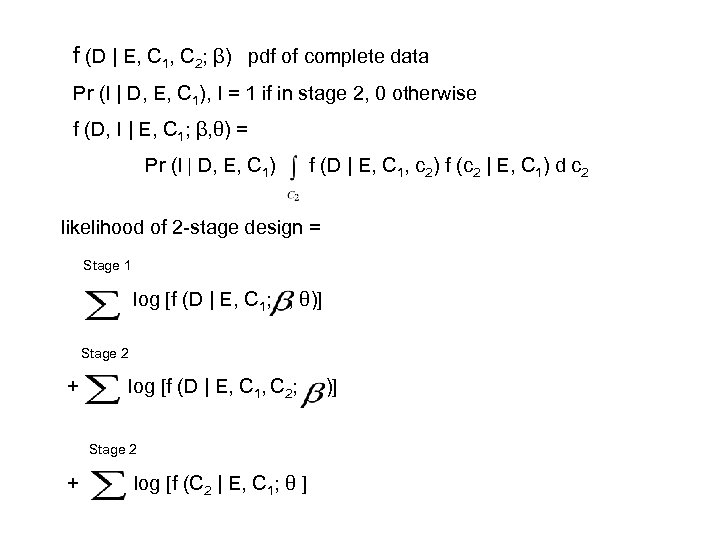 f (D | E, C 1, C 2; β) pdf of complete data Pr (I | D, E, C 1), I = 1 if in stage 2, 0 otherwise f (D, I | E, C 1; β, θ) = Pr (I | D, E, C 1) f (D | E, C 1, c 2) f (c 2 | E, C 1) d c 2 likelihood of 2 -stage design = Stage 1 log [f (D | E, C 1; , θ)] Stage 2 + log [f (D | E, C 1, C 2; Stage 2 + log [f (C 2 | E, C 1; θ ] )]
f (D | E, C 1, C 2; β) pdf of complete data Pr (I | D, E, C 1), I = 1 if in stage 2, 0 otherwise f (D, I | E, C 1; β, θ) = Pr (I | D, E, C 1) f (D | E, C 1, c 2) f (c 2 | E, C 1) d c 2 likelihood of 2 -stage design = Stage 1 log [f (D | E, C 1; , θ)] Stage 2 + log [f (D | E, C 1, C 2; Stage 2 + log [f (C 2 | E, C 1; θ ] )]
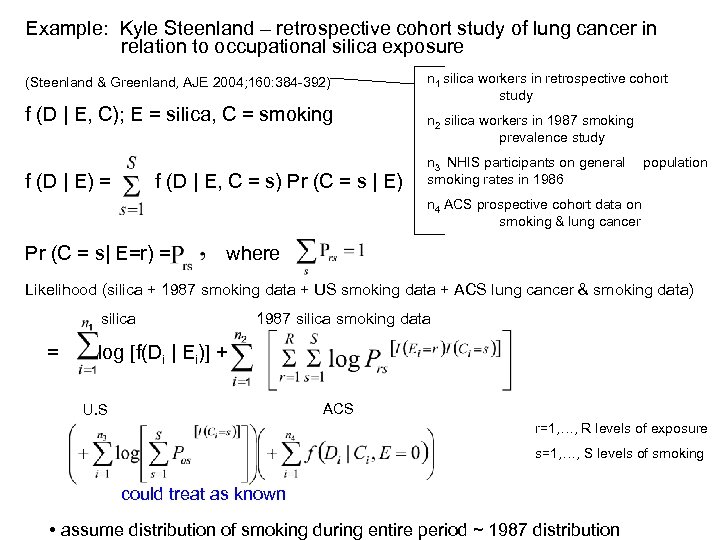 Example: Kyle Steenland – retrospective cohort study of lung cancer in relation to occupational silica exposure (Steenland & Greenland, AJE 2004; 160: 384 -392) f (D | E, C); E = silica, C = smoking f (D | E) = f (D | E, C = s) Pr (C = s | E) n 1 silica workers in retrospective cohort study n 2 silica workers in 1987 smoking prevalence study n 3 NHIS participants on general smoking rates in 1986 population n 4 ACS prospective cohort data on smoking & lung cancer Pr (C = s| E=r) = where Likelihood (silica + 1987 smoking data + US smoking data + ACS lung cancer & smoking data) silica = 1987 silica smoking data log [f(Di | Ei)] + ACS U. S r=1, …, R levels of exposure s=1, …, S levels of smoking could treat as known • assume distribution of smoking during entire period ~ 1987 distribution
Example: Kyle Steenland – retrospective cohort study of lung cancer in relation to occupational silica exposure (Steenland & Greenland, AJE 2004; 160: 384 -392) f (D | E, C); E = silica, C = smoking f (D | E) = f (D | E, C = s) Pr (C = s | E) n 1 silica workers in retrospective cohort study n 2 silica workers in 1987 smoking prevalence study n 3 NHIS participants on general smoking rates in 1986 population n 4 ACS prospective cohort data on smoking & lung cancer Pr (C = s| E=r) = where Likelihood (silica + 1987 smoking data + US smoking data + ACS lung cancer & smoking data) silica = 1987 silica smoking data log [f(Di | Ei)] + ACS U. S r=1, …, R levels of exposure s=1, …, S levels of smoking could treat as known • assume distribution of smoking during entire period ~ 1987 distribution
 Obstacles: software? Design software available; Offsets or weights in PROC GENMOD or PROC PHREG can be used for analysis training? funding? Result: The right answer? A better answer? Is it worth it?
Obstacles: software? Design software available; Offsets or weights in PROC GENMOD or PROC PHREG can be used for analysis training? funding? Result: The right answer? A better answer? Is it worth it?
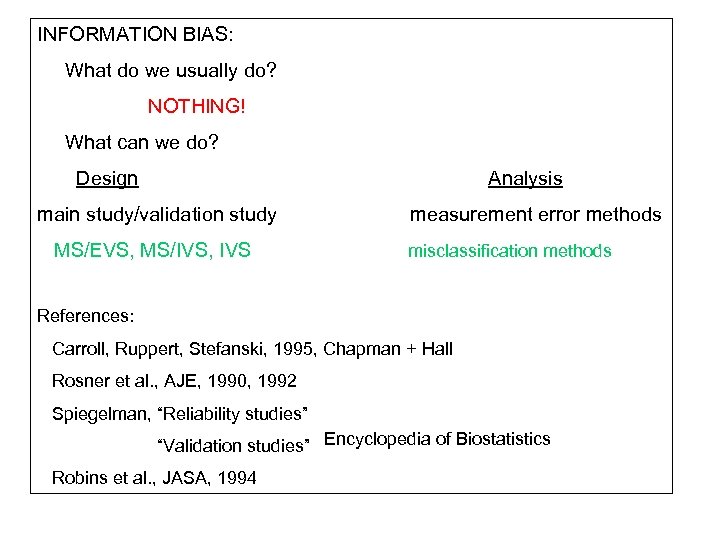 INFORMATION BIAS: What do we usually do? NOTHING! What can we do? Design Analysis main study/validation study MS/EVS, MS/IVS, IVS measurement error methods misclassification methods References: Carroll, Ruppert, Stefanski, 1995, Chapman + Hall Rosner et al. , AJE, 1990, 1992 Spiegelman, “Reliability studies” “Validation studies” Encyclopedia of Biostatistics Robins et al. , JASA, 1994
INFORMATION BIAS: What do we usually do? NOTHING! What can we do? Design Analysis main study/validation study MS/EVS, MS/IVS, IVS measurement error methods misclassification methods References: Carroll, Ruppert, Stefanski, 1995, Chapman + Hall Rosner et al. , AJE, 1990, 1992 Spiegelman, “Reliability studies” “Validation studies” Encyclopedia of Biostatistics Robins et al. , JASA, 1994
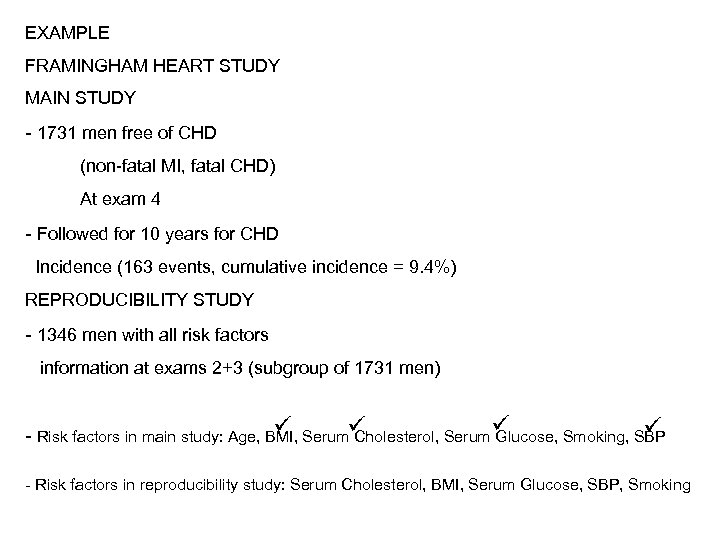 EXAMPLE FRAMINGHAM HEART STUDY MAIN STUDY - 1731 men free of CHD (non-fatal MI, fatal CHD) At exam 4 - Followed for 10 years for CHD Incidence (163 events, cumulative incidence = 9. 4%) REPRODUCIBILITY STUDY - 1346 men with all risk factors information at exams 2+3 (subgroup of 1731 men) - Risk factors in main study: Age, BMI, Serum Cholesterol, Serum Glucose, Smoking, SBP - Risk factors in reproducibility study: Serum Cholesterol, BMI, Serum Glucose, SBP, Smoking
EXAMPLE FRAMINGHAM HEART STUDY MAIN STUDY - 1731 men free of CHD (non-fatal MI, fatal CHD) At exam 4 - Followed for 10 years for CHD Incidence (163 events, cumulative incidence = 9. 4%) REPRODUCIBILITY STUDY - 1346 men with all risk factors information at exams 2+3 (subgroup of 1731 men) - Risk factors in main study: Age, BMI, Serum Cholesterol, Serum Glucose, Smoking, SBP - Risk factors in reproducibility study: Serum Cholesterol, BMI, Serum Glucose, SBP, Smoking
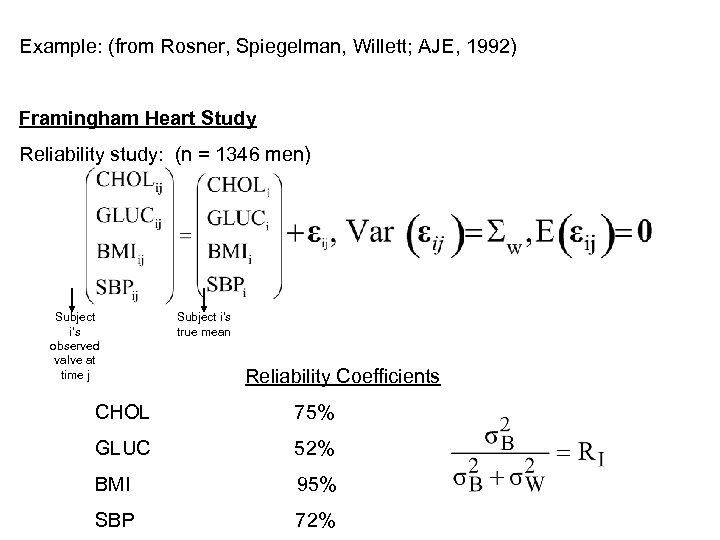 Example: (from Rosner, Spiegelman, Willett; AJE, 1992) Framingham Heart Study Reliability study: (n = 1346 men) Subject i’s observed valve at time j Subject i’s true mean Reliability Coefficients CHOL 75% GLUC 52% BMI 95% SBP 72%
Example: (from Rosner, Spiegelman, Willett; AJE, 1992) Framingham Heart Study Reliability study: (n = 1346 men) Subject i’s observed valve at time j Subject i’s true mean Reliability Coefficients CHOL 75% GLUC 52% BMI 95% SBP 72%
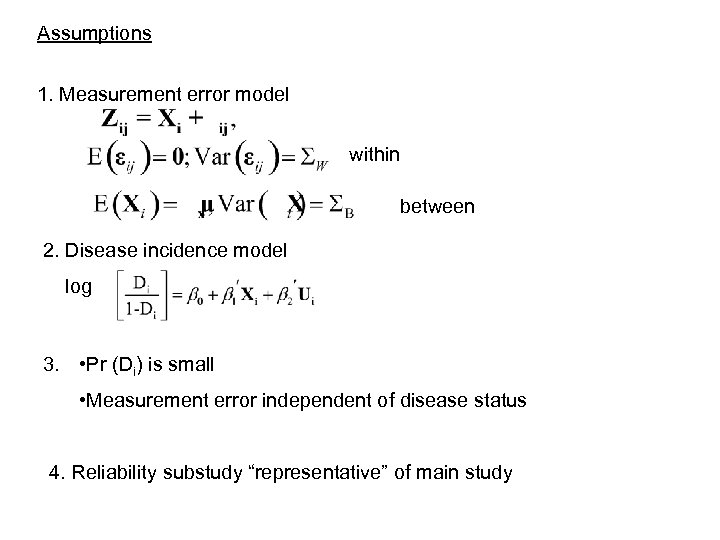 Assumptions 1. Measurement error model within between 2. Disease incidence model log 3. • Pr (Di) is small • Measurement error independent of disease status 4. Reliability substudy “representative” of main study
Assumptions 1. Measurement error model within between 2. Disease incidence model log 3. • Pr (Di) is small • Measurement error independent of disease status 4. Reliability substudy “representative” of main study
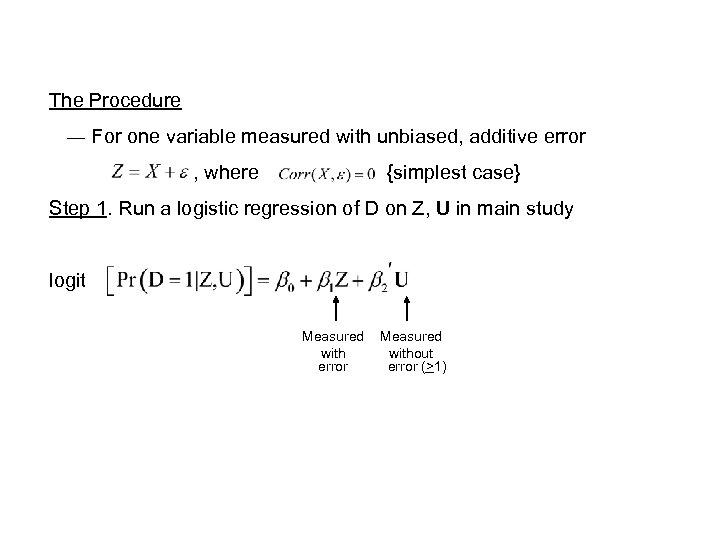 The Procedure ― For one variable measured with unbiased, additive error , where {simplest case} Step 1. Run a logistic regression of D on Z, U in main study logit Measured with error Measured without error (>1)
The Procedure ― For one variable measured with unbiased, additive error , where {simplest case} Step 1. Run a logistic regression of D on Z, U in main study logit Measured with error Measured without error (>1)
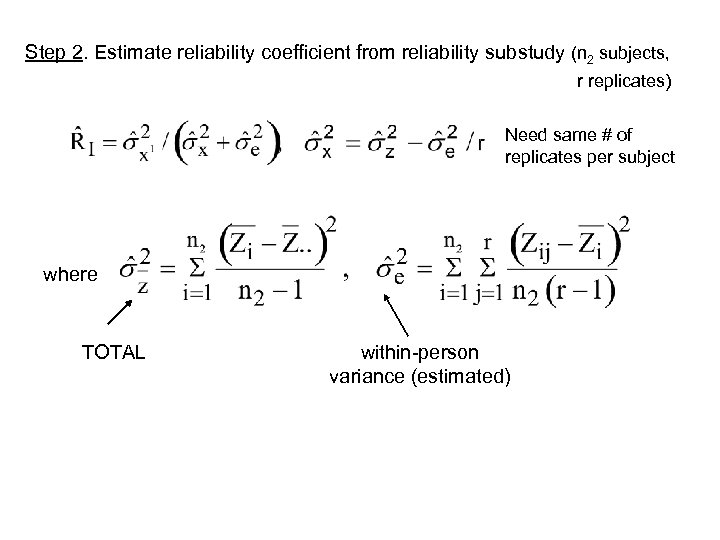 Step 2. Estimate reliability coefficient from reliability substudy (n 2 subjects, r replicates) Need same # of replicates per subject where TOTAL within-person variance (estimated)
Step 2. Estimate reliability coefficient from reliability substudy (n 2 subjects, r replicates) Need same # of replicates per subject where TOTAL within-person variance (estimated)
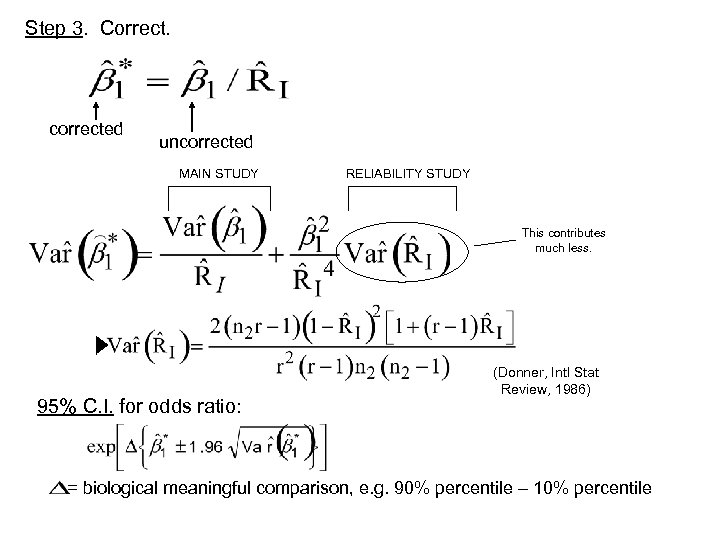 Step 3. Correct. corrected uncorrected MAIN STUDY RELIABILITY STUDY This contributes much less. 95% C. I. for odds ratio: (Donner, Intl Stat Review, 1986) = biological meaningful comparison, e. g. 90% percentile – 10% percentile
Step 3. Correct. corrected uncorrected MAIN STUDY RELIABILITY STUDY This contributes much less. 95% C. I. for odds ratio: (Donner, Intl Stat Review, 1986) = biological meaningful comparison, e. g. 90% percentile – 10% percentile
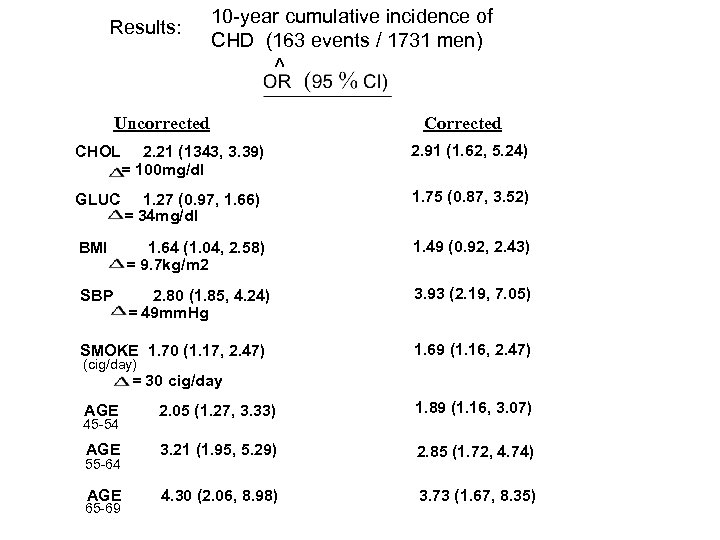 Results: 10 -year cumulative incidence of CHD (163 events / 1731 men) ^ Uncorrected CHOL 2. 21 (1343, 3. 39) = 100 mg/dl 2. 91 (1. 62, 5. 24) GLUC 1. 27 (0. 97, 1. 66) = 34 mg/dl 1. 75 (0. 87, 3. 52) BMI 1. 64 (1. 04, 2. 58) = 9. 7 kg/m 2 1. 49 (0. 92, 2. 43) SBP 2. 80 (1. 85, 4. 24) = 49 mm. Hg 3. 93 (2. 19, 7. 05) SMOKE 1. 70 (1. 17, 2. 47) (cig/day) 1. 69 (1. 16, 2. 47) = 30 cig/day AGE 2. 05 (1. 27, 3. 33) 1. 89 (1. 16, 3. 07) AGE 3. 21 (1. 95, 5. 29) 2. 85 (1. 72, 4. 74) AGE 4. 30 (2. 06, 8. 98) 3. 73 (1. 67, 8. 35) 45 -54 55 -64 65 -69
Results: 10 -year cumulative incidence of CHD (163 events / 1731 men) ^ Uncorrected CHOL 2. 21 (1343, 3. 39) = 100 mg/dl 2. 91 (1. 62, 5. 24) GLUC 1. 27 (0. 97, 1. 66) = 34 mg/dl 1. 75 (0. 87, 3. 52) BMI 1. 64 (1. 04, 2. 58) = 9. 7 kg/m 2 1. 49 (0. 92, 2. 43) SBP 2. 80 (1. 85, 4. 24) = 49 mm. Hg 3. 93 (2. 19, 7. 05) SMOKE 1. 70 (1. 17, 2. 47) (cig/day) 1. 69 (1. 16, 2. 47) = 30 cig/day AGE 2. 05 (1. 27, 3. 33) 1. 89 (1. 16, 3. 07) AGE 3. 21 (1. 95, 5. 29) 2. 85 (1. 72, 4. 74) AGE 4. 30 (2. 06, 8. 98) 3. 73 (1. 67, 8. 35) 45 -54 55 -64 65 -69
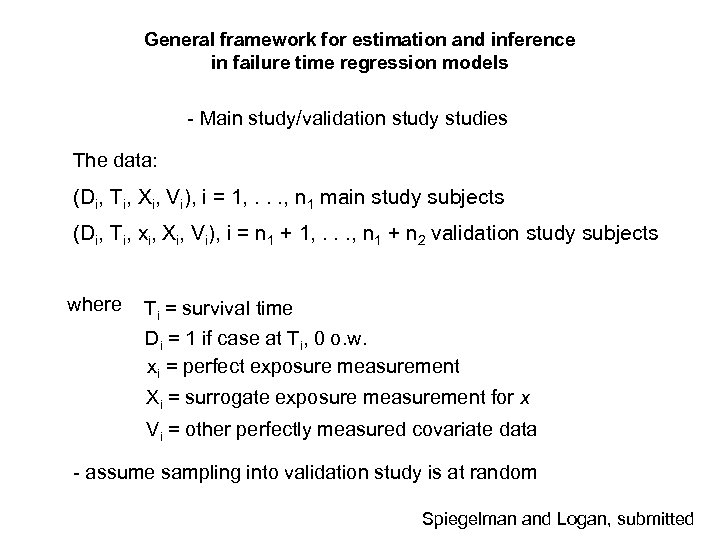 General framework for estimation and inference in failure time regression models - Main study/validation study studies The data: (Di, Ti, Xi, Vi), i = 1, . . . , n 1 main study subjects (Di, Ti, xi, Xi, Vi), i = n 1 + 1, . . . , n 1 + n 2 validation study subjects where Ti = survival time Di = 1 if case at Ti, 0 o. w. xi = perfect exposure measurement Xi = surrogate exposure measurement for x Vi = other perfectly measured covariate data - assume sampling into validation study is at random Spiegelman and Logan, submitted
General framework for estimation and inference in failure time regression models - Main study/validation study studies The data: (Di, Ti, Xi, Vi), i = 1, . . . , n 1 main study subjects (Di, Ti, xi, Xi, Vi), i = n 1 + 1, . . . , n 1 + n 2 validation study subjects where Ti = survival time Di = 1 if case at Ti, 0 o. w. xi = perfect exposure measurement Xi = surrogate exposure measurement for x Vi = other perfectly measured covariate data - assume sampling into validation study is at random Spiegelman and Logan, submitted
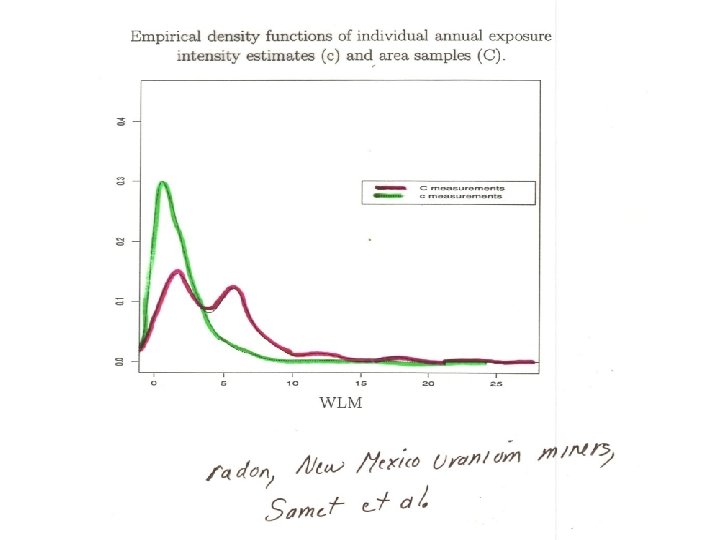
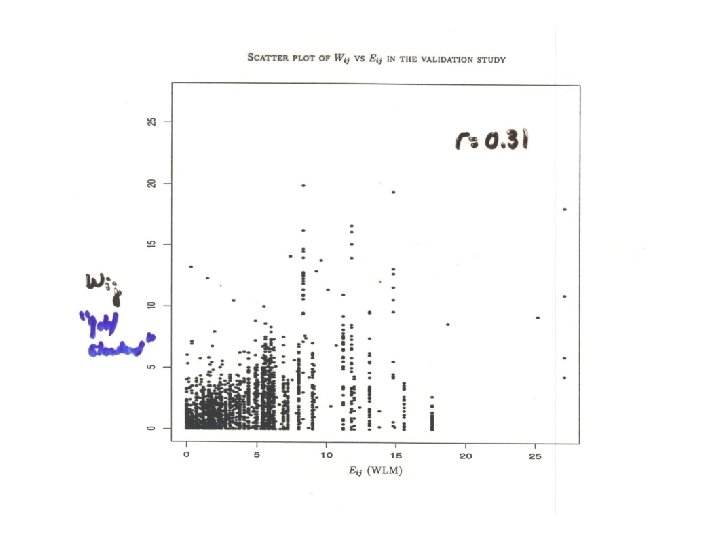
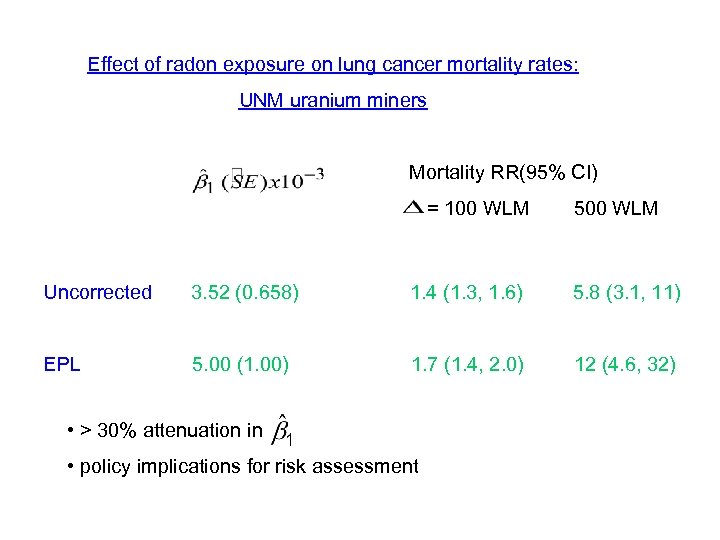 Effect of radon exposure on lung cancer mortality rates: UNM uranium miners Mortality RR(95% CI) = 100 WLM 500 WLM Uncorrected 3. 52 (0. 658) 1. 4 (1. 3, 1. 6) 5. 8 (3. 1, 11) EPL 5. 00 (1. 00) 1. 7 (1. 4, 2. 0) 12 (4. 6, 32) • > 30% attenuation in • policy implications for risk assessment
Effect of radon exposure on lung cancer mortality rates: UNM uranium miners Mortality RR(95% CI) = 100 WLM 500 WLM Uncorrected 3. 52 (0. 658) 1. 4 (1. 3, 1. 6) 5. 8 (3. 1, 11) EPL 5. 00 (1. 00) 1. 7 (1. 4, 2. 0) 12 (4. 6, 32) • > 30% attenuation in • policy implications for risk assessment
 Nutritional epidemiology: Tworoger SS, Eliassen AH, Rosner B, Sluss P, Hankinson SE. Plasma prolaction concentrations and risk of premenopausal breast cancer. Cancer Research, 2004; 64: 6814 -6819. Hankinson SE, Willett WC, Michaud DS, Manson JE, Colditz GA, Longcope C, Rosner B, Speizer FE. Plasma prolaction levels and subsequent risk of breast cancer in postmenopausal women. Journal of the National Cancer Institute 1999; 91: 629 -634. Smith-Warner SA, Spiegelman D, Adami H, Beeson L, van den Brandt P, Folsom A, Fraser G, Freudenheim J, Goldbohm R, Graham S, Kushi L, Miller A, Rohan T, Speizer FE, Toniolo P, Willett WC, Wolk A, Zeleniuch-Jacquotte A, Hunter DJ. Types of dietary fat and breast cancer: a pooled analysis of cohort studies. International Journal of Cancer 2001; 92: 767 -774. Holmes MD, Stampfer MJ, Wolf AM, Jones CP, Spiegelman D, Manson JE, Coldditz GA. Can behavioral risk factors explain the difference in body mass index between African-American and European-American women? Ethnicity and Disease 1999; 8: 331 -339. Rich-Edwards JW, Hu F, Michels K, Stampfer MJ, Manson JE, Rosner B, Willett WC. Breastfeeding in infancy and risk of cardiovascular disease in adult women. Epidemiology, 2004; 15: 550 -556. Koh-Banerjee P, Chu NF, Spiegelman D, Rosner B, Colditz GA, Willett WC, Rimm EB. Prospective study of the association of changes in dietary intake, physical activity, alcohol consumption, and smoking with 9 -year gain in wais circumference among 15, 587 men. Am J Clin Nutr 2003; 78: 719 -727. Koh-Banerjee P, Franz M, Sampson L, Liu S, Jacobs Jr. DR, Spiegelman D, Willett WC, Rimm EB. Changes in whole grain, bran and cereal fiber consumption in relation to 8 -year weight gain among men. Am J Clin Nutr, 2004; 5: 1237 -1245.
Nutritional epidemiology: Tworoger SS, Eliassen AH, Rosner B, Sluss P, Hankinson SE. Plasma prolaction concentrations and risk of premenopausal breast cancer. Cancer Research, 2004; 64: 6814 -6819. Hankinson SE, Willett WC, Michaud DS, Manson JE, Colditz GA, Longcope C, Rosner B, Speizer FE. Plasma prolaction levels and subsequent risk of breast cancer in postmenopausal women. Journal of the National Cancer Institute 1999; 91: 629 -634. Smith-Warner SA, Spiegelman D, Adami H, Beeson L, van den Brandt P, Folsom A, Fraser G, Freudenheim J, Goldbohm R, Graham S, Kushi L, Miller A, Rohan T, Speizer FE, Toniolo P, Willett WC, Wolk A, Zeleniuch-Jacquotte A, Hunter DJ. Types of dietary fat and breast cancer: a pooled analysis of cohort studies. International Journal of Cancer 2001; 92: 767 -774. Holmes MD, Stampfer MJ, Wolf AM, Jones CP, Spiegelman D, Manson JE, Coldditz GA. Can behavioral risk factors explain the difference in body mass index between African-American and European-American women? Ethnicity and Disease 1999; 8: 331 -339. Rich-Edwards JW, Hu F, Michels K, Stampfer MJ, Manson JE, Rosner B, Willett WC. Breastfeeding in infancy and risk of cardiovascular disease in adult women. Epidemiology, 2004; 15: 550 -556. Koh-Banerjee P, Chu NF, Spiegelman D, Rosner B, Colditz GA, Willett WC, Rimm EB. Prospective study of the association of changes in dietary intake, physical activity, alcohol consumption, and smoking with 9 -year gain in wais circumference among 15, 587 men. Am J Clin Nutr 2003; 78: 719 -727. Koh-Banerjee P, Franz M, Sampson L, Liu S, Jacobs Jr. DR, Spiegelman D, Willett WC, Rimm EB. Changes in whole grain, bran and cereal fiber consumption in relation to 8 -year weight gain among men. Am J Clin Nutr, 2004; 5: 1237 -1245.
 Environmental epidemiology Keshaviah AP, Weller EA, Spiegelman D. Occupational exposure to methyl tertiary-butyl ether in relation to key health symptom prevalence: the effect of measurement error correction. Environmetrics, 2002; 14: 573 -582. Thurston SW, Williams P, Hauser R, Hu H, Hernandez-Avila M, Spiegelman D. A comparison of regression calibration methods for measurement error in main study/internal validation study designs. Journal of Statistical Planning and Inference, 2005; 131: 175 -190. Fetal lead exposure in relation to birth weight; MS/IVS; bone lead vs. cord lead (r=0. 19) Weller EA, Milton DK, Eisen EA, Spiegelman D. Regression calibration for logistic regression with multiple surrogates for one exposure. Journal of Statistical Planning and Inference, 2007; 137: 449 -461. working fluids exposure in relation to lung function; Metal MS/EVS; job characteristics vs. personal monitors (r=0. 82) Horick N, Milton DK, Gold D, Weller E, Spiegelman D. Household dust endotoxin exposure and respiratory effects in infants: correction for measurement error bias. Environmental Health Perspectives, 2006; 114: 135 -140. Li R, Weller EA, Dockery DW, Neas LM, Spiegelman D. Association of indoor nitrogen dioxide with respiratory symptoms in children: the effect of measurement error correction with multiple surrogates. Journal of Exposure Analysis and Environmental Epidemiology, 2006; 16: 342 -350.
Environmental epidemiology Keshaviah AP, Weller EA, Spiegelman D. Occupational exposure to methyl tertiary-butyl ether in relation to key health symptom prevalence: the effect of measurement error correction. Environmetrics, 2002; 14: 573 -582. Thurston SW, Williams P, Hauser R, Hu H, Hernandez-Avila M, Spiegelman D. A comparison of regression calibration methods for measurement error in main study/internal validation study designs. Journal of Statistical Planning and Inference, 2005; 131: 175 -190. Fetal lead exposure in relation to birth weight; MS/IVS; bone lead vs. cord lead (r=0. 19) Weller EA, Milton DK, Eisen EA, Spiegelman D. Regression calibration for logistic regression with multiple surrogates for one exposure. Journal of Statistical Planning and Inference, 2007; 137: 449 -461. working fluids exposure in relation to lung function; Metal MS/EVS; job characteristics vs. personal monitors (r=0. 82) Horick N, Milton DK, Gold D, Weller E, Spiegelman D. Household dust endotoxin exposure and respiratory effects in infants: correction for measurement error bias. Environmental Health Perspectives, 2006; 114: 135 -140. Li R, Weller EA, Dockery DW, Neas LM, Spiegelman D. Association of indoor nitrogen dioxide with respiratory symptoms in children: the effect of measurement error correction with multiple surrogates. Journal of Exposure Analysis and Environmental Epidemiology, 2006; 16: 342 -350.
 SOFTWARE IS AVAILABLE! • http: /www. hsph. harvard. edu/facres/spglmn. html SAS macros for regression calibration (Rosner et al. , AJE, 1990, 1992; Spiegelman et al. , AJCN, 1997; Spiegelman et al, SIM, 2001) in main study/validation study designs • STATA (Carroll et al. SIMEX, regression calibration) So why are methods under-utilized? No validation data Insufficient training of statisticians & epidemiologists Either/or about assumptions
SOFTWARE IS AVAILABLE! • http: /www. hsph. harvard. edu/facres/spglmn. html SAS macros for regression calibration (Rosner et al. , AJE, 1990, 1992; Spiegelman et al. , AJCN, 1997; Spiegelman et al, SIM, 2001) in main study/validation study designs • STATA (Carroll et al. SIMEX, regression calibration) So why are methods under-utilized? No validation data Insufficient training of statisticians & epidemiologists Either/or about assumptions
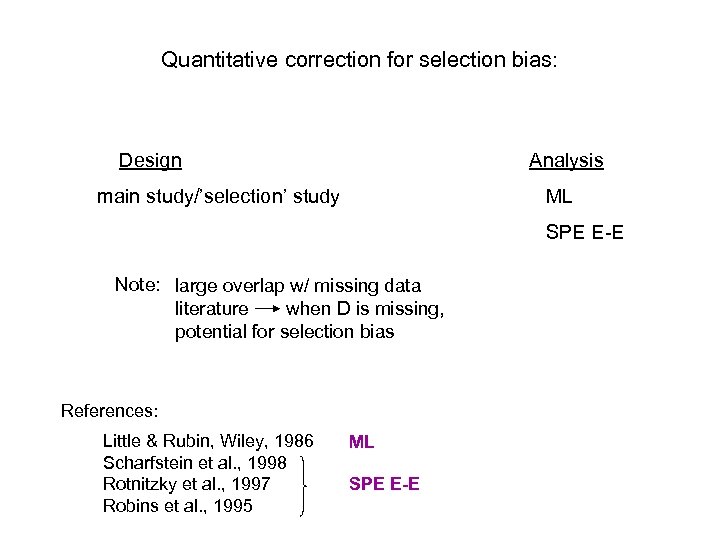 Quantitative correction for selection bias: Design Analysis main study/’selection’ study ML SPE E-E Note: large overlap w/ missing data literature when D is missing, potential for selection bias References: Little & Rubin, Wiley, 1986 Scharfstein et al. , 1998 Rotnitzky et al. , 1997 Robins et al. , 1995 ML SPE E-E
Quantitative correction for selection bias: Design Analysis main study/’selection’ study ML SPE E-E Note: large overlap w/ missing data literature when D is missing, potential for selection bias References: Little & Rubin, Wiley, 1986 Scharfstein et al. , 1998 Rotnitzky et al. , 1997 Robins et al. , 1995 ML SPE E-E
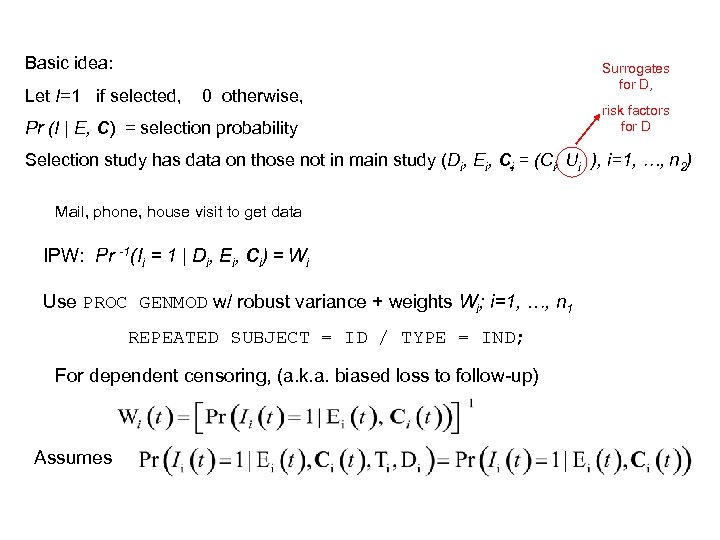 Basic idea: Let I=1 if selected, 0 otherwise, Pr (I | E, C) = selection probability Surrogates for D, risk factors for D Selection study has data on those not in main study (Di, Ei, Ci = (Ci, Ui ), i=1, …, n 2) Mail, phone, house visit to get data IPW: Pr -1(Ii = 1 | Di, Ei, Ci) = Wi Use PROC GENMOD w/ robust variance + weights Wi; i=1, …, n 1 REPEATED SUBJECT = ID / TYPE = IND; For dependent censoring, (a. k. a. biased loss to follow-up) Assumes
Basic idea: Let I=1 if selected, 0 otherwise, Pr (I | E, C) = selection probability Surrogates for D, risk factors for D Selection study has data on those not in main study (Di, Ei, Ci = (Ci, Ui ), i=1, …, n 2) Mail, phone, house visit to get data IPW: Pr -1(Ii = 1 | Di, Ei, Ci) = Wi Use PROC GENMOD w/ robust variance + weights Wi; i=1, …, n 1 REPEATED SUBJECT = ID / TYPE = IND; For dependent censoring, (a. k. a. biased loss to follow-up) Assumes
 ENAR 2007 Spring Meeting Double Sampling Designs for Addressing Loss to Follow-up In Estimating Mortality Ming-Wen An, Johns Hopkins University Constantine Frangakis, Johns Hopkins University Donald B. Rubin, Harvard University Constantin T. Yiannoutsos, Indiana University School of Medicine Loss to follow-up is an important challenge that arises when estimating mortality, and is particular concern in developing countries. In the absence of more active follow-up systems, resulting mortality estimates may be biased. One design approach to address this is ‘double sampling’, where a subset of patients who are lost to follow-up is chosen to be actively followed, often subject to resource constraints, with the goal of obtaining valid and efficient estimators. We demonstrate our results using data from Africa, which were collected to estimate HIV mortality as part of the evaluation of the President’s Emergency Plan for AIDS Relief (PEPFAR).
ENAR 2007 Spring Meeting Double Sampling Designs for Addressing Loss to Follow-up In Estimating Mortality Ming-Wen An, Johns Hopkins University Constantine Frangakis, Johns Hopkins University Donald B. Rubin, Harvard University Constantin T. Yiannoutsos, Indiana University School of Medicine Loss to follow-up is an important challenge that arises when estimating mortality, and is particular concern in developing countries. In the absence of more active follow-up systems, resulting mortality estimates may be biased. One design approach to address this is ‘double sampling’, where a subset of patients who are lost to follow-up is chosen to be actively followed, often subject to resource constraints, with the goal of obtaining valid and efficient estimators. We demonstrate our results using data from Africa, which were collected to estimate HIV mortality as part of the evaluation of the President’s Emergency Plan for AIDS Relief (PEPFAR).
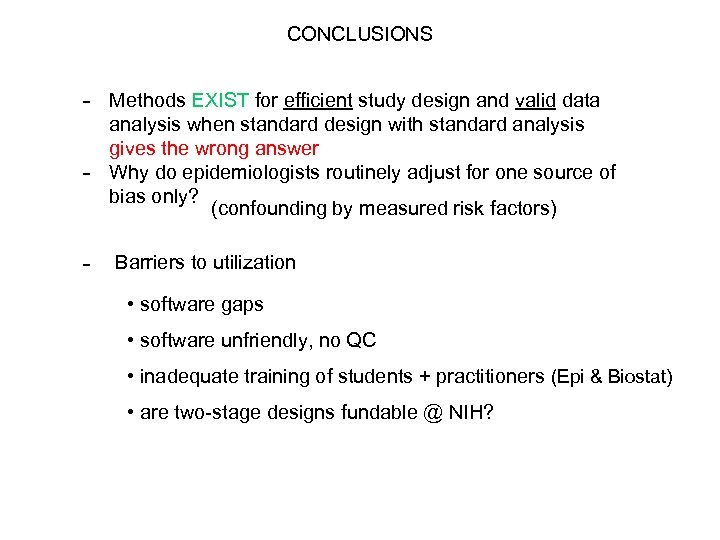 CONCLUSIONS - Methods EXIST for efficient study design and valid data analysis when standard design with standard analysis gives the wrong answer Why do epidemiologists routinely adjust for one source of bias only? (confounding by measured risk factors) Barriers to utilization • software gaps • software unfriendly, no QC • inadequate training of students + practitioners (Epi & Biostat) • are two-stage designs fundable @ NIH?
CONCLUSIONS - Methods EXIST for efficient study design and valid data analysis when standard design with standard analysis gives the wrong answer Why do epidemiologists routinely adjust for one source of bias only? (confounding by measured risk factors) Barriers to utilization • software gaps • software unfriendly, no QC • inadequate training of students + practitioners (Epi & Biostat) • are two-stage designs fundable @ NIH?


