e0050ad5f8aa6dfe03087ea709cba601.ppt
- Количество слайдов: 35
 How many picture frames can you make? DIRECTIONS: Find your new lab group, get the bag assigned to your group, and determine 1) How many picture frames you can make given the supplies provided: wooden splints = 1 side of the frame aluminum foil = glass hookeyes = hookeyes for stringing the wire on the back wire = hanging wire 2) How many of each other type of supplies you will need to buy. See if you can use DIMENSIONAL ANALYSIS to do this!! 3) The “EQUATION” or recipe for making a picture frame. 4) Bring me your group’s answers on paper as soon as you are finished!! Then sit down at your desk.
How many picture frames can you make? DIRECTIONS: Find your new lab group, get the bag assigned to your group, and determine 1) How many picture frames you can make given the supplies provided: wooden splints = 1 side of the frame aluminum foil = glass hookeyes = hookeyes for stringing the wire on the back wire = hanging wire 2) How many of each other type of supplies you will need to buy. See if you can use DIMENSIONAL ANALYSIS to do this!! 3) The “EQUATION” or recipe for making a picture frame. 4) Bring me your group’s answers on paper as soon as you are finished!! Then sit down at your desk.
 If you have 36 pieces of wood, how many picture frames can you make?
If you have 36 pieces of wood, how many picture frames can you make?
 If you have 36 pieces of wood, how many picture frames can you make? If you need 7 picture frames, how many pieces of glass do you need?
If you have 36 pieces of wood, how many picture frames can you make? If you need 7 picture frames, how many pieces of glass do you need?
 If you have 36 pieces of wood, how many picture frames can you make? If you need 7 picture frames, how many pieces of glass do you need? If you have 22 picture hanger hooks, how many picture frames can you mak
If you have 36 pieces of wood, how many picture frames can you make? If you need 7 picture frames, how many pieces of glass do you need? If you have 22 picture hanger hooks, how many picture frames can you mak
 If you have 36 pieces of wood, how many picture frames can you make? If you need 7 picture frames, how many pieces of glass do you need? If you have 22 picture hanger hooks, how many picture frames can you make? If you have 9 pieces of wire, how many picture frames can you make?
If you have 36 pieces of wood, how many picture frames can you make? If you need 7 picture frames, how many pieces of glass do you need? If you have 22 picture hanger hooks, how many picture frames can you make? If you have 9 pieces of wire, how many picture frames can you make?
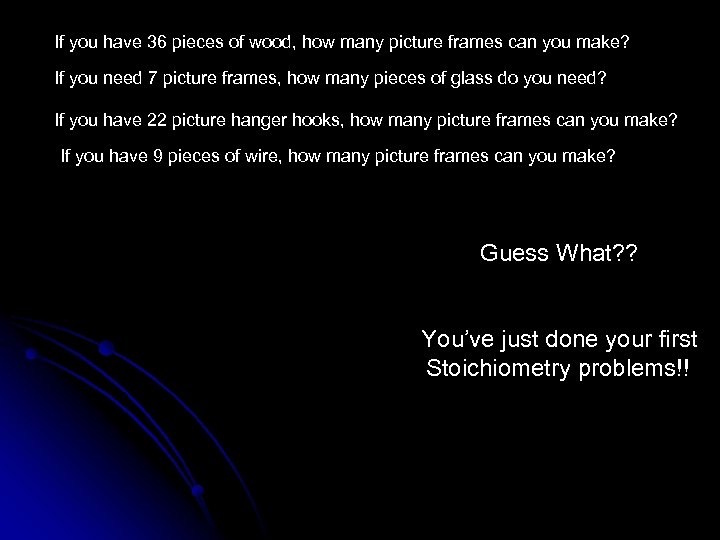 If you have 36 pieces of wood, how many picture frames can you make? If you need 7 picture frames, how many pieces of glass do you need? If you have 22 picture hanger hooks, how many picture frames can you make? If you have 9 pieces of wire, how many picture frames can you make? Guess What? ? You’ve just done your first Stoichiometry problems!!
If you have 36 pieces of wood, how many picture frames can you make? If you need 7 picture frames, how many pieces of glass do you need? If you have 22 picture hanger hooks, how many picture frames can you make? If you have 9 pieces of wire, how many picture frames can you make? Guess What? ? You’ve just done your first Stoichiometry problems!!
 If you have 36 pieces of wood, how many picture frames can you make? If you need 7 picture frames, how many pieces of glass do you need? If you have 22 picture hanger hooks, how many picture frames can you make? If you have 9 pieces of wire, how many picture frames can you make? Guess What? ? You’ve just done your first Stoichiometry problems!! 4 wood pieces + 1 piece of glass + 2 hooks + 1 wire = 1 frame!
If you have 36 pieces of wood, how many picture frames can you make? If you need 7 picture frames, how many pieces of glass do you need? If you have 22 picture hanger hooks, how many picture frames can you make? If you have 9 pieces of wire, how many picture frames can you make? Guess What? ? You’ve just done your first Stoichiometry problems!! 4 wood pieces + 1 piece of glass + 2 hooks + 1 wire = 1 frame!
 Stoichiometry Why is it important?
Stoichiometry Why is it important?
 Stoichiometry What would happen if too much or not enough “air” was produced during a crash?
Stoichiometry What would happen if too much or not enough “air” was produced during a crash?
 Picture Frames and Chemistry…Huh!? !? l l Just like picture frames are composed of parts in certain ratios, chemists have ratios as well Chemists call them reaction equations Furthermore, we use moles to count what we need Lastly, instead of wood, glass, wire, and hooks, we use chemical compounds as sodium azide, or oxygen and hydrogen!
Picture Frames and Chemistry…Huh!? !? l l Just like picture frames are composed of parts in certain ratios, chemists have ratios as well Chemists call them reaction equations Furthermore, we use moles to count what we need Lastly, instead of wood, glass, wire, and hooks, we use chemical compounds as sodium azide, or oxygen and hydrogen!
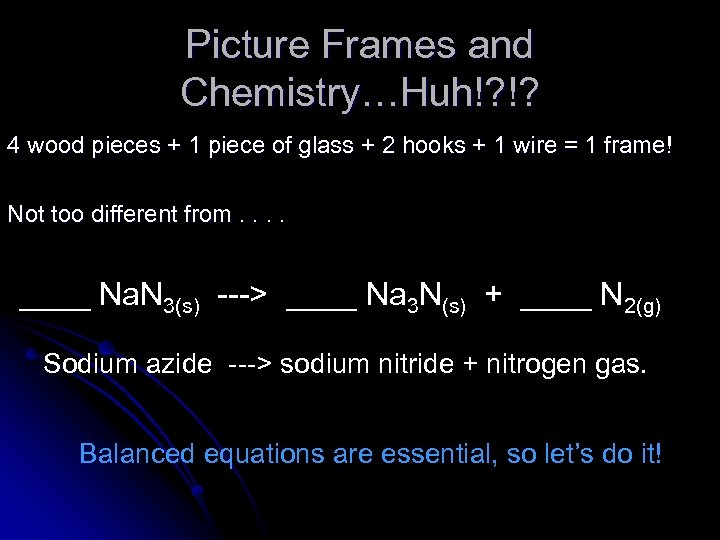 Picture Frames and Chemistry…Huh!? !? 4 wood pieces + 1 piece of glass + 2 hooks + 1 wire = 1 frame! Not too different from. . ____ Na. N 3(s) ---> ____ Na 3 N(s) + ____ N 2(g) Sodium azide ---> sodium nitride + nitrogen gas. Balanced equations are essential, so let’s do it!
Picture Frames and Chemistry…Huh!? !? 4 wood pieces + 1 piece of glass + 2 hooks + 1 wire = 1 frame! Not too different from. . ____ Na. N 3(s) ---> ____ Na 3 N(s) + ____ N 2(g) Sodium azide ---> sodium nitride + nitrogen gas. Balanced equations are essential, so let’s do it!
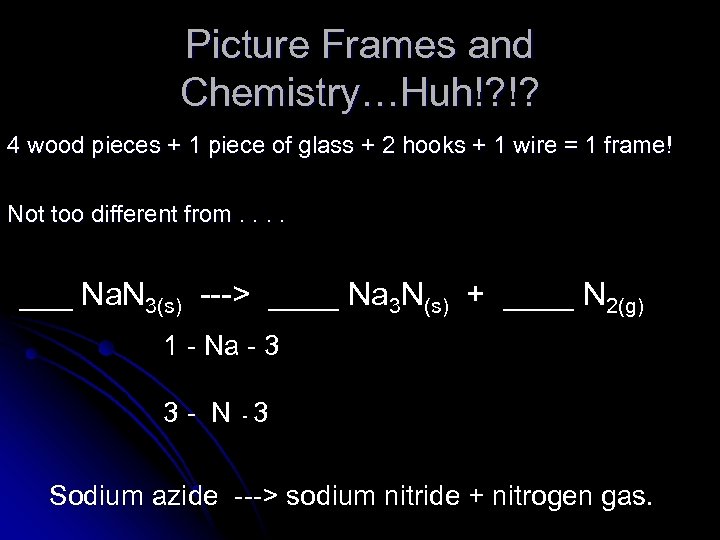 Picture Frames and Chemistry…Huh!? !? 4 wood pieces + 1 piece of glass + 2 hooks + 1 wire = 1 frame! Not too different from. . ___ Na. N 3(s) ---> ____ Na 3 N(s) + ____ N 2(g) 1 - Na - 3 3 - N -3 Sodium azide ---> sodium nitride + nitrogen gas.
Picture Frames and Chemistry…Huh!? !? 4 wood pieces + 1 piece of glass + 2 hooks + 1 wire = 1 frame! Not too different from. . ___ Na. N 3(s) ---> ____ Na 3 N(s) + ____ N 2(g) 1 - Na - 3 3 - N -3 Sodium azide ---> sodium nitride + nitrogen gas.
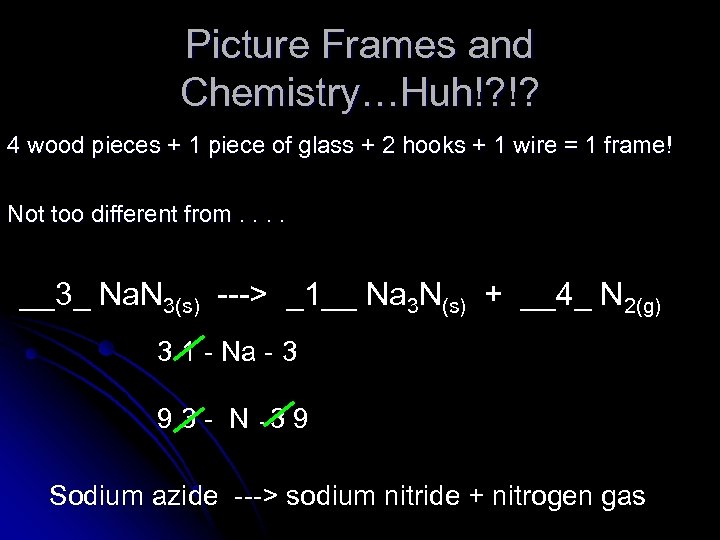 Picture Frames and Chemistry…Huh!? !? 4 wood pieces + 1 piece of glass + 2 hooks + 1 wire = 1 frame! Not too different from. . __3_ Na. N 3(s) ---> _1__ Na 3 N(s) + __4_ N 2(g) 3 1 - Na - 3 9 3 - N -3 9 Sodium azide ---> sodium nitride + nitrogen gas
Picture Frames and Chemistry…Huh!? !? 4 wood pieces + 1 piece of glass + 2 hooks + 1 wire = 1 frame! Not too different from. . __3_ Na. N 3(s) ---> _1__ Na 3 N(s) + __4_ N 2(g) 3 1 - Na - 3 9 3 - N -3 9 Sodium azide ---> sodium nitride + nitrogen gas
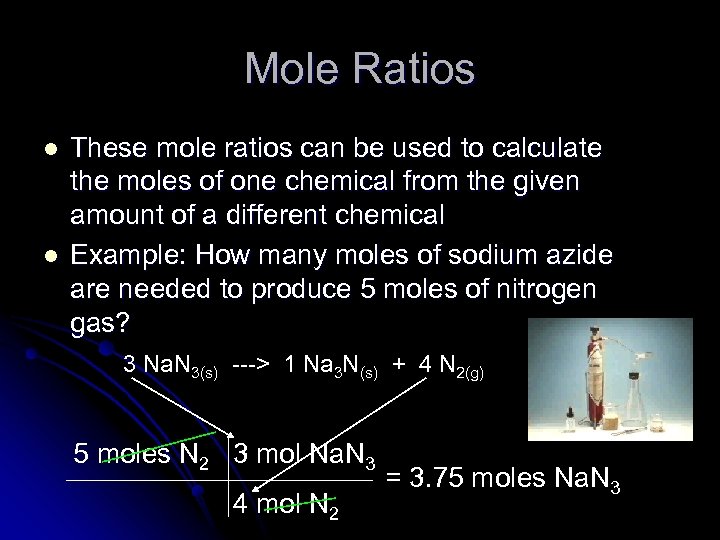 Mole Ratios l l These mole ratios can be used to calculate the moles of one chemical from the given amount of a different chemical Example: How many moles of sodium azide are needed to produce 5 moles of nitrogen gas? 3 Na. N 3(s) ---> 1 Na 3 N(s) + 4 N 2(g) 5 moles N 2 3 mol Na. N 3 4 mol N 2 = 3. 75 moles Na. N 3
Mole Ratios l l These mole ratios can be used to calculate the moles of one chemical from the given amount of a different chemical Example: How many moles of sodium azide are needed to produce 5 moles of nitrogen gas? 3 Na. N 3(s) ---> 1 Na 3 N(s) + 4 N 2(g) 5 moles N 2 3 mol Na. N 3 4 mol N 2 = 3. 75 moles Na. N 3
 Mole-Mole Conversions 3 Na. N 3(s) ---> 1 Na 3 N(s) + 4 N 2(g) Sodium azide ---> sodium nitride + nitrogen gas How many moles of sodium azide do you need to produce 2. 6 moles of nitrogen? l How many moles of sodium nitride will be produced if 2. 6 moles of nitrogen are produced? l
Mole-Mole Conversions 3 Na. N 3(s) ---> 1 Na 3 N(s) + 4 N 2(g) Sodium azide ---> sodium nitride + nitrogen gas How many moles of sodium azide do you need to produce 2. 6 moles of nitrogen? l How many moles of sodium nitride will be produced if 2. 6 moles of nitrogen are produced? l
 Mole-Mole Conversions 3 Na. N 3(s) ---> 1 Na 3 N(s) + 4 N 2(g) Sodium azide ---> sodium nitride + nitrogen gas l How many moles of sodium azide do you need to produce 2. 6 moles of nitrogen? 2. 6 moles N 2 3 moles Na. N 3 4 moles N 2 = 1. 95 moles Na. N 3
Mole-Mole Conversions 3 Na. N 3(s) ---> 1 Na 3 N(s) + 4 N 2(g) Sodium azide ---> sodium nitride + nitrogen gas l How many moles of sodium azide do you need to produce 2. 6 moles of nitrogen? 2. 6 moles N 2 3 moles Na. N 3 4 moles N 2 = 1. 95 moles Na. N 3
 Mole-Mole Conversions 3 Na. N 3(s) ---> 1 Na 3 N(s) + 4 N 2(g) Sodium azide ---> sodium nitride + nitrogen gas l How many moles of sodium nitride will be produced if 2. 6 moles of nitrogen are produced? 2. 6 moles N 2 1 moles Na 3 N 4 moles N 2 = 0. 65 moles Na 3 N
Mole-Mole Conversions 3 Na. N 3(s) ---> 1 Na 3 N(s) + 4 N 2(g) Sodium azide ---> sodium nitride + nitrogen gas l How many moles of sodium nitride will be produced if 2. 6 moles of nitrogen are produced? 2. 6 moles N 2 1 moles Na 3 N 4 moles N 2 = 0. 65 moles Na 3 N
 You. Tube ht tp: //www. youtube. com/watch? v=n. Yh. WH 1 HRqo 4 Practice l Write the balanced reaction for hydrogen gas reacting with oxygen gas. __ H 2 + ___ O 2 ___ H 2 O
You. Tube ht tp: //www. youtube. com/watch? v=n. Yh. WH 1 HRqo 4 Practice l Write the balanced reaction for hydrogen gas reacting with oxygen gas. __ H 2 + ___ O 2 ___ H 2 O
 http: //www. youtube. com/watch? v=n. Yh. WH 1 HRqo 4 Practice l Write the balanced reaction for hydrogen gas reacting with oxygen gas. 2 H 2 + O 2 2 H 2 O Right?
http: //www. youtube. com/watch? v=n. Yh. WH 1 HRqo 4 Practice l Write the balanced reaction for hydrogen gas reacting with oxygen gas. 2 H 2 + O 2 2 H 2 O Right?
 http: //www. youtube. com/watch? v=n. Yh. WH 1 HRqo 4 Practice l Write the balanced reaction for hydrogen gas reacting with oxygen gas. l l 2 H 2 + O 2 2 H 2 O How many moles of reactants are needed? What if we wanted 4 moles of water?
http: //www. youtube. com/watch? v=n. Yh. WH 1 HRqo 4 Practice l Write the balanced reaction for hydrogen gas reacting with oxygen gas. l l 2 H 2 + O 2 2 H 2 O How many moles of reactants are needed? What if we wanted 4 moles of water?
 http: //www. youtube. com/watch? v=n. Yh. WH 1 HRqo 4 Practice l Write the balanced reaction for hydrogen gas reacting with oxygen gas. l l l 2 H 2 + O 2 2 H 2 O How many moles of reactants are needed? What if we wanted 4 moles of water? What if we had 3 moles of oxygen, how much hydrogen would we need to react, and how much water would we get?
http: //www. youtube. com/watch? v=n. Yh. WH 1 HRqo 4 Practice l Write the balanced reaction for hydrogen gas reacting with oxygen gas. l l l 2 H 2 + O 2 2 H 2 O How many moles of reactants are needed? What if we wanted 4 moles of water? What if we had 3 moles of oxygen, how much hydrogen would we need to react, and how much water would we get?
 Mole-Mass Conversions l l Most of the time in chemistry, the amounts are given in grams instead of moles We still go through moles and use the mole ratio, but now we also use molar mass to get to grams l Example: How many grams of chlorine are required to react completely with 5. 00 moles of sodium to produce sodium chloride? 2 Na + Cl 2 2 Na. Cl 5. 00 moles Na 1 mol Cl 2 2 mol Na 70. 90 g Cl 2 1 mol Cl 2 = 177 g Cl 2
Mole-Mass Conversions l l Most of the time in chemistry, the amounts are given in grams instead of moles We still go through moles and use the mole ratio, but now we also use molar mass to get to grams l Example: How many grams of chlorine are required to react completely with 5. 00 moles of sodium to produce sodium chloride? 2 Na + Cl 2 2 Na. Cl 5. 00 moles Na 1 mol Cl 2 2 mol Na 70. 90 g Cl 2 1 mol Cl 2 = 177 g Cl 2
 Practice l Calculate the mass in grams of Iodine required to react completely with 0. 50 moles of aluminum.
Practice l Calculate the mass in grams of Iodine required to react completely with 0. 50 moles of aluminum.
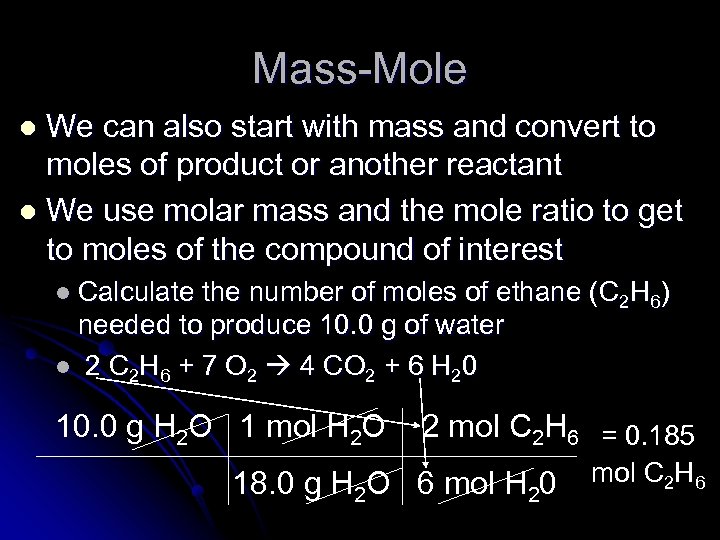 Mass-Mole We can also start with mass and convert to moles of product or another reactant l We use molar mass and the mole ratio to get to moles of the compound of interest l l Calculate the number of moles of ethane (C 2 H 6) needed to produce 10. 0 g of water l 2 C 2 H 6 + 7 O 2 4 CO 2 + 6 H 20 10. 0 g H 2 O 1 mol H 2 O 2 mol C 2 H 6 = 0. 185 18. 0 g H 2 O 6 mol H 20 mol C 2 H 6
Mass-Mole We can also start with mass and convert to moles of product or another reactant l We use molar mass and the mole ratio to get to moles of the compound of interest l l Calculate the number of moles of ethane (C 2 H 6) needed to produce 10. 0 g of water l 2 C 2 H 6 + 7 O 2 4 CO 2 + 6 H 20 10. 0 g H 2 O 1 mol H 2 O 2 mol C 2 H 6 = 0. 185 18. 0 g H 2 O 6 mol H 20 mol C 2 H 6
 Practice l Calculate how many moles of oxygen are required to make 10. 0 g of aluminum oxide
Practice l Calculate how many moles of oxygen are required to make 10. 0 g of aluminum oxide
 Chemistry l Looking at a reaction tells us how much of something you need to react with something else to get a product. Be sure you have a balanced reaction before you start! Example: 2 Na + Cl 2 2 Na. Cl l This reaction tells us that by mixing 2 moles of sodium with 1 mole of chlorine we will get 2 moles of sodium chloride l What if we wanted 4 moles of Na. Cl? 10 moles? 50 moles? l
Chemistry l Looking at a reaction tells us how much of something you need to react with something else to get a product. Be sure you have a balanced reaction before you start! Example: 2 Na + Cl 2 2 Na. Cl l This reaction tells us that by mixing 2 moles of sodium with 1 mole of chlorine we will get 2 moles of sodium chloride l What if we wanted 4 moles of Na. Cl? 10 moles? 50 moles? l
 Mass-Mass Conversions l Most often we are given a starting mass and want to find out the mass of a product we will get (called theoretical yield) or how much of another reactant we need to completely react with it (no leftover ingredients!)
Mass-Mass Conversions l Most often we are given a starting mass and want to find out the mass of a product we will get (called theoretical yield) or how much of another reactant we need to completely react with it (no leftover ingredients!)
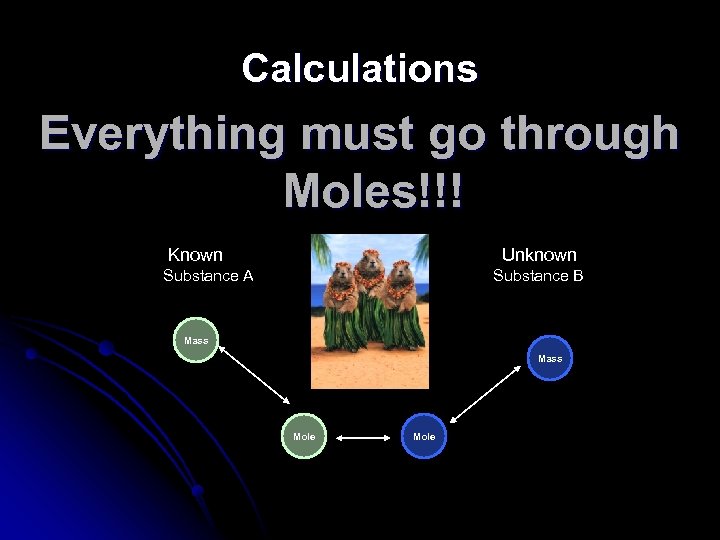 Calculations Everything must go through Moles!!! Known Unknown Substance A Substance B Mass Mole
Calculations Everything must go through Moles!!! Known Unknown Substance A Substance B Mass Mole
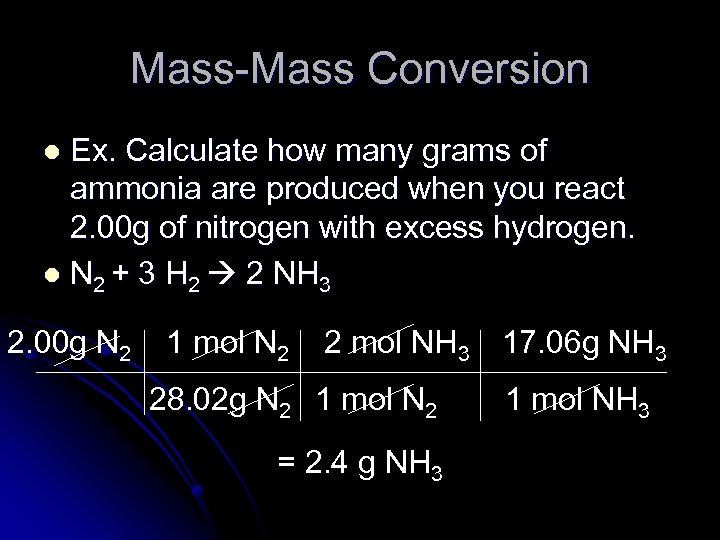 Mass-Mass Conversion Ex. Calculate how many grams of ammonia are produced when you react 2. 00 g of nitrogen with excess hydrogen. l N 2 + 3 H 2 2 NH 3 l 2. 00 g N 2 1 mol N 2 2 mol NH 3 28. 02 g N 2 1 mol N 2 = 2. 4 g NH 3 17. 06 g NH 3 1 mol NH 3
Mass-Mass Conversion Ex. Calculate how many grams of ammonia are produced when you react 2. 00 g of nitrogen with excess hydrogen. l N 2 + 3 H 2 2 NH 3 l 2. 00 g N 2 1 mol N 2 2 mol NH 3 28. 02 g N 2 1 mol N 2 = 2. 4 g NH 3 17. 06 g NH 3 1 mol NH 3
 Practice l How many grams of calcium nitride are produced when 2. 00 g of calcium reacts with an excess of nitrogen?
Practice l How many grams of calcium nitride are produced when 2. 00 g of calcium reacts with an excess of nitrogen?
 Amedeo Avogadro (1766 -1856) never knew his own number; it was named in his honor by a French scientist in 1909. its value was first estimated by Josef Loschmidt, an Austrian chemistry teacher, in 1895.
Amedeo Avogadro (1766 -1856) never knew his own number; it was named in his honor by a French scientist in 1909. its value was first estimated by Josef Loschmidt, an Austrian chemistry teacher, in 1895.
 How long would it take a computer that can count 10, 000 atoms per second to count a mole of atoms? A mole of marbles would cover the Earth, including The ocean how deeply? A mole of $100 bills stacked on top of each other would reach how far out in the solar system? (Careful, this may be a trick question!)
How long would it take a computer that can count 10, 000 atoms per second to count a mole of atoms? A mole of marbles would cover the Earth, including The ocean how deeply? A mole of $100 bills stacked on top of each other would reach how far out in the solar system? (Careful, this may be a trick question!)
 A: A computer that can count 10, 000 atoms per second would take 2, 000, 000 years to count 1 mole of a substance. How Big is a Mole? One mole of marbles would cover the entire Earth (oceans included) for a depth of three miles. One mole of $100 bills stacked one on top of another would reach from the Sun to Pluto and back 7. 5 million times.
A: A computer that can count 10, 000 atoms per second would take 2, 000, 000 years to count 1 mole of a substance. How Big is a Mole? One mole of marbles would cover the entire Earth (oceans included) for a depth of three miles. One mole of $100 bills stacked one on top of another would reach from the Sun to Pluto and back 7. 5 million times.
 How Big is a Mole? One mole of $100 bills stacked one on top of another would reach from the Sun to Pluto and back 7. 5 million times. How many light years (yes, light years!!) would it take light to travel the height of this stack of $100 dollar bills?
How Big is a Mole? One mole of $100 bills stacked one on top of another would reach from the Sun to Pluto and back 7. 5 million times. How many light years (yes, light years!!) would it take light to travel the height of this stack of $100 dollar bills?
 Q: Can you give me an example to put that number in perspective? A: A computer that can count 10, 000 atoms per second would take 2, 000, 000 years to count 1 mole of a substance. It would take light 9500 years to travel from the bottom to the top of a stack of 1 mole of $1 bills.
Q: Can you give me an example to put that number in perspective? A: A computer that can count 10, 000 atoms per second would take 2, 000, 000 years to count 1 mole of a substance. It would take light 9500 years to travel from the bottom to the top of a stack of 1 mole of $1 bills.


