28098caf601da9c8236ff2d35f66d30a.ppt
- Количество слайдов: 70
 Homogeneous Catalysis HMC-5 - 2013 Dr. K. R. Krishnamurthy National Centre for Catalysis Research Indian Institute of Technology, Madras Chennai-600036
Homogeneous Catalysis HMC-5 - 2013 Dr. K. R. Krishnamurthy National Centre for Catalysis Research Indian Institute of Technology, Madras Chennai-600036
 Homogeneous Catalysis- 5 Homogeneous Oxidation reactions Types of oxidation Wacker process Epoxidation Oxidation of cyclohexane Oxidation of p-Xylene
Homogeneous Catalysis- 5 Homogeneous Oxidation reactions Types of oxidation Wacker process Epoxidation Oxidation of cyclohexane Oxidation of p-Xylene
 Hydrocarbons: Saturated hydrocarbons Paraffins Isoparaffins Alicyclic (cyclohexane) Aromatics Alkyl aromatics Unsaturated hydrocarbons Olefins Oxidants: (Triplet /singlet) Alkynes Nitric acid Hypochlorites (Na. OCl, Ca. OCl 2) Ph. OI Objectives Peracids, Selectivity Peroxides (H 2 O 2, t-Butyl Atom efficiency hydroperoxide, etc. ) Eco-friedlyness Clean solvents/No solvents N 2 O Use of dioxygen Dioxygen (O 2)(air)
Hydrocarbons: Saturated hydrocarbons Paraffins Isoparaffins Alicyclic (cyclohexane) Aromatics Alkyl aromatics Unsaturated hydrocarbons Olefins Oxidants: (Triplet /singlet) Alkynes Nitric acid Hypochlorites (Na. OCl, Ca. OCl 2) Ph. OI Objectives Peracids, Selectivity Peroxides (H 2 O 2, t-Butyl Atom efficiency hydroperoxide, etc. ) Eco-friedlyness Clean solvents/No solvents N 2 O Use of dioxygen Dioxygen (O 2)(air)
 Homogeneous Oxidation Objectives Introduction of oxygen- Paraffins, Olefins, Aromatics, Naphthenes Conventional- Inorganic oxidising agents
Homogeneous Oxidation Objectives Introduction of oxygen- Paraffins, Olefins, Aromatics, Naphthenes Conventional- Inorganic oxidising agents
 Oxidizing agents Chemicals- Stoichiometric • Molecular oxygen, ozone, hydrogen peroxide, 40 -60% HNO 3, • Permanganates, dichromates, chromium trioxide, transition metal oxides. Catalysts • Metals: Cu, Ag, Pt, Pd; • Oxides: Cu. O+Cu 2 O, V 2 O 5, Co 2 O 3 • Mixed oxides: Bi 2 O 3. Mo. O 3, Co. O. WO 3, Molybdates
Oxidizing agents Chemicals- Stoichiometric • Molecular oxygen, ozone, hydrogen peroxide, 40 -60% HNO 3, • Permanganates, dichromates, chromium trioxide, transition metal oxides. Catalysts • Metals: Cu, Ag, Pt, Pd; • Oxides: Cu. O+Cu 2 O, V 2 O 5, Co 2 O 3 • Mixed oxides: Bi 2 O 3. Mo. O 3, Co. O. WO 3, Molybdates
 Oxidation of hydrocarbons Reaction mechanisms • Reduction-Oxidation- Insertion of lattice oxygen Reduction of metal oxide • Epoxidation- Oxygen insertion through activation • Free radical based- Initiation-Propagation-Termination
Oxidation of hydrocarbons Reaction mechanisms • Reduction-Oxidation- Insertion of lattice oxygen Reduction of metal oxide • Epoxidation- Oxygen insertion through activation • Free radical based- Initiation-Propagation-Termination
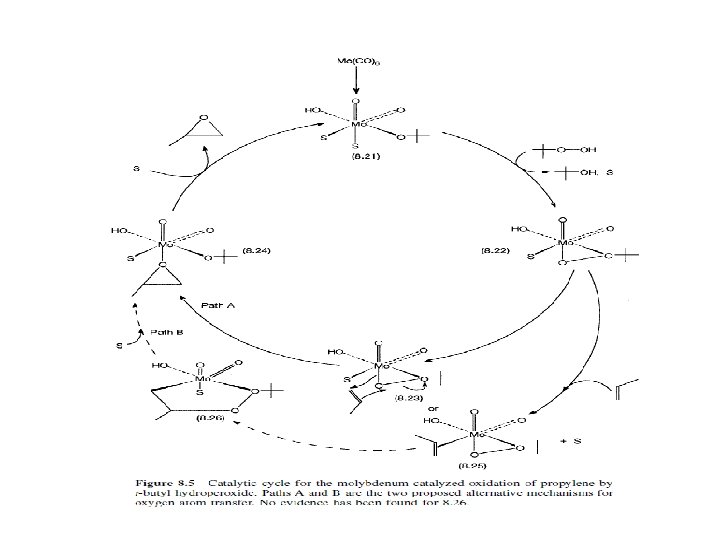
 Homogeneous Oxidation-Reaction Mechanisms 1. CH 2=CH 2 → CH 3 CHO Organometallic and Redox chemistry of Pd Nucleophilic attack by water on coordinated ethylene is the key step 2. Cyclohexane and p-xylene oxidation by air: Chain reaction of organic radicals Soluble Co and Mn ions catalyze the initiation step Auto-oxidation reaction involving dioxygen 3. Propylene to Propylene oxide (Epoxidation) Selective oxygen atom transfer chemistry; Oxygen source is organic hydroperoxide, e. g. , tert-butyl hydroperoxide
Homogeneous Oxidation-Reaction Mechanisms 1. CH 2=CH 2 → CH 3 CHO Organometallic and Redox chemistry of Pd Nucleophilic attack by water on coordinated ethylene is the key step 2. Cyclohexane and p-xylene oxidation by air: Chain reaction of organic radicals Soluble Co and Mn ions catalyze the initiation step Auto-oxidation reaction involving dioxygen 3. Propylene to Propylene oxide (Epoxidation) Selective oxygen atom transfer chemistry; Oxygen source is organic hydroperoxide, e. g. , tert-butyl hydroperoxide
 Motivation for research in selective oxidation processes
Motivation for research in selective oxidation processes
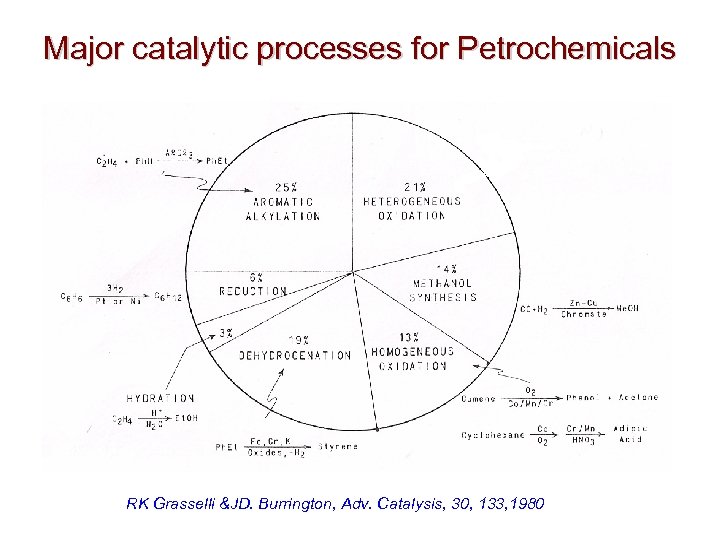 Major catalytic processes for Petrochemicals RK Grasselli &JD. Burrington, Adv. Catalysis, 30, 133, 1980
Major catalytic processes for Petrochemicals RK Grasselli &JD. Burrington, Adv. Catalysis, 30, 133, 1980
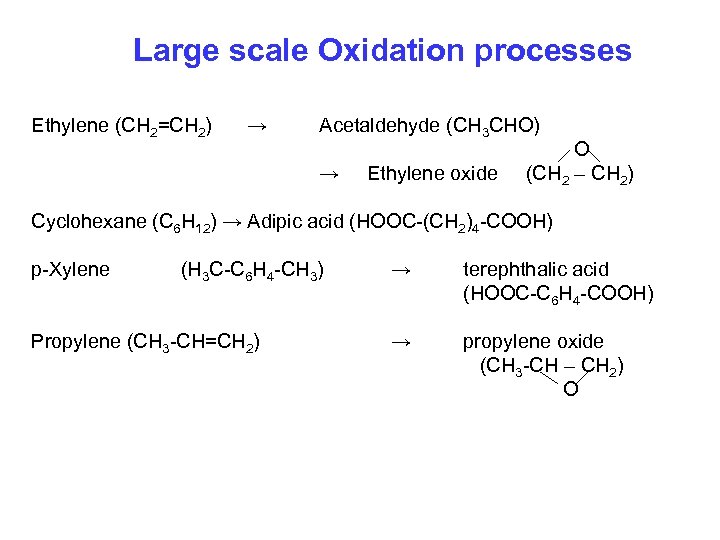 Large scale Oxidation processes Ethylene (CH 2=CH 2) → Acetaldehyde (CH 3 CHO) → Ethylene oxide O (CH 2 – CH 2) Cyclohexane (C 6 H 12) → Adipic acid (HOOC-(CH 2)4 -COOH) p-Xylene (H 3 C-C 6 H 4 -CH 3) Propylene (CH 3 -CH=CH 2) → terephthalic acid (HOOC-C 6 H 4 -COOH) → propylene oxide (CH 3 -CH – CH 2) O
Large scale Oxidation processes Ethylene (CH 2=CH 2) → Acetaldehyde (CH 3 CHO) → Ethylene oxide O (CH 2 – CH 2) Cyclohexane (C 6 H 12) → Adipic acid (HOOC-(CH 2)4 -COOH) p-Xylene (H 3 C-C 6 H 4 -CH 3) Propylene (CH 3 -CH=CH 2) → terephthalic acid (HOOC-C 6 H 4 -COOH) → propylene oxide (CH 3 -CH – CH 2) O
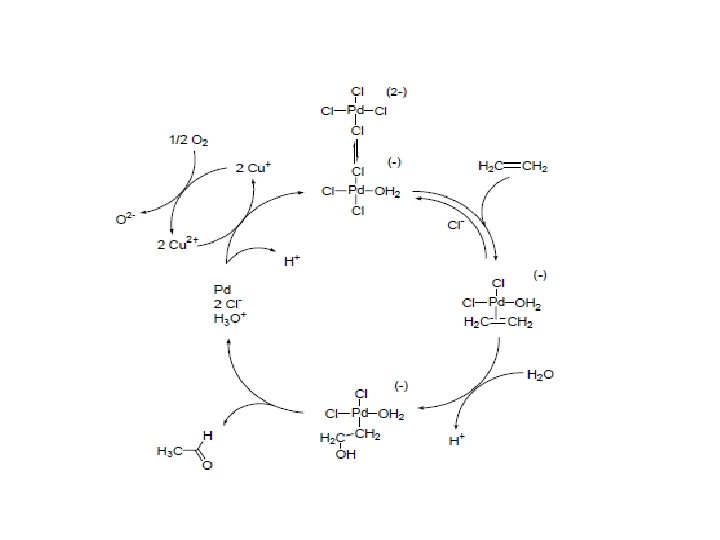
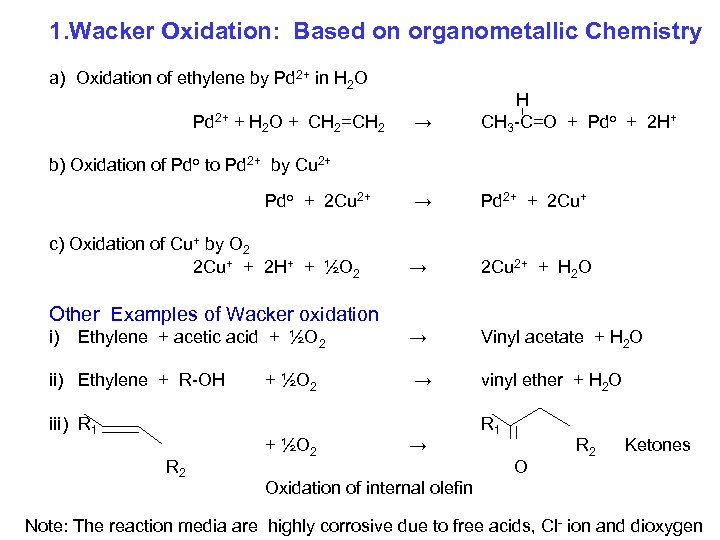 1. Wacker Oxidation: Based on organometallic Chemistry a) Oxidation of ethylene by Pd 2+ in H 2 O Pd 2+ + H 2 O + CH 2=CH 2 → H CH 3 -C=O + Pdo + 2 H+ → Pd 2+ + 2 Cu+ → 2 Cu 2+ + H 2 O → Vinyl acetate + H 2 O → vinyl ether + H 2 O b) Oxidation of Pdo to Pd 2+ by Cu 2+ Pdo + 2 Cu 2+ c) Oxidation of Cu+ by O 2 2 Cu+ + 2 H+ + ½O 2 Other Examples of Wacker oxidation i) Ethylene + acetic acid + ½O 2 ii) Ethylene + R-OH iii) R 1 R 2 + ½O 2 → R 1 O R 2 Ketones Oxidation of internal olefin Note: The reaction media are highly corrosive due to free acids, Cl- ion and dioxygen
1. Wacker Oxidation: Based on organometallic Chemistry a) Oxidation of ethylene by Pd 2+ in H 2 O Pd 2+ + H 2 O + CH 2=CH 2 → H CH 3 -C=O + Pdo + 2 H+ → Pd 2+ + 2 Cu+ → 2 Cu 2+ + H 2 O → Vinyl acetate + H 2 O → vinyl ether + H 2 O b) Oxidation of Pdo to Pd 2+ by Cu 2+ Pdo + 2 Cu 2+ c) Oxidation of Cu+ by O 2 2 Cu+ + 2 H+ + ½O 2 Other Examples of Wacker oxidation i) Ethylene + acetic acid + ½O 2 ii) Ethylene + R-OH iii) R 1 R 2 + ½O 2 → R 1 O R 2 Ketones Oxidation of internal olefin Note: The reaction media are highly corrosive due to free acids, Cl- ion and dioxygen
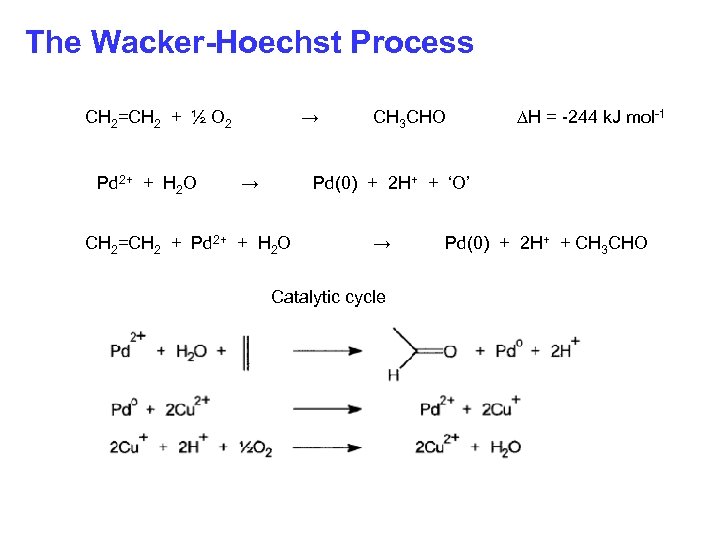 The Wacker-Hoechst Process CH 2=CH 2 + ½ O 2 Pd 2+ + H 2 O → → CH 3 CHO ∆H = -244 k. J mol-1 Pd(0) + 2 H+ + ‘O’ CH 2=CH 2 + Pd 2+ + H 2 O → Catalytic cycle Pd(0) + 2 H+ + CH 3 CHO
The Wacker-Hoechst Process CH 2=CH 2 + ½ O 2 Pd 2+ + H 2 O → → CH 3 CHO ∆H = -244 k. J mol-1 Pd(0) + 2 H+ + ‘O’ CH 2=CH 2 + Pd 2+ + H 2 O → Catalytic cycle Pd(0) + 2 H+ + CH 3 CHO
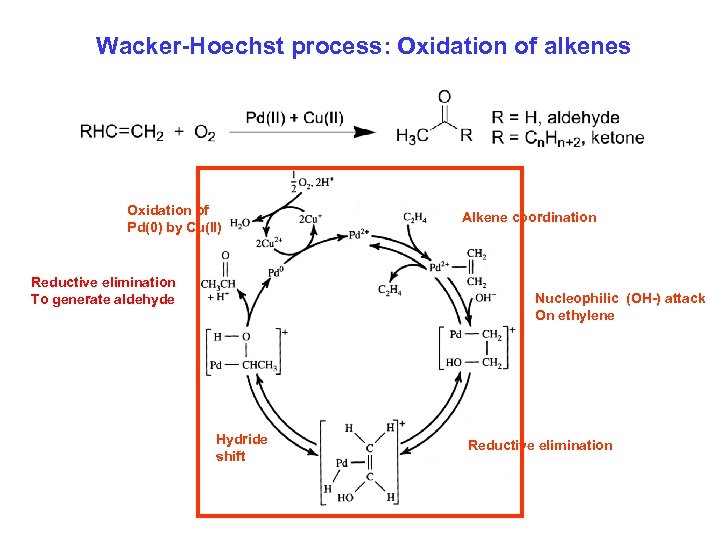 Wacker-Hoechst process: Oxidation alkenes Wacker-Hoechst process: Oxidation ofof alkenes Oxidation of Pd(0) by Cu(II) Reductive elimination To generate aldehyde Alkene coordination Nucleophilic (OH-) attack On ethylene Hydride shift Reductive elimination
Wacker-Hoechst process: Oxidation alkenes Wacker-Hoechst process: Oxidation ofof alkenes Oxidation of Pd(0) by Cu(II) Reductive elimination To generate aldehyde Alkene coordination Nucleophilic (OH-) attack On ethylene Hydride shift Reductive elimination
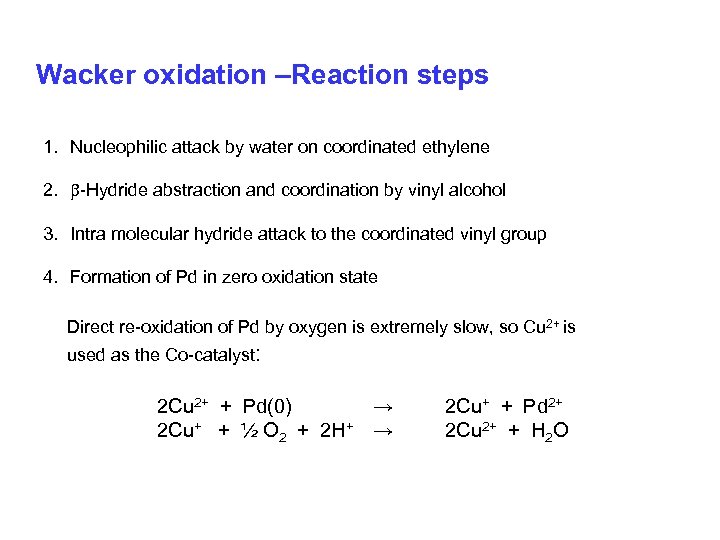 Wacker oxidation –Reaction steps 1. Nucleophilic attack by water on coordinated ethylene 2. -Hydride abstraction and coordination by vinyl alcohol 3. Intra molecular hydride attack to the coordinated vinyl group 4. Formation of Pd in zero oxidation state Direct re-oxidation of Pd by oxygen is extremely slow, so Cu 2+ is used as the Co-catalyst: 2 Cu 2+ + Pd(0) → 2 Cu+ + ½ O 2 + 2 H+ → 2 Cu+ + Pd 2+ 2 Cu 2+ + H 2 O
Wacker oxidation –Reaction steps 1. Nucleophilic attack by water on coordinated ethylene 2. -Hydride abstraction and coordination by vinyl alcohol 3. Intra molecular hydride attack to the coordinated vinyl group 4. Formation of Pd in zero oxidation state Direct re-oxidation of Pd by oxygen is extremely slow, so Cu 2+ is used as the Co-catalyst: 2 Cu 2+ + Pd(0) → 2 Cu+ + ½ O 2 + 2 H+ → 2 Cu+ + Pd 2+ 2 Cu 2+ + H 2 O
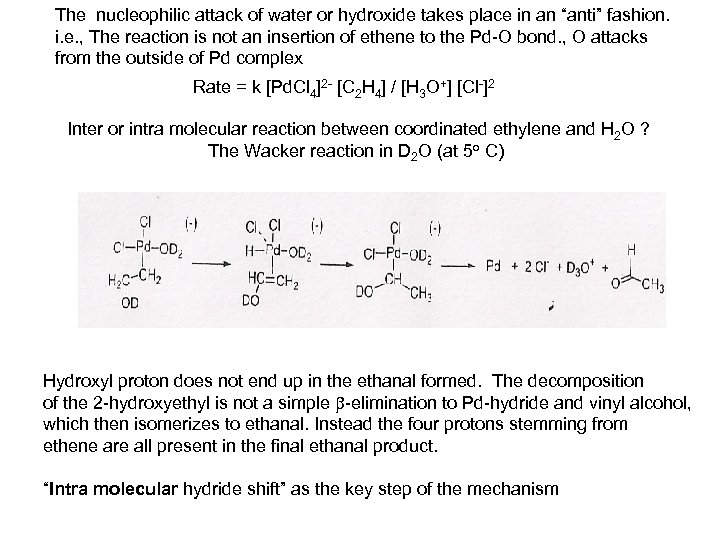 The nucleophilic attack of water or hydroxide takes place in an “anti” fashion. i. e. , The reaction is not an insertion of ethene to the Pd-O bond. , O attacks from the outside of Pd complex Rate = k [Pd. Cl 4]2 - [C 2 H 4] / [H 3 O+] [Cl-]2 Inter or intra molecular reaction between coordinated ethylene and H 2 O ? The Wacker reaction in D 2 O (at 5 o C) Hydroxyl proton does not end up in the ethanal formed. The decomposition of the 2 -hydroxyethyl is not a simple -elimination to Pd-hydride and vinyl alcohol, which then isomerizes to ethanal. Instead the four protons stemming from ethene are all present in the final ethanal product. “Intra molecular hydride shift” as the key step of the mechanism
The nucleophilic attack of water or hydroxide takes place in an “anti” fashion. i. e. , The reaction is not an insertion of ethene to the Pd-O bond. , O attacks from the outside of Pd complex Rate = k [Pd. Cl 4]2 - [C 2 H 4] / [H 3 O+] [Cl-]2 Inter or intra molecular reaction between coordinated ethylene and H 2 O ? The Wacker reaction in D 2 O (at 5 o C) Hydroxyl proton does not end up in the ethanal formed. The decomposition of the 2 -hydroxyethyl is not a simple -elimination to Pd-hydride and vinyl alcohol, which then isomerizes to ethanal. Instead the four protons stemming from ethene are all present in the final ethanal product. “Intra molecular hydride shift” as the key step of the mechanism
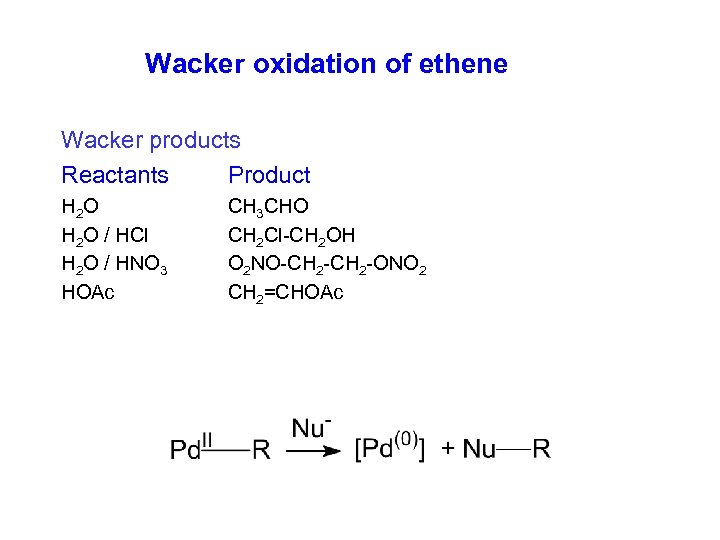 Wacker oxidation of ethene Wacker products Reactants Product H 2 O H 2 O / HCl H 2 O / HNO 3 HOAc CH 3 CHO CH 2 Cl-CH 2 OH O 2 NO-CH 2 -ONO 2 CH 2=CHOAc
Wacker oxidation of ethene Wacker products Reactants Product H 2 O H 2 O / HCl H 2 O / HNO 3 HOAc CH 3 CHO CH 2 Cl-CH 2 OH O 2 NO-CH 2 -ONO 2 CH 2=CHOAc
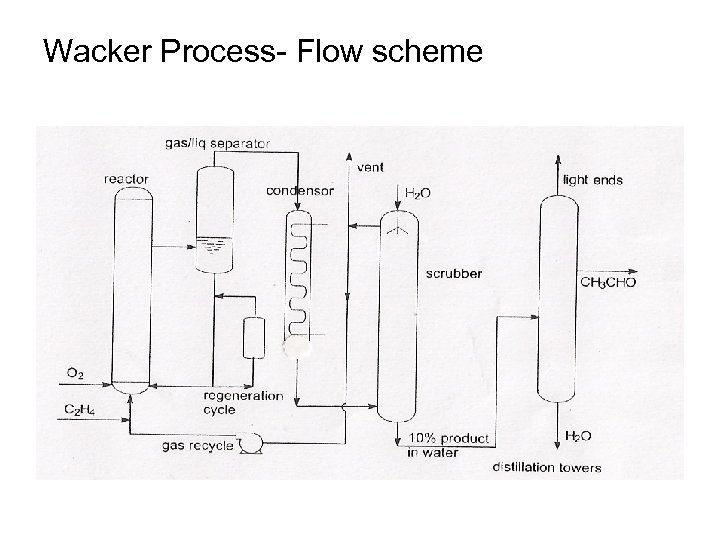 Wacker Process- Flow scheme
Wacker Process- Flow scheme
![Table 2. 2. Concepts that define the enviro-soundness of processes [4] 1. The E-factor Table 2. 2. Concepts that define the enviro-soundness of processes [4] 1. The E-factor](https://present5.com/presentation/28098caf601da9c8236ff2d35f66d30a/image-20.jpg) Table 2. 2. Concepts that define the enviro-soundness of processes [4] 1. The E-factor Industry Petroleum Bulk Chemicals Fine Chemicals Pharmaceuticals Product tonnage 106 -108 104 -106 102 -104 10 -103 Kg byproduct / Kg product (E-factor) <0. 1 <1 – 5 5 - >50 25 - >100 2. Environmental Quotient (EQ) = (E-factor x unfriendliness quotient, Q). Q can be 1 for Na. Cl and 100 – 1000 for heavy metal salts etc. 3. Atom Efficiency = Weight of desired product / weight of all products.
Table 2. 2. Concepts that define the enviro-soundness of processes [4] 1. The E-factor Industry Petroleum Bulk Chemicals Fine Chemicals Pharmaceuticals Product tonnage 106 -108 104 -106 102 -104 10 -103 Kg byproduct / Kg product (E-factor) <0. 1 <1 – 5 5 - >50 25 - >100 2. Environmental Quotient (EQ) = (E-factor x unfriendliness quotient, Q). Q can be 1 for Na. Cl and 100 – 1000 for heavy metal salts etc. 3. Atom Efficiency = Weight of desired product / weight of all products.
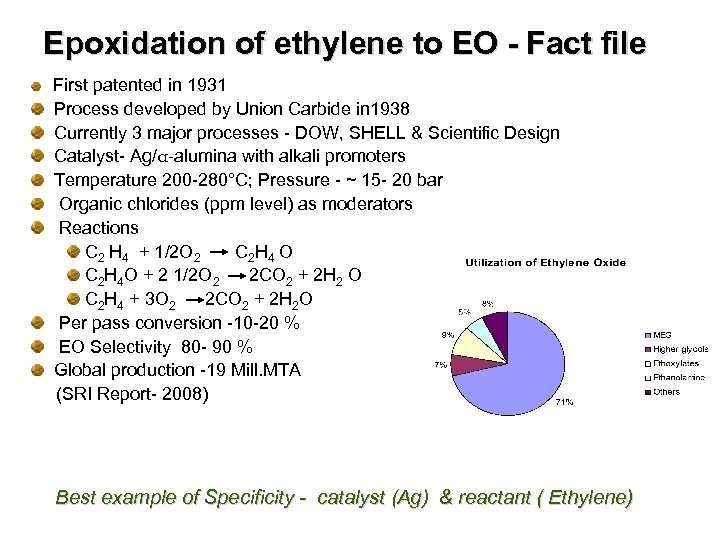 Epoxidation of ethylene to EO - Fact file First patented in 1931 Process developed by Union Carbide in 1938 Currently 3 major processes - DOW, SHELL & Scientific Design Catalyst- Ag/α-alumina with alkali promoters Temperature 200 -280°C; Pressure - ~ 15 - 20 bar Organic chlorides (ppm level) as moderators Reactions C 2 H 4 + 1/2 O 2 C 2 H 4 O C 2 H 4 O + 2 1/2 O 2 2 CO 2 + 2 H 2 O C 2 H 4 + 3 O 2 2 CO 2 + 2 H 2 O Per pass conversion -10 -20 % EO Selectivity 80 - 90 % Global production -19 Mill. MTA (SRI Report- 2008) Best example of Specificity - catalyst (Ag) & reactant ( Ethylene)
Epoxidation of ethylene to EO - Fact file First patented in 1931 Process developed by Union Carbide in 1938 Currently 3 major processes - DOW, SHELL & Scientific Design Catalyst- Ag/α-alumina with alkali promoters Temperature 200 -280°C; Pressure - ~ 15 - 20 bar Organic chlorides (ppm level) as moderators Reactions C 2 H 4 + 1/2 O 2 C 2 H 4 O C 2 H 4 O + 2 1/2 O 2 2 CO 2 + 2 H 2 O C 2 H 4 + 3 O 2 2 CO 2 + 2 H 2 O Per pass conversion -10 -20 % EO Selectivity 80 - 90 % Global production -19 Mill. MTA (SRI Report- 2008) Best example of Specificity - catalyst (Ag) & reactant ( Ethylene)
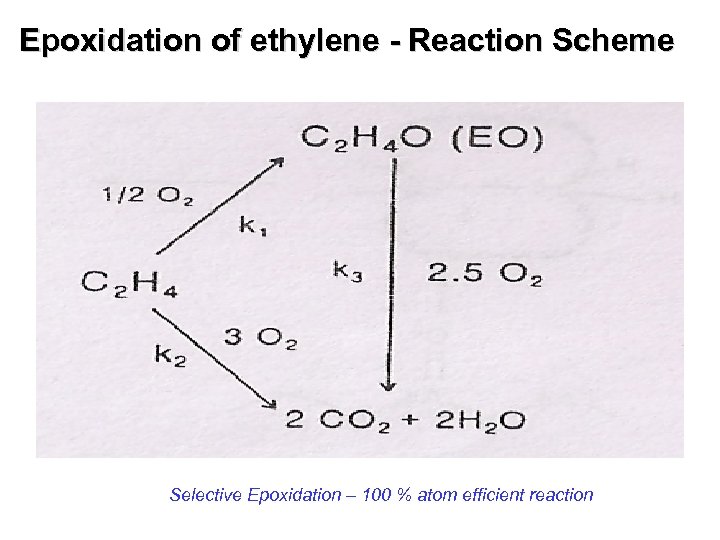 Epoxidation of ethylene - Reaction Scheme Selective Epoxidation – 100 % atom efficient reaction
Epoxidation of ethylene - Reaction Scheme Selective Epoxidation – 100 % atom efficient reaction
 Epoxidation The simplest example and one of the most important epoxide intermediates is ethylene oxide CH 2=CH 2 + ½ O 2 → Ag Catalyst→ CH 2 O ∆H = -1300 k. J mol-1 The reaction is highly exothermic. The oxidation by dioxygen also leads to formaldehyde, acetaldehyde and some CO 2 and H 2 O Ethylene does not have a great affinity to clean Ag surface, but when O 2 is preadosrbed on Ag, ethylene adsorbs rapidly. O 2 adsorbs on Ag diatomically and dissociatively and is relatively weekly adsorbed. Electrophilic attack of mono oxygen on the electrons of ethene Suppression of further oxidation is important. Conditions: 230 -270 o. C; 20 bar and ethylene, oxygen, CO 2 & ballast gas nitrogen/methane- explosion limits consideration Organic chloride in ppm levels introduced to moderate activity and maximize selectivity towards EO
Epoxidation The simplest example and one of the most important epoxide intermediates is ethylene oxide CH 2=CH 2 + ½ O 2 → Ag Catalyst→ CH 2 O ∆H = -1300 k. J mol-1 The reaction is highly exothermic. The oxidation by dioxygen also leads to formaldehyde, acetaldehyde and some CO 2 and H 2 O Ethylene does not have a great affinity to clean Ag surface, but when O 2 is preadosrbed on Ag, ethylene adsorbs rapidly. O 2 adsorbs on Ag diatomically and dissociatively and is relatively weekly adsorbed. Electrophilic attack of mono oxygen on the electrons of ethene Suppression of further oxidation is important. Conditions: 230 -270 o. C; 20 bar and ethylene, oxygen, CO 2 & ballast gas nitrogen/methane- explosion limits consideration Organic chloride in ppm levels introduced to moderate activity and maximize selectivity towards EO
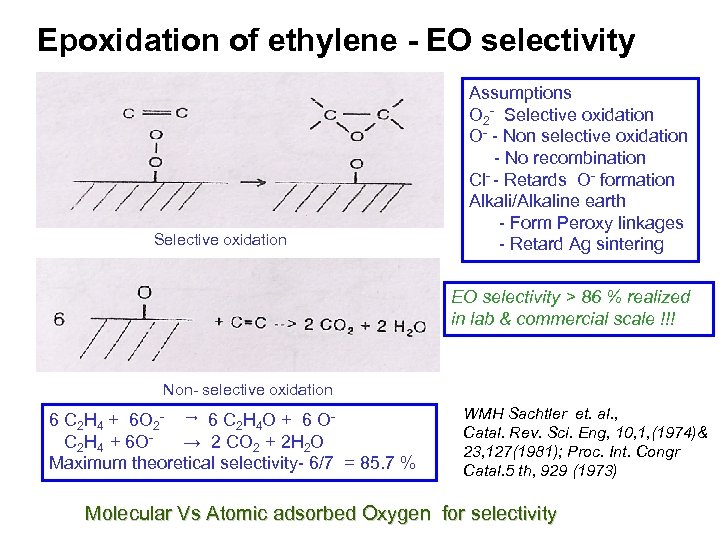 Epoxidation of ethylene - EO selectivity Selective oxidation Assumptions O 2 - Selective oxidation O- - Non selective oxidation - No recombination Cl- - Retards O- formation Alkali/Alkaline earth - Form Peroxy linkages - Retard Ag sintering EO selectivity > 86 % realized in lab & commercial scale !!! Non- selective oxidation 6 C 2 H 4 + 6 O 2 - → 6 C 2 H 4 O + 6 OC 2 H 4 + 6 O→ 2 CO 2 + 2 H 2 O Maximum theoretical selectivity- 6/7 = 85. 7 % WMH Sachtler et. al. , Catal. Rev. Sci. Eng, 10, 1, (1974)& 23, 127(1981); Proc. Int. Congr Catal. 5 th, 929 (1973) Molecular Vs Atomic adsorbed Oxygen for selectivity
Epoxidation of ethylene - EO selectivity Selective oxidation Assumptions O 2 - Selective oxidation O- - Non selective oxidation - No recombination Cl- - Retards O- formation Alkali/Alkaline earth - Form Peroxy linkages - Retard Ag sintering EO selectivity > 86 % realized in lab & commercial scale !!! Non- selective oxidation 6 C 2 H 4 + 6 O 2 - → 6 C 2 H 4 O + 6 OC 2 H 4 + 6 O→ 2 CO 2 + 2 H 2 O Maximum theoretical selectivity- 6/7 = 85. 7 % WMH Sachtler et. al. , Catal. Rev. Sci. Eng, 10, 1, (1974)& 23, 127(1981); Proc. Int. Congr Catal. 5 th, 929 (1973) Molecular Vs Atomic adsorbed Oxygen for selectivity
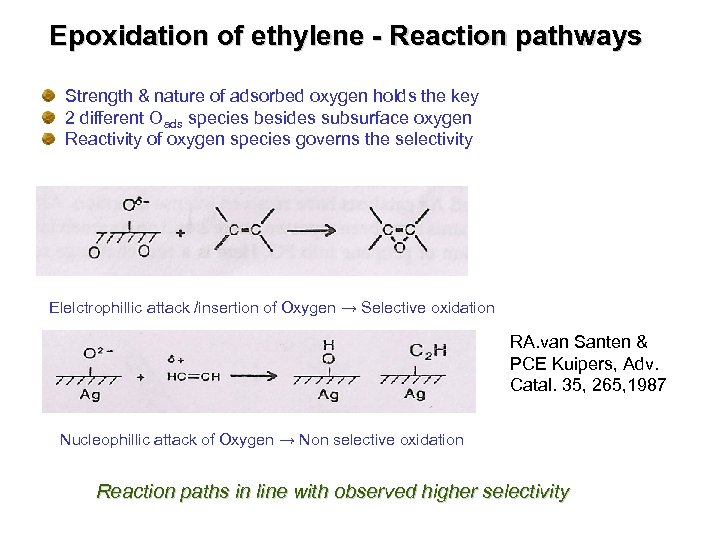 Epoxidation of ethylene - Reaction pathways Strength & nature of adsorbed oxygen holds the key 2 different Oads species besides subsurface oxygen Reactivity of oxygen species governs the selectivity Elelctrophillic attack /insertion of Oxygen → Selective oxidation RA. van Santen & PCE Kuipers, Adv. Catal. 35, 265, 1987 Nucleophillic attack of Oxygen → Non selective oxidation Reaction paths in line with observed higher selectivity
Epoxidation of ethylene - Reaction pathways Strength & nature of adsorbed oxygen holds the key 2 different Oads species besides subsurface oxygen Reactivity of oxygen species governs the selectivity Elelctrophillic attack /insertion of Oxygen → Selective oxidation RA. van Santen & PCE Kuipers, Adv. Catal. 35, 265, 1987 Nucleophillic attack of Oxygen → Non selective oxidation Reaction paths in line with observed higher selectivity
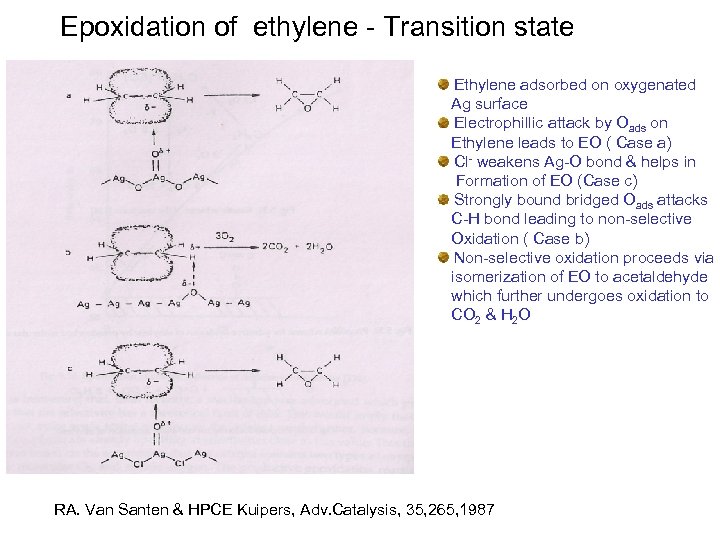 Epoxidation of ethylene - Transition state Ethylene adsorbed on oxygenated Ag surface Electrophillic attack by Oads on Ethylene leads to EO ( Case a) Cl- weakens Ag-O bond & helps in Formation of EO (Case c) Strongly bound bridged Oads attacks C-H bond leading to non-selective Oxidation ( Case b) Non-selective oxidation proceeds via isomerization of EO to acetaldehyde which further undergoes oxidation to CO 2 & H 2 O RA. Van Santen & HPCE Kuipers, Adv. Catalysis, 35, 265, 1987
Epoxidation of ethylene - Transition state Ethylene adsorbed on oxygenated Ag surface Electrophillic attack by Oads on Ethylene leads to EO ( Case a) Cl- weakens Ag-O bond & helps in Formation of EO (Case c) Strongly bound bridged Oads attacks C-H bond leading to non-selective Oxidation ( Case b) Non-selective oxidation proceeds via isomerization of EO to acetaldehyde which further undergoes oxidation to CO 2 & H 2 O RA. Van Santen & HPCE Kuipers, Adv. Catalysis, 35, 265, 1987
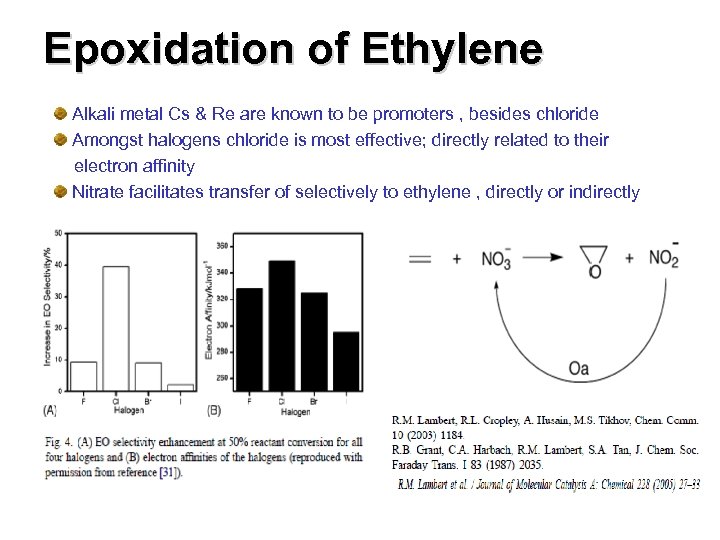 Epoxidation of Ethylene Alkali metal Cs & Re are known to be promoters , besides chloride Amongst halogens chloride is most effective; directly related to their electron affinity Nitrate facilitates transfer of selectively to ethylene , directly or indirectly
Epoxidation of Ethylene Alkali metal Cs & Re are known to be promoters , besides chloride Amongst halogens chloride is most effective; directly related to their electron affinity Nitrate facilitates transfer of selectively to ethylene , directly or indirectly
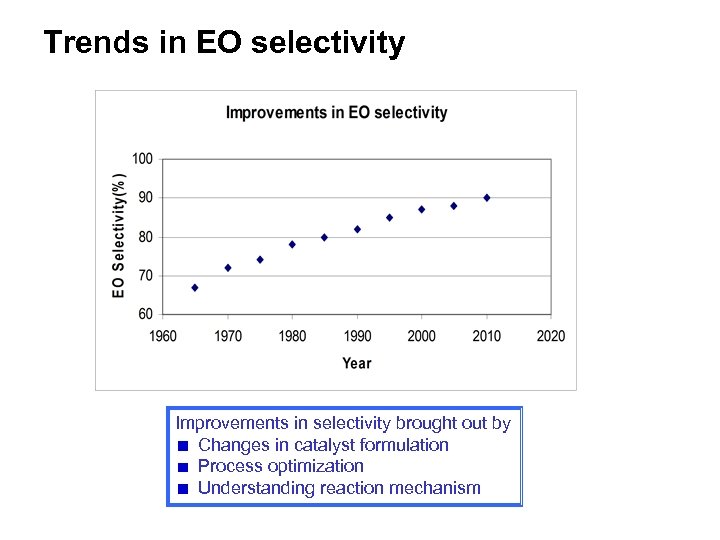 Trends in EO selectivity Improvements in selectivity brought out by Changes in catalyst formulation Process optimization Understanding reaction mechanism
Trends in EO selectivity Improvements in selectivity brought out by Changes in catalyst formulation Process optimization Understanding reaction mechanism
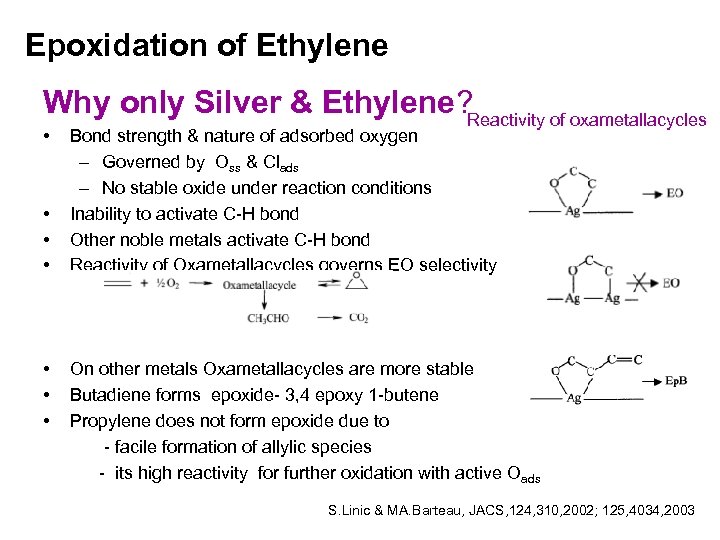 Epoxidation of Ethylene Why only Silver & Ethylene? Reactivity of oxametallacycles • • Bond strength & nature of adsorbed oxygen – Governed by Oss & Clads – No stable oxide under reaction conditions Inability to activate C-H bond Other noble metals activate C-H bond Reactivity of Oxametallacycles governs EO selectivity On other metals Oxametallacycles are more stable Butadiene forms epoxide- 3, 4 epoxy 1 -butene Propylene does not form epoxide due to - facile formation of allylic species - its high reactivity for further oxidation with active Oads S. Linic & MA. Barteau, JACS, 124, 310, 2002; 125, 4034, 2003
Epoxidation of Ethylene Why only Silver & Ethylene? Reactivity of oxametallacycles • • Bond strength & nature of adsorbed oxygen – Governed by Oss & Clads – No stable oxide under reaction conditions Inability to activate C-H bond Other noble metals activate C-H bond Reactivity of Oxametallacycles governs EO selectivity On other metals Oxametallacycles are more stable Butadiene forms epoxide- 3, 4 epoxy 1 -butene Propylene does not form epoxide due to - facile formation of allylic species - its high reactivity for further oxidation with active Oads S. Linic & MA. Barteau, JACS, 124, 310, 2002; 125, 4034, 2003
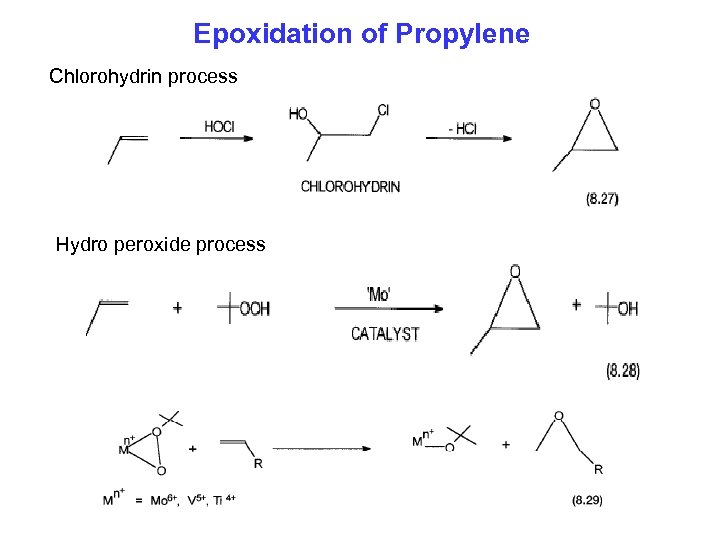 Epoxidation of Propylene Chlorohydrin process Hydro peroxide process
Epoxidation of Propylene Chlorohydrin process Hydro peroxide process
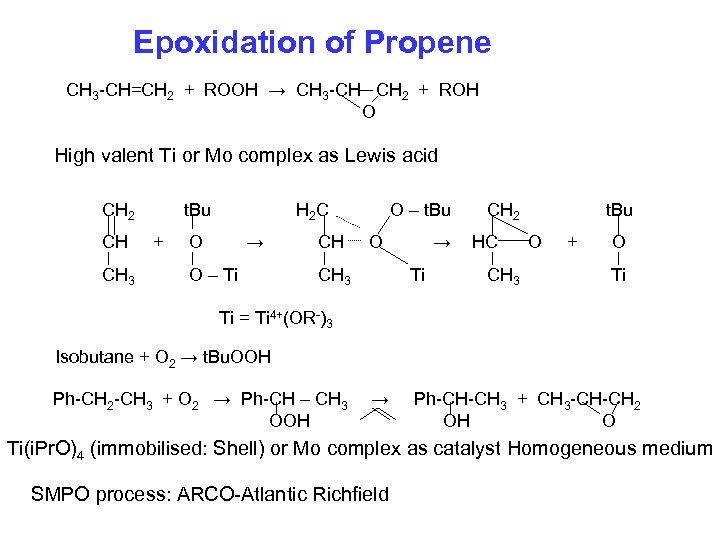 Epoxidation of Propene CH 3 -CH=CH 2 + ROOH → CH 3 -CH CH 2 + ROH O High valent Ti or Mo complex as Lewis acid CH 2 CH CH 3 t. Bu + H 2 C O → O – Ti CH O – t. Bu O CH 3 → Ti CH 2 HC CH 3 t. Bu O + O Ti Ti = Ti 4+(OR-)3 Isobutane + O 2 → t. Bu. OOH Ph-CH 2 -CH 3 + O 2 → Ph-CH – CH 3 OOH → Ph-CH-CH 3 + CH 3 -CH-CH 2 OH O Ti(i. Pr. O)4 (immobilised: Shell) or Mo complex as catalyst Homogeneous medium SMPO process: ARCO-Atlantic Richfield
Epoxidation of Propene CH 3 -CH=CH 2 + ROOH → CH 3 -CH CH 2 + ROH O High valent Ti or Mo complex as Lewis acid CH 2 CH CH 3 t. Bu + H 2 C O → O – Ti CH O – t. Bu O CH 3 → Ti CH 2 HC CH 3 t. Bu O + O Ti Ti = Ti 4+(OR-)3 Isobutane + O 2 → t. Bu. OOH Ph-CH 2 -CH 3 + O 2 → Ph-CH – CH 3 OOH → Ph-CH-CH 3 + CH 3 -CH-CH 2 OH O Ti(i. Pr. O)4 (immobilised: Shell) or Mo complex as catalyst Homogeneous medium SMPO process: ARCO-Atlantic Richfield
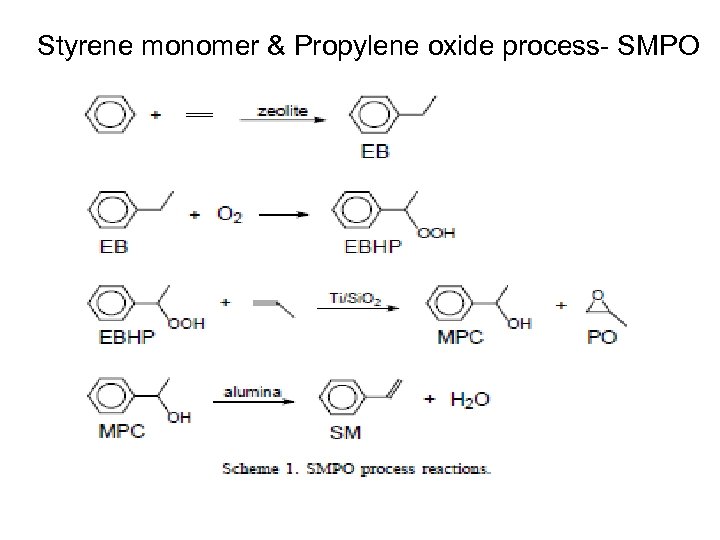 Styrene monomer & Propylene oxide process- SMPO
Styrene monomer & Propylene oxide process- SMPO
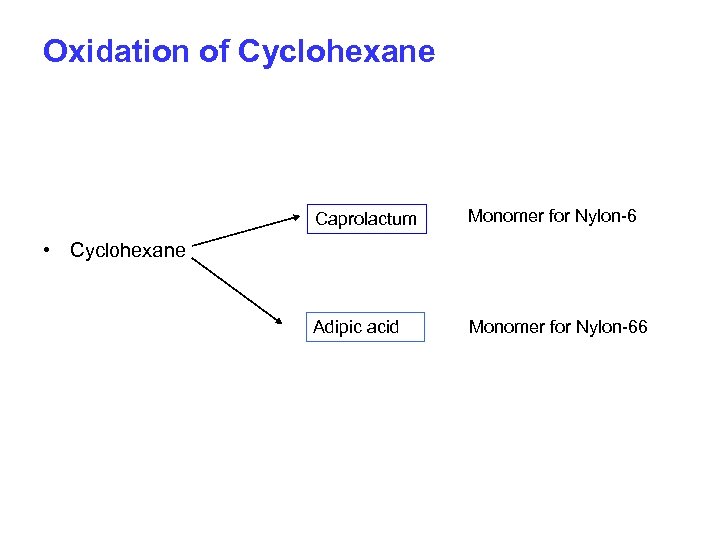 Oxidation of Cyclohexane Caprolactum Monomer for Nylon-6 Adipic acid Monomer for Nylon-66 • Cyclohexane
Oxidation of Cyclohexane Caprolactum Monomer for Nylon-6 Adipic acid Monomer for Nylon-66 • Cyclohexane
 3. Cyclohexane to Adipic acid & Caprolactum
3. Cyclohexane to Adipic acid & Caprolactum
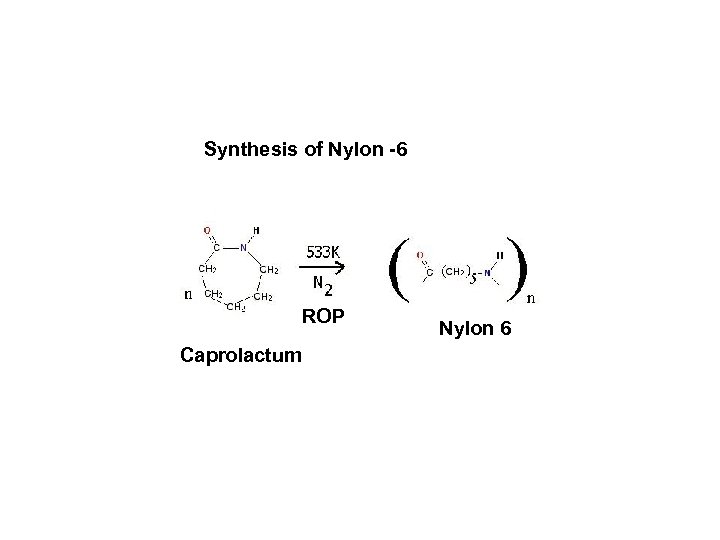 Synthesis of Nylon -6 ROP Caprolactum Nylon 6
Synthesis of Nylon -6 ROP Caprolactum Nylon 6
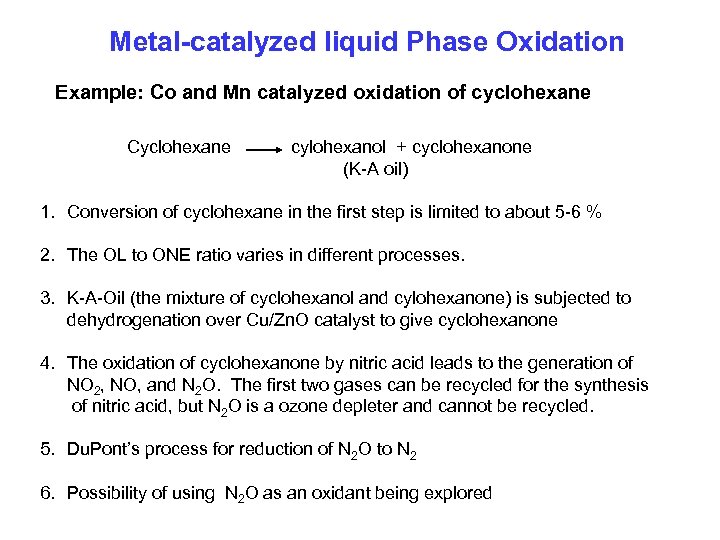 Metal-catalyzed liquid Phase Oxidation Example: Co and Mn catalyzed oxidation of cyclohexane Cyclohexane cylohexanol + cyclohexanone (K-A oil) 1. Conversion of cyclohexane in the first step is limited to about 5 -6 % 2. The OL to ONE ratio varies in different processes. 3. K-A-Oil (the mixture of cyclohexanol and cylohexanone) is subjected to dehydrogenation over Cu/Zn. O catalyst to give cyclohexanone 4. The oxidation of cyclohexanone by nitric acid leads to the generation of NO 2, NO, and N 2 O. The first two gases can be recycled for the synthesis of nitric acid, but N 2 O is a ozone depleter and cannot be recycled. 5. Du. Pont’s process for reduction of N 2 O to N 2 6. Possibility of using N 2 O as an oxidant being explored
Metal-catalyzed liquid Phase Oxidation Example: Co and Mn catalyzed oxidation of cyclohexane Cyclohexane cylohexanol + cyclohexanone (K-A oil) 1. Conversion of cyclohexane in the first step is limited to about 5 -6 % 2. The OL to ONE ratio varies in different processes. 3. K-A-Oil (the mixture of cyclohexanol and cylohexanone) is subjected to dehydrogenation over Cu/Zn. O catalyst to give cyclohexanone 4. The oxidation of cyclohexanone by nitric acid leads to the generation of NO 2, NO, and N 2 O. The first two gases can be recycled for the synthesis of nitric acid, but N 2 O is a ozone depleter and cannot be recycled. 5. Du. Pont’s process for reduction of N 2 O to N 2 6. Possibility of using N 2 O as an oxidant being explored
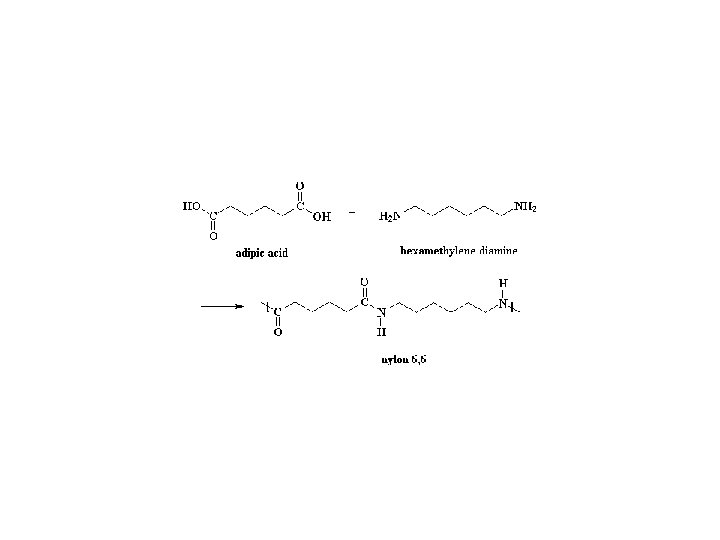
 Production of adipic acid Two step process STEP. 1 Oxidation of Cyclohexane to Cyclohexanol + Cyclohexanone Cobalt Aectate Naphthenate Octanoate 423 -473 K, 115 -175 PSIG 10 % conversion, 70 -90 -% selectivity for KA-Oil STEP. 2 Oxidation of KA-Oil to Adipic acid 50 -60% HNO 3 / Cu 2+ & V 5+ 1 -3 Atmos, 233 -253 K 80 -90% yield of AA
Production of adipic acid Two step process STEP. 1 Oxidation of Cyclohexane to Cyclohexanol + Cyclohexanone Cobalt Aectate Naphthenate Octanoate 423 -473 K, 115 -175 PSIG 10 % conversion, 70 -90 -% selectivity for KA-Oil STEP. 2 Oxidation of KA-Oil to Adipic acid 50 -60% HNO 3 / Cu 2+ & V 5+ 1 -3 Atmos, 233 -253 K 80 -90% yield of AA
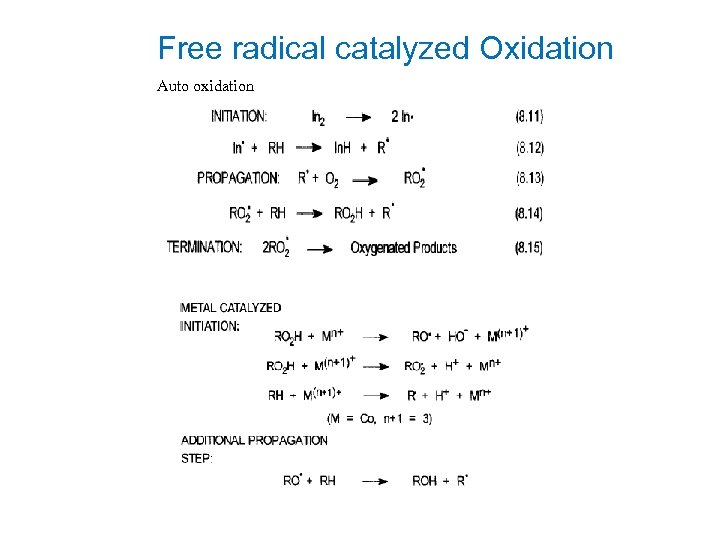 Free radical catalyzed Oxidation Auto oxidation
Free radical catalyzed Oxidation Auto oxidation
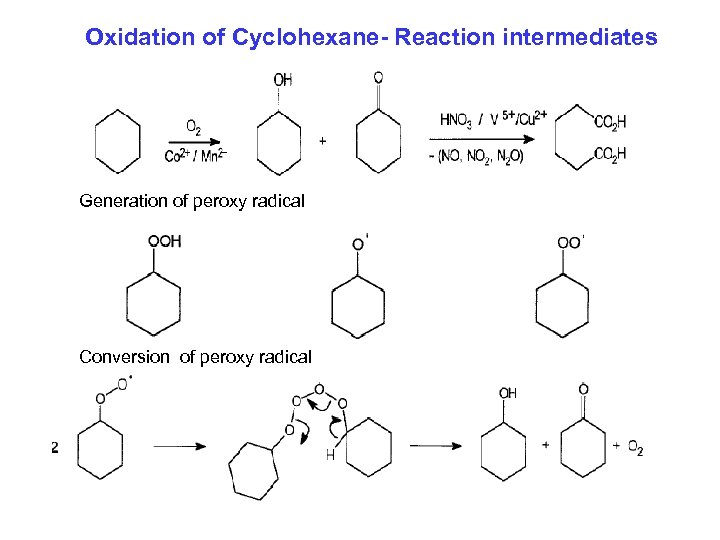 Oxidation of Cyclohexane- Reaction intermediates Generation of peroxy radical Conversion of peroxy radical
Oxidation of Cyclohexane- Reaction intermediates Generation of peroxy radical Conversion of peroxy radical
 KA Oil to Adipic acid
KA Oil to Adipic acid
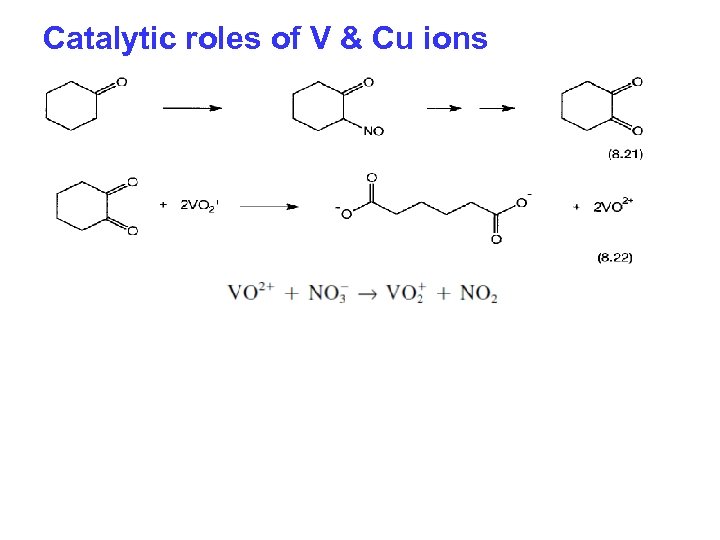 Catalytic roles of V & Cu ions
Catalytic roles of V & Cu ions
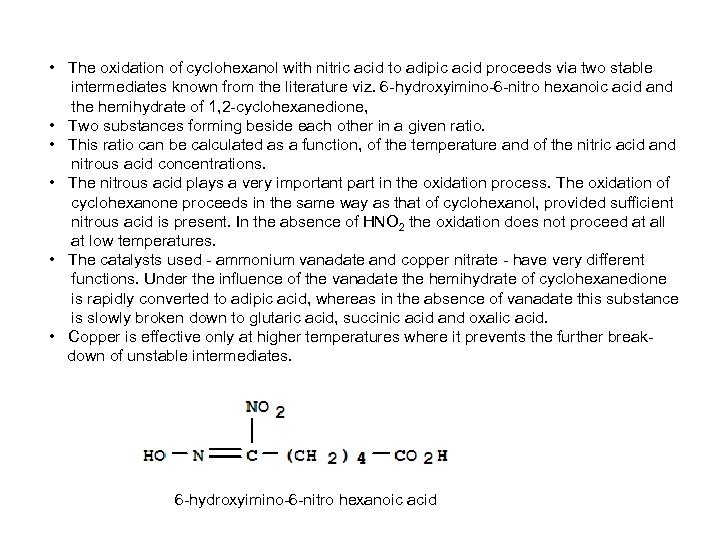 • The oxidation of cyclohexanol with nitric acid to adipic acid proceeds via two stable intermediates known from the literature viz. 6 -hydroxyimino-6 -nitro hexanoic acid and the hemihydrate of 1, 2 -cyclohexanedione, • Two substances forming beside each other in a given ratio. • This ratio can be calculated as a function, of the temperature and of the nitric acid and nitrous acid concentrations. • The nitrous acid plays a very important part in the oxidation process. The oxidation of cyclohexanone proceeds in the same way as that of cyclohexanol, provided sufficient nitrous acid is present. In the absence of HNO 2 the oxidation does not proceed at all at low temperatures. • The catalysts used - ammonium vanadate and copper nitrate - have very different functions. Under the influence of the vanadate the hemihydrate of cyclohexanedione is rapidly converted to adipic acid, whereas in the absence of vanadate this substance is slowly broken down to glutaric acid, succinic acid and oxalic acid. • Copper is effective only at higher temperatures where it prevents the further breakdown of unstable intermediates. 6 -hydroxyimino-6 -nitro hexanoic acid
• The oxidation of cyclohexanol with nitric acid to adipic acid proceeds via two stable intermediates known from the literature viz. 6 -hydroxyimino-6 -nitro hexanoic acid and the hemihydrate of 1, 2 -cyclohexanedione, • Two substances forming beside each other in a given ratio. • This ratio can be calculated as a function, of the temperature and of the nitric acid and nitrous acid concentrations. • The nitrous acid plays a very important part in the oxidation process. The oxidation of cyclohexanone proceeds in the same way as that of cyclohexanol, provided sufficient nitrous acid is present. In the absence of HNO 2 the oxidation does not proceed at all at low temperatures. • The catalysts used - ammonium vanadate and copper nitrate - have very different functions. Under the influence of the vanadate the hemihydrate of cyclohexanedione is rapidly converted to adipic acid, whereas in the absence of vanadate this substance is slowly broken down to glutaric acid, succinic acid and oxalic acid. • Copper is effective only at higher temperatures where it prevents the further breakdown of unstable intermediates. 6 -hydroxyimino-6 -nitro hexanoic acid
 Production of adipic acid: N 2 O issue Nitric acid oxidation of KA (cyclohexanone) Oxidation chemistry controlled by nitrous acid in equilibrium with NO, NO 2, HNO 3 and H 2 O in reaction mixture; Reaction pathway through Nitrolic acid (Nitro-6 -hydroxyimino hexanoic acid), which is hydrolyzed (slow step) and N 2 O is formed by further reactions of N-containing products of hydrolysis; NO and NO 2 are adsorbed and converted back to nitric acid, but N 2 O cannot be recovered in this manner; 0. 15 to 0. 3 tons of N 2 O per ton of adipic acid!
Production of adipic acid: N 2 O issue Nitric acid oxidation of KA (cyclohexanone) Oxidation chemistry controlled by nitrous acid in equilibrium with NO, NO 2, HNO 3 and H 2 O in reaction mixture; Reaction pathway through Nitrolic acid (Nitro-6 -hydroxyimino hexanoic acid), which is hydrolyzed (slow step) and N 2 O is formed by further reactions of N-containing products of hydrolysis; NO and NO 2 are adsorbed and converted back to nitric acid, but N 2 O cannot be recovered in this manner; 0. 15 to 0. 3 tons of N 2 O per ton of adipic acid!
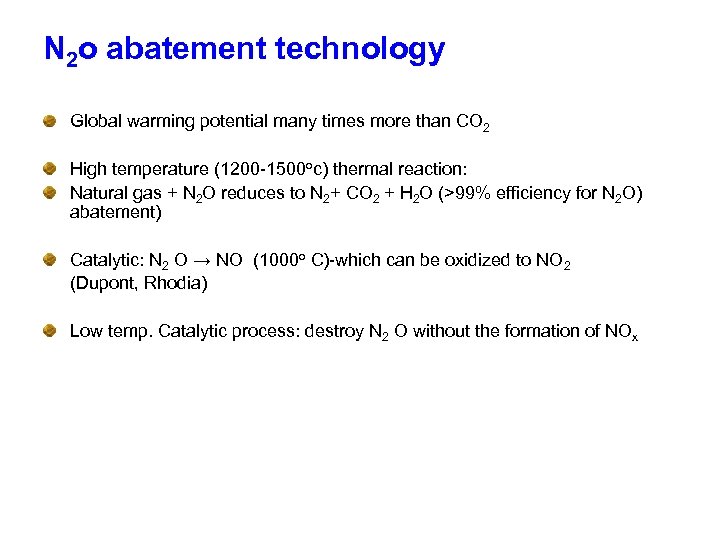 N 2 o abatement technology Global warming potential many times more than CO 2 High temperature (1200 -1500 oc) thermal reaction: Natural gas + N 2 O reduces to N 2+ CO 2 + H 2 O (>99% efficiency for N 2 O) abatement) Catalytic: N 2 O → NO (1000 o C)-which can be oxidized to NO 2 (Dupont, Rhodia) Low temp. Catalytic process: destroy N 2 O without the formation of NOx
N 2 o abatement technology Global warming potential many times more than CO 2 High temperature (1200 -1500 oc) thermal reaction: Natural gas + N 2 O reduces to N 2+ CO 2 + H 2 O (>99% efficiency for N 2 O) abatement) Catalytic: N 2 O → NO (1000 o C)-which can be oxidized to NO 2 (Dupont, Rhodia) Low temp. Catalytic process: destroy N 2 O without the formation of NOx
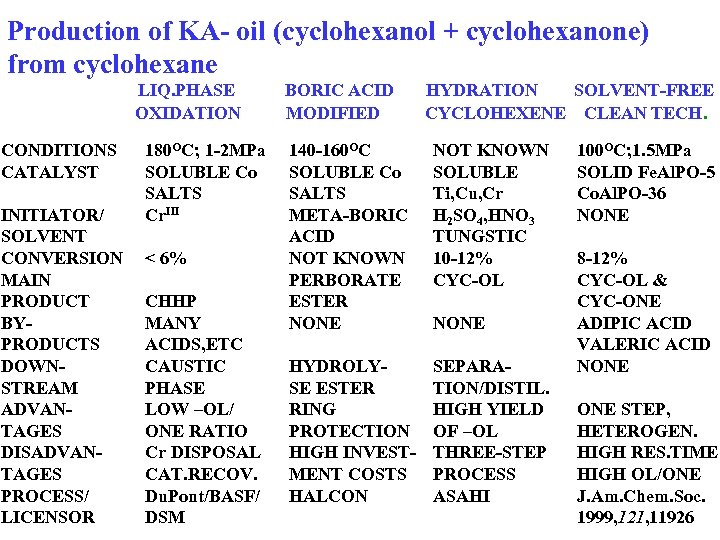 Production of KA- oil (cyclohexanol + cyclohexanone) from cyclohexane LIQ. PHASE OXIDATION CONDITIONS CATALYST INITIATOR/ SOLVENT CONVERSION MAIN PRODUCT BYPRODUCTS DOWNSTREAM ADVANTAGES DISADVANTAGES PROCESS/ LICENSOR 180 OC; 1 -2 MPa SOLUBLE Co SALTS Cr. III < 6% CHHP MANY ACIDS, ETC CAUSTIC PHASE LOW –OL/ ONE RATIO Cr DISPOSAL CAT. RECOV. Du. Pont/BASF/ DSM BORIC ACID MODIFIED HYDRATION SOLVENT-FREE CYCLOHEXENE CLEAN TECH. 140 -160 OC SOLUBLE Co SALTS META-BORIC ACID NOT KNOWN PERBORATE ESTER NONE NOT KNOWN SOLUBLE Ti, Cu, Cr H 2 SO 4, HNO 3 TUNGSTIC 10 -12% CYC-OL HYDROLYSE ESTER RING PROTECTION HIGH INVESTMENT COSTS HALCON SEPARATION/DISTIL. HIGH YIELD OF –OL THREE-STEP PROCESS ASAHI NONE 100 OC; 1. 5 MPa SOLID Fe. Al. PO-5 Co. Al. PO-36 NONE 8 -12% CYC-OL & CYC-ONE ADIPIC ACID VALERIC ACID NONE STEP, HETEROGEN. HIGH RES. TIME HIGH OL/ONE J. Am. Chem. Soc. 1999, 121, 11926
Production of KA- oil (cyclohexanol + cyclohexanone) from cyclohexane LIQ. PHASE OXIDATION CONDITIONS CATALYST INITIATOR/ SOLVENT CONVERSION MAIN PRODUCT BYPRODUCTS DOWNSTREAM ADVANTAGES DISADVANTAGES PROCESS/ LICENSOR 180 OC; 1 -2 MPa SOLUBLE Co SALTS Cr. III < 6% CHHP MANY ACIDS, ETC CAUSTIC PHASE LOW –OL/ ONE RATIO Cr DISPOSAL CAT. RECOV. Du. Pont/BASF/ DSM BORIC ACID MODIFIED HYDRATION SOLVENT-FREE CYCLOHEXENE CLEAN TECH. 140 -160 OC SOLUBLE Co SALTS META-BORIC ACID NOT KNOWN PERBORATE ESTER NONE NOT KNOWN SOLUBLE Ti, Cu, Cr H 2 SO 4, HNO 3 TUNGSTIC 10 -12% CYC-OL HYDROLYSE ESTER RING PROTECTION HIGH INVESTMENT COSTS HALCON SEPARATION/DISTIL. HIGH YIELD OF –OL THREE-STEP PROCESS ASAHI NONE 100 OC; 1. 5 MPa SOLID Fe. Al. PO-5 Co. Al. PO-36 NONE 8 -12% CYC-OL & CYC-ONE ADIPIC ACID VALERIC ACID NONE STEP, HETEROGEN. HIGH RES. TIME HIGH OL/ONE J. Am. Chem. Soc. 1999, 121, 11926
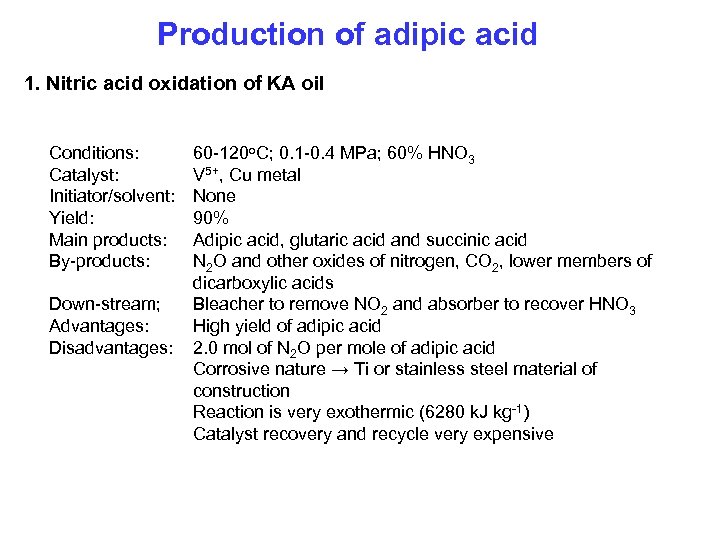 Production of adipic acid 1. Nitric acid oxidation of KA oil Conditions: Catalyst: Initiator/solvent: Yield: Main products: By-products: Down-stream; Advantages: Disadvantages: 60 -120 o. C; 0. 1 -0. 4 MPa; 60% HNO 3 V 5+, Cu metal None 90% Adipic acid, glutaric acid and succinic acid N 2 O and other oxides of nitrogen, CO 2, lower members of dicarboxylic acids Bleacher to remove NO 2 and absorber to recover HNO 3 High yield of adipic acid 2. 0 mol of N 2 O per mole of adipic acid Corrosive nature → Ti or stainless steel material of construction Reaction is very exothermic (6280 k. J kg-1) Catalyst recovery and recycle very expensive
Production of adipic acid 1. Nitric acid oxidation of KA oil Conditions: Catalyst: Initiator/solvent: Yield: Main products: By-products: Down-stream; Advantages: Disadvantages: 60 -120 o. C; 0. 1 -0. 4 MPa; 60% HNO 3 V 5+, Cu metal None 90% Adipic acid, glutaric acid and succinic acid N 2 O and other oxides of nitrogen, CO 2, lower members of dicarboxylic acids Bleacher to remove NO 2 and absorber to recover HNO 3 High yield of adipic acid 2. 0 mol of N 2 O per mole of adipic acid Corrosive nature → Ti or stainless steel material of construction Reaction is very exothermic (6280 k. J kg-1) Catalyst recovery and recycle very expensive
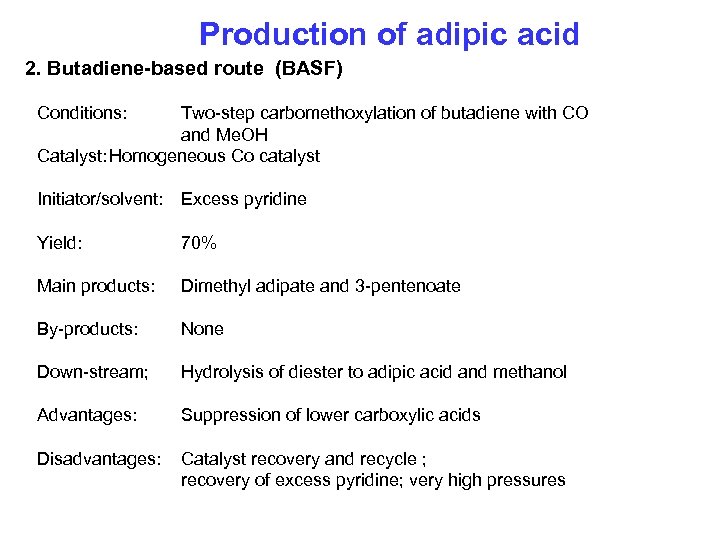 Production of adipic acid 2. Butadiene-based route (BASF) Conditions: Two-step carbomethoxylation of butadiene with CO and Me. OH Catalyst: Homogeneous Co catalyst Initiator/solvent: Excess pyridine Yield: 70% Main products: Dimethyl adipate and 3 -pentenoate By-products: None Down-stream; Hydrolysis of diester to adipic acid and methanol Advantages: Suppression of lower carboxylic acids Disadvantages: Catalyst recovery and recycle ; recovery of excess pyridine; very high pressures
Production of adipic acid 2. Butadiene-based route (BASF) Conditions: Two-step carbomethoxylation of butadiene with CO and Me. OH Catalyst: Homogeneous Co catalyst Initiator/solvent: Excess pyridine Yield: 70% Main products: Dimethyl adipate and 3 -pentenoate By-products: None Down-stream; Hydrolysis of diester to adipic acid and methanol Advantages: Suppression of lower carboxylic acids Disadvantages: Catalyst recovery and recycle ; recovery of excess pyridine; very high pressures
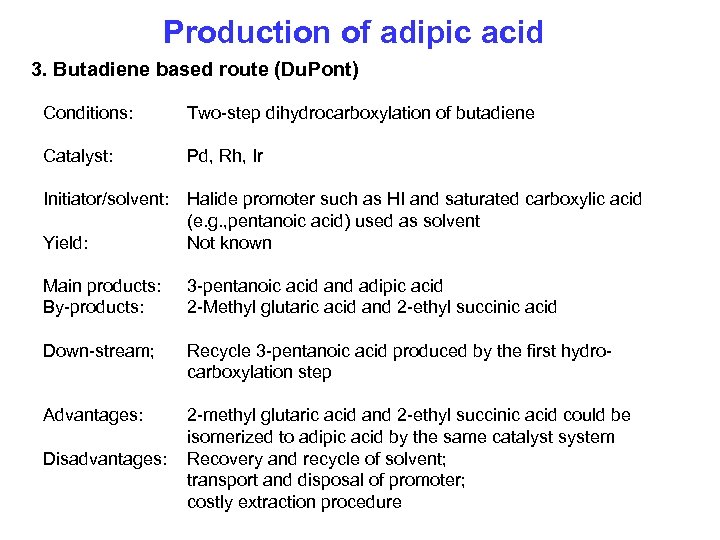 Production of adipic acid 3. Butadiene based route (Du. Pont) Conditions: Two-step dihydrocarboxylation of butadiene Catalyst: Pd, Rh, Ir Initiator/solvent: Yield: Halide promoter such as HI and saturated carboxylic acid (e. g. , pentanoic acid) used as solvent Not known Main products: By-products: 3 -pentanoic acid and adipic acid 2 -Methyl glutaric acid and 2 -ethyl succinic acid Down-stream; Recycle 3 -pentanoic acid produced by the first hydrocarboxylation step Advantages: 2 -methyl glutaric acid and 2 -ethyl succinic acid could be isomerized to adipic acid by the same catalyst system Recovery and recycle of solvent; transport and disposal of promoter; costly extraction procedure Disadvantages:
Production of adipic acid 3. Butadiene based route (Du. Pont) Conditions: Two-step dihydrocarboxylation of butadiene Catalyst: Pd, Rh, Ir Initiator/solvent: Yield: Halide promoter such as HI and saturated carboxylic acid (e. g. , pentanoic acid) used as solvent Not known Main products: By-products: 3 -pentanoic acid and adipic acid 2 -Methyl glutaric acid and 2 -ethyl succinic acid Down-stream; Recycle 3 -pentanoic acid produced by the first hydrocarboxylation step Advantages: 2 -methyl glutaric acid and 2 -ethyl succinic acid could be isomerized to adipic acid by the same catalyst system Recovery and recycle of solvent; transport and disposal of promoter; costly extraction procedure Disadvantages:
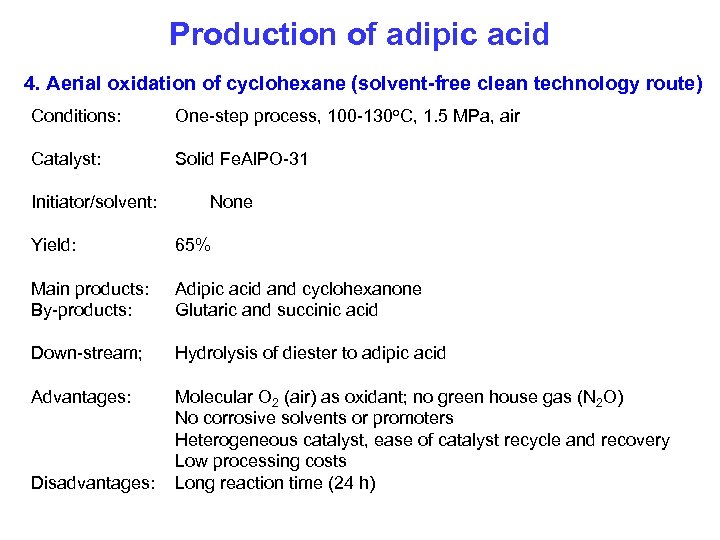 Production of adipic acid 4. Aerial oxidation of cyclohexane (solvent-free clean technology route) Conditions: One-step process, 100 -130 o. C, 1. 5 MPa, air Catalyst: Solid Fe. Al. PO-31 Initiator/solvent: None Yield: 65% Main products: By-products: Adipic acid and cyclohexanone Glutaric and succinic acid Down-stream; Hydrolysis of diester to adipic acid Advantages: Molecular O 2 (air) as oxidant; no green house gas (N 2 O) No corrosive solvents or promoters Heterogeneous catalyst, ease of catalyst recycle and recovery Low processing costs Long reaction time (24 h) Disadvantages:
Production of adipic acid 4. Aerial oxidation of cyclohexane (solvent-free clean technology route) Conditions: One-step process, 100 -130 o. C, 1. 5 MPa, air Catalyst: Solid Fe. Al. PO-31 Initiator/solvent: None Yield: 65% Main products: By-products: Adipic acid and cyclohexanone Glutaric and succinic acid Down-stream; Hydrolysis of diester to adipic acid Advantages: Molecular O 2 (air) as oxidant; no green house gas (N 2 O) No corrosive solvents or promoters Heterogeneous catalyst, ease of catalyst recycle and recovery Low processing costs Long reaction time (24 h) Disadvantages:
 Cyclohexane to adipic acid Co 2+/Mn 2+ catalyzed oxidation of CYCLOHEXANE, Liquid phase reaction; the free radical intermediate is more active than cyclohexane , Hence conversion is restricted to 3 -8 mol% Alternative technologies for production of KA oil: H 3 BO 3 as catalyst, borate ester (Halcon Process); CH= by selective partial hydrogenation of benzene by aqueous Ru catalyst, followed by hydration of CH= using ZSM-5 catalyst (Asahi Chemicals); Vapour or liquid phase hydrogenation of phenol using Pd/Al 2 O 3 catalyst Benzene to phenol using N 2 O (Fe-ZSM-5, one-step, vapour phase) (Solutia/Monsanto)
Cyclohexane to adipic acid Co 2+/Mn 2+ catalyzed oxidation of CYCLOHEXANE, Liquid phase reaction; the free radical intermediate is more active than cyclohexane , Hence conversion is restricted to 3 -8 mol% Alternative technologies for production of KA oil: H 3 BO 3 as catalyst, borate ester (Halcon Process); CH= by selective partial hydrogenation of benzene by aqueous Ru catalyst, followed by hydration of CH= using ZSM-5 catalyst (Asahi Chemicals); Vapour or liquid phase hydrogenation of phenol using Pd/Al 2 O 3 catalyst Benzene to phenol using N 2 O (Fe-ZSM-5, one-step, vapour phase) (Solutia/Monsanto)

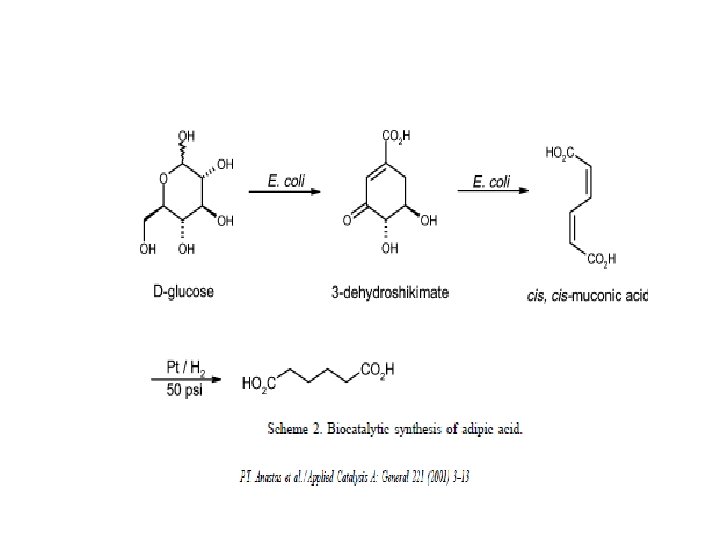
 Alternative routes to adipic acid Methyl acrylate → dimerized to dimethyl adipate Dimerization of acrylonitrile to adiponitrile (propylene as source) Air/oxygen oxidation of cyclohexane, cyclohexanol or n-hexane Oxidation of cyclohexane and/or cyclohexanol using H 2 O 2 “Green” route Renewable glucose to adipic acid via the formation of muconic acid
Alternative routes to adipic acid Methyl acrylate → dimerized to dimethyl adipate Dimerization of acrylonitrile to adiponitrile (propylene as source) Air/oxygen oxidation of cyclohexane, cyclohexanol or n-hexane Oxidation of cyclohexane and/or cyclohexanol using H 2 O 2 “Green” route Renewable glucose to adipic acid via the formation of muconic acid
 Adipic acid
Adipic acid
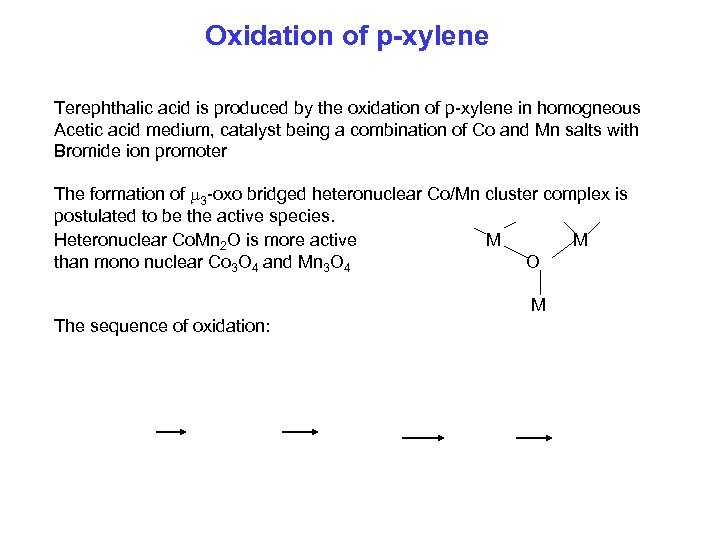 Oxidation of p-xylene Terephthalic acid is produced by the oxidation of p-xylene in homogneous Acetic acid medium, catalyst being a combination of Co and Mn salts with Bromide ion promoter The formation of 3 -oxo bridged heteronuclear Co/Mn cluster complex is postulated to be the active species. Heteronuclear Co. Mn 2 O is more active M M than mono nuclear Co 3 O 4 and Mn 3 O 4 O M The sequence of oxidation:
Oxidation of p-xylene Terephthalic acid is produced by the oxidation of p-xylene in homogneous Acetic acid medium, catalyst being a combination of Co and Mn salts with Bromide ion promoter The formation of 3 -oxo bridged heteronuclear Co/Mn cluster complex is postulated to be the active species. Heteronuclear Co. Mn 2 O is more active M M than mono nuclear Co 3 O 4 and Mn 3 O 4 O M The sequence of oxidation:
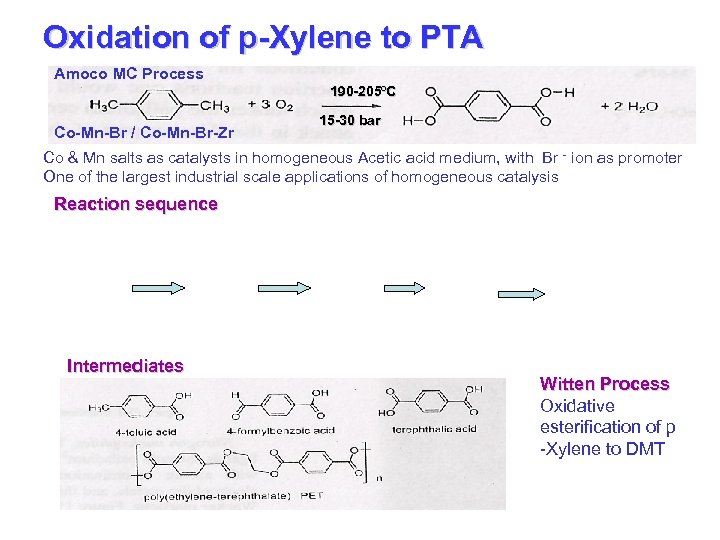 Oxidation of p-Xylene to PTA Amoco MC Process 190 -205ºC Co-Mn-Br / Co-Mn-Br-Zr 15 -30 bar Co & Mn salts as catalysts in homogeneous Acetic acid medium, with Br - ion as promoter One of the largest industrial scale applications of homogeneous catalysis Reaction sequence Intermediates Witten Process Oxidative esterification of p -Xylene to DMT
Oxidation of p-Xylene to PTA Amoco MC Process 190 -205ºC Co-Mn-Br / Co-Mn-Br-Zr 15 -30 bar Co & Mn salts as catalysts in homogeneous Acetic acid medium, with Br - ion as promoter One of the largest industrial scale applications of homogeneous catalysis Reaction sequence Intermediates Witten Process Oxidative esterification of p -Xylene to DMT
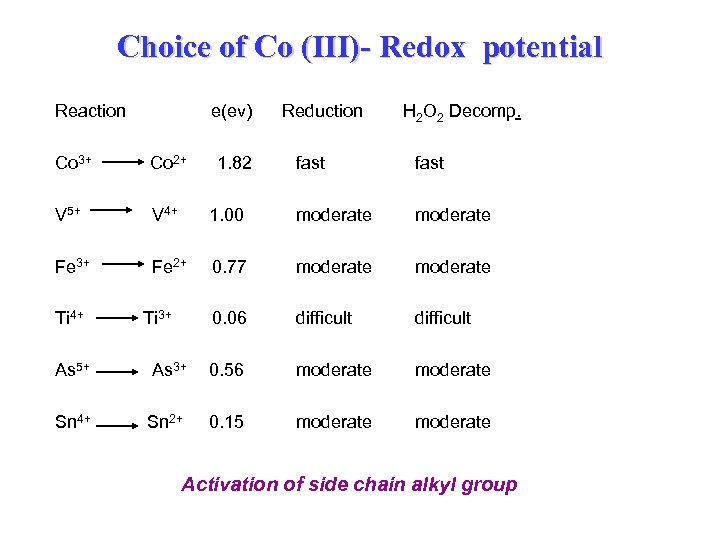 Choice of Co (III)- Redox potential Reaction e(ev) Co 3+ Co 2+ V 5+ V 4+ Fe 3+ Fe 2+ Ti 4+ H 2 O 2 Decomp. fast 1. 00 moderate 0. 77 moderate 0. 06 Ti 3+ 1. 82 Reduction difficult As 5+ As 3+ 0. 56 moderate Sn 4+ Sn 2+ 0. 15 moderate Activation of side chain alkyl group
Choice of Co (III)- Redox potential Reaction e(ev) Co 3+ Co 2+ V 5+ V 4+ Fe 3+ Fe 2+ Ti 4+ H 2 O 2 Decomp. fast 1. 00 moderate 0. 77 moderate 0. 06 Ti 3+ 1. 82 Reduction difficult As 5+ As 3+ 0. 56 moderate Sn 4+ Sn 2+ 0. 15 moderate Activation of side chain alkyl group
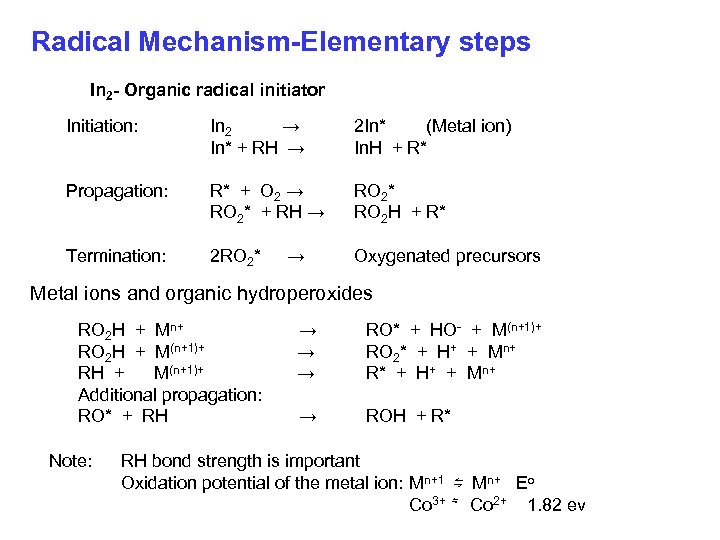 Radical Mechanism-Elementary steps In 2 - Organic radical initiator Initiation: In 2 → In* + RH → 2 In* (Metal ion) In. H + R* Propagation: R* + O 2 → RO 2* + RH → RO 2* RO 2 H + R* Termination: 2 RO 2* Oxygenated precursors → Metal ions and organic hydroperoxides RO 2 H + Mn+ RO 2 H + M(n+1)+ RH + M(n+1)+ Additional propagation: RO* + RH Note: → → → RO* + HO- + M(n+1)+ RO 2* + H+ + Mn+ R* + H+ + Mn+ → ROH + R* RH bond strength is important Oxidation potential of the metal ion: Mn+1 ⇋ Mn+ Eo Co 3+ ⇋ Co 2+ 1. 82 ev
Radical Mechanism-Elementary steps In 2 - Organic radical initiator Initiation: In 2 → In* + RH → 2 In* (Metal ion) In. H + R* Propagation: R* + O 2 → RO 2* + RH → RO 2* RO 2 H + R* Termination: 2 RO 2* Oxygenated precursors → Metal ions and organic hydroperoxides RO 2 H + Mn+ RO 2 H + M(n+1)+ RH + M(n+1)+ Additional propagation: RO* + RH Note: → → → RO* + HO- + M(n+1)+ RO 2* + H+ + Mn+ R* + H+ + Mn+ → ROH + R* RH bond strength is important Oxidation potential of the metal ion: Mn+1 ⇋ Mn+ Eo Co 3+ ⇋ Co 2+ 1. 82 ev
 p-Xylene oxidation- Catalyst system Co/Mn/Br - Co & Mn as acetates & Br as HBr, NH 4 Br, Tetrabromoethane Improved catalyst system- Co/Mn/Br/Zr Active species- MIIIMII[Br-(OOCR)1. 2] Co 3+ when bound to RCOO- is a powerful oxidizing agent Mn 2+ less active than Co 3+- Synergistic effect of Co & Mn Co-Mn pair facilitates formation of Br. Reaction of Co 2+ peracid to give Co 3+ oxidizes Mn 2+ to Mn 3+ Co(III) + Mn(II) Co(II)+ Mn(III) Mn 3+ oxidizes Br- to Br. 3+ Co 2+ (E = 1. 92 V) Mn(III) + Br. Mn(II) + Br. Co 2+ + e ↔ 3+ + e-(E = 1. 2 V) Mn ↔ Mn Br. generates another HC radical Br ↔ Br. + e- (E = 1. 06 V) Cl↔ Cl. + e- (E = 1. 36 V) R-H +Br. R. + HBr Dimeric Co 2+-Co 3+ pairs, once formed are inactive Zr retards formation of dimers by complexation with Co 3+
p-Xylene oxidation- Catalyst system Co/Mn/Br - Co & Mn as acetates & Br as HBr, NH 4 Br, Tetrabromoethane Improved catalyst system- Co/Mn/Br/Zr Active species- MIIIMII[Br-(OOCR)1. 2] Co 3+ when bound to RCOO- is a powerful oxidizing agent Mn 2+ less active than Co 3+- Synergistic effect of Co & Mn Co-Mn pair facilitates formation of Br. Reaction of Co 2+ peracid to give Co 3+ oxidizes Mn 2+ to Mn 3+ Co(III) + Mn(II) Co(II)+ Mn(III) Mn 3+ oxidizes Br- to Br. 3+ Co 2+ (E = 1. 92 V) Mn(III) + Br. Mn(II) + Br. Co 2+ + e ↔ 3+ + e-(E = 1. 2 V) Mn ↔ Mn Br. generates another HC radical Br ↔ Br. + e- (E = 1. 06 V) Cl↔ Cl. + e- (E = 1. 36 V) R-H +Br. R. + HBr Dimeric Co 2+-Co 3+ pairs, once formed are inactive Zr retards formation of dimers by complexation with Co 3+
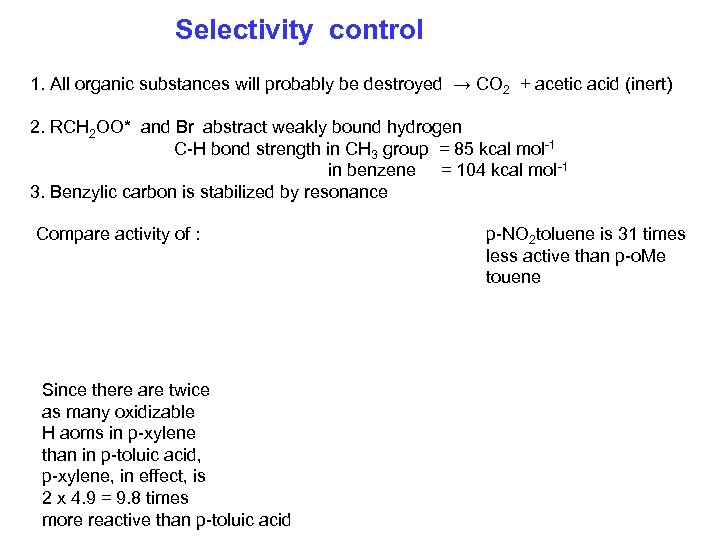 Selectivity control 1. All organic substances will probably be destroyed → CO 2 + acetic acid (inert) 2. RCH 2 OO* and Br abstract weakly bound hydrogen C-H bond strength in CH 3 group = 85 kcal mol-1 in benzene = 104 kcal mol-1 3. Benzylic carbon is stabilized by resonance Compare activity of : Since there are twice as many oxidizable H aoms in p-xylene than in p-toluic acid, p-xylene, in effect, is 2 x 4. 9 = 9. 8 times more reactive than p-toluic acid p-NO 2 toluene is 31 times less active than p-o. Me touene
Selectivity control 1. All organic substances will probably be destroyed → CO 2 + acetic acid (inert) 2. RCH 2 OO* and Br abstract weakly bound hydrogen C-H bond strength in CH 3 group = 85 kcal mol-1 in benzene = 104 kcal mol-1 3. Benzylic carbon is stabilized by resonance Compare activity of : Since there are twice as many oxidizable H aoms in p-xylene than in p-toluic acid, p-xylene, in effect, is 2 x 4. 9 = 9. 8 times more reactive than p-toluic acid p-NO 2 toluene is 31 times less active than p-o. Me touene
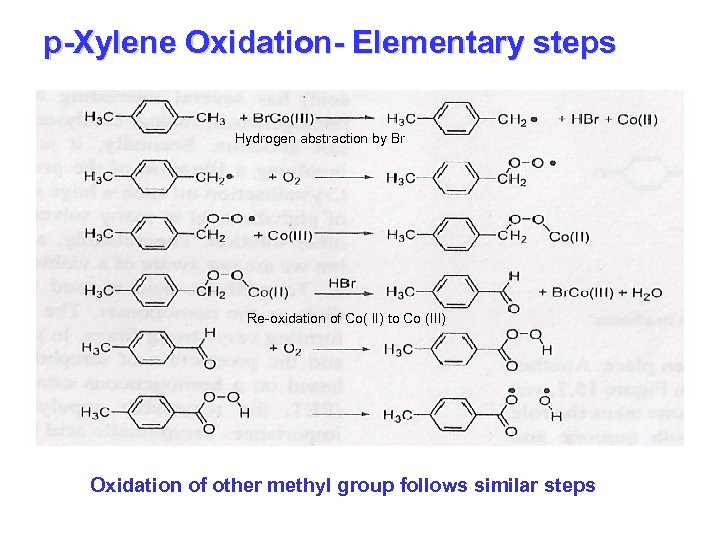 p-Xylene Oxidation- Elementary steps Hydrogen abstraction by Br Re-oxidation of Co( II) to Co (III) Oxidation of other methyl group follows similar steps
p-Xylene Oxidation- Elementary steps Hydrogen abstraction by Br Re-oxidation of Co( II) to Co (III) Oxidation of other methyl group follows similar steps
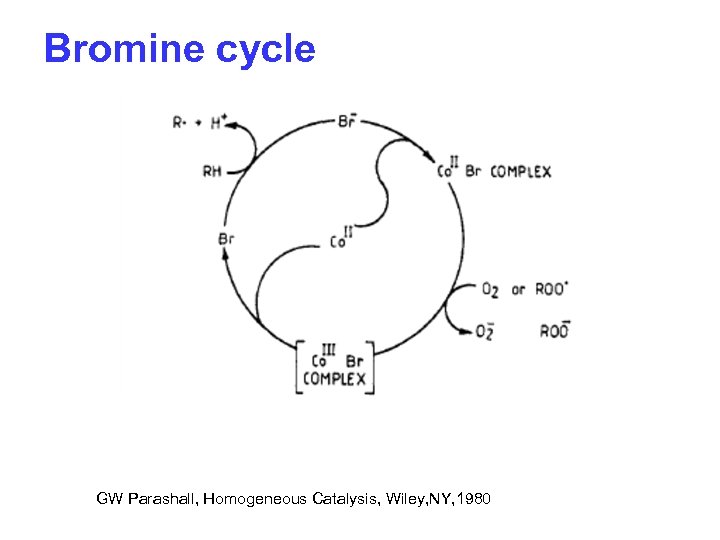 Bromine cycle GW Parashall, Homogeneous Catalysis, Wiley, NY, 1980
Bromine cycle GW Parashall, Homogeneous Catalysis, Wiley, NY, 1980
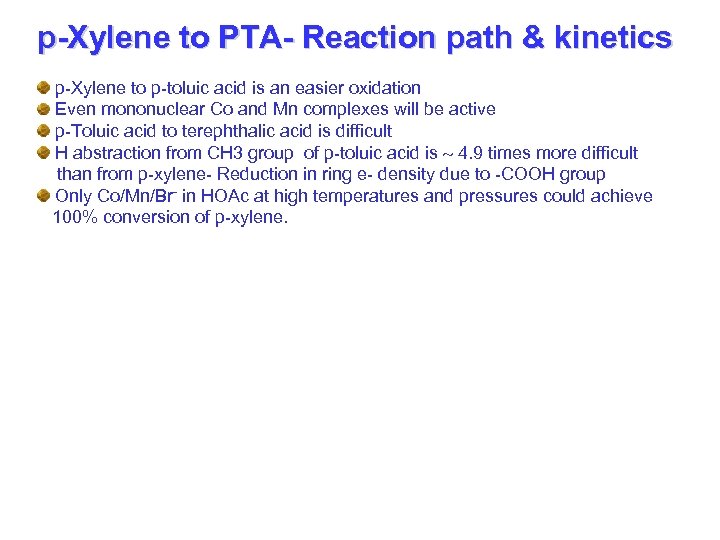 p-Xylene to PTA- Reaction path & kinetics p-Xylene to p-toluic acid is an easier oxidation Even mononuclear Co and Mn complexes will be active p-Toluic acid to terephthalic acid is difficult H abstraction from CH 3 group of p-toluic acid is 4. 9 times more difficult than from p-xylene- Reduction in ring e- density due to -COOH group Only Co/Mn/Br- in HOAc at high temperatures and pressures could achieve 100% conversion of p-xylene.
p-Xylene to PTA- Reaction path & kinetics p-Xylene to p-toluic acid is an easier oxidation Even mononuclear Co and Mn complexes will be active p-Toluic acid to terephthalic acid is difficult H abstraction from CH 3 group of p-toluic acid is 4. 9 times more difficult than from p-xylene- Reduction in ring e- density due to -COOH group Only Co/Mn/Br- in HOAc at high temperatures and pressures could achieve 100% conversion of p-xylene.
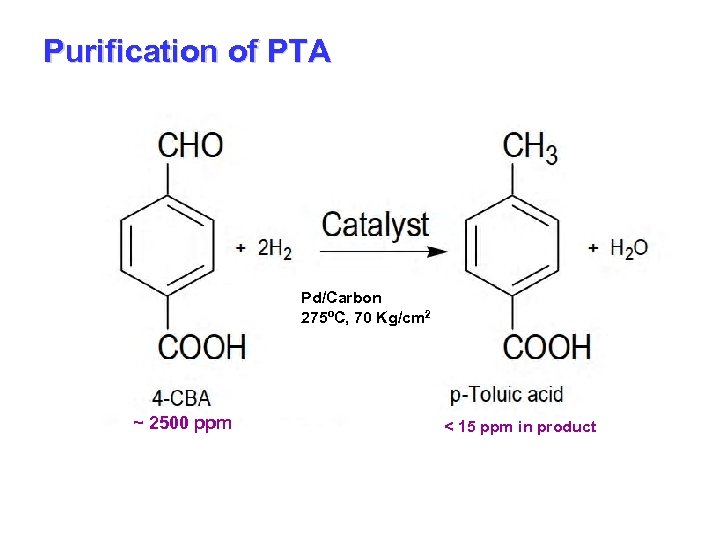 Purification of PTA Pd/Carbon 275ºC, 70 Kg/cm 2 ~ 2500 ppm < 15 ppm in product
Purification of PTA Pd/Carbon 275ºC, 70 Kg/cm 2 ~ 2500 ppm < 15 ppm in product
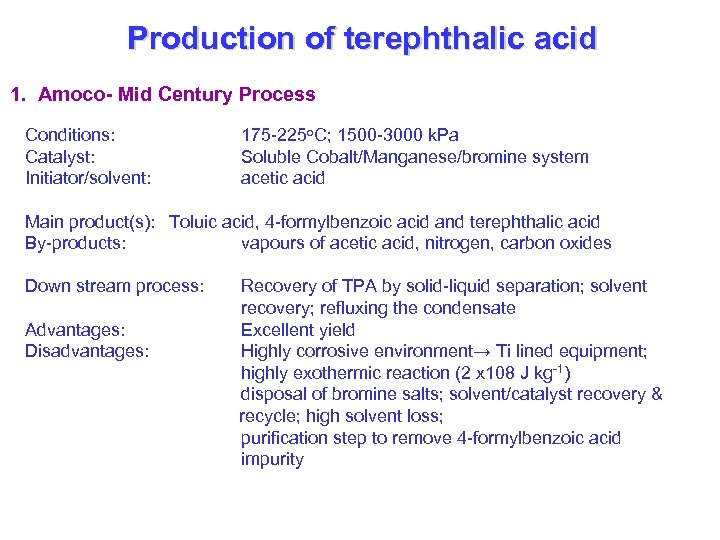 Production of terephthalic acid 1. Amoco- Mid Century Process Conditions: Catalyst: Initiator/solvent: 175 -225 o. C; 1500 -3000 k. Pa Soluble Cobalt/Manganese/bromine system acetic acid Main product(s): Toluic acid, 4 -formylbenzoic acid and terephthalic acid By-products: vapours of acetic acid, nitrogen, carbon oxides Down stream process: Advantages: Disadvantages: Recovery of TPA by solid-liquid separation; solvent recovery; refluxing the condensate Excellent yield Highly corrosive environment→ Ti lined equipment; highly exothermic reaction (2 x 108 J kg-1) disposal of bromine salts; solvent/catalyst recovery & recycle; high solvent loss; purification step to remove 4 -formylbenzoic acid impurity
Production of terephthalic acid 1. Amoco- Mid Century Process Conditions: Catalyst: Initiator/solvent: 175 -225 o. C; 1500 -3000 k. Pa Soluble Cobalt/Manganese/bromine system acetic acid Main product(s): Toluic acid, 4 -formylbenzoic acid and terephthalic acid By-products: vapours of acetic acid, nitrogen, carbon oxides Down stream process: Advantages: Disadvantages: Recovery of TPA by solid-liquid separation; solvent recovery; refluxing the condensate Excellent yield Highly corrosive environment→ Ti lined equipment; highly exothermic reaction (2 x 108 J kg-1) disposal of bromine salts; solvent/catalyst recovery & recycle; high solvent loss; purification step to remove 4 -formylbenzoic acid impurity
 Production of terephthalic acid 2. Oxidation with an activator and/or bromine in acetic acid (Eastman Chemical; Mobil Chemicals) Conditions: Catalyst: Initiator/solvent: 120 -140 o. C; 1500 -3000 k. Pa Soluble Cobalt/Manganese Acetaldehyde, 2 -butanone, bromine, acetic acid Main product(s): By-products: 4 -formylbenzoic acid and terephthalic acid Vapours of acetic acid Down stream process: Crude TPA leached using excess acetic acid followed by sublimation and centrifugation Advantages: Ti-lined vessels are not needed Disadvantages: Costly activators; catalyst recovery and recycle; purification step, solvent recovery, recycle and disposal
Production of terephthalic acid 2. Oxidation with an activator and/or bromine in acetic acid (Eastman Chemical; Mobil Chemicals) Conditions: Catalyst: Initiator/solvent: 120 -140 o. C; 1500 -3000 k. Pa Soluble Cobalt/Manganese Acetaldehyde, 2 -butanone, bromine, acetic acid Main product(s): By-products: 4 -formylbenzoic acid and terephthalic acid Vapours of acetic acid Down stream process: Crude TPA leached using excess acetic acid followed by sublimation and centrifugation Advantages: Ti-lined vessels are not needed Disadvantages: Costly activators; catalyst recovery and recycle; purification step, solvent recovery, recycle and disposal
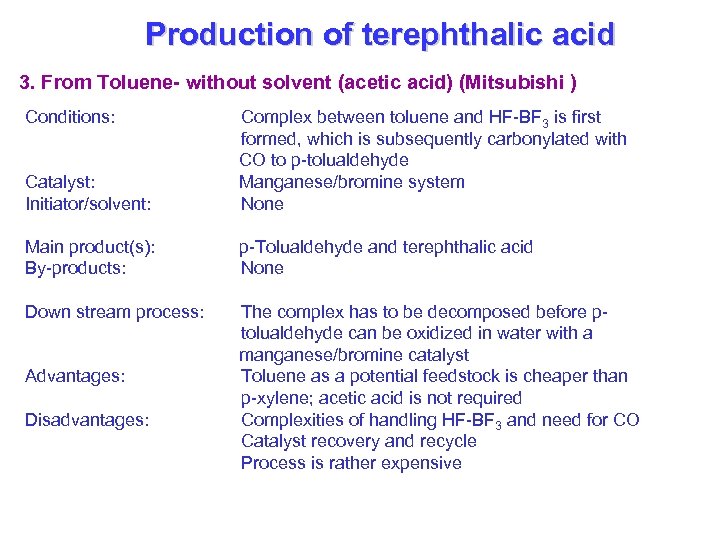 Production of terephthalic acid 3. From Toluene- without solvent (acetic acid) (Mitsubishi ) Conditions: Catalyst: Initiator/solvent: Complex between toluene and HF-BF 3 is first formed, which is subsequently carbonylated with CO to p-tolualdehyde Manganese/bromine system None Main product(s): By-products: p-Tolualdehyde and terephthalic acid None Down stream process: The complex has to be decomposed before ptolualdehyde can be oxidized in water with a manganese/bromine catalyst Toluene as a potential feedstock is cheaper than p-xylene; acetic acid is not required Complexities of handling HF-BF 3 and need for CO Catalyst recovery and recycle Process is rather expensive Advantages: Disadvantages:
Production of terephthalic acid 3. From Toluene- without solvent (acetic acid) (Mitsubishi ) Conditions: Catalyst: Initiator/solvent: Complex between toluene and HF-BF 3 is first formed, which is subsequently carbonylated with CO to p-tolualdehyde Manganese/bromine system None Main product(s): By-products: p-Tolualdehyde and terephthalic acid None Down stream process: The complex has to be decomposed before ptolualdehyde can be oxidized in water with a manganese/bromine catalyst Toluene as a potential feedstock is cheaper than p-xylene; acetic acid is not required Complexities of handling HF-BF 3 and need for CO Catalyst recovery and recycle Process is rather expensive Advantages: Disadvantages:
 Production of terephthalic acid 4. Liquid phase oxidation of p-xylene in air (Solvent-free clean technology route) Conditions: 130 -150 o. C; 2. 5 MPa Catalyst: Initiator/solvent: Solid Co. Al. PO-36 None Main product(s): Toluic acid, 4 -formylbenzoic acid and terephthalic acid By-products: None Down stream process: Esterification of terephthalic acid Advantages: No need for corrosive solvents, activators and bromine; heterogeneous catalyst, ease of separation and recycle Low yield: high residence times. purification step to remove 4 -formylbenzoic acid Disadvantages:
Production of terephthalic acid 4. Liquid phase oxidation of p-xylene in air (Solvent-free clean technology route) Conditions: 130 -150 o. C; 2. 5 MPa Catalyst: Initiator/solvent: Solid Co. Al. PO-36 None Main product(s): Toluic acid, 4 -formylbenzoic acid and terephthalic acid By-products: None Down stream process: Esterification of terephthalic acid Advantages: No need for corrosive solvents, activators and bromine; heterogeneous catalyst, ease of separation and recycle Low yield: high residence times. purification step to remove 4 -formylbenzoic acid Disadvantages:


