ea73e237738421d7ac798e7c372ff882.ppt
- Количество слайдов: 37
 Homework # 5 Gay Lussac’s Law 1. Data + units 2. Set up the problem + units 3. Answer + units
Homework # 5 Gay Lussac’s Law 1. Data + units 2. Set up the problem + units 3. Answer + units
 Aim 5: How can you describe Gay- Lussac’s Law?
Aim 5: How can you describe Gay- Lussac’s Law?
 Gay-Lussac’s Law • Discovered in 1802 by Joseph Gay-Lussac
Gay-Lussac’s Law • Discovered in 1802 by Joseph Gay-Lussac
 The tank contains a gas ……. .
The tank contains a gas ……. .
 How much propane is in the tank? Propane tanks are widely used with barbeque grills. But it’s not fun to find out half-way through your grilling that you’ve run out of gas. You can buy gauges that measure the pressure inside the tank to see how much is left. The gauge measures pressure and will register a higher pressure on a hot day than it will on a cold day. So you need to take the air temperature into account when you decide whether or not to refill the tank before your next cook-out.
How much propane is in the tank? Propane tanks are widely used with barbeque grills. But it’s not fun to find out half-way through your grilling that you’ve run out of gas. You can buy gauges that measure the pressure inside the tank to see how much is left. The gauge measures pressure and will register a higher pressure on a hot day than it will on a cold day. So you need to take the air temperature into account when you decide whether or not to refill the tank before your next cook-out.
 Suppose a gas is kept in a closed, rigid container. If the temperature of the gas is increased, what happens ?
Suppose a gas is kept in a closed, rigid container. If the temperature of the gas is increased, what happens ?
 Constant Volume:
Constant Volume:
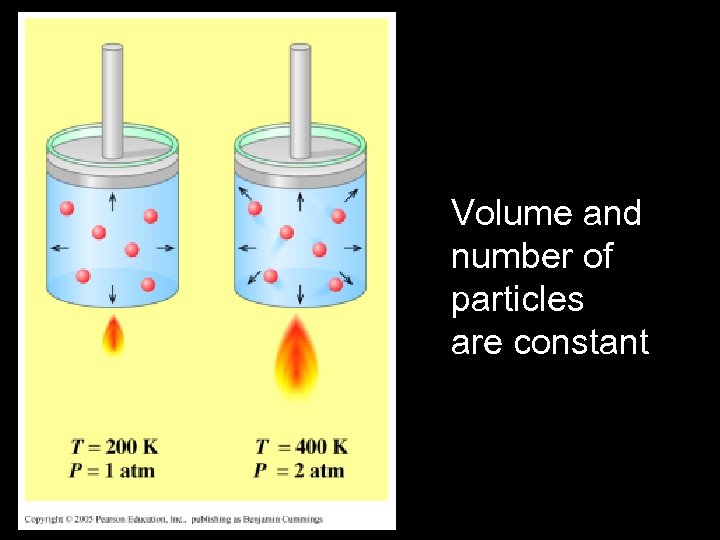 Volume and number of particles are constant
Volume and number of particles are constant
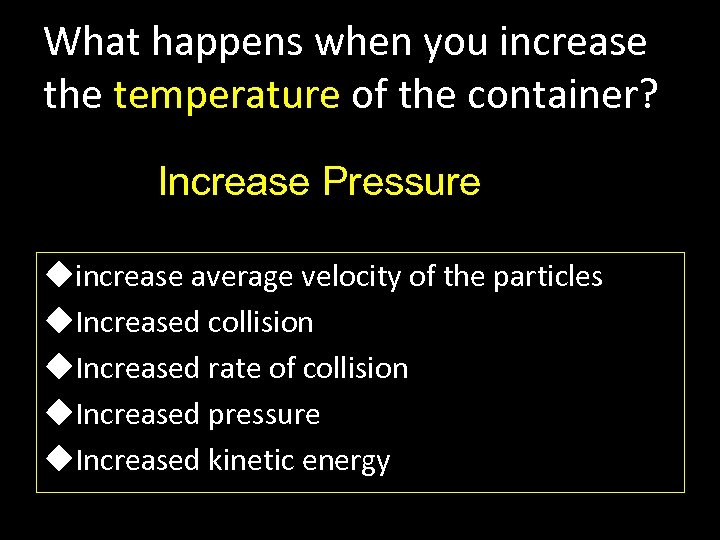 What happens when you increase the temperature of the container? Increase Pressure uincrease average velocity of the particles u. Increased collision u. Increased rate of collision u. Increased pressure u. Increased kinetic energy
What happens when you increase the temperature of the container? Increase Pressure uincrease average velocity of the particles u. Increased collision u. Increased rate of collision u. Increased pressure u. Increased kinetic energy
 At a Constant Volume
At a Constant Volume
 At a Constant Volume
At a Constant Volume
 Think about a SCUBA tank where volume is constant. What happens to the molecular kinetics inside of it when you heat the tank? Since the particles are moving faster the number of collisions with the side of the tank increases, increasing the pressure.
Think about a SCUBA tank where volume is constant. What happens to the molecular kinetics inside of it when you heat the tank? Since the particles are moving faster the number of collisions with the side of the tank increases, increasing the pressure.
 1. Gay-Lussac Law • For a gas confined at a constant volume, the pressure and temperature are directly related.
1. Gay-Lussac Law • For a gas confined at a constant volume, the pressure and temperature are directly related.
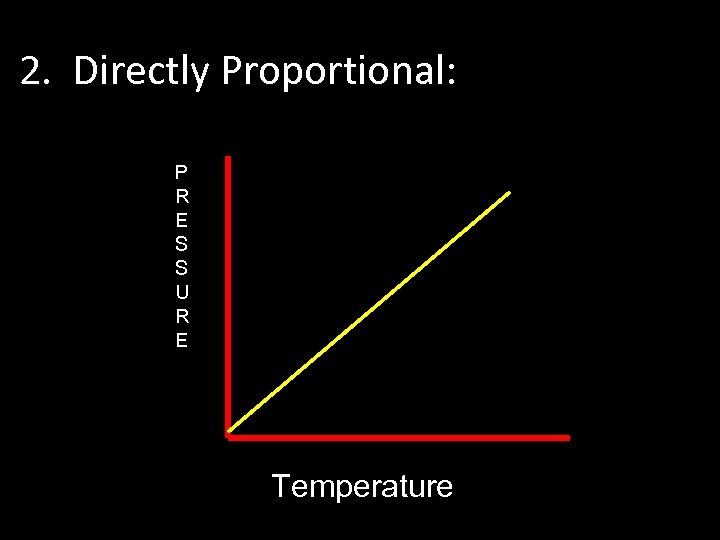 2. Directly Proportional: P R E S S U R E Temperature
2. Directly Proportional: P R E S S U R E Temperature
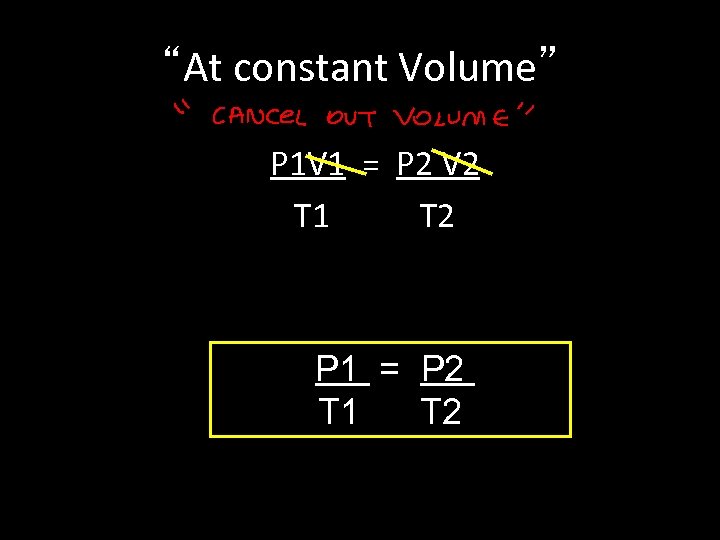 “At constant Volume” P 1 V 1 = P 2 V 2 T 1 T 2 P 1 = P 2 T 1 T 2
“At constant Volume” P 1 V 1 = P 2 V 2 T 1 T 2 P 1 = P 2 T 1 T 2
 Tell some units of pressure • • Atm Kpa mm. Hg Torr
Tell some units of pressure • • Atm Kpa mm. Hg Torr
 How do yo change 300 Kpa to atm? 300 Kpa x 1 atm 101. 3 KPA
How do yo change 300 Kpa to atm? 300 Kpa x 1 atm 101. 3 KPA
 Why use kelvin scale for gas law?
Why use kelvin scale for gas law?
 How do you change 100 degrees Celsius to Kelvin? • Table T • K= OC +273
How do you change 100 degrees Celsius to Kelvin? • Table T • K= OC +273
 When you multiply or divide, how do you determine the number of significant figures in your answer? 300. X 2350 = 3 SF 1200 x 40 =1 SF
When you multiply or divide, how do you determine the number of significant figures in your answer? 300. X 2350 = 3 SF 1200 x 40 =1 SF
 1. Determine the pressure change when a constant volume of gas at 1. 00 atm is heated from 20. 0 °C to 30. 0 °C. P 1 = P 2 T 1 T 2 Data P 1= 1. 00 atm (3 sf) T 1 = 20. 0 °C = 293. 0 K (1 decimal) P 2 = ? T 2 = 30. 0 °C = 303. 0 K (1 decimal)
1. Determine the pressure change when a constant volume of gas at 1. 00 atm is heated from 20. 0 °C to 30. 0 °C. P 1 = P 2 T 1 T 2 Data P 1= 1. 00 atm (3 sf) T 1 = 20. 0 °C = 293. 0 K (1 decimal) P 2 = ? T 2 = 30. 0 °C = 303. 0 K (1 decimal)
 1 st Method: replace the data in the formula
1 st Method: replace the data in the formula
 P 1 = P 2 T 1 T 2 Data P 1= 1. 00 atm P 2 = ? T 1 = 293. 0 K T 2 = 303. 0 K 1. 00 atm = 293. 0 K P 2= 1. 03 atm P 2 303. 0 K
P 1 = P 2 T 1 T 2 Data P 1= 1. 00 atm P 2 = ? T 1 = 293. 0 K T 2 = 303. 0 K 1. 00 atm = 293. 0 K P 2= 1. 03 atm P 2 303. 0 K
 2 nd Method: Practice algebra! • Solve P 2
2 nd Method: Practice algebra! • Solve P 2
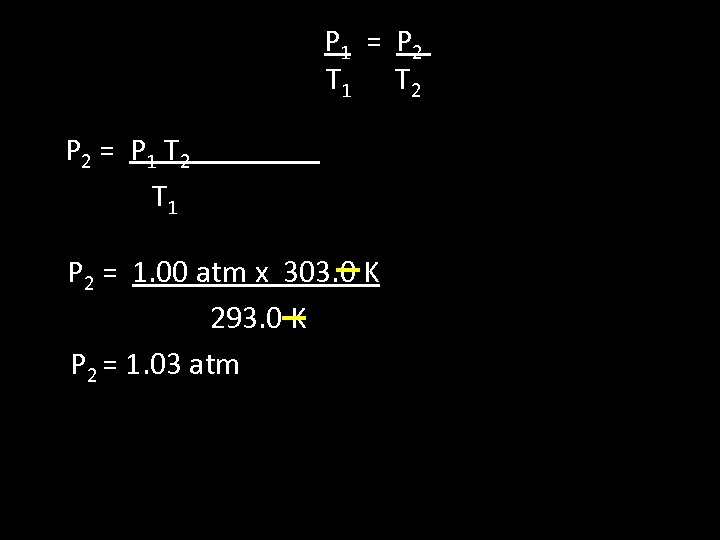 P 1 = P 2 T 1 T 2 P 2 = P 1 T 2 T 1 P 2 = 1. 00 atm x 303. 0 K 293. 0 K P 2 = 1. 03 atm
P 1 = P 2 T 1 T 2 P 2 = P 1 T 2 T 1 P 2 = 1. 00 atm x 303. 0 K 293. 0 K P 2 = 1. 03 atm
 I prefer that you use the first method : . )
I prefer that you use the first method : . )
 What does STP mean in a pressure and temperature question? • Use table A to answer this question.
What does STP mean in a pressure and temperature question? • Use table A to answer this question.
 2. A gas has a pressure of 0. 370 atm at 50. 0 °C. What is the pressure at standard temperature? Data P 1= 0. 370 atm T 1 = 50. 0 °C = 323. 0 K P 2 = ? T 2 = 273 K (at standard temperature : table A)
2. A gas has a pressure of 0. 370 atm at 50. 0 °C. What is the pressure at standard temperature? Data P 1= 0. 370 atm T 1 = 50. 0 °C = 323. 0 K P 2 = ? T 2 = 273 K (at standard temperature : table A)
 P 2 = P 1 T 2 T 1 P 2 = 0. 370 atm x 273 K 323 K P 2 =0. 313 atm
P 2 = P 1 T 2 T 1 P 2 = 0. 370 atm x 273 K 323 K P 2 =0. 313 atm
 3. If a gas in a closed container is pressurized from 15. 0 atmospheres to 16. 0 atmospheres and its original temperature was 25. 0 °C, what would the final temperature of the gas be? Data P 1= 15. 0 atm T 1 = 25 °C = 298 K P 2 = 16. 0 atm T 2 =
3. If a gas in a closed container is pressurized from 15. 0 atmospheres to 16. 0 atmospheres and its original temperature was 25. 0 °C, what would the final temperature of the gas be? Data P 1= 15. 0 atm T 1 = 25 °C = 298 K P 2 = 16. 0 atm T 2 =
 T 2 = P 2 T 1 P 1 T 2 = 16. 0 atm x 298 K 15 atm T 2 = 317. 87 K
T 2 = P 2 T 1 P 1 T 2 = 16. 0 atm x 298 K 15 atm T 2 = 317. 87 K
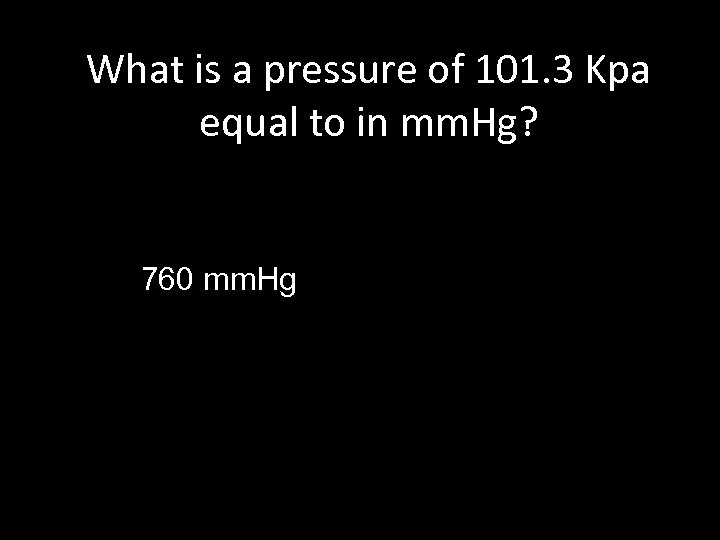 What is a pressure of 101. 3 Kpa equal to in mm. Hg? 760 mm. Hg
What is a pressure of 101. 3 Kpa equal to in mm. Hg? 760 mm. Hg
 4. A gas has a pressure of 699. 0 mm Hg at 40. 0 °C. What is the temperature at standard pressure? Data P 1= 699. 0 mm Hg T 1 = 40. 0 °C = 313 K P 2 = 760. 0 mm Hg (at standard pressure) T 2 = ? *1 atm= 101. 3 Kpa= 760 mm Hg
4. A gas has a pressure of 699. 0 mm Hg at 40. 0 °C. What is the temperature at standard pressure? Data P 1= 699. 0 mm Hg T 1 = 40. 0 °C = 313 K P 2 = 760. 0 mm Hg (at standard pressure) T 2 = ? *1 atm= 101. 3 Kpa= 760 mm Hg
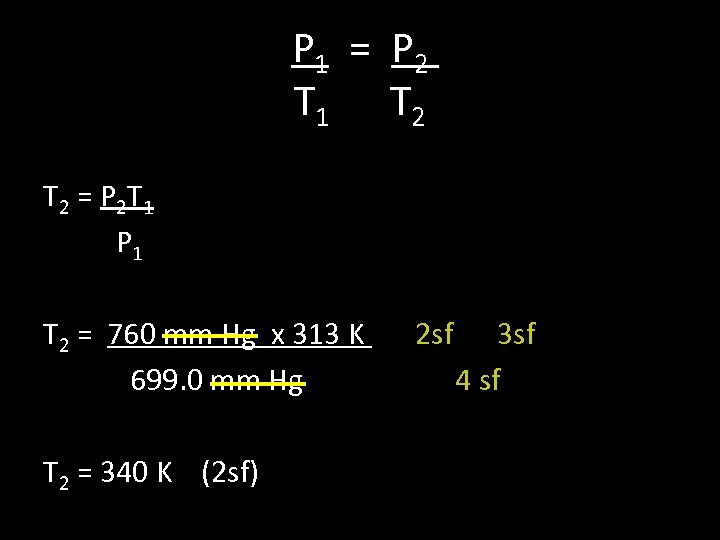 P 1 = P 2 T 1 T 2 = P 2 T 1 P 1 T 2 = 760 mm Hg x 313 K 2 sf 3 sf 699. 0 mm Hg 4 sf T 2 = 340 K (2 sf)
P 1 = P 2 T 1 T 2 = P 2 T 1 P 1 T 2 = 760 mm Hg x 313 K 2 sf 3 sf 699. 0 mm Hg 4 sf T 2 = 340 K (2 sf)
 5. If a gas is cooled from 323. 0 K to 273. 15 K and the volume is kept constant what final pressure would result if the original pressure was 750. 0 mm Hg? Data P 1= 750. 0 mm Hg T 1 = 320 K P 2 = ? T 2 = 273. 15 K
5. If a gas is cooled from 323. 0 K to 273. 15 K and the volume is kept constant what final pressure would result if the original pressure was 750. 0 mm Hg? Data P 1= 750. 0 mm Hg T 1 = 320 K P 2 = ? T 2 = 273. 15 K
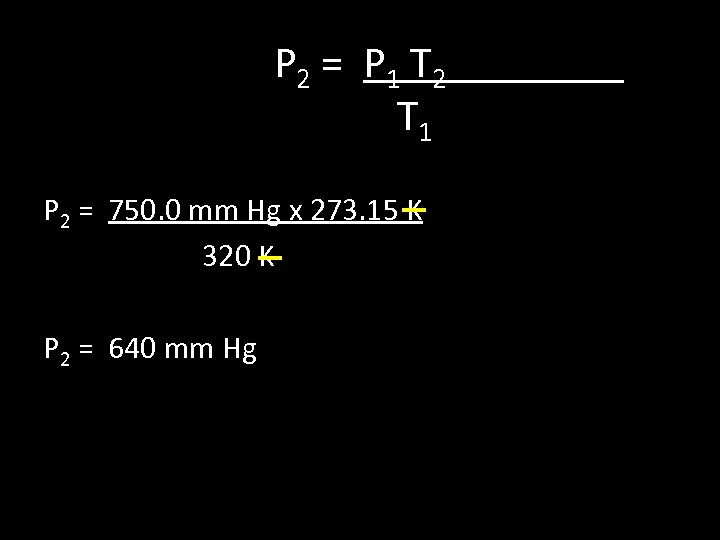 P 2 = P 1 T 2 T 1 P 2 = 750. 0 mm Hg x 273. 15 K 320 K P 2 = 640 mm Hg
P 2 = P 1 T 2 T 1 P 2 = 750. 0 mm Hg x 273. 15 K 320 K P 2 = 640 mm Hg
 End of the show
End of the show


