02a14d386ff2232b8e4f97cf68116a31.ppt
- Количество слайдов: 41

Homework # 10 1. Worksheet: Quantum Numbers 2. Assign quantum numbers to the valence electron of a lithium. 3. Assign quantum numbers for the tenth electron to fill a neon atom.

Aim 10: How can you describe the quantum mechanical model?
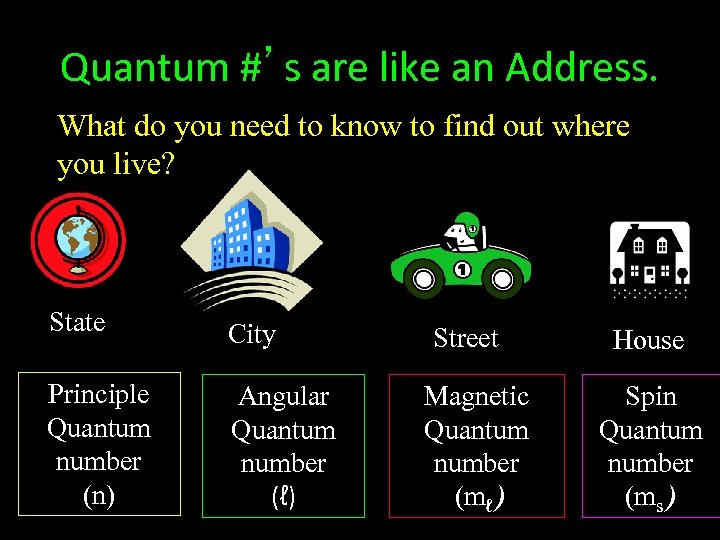
Quantum #’s are like an Address. What do you need to know to find out where you live? State City Principle Quantum number (n) Angular Quantum number (ℓ) Street Magnetic Quantum number (mℓ) House Spin Quantum number (ms)

1. Quantum Numbers • Modern atomic theory states that any electron in an atom can be completely describe by four quantum numbers: n, l, ml , ms
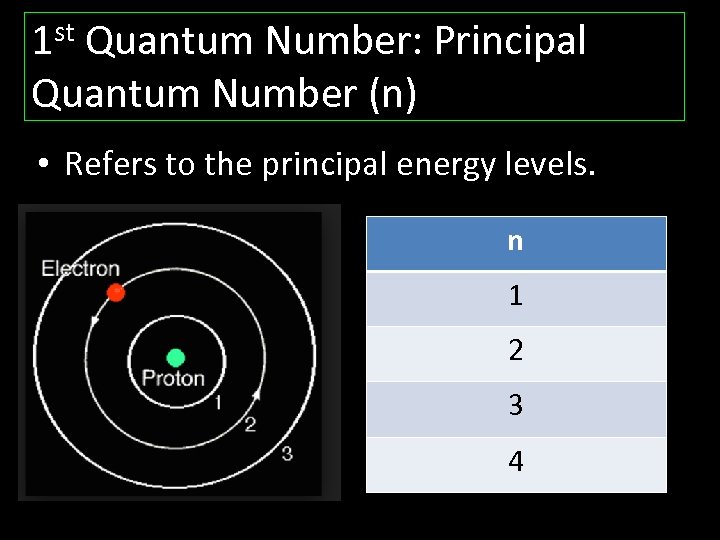
1 st Quantum Number: Principal Quantum Number (n) • Refers to the principal energy levels. n 1 2 3 4

2 nd Quantum Number: Azimuthal or Angular Momentum Quantum Number (ℓ)
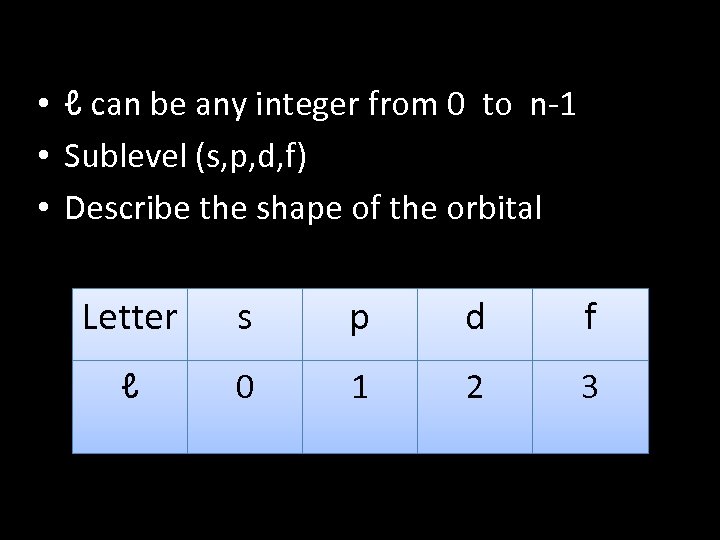
• ℓ can be any integer from 0 to n-1 • Sublevel (s, p, d, f) • Describe the shape of the orbital Letter s p d f ℓ 0 1 2 3

letter s p d f ℓ 0 1 2 3 Example: n = 3 ℓ = n-1 ℓ = 3 -1 ℓ=2

S, P, D, F s: Sharp p: Principal d: Diffuse f: Fundamental The s, p, d, and f stand for "sharp, " "principal, " "diffuse, " and "fundamental, " respectively, and are so named because they categorize the spectral lines generated by those types of orbitals: Electron configuration
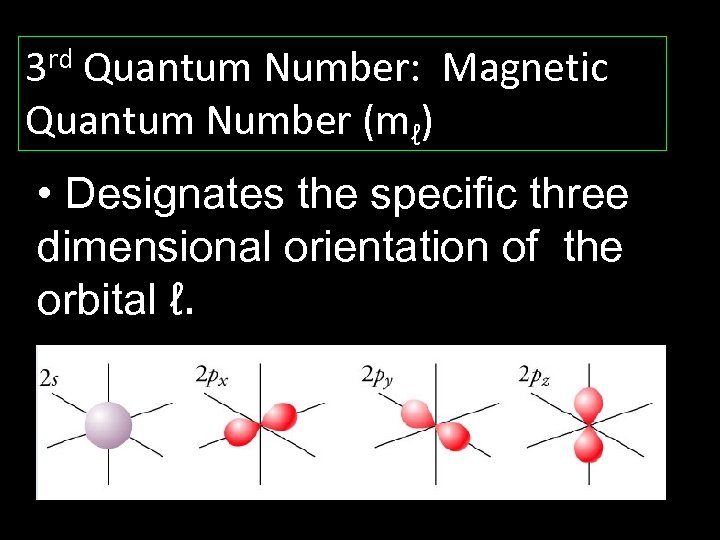
3 rd Quantum Number: Magnetic Quantum Number (mℓ) • Designates the specific three dimensional orientation of the orbital ℓ.
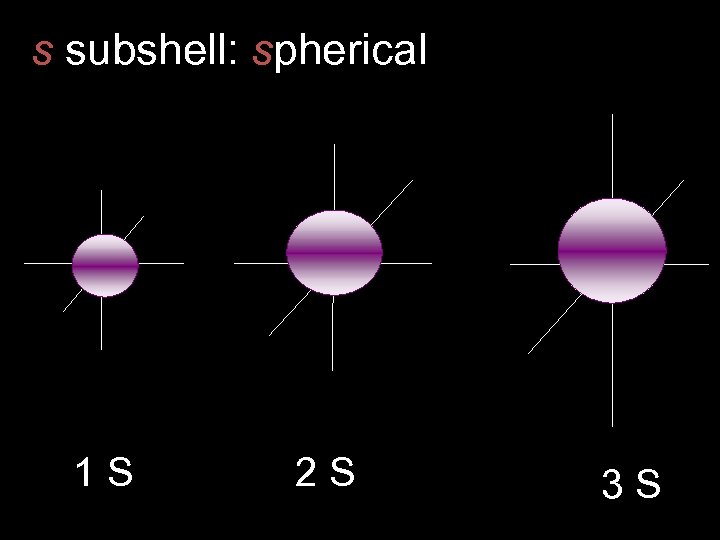
s subshell: spherical 1 S 2 S 3 S
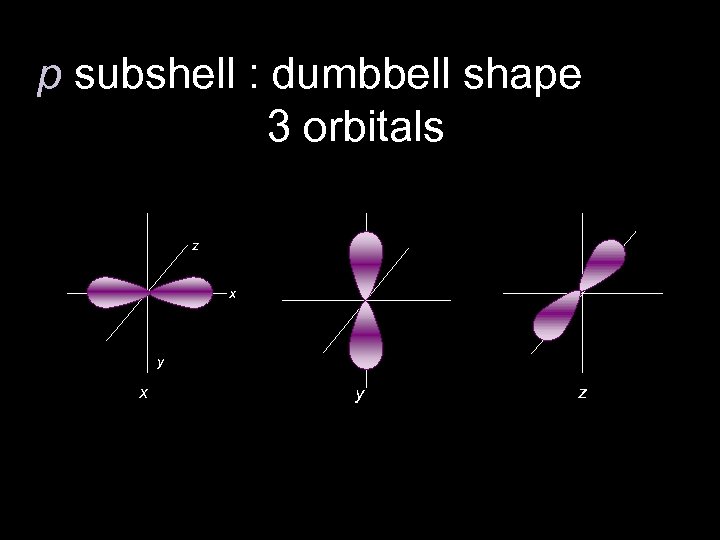
p subshell : dumbbell shape 3 orbitals z x y z
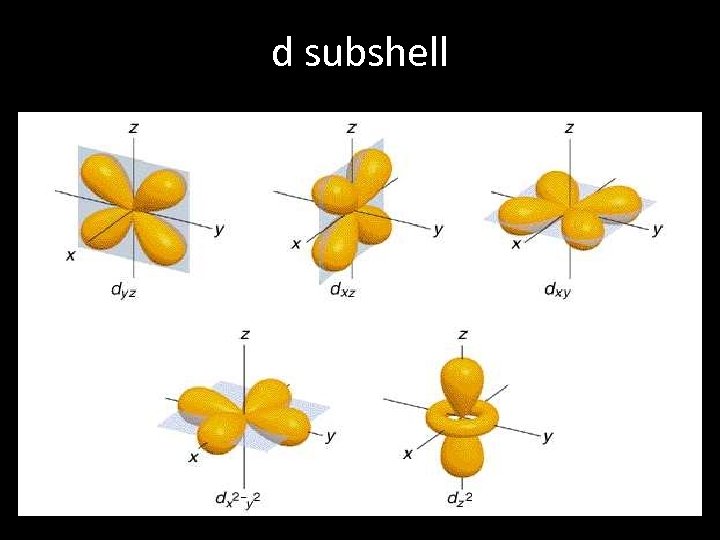
d subshell

mℓ can be any integer from: - ℓ to + ℓ Example: ℓ= 2 mℓ = -2, -1, 0, +1, +2
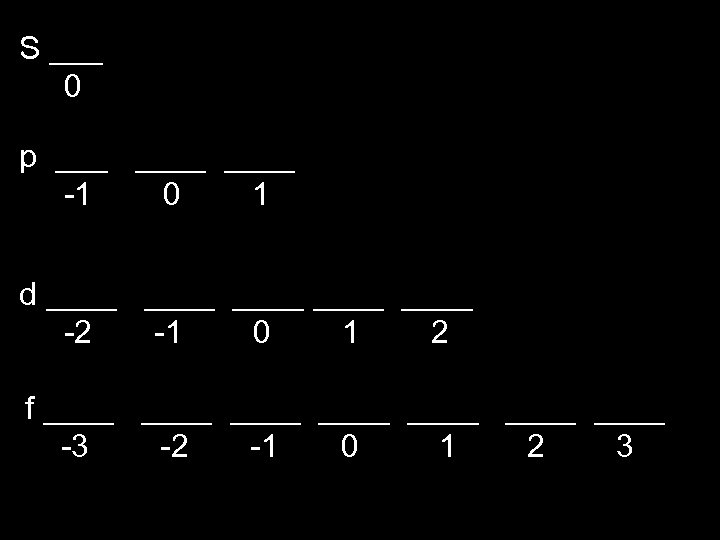
S ___ 0 p ____ -1 0 1 d ____ ____ -2 -1 0 1 2 f ____ ____ -3 -2 -1 0 1 2 3
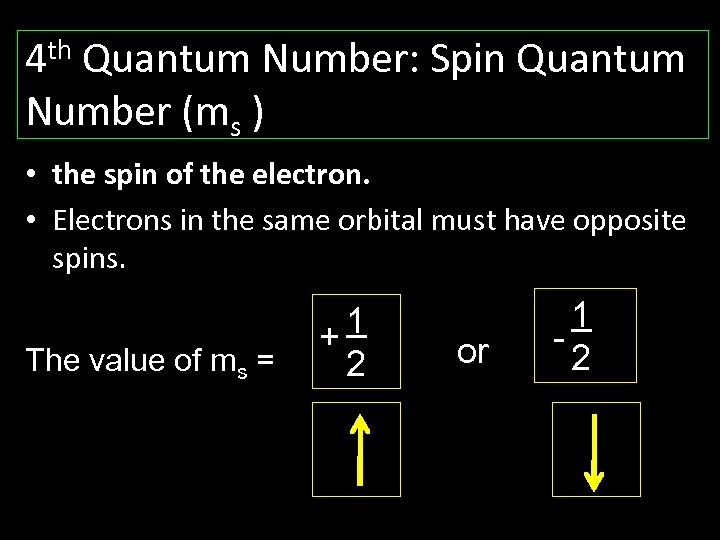
th 4 Quantum Number: Spin Quantum Number (ms ) • the spin of the electron. • Electrons in the same orbital must have opposite spins. The value of ms = 1 + 2 or 1 2
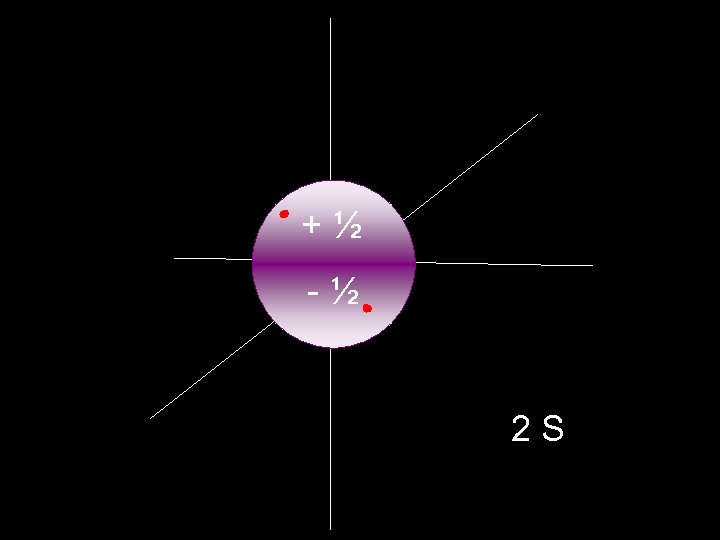
+½ -½ 2 S
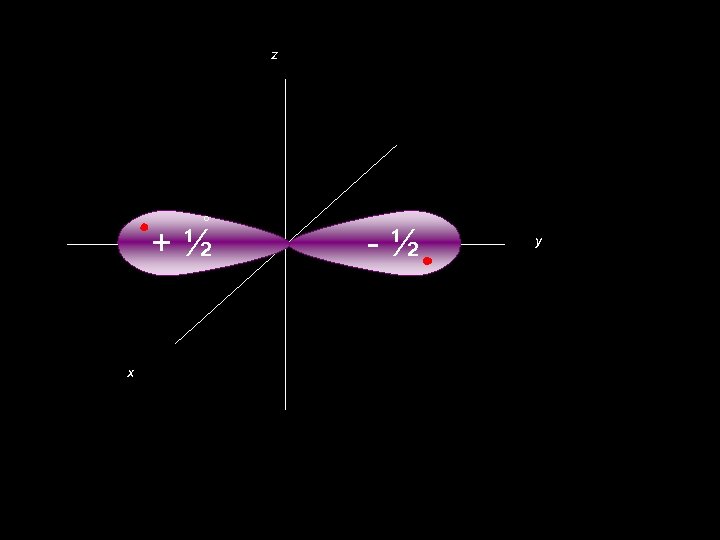
z ° +½ x -½ y
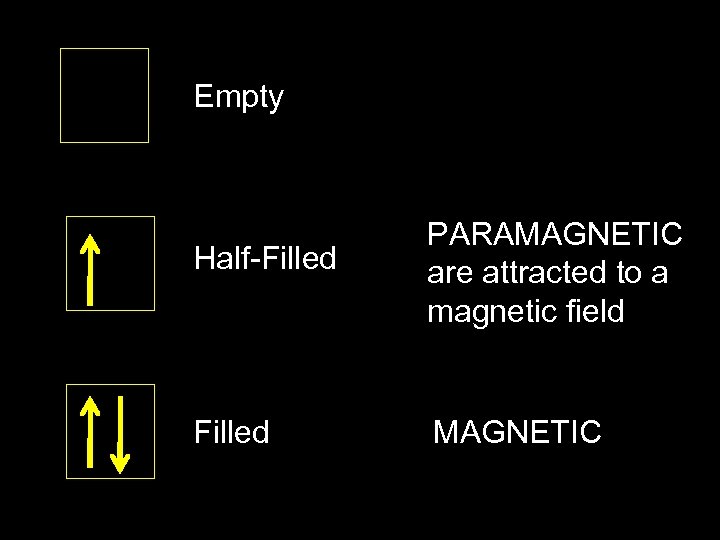
Empty Half-Filled PARAMAGNETIC are attracted to a magnetic field Filled MAGNETIC

The Pauli exclusion principle (Wolfgang Pauli, Nobel Prize 1945) states that: no two electrons in the same atom can have identical values for all four of their quantum numbers. What this means is that no more than two electrons can occupy the same orbital, and that two electrons in the same orbital must have opposite spins.

Summary
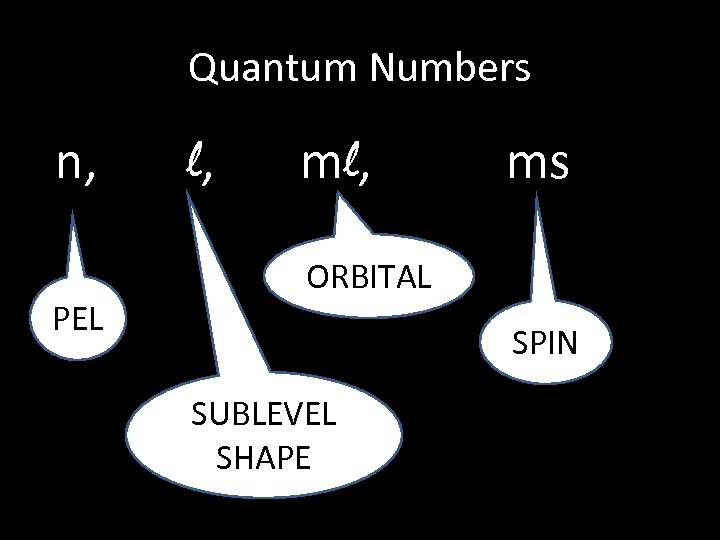
Quantum Numbers n, PEL l, ms ORBITAL SPIN SUBLEVEL SHAPE

l = sublevel (s, p, d, f) S=0 P=1 d=2 F=3
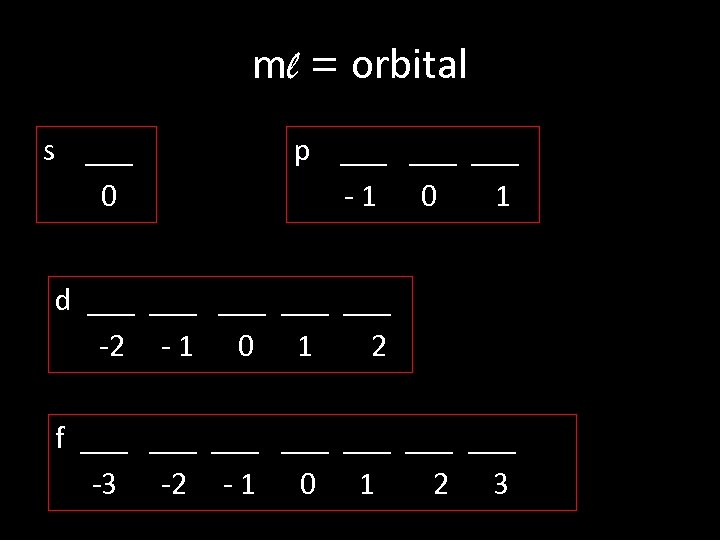
ml = orbital s ___ 0 p ___ ___ -1 0 1 d ___ ___ ___ -2 - 1 0 1 2 f ___ ___ -3 -2 - 1 0 1 2 3
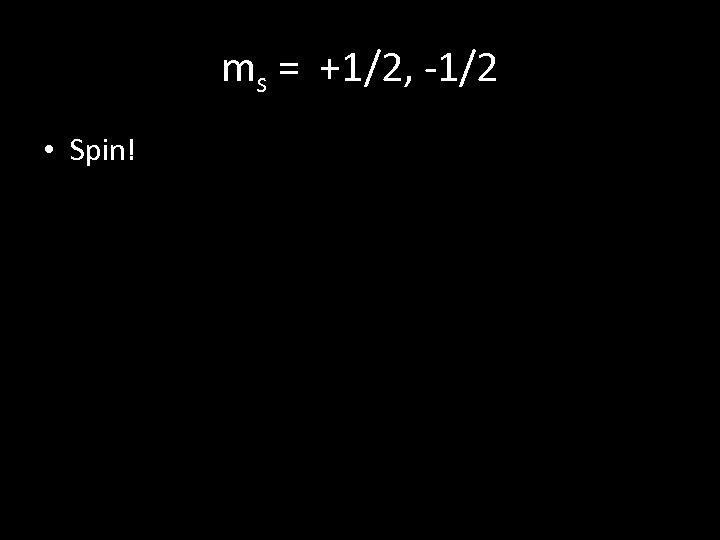
ms = +1/2, -1/2 • Spin!

TEST YOUR UNDERSTANDING
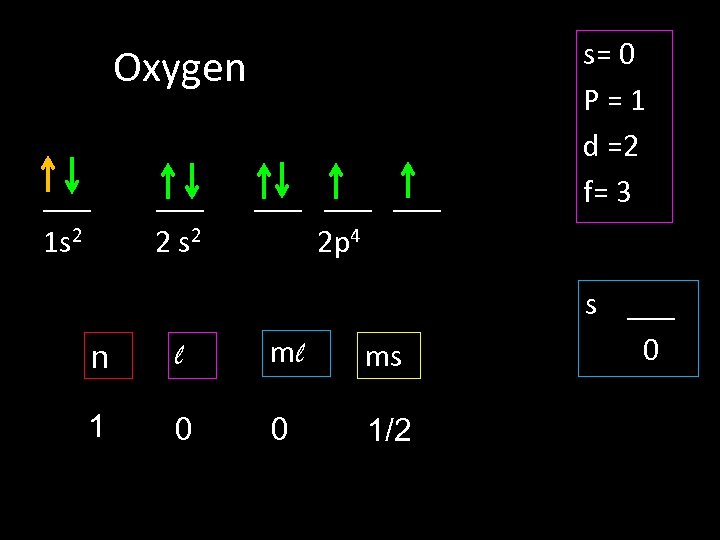
Oxygen ___ 1 s 2 ___ 2 s 2 ___ ___ 2 p 4 n l ml ms 1 0 0 1/2 s= 0 P=1 d =2 f= 3 s ___ 0
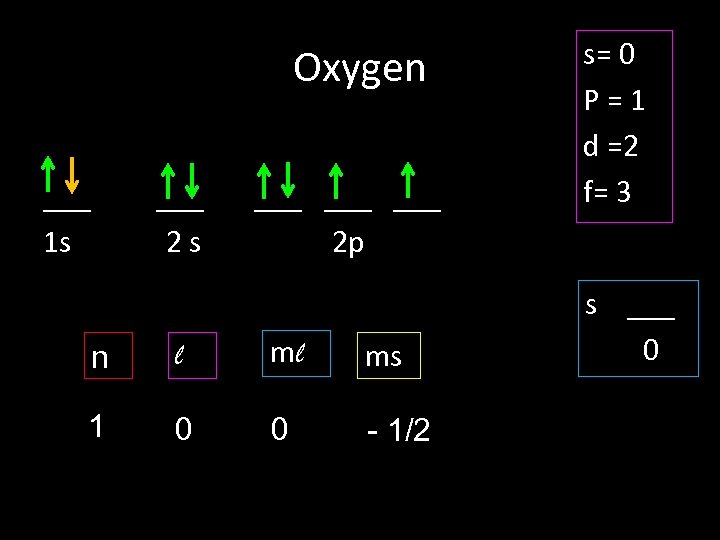
Oxygen ___ 1 s ___ 2 s ___ ___ 2 p n l ml ms 1 0 0 - 1/2 s= 0 P=1 d =2 f= 3 s ___ 0
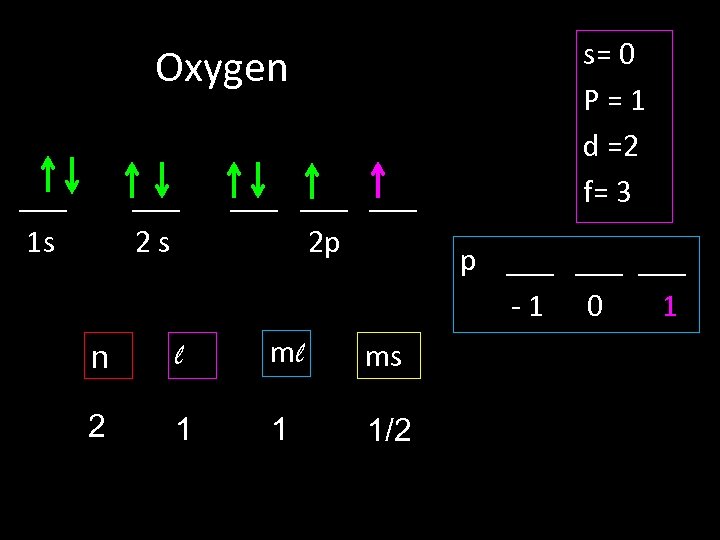
Oxygen ___ 1 s ___ 2 s ___ ___ 2 p n l ml ms 2 1 1 1/2 s= 0 P=1 d =2 f= 3 p ___ ___ -1 0 1
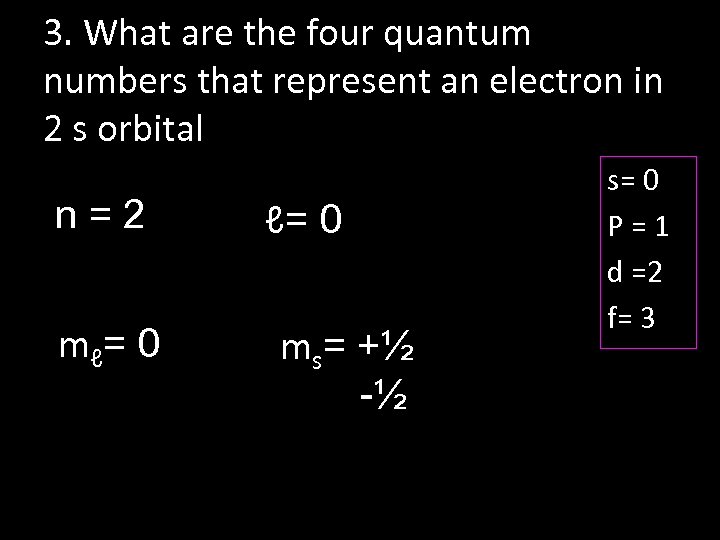
3. What are the four quantum numbers that represent an electron in 2 s orbital n=2 m ℓ= 0 ms= +½ -½ s= 0 P=1 d =2 f= 3
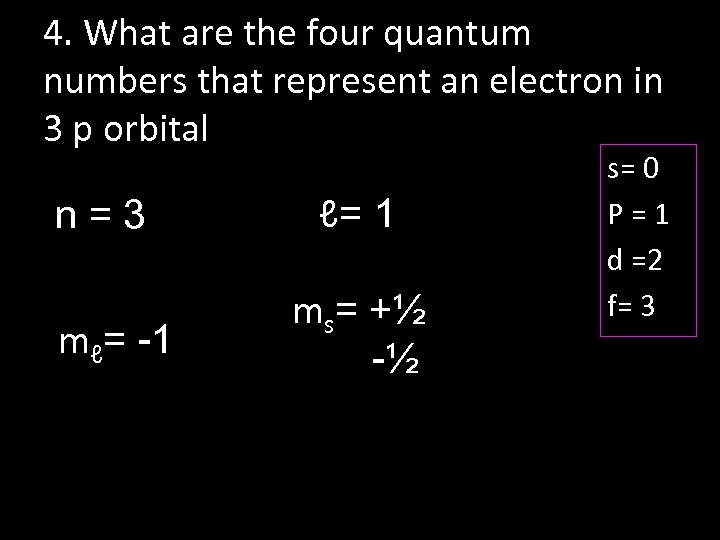
4. What are the four quantum numbers that represent an electron in 3 p orbital n=3 mℓ= -1 ℓ= 1 ms= +½ -½ s= 0 P=1 d =2 f= 3
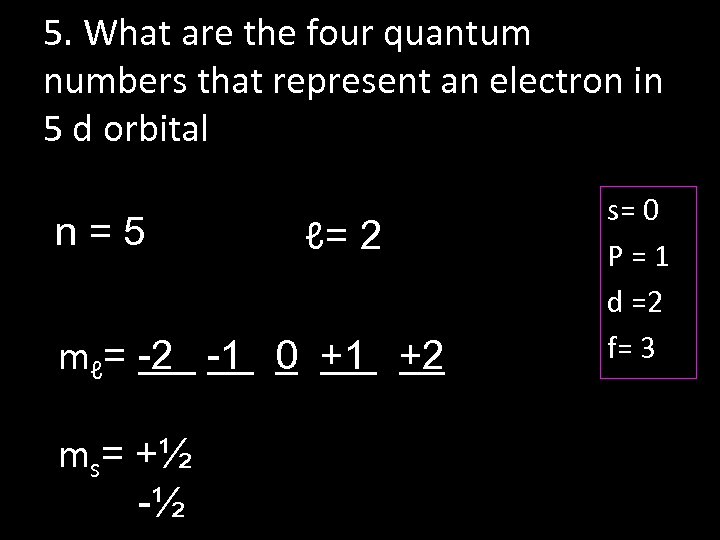
5. What are the four quantum numbers that represent an electron in 5 d orbital n=5 ℓ= 2 mℓ= -2 -1 0 +1 +2 ms= +½ -½ s= 0 P=1 d =2 f= 3

6. What are the four quantum numbers that represent an electron in 4 f orbital s= 0 n=4 ℓ= 3 P=1 d =2 f= 3 mℓ= - 3 -2 -1 0 +1 +2 + 3 ms= +½ -½
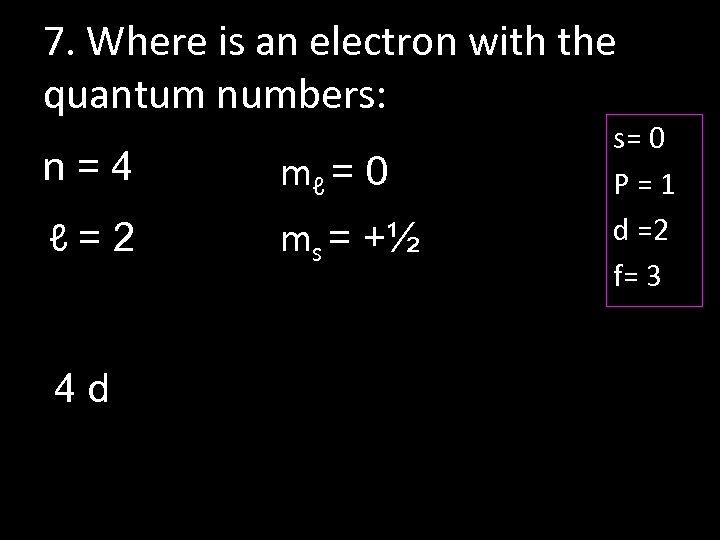
7. Where is an electron with the quantum numbers: n=4 mℓ = 0 ℓ=2 ms = +½ 4 d s= 0 P=1 d =2 f= 3

8. Where is an electron with the quantum numbers: n=2 m ℓ= 0 ms= -½ 2 s 2
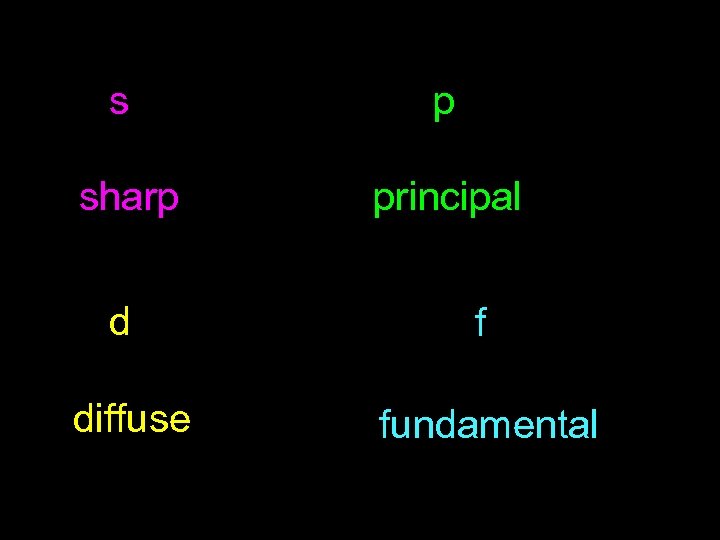
s p sharp principal d f diffuse fundamental
![9. Assign quantum numbers for the tenth electron to fill a neon atom. [He] 9. Assign quantum numbers for the tenth electron to fill a neon atom. [He]](https://present5.com/presentation/02a14d386ff2232b8e4f97cf68116a31/image-37.jpg)
9. Assign quantum numbers for the tenth electron to fill a neon atom. [He] 2 s 2 2 p 6 1 2 2 s 1 s 2 2 p 6 s p p p
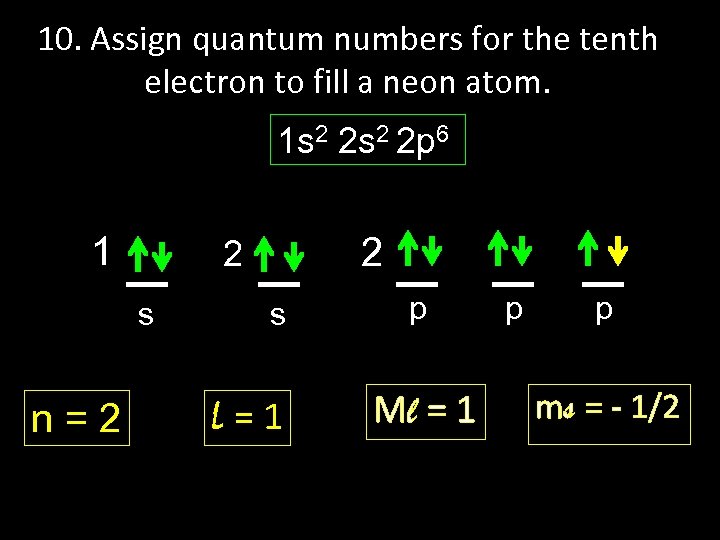
10. Assign quantum numbers for the tenth electron to fill a neon atom. 1 s 2 2 p 6 1 s n=2 2 2 s L=1 p Ml = 1 p p ms = - 1/2

For each of the quantum numbers, state which one is invalid and why?

Practice: MCQ Questions http: //www. ualr. edu/rebelford/chem 1402/q 14 02/X 3/c 7/7 -3. htm#JUMP_11
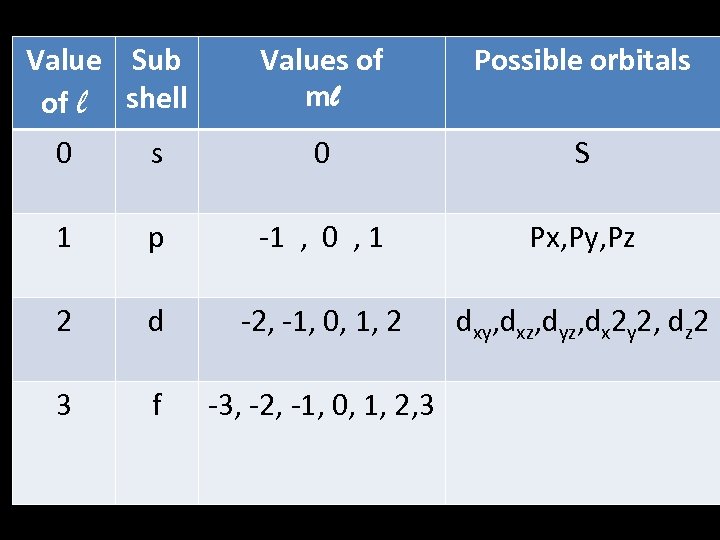
Value Sub of l shell Values of ml Possible orbitals 0 S 1 p -1 , 0 , 1 Px, Py, Pz 2 d -2, -1, 0, 1, 2 dxy, dxz, dyz, dx 2 y 2, dz 2 3 f -3, -2, -1, 0, 1, 2, 3
02a14d386ff2232b8e4f97cf68116a31.ppt