09463079651eb28c7ce0fd3ae4faa4c5.ppt
- Количество слайдов: 90
 HIV INFECTION AND THE ACQUIRED IMMUNODEFICIENCY SYNDROME (AIDS)
HIV INFECTION AND THE ACQUIRED IMMUNODEFICIENCY SYNDROME (AIDS)
 HISTORICAL PERSPECTIVES OF AIDS 1981 Recognition of Pneumocystis carinii pneumonia (PCP) and Kaposi’s sarcoma (KS) in young healthy men in NYC and Los Angeles 1982 GRID to AIDS by CDC 1983 Isolation of Lymphadenopathy-Associated Virus (LAV) by Pasteur Institute (Luc Montagnier) 1984 Isolation of Human T-Lymphotrophic Virus , Type III (HTLV-III) by NCI/NIH (Robert Gallo) 1986 Recommendation of the name Human Immunodeficiency Virus (HIV) by an international subcommittee on virus taxonomy
HISTORICAL PERSPECTIVES OF AIDS 1981 Recognition of Pneumocystis carinii pneumonia (PCP) and Kaposi’s sarcoma (KS) in young healthy men in NYC and Los Angeles 1982 GRID to AIDS by CDC 1983 Isolation of Lymphadenopathy-Associated Virus (LAV) by Pasteur Institute (Luc Montagnier) 1984 Isolation of Human T-Lymphotrophic Virus , Type III (HTLV-III) by NCI/NIH (Robert Gallo) 1986 Recommendation of the name Human Immunodeficiency Virus (HIV) by an international subcommittee on virus taxonomy



 HUMAN IMMUNODEFICIENCY VIRUSES (HIV) * Classification * Retroviridae (family) * Lentivirus (genus) * Characteristics * 100 nm in diameter * Genome of 2 single strands of RNA * Nine genes * Reverse transcriptase * RNA-dependent DNA polymerase * Transcribes RNA into DNA
HUMAN IMMUNODEFICIENCY VIRUSES (HIV) * Classification * Retroviridae (family) * Lentivirus (genus) * Characteristics * 100 nm in diameter * Genome of 2 single strands of RNA * Nine genes * Reverse transcriptase * RNA-dependent DNA polymerase * Transcribes RNA into DNA
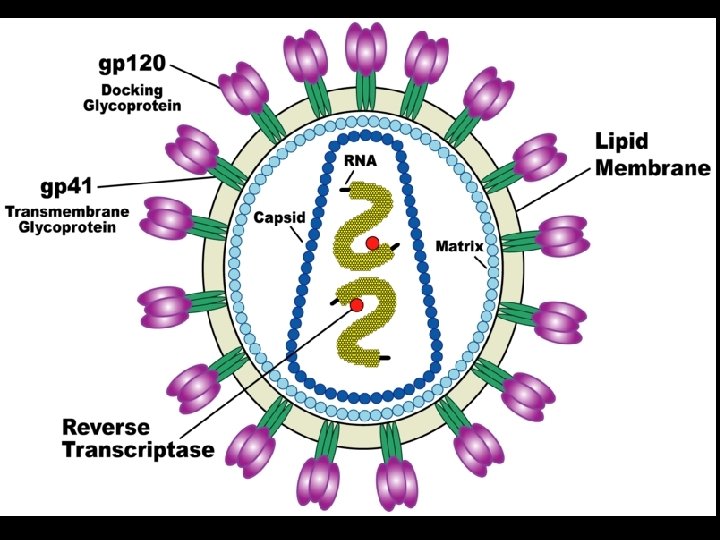
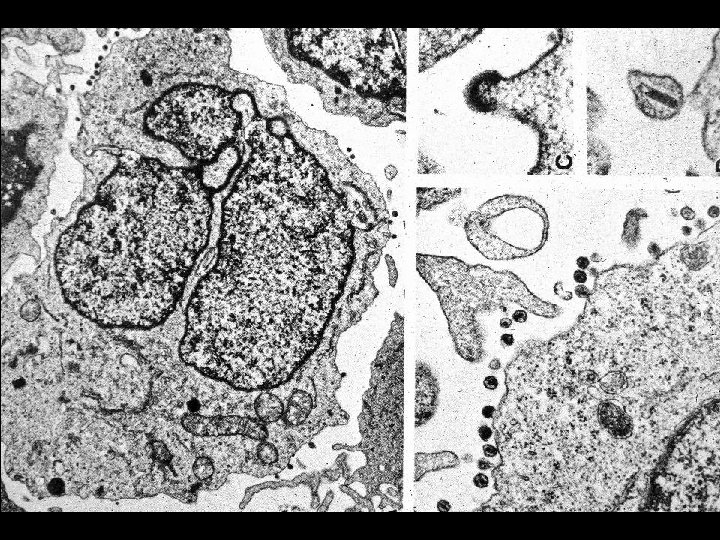
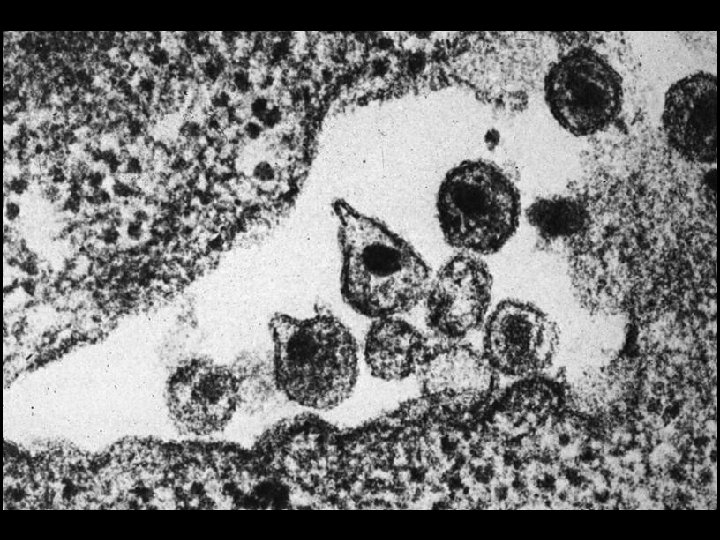
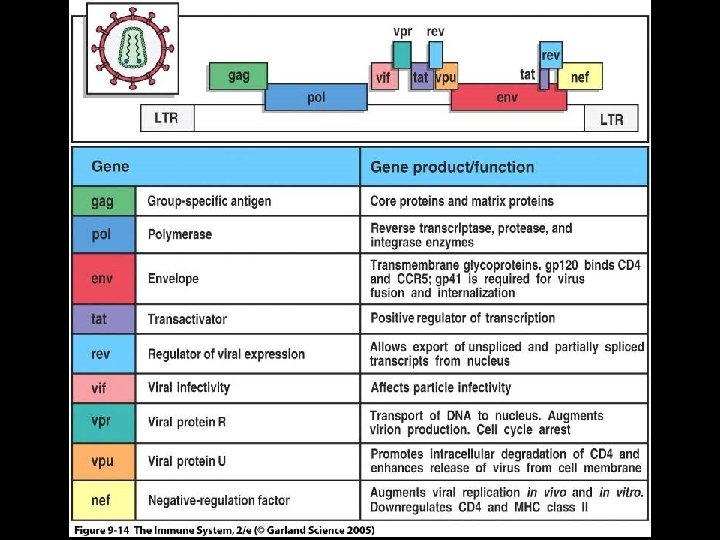
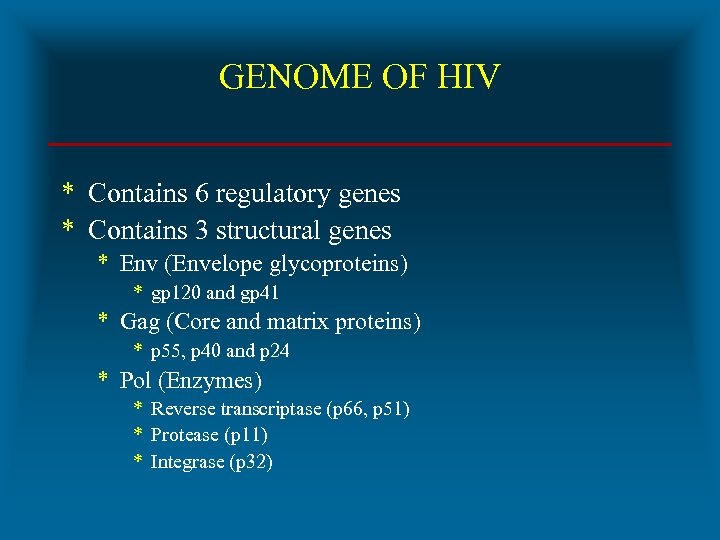 GENOME OF HIV * Contains 6 regulatory genes * Contains 3 structural genes * Env (Envelope glycoproteins) * gp 120 and gp 41 * Gag (Core and matrix proteins) * p 55, p 40 and p 24 * Pol (Enzymes) * Reverse transcriptase (p 66, p 51) * Protease (p 11) * Integrase (p 32)
GENOME OF HIV * Contains 6 regulatory genes * Contains 3 structural genes * Env (Envelope glycoproteins) * gp 120 and gp 41 * Gag (Core and matrix proteins) * p 55, p 40 and p 24 * Pol (Enzymes) * Reverse transcriptase (p 66, p 51) * Protease (p 11) * Integrase (p 32)
 HUMAN RETROVIRIDAE (EXOGENOUS RETROVIRUSES) * Seven genera * Alpha, Beta, Gamma, Delta, Epsilon, Lenti and Spuma * Deltavirus * Human T-lymphotropic virus, type I (HTLV-1) * Human T-lymphotropic virus, type II (HTLV-II) * Lentivirus * Human immunodeficiency virus, type 1 (HIV-1) * Human immunodeficiency virus, type 2 (HIV-2)
HUMAN RETROVIRIDAE (EXOGENOUS RETROVIRUSES) * Seven genera * Alpha, Beta, Gamma, Delta, Epsilon, Lenti and Spuma * Deltavirus * Human T-lymphotropic virus, type I (HTLV-1) * Human T-lymphotropic virus, type II (HTLV-II) * Lentivirus * Human immunodeficiency virus, type 1 (HIV-1) * Human immunodeficiency virus, type 2 (HIV-2)
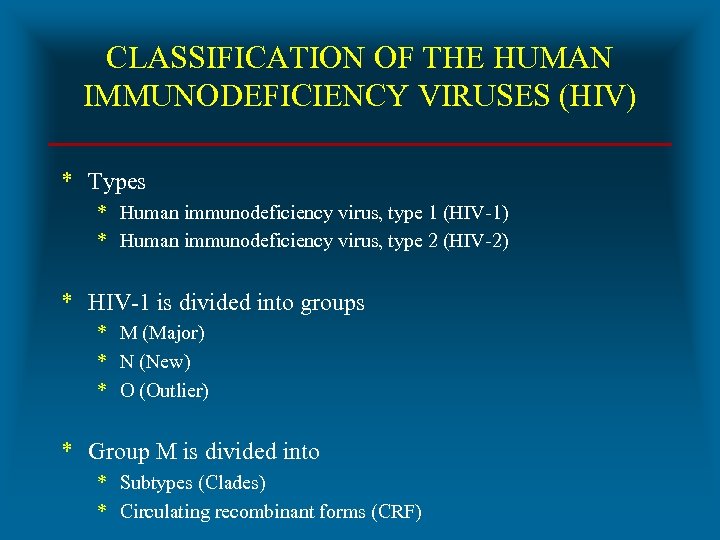 CLASSIFICATION OF THE HUMAN IMMUNODEFICIENCY VIRUSES (HIV) * Types * Human immunodeficiency virus, type 1 (HIV-1) * Human immunodeficiency virus, type 2 (HIV-2) * HIV-1 is divided into groups * M (Major) * N (New) * O (Outlier) * Group M is divided into * Subtypes (Clades) * Circulating recombinant forms (CRF)
CLASSIFICATION OF THE HUMAN IMMUNODEFICIENCY VIRUSES (HIV) * Types * Human immunodeficiency virus, type 1 (HIV-1) * Human immunodeficiency virus, type 2 (HIV-2) * HIV-1 is divided into groups * M (Major) * N (New) * O (Outlier) * Group M is divided into * Subtypes (Clades) * Circulating recombinant forms (CRF)
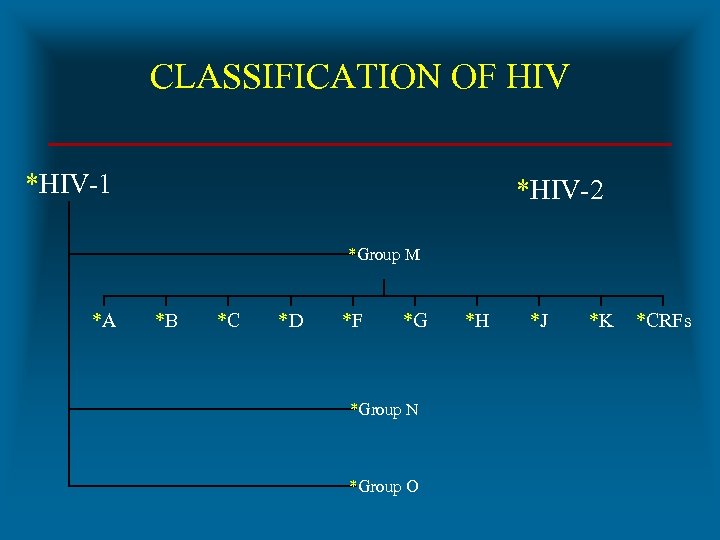 CLASSIFICATION OF HIV *HIV-1 *HIV-2 *Group M *A *B *C *D *F *G *Group N *Group O *H *J *K *CRFs
CLASSIFICATION OF HIV *HIV-1 *HIV-2 *Group M *A *B *C *D *F *G *Group N *Group O *H *J *K *CRFs
 ORIGIN OF HUMAN IMMUNODEFICIENCY VIRUSES * Existed as monkey virus in equatorial Africa * HIV-1 * Chimpanzee (Pan troglodytes) * HIV-2 * Sooty Mangabey (Cercocebus atys) * Transition from monkeys to humans * When - Circa 1908 * Molecular phylogenetics * How – Hunter theory
ORIGIN OF HUMAN IMMUNODEFICIENCY VIRUSES * Existed as monkey virus in equatorial Africa * HIV-1 * Chimpanzee (Pan troglodytes) * HIV-2 * Sooty Mangabey (Cercocebus atys) * Transition from monkeys to humans * When - Circa 1908 * Molecular phylogenetics * How – Hunter theory


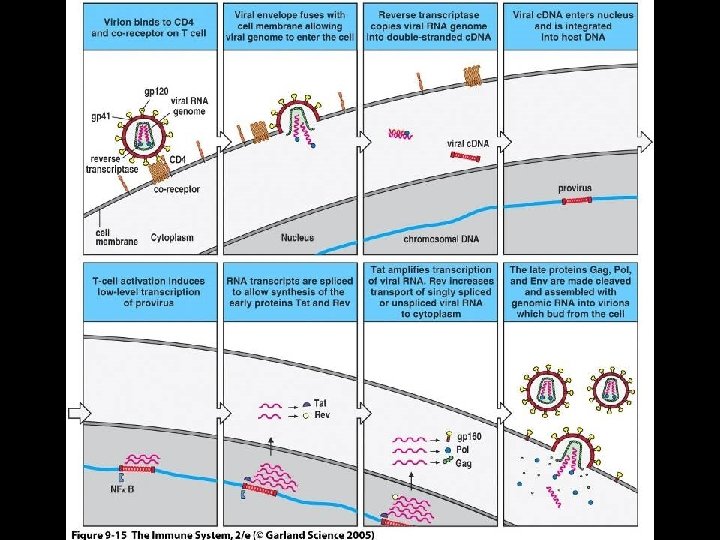
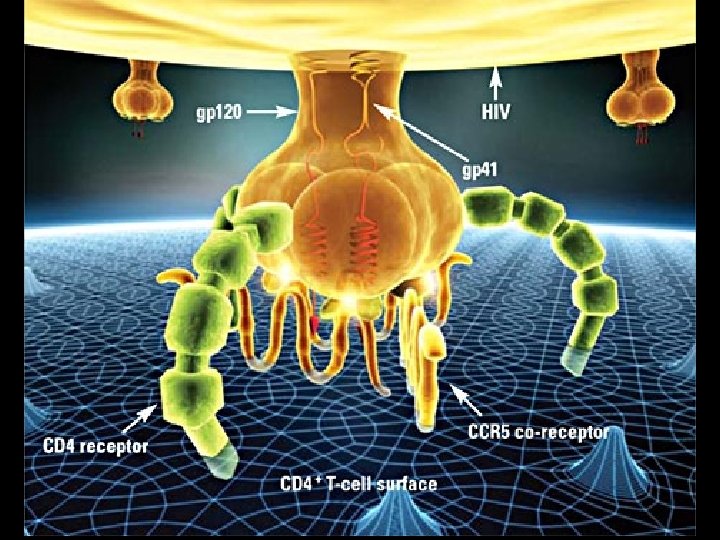
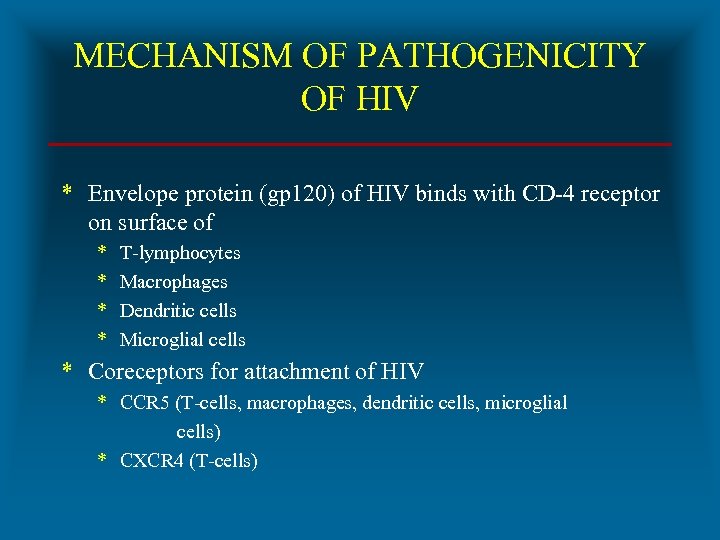 MECHANISM OF PATHOGENICITY OF HIV * Envelope protein (gp 120) of HIV binds with CD-4 receptor on surface of * * T-lymphocytes Macrophages Dendritic cells Microglial cells * Coreceptors for attachment of HIV * CCR 5 (T-cells, macrophages, dendritic cells, microglial cells) * CXCR 4 (T-cells)
MECHANISM OF PATHOGENICITY OF HIV * Envelope protein (gp 120) of HIV binds with CD-4 receptor on surface of * * T-lymphocytes Macrophages Dendritic cells Microglial cells * Coreceptors for attachment of HIV * CCR 5 (T-cells, macrophages, dendritic cells, microglial cells) * CXCR 4 (T-cells)
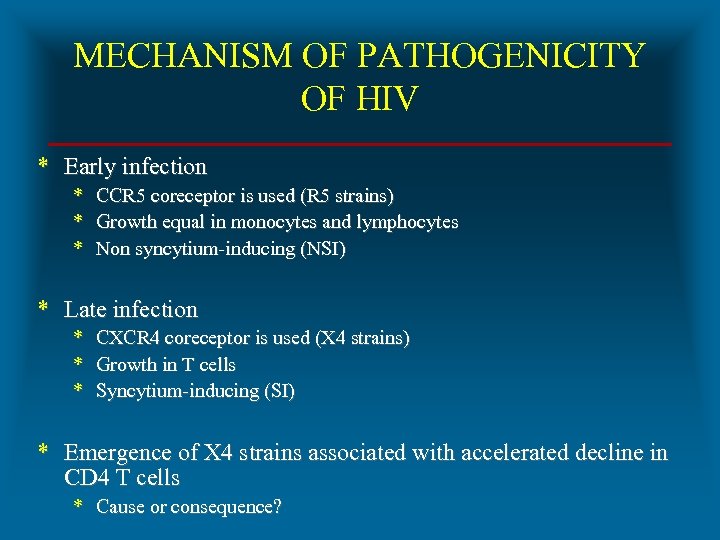 MECHANISM OF PATHOGENICITY OF HIV * Early infection * CCR 5 coreceptor is used (R 5 strains) * Growth equal in monocytes and lymphocytes * Non syncytium-inducing (NSI) * Late infection * CXCR 4 coreceptor is used (X 4 strains) * Growth in T cells * Syncytium-inducing (SI) * Emergence of X 4 strains associated with accelerated decline in CD 4 T cells * Cause or consequence?
MECHANISM OF PATHOGENICITY OF HIV * Early infection * CCR 5 coreceptor is used (R 5 strains) * Growth equal in monocytes and lymphocytes * Non syncytium-inducing (NSI) * Late infection * CXCR 4 coreceptor is used (X 4 strains) * Growth in T cells * Syncytium-inducing (SI) * Emergence of X 4 strains associated with accelerated decline in CD 4 T cells * Cause or consequence?
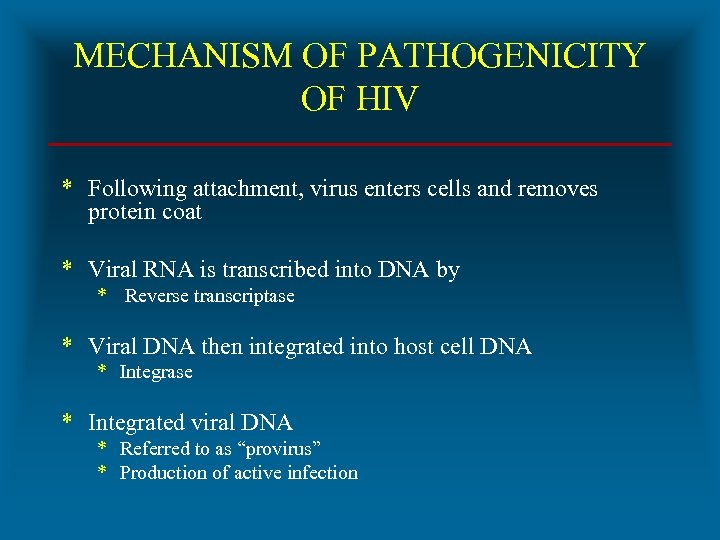 MECHANISM OF PATHOGENICITY OF HIV * Following attachment, virus enters cells and removes protein coat * Viral RNA is transcribed into DNA by * Reverse transcriptase * Viral DNA then integrated into host cell DNA * Integrase * Integrated viral DNA * Referred to as “provirus” * Production of active infection
MECHANISM OF PATHOGENICITY OF HIV * Following attachment, virus enters cells and removes protein coat * Viral RNA is transcribed into DNA by * Reverse transcriptase * Viral DNA then integrated into host cell DNA * Integrase * Integrated viral DNA * Referred to as “provirus” * Production of active infection
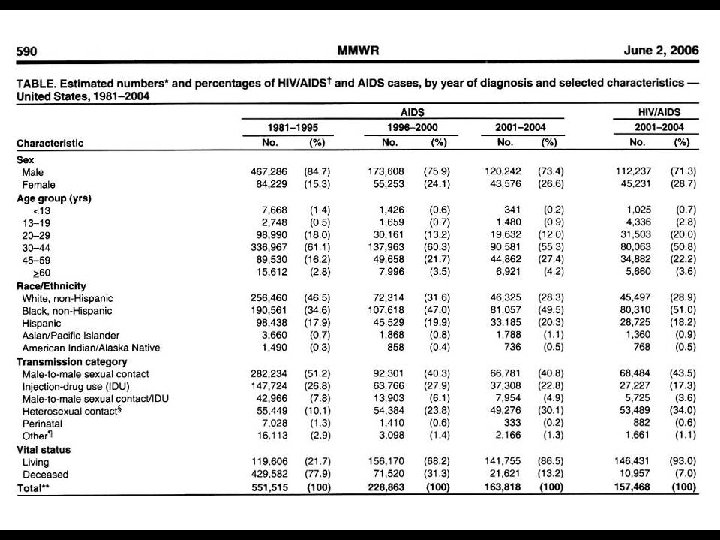
 EPIDEMIOLOGY OF HIV INFECTION AND AIDS * Since 1981, 65 million people worldwide have contracted HIV * > 25 million deaths * 87% of HIV cases in developing nations * 64% in sub-Saharan Africa * 23% in southern and Southeast Asia * Since 1981, 1. 5 million people in the U. S. have contracted HIV * Approximately 576, 000 deaths * In 2009, 56 K new cases in the U. S.
EPIDEMIOLOGY OF HIV INFECTION AND AIDS * Since 1981, 65 million people worldwide have contracted HIV * > 25 million deaths * 87% of HIV cases in developing nations * 64% in sub-Saharan Africa * 23% in southern and Southeast Asia * Since 1981, 1. 5 million people in the U. S. have contracted HIV * Approximately 576, 000 deaths * In 2009, 56 K new cases in the U. S.
 TRANSMISSION OF HIV INFECTION AND AIDS * Sexual intercourse with infected person * Homosexual (MSM) * Heterosexual * Bisexual * Children born to infected mothers * Perinatal * IV drug addicts sharing contaminated syringes/needles * Transfusion of blood and blood products * Transfusion recipients * Hemophiliacs * Occupational exposure in health-care setting
TRANSMISSION OF HIV INFECTION AND AIDS * Sexual intercourse with infected person * Homosexual (MSM) * Heterosexual * Bisexual * Children born to infected mothers * Perinatal * IV drug addicts sharing contaminated syringes/needles * Transfusion of blood and blood products * Transfusion recipients * Hemophiliacs * Occupational exposure in health-care setting
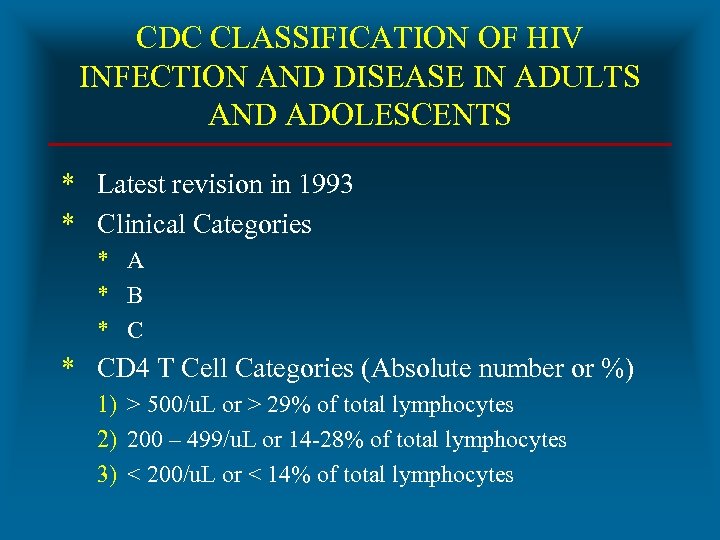 CDC CLASSIFICATION OF HIV INFECTION AND DISEASE IN ADULTS AND ADOLESCENTS * Latest revision in 1993 * Clinical Categories * A * B * CD 4 T Cell Categories (Absolute number or %) 1) > 500/u. L or > 29% of total lymphocytes 2) 200 – 499/u. L or 14 -28% of total lymphocytes 3) < 200/u. L or < 14% of total lymphocytes
CDC CLASSIFICATION OF HIV INFECTION AND DISEASE IN ADULTS AND ADOLESCENTS * Latest revision in 1993 * Clinical Categories * A * B * CD 4 T Cell Categories (Absolute number or %) 1) > 500/u. L or > 29% of total lymphocytes 2) 200 – 499/u. L or 14 -28% of total lymphocytes 3) < 200/u. L or < 14% of total lymphocytes
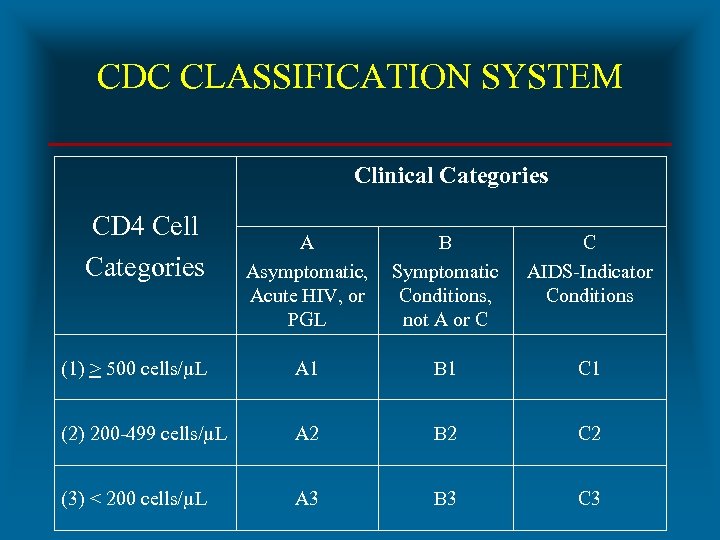 CDC CLASSIFICATION SYSTEM Clinical Categories CD 4 Cell Categories A Asymptomatic, Acute HIV, or PGL B Symptomatic Conditions, not A or C C AIDS-Indicator Conditions (1) > 500 cells/µL A 1 B 1 C 1 (2) 200 -499 cells/µL A 2 B 2 C 2 (3) < 200 cells/µL A 3 B 3 C 3
CDC CLASSIFICATION SYSTEM Clinical Categories CD 4 Cell Categories A Asymptomatic, Acute HIV, or PGL B Symptomatic Conditions, not A or C C AIDS-Indicator Conditions (1) > 500 cells/µL A 1 B 1 C 1 (2) 200 -499 cells/µL A 2 B 2 C 2 (3) < 200 cells/µL A 3 B 3 C 3
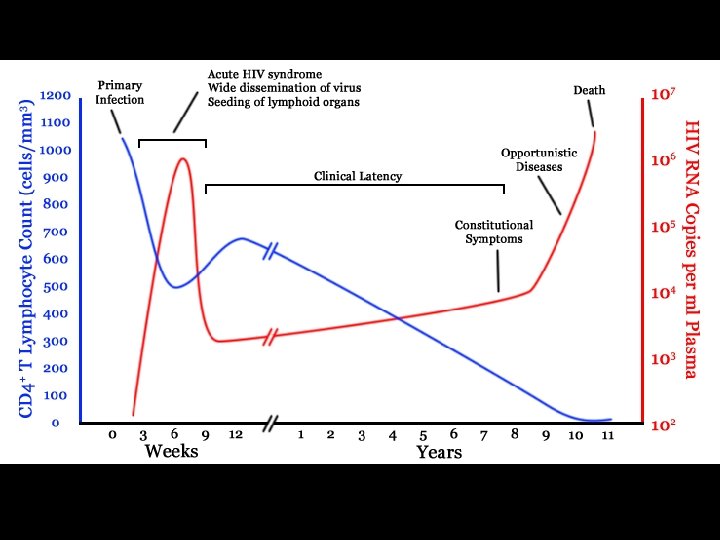
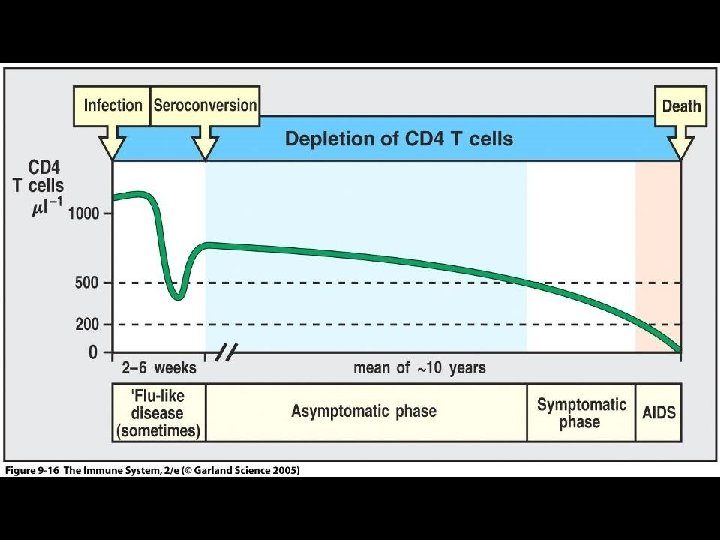
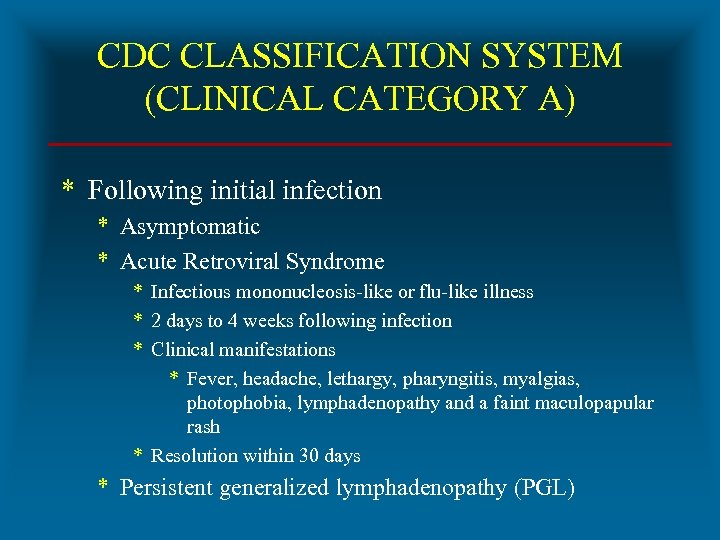 CDC CLASSIFICATION SYSTEM (CLINICAL CATEGORY A) * Following initial infection * Asymptomatic * Acute Retroviral Syndrome * Infectious mononucleosis-like or flu-like illness * 2 days to 4 weeks following infection * Clinical manifestations * Fever, headache, lethargy, pharyngitis, myalgias, photophobia, lymphadenopathy and a faint maculopapular rash * Resolution within 30 days * Persistent generalized lymphadenopathy (PGL)
CDC CLASSIFICATION SYSTEM (CLINICAL CATEGORY A) * Following initial infection * Asymptomatic * Acute Retroviral Syndrome * Infectious mononucleosis-like or flu-like illness * 2 days to 4 weeks following infection * Clinical manifestations * Fever, headache, lethargy, pharyngitis, myalgias, photophobia, lymphadenopathy and a faint maculopapular rash * Resolution within 30 days * Persistent generalized lymphadenopathy (PGL)
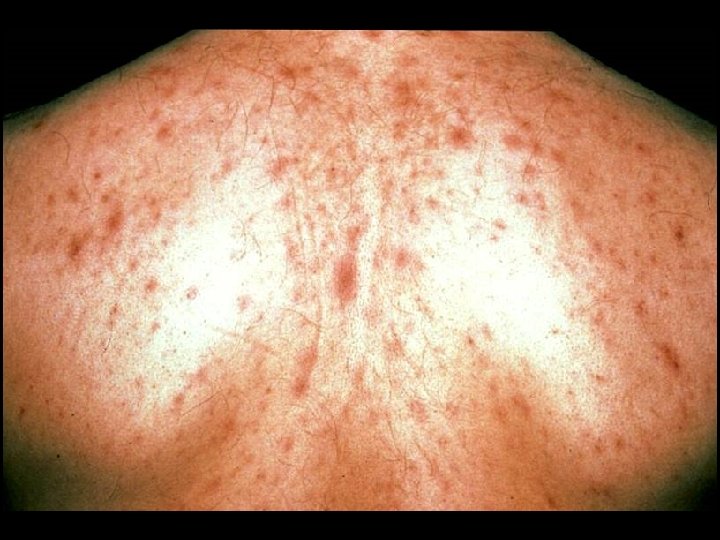
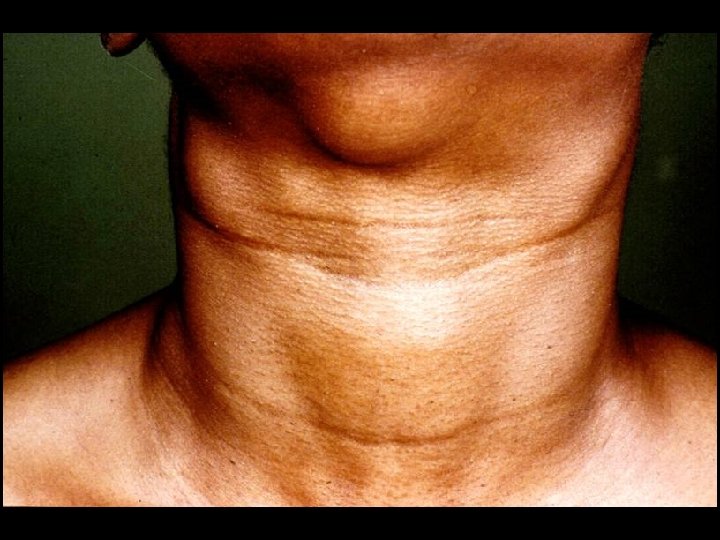
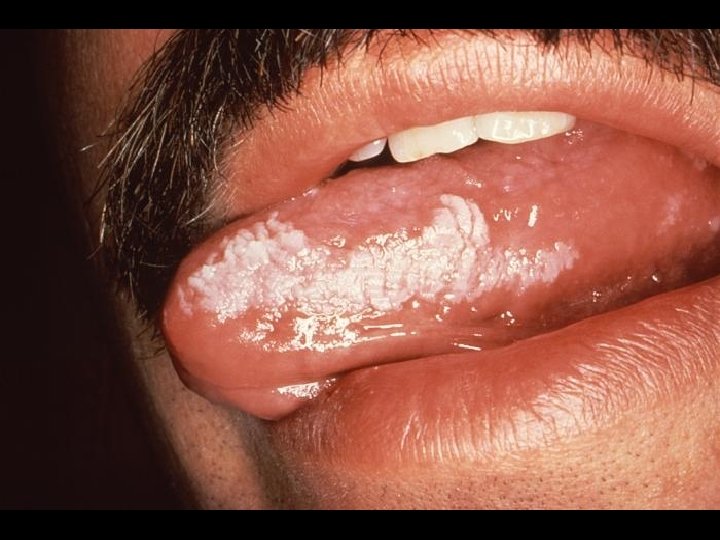
 CDC CLASSIFICATION SYSTEM (CLINICAL CATEGORY B) * Symptomatic conditions not meeting conditions of clinical categories A or C * Herpes zoster (shingles) * Oropharyngeal Candidiasis (thrush) * Candida albicans * Vulvovaginal candidiasis * Bacillary angiomatosis * Bartonella henselae * Peripheral neuropathy * Idiopathic thrombocytopenic purpura (ITP) * Hairy leukoplakia (oral)
CDC CLASSIFICATION SYSTEM (CLINICAL CATEGORY B) * Symptomatic conditions not meeting conditions of clinical categories A or C * Herpes zoster (shingles) * Oropharyngeal Candidiasis (thrush) * Candida albicans * Vulvovaginal candidiasis * Bacillary angiomatosis * Bartonella henselae * Peripheral neuropathy * Idiopathic thrombocytopenic purpura (ITP) * Hairy leukoplakia (oral)
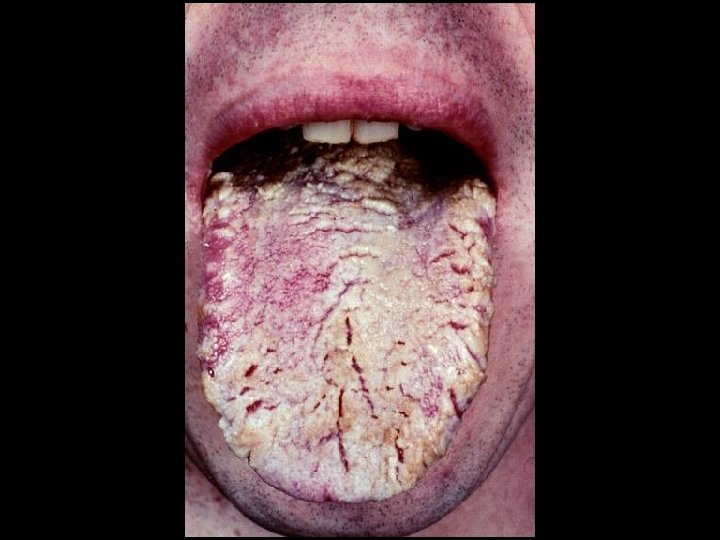
 CDC CLASSIFICATION SYSTEM (CLINICAL CATEGORY C) * Acquired Immunodeficiency Syndrome (AIDS) Defining Conditions * * * Esophageal Candidiasis Cryptosporidiosis Pneumocystis jiroveci (carinii) pneumonia Tuberculosis (pulmonary or extrapulmonary) Disseminated Mycobacterium avium complex (MAC) disease * Histoplasmosis (disseminated or extrapulmonary)
CDC CLASSIFICATION SYSTEM (CLINICAL CATEGORY C) * Acquired Immunodeficiency Syndrome (AIDS) Defining Conditions * * * Esophageal Candidiasis Cryptosporidiosis Pneumocystis jiroveci (carinii) pneumonia Tuberculosis (pulmonary or extrapulmonary) Disseminated Mycobacterium avium complex (MAC) disease * Histoplasmosis (disseminated or extrapulmonary)
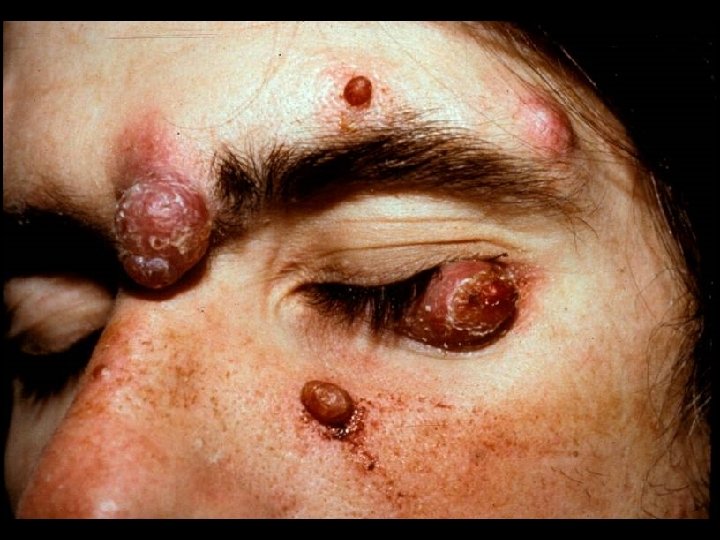
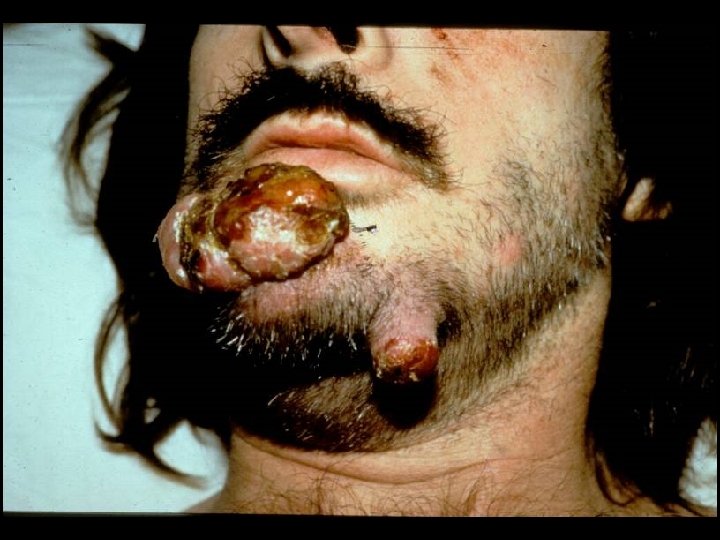
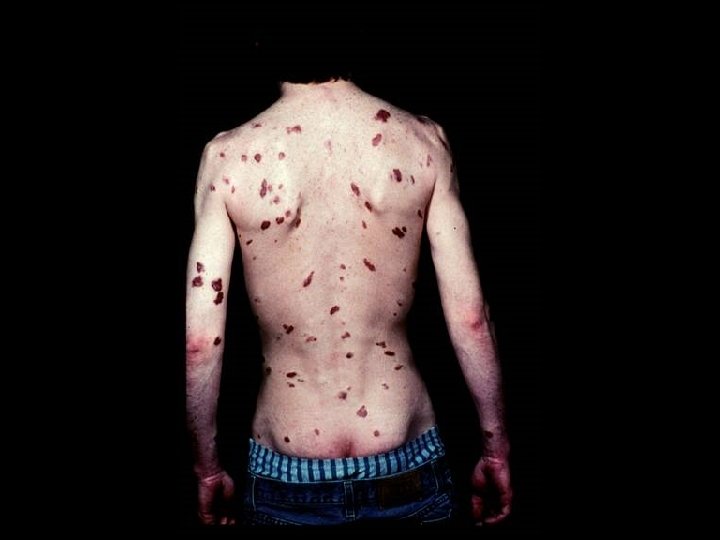
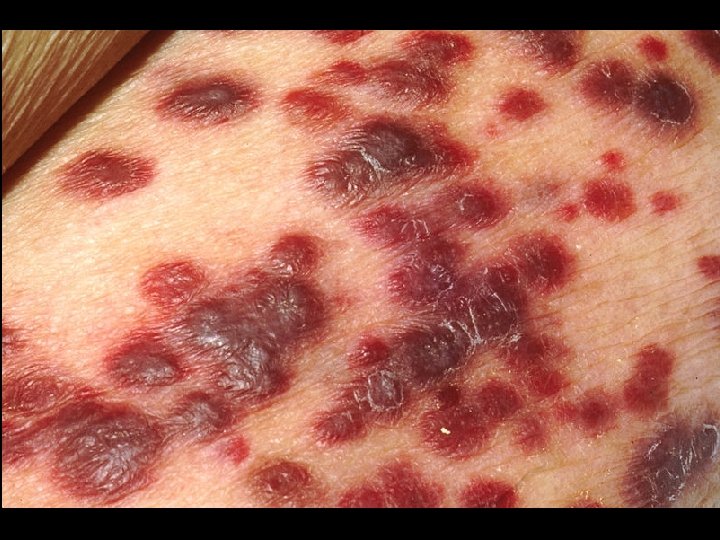
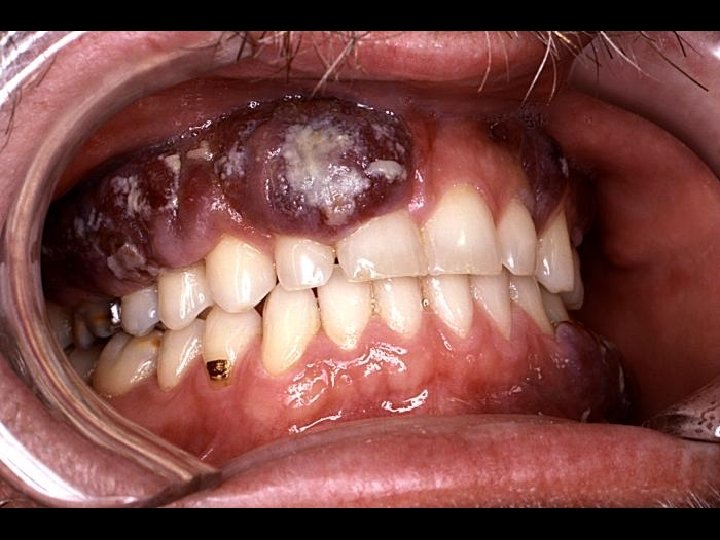
 HIV INFECTION IN ADULTS (CLINICAL CATEGORY C) * Acquired Immunodeficiency Syndrome (AIDS) Defining Conditions * * * HIV wasting syndrome Cryptococcal meningitis Cytomegalovirus retinitis Cerebral Toxoplasmosis Progressive multifocal leukoencephalopathy (PML) * JC virus * Kaposi’s sarcoma * Human herpesvirus type 8 (HHV-8)
HIV INFECTION IN ADULTS (CLINICAL CATEGORY C) * Acquired Immunodeficiency Syndrome (AIDS) Defining Conditions * * * HIV wasting syndrome Cryptococcal meningitis Cytomegalovirus retinitis Cerebral Toxoplasmosis Progressive multifocal leukoencephalopathy (PML) * JC virus * Kaposi’s sarcoma * Human herpesvirus type 8 (HHV-8)
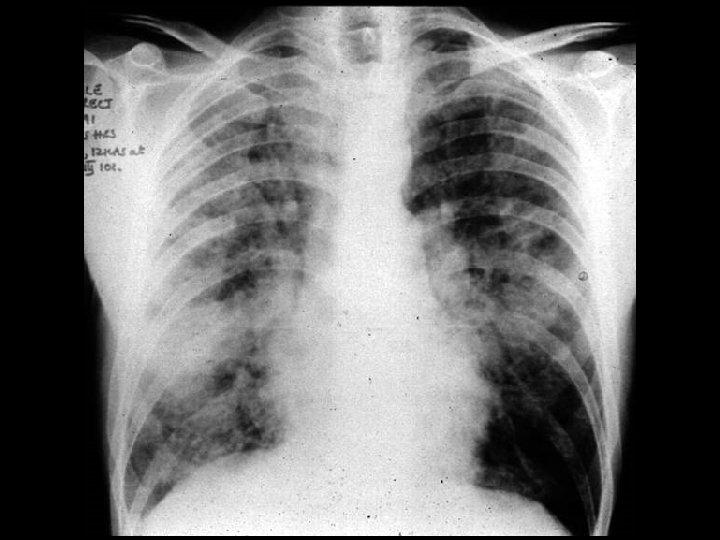
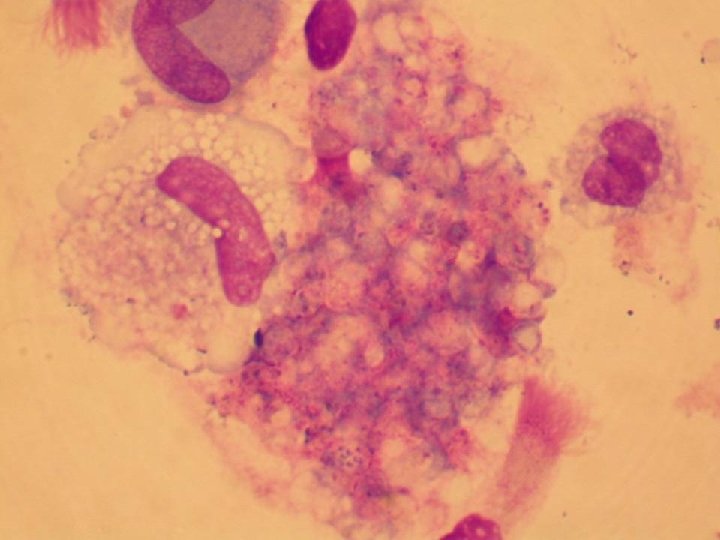
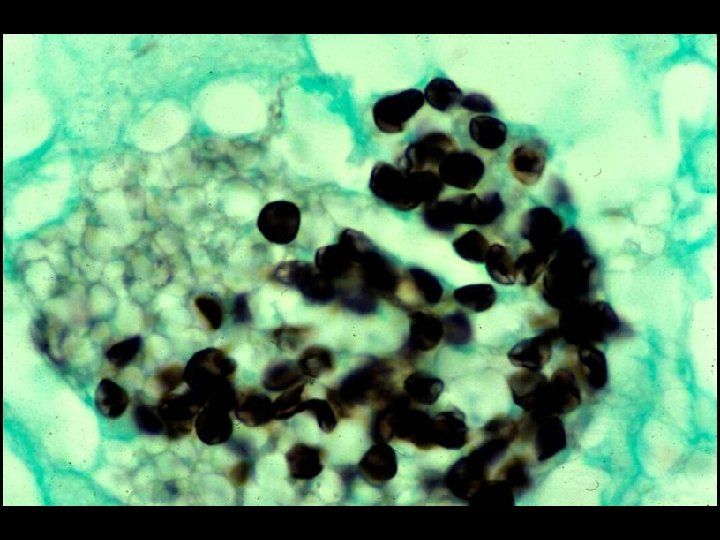
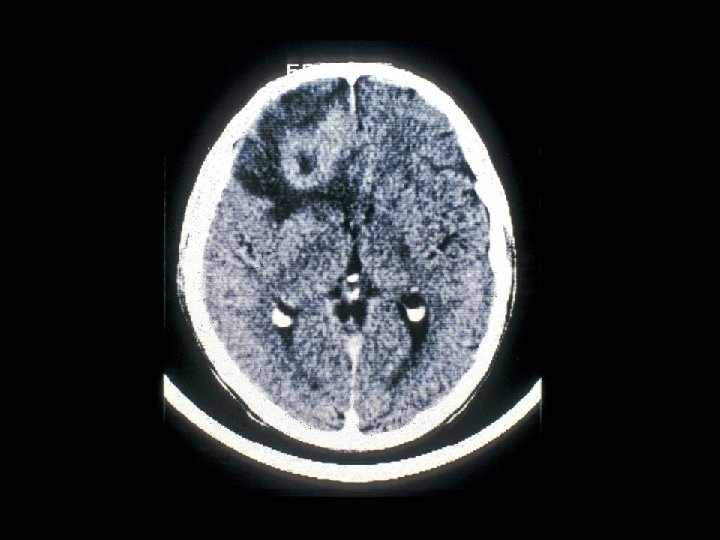
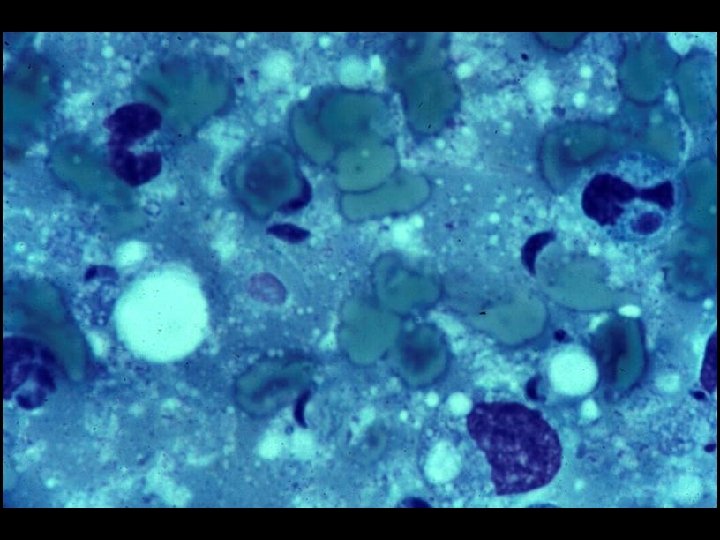
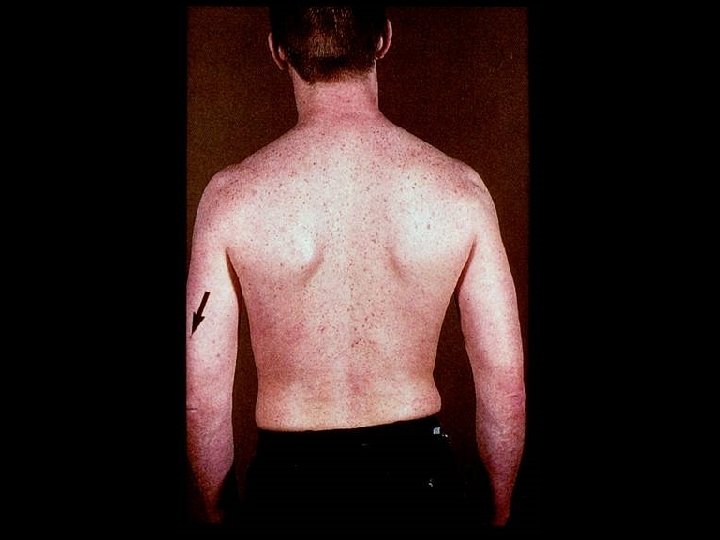
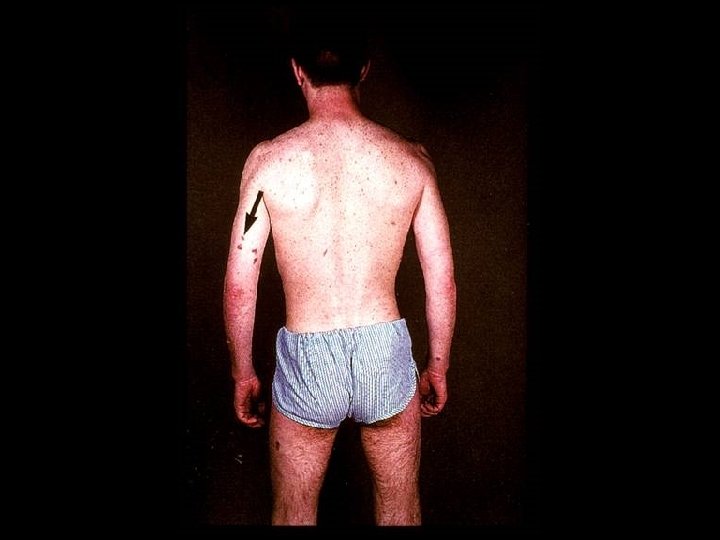
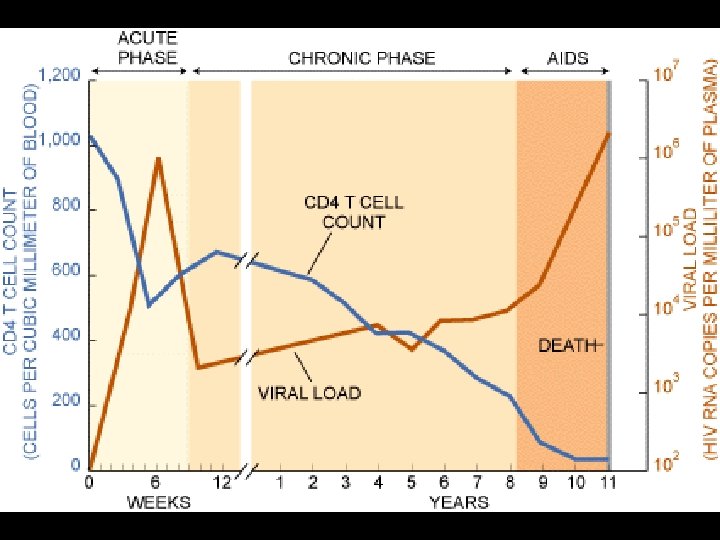
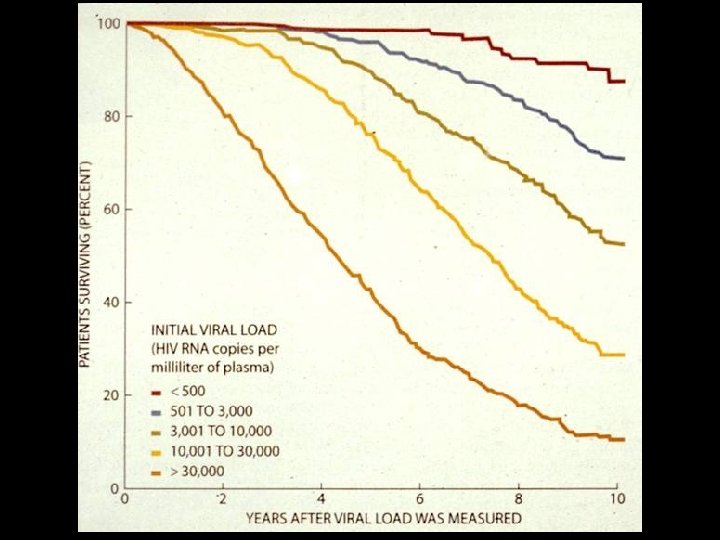
 PROGNOSIS AND MONITORING OF HIV TREATMENT AND DISEASE * CD 4 T cell count (Immunological response) * Absolute number * Best indicator for patients with counts < 200 cells/u. L * Percent * Best indicator for patients with counts > 200 cells/u. L * HIV-1 RNA (Viral load) (Virological response) * Discordant immunological and virological responses exist
PROGNOSIS AND MONITORING OF HIV TREATMENT AND DISEASE * CD 4 T cell count (Immunological response) * Absolute number * Best indicator for patients with counts < 200 cells/u. L * Percent * Best indicator for patients with counts > 200 cells/u. L * HIV-1 RNA (Viral load) (Virological response) * Discordant immunological and virological responses exist
 TREATMENT OF HIV INFECTION AND DISEASE * Anti-retroviral drugs do not cure HIV infection or disease * Suppression of virus to undetectable levels * Suppression of virus * Drugs must be taken continuously * Patients remain infectious * Mutation rate in HIV is high and resistance develops * Recommendation for combination therapy * Combination of drugs from two or more classes * Highly Active Anti-Retroviral Therapy (HAART)
TREATMENT OF HIV INFECTION AND DISEASE * Anti-retroviral drugs do not cure HIV infection or disease * Suppression of virus to undetectable levels * Suppression of virus * Drugs must be taken continuously * Patients remain infectious * Mutation rate in HIV is high and resistance develops * Recommendation for combination therapy * Combination of drugs from two or more classes * Highly Active Anti-Retroviral Therapy (HAART)
 TREATMENT OF HIV INECTION AND DISEASE * Classes of anti-retroviral drugs * Reverse Transcriptase Inhibitors (RTI’s) * Nucleoside * Nucleotide * Non-nucleoside * Protease Inhibitors (PIs) * Fusion or Entry Inhibitors * Act on gp 41 or CCR 5 coreceptor * Integrase Inhibitors * Fixed dose combinations * Drugs from two or more classes into a single product
TREATMENT OF HIV INECTION AND DISEASE * Classes of anti-retroviral drugs * Reverse Transcriptase Inhibitors (RTI’s) * Nucleoside * Nucleotide * Non-nucleoside * Protease Inhibitors (PIs) * Fusion or Entry Inhibitors * Act on gp 41 or CCR 5 coreceptor * Integrase Inhibitors * Fixed dose combinations * Drugs from two or more classes into a single product
 TREATMENT OF HIV INECTION AND DISEASE * Reverse transcriptase inhibitors * Nucleoside analog (NARTI, NRTI) * * * Converted into nucleotide Incorporated into and stops viral DNA synthesis Zidovudine (Retrovir) * Nucleotide analog (Nt. ARTI, Nt. RTI) * Incorporated into and stops viral DNA synthesis * Tenofir (Viread) * Non-nucleoside (NNRTI) * Not incorporated into viral DNA * Binds to enzyme and inhibits function * Nevirapine (Viramune)
TREATMENT OF HIV INECTION AND DISEASE * Reverse transcriptase inhibitors * Nucleoside analog (NARTI, NRTI) * * * Converted into nucleotide Incorporated into and stops viral DNA synthesis Zidovudine (Retrovir) * Nucleotide analog (Nt. ARTI, Nt. RTI) * Incorporated into and stops viral DNA synthesis * Tenofir (Viread) * Non-nucleoside (NNRTI) * Not incorporated into viral DNA * Binds to enzyme and inhibits function * Nevirapine (Viramune)
 TREATMENT OF HIV INECTION AND DISEASE * Goals of HAART * Suppression of HIV * Decrease viral load * Reduce potential for resistance to anti-viral agents * Immune system reconstitution * Restore CD 4 T cell population * Immune system reconstitution * Most successful with high baseline CD 4 count at HAART initiation * Increase of 50 to 150 cells per year
TREATMENT OF HIV INECTION AND DISEASE * Goals of HAART * Suppression of HIV * Decrease viral load * Reduce potential for resistance to anti-viral agents * Immune system reconstitution * Restore CD 4 T cell population * Immune system reconstitution * Most successful with high baseline CD 4 count at HAART initiation * Increase of 50 to 150 cells per year
 TREATMENT OF HIV INECTION AND DISEASE * HAART negatives * High cost, medication fatigue, adherence to complicated drug regimens, adverse events, names for anti-retroviral drugs * HAART Interruption * Minimize negatives using structured treatment interruption (STI) * 6 months of IL-2 without HAART * Safety (unclear) and efficacy (inferior) * HAART associated with * Immune reconstitution syndrome
TREATMENT OF HIV INECTION AND DISEASE * HAART negatives * High cost, medication fatigue, adherence to complicated drug regimens, adverse events, names for anti-retroviral drugs * HAART Interruption * Minimize negatives using structured treatment interruption (STI) * 6 months of IL-2 without HAART * Safety (unclear) and efficacy (inferior) * HAART associated with * Immune reconstitution syndrome
 IMMUNE RECONSTITUTION SYNDROME (IRS) * Immune reconstitution inflammatory syndrome (IRIS) * Strong response by recovering immune system to latent or active infections * Risk factors for IRIS following HAART * * * CD 4 percent of < 15% CD 4 count of < 100 cell/u. L High rate of increase of CD 4 count * Most commonly associated with * * Pneumocystis pneumonia Cytomegalovirus disease Herpes zoster Mycobacterium avium complex (MAC) disease * Tuberculosis
IMMUNE RECONSTITUTION SYNDROME (IRS) * Immune reconstitution inflammatory syndrome (IRIS) * Strong response by recovering immune system to latent or active infections * Risk factors for IRIS following HAART * * * CD 4 percent of < 15% CD 4 count of < 100 cell/u. L High rate of increase of CD 4 count * Most commonly associated with * * Pneumocystis pneumonia Cytomegalovirus disease Herpes zoster Mycobacterium avium complex (MAC) disease * Tuberculosis
 IMMUNE RECONSTITUTION SYNDROME * Important to distinguish between IRIS and clinical failure * Clinical failure * Disease progression with development of OI or malignancy when drugs given for sufficient time * IRIS * Seen within first several weeks of therapy when a latent or active infection is present
IMMUNE RECONSTITUTION SYNDROME * Important to distinguish between IRIS and clinical failure * Clinical failure * Disease progression with development of OI or malignancy when drugs given for sufficient time * IRIS * Seen within first several weeks of therapy when a latent or active infection is present
 IMMUNE RECONSTITUTION SYNDROME * Management options * Inflammatory reaction treated with * Steroids * Non-steroidal anti-inflammatory drugs (NSAIDS) * Antimicrobial agents directed at the infectious agent * Antiretroviral therapy * Withhold or continue (? )
IMMUNE RECONSTITUTION SYNDROME * Management options * Inflammatory reaction treated with * Steroids * Non-steroidal anti-inflammatory drugs (NSAIDS) * Antimicrobial agents directed at the infectious agent * Antiretroviral therapy * Withhold or continue (? )
 NAMING ANTI-RETROVIRAL DRUGS * Anti-retroviral drugs have at least 3 names * Abbreviation * Research or chemical name * Generic name * Trade name * Example * * Abbreviation (Research/Chemical) Abbreviation (Generic name) Generic Trade AZT ZDV Zidovudine Retrovir
NAMING ANTI-RETROVIRAL DRUGS * Anti-retroviral drugs have at least 3 names * Abbreviation * Research or chemical name * Generic name * Trade name * Example * * Abbreviation (Research/Chemical) Abbreviation (Generic name) Generic Trade AZT ZDV Zidovudine Retrovir
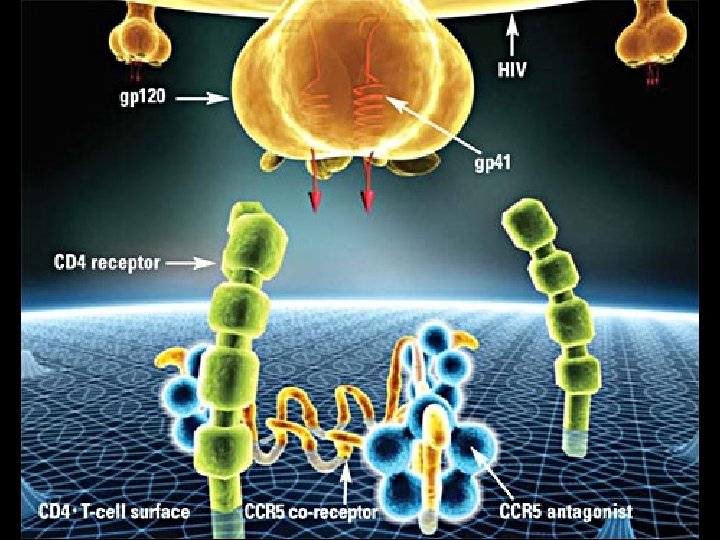
 MARAVORIC (MVC / SELZENTRY) * First in new class anti-retroviral drug * CCR 5 co-receptor antagonist (entry inhibitor) * Indicated for CCR 5 tropic HIV-1 showing resistance to multiple ant -retroviral drugs * Black box warning * Hepatotoxicity * Systemic allergic reaction * Pruritic rash, eosinophilia, elevated Ig. E * FDA approval on August 8, 2007 * Requires tropism testing
MARAVORIC (MVC / SELZENTRY) * First in new class anti-retroviral drug * CCR 5 co-receptor antagonist (entry inhibitor) * Indicated for CCR 5 tropic HIV-1 showing resistance to multiple ant -retroviral drugs * Black box warning * Hepatotoxicity * Systemic allergic reaction * Pruritic rash, eosinophilia, elevated Ig. E * FDA approval on August 8, 2007 * Requires tropism testing
 HIV CO-RECEPTOR TROPISM ASSAY * Trofile ™ (Monogram Bioscience) * FDA approval on August 6, 2007 * In vitro diagnostic assay * Determines tropism of patient’s HIV * CCR 5 * CXCR 4 * D/M (dual / mixed) * Trofile ™ assay * * Specimen is EDTA plasma Viral load of 1, 000 copies/m. L TAT of 14 days Cost $$$$$
HIV CO-RECEPTOR TROPISM ASSAY * Trofile ™ (Monogram Bioscience) * FDA approval on August 6, 2007 * In vitro diagnostic assay * Determines tropism of patient’s HIV * CCR 5 * CXCR 4 * D/M (dual / mixed) * Trofile ™ assay * * Specimen is EDTA plasma Viral load of 1, 000 copies/m. L TAT of 14 days Cost $$$$$
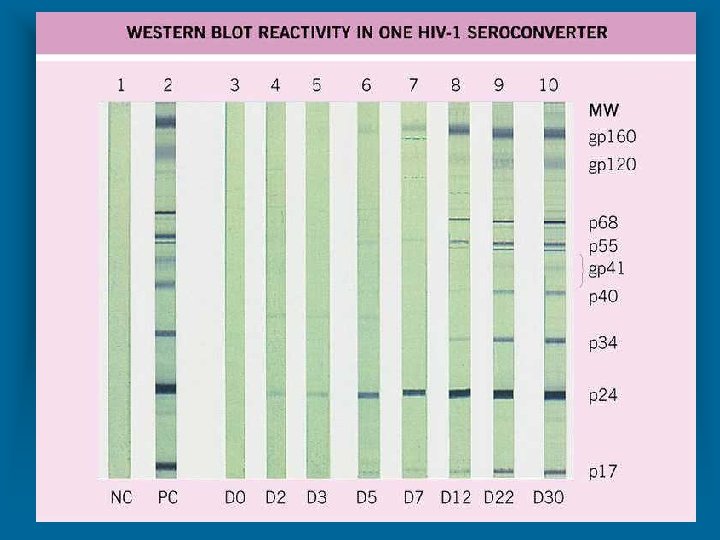
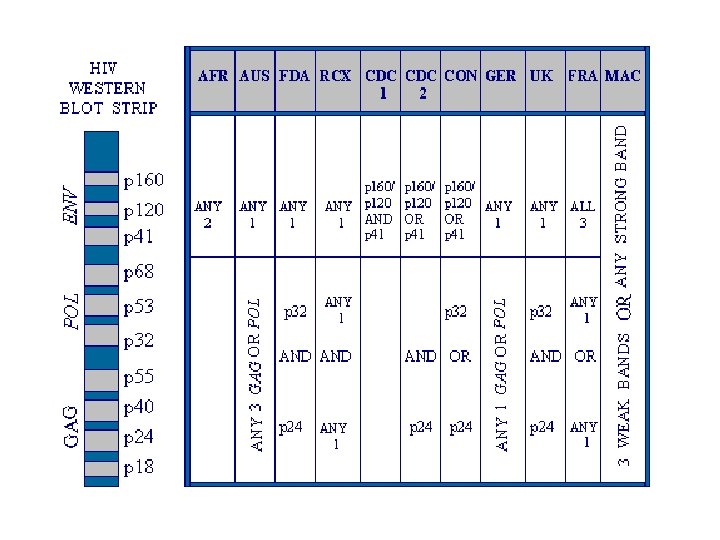
 LABORATORY DIAGNOSIS OF HIV INFECTION * Standard algorithm consists of using two tests for the detection of antibody to HIV-1/2 * Screening * Enzyme immunoassay (EIA) or * Enzyme-linked Immunosorbent Assay (ELISA) * High sensitivity * Confirmation * Western blot (WB) * High specificity * Sensitivity is “positivity in disease” * Specificity is “negativity in disease”
LABORATORY DIAGNOSIS OF HIV INFECTION * Standard algorithm consists of using two tests for the detection of antibody to HIV-1/2 * Screening * Enzyme immunoassay (EIA) or * Enzyme-linked Immunosorbent Assay (ELISA) * High sensitivity * Confirmation * Western blot (WB) * High specificity * Sensitivity is “positivity in disease” * Specificity is “negativity in disease”
 LABORATORY DIAGNOSIS OF HIV INFECTION (STANDARD ALGORITHM) * Specimens “initially reactive” by EIA / ELISA are retested in duplicate * One or both repeat tests positive, specimens are considered “repeatedly reactive” for antibody * Specimens “repeatedly reactive” by EIA / ELISA then tested by Western Blot (WB) assay * Specimens “reactive” for both EIA / ELISA and WB are considered “positive” for HIV infection * Seroconversion * From infection to antibody
LABORATORY DIAGNOSIS OF HIV INFECTION (STANDARD ALGORITHM) * Specimens “initially reactive” by EIA / ELISA are retested in duplicate * One or both repeat tests positive, specimens are considered “repeatedly reactive” for antibody * Specimens “repeatedly reactive” by EIA / ELISA then tested by Western Blot (WB) assay * Specimens “reactive” for both EIA / ELISA and WB are considered “positive” for HIV infection * Seroconversion * From infection to antibody
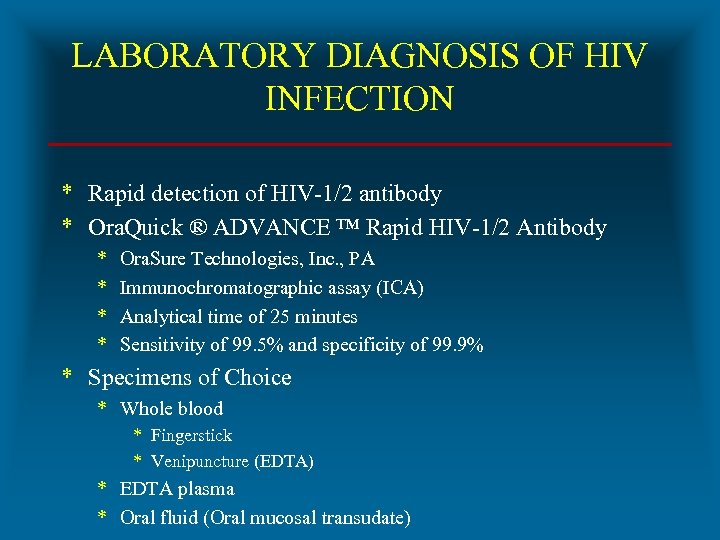 LABORATORY DIAGNOSIS OF HIV INFECTION * Rapid detection of HIV-1/2 antibody * Ora. Quick ® ADVANCE ™ Rapid HIV-1/2 Antibody * * Ora. Sure Technologies, Inc. , PA Immunochromatographic assay (ICA) Analytical time of 25 minutes Sensitivity of 99. 5% and specificity of 99. 9% * Specimens of Choice * Whole blood * Fingerstick * Venipuncture (EDTA) * EDTA plasma * Oral fluid (Oral mucosal transudate)
LABORATORY DIAGNOSIS OF HIV INFECTION * Rapid detection of HIV-1/2 antibody * Ora. Quick ® ADVANCE ™ Rapid HIV-1/2 Antibody * * Ora. Sure Technologies, Inc. , PA Immunochromatographic assay (ICA) Analytical time of 25 minutes Sensitivity of 99. 5% and specificity of 99. 9% * Specimens of Choice * Whole blood * Fingerstick * Venipuncture (EDTA) * EDTA plasma * Oral fluid (Oral mucosal transudate)
 CLINICAL USE OF ORAQUICK ® RAPID HIV-1/2 ASSAY * Rapid screening * HCW with potential HIV exposure * Pregnant females with unknown HIV status at time of delivery * New HIV clinic patients * Same day screening * All other patients * Reporting of Results * Negative for HIV-1/2 Antibodies * Preliminary Positive for HIV-1/2 Antibodies. Confirmation by Western Blot testing to follow.
CLINICAL USE OF ORAQUICK ® RAPID HIV-1/2 ASSAY * Rapid screening * HCW with potential HIV exposure * Pregnant females with unknown HIV status at time of delivery * New HIV clinic patients * Same day screening * All other patients * Reporting of Results * Negative for HIV-1/2 Antibodies * Preliminary Positive for HIV-1/2 Antibodies. Confirmation by Western Blot testing to follow.



 LABORATORY DIAGNOSIS OF HIV INFECTION * Detection of HIV Core Antigen (p 24) * Serum or CSF * Methods * EIA or ELISA (Non-ICD) * EIA or ELISA (immune complex dissociation) * Positives confirmed by neutralization * Clinical Use * Early diagnosis before antibody response * Monitor effectiveness of therapy * Marker of disease progression
LABORATORY DIAGNOSIS OF HIV INFECTION * Detection of HIV Core Antigen (p 24) * Serum or CSF * Methods * EIA or ELISA (Non-ICD) * EIA or ELISA (immune complex dissociation) * Positives confirmed by neutralization * Clinical Use * Early diagnosis before antibody response * Monitor effectiveness of therapy * Marker of disease progression
 LABORATORY DIAGNOSIS OF HIV INFECTION * Detection of proviral DNA * EDTA whole blood * Method * Polymerase chain reaction (PCR) * Clinical Use * Diagnosis of infection in neonates of HIV positive mothers * Early diagnosis before antibody response
LABORATORY DIAGNOSIS OF HIV INFECTION * Detection of proviral DNA * EDTA whole blood * Method * Polymerase chain reaction (PCR) * Clinical Use * Diagnosis of infection in neonates of HIV positive mothers * Early diagnosis before antibody response
 LABORATORY PROGNOSIS OF HIV INFECTION * Quantitation of HIV-1 RNA (Viral load) * EDTA plasma * Methods * Reverse Transcriptase – PCR (RT-PCR) * Branched chain DNA (b. DNA) * Clinical Use * Determination of amount of free virus (Viral load) * Predicting progression and outcome of infection * Assessing efficacy of antiviral therapy
LABORATORY PROGNOSIS OF HIV INFECTION * Quantitation of HIV-1 RNA (Viral load) * EDTA plasma * Methods * Reverse Transcriptase – PCR (RT-PCR) * Branched chain DNA (b. DNA) * Clinical Use * Determination of amount of free virus (Viral load) * Predicting progression and outcome of infection * Assessing efficacy of antiviral therapy
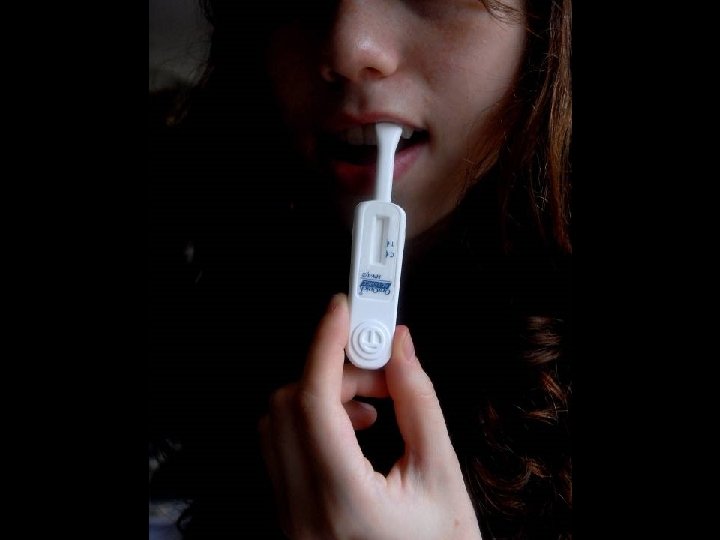
 LABORATORY DIAGNOSIS OF HIV INFECTION BY ORAL FLUID TESTING * Ora. Quick ® ADVANCE ™ Rapid HIV-1/2 Antibody Test * Pad on test device used to swab between upper and lower outer gums and cheek * Pad is stored in preservative vial and sent for ICA testing * Advantages * Reduces occupational exposure * Patient appeal
LABORATORY DIAGNOSIS OF HIV INFECTION BY ORAL FLUID TESTING * Ora. Quick ® ADVANCE ™ Rapid HIV-1/2 Antibody Test * Pad on test device used to swab between upper and lower outer gums and cheek * Pad is stored in preservative vial and sent for ICA testing * Advantages * Reduces occupational exposure * Patient appeal
 LABORATORY DIAGNOSIS OF HIV INFECTION BY URINE TESTING * Calypte HIV-1 Urine EIA (Calypte Biomedical, Berkeley, CA) * FDA approval for EIA (1996) and Western Blot (1998) * Sensitivity and specificity * Lower compared to blood and oral fluid * Question * Ig. G in urine * Calypte ® Aware ™ HIV-1/2 Urine Rapid Test * Available outside US * Advantages * Reduces occupational exposure * Patient appeal
LABORATORY DIAGNOSIS OF HIV INFECTION BY URINE TESTING * Calypte HIV-1 Urine EIA (Calypte Biomedical, Berkeley, CA) * FDA approval for EIA (1996) and Western Blot (1998) * Sensitivity and specificity * Lower compared to blood and oral fluid * Question * Ig. G in urine * Calypte ® Aware ™ HIV-1/2 Urine Rapid Test * Available outside US * Advantages * Reduces occupational exposure * Patient appeal
 THE IMMUNOLOGY OF HIV INFECTION * Interactions between HIV and human immune system are extremely complex * HIV subverts immune system by * Infecting CD 4 T cells and inducing quantitative and qualitative dysfunction * Hyperactivating B cells with resulting hypergammaglobulinemia * Inducing cytokine system to own replicative advantage * There are no known correlates of protective immunity
THE IMMUNOLOGY OF HIV INFECTION * Interactions between HIV and human immune system are extremely complex * HIV subverts immune system by * Infecting CD 4 T cells and inducing quantitative and qualitative dysfunction * Hyperactivating B cells with resulting hypergammaglobulinemia * Inducing cytokine system to own replicative advantage * There are no known correlates of protective immunity
 MECHANISMS OF CD 4 T-CELL DEPLETION * Direct killing of infected T cells * Increased rate of apoptosis in infected T cells * Molecule associated with apoptosis (PD-1) is over-expressed in chronic viremia * Syncytia formation * Fusion of infected and non-infected T cells * Killing of infected CD 4 cells by CD 8 cells
MECHANISMS OF CD 4 T-CELL DEPLETION * Direct killing of infected T cells * Increased rate of apoptosis in infected T cells * Molecule associated with apoptosis (PD-1) is over-expressed in chronic viremia * Syncytia formation * Fusion of infected and non-infected T cells * Killing of infected CD 4 cells by CD 8 cells
 KILLING OF INFECTED CD 4 CELLS BY CD 8 CELLS – ALTERNATIVE VIEW * Mechanism that keeps HIV in check in long term non-progressors (LTNPs) * Long term non-progressors (LTNPs) * Carry the virus but do not get AIDS * Have 20 times more CD 8 T cells than progressors * Function of CD 8 T cell surplus * Up-regulate production (2 X rate of progressors) of 2 killer proteins * Perforin * Granzyme B
KILLING OF INFECTED CD 4 CELLS BY CD 8 CELLS – ALTERNATIVE VIEW * Mechanism that keeps HIV in check in long term non-progressors (LTNPs) * Long term non-progressors (LTNPs) * Carry the virus but do not get AIDS * Have 20 times more CD 8 T cells than progressors * Function of CD 8 T cell surplus * Up-regulate production (2 X rate of progressors) of 2 killer proteins * Perforin * Granzyme B
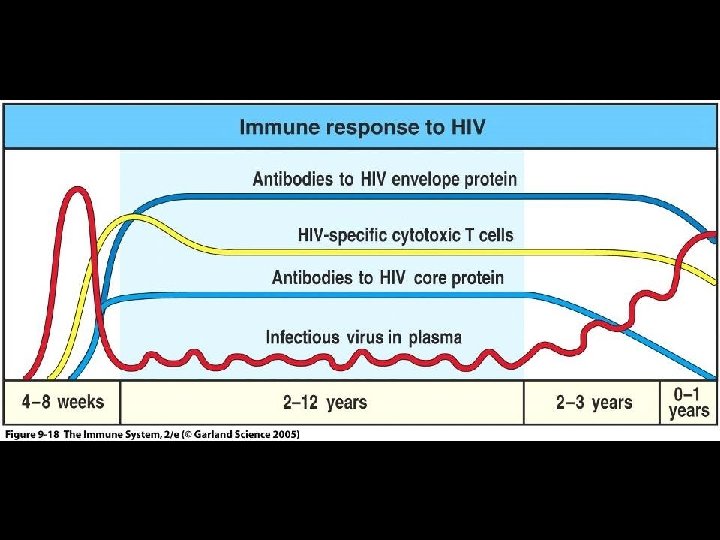
 IMMUNE DYSFUNCTION DURING HIV INFECTION - SUMMARY * HIV infection is multifactorial process capable of disarming immune system by direct and indirect mechanisms * Certain chemokine receptors function as necessary coreceptors for entry of HIV into cells * Central Paradox * Progression of HIV disease in setting of vigorous immune response * Lack of correlates of protective immunity are major obstacle to immunotherapy and vaccine development
IMMUNE DYSFUNCTION DURING HIV INFECTION - SUMMARY * HIV infection is multifactorial process capable of disarming immune system by direct and indirect mechanisms * Certain chemokine receptors function as necessary coreceptors for entry of HIV into cells * Central Paradox * Progression of HIV disease in setting of vigorous immune response * Lack of correlates of protective immunity are major obstacle to immunotherapy and vaccine development