27bb7ac722355cc6fda8319bed09411f.ppt
- Количество слайдов: 27
 HIV Drug Resistance Training Module 5: Sequencing Procedures: Commercial and In-House 1
HIV Drug Resistance Training Module 5: Sequencing Procedures: Commercial and In-House 1
 Topics § § § Overview of Genotyping Procedures Comparison of Three Different Procedures Application of Concepts 2
Topics § § § Overview of Genotyping Procedures Comparison of Three Different Procedures Application of Concepts 2
 Objectives § § § Identify individual lab needs for sequencing. Identify differences among the procedures. Based on the differences among the procedures, identify the procedure that best meets the lab’s needs. 3
Objectives § § § Identify individual lab needs for sequencing. Identify differences among the procedures. Based on the differences among the procedures, identify the procedure that best meets the lab’s needs. 3
 overview of genotyping procedures What systems are available for HIV DR genotyping? What do these systems require? 4
overview of genotyping procedures What systems are available for HIV DR genotyping? What do these systems require? 4
 HIV DR Genotyping Systems § US FDA-approved HIV-1 genotyping systems: – Viro. Seq™ HIV-1 genotyping system – TRUGENE® HIV-1 genotyping system § In-house, or “home-brew” genotyping systems 5
HIV DR Genotyping Systems § US FDA-approved HIV-1 genotyping systems: – Viro. Seq™ HIV-1 genotyping system – TRUGENE® HIV-1 genotyping system § In-house, or “home-brew” genotyping systems 5
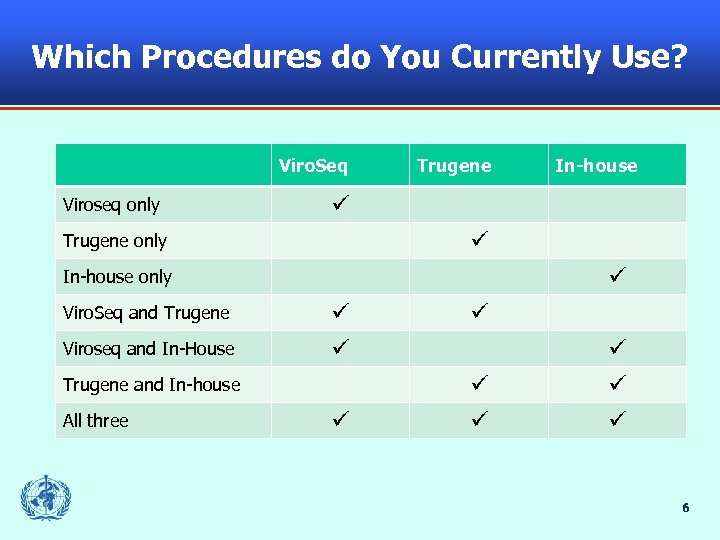 Which Procedures do You Currently Use? Viro. Seq Viroseq only Trugene only In-house only Viro. Seq and Trugene Viroseq and In-House Trugene and In-house All three In-house 6
Which Procedures do You Currently Use? Viro. Seq Viroseq only Trugene only In-house only Viro. Seq and Trugene Viroseq and In-House Trugene and In-house All three In-house 6
 US FDA-approved Systems § Characteristics: – Approved for HIV-1 subtype B viral DR genotyping * – Labor-intensive and very expensive § Requirements: – – – Specific instruments Specimen: EDTA plasma Viral load level: ≥ 1000 -2000 copies/ml of plasma Extensive training for technicians Laboratory Biosafety level II working environment for sample preparation * They have been demonstrated to work well for non-B subtypes as well, although with less certainty around performance characteristics. 7
US FDA-approved Systems § Characteristics: – Approved for HIV-1 subtype B viral DR genotyping * – Labor-intensive and very expensive § Requirements: – – – Specific instruments Specimen: EDTA plasma Viral load level: ≥ 1000 -2000 copies/ml of plasma Extensive training for technicians Laboratory Biosafety level II working environment for sample preparation * They have been demonstrated to work well for non-B subtypes as well, although with less certainty around performance characteristics. 7
 comparison of three different procedures What are the procedures used by Viro. Seq™, Tru. Gene®, and inhouse assays? What factors should be considered in choosing one procedure over another? 8
comparison of three different procedures What are the procedures used by Viro. Seq™, Tru. Gene®, and inhouse assays? What factors should be considered in choosing one procedure over another? 8
 Option One: Viro. Seq™ HIV-1 Genotyping § Detects HIV-1 subtype B viral resistance – in plasma samples – collected in EDTA tubes – with a viral load ranging from 2, 000 to >750, 000 copies/ml. § Genotypes the entire HIV-1 protease coding region (codons 1 -99) and codons 1 -335 of reverse transcriptase. 9
Option One: Viro. Seq™ HIV-1 Genotyping § Detects HIV-1 subtype B viral resistance – in plasma samples – collected in EDTA tubes – with a viral load ranging from 2, 000 to >750, 000 copies/ml. § Genotypes the entire HIV-1 protease coding region (codons 1 -99) and codons 1 -335 of reverse transcriptase. 9
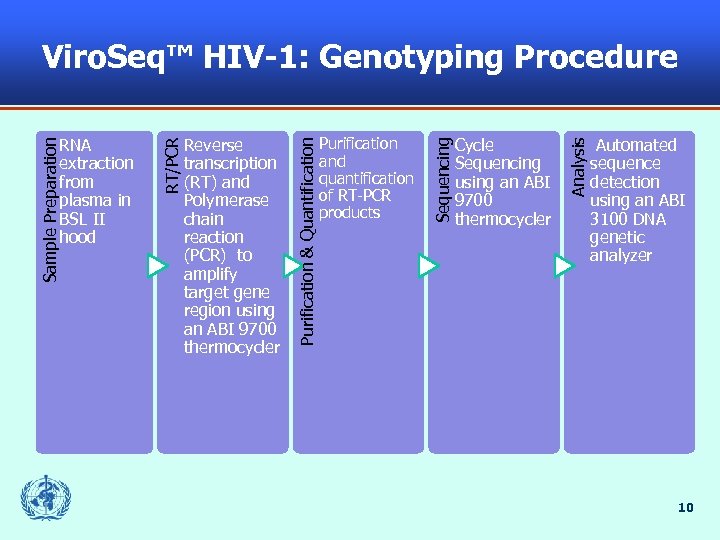 Cycle Sequencing using an ABI 9700 thermocycler Analysis Purification and quantification of RT-PCR products Sequencing Reverse transcription (RT) and Polymerase chain reaction (PCR) to amplify target gene region using an ABI 9700 thermocycler Purification & Quantification Sample Preparation RNA extraction from plasma in BSL II hood RT/PCR Viro. Seq™ HIV-1: Genotyping Procedure Automated sequence detection using an ABI 3100 DNA genetic analyzer 10
Cycle Sequencing using an ABI 9700 thermocycler Analysis Purification and quantification of RT-PCR products Sequencing Reverse transcription (RT) and Polymerase chain reaction (PCR) to amplify target gene region using an ABI 9700 thermocycler Purification & Quantification Sample Preparation RNA extraction from plasma in BSL II hood RT/PCR Viro. Seq™ HIV-1: Genotyping Procedure Automated sequence detection using an ABI 3100 DNA genetic analyzer 10
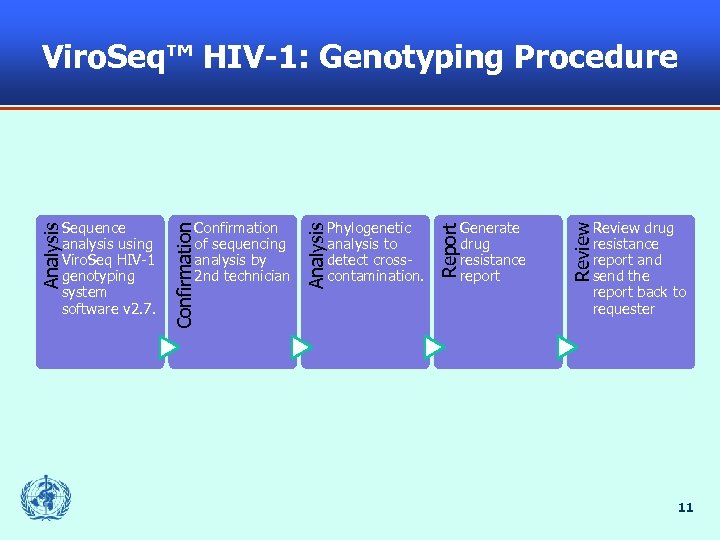 Viro. Seq™ HIV-1: Genotyping Procedure Review drug resistance report and send the report back to requester Review Generate drug resistance report Report Phylogenetic analysis to detect crosscontamination. Analysis Confirmation of sequencing analysis by 2 nd technician Confirmation Analysis Sequence analysis using Viro. Seq HIV-1 genotyping system software v 2. 7. 11
Viro. Seq™ HIV-1: Genotyping Procedure Review drug resistance report and send the report back to requester Review Generate drug resistance report Report Phylogenetic analysis to detect crosscontamination. Analysis Confirmation of sequencing analysis by 2 nd technician Confirmation Analysis Sequence analysis using Viro. Seq HIV-1 genotyping system software v 2. 7. 11
 Option Two: TRUGENE® HIV-1 Genotyping § § § Semi-automated technique Covers PR (codons 4 -99) and RT (codons 38 -248) Viral RNA extracted from plasma – Plasma viral load ≥ 1, 000 copies/ml § § c. DNA amplified in a single-tube RT-PCR reaction Sequenced with a modified Sanger technique – Dye-primer (not dye-labeled dd. NTPs) – PR and RT sequences non-contiguous Semi-automated data analysis, alignment and interpretation § Specific chemical waste disposal required (acrylamide) § 12
Option Two: TRUGENE® HIV-1 Genotyping § § § Semi-automated technique Covers PR (codons 4 -99) and RT (codons 38 -248) Viral RNA extracted from plasma – Plasma viral load ≥ 1, 000 copies/ml § § c. DNA amplified in a single-tube RT-PCR reaction Sequenced with a modified Sanger technique – Dye-primer (not dye-labeled dd. NTPs) – PR and RT sequences non-contiguous Semi-automated data analysis, alignment and interpretation § Specific chemical waste disposal required (acrylamide) § 12
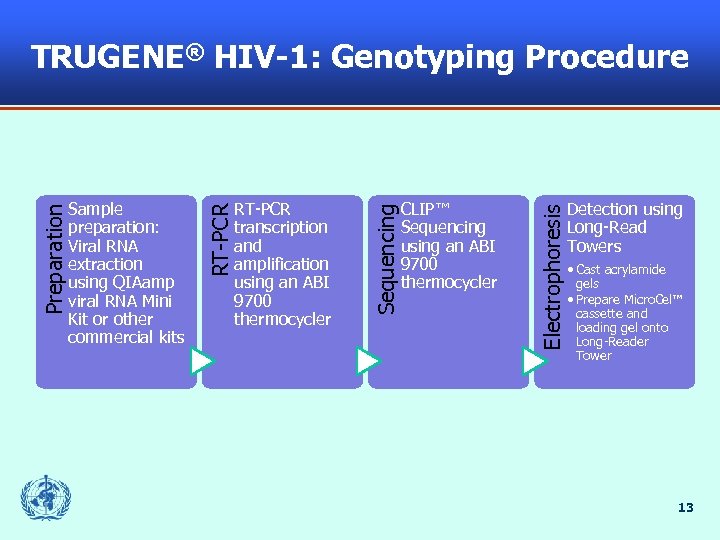 CLIP™ Sequencing using an ABI 9700 thermocycler Electrophoresis RT-PCR transcription and amplification using an ABI 9700 thermocycler Sequencing Sample preparation: Viral RNA extraction using QIAamp viral RNA Mini Kit or other commercial kits RT-PCR Preparation TRUGENE® HIV-1: Genotyping Procedure Detection using Long-Read Towers • Cast acrylamide gels • Prepare Micro. Cel™ cassette and loading gel onto Long-Reader Tower 13
CLIP™ Sequencing using an ABI 9700 thermocycler Electrophoresis RT-PCR transcription and amplification using an ABI 9700 thermocycler Sequencing Sample preparation: Viral RNA extraction using QIAamp viral RNA Mini Kit or other commercial kits RT-PCR Preparation TRUGENE® HIV-1: Genotyping Procedure Detection using Long-Read Towers • Cast acrylamide gels • Prepare Micro. Cel™ cassette and loading gel onto Long-Reader Tower 13
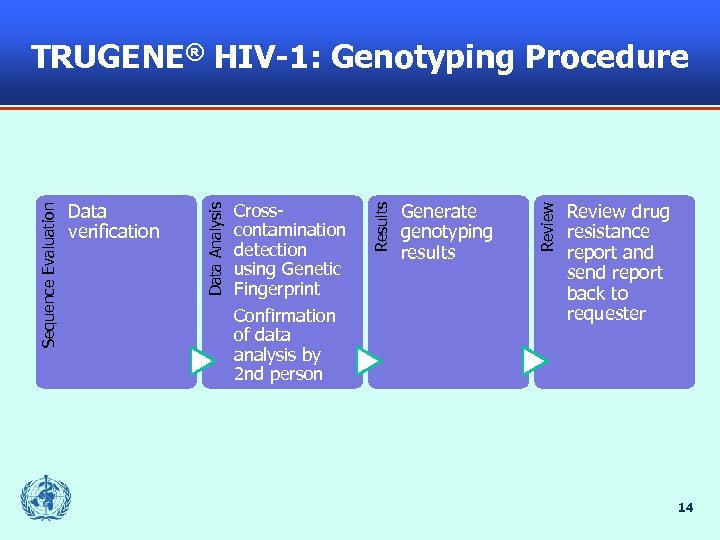 Generate genotyping results Review Crosscontamination detection using Genetic Fingerprint Confirmation of data analysis by 2 nd person Results Data verification Data Analysis Sequence Evaluation TRUGENE® HIV-1: Genotyping Procedure Review drug resistance report and send report back to requester 14
Generate genotyping results Review Crosscontamination detection using Genetic Fingerprint Confirmation of data analysis by 2 nd person Results Data verification Data Analysis Sequence Evaluation TRUGENE® HIV-1: Genotyping Procedure Review drug resistance report and send report back to requester 14
 Home-brew/In-house Genotyping Assays § § Many home-brew genotyping assays Differences: – Detection of HIV-1 viral resistance for non-B subtypes and circulating recombinant forms (CRFs) – Specimen type: plasma, dried blood spots (DBS), or dried plasma/serum spots (DPS/DSS) – Boundaries of the protease and the reverse transcriptase regions of the pol gene that are sequenced – Sensitivity (viral load requirement) – Other performance characteristics (validation) – Cost and turn-around time 15
Home-brew/In-house Genotyping Assays § § Many home-brew genotyping assays Differences: – Detection of HIV-1 viral resistance for non-B subtypes and circulating recombinant forms (CRFs) – Specimen type: plasma, dried blood spots (DBS), or dried plasma/serum spots (DPS/DSS) – Boundaries of the protease and the reverse transcriptase regions of the pol gene that are sequenced – Sensitivity (viral load requirement) – Other performance characteristics (validation) – Cost and turn-around time 15
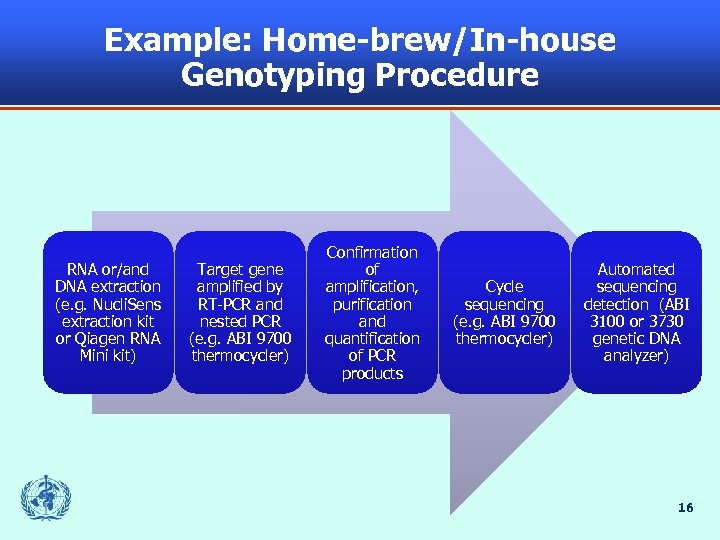 Example: Home-brew/In-house Genotyping Procedure RNA or/and DNA extraction (e. g. Nucli. Sens extraction kit or Qiagen RNA Mini kit) Target gene amplified by RT-PCR and nested PCR (e. g. ABI 9700 thermocycler) Confirmation of amplification, purification and quantification of PCR products Cycle sequencing (e. g. ABI 9700 thermocycler) Automated sequencing detection (ABI 3100 or 3730 genetic DNA analyzer) 16
Example: Home-brew/In-house Genotyping Procedure RNA or/and DNA extraction (e. g. Nucli. Sens extraction kit or Qiagen RNA Mini kit) Target gene amplified by RT-PCR and nested PCR (e. g. ABI 9700 thermocycler) Confirmation of amplification, purification and quantification of PCR products Cycle sequencing (e. g. ABI 9700 thermocycler) Automated sequencing detection (ABI 3100 or 3730 genetic DNA analyzer) 16
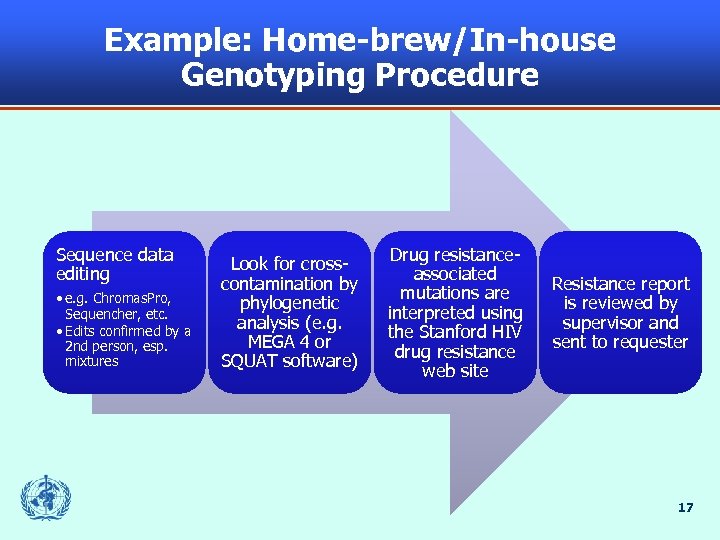 Example: Home-brew/In-house Genotyping Procedure Sequence data editing • e. g. Chromas. Pro, Sequencher, etc. • Edits confirmed by a 2 nd person, esp. mixtures Look for crosscontamination by phylogenetic analysis (e. g. MEGA 4 or SQUAT software) Drug resistanceassociated mutations are interpreted using the Stanford HIV drug resistance web site Resistance report is reviewed by supervisor and sent to requester 17
Example: Home-brew/In-house Genotyping Procedure Sequence data editing • e. g. Chromas. Pro, Sequencher, etc. • Edits confirmed by a 2 nd person, esp. mixtures Look for crosscontamination by phylogenetic analysis (e. g. MEGA 4 or SQUAT software) Drug resistanceassociated mutations are interpreted using the Stanford HIV drug resistance web site Resistance report is reviewed by supervisor and sent to requester 17
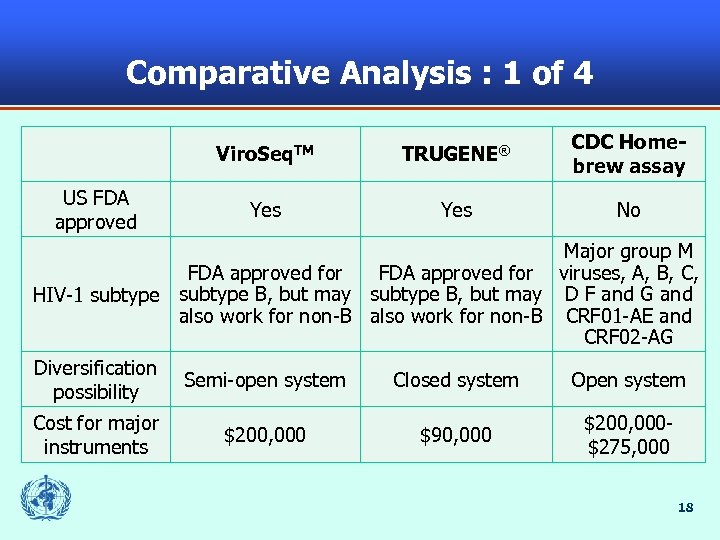 Comparative Analysis : 1 of 4 Viro. Seq. TM US FDA approved TRUGENE® CDC Homebrew assay Yes No Major group M FDA approved for viruses, A, B, C, HIV-1 subtype B, but may D F and G and also work for non-B CRF 01 -AE and CRF 02 -AG Diversification possibility Semi-open system Closed system Open system Cost for major instruments $200, 000 $90, 000 $200, 000$275, 000 18
Comparative Analysis : 1 of 4 Viro. Seq. TM US FDA approved TRUGENE® CDC Homebrew assay Yes No Major group M FDA approved for viruses, A, B, C, HIV-1 subtype B, but may D F and G and also work for non-B CRF 01 -AE and CRF 02 -AG Diversification possibility Semi-open system Closed system Open system Cost for major instruments $200, 000 $90, 000 $200, 000$275, 000 18
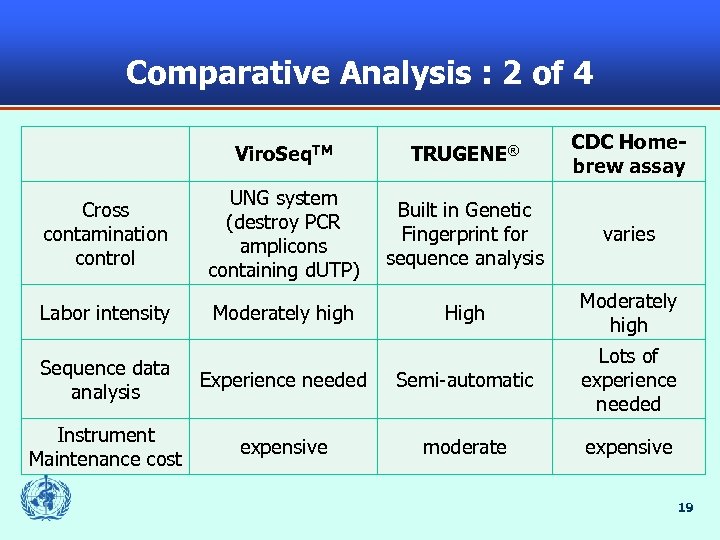 Comparative Analysis : 2 of 4 Viro. Seq. TM TRUGENE® CDC Homebrew assay Cross contamination control UNG system (destroy PCR amplicons containing d. UTP) Built in Genetic Fingerprint for sequence analysis varies Labor intensity Moderately high High Moderately high Sequence data analysis Experience needed Semi-automatic Lots of experience needed Instrument Maintenance cost expensive moderate expensive 19
Comparative Analysis : 2 of 4 Viro. Seq. TM TRUGENE® CDC Homebrew assay Cross contamination control UNG system (destroy PCR amplicons containing d. UTP) Built in Genetic Fingerprint for sequence analysis varies Labor intensity Moderately high High Moderately high Sequence data analysis Experience needed Semi-automatic Lots of experience needed Instrument Maintenance cost expensive moderate expensive 19
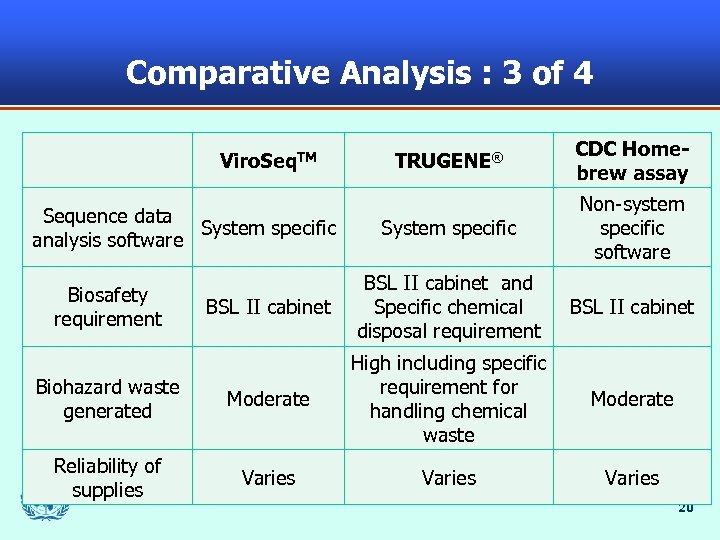 Comparative Analysis : 3 of 4 TRUGENE® CDC Homebrew assay System specific Non-system specific software BSL II cabinet and Specific chemical disposal requirement BSL II cabinet Biohazard waste generated Moderate High including specific requirement for handling chemical waste Moderate Reliability of supplies Varies Viro. Seq. TM Sequence data System specific analysis software Biosafety requirement 20
Comparative Analysis : 3 of 4 TRUGENE® CDC Homebrew assay System specific Non-system specific software BSL II cabinet and Specific chemical disposal requirement BSL II cabinet Biohazard waste generated Moderate High including specific requirement for handling chemical waste Moderate Reliability of supplies Varies Viro. Seq. TM Sequence data System specific analysis software Biosafety requirement 20
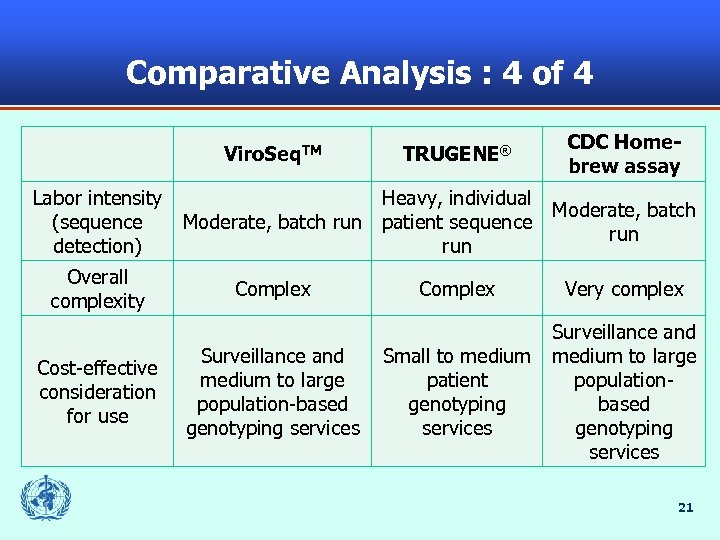 Comparative Analysis : 4 of 4 Viro. Seq. TM Labor intensity (sequence detection) Overall complexity Cost-effective consideration for use TRUGENE® CDC Homebrew assay Heavy, individual Moderate, batch run patient sequence run Complex Surveillance and medium to large population-based genotyping services Complex Very complex Small to medium patient genotyping services Surveillance and medium to large populationbased genotyping services 21
Comparative Analysis : 4 of 4 Viro. Seq. TM Labor intensity (sequence detection) Overall complexity Cost-effective consideration for use TRUGENE® CDC Homebrew assay Heavy, individual Moderate, batch run patient sequence run Complex Surveillance and medium to large population-based genotyping services Complex Very complex Small to medium patient genotyping services Surveillance and medium to large populationbased genotyping services 21
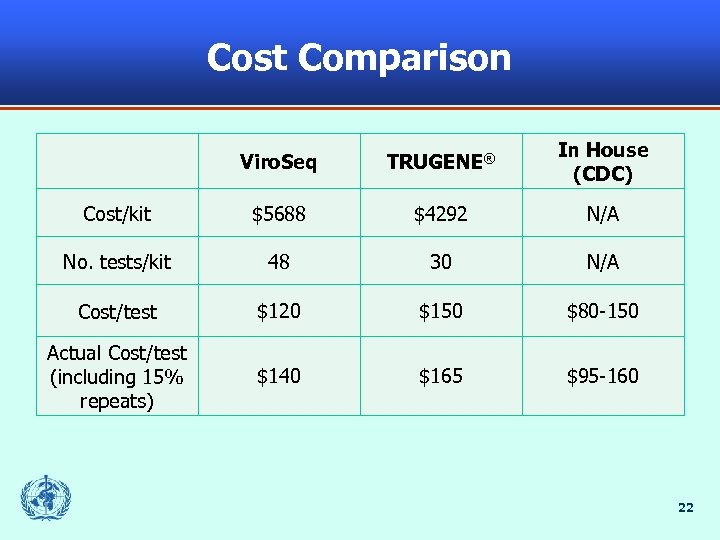 Cost Comparison Viro. Seq TRUGENE® In House (CDC) Cost/kit $5688 $4292 N/A No. tests/kit 48 30 N/A Cost/test $120 $150 $80 -150 Actual Cost/test (including 15% repeats) $140 $165 $95 -160 22
Cost Comparison Viro. Seq TRUGENE® In House (CDC) Cost/kit $5688 $4292 N/A No. tests/kit 48 30 N/A Cost/test $120 $150 $80 -150 Actual Cost/test (including 15% repeats) $140 $165 $95 -160 22
 Summary of HIV-1 Genotyping Procedures Two US FDA-approved genotyping systems Many home-brew genotyping systems All are conventional viral population-based sequencing assays with a minor viral population detection limit of ≥ 20% § Complex assays - need highly skilled staff, dedicated instrumentation and infrastructure § Test and instrument maintenance are expensive § § § 23
Summary of HIV-1 Genotyping Procedures Two US FDA-approved genotyping systems Many home-brew genotyping systems All are conventional viral population-based sequencing assays with a minor viral population detection limit of ≥ 20% § Complex assays - need highly skilled staff, dedicated instrumentation and infrastructure § Test and instrument maintenance are expensive § § § 23
 application of concepts Which type of procedure is most appropriate for our national lab? 24
application of concepts Which type of procedure is most appropriate for our national lab? 24
 Discussion What factors are most important in choosing a procedure? § Which procedure (if any) meets your needs best? § What issues do you need to resolve in order to obtain and use the procedure you have selected? § 25
Discussion What factors are most important in choosing a procedure? § Which procedure (if any) meets your needs best? § What issues do you need to resolve in order to obtain and use the procedure you have selected? § 25
 Reflection § § Which procedure will we use (if any)? What must we do as “next steps? ” 26
Reflection § § Which procedure will we use (if any)? What must we do as “next steps? ” 26
 Summary Overview of Genotyping Procedures Comparison of Three Different Procedures Application of Concepts 27
Summary Overview of Genotyping Procedures Comparison of Three Different Procedures Application of Concepts 27


