c5d21a64bf801bccbd88e590e55ab0e2.ppt
- Количество слайдов: 44
 HIV Drug Resistance Training Module 10 Standard Operating Procedures (SOPs) 1
HIV Drug Resistance Training Module 10 Standard Operating Procedures (SOPs) 1
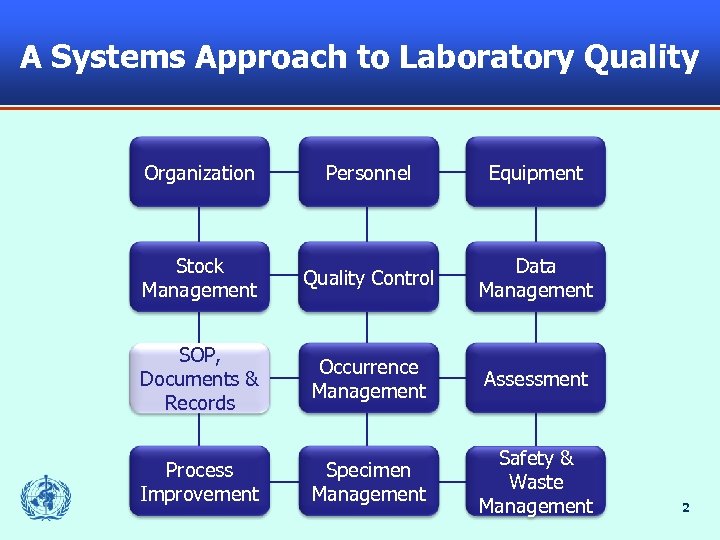 A Systems Approach to Laboratory Quality Organization Personnel Equipment Stock Management Quality Control Data Management SOP, Documents & Records Occurrence Management Assessment Process Improvement Specimen Management Safety & Waste Management 2
A Systems Approach to Laboratory Quality Organization Personnel Equipment Stock Management Quality Control Data Management SOP, Documents & Records Occurrence Management Assessment Process Improvement Specimen Management Safety & Waste Management 2
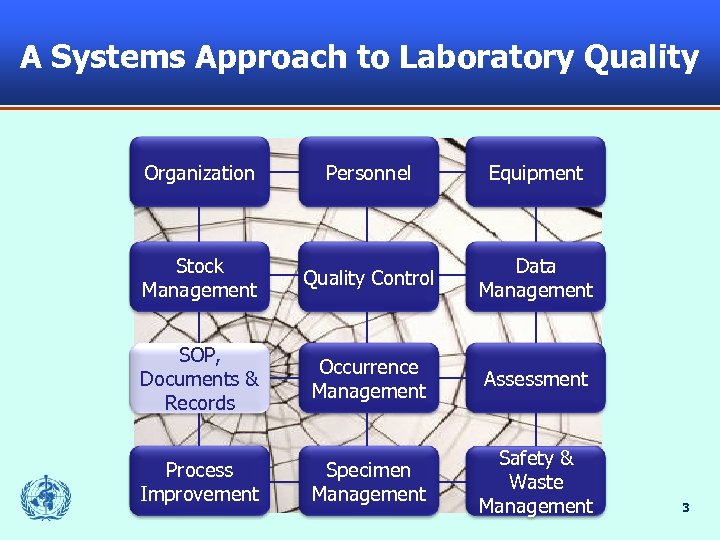 A Systems Approach to Laboratory Quality Organization Personnel Equipment Stock Management Quality Control Data Management SOP, Documents & Records Occurrence Management Assessment Process Improvement Specimen Management Safety & Waste Management 3
A Systems Approach to Laboratory Quality Organization Personnel Equipment Stock Management Quality Control Data Management SOP, Documents & Records Occurrence Management Assessment Process Improvement Specimen Management Safety & Waste Management 3
 Topics § § Processes and Procedures Documenting Processes and Procedures Forms, Manuals, and Job Aids Managing Documents 4
Topics § § Processes and Procedures Documenting Processes and Procedures Forms, Manuals, and Job Aids Managing Documents 4
 Objectives § § § Identify purpose of SOPs and related documentation. Identify components of SOPs. Identify the SOPs that are needed to ensure the quality of testing. Given a procedure, demonstrate how to write an SOP. Describe how to manage SOPs and related documents in the lab. 5
Objectives § § § Identify purpose of SOPs and related documentation. Identify components of SOPs. Identify the SOPs that are needed to ensure the quality of testing. Given a procedure, demonstrate how to write an SOP. Describe how to manage SOPs and related documents in the lab. 5
 processes and procedures What is a process? What is a procedure? Why do we need to document both? 6
processes and procedures What is a process? What is a procedure? Why do we need to document both? 6
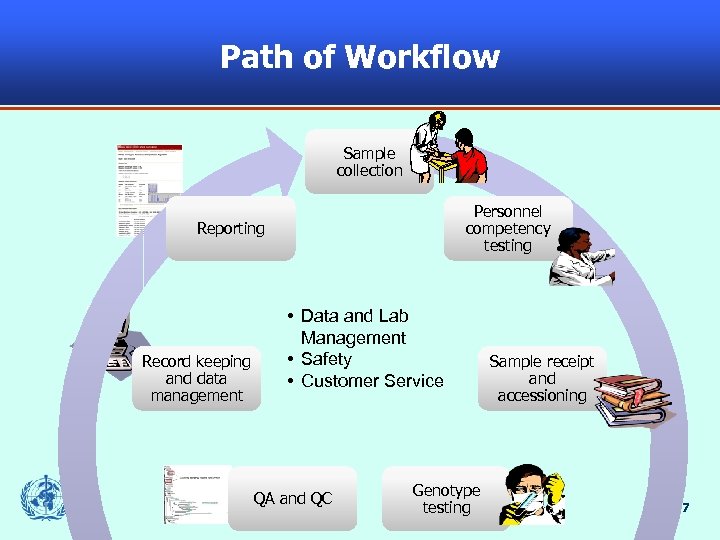 Path of Workflow Sample collection Personnel competency testing Reporting Record keeping and data management • Data and Lab Management • Safety • Customer Service QA and QC Genotype testing Sample receipt and accessioning 7
Path of Workflow Sample collection Personnel competency testing Reporting Record keeping and data management • Data and Lab Management • Safety • Customer Service QA and QC Genotype testing Sample receipt and accessioning 7
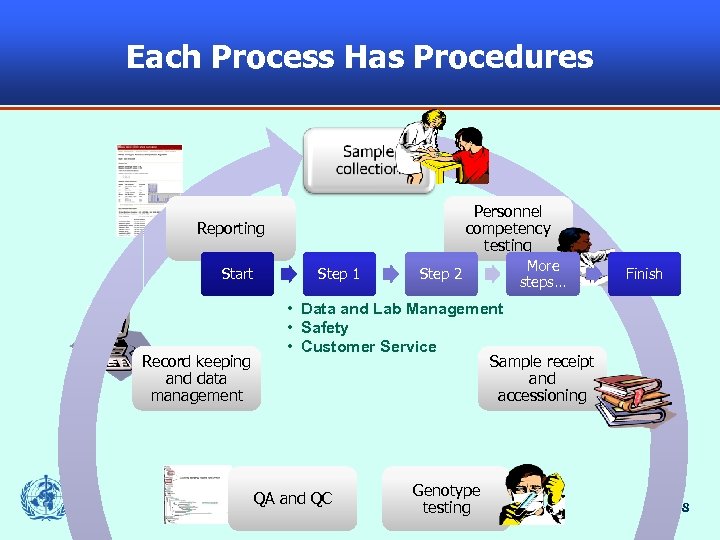 Each Process Has Procedures Sample collection Personnel competency testing Reporting Start Record keeping and data management Step 1 Step 2 More steps… Finish • Data and Lab Management • Safety • Customer Service Sample receipt and accessioning QA and QC Genotype testing 8
Each Process Has Procedures Sample collection Personnel competency testing Reporting Start Record keeping and data management Step 1 Step 2 More steps… Finish • Data and Lab Management • Safety • Customer Service Sample receipt and accessioning QA and QC Genotype testing 8
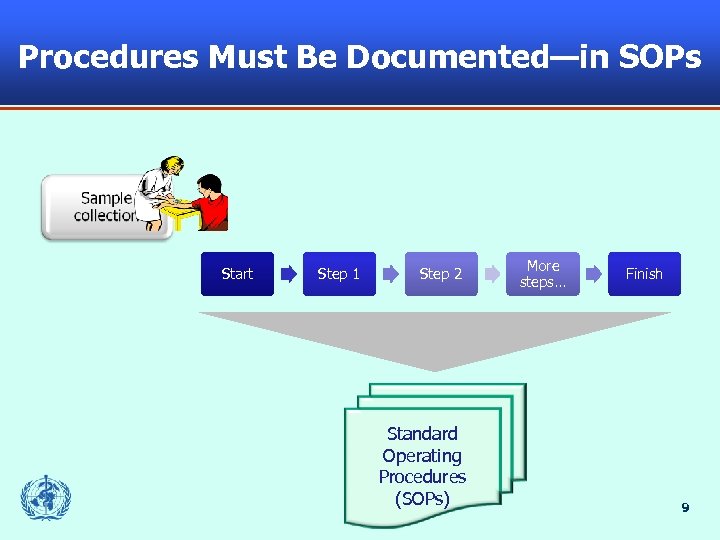 Procedures Must Be Documented—in SOPs Start Step 1 Step 2 Standard Operating Procedures (SOPs) More steps… Finish 9
Procedures Must Be Documented—in SOPs Start Step 1 Step 2 Standard Operating Procedures (SOPs) More steps… Finish 9
 Process and Procedure § Process documents: – – § Show everything fits together Flow of work Across procedures Across time – – Directions on how to perform a task One task Clear and accurate What the operator needs to do and how Procedure documents: 10
Process and Procedure § Process documents: – – § Show everything fits together Flow of work Across procedures Across time – – Directions on how to perform a task One task Clear and accurate What the operator needs to do and how Procedure documents: 10
 Why Processes and Procedures are Needed Can be used for training and competence assessment programs § Personnel can become competent more quickly § Way to identify, label, and correct process problems § 11
Why Processes and Procedures are Needed Can be used for training and competence assessment programs § Personnel can become competent more quickly § Way to identify, label, and correct process problems § 11
 Standard Operating Procedures § Purpose: to ensure reproducible and correct performance of laboratory tests – Reduce inter-operator and inter-run variation § § Made specific for each laboratory Controlled document: need a regulated system for – – – Preparation Approval Distribution Revision Training 12
Standard Operating Procedures § Purpose: to ensure reproducible and correct performance of laboratory tests – Reduce inter-operator and inter-run variation § § Made specific for each laboratory Controlled document: need a regulated system for – – – Preparation Approval Distribution Revision Training 12
 Discussion § § § What is a process? What is a procedure? Why do we need to document both? 13
Discussion § § § What is a process? What is a procedure? Why do we need to document both? 13
 documenting processes and procedures What should a process document include? What types of SOPs are there? What SOPs does a genotyping lab need? How can each lab write SOPs that work for it? 14
documenting processes and procedures What should a process document include? What types of SOPs are there? What SOPs does a genotyping lab need? How can each lab write SOPs that work for it? 14
 Essential Process Documents for HIV DR Labs § § § Document approval process Document management process New assay validation process New employee training process Genotyping process 15
Essential Process Documents for HIV DR Labs § § § Document approval process Document management process New assay validation process New employee training process Genotyping process 15
 Template for Process Documents § Title – Clearly states the intent of the document – Include the word “process” § Purpose – State purpose of document (optional: rationale, theory, or historical background) § Process: Flowchart or Table – Main part of document – Shows/describes sequence of activities and outcome § Supporting Documents – Related procedures (SOPs) 16
Template for Process Documents § Title – Clearly states the intent of the document – Include the word “process” § Purpose – State purpose of document (optional: rationale, theory, or historical background) § Process: Flowchart or Table – Main part of document – Shows/describes sequence of activities and outcome § Supporting Documents – Related procedures (SOPs) 16
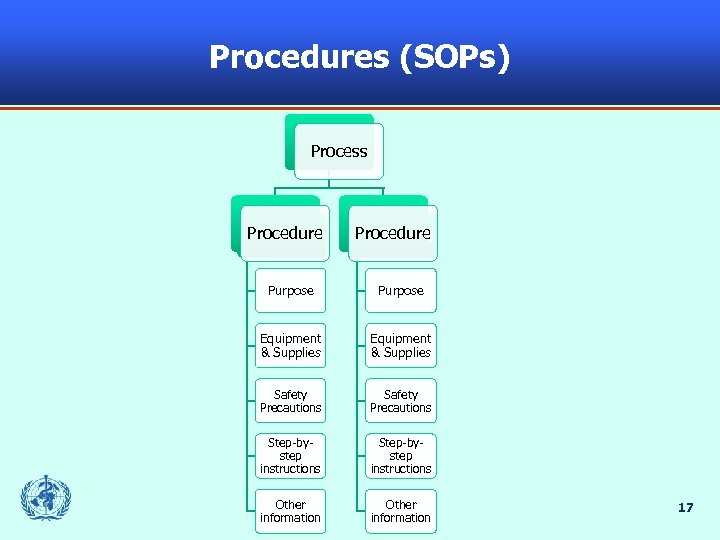 Procedures (SOPs) Process Procedure Purpose Equipment & Supplies Safety Precautions Step-bystep instructions Other information 17
Procedures (SOPs) Process Procedure Purpose Equipment & Supplies Safety Precautions Step-bystep instructions Other information 17
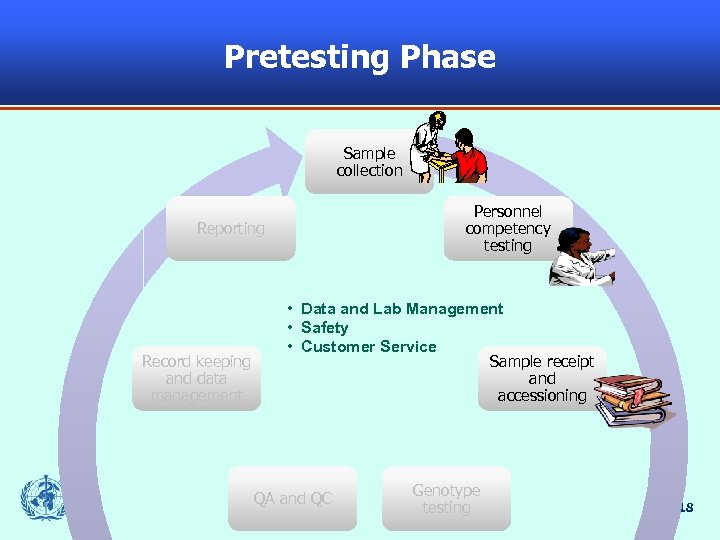 Pretesting Phase Sample collection Personnel competency testing Reporting Record keeping and data management • Data and Lab Management • Safety • Customer Service Sample receipt and accessioning QA and QC Genotype testing 18
Pretesting Phase Sample collection Personnel competency testing Reporting Record keeping and data management • Data and Lab Management • Safety • Customer Service Sample receipt and accessioning QA and QC Genotype testing 18
 Pretesting Procedures § Essential for HIV DR Labs: – Specimen management: collection, labelling, receipt, assessment, storage and shipping • Criteria for unacceptable samples and follow up action • Any storage or processing before the test • Any preparations for samples transported to other laboratories for testing – Equipment maintenance § Other Helpful Procedures: – Test requesting – Sample collection and labeling – Sample transport 19
Pretesting Procedures § Essential for HIV DR Labs: – Specimen management: collection, labelling, receipt, assessment, storage and shipping • Criteria for unacceptable samples and follow up action • Any storage or processing before the test • Any preparations for samples transported to other laboratories for testing – Equipment maintenance § Other Helpful Procedures: – Test requesting – Sample collection and labeling – Sample transport 19
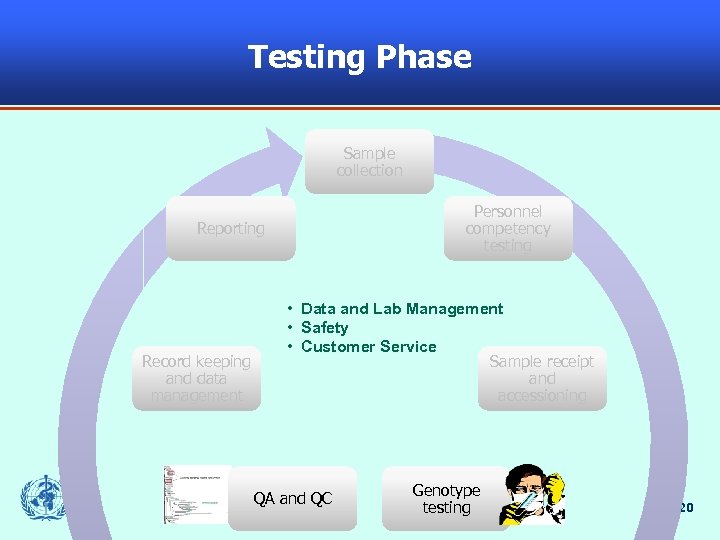 Testing Phase Sample collection Personnel competency testing Reporting Record keeping and data management • Data and Lab Management • Safety • Customer Service Sample receipt and accessioning QA and QC Genotype testing 20
Testing Phase Sample collection Personnel competency testing Reporting Record keeping and data management • Data and Lab Management • Safety • Customer Service Sample receipt and accessioning QA and QC Genotype testing 20
 Testing Procedures § Essential for HIV DR Labs: – Unidirectional Laboratory workflow – Properly Handling and manipulation of infectious human material and biohazardous waste disposal – All steps of sequencing procedure, including editing and interpretation – Internal quality control • For commercial tests, can use manufacturer’s manuals or write own procedures Copied from previous slide. • If manufacturer’s procedures are modified: ØDocument changes ØVerify adapted procedure 21
Testing Procedures § Essential for HIV DR Labs: – Unidirectional Laboratory workflow – Properly Handling and manipulation of infectious human material and biohazardous waste disposal – All steps of sequencing procedure, including editing and interpretation – Internal quality control • For commercial tests, can use manufacturer’s manuals or write own procedures Copied from previous slide. • If manufacturer’s procedures are modified: ØDocument changes ØVerify adapted procedure 21
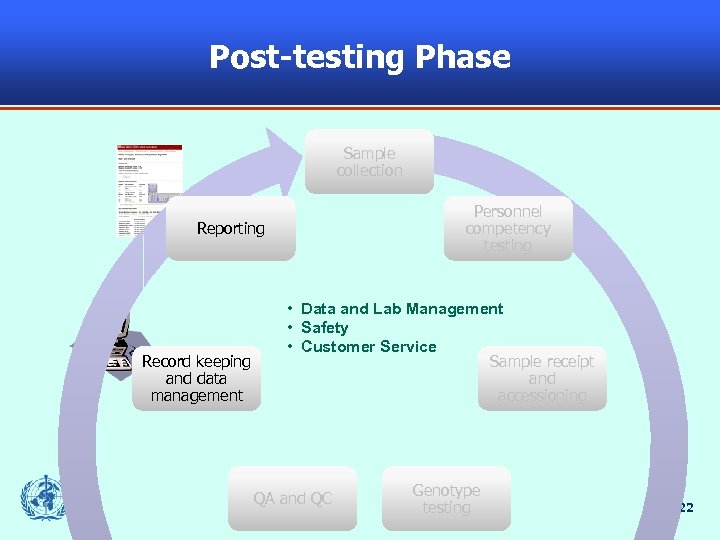 Post-testing Phase Sample collection Personnel competency testing Reporting Record keeping and data management • Data and Lab Management • Safety • Customer Service Sample receipt and accessioning QA and QC Genotype testing 22
Post-testing Phase Sample collection Personnel competency testing Reporting Record keeping and data management • Data and Lab Management • Safety • Customer Service Sample receipt and accessioning QA and QC Genotype testing 22
 Post-testing Procedures § Essential for HIV DR Labs: – Detection of containments • Negative controls and what to do if they are positive • Phylogenetic testing for cross-contamination (SQUAT) • Decontamination procedures – Data management • Archiving sequence data and report documents • Data storage and retrieval procedures 23
Post-testing Procedures § Essential for HIV DR Labs: – Detection of containments • Negative controls and what to do if they are positive • Phylogenetic testing for cross-contamination (SQUAT) • Decontamination procedures – Data management • Archiving sequence data and report documents • Data storage and retrieval procedures 23
 Post-testing Procedures § Other Helpful Procedures (Data Management): – Electronic transfer of data from an instrument into a computer system – Manual entry of data into a computer system – Manually reporting results on paper report forms – Entering test results into the laboratory’s information system (include each prompt in the computer program sequence) – Correcting results: how to change results that have been erroneously entered into the reporting system and are thus available for review and use by clinicians and caregivers. – Supervisory and/or medical review of examination 24 results, where such review is required.
Post-testing Procedures § Other Helpful Procedures (Data Management): – Electronic transfer of data from an instrument into a computer system – Manual entry of data into a computer system – Manually reporting results on paper report forms – Entering test results into the laboratory’s information system (include each prompt in the computer program sequence) – Correcting results: how to change results that have been erroneously entered into the reporting system and are thus available for review and use by clinicians and caregivers. – Supervisory and/or medical review of examination 24 results, where such review is required.
 Post-testing Procedures § Other Helpful Procedures (cont): – Sample retention: • Post-examination procedures for sample retention need to include step-by-step instructions for archiving sample materials in such a way as to be readily retrievable when needed. • Schedule for the duration of sample retention as defined by regulatory or accreditation requirements, and organizational needs. • Disposal of samples after they have exceeded their established retention times. 25
Post-testing Procedures § Other Helpful Procedures (cont): – Sample retention: • Post-examination procedures for sample retention need to include step-by-step instructions for archiving sample materials in such a way as to be readily retrievable when needed. • Schedule for the duration of sample retention as defined by regulatory or accreditation requirements, and organizational needs. • Disposal of samples after they have exceeded their established retention times. 25
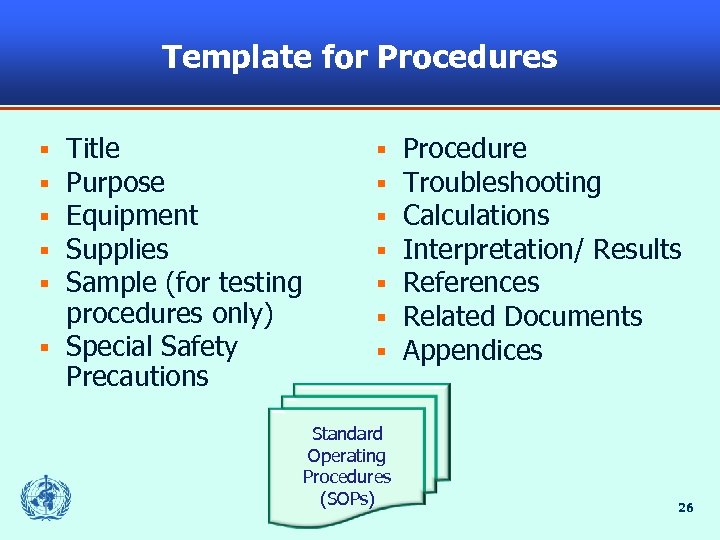 Template for Procedures Title Purpose Equipment Supplies Sample (for testing procedures only) § Special Safety Precautions § § § Standard Operating Procedures (SOPs) Procedure Troubleshooting Calculations Interpretation/ Results References Related Documents Appendices 26
Template for Procedures Title Purpose Equipment Supplies Sample (for testing procedures only) § Special Safety Precautions § § § Standard Operating Procedures (SOPs) Procedure Troubleshooting Calculations Interpretation/ Results References Related Documents Appendices 26
 Discussion § § What should a process document include? What types of SOPs are there? What SOPs does a genotyping lab need? How can each lab write SOPs that work for it? 27
Discussion § § What should a process document include? What types of SOPs are there? What SOPs does a genotyping lab need? How can each lab write SOPs that work for it? 27
 forms, manuals, and job aids What other types of documents help with process control? 28
forms, manuals, and job aids What other types of documents help with process control? 28
 Form Documents § § § Blank documents (paper or computer) Record results of a given procedure Forms include: – – – § Title that describes the form’s purpose Facility name and location Effective Date Fields for information Identifying information to link it to a procedure • Form ABC-100 -F 01 is with Procedure ABC-100 -P 01) Guidelines: – Include properly completed in procedures manual – Note needed forms in Appendices section of SOPs 29
Form Documents § § § Blank documents (paper or computer) Record results of a given procedure Forms include: – – – § Title that describes the form’s purpose Facility name and location Effective Date Fields for information Identifying information to link it to a procedure • Form ABC-100 -F 01 is with Procedure ABC-100 -P 01) Guidelines: – Include properly completed in procedures manual – Note needed forms in Appendices section of SOPs 29
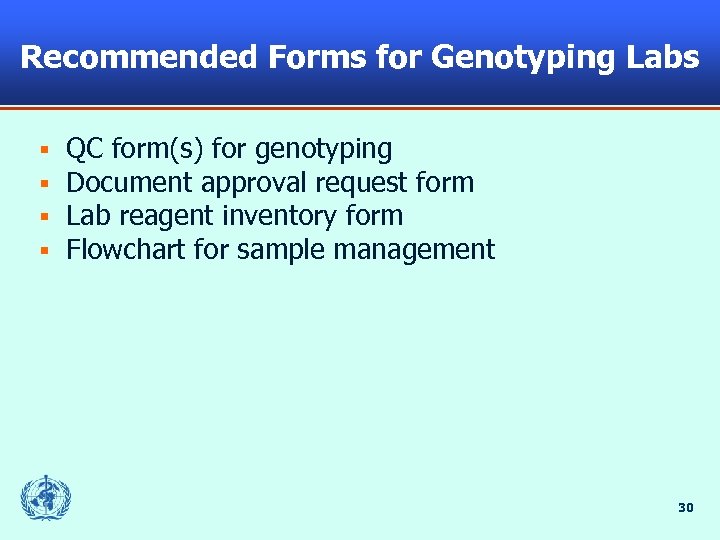 Recommended Forms for Genotyping Labs § § QC form(s) for genotyping Document approval request form Lab reagent inventory form Flowchart for sample management 30
Recommended Forms for Genotyping Labs § § QC form(s) for genotyping Document approval request form Lab reagent inventory form Flowchart for sample management 30
 Procedure Manuals § Procedure manuals should be organized in a way that can be easily followed by laboratory personnel and should contain the following elements: – – Table of contents Process descriptions (optional) Procedures Associated Forms 31
Procedure Manuals § Procedure manuals should be organized in a way that can be easily followed by laboratory personnel and should contain the following elements: – – Table of contents Process descriptions (optional) Procedures Associated Forms 31
 Job Aids § Examples: – Instruction sheets – Wall charts – Instructions posted on equipment § Qualities of good job aids: – 3 C’s: Current, complete, and correct – Traceable to the “parent” document – Effective date is listed. § If some of the above qualities are missing, start the document change process. 32
Job Aids § Examples: – Instruction sheets – Wall charts – Instructions posted on equipment § Qualities of good job aids: – 3 C’s: Current, complete, and correct – Traceable to the “parent” document – Effective date is listed. § If some of the above qualities are missing, start the document change process. 32
 Discussion § Besides SOPs, what other types of documents help with process control? 33
Discussion § Besides SOPs, what other types of documents help with process control? 33
 managing documents Why is it important to control how documents are created, updated, copied, and stored? What are some general guidelines for document management? 34
managing documents Why is it important to control how documents are created, updated, copied, and stored? What are some general guidelines for document management? 34
 Document Management § A Document Management System ensures – Approved format – Current, approved version Quality Assurance Manager is responsible for the assignment and tracking of all documentation § System may be § – Paper-based – Electronic-based 35
Document Management § A Document Management System ensures – Approved format – Current, approved version Quality Assurance Manager is responsible for the assignment and tracking of all documentation § System may be § – Paper-based – Electronic-based 35
 Document Management System: Purpose § § § Keep everyone up to date Get rid of old, outdated information Standardize procedures § Maintain quality of laboratory services and results 36
Document Management System: Purpose § § § Keep everyone up to date Get rid of old, outdated information Standardize procedures § Maintain quality of laboratory services and results 36
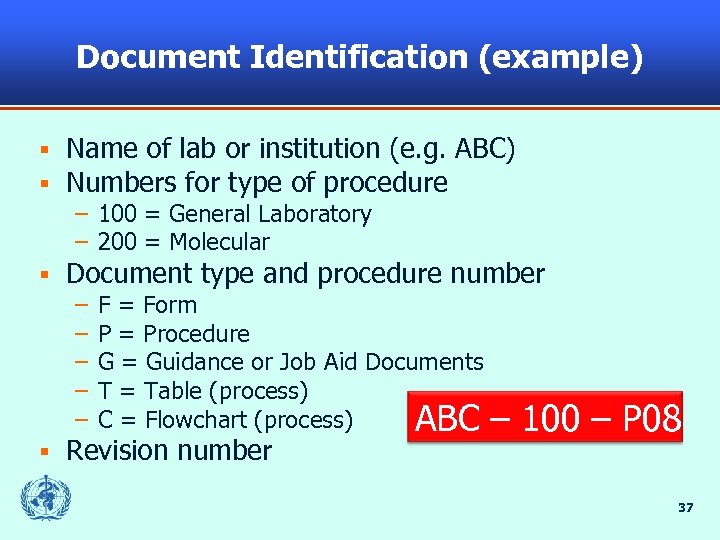 Document Identification (example) § § Name of lab or institution (e. g. ABC) Numbers for type of procedure – 100 = General Laboratory – 200 = Molecular § Document type and procedure number – – – § F = Form P = Procedure G = Guidance or Job Aid Documents T = Table (process) C = Flowchart (process) ABC – Revision number 100 – P 08 37
Document Identification (example) § § Name of lab or institution (e. g. ABC) Numbers for type of procedure – 100 = General Laboratory – 200 = Molecular § Document type and procedure number – – – § F = Form P = Procedure G = Guidance or Job Aid Documents T = Table (process) C = Flowchart (process) ABC – Revision number 100 – P 08 37
 Review and Approval of New Documents Requestor completes approval request form Lab supervisor(s) reviews document Lab Director approves all new procedures and any major modifications. § The Quality Assurance Manager maintains review and approval documentation in the master file hard copy binder § § § 38
Review and Approval of New Documents Requestor completes approval request form Lab supervisor(s) reviews document Lab Director approves all new procedures and any major modifications. § The Quality Assurance Manager maintains review and approval documentation in the master file hard copy binder § § § 38
 Archiving, Storage, and Retention of Documents § Master file: – – § Stored by the Quality Assurance Manager Electronic Current version All previous versions , clearly identified • RETIRED in bold on the upper right hand corner of the header OR • Watermarked diagonally across the page in order Hard copy – Stored by the Quality Assurance Manager – In Master File Hard Copy Binder 39
Archiving, Storage, and Retention of Documents § Master file: – – § Stored by the Quality Assurance Manager Electronic Current version All previous versions , clearly identified • RETIRED in bold on the upper right hand corner of the header OR • Watermarked diagonally across the page in order Hard copy – Stored by the Quality Assurance Manager – In Master File Hard Copy Binder 39
 Discussion Why is it important to control how documents are created, updated, copied, and stored? § What are some general guidelines for document management? § 40
Discussion Why is it important to control how documents are created, updated, copied, and stored? § What are some general guidelines for document management? § 40
 Reflection What are the strengths and weaknesses of our current processes and procedures? § Where are we vulnerable to errors? § How can we improve our documentation to reduce errors? § 41
Reflection What are the strengths and weaknesses of our current processes and procedures? § Where are we vulnerable to errors? § How can we improve our documentation to reduce errors? § 41
 Summary ü ü Processes and Procedures Documenting Processes and Procedures Forms, Manuals, and Job Aids Managing Documents 42
Summary ü ü Processes and Procedures Documenting Processes and Procedures Forms, Manuals, and Job Aids Managing Documents 42
 Practice: Create an SOP for Making Coffee 43
Practice: Create an SOP for Making Coffee 43
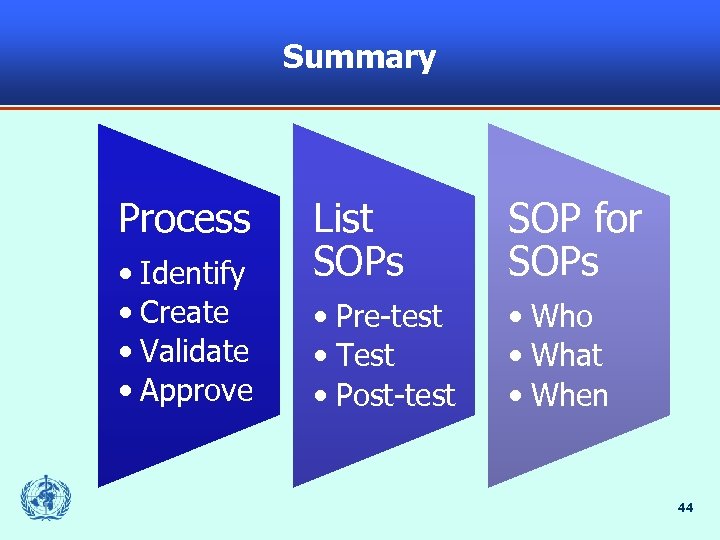 Summary Process • Identify • Create • Validate • Approve List SOPs SOP for SOPs • Pre-test • Test • Post-test • Who • What • When 44
Summary Process • Identify • Create • Validate • Approve List SOPs SOP for SOPs • Pre-test • Test • Post-test • Who • What • When 44


