hiv.ppt
- Количество слайдов: 29
 HIV and Pregnancy: Prevention of Mother-to-Child Transmission Advances in Maternal and Neonatal Health HIV and Pregnancy
HIV and Pregnancy: Prevention of Mother-to-Child Transmission Advances in Maternal and Neonatal Health HIV and Pregnancy
 Session Objectives l To discuss best practice for antenatal, intrapartum and postpartum care of the HIV-positive mother to reduce motherto-child transmission l To review the evidence supporting these practices HIV and Pregnancy 2
Session Objectives l To discuss best practice for antenatal, intrapartum and postpartum care of the HIV-positive mother to reduce motherto-child transmission l To review the evidence supporting these practices HIV and Pregnancy 2
 HIV-Related Counseling Issues During Pregnancy l Educate/counsel regarding HIV and pregnancy before pregnancy: l Impact of HIV on pregnancy and pregnancy on HIV l Maternal health l Long-term health of mother and care for children l Perinatal transmission l Use of antiretrovirals and other drugs in pregnancy HIV and Pregnancy 3
HIV-Related Counseling Issues During Pregnancy l Educate/counsel regarding HIV and pregnancy before pregnancy: l Impact of HIV on pregnancy and pregnancy on HIV l Maternal health l Long-term health of mother and care for children l Perinatal transmission l Use of antiretrovirals and other drugs in pregnancy HIV and Pregnancy 3
 Pregnancy Effects on HIV l In all women, the absolute CD 4 count decreases no matter whether HIV-positive or negative (pregnancy does not make HIV worse) l In HIV-positive women, percentage of CD 4 cells should not change and viral load should not change because of pregnancy HIV and Pregnancy 4
Pregnancy Effects on HIV l In all women, the absolute CD 4 count decreases no matter whether HIV-positive or negative (pregnancy does not make HIV worse) l In HIV-positive women, percentage of CD 4 cells should not change and viral load should not change because of pregnancy HIV and Pregnancy 4
 Adverse Pregnancy Outcomes and Relationship to HIV Infection Pregnancy Outcome Relationship to HIV Infection Spontaneous abortion Limited data, but evidence of possible increased risk Stillbirth No association noted in developed countries; evidence of increased risk in developing countries Perinatal mortality No association noted in developed countries, but data limited; evidence of increased risk in developing countries Newborn mortality Limited data in developed countries; evidence of increased risk in developing countries Intra-uterine growth retardation Evidence of possible increased risk Anderson 2001. HIV and Pregnancy 5
Adverse Pregnancy Outcomes and Relationship to HIV Infection Pregnancy Outcome Relationship to HIV Infection Spontaneous abortion Limited data, but evidence of possible increased risk Stillbirth No association noted in developed countries; evidence of increased risk in developing countries Perinatal mortality No association noted in developed countries, but data limited; evidence of increased risk in developing countries Newborn mortality Limited data in developed countries; evidence of increased risk in developing countries Intra-uterine growth retardation Evidence of possible increased risk Anderson 2001. HIV and Pregnancy 5
 Adverse Pregnancy Outcomes and Relationship to HIV Infection (continued) Pregnancy Outcome Relationship to HIV Infection Low birth weight Evidence of possible increased risk Preterm delivery Evidence of possible increased risk, especially w/ more advanced disease Pre-eclampsia No data Gestational diabetes No data Amnionitis Limited data; more recent studies do not suggest an increased risk; some earlier studies found increased histologic placental inflammation, particularly in those with preterm deliveries Oligohydramnios Minimal data Fetal malformation No evidence of increased risk Anderson 2001. HIV and Pregnancy 6
Adverse Pregnancy Outcomes and Relationship to HIV Infection (continued) Pregnancy Outcome Relationship to HIV Infection Low birth weight Evidence of possible increased risk Preterm delivery Evidence of possible increased risk, especially w/ more advanced disease Pre-eclampsia No data Gestational diabetes No data Amnionitis Limited data; more recent studies do not suggest an increased risk; some earlier studies found increased histologic placental inflammation, particularly in those with preterm deliveries Oligohydramnios Minimal data Fetal malformation No evidence of increased risk Anderson 2001. HIV and Pregnancy 6
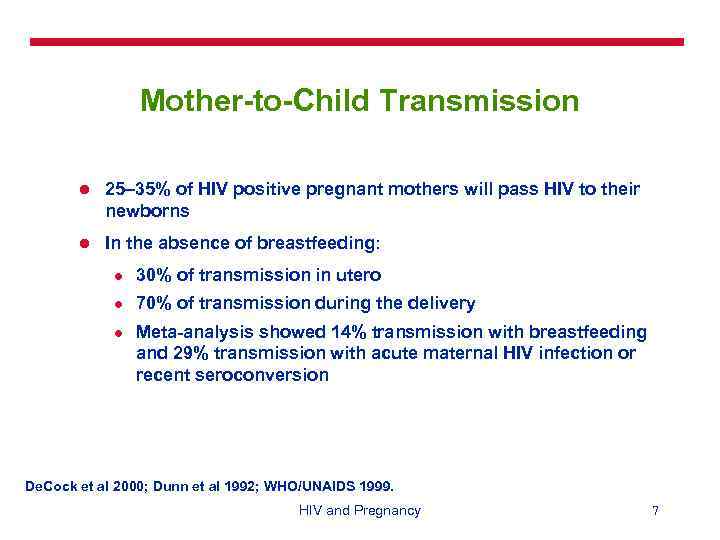 Mother-to-Child Transmission l 25– 35% of HIV positive pregnant mothers will pass HIV to their newborns l In the absence of breastfeeding: l 30% of transmission in utero l 70% of transmission during the delivery l Meta-analysis showed 14% transmission with breastfeeding and 29% transmission with acute maternal HIV infection or recent seroconversion De. Cock et al 2000; Dunn et al 1992; WHO/UNAIDS 1999. HIV and Pregnancy 7
Mother-to-Child Transmission l 25– 35% of HIV positive pregnant mothers will pass HIV to their newborns l In the absence of breastfeeding: l 30% of transmission in utero l 70% of transmission during the delivery l Meta-analysis showed 14% transmission with breastfeeding and 29% transmission with acute maternal HIV infection or recent seroconversion De. Cock et al 2000; Dunn et al 1992; WHO/UNAIDS 1999. HIV and Pregnancy 7
 Risk Factors for Mother-to-Child Transmission l Viral load (HIV-RNA level) l STDs and other coinfections l Genital tract viral load l Antiretroviral agents l CD 4 cell count l Preterm delivery l Clinical stage of HIV l Placental disruption l Unprotected sex with multiple partners l Invasive fetal monitoring l Duration of membrane rupture l Vaginal delivery vs. cesarean section l Breastfeeding l Smoking cigarettes l Substance abuse l Vitamin A deficiency Anderson 2001. HIV and Pregnancy 8
Risk Factors for Mother-to-Child Transmission l Viral load (HIV-RNA level) l STDs and other coinfections l Genital tract viral load l Antiretroviral agents l CD 4 cell count l Preterm delivery l Clinical stage of HIV l Placental disruption l Unprotected sex with multiple partners l Invasive fetal monitoring l Duration of membrane rupture l Vaginal delivery vs. cesarean section l Breastfeeding l Smoking cigarettes l Substance abuse l Vitamin A deficiency Anderson 2001. HIV and Pregnancy 8
 Interventions to Reduce Mother-to-Child Transmission l HIV testing in pregnancy l Antenatal care l Antiretroviral agents l Obstetric interventions Avoid amniotomy l Avoid procedures: Forceps/vacuum extractor, scalp electrode, scalp blood sampling l Restrict episiotomy l Elective cesarean section l Remember infection prevention practices l Newborn feeding: Breastmilk vs. formula l HIV and Pregnancy 9
Interventions to Reduce Mother-to-Child Transmission l HIV testing in pregnancy l Antenatal care l Antiretroviral agents l Obstetric interventions Avoid amniotomy l Avoid procedures: Forceps/vacuum extractor, scalp electrode, scalp blood sampling l Restrict episiotomy l Elective cesarean section l Remember infection prevention practices l Newborn feeding: Breastmilk vs. formula l HIV and Pregnancy 9
 HIV Testing during Pregnancy l Advantages: l Possible treatment of mother l Reduce risk of mother-to-child transmission l Future family planning issues l Precautions against further spread l If negative, advise about HIV prevention Counseling is important! HIV and Pregnancy 10
HIV Testing during Pregnancy l Advantages: l Possible treatment of mother l Reduce risk of mother-to-child transmission l Future family planning issues l Precautions against further spread l If negative, advise about HIV prevention Counseling is important! HIV and Pregnancy 10
 Antenatal Care l Most HIV-infected women will be asymptomatic l Watch for signs/symptoms of AIDS and pregnancy-related complications l Unless complication develops, no need to increase number of visits l Treat STDs and other coinfections l Counsel against unprotected intercourse l Avoid invasive procedures and external cephalic version l Give antiretroviral agents, if available l Counsel about nutrition HIV and Pregnancy 11
Antenatal Care l Most HIV-infected women will be asymptomatic l Watch for signs/symptoms of AIDS and pregnancy-related complications l Unless complication develops, no need to increase number of visits l Treat STDs and other coinfections l Counsel against unprotected intercourse l Avoid invasive procedures and external cephalic version l Give antiretroviral agents, if available l Counsel about nutrition HIV and Pregnancy 11
 Antiretrovirals l Zidovudine (ZDV): l Long course l Short course l Nevirapine l ZDV/lamivudine (ZDV/3 TC) HIV and Pregnancy 12
Antiretrovirals l Zidovudine (ZDV): l Long course l Short course l Nevirapine l ZDV/lamivudine (ZDV/3 TC) HIV and Pregnancy 12
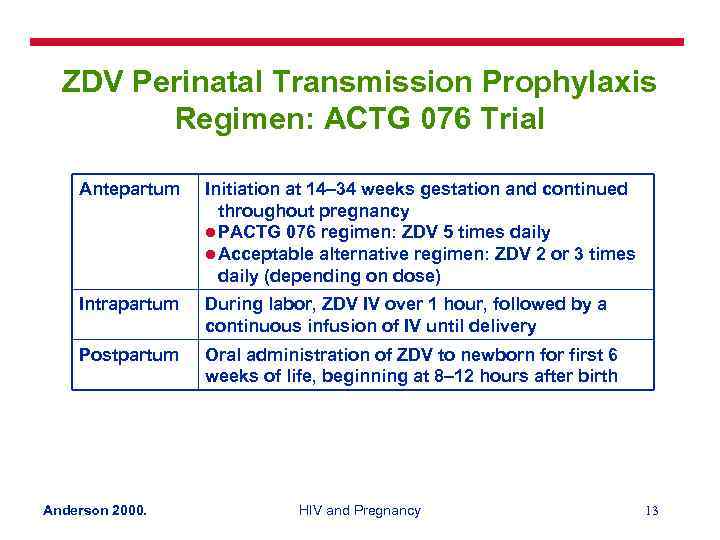 ZDV Perinatal Transmission Prophylaxis Regimen: ACTG 076 Trial Antepartum Initiation at 14– 34 weeks gestation and continued throughout pregnancy l PACTG 076 regimen: ZDV 5 times daily l Acceptable alternative regimen: ZDV 2 or 3 times daily (depending on dose) Intrapartum During labor, ZDV IV over 1 hour, followed by a continuous infusion of IV until delivery Postpartum Oral administration of ZDV to newborn for first 6 weeks of life, beginning at 8– 12 hours after birth Anderson 2000. HIV and Pregnancy 13
ZDV Perinatal Transmission Prophylaxis Regimen: ACTG 076 Trial Antepartum Initiation at 14– 34 weeks gestation and continued throughout pregnancy l PACTG 076 regimen: ZDV 5 times daily l Acceptable alternative regimen: ZDV 2 or 3 times daily (depending on dose) Intrapartum During labor, ZDV IV over 1 hour, followed by a continuous infusion of IV until delivery Postpartum Oral administration of ZDV to newborn for first 6 weeks of life, beginning at 8– 12 hours after birth Anderson 2000. HIV and Pregnancy 13
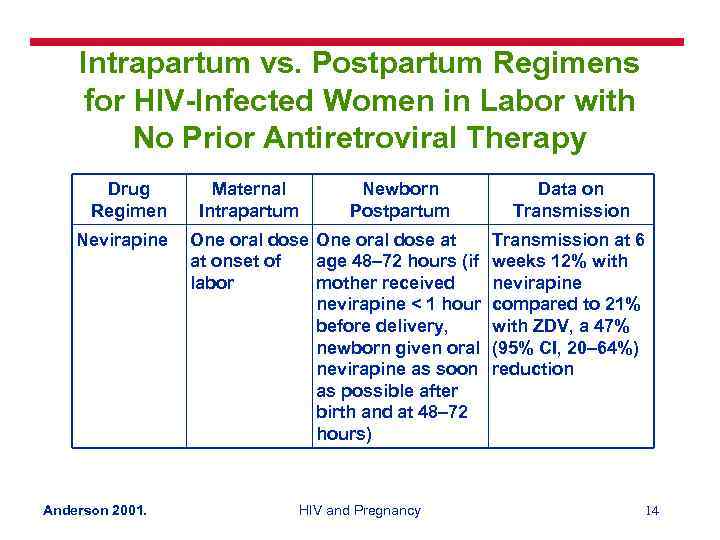 Intrapartum vs. Postpartum Regimens for HIV-Infected Women in Labor with No Prior Antiretroviral Therapy Drug Regimen Nevirapine Anderson 2001. Maternal Intrapartum Newborn Postpartum One oral dose at at onset of age 48– 72 hours (if labor mother received nevirapine < 1 hour before delivery, newborn given oral nevirapine as soon as possible after birth and at 48– 72 hours) HIV and Pregnancy Data on Transmission at 6 weeks 12% with nevirapine compared to 21% with ZDV, a 47% (95% CI, 20– 64%) reduction 14
Intrapartum vs. Postpartum Regimens for HIV-Infected Women in Labor with No Prior Antiretroviral Therapy Drug Regimen Nevirapine Anderson 2001. Maternal Intrapartum Newborn Postpartum One oral dose at at onset of age 48– 72 hours (if labor mother received nevirapine < 1 hour before delivery, newborn given oral nevirapine as soon as possible after birth and at 48– 72 hours) HIV and Pregnancy Data on Transmission at 6 weeks 12% with nevirapine compared to 21% with ZDV, a 47% (95% CI, 20– 64%) reduction 14
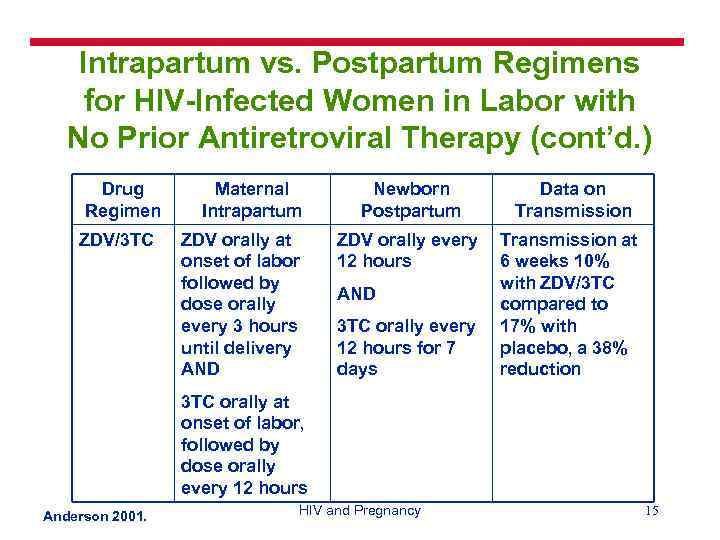 Intrapartum vs. Postpartum Regimens for HIV-Infected Women in Labor with No Prior Antiretroviral Therapy (cont’d. ) Drug Regimen ZDV/3 TC Maternal Intrapartum ZDV orally at onset of labor followed by dose orally every 3 hours until delivery AND Newborn Postpartum Data on Transmission ZDV orally every 12 hours Transmission at 6 weeks 10% with ZDV/3 TC compared to 17% with placebo, a 38% reduction AND 3 TC orally every 12 hours for 7 days 3 TC orally at onset of labor, followed by dose orally every 12 hours Anderson 2001. HIV and Pregnancy 15
Intrapartum vs. Postpartum Regimens for HIV-Infected Women in Labor with No Prior Antiretroviral Therapy (cont’d. ) Drug Regimen ZDV/3 TC Maternal Intrapartum ZDV orally at onset of labor followed by dose orally every 3 hours until delivery AND Newborn Postpartum Data on Transmission ZDV orally every 12 hours Transmission at 6 weeks 10% with ZDV/3 TC compared to 17% with placebo, a 38% reduction AND 3 TC orally every 12 hours for 7 days 3 TC orally at onset of labor, followed by dose orally every 12 hours Anderson 2001. HIV and Pregnancy 15
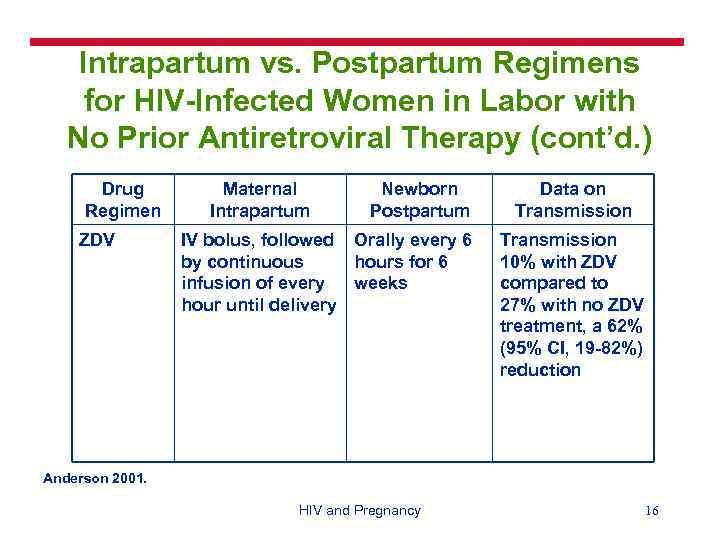 Intrapartum vs. Postpartum Regimens for HIV-Infected Women in Labor with No Prior Antiretroviral Therapy (cont’d. ) Drug Regimen ZDV Maternal Intrapartum Newborn Postpartum IV bolus, followed Orally every 6 by continuous hours for 6 infusion of every weeks hour until delivery Data on Transmission 10% with ZDV compared to 27% with no ZDV treatment, a 62% (95% CI, 19 -82%) reduction Anderson 2001. HIV and Pregnancy 16
Intrapartum vs. Postpartum Regimens for HIV-Infected Women in Labor with No Prior Antiretroviral Therapy (cont’d. ) Drug Regimen ZDV Maternal Intrapartum Newborn Postpartum IV bolus, followed Orally every 6 by continuous hours for 6 infusion of every weeks hour until delivery Data on Transmission 10% with ZDV compared to 27% with no ZDV treatment, a 62% (95% CI, 19 -82%) reduction Anderson 2001. HIV and Pregnancy 16
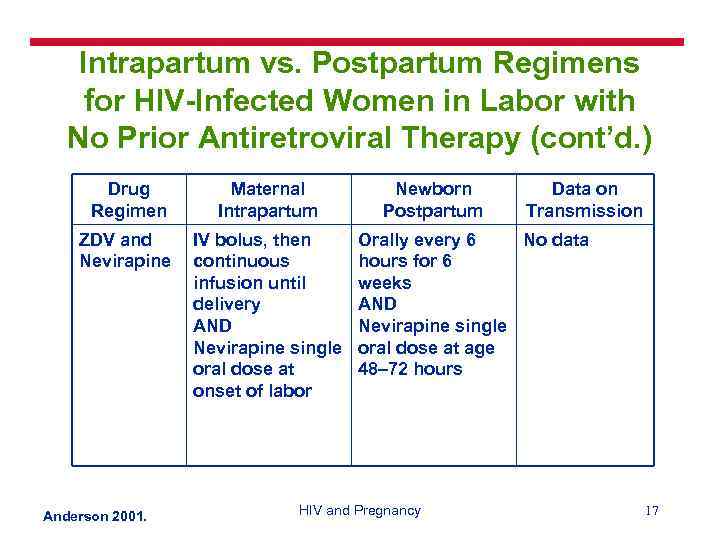 Intrapartum vs. Postpartum Regimens for HIV-Infected Women in Labor with No Prior Antiretroviral Therapy (cont’d. ) Drug Regimen Maternal Intrapartum ZDV and Nevirapine IV bolus, then continuous infusion until delivery AND Nevirapine single oral dose at onset of labor Anderson 2001. Newborn Postpartum Data on Transmission Orally every 6 No data hours for 6 weeks AND Nevirapine single oral dose at age 48– 72 hours HIV and Pregnancy 17
Intrapartum vs. Postpartum Regimens for HIV-Infected Women in Labor with No Prior Antiretroviral Therapy (cont’d. ) Drug Regimen Maternal Intrapartum ZDV and Nevirapine IV bolus, then continuous infusion until delivery AND Nevirapine single oral dose at onset of labor Anderson 2001. Newborn Postpartum Data on Transmission Orally every 6 No data hours for 6 weeks AND Nevirapine single oral dose at age 48– 72 hours HIV and Pregnancy 17
 Obstetric Procedures Because of increased fetal exposure to infected maternal blood and secretions, increased transmission may come from: l Amniotomy l Fetal scalp electrode/sampling l Forceps/vacuum extractor l Episiotomy l Vaginal tears HIV and Pregnancy 18
Obstetric Procedures Because of increased fetal exposure to infected maternal blood and secretions, increased transmission may come from: l Amniotomy l Fetal scalp electrode/sampling l Forceps/vacuum extractor l Episiotomy l Vaginal tears HIV and Pregnancy 18
 Delivery: Cesarean vs. Vaginal Birth l Risk of mother-to-child transmission increased 2% each hour after membranes have been ruptured l Cesarean section before labor and/or rupture of membranes reduces risk of mother-to-child transmission by 50– 80% compared with other modes of delivery in women on no antiretroviral therapy or on ZDV alone l No evidence of benefit with cesarean section after onset of labor or membranes have been ruptured l Cesarean section, however, increases morbidity and possible mortality to mother l Give antibiotic prophylaxis for cesarean section in HIV-infected women International Perinatal HIV Group 1999; HIV and Pregnancy Semprini 1995. 19
Delivery: Cesarean vs. Vaginal Birth l Risk of mother-to-child transmission increased 2% each hour after membranes have been ruptured l Cesarean section before labor and/or rupture of membranes reduces risk of mother-to-child transmission by 50– 80% compared with other modes of delivery in women on no antiretroviral therapy or on ZDV alone l No evidence of benefit with cesarean section after onset of labor or membranes have been ruptured l Cesarean section, however, increases morbidity and possible mortality to mother l Give antibiotic prophylaxis for cesarean section in HIV-infected women International Perinatal HIV Group 1999; HIV and Pregnancy Semprini 1995. 19
 Recommended Infection Prevention Practices l Needles: l Take care! Minimal use l Suturing: Use appropriate needle and holder l Care with recapping and disposal l Wear gloves, wash hands with soap immediately after contact with blood and body fluids l Cover incisions with watertight dressings for first 24 hours HIV and Pregnancy 20
Recommended Infection Prevention Practices l Needles: l Take care! Minimal use l Suturing: Use appropriate needle and holder l Care with recapping and disposal l Wear gloves, wash hands with soap immediately after contact with blood and body fluids l Cover incisions with watertight dressings for first 24 hours HIV and Pregnancy 20
 Recommended Infection Prevention Practices (continued) l Use: l Plastic aprons for delivery l Goggles and gloves for delivery and surgery l Long gloves for placenta removal l Dispose of blood, placenta and waste safely l PROTECT YOURSELF! HIV and Pregnancy 21
Recommended Infection Prevention Practices (continued) l Use: l Plastic aprons for delivery l Goggles and gloves for delivery and surgery l Long gloves for placenta removal l Dispose of blood, placenta and waste safely l PROTECT YOURSELF! HIV and Pregnancy 21
 Newborn l Wash newborn after birth, especially face l Avoid hypothermia l Give antiretroviral agents, if available HIV and Pregnancy 22
Newborn l Wash newborn after birth, especially face l Avoid hypothermia l Give antiretroviral agents, if available HIV and Pregnancy 22
 Breasfeeding Issues l Warmth for newborn l Nutrition for newborn l Protection against other infections l Safety – unclean water, diarrheal diseases l Risk of HIV transmission l Contraception for mother l Cost HIV and Pregnancy 23
Breasfeeding Issues l Warmth for newborn l Nutrition for newborn l Protection against other infections l Safety – unclean water, diarrheal diseases l Risk of HIV transmission l Contraception for mother l Cost HIV and Pregnancy 23
 Breastfeeding Recommendations If the woman is: l HIV-negative or does not know her HIV status, promote exclusive breastfeeding for 6 months l HIV-positive and chooses to use replacements feedings, counsel on the safe and appropriate use of formula l HIV-positive and chooses to breastfeed, promote exclusive breastfeeding for 6 months HIV and Pregnancy 24
Breastfeeding Recommendations If the woman is: l HIV-negative or does not know her HIV status, promote exclusive breastfeeding for 6 months l HIV-positive and chooses to use replacements feedings, counsel on the safe and appropriate use of formula l HIV-positive and chooses to breastfeed, promote exclusive breastfeeding for 6 months HIV and Pregnancy 24
 South Africa Breastfeeding Trial: Objective and Design l Objective: To assess whether pattern of breastfeeding is a critical determinant of early mother-to-child transmission of HIV l 549 HIV-infected women studied l Compared newborns at 3 months that had been: l Exclusively breastfed l Breastfed and formula-fed l Never breastfed Coutsoudis et al 1999. HIV and Pregnancy 25
South Africa Breastfeeding Trial: Objective and Design l Objective: To assess whether pattern of breastfeeding is a critical determinant of early mother-to-child transmission of HIV l 549 HIV-infected women studied l Compared newborns at 3 months that had been: l Exclusively breastfed l Breastfed and formula-fed l Never breastfed Coutsoudis et al 1999. HIV and Pregnancy 25
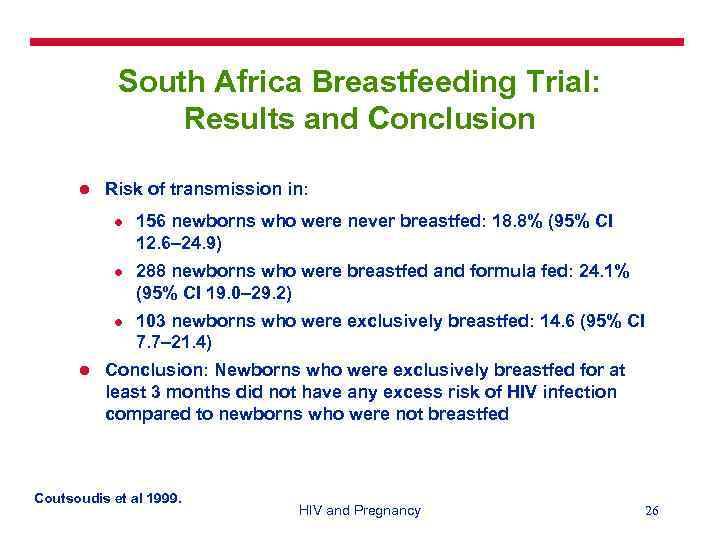 South Africa Breastfeeding Trial: Results and Conclusion l Risk of transmission in: l l 156 newborns who were never breastfed: 18. 8% (95% CI 12. 6– 24. 9) 288 newborns who were breastfed and formula fed: 24. 1% (95% CI 19. 0– 29. 2) 103 newborns who were exclusively breastfed: 14. 6 (95% CI 7. 7– 21. 4) Conclusion: Newborns who were exclusively breastfed for at least 3 months did not have any excess risk of HIV infection compared to newborns who were not breastfed Coutsoudis et al 1999. HIV and Pregnancy 26
South Africa Breastfeeding Trial: Results and Conclusion l Risk of transmission in: l l 156 newborns who were never breastfed: 18. 8% (95% CI 12. 6– 24. 9) 288 newborns who were breastfed and formula fed: 24. 1% (95% CI 19. 0– 29. 2) 103 newborns who were exclusively breastfed: 14. 6 (95% CI 7. 7– 21. 4) Conclusion: Newborns who were exclusively breastfed for at least 3 months did not have any excess risk of HIV infection compared to newborns who were not breastfed Coutsoudis et al 1999. HIV and Pregnancy 26
 Conclusion l Voluntary counseling and testing l Antenatal, intrapartum and postpartum care to mother can decrease risk of mother-to-child transmission l l Antiretroviral therapy can also reduce risk of transmission Newborn care: Feeding HIV and Pregnancy 27
Conclusion l Voluntary counseling and testing l Antenatal, intrapartum and postpartum care to mother can decrease risk of mother-to-child transmission l l Antiretroviral therapy can also reduce risk of transmission Newborn care: Feeding HIV and Pregnancy 27
 References Anderson J (ed). 2001. A Guide to the Clinical Care of Women with HIV, 2 nd ed. U. S. Department of Health and Human Services, Health Resources and Services Administration: Rockville, Maryland. Coutsoudis A et al. 1999. Influence of infant-feeding patterns on early mother-to-child transmission of HIV-1 in Durban, South Africa: A prospective cohort study. Lancet 354: 471– 476. De. Cock K et al. 2000. Prevention of mother-to-child transmission in resource-poor countries: Translating research into policy and practice. J Am Med Assoc 283(9): 1175– 1182. Dunn D et al. 1992. Risk of HIV-1 transmission through breastfeeding. Lancet 340(8819): 585– 588. Gray G. 2000. The PETRA study: Early and late efficacy of three short ZDV/3 TC combinations regimens to prevent mother-to-child transmission of HIV-1. XIII International AIDS Conference, Durban, South Africa. HIV and Pregnancy 28
References Anderson J (ed). 2001. A Guide to the Clinical Care of Women with HIV, 2 nd ed. U. S. Department of Health and Human Services, Health Resources and Services Administration: Rockville, Maryland. Coutsoudis A et al. 1999. Influence of infant-feeding patterns on early mother-to-child transmission of HIV-1 in Durban, South Africa: A prospective cohort study. Lancet 354: 471– 476. De. Cock K et al. 2000. Prevention of mother-to-child transmission in resource-poor countries: Translating research into policy and practice. J Am Med Assoc 283(9): 1175– 1182. Dunn D et al. 1992. Risk of HIV-1 transmission through breastfeeding. Lancet 340(8819): 585– 588. Gray G. 2000. The PETRA study: Early and late efficacy of three short ZDV/3 TC combinations regimens to prevent mother-to-child transmission of HIV-1. XIII International AIDS Conference, Durban, South Africa. HIV and Pregnancy 28
 References (continued) International Perinatal HIV Group. 1999. The mode of delivery and the risk of vertical transmission of human immunodeficiency virus type 1. N Engl J Med 340(14): 977– 987. Mandelbrot L et al. 1996. Obstetric factors and mother-to-child transmission of human immunodeficiency virus type 1: The French perinatal cohorts. Amer J Obstet Gynecol 175(3 pt 1): 661– 667. Semprini AE et al. 1995. The incidence of complications after cesarean section in 156 women. AIDS 9: 913– 917. Shaffer N et al. 1999. Short-course ZDV for perinatal HIV-1 transmission in Bangkok, Thailand: A randomized controlled trial. Lancet 353: 773– 780. Sperling RS et al. 1996. Maternal viral load, ZDV treatment, and the risk of transmission of HIV type 1 from mother to infant. N Engl J Med 335(22): 1621– 1629. UNICEF/UNAIDS/WHO Technical Consultation on HIV and Infant Feeding. 1998. HIV and Infant Feeding: Implementation of Guidelines. WHO: Geneva. World Health Organization (WHO)/Joint United Nations Programme on HIV/AIDS (UNAIDS). 1999. HIV In Pregnancy: A Review. WHO/UNAIDS: Geneva. HIV and Pregnancy 29
References (continued) International Perinatal HIV Group. 1999. The mode of delivery and the risk of vertical transmission of human immunodeficiency virus type 1. N Engl J Med 340(14): 977– 987. Mandelbrot L et al. 1996. Obstetric factors and mother-to-child transmission of human immunodeficiency virus type 1: The French perinatal cohorts. Amer J Obstet Gynecol 175(3 pt 1): 661– 667. Semprini AE et al. 1995. The incidence of complications after cesarean section in 156 women. AIDS 9: 913– 917. Shaffer N et al. 1999. Short-course ZDV for perinatal HIV-1 transmission in Bangkok, Thailand: A randomized controlled trial. Lancet 353: 773– 780. Sperling RS et al. 1996. Maternal viral load, ZDV treatment, and the risk of transmission of HIV type 1 from mother to infant. N Engl J Med 335(22): 1621– 1629. UNICEF/UNAIDS/WHO Technical Consultation on HIV and Infant Feeding. 1998. HIV and Infant Feeding: Implementation of Guidelines. WHO: Geneva. World Health Organization (WHO)/Joint United Nations Programme on HIV/AIDS (UNAIDS). 1999. HIV In Pregnancy: A Review. WHO/UNAIDS: Geneva. HIV and Pregnancy 29


