f9e33b99e4a1ac9ba2683626f3f75be2.ppt
- Количество слайдов: 23
 HIT Policy Committee Information Exchange Workgroup Recommendations on Standardizing Electronic Laboratory Transactions Deven Mc. Graw, Chair Center for Democracy & Technology Micky Tripathi, Co-Chair Massachusetts e. Health Collaborative December 15, 2009
HIT Policy Committee Information Exchange Workgroup Recommendations on Standardizing Electronic Laboratory Transactions Deven Mc. Graw, Chair Center for Democracy & Technology Micky Tripathi, Co-Chair Massachusetts e. Health Collaborative December 15, 2009
 Information Exchange Workgroup Members Co-Chairs: • Deven Mc. Graw, Center for Democracy & Technology • Micky Tripathi, Massachusetts e. Health Collaborative Members: • Connie White Delaney, University of Minnesota, School of Nursing • Paul Egerman, Retired • Judith Faulkner, Epic Systems Corp. • Jonah Frohlich, California Health & Human Services Agency • Dave Goetz, Tennessee Department of Finance and Administration • Gayle Harrell, Former Florida State Legislator • Charles Kennedy, Well. Point, Inc. • Michael Klag, Johns Hopkins University, Bloomberg School of Public Health • Martin Laventure, Minnesota Public Health • Frank Nemec, Gastroenterology Associates, Inc. • Steve Stack, American Medical Association • Latanya Sweeney, Carnegie-Mellon University ONC Lead: • Kelly Cronin 2
Information Exchange Workgroup Members Co-Chairs: • Deven Mc. Graw, Center for Democracy & Technology • Micky Tripathi, Massachusetts e. Health Collaborative Members: • Connie White Delaney, University of Minnesota, School of Nursing • Paul Egerman, Retired • Judith Faulkner, Epic Systems Corp. • Jonah Frohlich, California Health & Human Services Agency • Dave Goetz, Tennessee Department of Finance and Administration • Gayle Harrell, Former Florida State Legislator • Charles Kennedy, Well. Point, Inc. • Michael Klag, Johns Hopkins University, Bloomberg School of Public Health • Martin Laventure, Minnesota Public Health • Frank Nemec, Gastroenterology Associates, Inc. • Steve Stack, American Medical Association • Latanya Sweeney, Carnegie-Mellon University ONC Lead: • Kelly Cronin 2
 Executive Summary • Electronic laboratory ordering and results delivery are key elements of meaningful use, and key enablers of better patient care through health IT – Electronic lab results delivery (along with e. RX) is a pillar of 2011 ambulatory MU requirements – Quality reporting practically impossible without standards-based lab transactions • Many obstacles exist to rapid and widespread penetration of standards-based lab transactions: business, technical, regulatory – Lab market is highly decentralized – Little market imperative and no regulatory requirements exist for messaging or vocabulary standards • Federal government has a number of levers to influence the market, though decentralized nature of the market requires orchestration of a variety of levers – ARRA levers: certification, MU requirements, cooperative agreements, NHIN governance – Regulatory levers: CLIA authority – Contracting levers: CMS and FEHB contracts • IE Workgroup has a number of recommendations that, taken together, will facilitate more rapid adoption of electronic lab transactions 3
Executive Summary • Electronic laboratory ordering and results delivery are key elements of meaningful use, and key enablers of better patient care through health IT – Electronic lab results delivery (along with e. RX) is a pillar of 2011 ambulatory MU requirements – Quality reporting practically impossible without standards-based lab transactions • Many obstacles exist to rapid and widespread penetration of standards-based lab transactions: business, technical, regulatory – Lab market is highly decentralized – Little market imperative and no regulatory requirements exist for messaging or vocabulary standards • Federal government has a number of levers to influence the market, though decentralized nature of the market requires orchestration of a variety of levers – ARRA levers: certification, MU requirements, cooperative agreements, NHIN governance – Regulatory levers: CLIA authority – Contracting levers: CMS and FEHB contracts • IE Workgroup has a number of recommendations that, taken together, will facilitate more rapid adoption of electronic lab transactions 3
 Meaningful Use Requirements Electronic lab results delivery key to MU fulfillment starting in 2011 – – 2011 – structured labs requirement; quality reporting 2013 – electronic ordering Standards not necessarily required for either of these requirements, however – – Without standardization will be too costly and too difficult to achieve Quality measures and clinical decision support rely on standardized nomenclatures 4
Meaningful Use Requirements Electronic lab results delivery key to MU fulfillment starting in 2011 – – 2011 – structured labs requirement; quality reporting 2013 – electronic ordering Standards not necessarily required for either of these requirements, however – – Without standardization will be too costly and too difficult to achieve Quality measures and clinical decision support rely on standardized nomenclatures 4
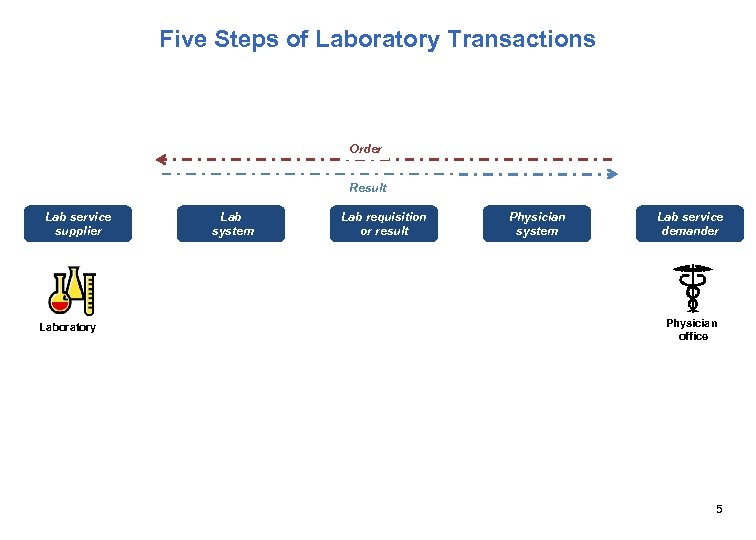 Five Steps of Laboratory Transactions Order Result Lab service supplier Laboratory Lab system Lab requisition or result Physician system Lab service demander Physician office 5
Five Steps of Laboratory Transactions Order Result Lab service supplier Laboratory Lab system Lab requisition or result Physician system Lab service demander Physician office 5
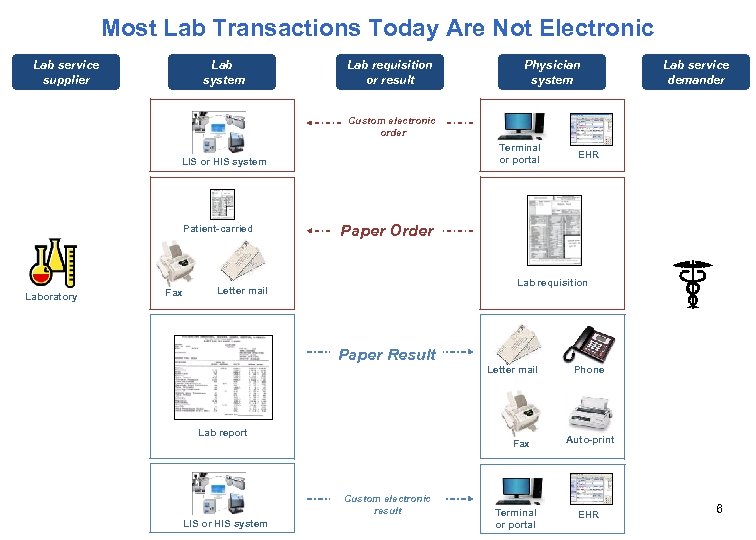 Most Lab Transactions Today Are Not Electronic Lab service supplier Lab system Lab requisition or result Physician system Lab service demander Custom electronic order Terminal or portal LIS or HIS system Patient-carried Laboratory Fax EHR Paper Order Lab requisition Letter mail Physician office Paper Result Letter mail Lab report Fax Custom electronic result LIS or HIS system Terminal or portal Phone Auto-print EHR 6
Most Lab Transactions Today Are Not Electronic Lab service supplier Lab system Lab requisition or result Physician system Lab service demander Custom electronic order Terminal or portal LIS or HIS system Patient-carried Laboratory Fax EHR Paper Order Lab requisition Letter mail Physician office Paper Result Letter mail Lab report Fax Custom electronic result LIS or HIS system Terminal or portal Phone Auto-print EHR 6
 IE Workgroup Hearing Revealed Many Systemic Issues. . . Business Technical • CLIA recognizes over 200 K labs • Messaging and vocabulary standards exist but are not monitored or enforced • Over 75% of laboratory tests conducted by hospitals and local labs • HL 7 2. 5. 1 released in 2007 but still not widely used • Most lab results delivered through paper-based means (letter, fax) • Allowable variations in HL 7 standards keeps costs high; high number of optional fields • Lab interfaces cost $5 -$25 K each • Hospital labs usually use local legacy codes; national labs closer to conforming with LOINC, but still have internal legacy-based variations • Labs typically pay EHR vendors for lab interfaces • Users typically do not pay for lab interface or delivery as long as they are high enough volume users • Interface approval process can take months, which lengthens interface time and cost Weak market incentives prevent rapid growth in standards-based lab interfacing; interfaces still not replicable and are thus timeconsuming and costly • LOINC and SNOMED still too complex for routine ambulatory implementation – not “clinically relevant” Regulatory • CLIA regulation that holds laboratory responsible for how results appear in the EHR is being interpreted differently by various lab companies and hospitals • CLIA Requirement 42 CFR 493. 1291: “The laboratory must have an adequate manual or electronic system(s) in place to ensure test results and other patient-specific data are accurately and reliably sent from the point of data entry to final report destination” • Conversion to HL 7 2. 5. 1 and LOINC would require investment by most hospitals for interface development and upgrading of systems • No regulatory requirements or market imperatives currently exist for messaging or vocabulary standards either on the receiving or on the transmitting ends • No standard automated way to update compendiums; usually happens manually after fax notification • Patient access to lab results not established in law or regulation High degree of allowable variation in current messaging and vocabulary standards makes interfacing time-consuming and costly Interpretation of legal requirements across states and lab sources varies widely; no requirements exist for messaging or vocabulary standards, and no ability to monitor/enforce standards 7
IE Workgroup Hearing Revealed Many Systemic Issues. . . Business Technical • CLIA recognizes over 200 K labs • Messaging and vocabulary standards exist but are not monitored or enforced • Over 75% of laboratory tests conducted by hospitals and local labs • HL 7 2. 5. 1 released in 2007 but still not widely used • Most lab results delivered through paper-based means (letter, fax) • Allowable variations in HL 7 standards keeps costs high; high number of optional fields • Lab interfaces cost $5 -$25 K each • Hospital labs usually use local legacy codes; national labs closer to conforming with LOINC, but still have internal legacy-based variations • Labs typically pay EHR vendors for lab interfaces • Users typically do not pay for lab interface or delivery as long as they are high enough volume users • Interface approval process can take months, which lengthens interface time and cost Weak market incentives prevent rapid growth in standards-based lab interfacing; interfaces still not replicable and are thus timeconsuming and costly • LOINC and SNOMED still too complex for routine ambulatory implementation – not “clinically relevant” Regulatory • CLIA regulation that holds laboratory responsible for how results appear in the EHR is being interpreted differently by various lab companies and hospitals • CLIA Requirement 42 CFR 493. 1291: “The laboratory must have an adequate manual or electronic system(s) in place to ensure test results and other patient-specific data are accurately and reliably sent from the point of data entry to final report destination” • Conversion to HL 7 2. 5. 1 and LOINC would require investment by most hospitals for interface development and upgrading of systems • No regulatory requirements or market imperatives currently exist for messaging or vocabulary standards either on the receiving or on the transmitting ends • No standard automated way to update compendiums; usually happens manually after fax notification • Patient access to lab results not established in law or regulation High degree of allowable variation in current messaging and vocabulary standards makes interfacing time-consuming and costly Interpretation of legal requirements across states and lab sources varies widely; no requirements exist for messaging or vocabulary standards, and no ability to monitor/enforce standards 7
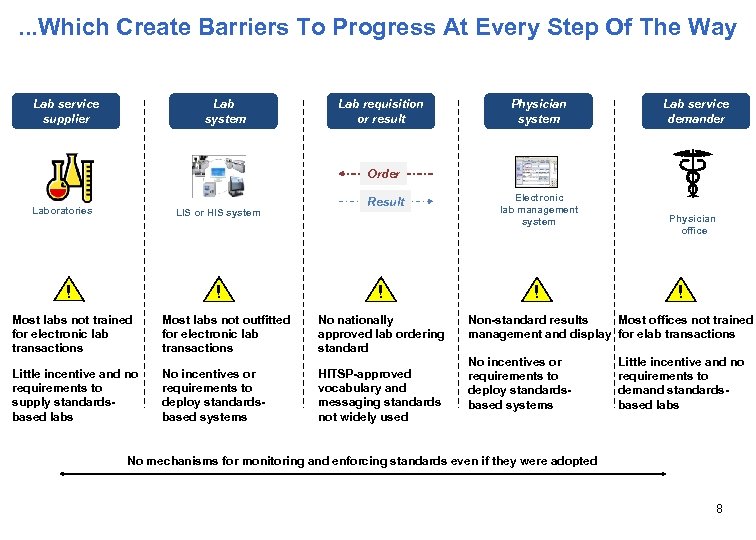 . . . Which Create Barriers To Progress At Every Step Of The Way Lab service supplier Lab system Lab requisition or result Physician system Lab service demander Order Laboratories LIS or HIS system ! ! Result ! Most labs not trained for electronic lab transactions Most labs not outfitted for electronic lab transactions No nationally approved lab ordering standard Little incentive and no requirements to supply standardsbased labs No incentives or requirements to deploy standardsbased systems HITSP-approved vocabulary and messaging standards not widely used Electronic lab management system ! Physician office ! Non-standard results Most offices not trained management and display for elab transactions No incentives or requirements to deploy standardsbased systems Little incentive and no requirements to demand standardsbased labs No mechanisms for monitoring and enforcing standards even if they were adopted 8
. . . Which Create Barriers To Progress At Every Step Of The Way Lab service supplier Lab system Lab requisition or result Physician system Lab service demander Order Laboratories LIS or HIS system ! ! Result ! Most labs not trained for electronic lab transactions Most labs not outfitted for electronic lab transactions No nationally approved lab ordering standard Little incentive and no requirements to supply standardsbased labs No incentives or requirements to deploy standardsbased systems HITSP-approved vocabulary and messaging standards not widely used Electronic lab management system ! Physician office ! Non-standard results Most offices not trained management and display for elab transactions No incentives or requirements to deploy standardsbased systems Little incentive and no requirements to demand standardsbased labs No mechanisms for monitoring and enforcing standards even if they were adopted 8
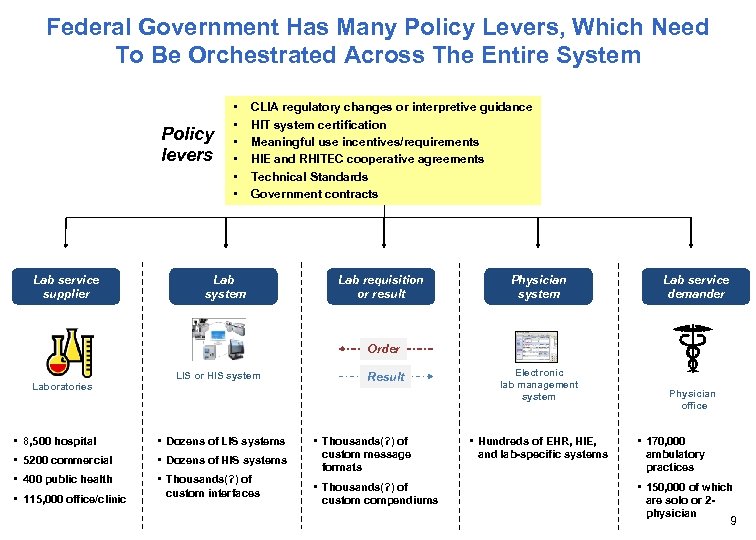 Federal Government Has Many Policy Levers, Which Need To Be Orchestrated Across The Entire System Policy levers Lab service supplier • • • CLIA regulatory changes or interpretive guidance HIT system certification Meaningful use incentives/requirements HIE and RHITEC cooperative agreements Technical Standards Government contracts Lab system Lab requisition or result Physician system Lab service demander Order Laboratories LIS or HIS system • 8, 500 hospital • Dozens of LIS systems • 5200 commercial • Dozens of HIS systems • 400 public health • Thousands(? ) of custom interfaces • 115, 000 office/clinic Result • Thousands(? ) of custom message formats • Thousands(? ) of custom compendiums Electronic lab management system • Hundreds of EHR, HIE, and lab-specific systems Physician office • 170, 000 ambulatory practices • 150, 000 of which are solo or 2 physician 9
Federal Government Has Many Policy Levers, Which Need To Be Orchestrated Across The Entire System Policy levers Lab service supplier • • • CLIA regulatory changes or interpretive guidance HIT system certification Meaningful use incentives/requirements HIE and RHITEC cooperative agreements Technical Standards Government contracts Lab system Lab requisition or result Physician system Lab service demander Order Laboratories LIS or HIS system • 8, 500 hospital • Dozens of LIS systems • 5200 commercial • Dozens of HIS systems • 400 public health • Thousands(? ) of custom interfaces • 115, 000 office/clinic Result • Thousands(? ) of custom message formats • Thousands(? ) of custom compendiums Electronic lab management system • Hundreds of EHR, HIE, and lab-specific systems Physician office • 170, 000 ambulatory practices • 150, 000 of which are solo or 2 physician 9
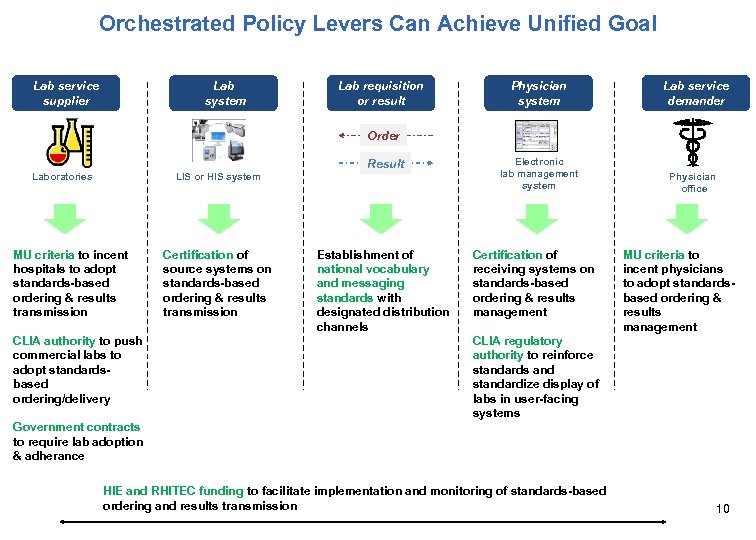 Orchestrated Policy Levers Can Achieve Unified Goal Lab service supplier Lab system Lab requisition or result Physician system Lab service demander Electronic lab management system Physician office Order Result Laboratories LIS or HIS system MU criteria to incent hospitals to adopt standards-based ordering & results transmission CLIA authority to push commercial labs to adopt standardsbased ordering/delivery Certification of source systems on standards-based ordering & results transmission Establishment of national vocabulary and messaging standards with designated distribution channels Certification of receiving systems on standards-based ordering & results management MU criteria to incent physicians to adopt standardsbased ordering & results management CLIA regulatory authority to reinforce standards and standardize display of labs in user-facing systems Government contracts to require lab adoption & adherance HIE and RHITEC funding to facilitate implementation and monitoring of standards-based ordering and results transmission 10
Orchestrated Policy Levers Can Achieve Unified Goal Lab service supplier Lab system Lab requisition or result Physician system Lab service demander Electronic lab management system Physician office Order Result Laboratories LIS or HIS system MU criteria to incent hospitals to adopt standards-based ordering & results transmission CLIA authority to push commercial labs to adopt standardsbased ordering/delivery Certification of source systems on standards-based ordering & results transmission Establishment of national vocabulary and messaging standards with designated distribution channels Certification of receiving systems on standards-based ordering & results management MU criteria to incent physicians to adopt standardsbased ordering & results management CLIA regulatory authority to reinforce standards and standardize display of labs in user-facing systems Government contracts to require lab adoption & adherance HIE and RHITEC funding to facilitate implementation and monitoring of standards-based ordering and results transmission 10
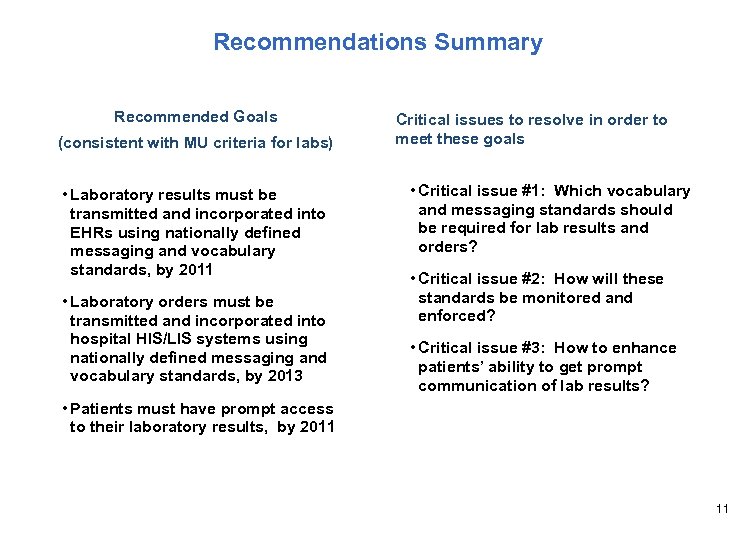 Recommendations Summary Recommended Goals (consistent with MU criteria for labs) • Laboratory results must be transmitted and incorporated into EHRs using nationally defined messaging and vocabulary standards, by 2011 • Laboratory orders must be transmitted and incorporated into hospital HIS/LIS systems using nationally defined messaging and vocabulary standards, by 2013 Critical issues to resolve in order to meet these goals • Critical issue #1: Which vocabulary and messaging standards should be required for lab results and orders? • Critical issue #2: How will these standards be monitored and enforced? • Critical issue #3: How to enhance patients’ ability to get prompt communication of lab results? • Patients must have prompt access to their laboratory results, by 2011 11
Recommendations Summary Recommended Goals (consistent with MU criteria for labs) • Laboratory results must be transmitted and incorporated into EHRs using nationally defined messaging and vocabulary standards, by 2011 • Laboratory orders must be transmitted and incorporated into hospital HIS/LIS systems using nationally defined messaging and vocabulary standards, by 2013 Critical issues to resolve in order to meet these goals • Critical issue #1: Which vocabulary and messaging standards should be required for lab results and orders? • Critical issue #2: How will these standards be monitored and enforced? • Critical issue #3: How to enhance patients’ ability to get prompt communication of lab results? • Patients must have prompt access to their laboratory results, by 2011 11
 CRITICAL ISSUE #1: Which Vocabulary and Messaging Standards Should be Required? Discussion The Standards Committee in September approved specific messaging and vocabulary standards for lab results in 2011, with temporary exceptions for local codes and pre-existing lab interfaces in 2011 and 2012. This development was not flagged for us by the stakeholders testifying at the hearing, so the full impact of the Standards Committee recommendations may not be widely understood. Current work underway through the Standards Committee and HITSP will address other standards issues identified by the IE WG • Identification of nationally-approved standards for electronic ordering • Identification of nationally-approved standard for electronic updating of lab dictionaries • Improved implementation assistance, clear communication of requirements, and education are needed for lab vocabulary standards to be widely adopted. 12
CRITICAL ISSUE #1: Which Vocabulary and Messaging Standards Should be Required? Discussion The Standards Committee in September approved specific messaging and vocabulary standards for lab results in 2011, with temporary exceptions for local codes and pre-existing lab interfaces in 2011 and 2012. This development was not flagged for us by the stakeholders testifying at the hearing, so the full impact of the Standards Committee recommendations may not be widely understood. Current work underway through the Standards Committee and HITSP will address other standards issues identified by the IE WG • Identification of nationally-approved standards for electronic ordering • Identification of nationally-approved standard for electronic updating of lab dictionaries • Improved implementation assistance, clear communication of requirements, and education are needed for lab vocabulary standards to be widely adopted. 12
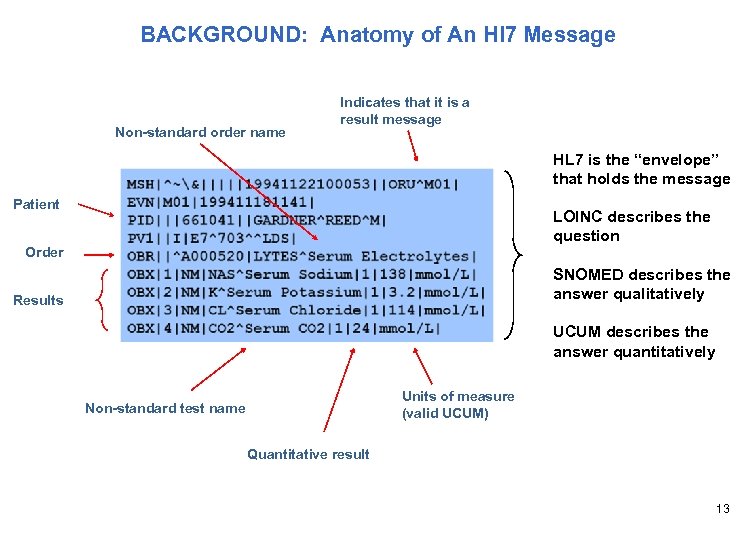 BACKGROUND: Anatomy of An Hl 7 Message Non-standard order name Indicates that it is a result message HL 7 is the “envelope” that holds the message Patient LOINC describes the question Order SNOMED describes the answer qualitatively Results UCUM describes the answer quantitatively Units of measure (valid UCUM) Non-standard test name Quantitative result 13
BACKGROUND: Anatomy of An Hl 7 Message Non-standard order name Indicates that it is a result message HL 7 is the “envelope” that holds the message Patient LOINC describes the question Order SNOMED describes the answer qualitatively Results UCUM describes the answer quantitatively Units of measure (valid UCUM) Non-standard test name Quantitative result 13
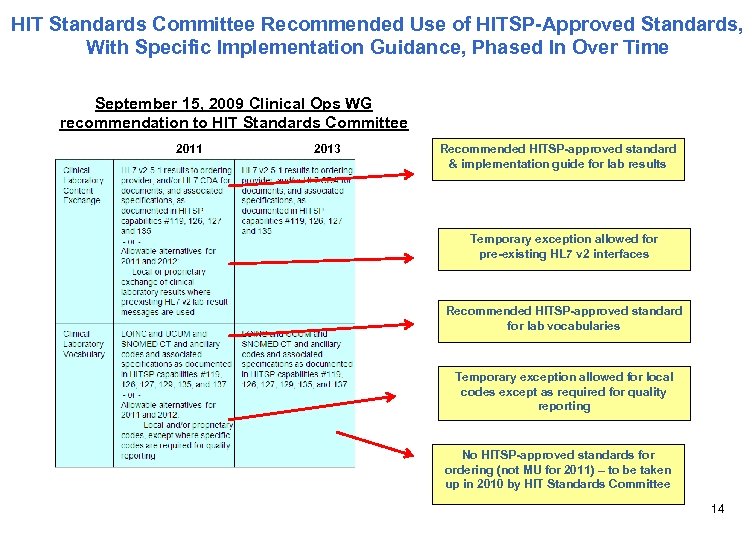 HIT Standards Committee Recommended Use of HITSP-Approved Standards, With Specific Implementation Guidance, Phased In Over Time September 15, 2009 Clinical Ops WG recommendation to HIT Standards Committee 2011 2013 Recommended HITSP-approved standard & implementation guide for lab results Temporary exception allowed for pre-existing HL 7 v 2 interfaces Recommended HITSP-approved standard for lab vocabularies Temporary exception allowed for local codes except as required for quality reporting No HITSP-approved standards for ordering (not MU for 2011) – to be taken up in 2010 by HIT Standards Committee 14
HIT Standards Committee Recommended Use of HITSP-Approved Standards, With Specific Implementation Guidance, Phased In Over Time September 15, 2009 Clinical Ops WG recommendation to HIT Standards Committee 2011 2013 Recommended HITSP-approved standard & implementation guide for lab results Temporary exception allowed for pre-existing HL 7 v 2 interfaces Recommended HITSP-approved standard for lab vocabularies Temporary exception allowed for local codes except as required for quality reporting No HITSP-approved standards for ordering (not MU for 2011) – to be taken up in 2010 by HIT Standards Committee 14
 CRITICAL ISSUE #1: Which vocabulary and messaging standards should be required? Recommendations ONC should require national standards for messaging, vocabulary, and measure codes, and create means for widespread availability of authorized implementation guides and code-sets. 15
CRITICAL ISSUE #1: Which vocabulary and messaging standards should be required? Recommendations ONC should require national standards for messaging, vocabulary, and measure codes, and create means for widespread availability of authorized implementation guides and code-sets. 15
 CRITICAL ISSUE #1: Which vocabulary and messaging standards should be required? Hit the Essentials in 2011 • Make mandatory the Standards Committee recommendations proposed in September 2009 but without the allowable alternatives for local HL 7 interfaces and local codes in 2011. To us this means: – Required vocabulary standards (LOINC, UCUM, SNOMED CT) cover “most frequent” results, and define a simplified “clinically relevant” vocabulary subset corresponding to those results – Required HL 7 messaging standards focus specifically on meaningful use transactions for treatment, quality measurement, and public health – Reduced optional fields • Standard should be mandatory, but meaningful use compliance measures may need to be flexible to allow for time to incorporate system changes More Comprehensive approach in 2013 • The Standards Committee should define simplified “clinically relevant” lab order compendium for a national laboratory vocabulary. The Committee also should simplify other lab vocabulary standards in subsets and value sets that are “clinically relevant”. (We understand this work is already underway. ) 16
CRITICAL ISSUE #1: Which vocabulary and messaging standards should be required? Hit the Essentials in 2011 • Make mandatory the Standards Committee recommendations proposed in September 2009 but without the allowable alternatives for local HL 7 interfaces and local codes in 2011. To us this means: – Required vocabulary standards (LOINC, UCUM, SNOMED CT) cover “most frequent” results, and define a simplified “clinically relevant” vocabulary subset corresponding to those results – Required HL 7 messaging standards focus specifically on meaningful use transactions for treatment, quality measurement, and public health – Reduced optional fields • Standard should be mandatory, but meaningful use compliance measures may need to be flexible to allow for time to incorporate system changes More Comprehensive approach in 2013 • The Standards Committee should define simplified “clinically relevant” lab order compendium for a national laboratory vocabulary. The Committee also should simplify other lab vocabulary standards in subsets and value sets that are “clinically relevant”. (We understand this work is already underway. ) 16
 CRITICAL ISSUE #2: How will these standards be monitored and enforced? Discussion Monitoring and enforcement of lab messaging and vocabulary standards is made difficult by: • Lack of certification and testing of lab receiving and transmitting systems • Lack of requirements to use such standards • Regulatory organizations not “in stream” of day-to-day transactions Commercial labs are not subject to MU requirements, so alignment of CLIA authorities is essential Monitoring and enforcement of standards-based lab transactions will be critical to success moving forward since current business processes have no “built-in” mechanism for monitoring and enforcing lab transactions. 17
CRITICAL ISSUE #2: How will these standards be monitored and enforced? Discussion Monitoring and enforcement of lab messaging and vocabulary standards is made difficult by: • Lack of certification and testing of lab receiving and transmitting systems • Lack of requirements to use such standards • Regulatory organizations not “in stream” of day-to-day transactions Commercial labs are not subject to MU requirements, so alignment of CLIA authorities is essential Monitoring and enforcement of standards-based lab transactions will be critical to success moving forward since current business processes have no “built-in” mechanism for monitoring and enforcing lab transactions. 17
 CRITICAL ISSUE #2: How will these standards be monitored and enforced? Recommendations (1) Enforcement regarding EHRs and Hospital Laboratories • Certification: Use of these standards by providers should be required for meaningful use (for receipt of results in 2011 and for orders in 2013). Thus, EHR (and EHR components pulled together by HIEs) certification requirements for 2011 should include the ability to incorporate lab results using these standards, and to transmit orders in accordance with these standards by 2013. Such certification should cover the interface with the laboratory, and include testing of the system/components and the interface to ensure compliance • Hospital MU requirements: Include standards-based laboratory results transmission to ambulatory physicians as a hospital (inpatient) meaningful use requirement for 2011. Hospitals should then be required to have their in-house HIS, laboratory, or interfacing systems certified to transmit according to the same messaging and vocabulary standards that ambulatory physicians and EHRs are required to receive. Allow standards-based delivery through certified HIE products (interoperability components) to satisfy this certification requirement. 18
CRITICAL ISSUE #2: How will these standards be monitored and enforced? Recommendations (1) Enforcement regarding EHRs and Hospital Laboratories • Certification: Use of these standards by providers should be required for meaningful use (for receipt of results in 2011 and for orders in 2013). Thus, EHR (and EHR components pulled together by HIEs) certification requirements for 2011 should include the ability to incorporate lab results using these standards, and to transmit orders in accordance with these standards by 2013. Such certification should cover the interface with the laboratory, and include testing of the system/components and the interface to ensure compliance • Hospital MU requirements: Include standards-based laboratory results transmission to ambulatory physicians as a hospital (inpatient) meaningful use requirement for 2011. Hospitals should then be required to have their in-house HIS, laboratory, or interfacing systems certified to transmit according to the same messaging and vocabulary standards that ambulatory physicians and EHRs are required to receive. Allow standards-based delivery through certified HIE products (interoperability components) to satisfy this certification requirement. 18
 CRITICAL ISSUE #2: How will these standards be monitored and enforced? Recommendations (2) Enforcement for Laboratories: • CMS CLIA Office should issue a survey and certification letter for laboratories that would include: - Interpretive guidance for presentation of lab information in user-facing applications (EHRs, HIEs, and PHRs) - Interpretive guidance for interfacing reflecting the messaging and vocabulary standards set forth above - Best practices reinforcing the above guidance • The sending of results using these messaging and vocabulary standards should be deemed acceptable for meeting the criteria of presentation of lab results in user facing applications • This survey and certification letter should deem EHR certification as demonstration of adherence to the guidance, which should eliminate the need for labs to test each EHR implementation. 19
CRITICAL ISSUE #2: How will these standards be monitored and enforced? Recommendations (2) Enforcement for Laboratories: • CMS CLIA Office should issue a survey and certification letter for laboratories that would include: - Interpretive guidance for presentation of lab information in user-facing applications (EHRs, HIEs, and PHRs) - Interpretive guidance for interfacing reflecting the messaging and vocabulary standards set forth above - Best practices reinforcing the above guidance • The sending of results using these messaging and vocabulary standards should be deemed acceptable for meeting the criteria of presentation of lab results in user facing applications • This survey and certification letter should deem EHR certification as demonstration of adherence to the guidance, which should eliminate the need for labs to test each EHR implementation. 19
 CRITICAL ISSUE #2: How will these standards be monitored and enforced? Recommendations (3) Alignment of national and state rules and enforcement: • Require State HIT Coordinator to work with state CLIA administrators to align statelevel lab approach with national CLIA, standards, and certification requirements. • Request NGA analysis of state variation in CLIA rules and enforcement, and recommendations on what policy levers state governments have on lab rules and enforcement. 20
CRITICAL ISSUE #2: How will these standards be monitored and enforced? Recommendations (3) Alignment of national and state rules and enforcement: • Require State HIT Coordinator to work with state CLIA administrators to align statelevel lab approach with national CLIA, standards, and certification requirements. • Request NGA analysis of state variation in CLIA rules and enforcement, and recommendations on what policy levers state governments have on lab rules and enforcement. 20
 CRITICAL ISSUE #2: How will these standards be monitored and enforced? Recommendation (4) Monitoring and enforcement for Labs: Monitoring and enforcement are critical to achieving a breakthrough in electronic laboratory transactions. ONC should develop an approach for ensuring ongoing adherence to national standards ONC has a variety of ways that this could be accomplished, some of which might be: • Require regular testing of source system through national test harnesses created for certification programs • Require state HIEs/HIOs as part of State-level HIE Cooperative Agreements to require the use of the national standards in any relevant services funded by the Cooperative Agreement (applicable to those who exchange lab data through HIEs, including hospital and state regional labs). (Note that HIEs/HIOs could also create lab transaction monitoring capabilities, such as interface testing platforms and periodic testing processes, and offer them as a service. ) • Require Regional Health IT Extension Centers through their Cooperative Agreements to require the use of national standards in their procurements. RHITECs can also help with education and clear communication of standards. 21
CRITICAL ISSUE #2: How will these standards be monitored and enforced? Recommendation (4) Monitoring and enforcement for Labs: Monitoring and enforcement are critical to achieving a breakthrough in electronic laboratory transactions. ONC should develop an approach for ensuring ongoing adherence to national standards ONC has a variety of ways that this could be accomplished, some of which might be: • Require regular testing of source system through national test harnesses created for certification programs • Require state HIEs/HIOs as part of State-level HIE Cooperative Agreements to require the use of the national standards in any relevant services funded by the Cooperative Agreement (applicable to those who exchange lab data through HIEs, including hospital and state regional labs). (Note that HIEs/HIOs could also create lab transaction monitoring capabilities, such as interface testing platforms and periodic testing processes, and offer them as a service. ) • Require Regional Health IT Extension Centers through their Cooperative Agreements to require the use of national standards in their procurements. RHITECs can also help with education and clear communication of standards. 21
 CRITICAL ISSUE #2: How will these standards be monitored and enforced? Recommendation (5) Enforce and enhance federal contractual and subregulatory provisions: Enforcement • Existing CMS call letter requirements that require health plans to seek contracted laboratory adoption of recognized lab result interoperability standards should be the subject of guidance letters, compliance audits and corrective action plans • Existing FEHB carrier requirements to implement recognized lab result interoperability standards should be the subject of an enforcement program (States could also impose such requirements as part of state employee health benefit plans. ) Enhancement • When electronic systems are used, laboratory providers should be required to implement federal lab interoperability standards consistent with MU as a condition of participation in CMS programs including Medicare and Medicaid • Federal contract provisions requiring adoption and implementation of clinical laboratory interoperability standards should be aligned with MU requirements and made consistent across federal departments including OPM, HHS, Do. D, VA and other relevant entities, so that providers, labs and others who voluntarily contract with federal agencies are not subjected to varying requirements 22
CRITICAL ISSUE #2: How will these standards be monitored and enforced? Recommendation (5) Enforce and enhance federal contractual and subregulatory provisions: Enforcement • Existing CMS call letter requirements that require health plans to seek contracted laboratory adoption of recognized lab result interoperability standards should be the subject of guidance letters, compliance audits and corrective action plans • Existing FEHB carrier requirements to implement recognized lab result interoperability standards should be the subject of an enforcement program (States could also impose such requirements as part of state employee health benefit plans. ) Enhancement • When electronic systems are used, laboratory providers should be required to implement federal lab interoperability standards consistent with MU as a condition of participation in CMS programs including Medicare and Medicaid • Federal contract provisions requiring adoption and implementation of clinical laboratory interoperability standards should be aligned with MU requirements and made consistent across federal departments including OPM, HHS, Do. D, VA and other relevant entities, so that providers, labs and others who voluntarily contract with federal agencies are not subjected to varying requirements 22
 CRITICAL ISSUE #3: How to ensure that patients can get prompt access to lab results? Discussion A study published in the Archives of Internal Medicine in June 2009 demonstrated that patients don’t always receive their lab results, even in cases where the results are clinically significant. The workgroup received testimony recommending changes in the law to allow patients to receive laboratory results directly from the lab (which occurs today only in states that permit patients to directly receive lab test results). Under the Policy Committee’s Meaningful Use Matrix, objectives for 2011 include providing patients with timely electronic access to, and upon request an electronic copy of, their health information, specifically including lab results. ARRA also requires that providers using electronic health records provide patients with electronic copies upon request – and patients can have that copy sent directly to another person or entity (such as a PHR). To ensure patients are getting timely access to lab results, Committee should consider whether the CMS meaningful use rule in 2011 and ONC interim final standards rule facilitate patient electronic receipt of lab results. 23
CRITICAL ISSUE #3: How to ensure that patients can get prompt access to lab results? Discussion A study published in the Archives of Internal Medicine in June 2009 demonstrated that patients don’t always receive their lab results, even in cases where the results are clinically significant. The workgroup received testimony recommending changes in the law to allow patients to receive laboratory results directly from the lab (which occurs today only in states that permit patients to directly receive lab test results). Under the Policy Committee’s Meaningful Use Matrix, objectives for 2011 include providing patients with timely electronic access to, and upon request an electronic copy of, their health information, specifically including lab results. ARRA also requires that providers using electronic health records provide patients with electronic copies upon request – and patients can have that copy sent directly to another person or entity (such as a PHR). To ensure patients are getting timely access to lab results, Committee should consider whether the CMS meaningful use rule in 2011 and ONC interim final standards rule facilitate patient electronic receipt of lab results. 23


