b063e24de0aa63a7f4ca57f7831a8301.ppt
- Количество слайдов: 56

Highlights From Berlin 2011 clinicaloptions. com/hepatitis Highlights From Berlin 2011 CCO Independent Conference Coverage of the 46 th Annual Meeting of the European Association for the Study of the Liver* March 30 - April 3, 2011 Berlin, Germany *CCO is an independent medical education company that provides state-of-the-art medical information to healthcare professionals through conference coverage and other educational programs. This program is supported by educational grants from This program is supported by an educational grant from

Highlights From Berlin 2011 clinicaloptions. com/hepatitis About These Slides Our thanks to the presenters who gave permission to include their original data Users are encouraged to use these slides in their own noncommercial presentations, but we ask that content and attribution not be changed. Users are asked to honor this intent These slides may not be published or posted online without permission from Clinical Care Options (email permissions@clinicaloptions. com) Disclaimer The materials published on the Clinical Care Options Web site reflect the views of the authors of the CCO material, not those of Clinical Care Options, LLC, the CME providers, or the companies providing educational grants. The materials may discuss uses and dosages for therapeutic products that have not been approved by the United States Food and Drug Administration. A qualified healthcare professional should be consulted before using any therapeutic product discussed. Readers should verify all information and data before treating patients or using any therapies described in these materials.

Highlights From Berlin 2011 clinicaloptions. com/hepatitis Faculty Nezam H. Afdhal, MD, FRCPI Associate Professor of Medicine Harvard Medical School Chief of Hepatology Beth Israel Deaconess Medical Center Boston, Massachusetts Paul Martin, MD Professor of Medicine Chief, Division of Hepatology Center for Liver Disease University of Miami School of Medicine Miami, Florida

Highlights From Berlin 2011 clinicaloptions. com/hepatitis Disclosures Nezam H. Afdhal, MD, FRCPI, has disclosed that he has received consulting fees and contracted research with Glaxo. Smith. Kline, Merck, Pharmasset, Spring Bank, and Vertex. Paul Martin, MD, has disclosed that he has received consulting fees from Bristol-Myers Squibb, Genentech, Merck, Salix, and Vertex; fees for non-CME services from Bristol-Myers Squibb and Vertex; and contracted research with Bristol-Myers Squibb, Gilead Sciences, Genentech, and Vertex.

Phase III Studies of Protease Inhibitors
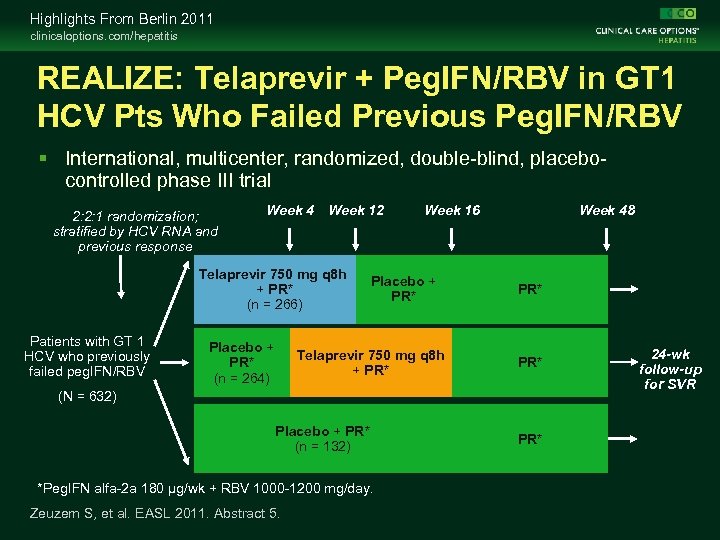
Highlights From Berlin 2011 clinicaloptions. com/hepatitis REALIZE: Telaprevir + Peg. IFN/RBV in GT 1 HCV Pts Who Failed Previous Peg. IFN/RBV International, multicenter, randomized, double-blind, placebocontrolled phase III trial 2: 2: 1 randomization; stratified by HCV RNA and previous response Week 4 Week 12 Telaprevir 750 mg q 8 h + PR* (n = 266) Patients with GT 1 HCV who previously failed peg. IFN/RBV Placebo + PR* (n = 264) Week 16 Placebo + PR* Telaprevir 750 mg q 8 h + PR* Week 48 PR* (N = 632) Placebo + PR* (n = 132) *Peg. IFN alfa-2 a 180 µg/wk + RBV 1000 -1200 mg/day. Zeuzem S, et al. EASL 2011. Abstract 5. PR* 24 -wk follow-up for SVR
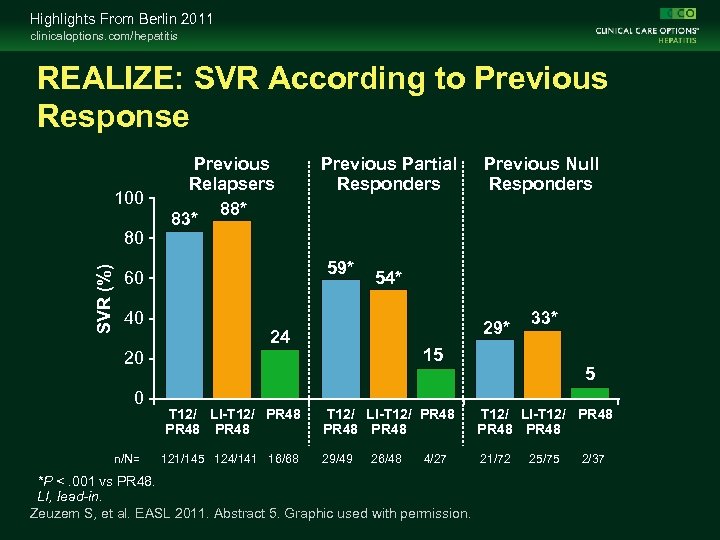
Highlights From Berlin 2011 clinicaloptions. com/hepatitis REALIZE: SVR According to Previous Response 100 SVR (%) 80 Previous Relapsers 88* 83* 59* 60 40 Previous Partial Responders 54* 29* 24 n/N= T 12/ LI-T 12/ PR 48 121/145 124/141 16/68 33* 15 20 0 Previous Null Responders 5 T 12/ LI-T 12/ PR 48 PR 48 29/49 21/72 26/48 4/27 *P <. 001 vs PR 48. LI, lead-in. Zeuzem S, et al. EASL 2011. Abstract 5. Graphic used with permission. 25/75 2/37
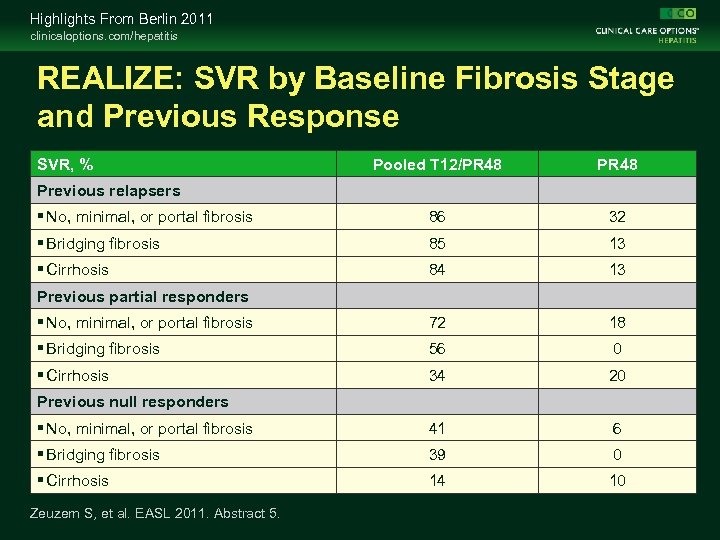
Highlights From Berlin 2011 clinicaloptions. com/hepatitis REALIZE: SVR by Baseline Fibrosis Stage and Previous Response SVR, % Pooled T 12/PR 48 No, minimal, or portal fibrosis 86 32 Bridging fibrosis 85 13 Cirrhosis 84 13 No, minimal, or portal fibrosis 72 18 Bridging fibrosis 56 0 Cirrhosis 34 20 No, minimal, or portal fibrosis 41 6 Bridging fibrosis 39 0 Cirrhosis 14 10 Previous relapsers Previous partial responders Previous null responders Zeuzem S, et al. EASL 2011. Abstract 5.
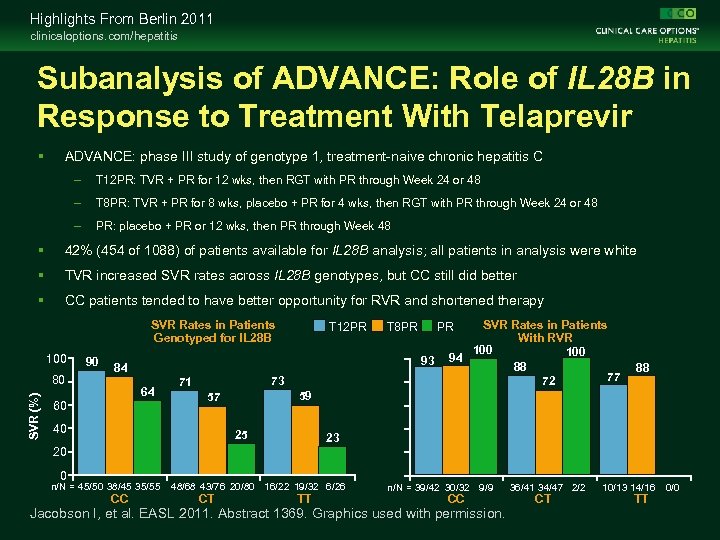
Highlights From Berlin 2011 clinicaloptions. com/hepatitis Subanalysis of ADVANCE: Role of IL 28 B in Response to Treatment With Telaprevir ADVANCE: phase III study of genotype 1, treatment-naive chronic hepatitis C – T 12 PR: TVR + PR for 12 wks, then RGT with PR through Week 24 or 48 – T 8 PR: TVR + PR for 8 wks, placebo + PR for 4 wks, then RGT with PR through Week 24 or 48 – PR: placebo + PR or 12 wks, then PR through Week 48 42% (454 of 1088) of patients available for IL 28 B analysis; all patients in analysis were white TVR increased SVR rates across IL 28 B genotypes, but CC still did better CC patients tended to have better opportunity for RVR and shortened therapy SVR Rates in Patients Genotyped for IL 28 B 100 SVR (%) 80 90 T 12 PR T 8 PR 93 84 64 60 73 71 88 59 57 40 SVR Rates in Patients With RVR 100 94 88 77 72 PR 25 23 20 0 n/N = 45/50 38/45 35/55 CC 48/68 43/76 20/80 16/22 19/32 6/26 CT TT n/N = 39/42 30/32 9/9 CC Jacobson I, et al. EASL 2011. Abstract 1369. Graphics used with permission. 36/41 34/47 2/2 CT 10/13 14/16 TT 0/0
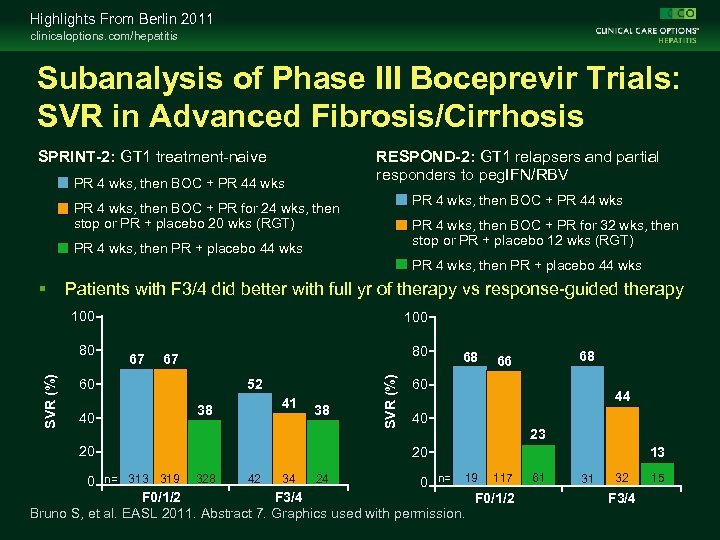
Highlights From Berlin 2011 clinicaloptions. com/hepatitis Subanalysis of Phase III Boceprevir Trials: SVR in Advanced Fibrosis/Cirrhosis SPRINT-2: GT 1 treatment-naive RESPOND-2: GT 1 relapsers and partial responders to peg. IFN/RBV PR 4 wks, then BOC + PR 44 wks PR 4 wks, then BOC + PR for 24 wks, then stop or PR + placebo 20 wks (RGT) PR 4 wks, then BOC + PR for 32 wks, then stop or PR + placebo 12 wks (RGT) PR 4 wks, then PR + placebo 44 wks Patients with F 3/4 did better with full yr of therapy vs response-guided therapy 100 67 80 67 60 40 20 52 38 41 38 SVR (%) 80 100 68 68 66 60 44 40 23 20 42 34 24 0 n= 313 319 328 0 n= 19 117 F 0/1/2 F 3/4 F 0/1/2 Bruno S, et al. EASL 2011. Abstract 7. Graphics used with permission. 13 61 31 32 F 3/4 15
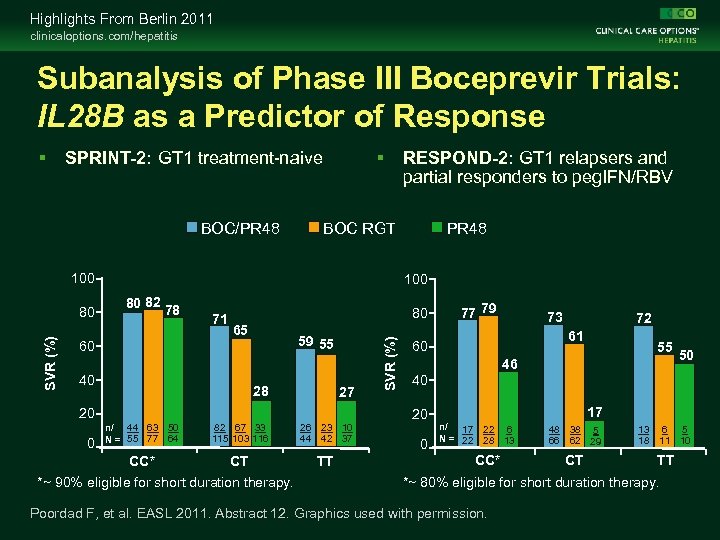
Highlights From Berlin 2011 clinicaloptions. com/hepatitis Subanalysis of Phase III Boceprevir Trials: IL 28 B as a Predictor of Response SPRINT-2: GT 1 treatment-naive BOC/PR 48 BOC RGT 100 80 82 78 71 80 65 59 55 60 40 28 27 20 82 67 33 115 103 116 CT *~ 90% eligible for short duration therapy. 26 44 23 10 42 37 TT 77 79 73 72 61 60 55 46 50 40 20 n/ 44 63 50 N = 55 77 64 CC* 0 PR 48 100 SVR (%) 80 RESPOND-2: GT 1 relapsers and partial responders to peg. IFN/RBV 0 17 n/ 17 N = 22 22 6 28 13 CC* 48 38 5 66 62 29 CT 13 18 6 5 11 10 TT *~ 80% eligible for short duration therapy. Poordad F, et al. EASL 2011. Abstract 12. Graphics used with permission.
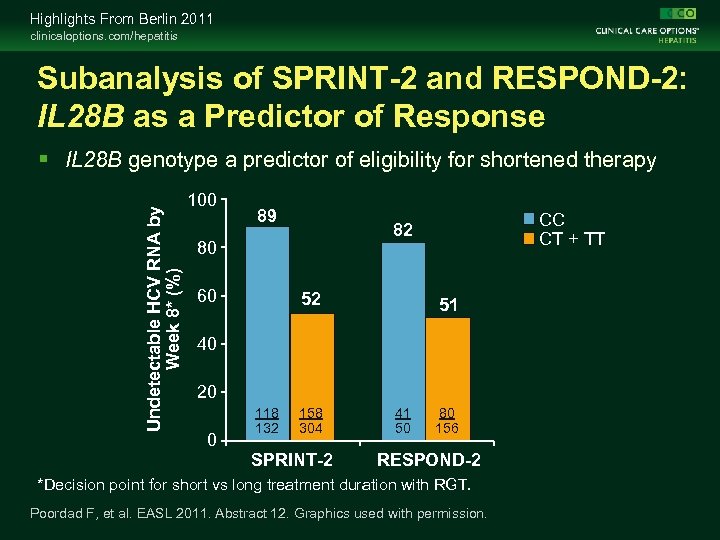
Highlights From Berlin 2011 clinicaloptions. com/hepatitis Subanalysis of SPRINT-2 and RESPOND-2: IL 28 B as a Predictor of Response Undetectable HCV RNA by Week 8* (%) IL 28 B genotype a predictor of eligibility for shortened therapy 100 89 80 60 CC CT + TT 82 52 51 40 20 0 118 132 158 304 SPRINT-2 41 50 80 156 RESPOND-2 *Decision point for short vs long treatment duration with RGT. Poordad F, et al. EASL 2011. Abstract 12. Graphics used with permission.
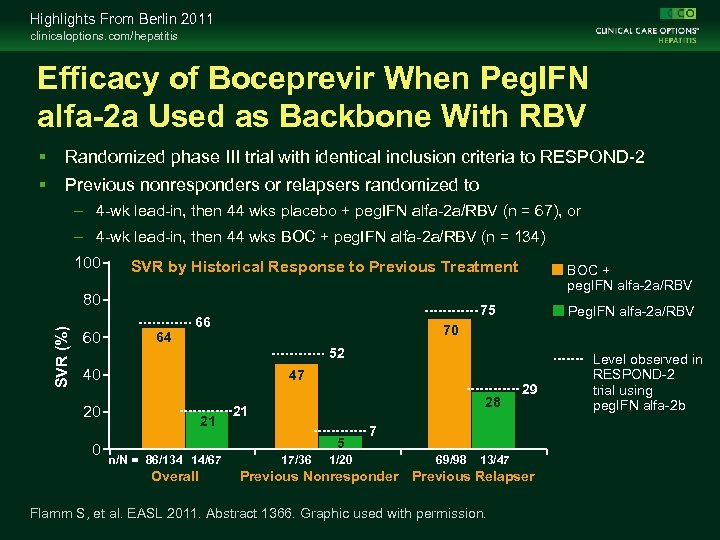
Highlights From Berlin 2011 clinicaloptions. com/hepatitis Efficacy of Boceprevir When Peg. IFN alfa-2 a Used as Backbone With RBV Randomized phase III trial with identical inclusion criteria to RESPOND-2 Previous nonresponders or relapsers randomized to – 4 -wk lead-in, then 44 wks placebo + peg. IFN alfa-2 a/RBV (n = 67), or – 4 -wk lead-in, then 44 wks BOC + peg. IFN alfa-2 a/RBV (n = 134) 100 SVR by Historical Response to Previous Treatment SVR (%) 80 60 64 75 66 Peg. IFN alfa-2 a/RBV 70 52 40 47 20 0 BOC + peg. IFN alfa-2 a/RBV 21 28 21 5 n/N = 86/134 14/67 Overall 17/36 1/20 29 7 69/98 13/47 Previous Nonresponder Previous Relapser Flamm S, et al. EASL 2011. Abstract 1366. Graphic used with permission. Level observed in RESPOND-2 trial using peg. IFN alfa-2 b

Novel HCV Agents in Treatment-Naive Patients
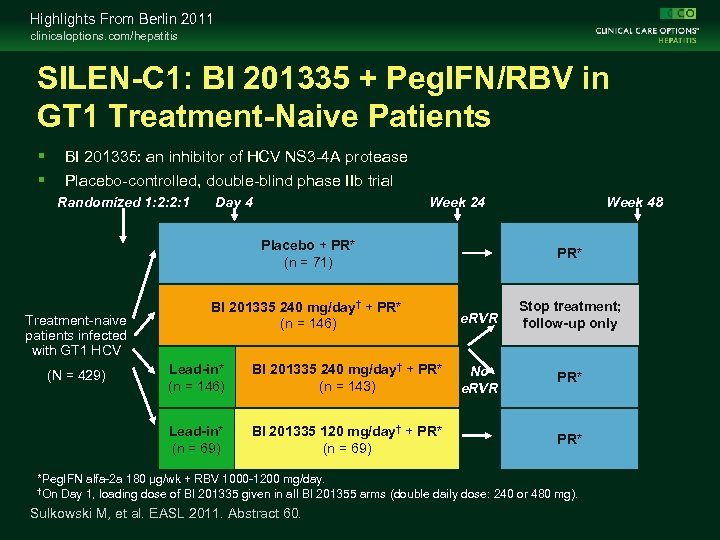
Highlights From Berlin 2011 clinicaloptions. com/hepatitis SILEN-C 1: BI 201335 + Peg. IFN/RBV in GT 1 Treatment-Naive Patients BI 201335: an inhibitor of HCV NS 3 -4 A protease Placebo-controlled, double-blind phase IIb trial Randomized 1: 2: 2: 1 Day 4 Week 24 Placebo + PR* (n = 71) Treatment-naive patients infected with GT 1 HCV BI 201335 240 mg/day† + PR* (n = 146) Lead-in* (n = 146) BI 201335 240 mg/day† + PR* (n = 143) Lead-in* (n = 69) (N = 429) BI 201335 120 mg/day† + PR* (n = 69) Week 48 PR* e. RVR Stop treatment; follow-up only No e. RVR PR* *Peg. IFN alfa-2 a 180 μg/wk + RBV 1000 -1200 mg/day. †On Day 1, loading dose of BI 201335 given in all BI 201355 arms (double daily dose: 240 or 480 mg). Sulkowski M, et al. EASL 2011. Abstract 60.
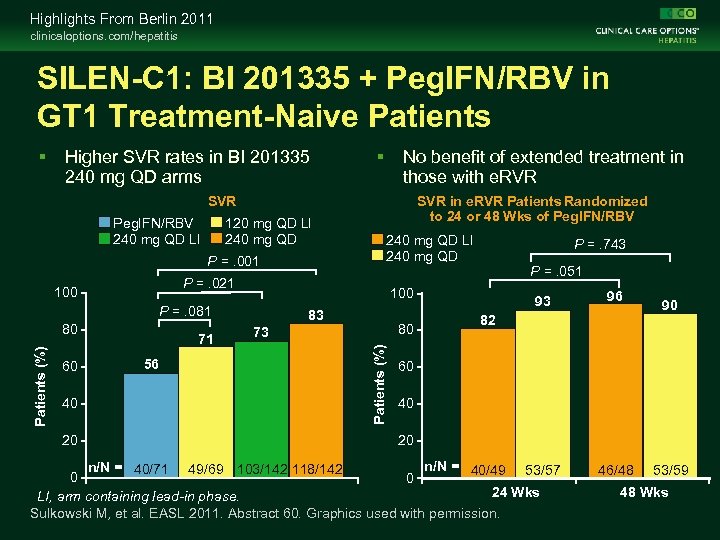
Highlights From Berlin 2011 clinicaloptions. com/hepatitis SILEN-C 1: BI 201335 + Peg. IFN/RBV in GT 1 Treatment-Naive Patients Higher SVR rates in BI 201335 240 mg QD arms No benefit of extended treatment in those with e. RVR SVR Peg. IFN/RBV 240 mg QD LI 120 mg QD LI 240 mg QD P =. 001 20 71 56 83 80 73 Patients (%) 80 P =. 743 P =. 051 100 P =. 081 40 240 mg QD LI 240 mg QD P =. 021 100 60 SVR in e. RVR Patients Randomized to 24 or 48 Wks of Peg. IFN/RBV 93 96 82 90 60 40 20 n/N = 40/49 53/57 n/N = 40/71 49/69 103/142 118/142 0 0 24 Wks LI, arm containing lead-in phase. Sulkowski M, et al. EASL 2011. Abstract 60. Graphics used with permission. 46/48 53/59 48 Wks
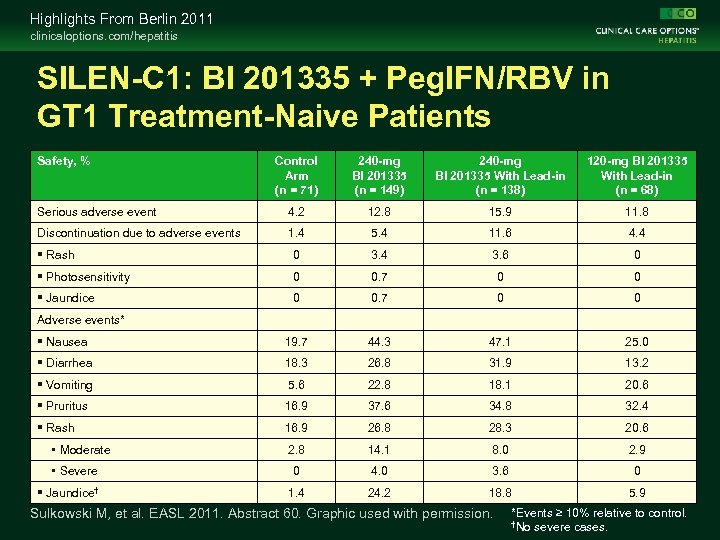
Highlights From Berlin 2011 clinicaloptions. com/hepatitis SILEN-C 1: BI 201335 + Peg. IFN/RBV in GT 1 Treatment-Naive Patients Safety, % Control Arm (n = 71) 240 -mg BI 201335 (n = 149) 240 -mg BI 201335 With Lead-in (n = 138) 120 -mg BI 201335 With Lead-in (n = 68) Serious adverse event 4. 2 12. 8 15. 9 11. 8 Discontinuation due to adverse events 1. 4 5. 4 11. 6 4. 4 Rash 0 3. 4 3. 6 0 Photosensitivity 0 0. 7 0 0 Jaundice 0 0. 7 0 0 Nausea 19. 7 44. 3 47. 1 25. 0 Diarrhea 18. 3 26. 8 31. 9 13. 2 Vomiting 5. 6 22. 8 18. 1 20. 6 Pruritus 16. 9 37. 6 34. 8 32. 4 Rash 16. 9 26. 8 28. 3 20. 6 2. 8 14. 1 8. 0 2. 9 0 4. 0 3. 6 0 1. 4 24. 2 18. 8 5. 9 Adverse events* • Moderate • Severe Jaundice† Sulkowski M, et al. EASL 2011. Abstract 60. Graphic used with permission. *Events ≥ 10% relative to control. †No severe cases.
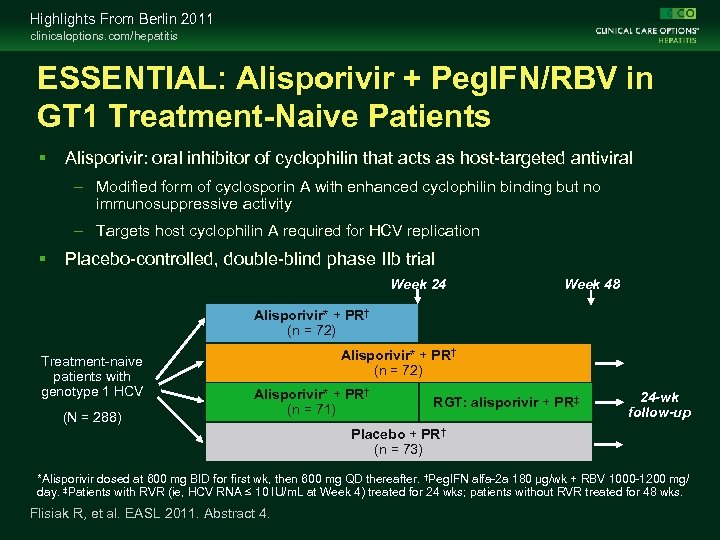
Highlights From Berlin 2011 clinicaloptions. com/hepatitis ESSENTIAL: Alisporivir + Peg. IFN/RBV in GT 1 Treatment-Naive Patients Alisporivir: oral inhibitor of cyclophilin that acts as host-targeted antiviral – Modified form of cyclosporin A with enhanced cyclophilin binding but no immunosuppressive activity – Targets host cyclophilin A required for HCV replication Placebo-controlled, double-blind phase IIb trial Week 24 Week 48 Alisporivir* + PR† (n = 72) Treatment-naive patients with genotype 1 HCV (N = 288) Alisporivir* + PR† (n = 72) Alisporivir* + PR† (n = 71) RGT: alisporivir + PR‡ 24 -wk follow-up Placebo + PR† (n = 73) *Alisporivir dosed at 600 mg BID for first wk, then 600 mg QD thereafter. †Peg. IFN alfa-2 a 180 µg/wk + RBV 1000 -1200 mg/ day. ‡Patients with RVR (ie, HCV RNA ≤ 10 IU/m. L at Week 4) treated for 24 wks; patients without RVR treated for 48 wks. Flisiak R, et al. EASL 2011. Abstract 4.
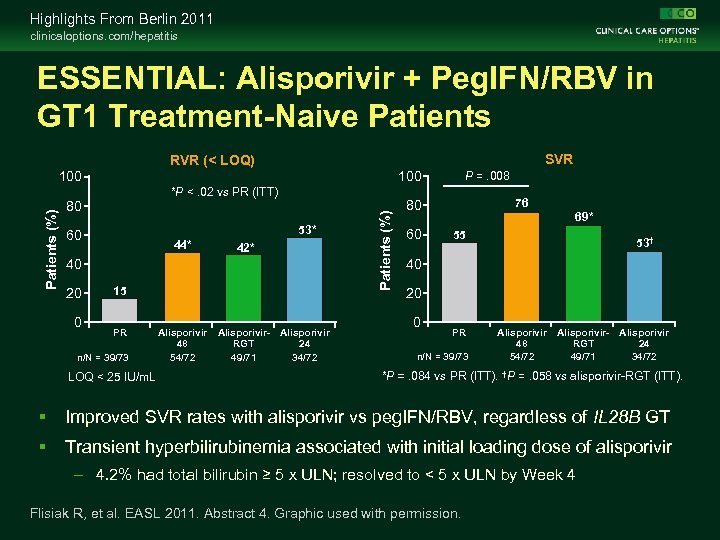
Highlights From Berlin 2011 clinicaloptions. com/hepatitis ESSENTIAL: Alisporivir + Peg. IFN/RBV in GT 1 Treatment-Naive Patients SVR RVR (< LOQ) 100 *P <. 02 vs PR (ITT) 80 53* 60 44* 42* 40 20 0 15 PR n/N = 39/73 LOQ < 25 IU/m. L Alisporivir 48 54/72 Alisporivir- Alisporivir RGT 24 49/71 34/72 Patients (%) 100 P =. 008 76 80 60 69* 55 53† 40 20 0 PR n/N = 39/73 Alisporivir 48 54/72 Alisporivir- Alisporivir RGT 24 49/71 34/72 *P =. 084 vs PR (ITT). †P =. 058 vs alisporivir-RGT (ITT). Improved SVR rates with alisporivir vs peg. IFN/RBV, regardless of IL 28 B GT Transient hyperbilirubinemia associated with initial loading dose of alisporivir – 4. 2% had total bilirubin ≥ 5 x ULN; resolved to < 5 x ULN by Week 4 Flisiak R, et al. EASL 2011. Abstract 4. Graphic used with permission.
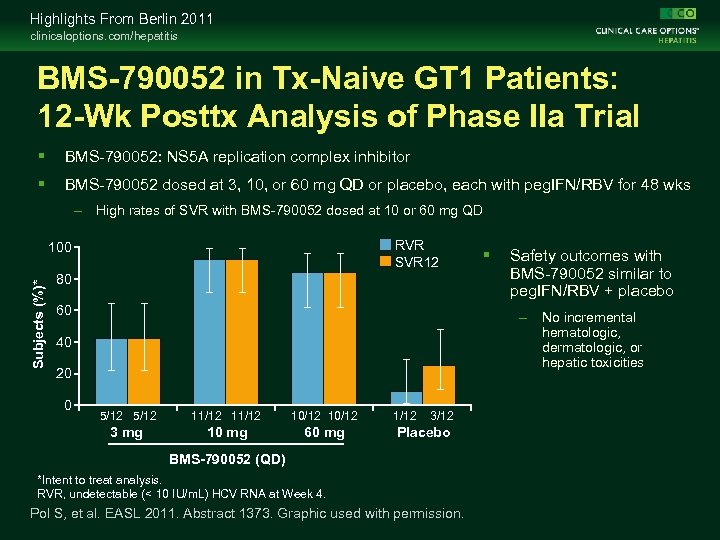
Highlights From Berlin 2011 clinicaloptions. com/hepatitis BMS-790052 in Tx-Naive GT 1 Patients: 12 -Wk Posttx Analysis of Phase IIa Trial BMS-790052: NS 5 A replication complex inhibitor BMS-790052 dosed at 3, 10, or 60 mg QD or placebo, each with peg. IFN/RBV for 48 wks – High rates of SVR with BMS-790052 dosed at 10 or 60 mg QD RVR SVR 12 Subjects (%)* 100 80 60 Safety outcomes with BMS-790052 similar to peg. IFN/RBV + placebo – No incremental hematologic, dermatologic, or hepatic toxicities 40 20 0 5/12 11/12 10/12 3 mg 10 mg 60 mg 1/12 3/12 Placebo BMS-790052 (QD) *Intent to treat analysis. RVR, undetectable (< 10 IU/m. L) HCV RNA at Week 4. Pol S, et al. EASL 2011. Abstract 1373. Graphic used with permission.
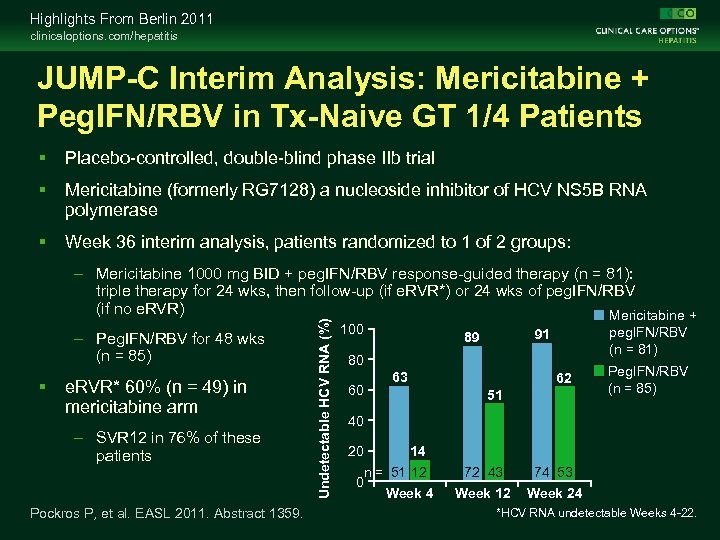
Highlights From Berlin 2011 clinicaloptions. com/hepatitis JUMP-C Interim Analysis: Mericitabine + Peg. IFN/RBV in Tx-Naive GT 1/4 Patients Placebo-controlled, double-blind phase IIb trial Mericitabine (formerly RG 7128) a nucleoside inhibitor of HCV NS 5 B RNA polymerase Week 36 interim analysis, patients randomized to 1 of 2 groups: – Peg. IFN/RBV for 48 wks (n = 85) e. RVR* 60% (n = 49) in mericitabine arm – SVR 12 in 76% of these patients Pockros P, et al. EASL 2011. Abstract 1359. Undetectable HCV RNA (%) – Mericitabine 1000 mg BID + peg. IFN/RBV response-guided therapy (n = 81): triple therapy for 24 wks, then follow-up (if e. RVR*) or 24 wks of peg. IFN/RBV (if no e. RVR) Mericitabine + 100 peg. IFN/RBV (n = 81) 91 89 80 60 63 62 51 Peg. IFN/RBV (n = 85) 40 20 14 n = 51 12 0 Week 4 72 43 74 53 Week 12 Week 24 *HCV RNA undetectable Weeks 4 -22.
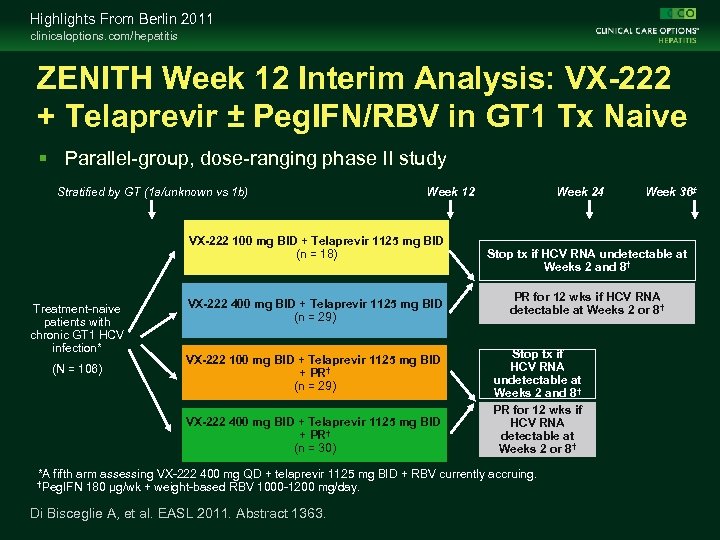
Highlights From Berlin 2011 clinicaloptions. com/hepatitis ZENITH Week 12 Interim Analysis: VX-222 + Telaprevir ± Peg. IFN/RBV in GT 1 Tx Naive Parallel-group, dose-ranging phase II study Stratified by GT (1 a/unknown vs 1 b) Week 12 VX-222 100 mg BID + Telaprevir 1125 mg BID (n = 18) Treatment-naive patients with chronic GT 1 HCV infection* (N = 106) VX-222 400 mg BID + Telaprevir 1125 mg BID (n = 29) VX-222 100 mg BID + Telaprevir 1125 mg BID + PR† (n = 29) VX-222 400 mg BID + Telaprevir 1125 mg BID + PR† (n = 30) Week 24 Stop tx if HCV RNA undetectable at Weeks 2 and 8† PR for 12 wks if HCV RNA detectable at Weeks 2 or 8† *A fifth arm assessing VX-222 400 mg QD + telaprevir 1125 mg BID + RBV currently accruing. †Peg. IFN 180 µg/wk + weight-based RBV 1000 -1200 mg/day. Di Bisceglie A, et al. EASL 2011. Abstract 1363. Week 36‡
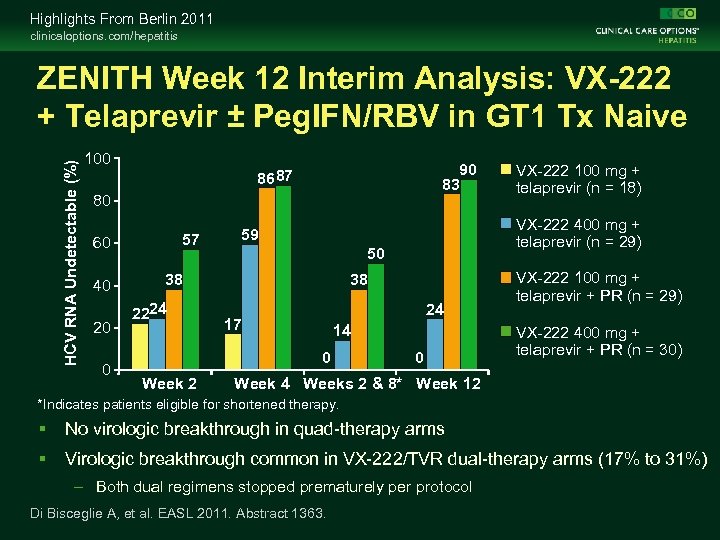
Highlights From Berlin 2011 clinicaloptions. com/hepatitis HCV RNA Undetectable (%) ZENITH Week 12 Interim Analysis: VX-222 + Telaprevir ± Peg. IFN/RBV in GT 1 Tx Naive 100 90 83 8687 80 57 60 40 20 0 2224 50 38 24 17 14 0 Week 2 VX-222 400 mg + telaprevir (n = 29) 59 38 VX-222 100 mg + telaprevir (n = 18) 0 VX-222 100 mg + telaprevir + PR (n = 29) VX-222 400 mg + telaprevir + PR (n = 30) Week 4 Weeks 2 & 8* Week 12 *Indicates patients eligible for shortened therapy. No virologic breakthrough in quad-therapy arms Virologic breakthrough common in VX-222/TVR dual-therapy arms (17% to 31%) – Both dual regimens stopped prematurely per protocol Di Bisceglie A, et al. EASL 2011. Abstract 1363.
Highlights From Berlin 2011 clinicaloptions. com/hepatitis EMERGE Week 12 Results: Peg. IFN Lambda vs Peg. IFN alfa-2 a in GT 1 -4 Tx-Naive Pts Phase IIb trial Genotyping at baseline Week 24 genotypes 2, 3 Week 48 genotypes 1, 4 Peg. IFN lambda 120 μg/wk + RBV (n = 128) Interferon-naive pts Peg. IFN lambda 180 μg/wk + RBV (n = 131) (N = 526) Peg. IFN lambda 240 μg/wk + RBV (n = 134) Peg. IFN alfa-2 a 180 μg/wk + RBV* (n = 133) *RBV dosed at 800 mg/day for genotype 2/3 and 1000 -1200 mg/day for genotype 1/4 patients. Zeuzem S, et al. EASL 2011. Abstract 1360.
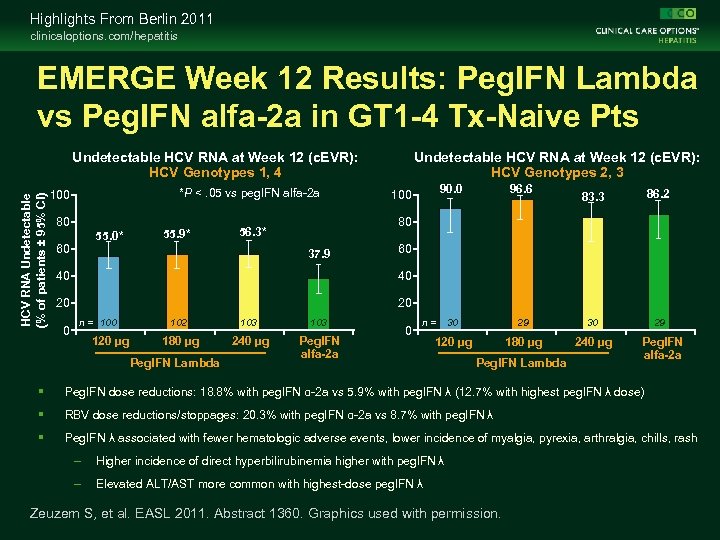
Highlights From Berlin 2011 clinicaloptions. com/hepatitis EMERGE Week 12 Results: Peg. IFN Lambda vs Peg. IFN alfa-2 a in GT 1 -4 Tx-Naive Pts HCV RNA Undetectable (% of patients ± 95% CI) Undetectable HCV RNA at Week 12 (c. EVR): HCV Genotypes 1, 4 *P <. 05 vs peg. IFN alfa-2 a 100 80 55. 0* 60 55. 9* Undetectable HCV RNA at Week 12 (c. EVR): HCV Genotypes 2, 3 90. 0 96. 6 30 120 µg 100 83. 3 86. 2 29 30 29 180 µg 240 µg 80 56. 3* 37. 9 60 40 40 20 20 0 n = 100 120 µg 102 103 180 µg 240 µg Peg. IFN alfa-2 a Peg. IFN Lambda 0 n= Peg. IFN Lambda Peg. IFN alfa-2 a Peg. IFN dose reductions: 18. 8% with peg. IFN α-2 a vs 5. 9% with peg. IFN λ (12. 7% with highest peg. IFN λ dose) RBV dose reductions/stoppages: 20. 3% with peg. IFN α-2 a vs 8. 7% with peg. IFN λ Peg. IFN λ associated with fewer hematologic adverse events, lower incidence of myalgia, pyrexia, arthralgia, chills, rash – Higher incidence of direct hyperbilirubinemia higher with peg. IFN λ – Elevated ALT/AST more common with highest-dose peg. IFN λ Zeuzem S, et al. EASL 2011. Abstract 1360. Graphics used with permission.
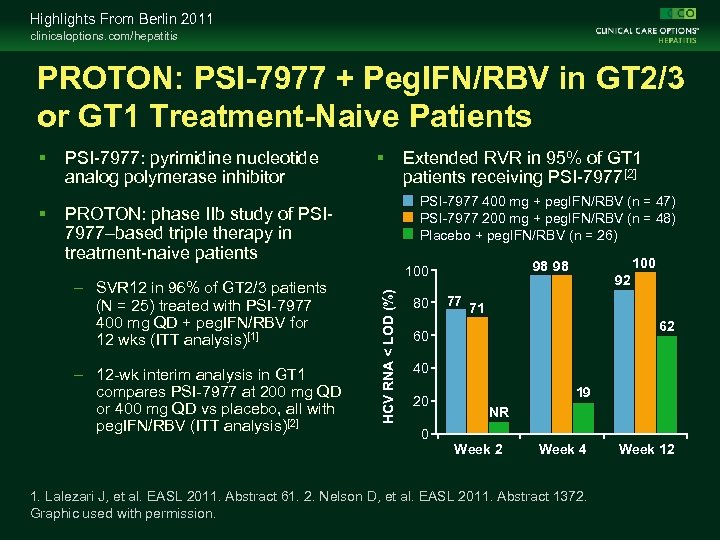
Highlights From Berlin 2011 clinicaloptions. com/hepatitis PROTON: PSI-7977 + Peg. IFN/RBV in GT 2/3 or GT 1 Treatment-Naive Patients PSI-7977: pyrimidine nucleotide analog polymerase inhibitor PROTON: phase IIb study of PSI 7977–based triple therapy in treatment-naive patients – SVR 12 in 96% of GT 2/3 patients (N = 25) treated with PSI-7977 400 mg QD + peg. IFN/RBV for 12 wks (ITT analysis)[1] – 12 -wk interim analysis in GT 1 compares PSI-7977 at 200 mg QD or 400 mg QD vs placebo, all with peg. IFN/RBV (ITT analysis)[2] Extended RVR in 95% of GT 1 patients receiving PSI-7977[2] PSI-7977 400 mg + peg. IFN/RBV (n = 47) PSI-7977 200 mg + peg. IFN/RBV (n = 48) Placebo + peg. IFN/RBV (n = 26) 80 100 98 98 100 HCV RNA < LOD (%) 77 92 71 62 60 40 20 19 NR 0 Week 2 Week 4 1. Lalezari J, et al. EASL 2011. Abstract 61. 2. Nelson D, et al. EASL 2011. Abstract 1372. Graphic used with permission. Week 12
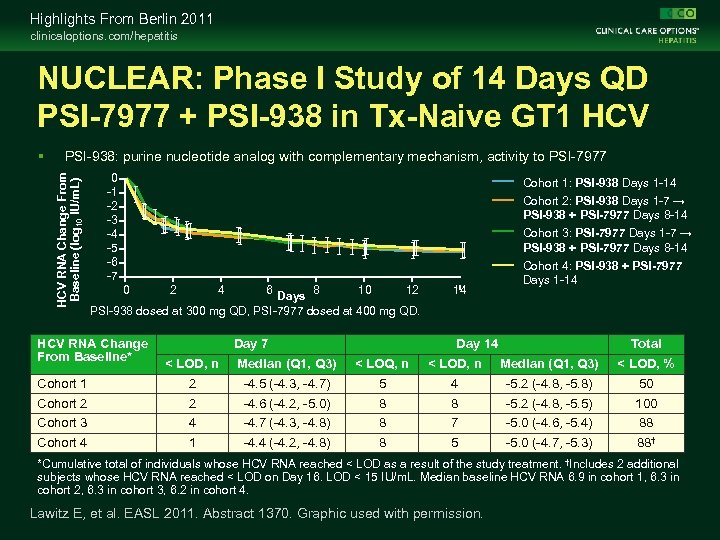
Highlights From Berlin 2011 clinicaloptions. com/hepatitis NUCLEAR: Phase I Study of 14 Days QD PSI-7977 + PSI-938 in Tx-Naive GT 1 HCV PSI-938: purine nucleotide analog with complementary mechanism, activity to PSI-7977 HCV RNA Change From Baseline (log 10 IU/m. L) 0 -1 -2 -3 -4 -5 -6 -7 0 8 10 12 Days PSI-938 dosed at 300 mg QD, PSI-7977 dosed at 400 mg QD. HCV RNA Change From Baseline* 2 4 6 Day 7 14 Cohort 1: PSI-938 Days 1 -14 Cohort 2: PSI-938 Days 1 -7 → PSI-938 + PSI-7977 Days 8 -14 Cohort 3: PSI-7977 Days 1 -7 → PSI-938 + PSI-7977 Days 8 -14 Cohort 4: PSI-938 + PSI-7977 Days 1 -14 Day 14 Total < LOD, n Median (Q 1, Q 3) < LOQ, n < LOD, n Median (Q 1, Q 3) < LOD, % Cohort 1 2 -4. 5 (-4. 3, -4. 7) 5 4 -5. 2 (-4. 8, -5. 8) 50 Cohort 2 2 -4. 6 (-4. 2, -5. 0) 8 8 -5. 2 (-4. 8, -5. 5) 100 Cohort 3 4 -4. 7 (-4. 3, -4. 8) 8 7 -5. 0 (-4. 6, -5. 4) 88 Cohort 4 1 -4. 4 (-4. 2, -4. 8) 8 5 -5. 0 (-4. 7, -5. 3) 88† *Cumulative total of individuals whose HCV RNA reached < LOD as a result of the study treatment. †Includes 2 additional subjects whose HCV RNA reached < LOD on Day 16. LOD < 15 IU/m. L. Median baseline HCV RNA 6. 9 in cohort 1, 6. 3 in cohort 2, 6. 3 in cohort 3, 6. 2 in cohort 4. Lawitz E, et al. EASL 2011. Abstract 1370. Graphic used with permission.
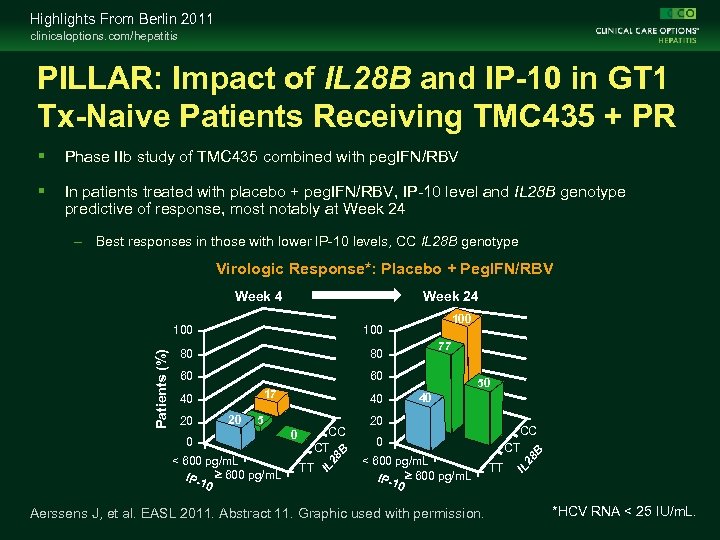
Highlights From Berlin 2011 clinicaloptions. com/hepatitis PILLAR: Impact of IL 28 B and IP-10 in GT 1 Tx-Naive Patients Receiving TMC 435 + PR Phase IIb study of TMC 435 combined with peg. IFN/RBV In patients treated with placebo + peg. IFN/RBV, IP-10 level and IL 28 B genotype predictive of response, most notably at Week 24 – Best responses in those with lower IP-10 levels, CC IL 28 B genotype Virologic Response*: Placebo + Peg. IFN/RBV Week 24 80 60 60 17 20 20 40 5 0 < 600 pg/m. L IP-1 ³ 600 pg/m. L 0 0 CC CT TT 28 B 40 77 50 40 20 CC CT 0 < 600 pg/m. L IP-1 ³ 600 pg/m. L 0 Aerssens J, et al. EASL 2011. Abstract 11. Graphic used with permission. TT 28 B 80 100 IL Patients (%) 100 IL Week 4 *HCV RNA < 25 IU/m. L.
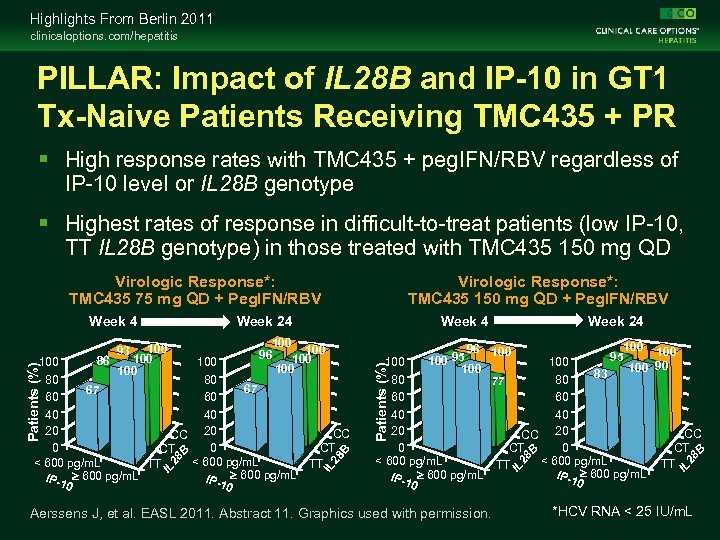
Highlights From Berlin 2011 clinicaloptions. com/hepatitis PILLAR: Impact of IL 28 B and IP-10 in GT 1 Tx-Naive Patients Receiving TMC 435 + PR High response rates with TMC 435 + peg. IFN/RBV regardless of IP-10 level or IL 28 B genotype Highest rates of response in difficult-to-treat patients (low IP-10, TT IL 28 B genotype) in those treated with TMC 435 150 mg QD IL 80 60 40 20 0 < 600 pg/m. L N=1 IP-1 ³ 600 pg/m. L 0 Aerssens J, et al. EASL 2011. Abstract 11. Graphics used with permission. 100 95 100 100 90 83 80 60 40 CC 20 CT 0 < 600 pg/m. L N=1 TT IP-1 ³ 600 pg/m. L 0 CC CT TT 28 B CC CT TT 96 100 95 100 77 28 B 80 80 67 67 60 60 40 40 20 CC 20 0 0 CT N=1 < 600 pg/m. L TT IP-1 ³ 600 pg/m. L 0 0 100 Week 24 IL 100 100 96 100 Patients (%) 100 86 100 93 Week 4 28 B Patients (%) 100 Week 24 IL Week 4 Virologic Response*: TMC 435 150 mg QD + Peg. IFN/RBV IL Virologic Response*: TMC 435 75 mg QD + Peg. IFN/RBV *HCV RNA < 25 IU/m. L

Novel HCV Agents in Treatment-Experienced Patients
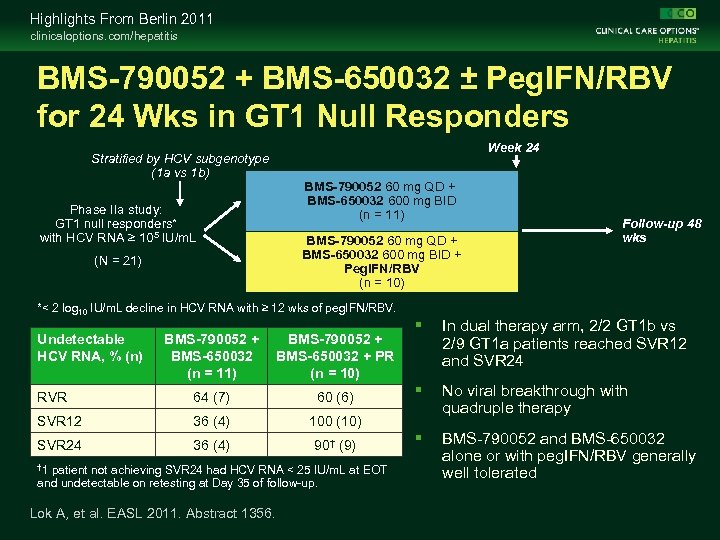
Highlights From Berlin 2011 clinicaloptions. com/hepatitis BMS-790052 + BMS-650032 ± Peg. IFN/RBV for 24 Wks in GT 1 Null Responders Stratified by HCV subgenotype (1 a vs 1 b) Phase IIa study: GT 1 null responders* with HCV RNA ≥ 105 IU/m. L (N = 21) Week 24 BMS-790052 60 mg QD + BMS-650032 600 mg BID (n = 11) BMS-790052 60 mg QD + BMS-650032 600 mg BID + Peg. IFN/RBV (n = 10) Follow-up 48 wks *< 2 log 10 IU/m. L decline in HCV RNA with ≥ 12 wks of peg. IFN/RBV. Undetectable HCV RNA, % (n) BMS-790052 + BMS-650032 (n = 11) BMS-790052 + BMS-650032 + PR (n = 10) RVR 64 (7) 60 (6) SVR 12 36 (4) 100 (10) SVR 24 36 (4) 90† (9) † 1 patient not achieving SVR 24 had HCV RNA < 25 IU/m. L at EOT and undetectable on retesting at Day 35 of follow-up. Lok A, et al. EASL 2011. Abstract 1356. In dual therapy arm, 2/2 GT 1 b vs 2/9 GT 1 a patients reached SVR 12 and SVR 24 No viral breakthrough with quadruple therapy BMS-790052 and BMS-650032 alone or with peg. IFN/RBV generally well tolerated
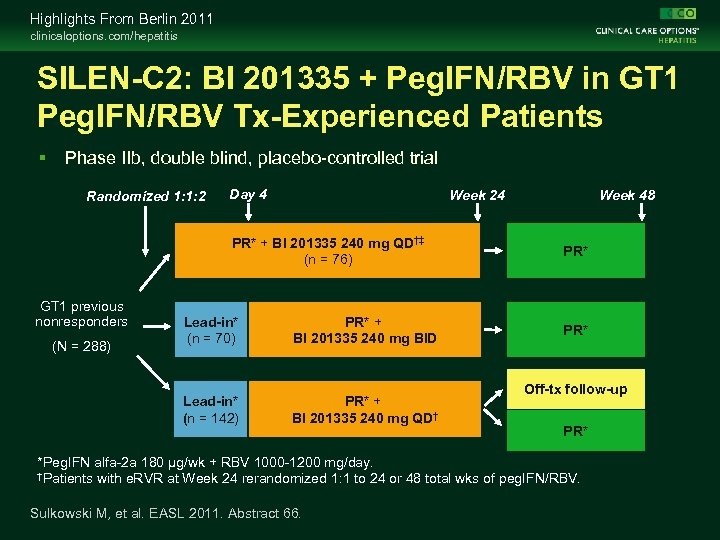
Highlights From Berlin 2011 clinicaloptions. com/hepatitis SILEN-C 2: BI 201335 + Peg. IFN/RBV in GT 1 Peg. IFN/RBV Tx-Experienced Patients Phase IIb, double blind, placebo-controlled trial Randomized 1: 1: 2 Day 4 Week 24 PR* + BI 201335 240 mg QD†‡ (n = 76) GT 1 previous nonresponders PR* + BI 201335 240 mg BID Lead-in* (n = 142) (N = 288) Lead-in* (n = 70) PR* + BI 201335 240 mg QD† Week 48 PR* Off-tx follow-up PR* *Peg. IFN alfa-2 a 180 μg/wk + RBV 1000 -1200 mg/day. †Patients with e. RVR at Week 24 rerandomized 1: 1 to 24 or 48 total wks of peg. IFN/RBV. Sulkowski M, et al. EASL 2011. Abstract 66.
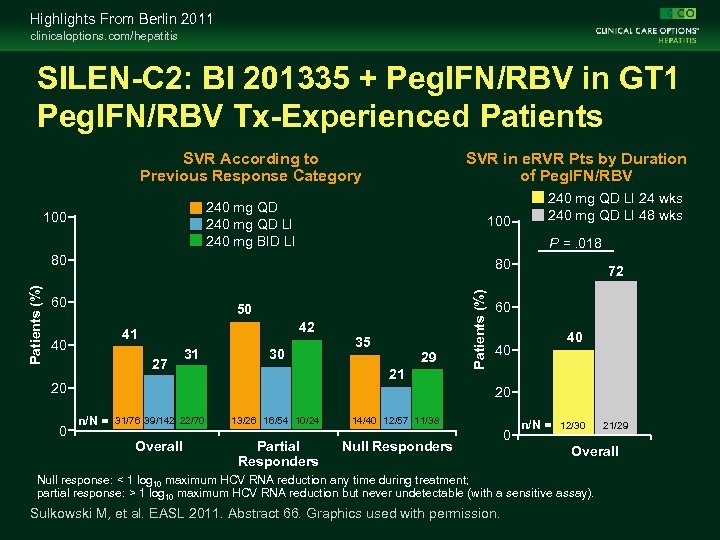
Highlights From Berlin 2011 clinicaloptions. com/hepatitis SILEN-C 2: BI 201335 + Peg. IFN/RBV in GT 1 Peg. IFN/RBV Tx-Experienced Patients SVR in e. RVR Pts by Duration of Peg. IFN/RBV SVR According to Previous Response Category 240 mg QD LI 240 mg BID LI 100 P =. 018 80 60 50 42 41 27 31 30 29 21 20 0 35 Patients (%) 80 40 240 mg QD LI 24 wks 240 mg QD LI 48 wks 72 60 40 40 20 n/N = 31/76 39/142 22/70 Overall 13/26 16/54 10/24 Partial Responders 14/40 12/57 11/38 Null Responders 0 n/N = 12/30 Overall Null response: < 1 log 10 maximum HCV RNA reduction any time during treatment; partial response: > 1 log 10 maximum HCV RNA reduction but never undetectable (with a sensitive assay). Sulkowski M, et al. EASL 2011. Abstract 66. Graphics used with permission. 21/29
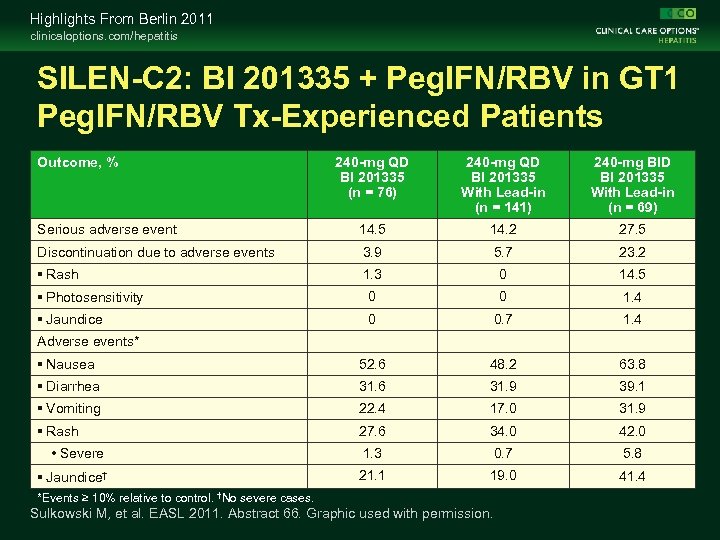
Highlights From Berlin 2011 clinicaloptions. com/hepatitis SILEN-C 2: BI 201335 + Peg. IFN/RBV in GT 1 Peg. IFN/RBV Tx-Experienced Patients Outcome, % 240 -mg QD BI 201335 (n = 76) 240 -mg QD BI 201335 With Lead-in (n = 141) 240 -mg BID BI 201335 With Lead-in (n = 69) Serious adverse event 14. 5 14. 2 27. 5 Discontinuation due to adverse events 3. 9 5. 7 23. 2 Rash 1. 3 0 14. 5 Photosensitivity 0 0 1. 4 Jaundice 0 0. 7 1. 4 Nausea 52. 6 48. 2 63. 8 Diarrhea 31. 6 31. 9 39. 1 Vomiting 22. 4 17. 0 31. 9 Rash 27. 6 34. 0 42. 0 • Severe 1. 3 0. 7 5. 8 Jaundice† 21. 1 19. 0 41. 4 Adverse events* *Events ≥ 10% relative to control. †No severe cases. Sulkowski M, et al. EASL 2011. Abstract 66. Graphic used with permission.
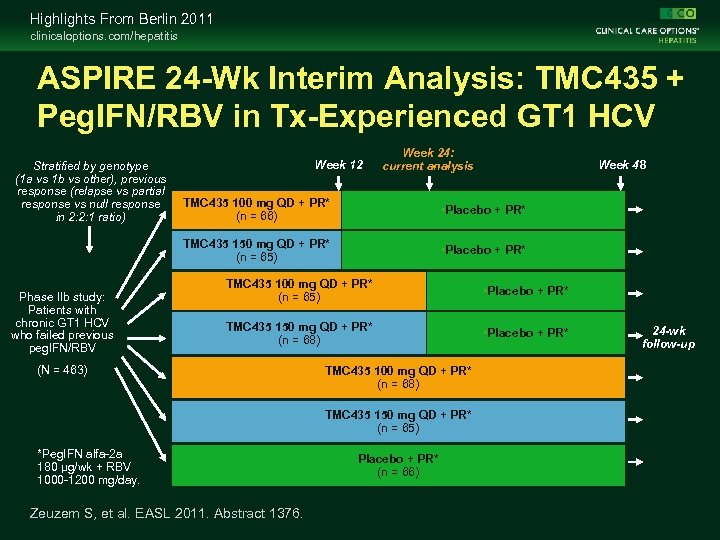
Highlights From Berlin 2011 clinicaloptions. com/hepatitis ASPIRE 24 -Wk Interim Analysis: TMC 435 + Peg. IFN/RBV in Tx-Experienced GT 1 HCV Stratified by genotype (1 a vs 1 b vs other), previous response (relapse vs partial response vs null response in 2: 2: 1 ratio) Week 12 Week 24: current analysis Week 48 • Placebo + PR* TMC 435 150 mg QD + PR* (n = 65) Phase IIb study: Patients with chronic GT 1 HCV who failed previous peg. IFN/RBV TMC 435 100 mg QD + PR* (n = 66) • Placebo + PR* TMC 435 100 mg QD + PR* (n = 65) • Placebo + PR* TMC 435 150 mg QD + PR* (n = 68) • Placebo + PR* (N = 463) TMC 435 100 mg QD + PR* (n = 68) TMC 435 150 mg QD + PR* (n = 65) *Peg. IFN alfa-2 a 180 µg/wk + RBV 1000 -1200 mg/day. Zeuzem S, et al. EASL 2011. Abstract 1376. Placebo + PR* (n = 66) 24 -wk follow-up
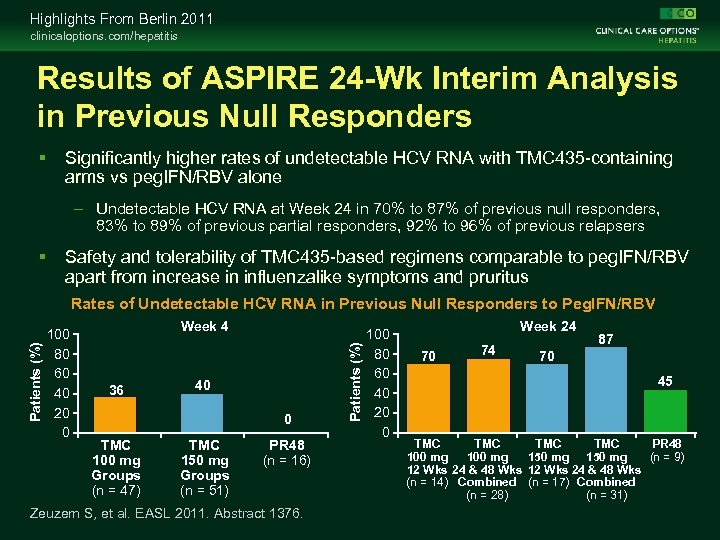
Highlights From Berlin 2011 clinicaloptions. com/hepatitis Results of ASPIRE 24 -Wk Interim Analysis in Previous Null Responders Significantly higher rates of undetectable HCV RNA with TMC 435 -containing arms vs peg. IFN/RBV alone – Undetectable HCV RNA at Week 24 in 70% to 87% of previous null responders, 83% to 89% of previous partial responders, 92% to 96% of previous relapsers Safety and tolerability of TMC 435 -based regimens comparable to peg. IFN/RBV apart from increase in influenzalike symptoms and pruritus 100 80 60 40 20 0 Week 4 36 40 0 TMC 100 mg Groups (n = 47) TMC 150 mg Groups (n = 51) PR 48 (n = 16) Zeuzem S, et al. EASL 2011. Abstract 1376. Patients (%) Rates of Undetectable HCV RNA in Previous Null Responders to Peg. IFN/RBV 100 80 60 40 20 0 Week 24 70 74 87 70 45 TMC TMC PR 48 100 mg 150 mg (n = 9) 12 Wks 24 & 48 Wks (n = 14) Combined (n = 17) Combined (n = 28) (n = 31)

Hepatitis B
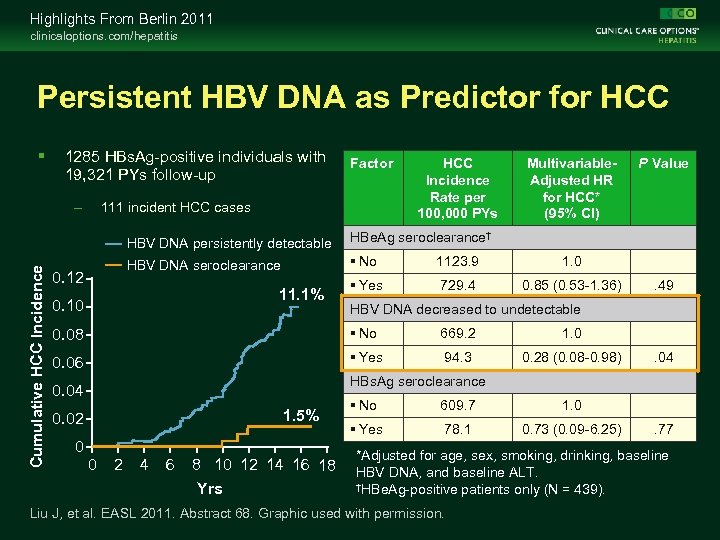
Highlights From Berlin 2011 clinicaloptions. com/hepatitis Persistent HBV DNA as Predictor for HCC 1285 HBs. Ag-positive individuals with 19, 321 PYs follow-up – Factor 111 incident HCC cases HCC Incidence Rate per 100, 000 PYs Multivariable. Adjusted HR for HCC* (95% CI) Cumulative HCC Incidence HBV DNA persistently detectable 0. 12 HBe. Ag seroclearance† HBV DNA seroclearance No 1123. 9 1. 0 Yes 729. 4 0. 85 (0. 53 -1. 36) P Value 11. 1% 0. 10 . 49 HBV DNA decreased to undetectable 0. 08 No 669. 2 1. 0 0. 06 Yes 94. 3 0. 28 (0. 08 -0. 98) . 04 HBs. Ag seroclearance 0. 04 1. 5% 0. 02 0 0 2 4 6 8 10 12 14 16 18 Yrs No 609. 7 1. 0 Yes 78. 1 0. 73 (0. 09 -6. 25) . 77 *Adjusted for age, sex, smoking, drinking, baseline HBV DNA, and baseline ALT. †HBe. Ag-positive patients only (N = 439). Liu J, et al. EASL 2011. Abstract 68. Graphic used with permission.
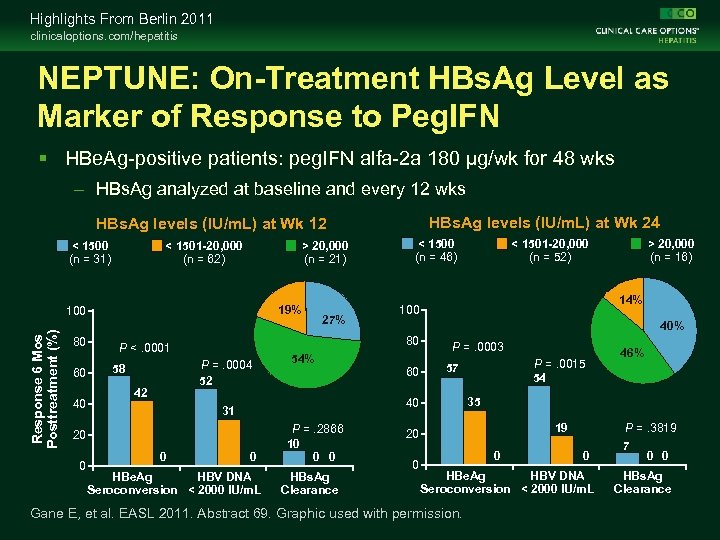
Highlights From Berlin 2011 clinicaloptions. com/hepatitis NEPTUNE: On-Treatment HBs. Ag Level as Marker of Response to Peg. IFN HBe. Ag-positive patients: peg. IFN alfa-2 a 180 µg/wk for 48 wks – HBs. Ag analyzed at baseline and every 12 wks HBs. Ag levels (IU/m. L) at Wk 24 HBs. Ag levels (IU/m. L) at Wk 12 < 1500 (n = 31) < 1501 -20, 000 (n = 62) > 20, 000 (n = 21) 19% Response 6 Mos Posttreatment (%) 100 80 60 40 P =. 0004 52 42 0 0 HBe. Ag HBV DNA Seroconversion < 2000 IU/m. L P =. 2866 10 0 0 HBs. Ag Clearance > 20, 000 (n = 16) 14% 40% 60 P =. 0003 P =. 0015 54 57 19 20 0 46% 35 40 31 20 0 54% < 1501 -20, 000 (n = 52) 100 80 P <. 0001 58 27% < 1500 (n = 46) 0 0 HBe. Ag HBV DNA Seroconversion < 2000 IU/m. L Gane E, et al. EASL 2011. Abstract 69. Graphic used with permission. P =. 3819 7 0 0 HBs. Ag Clearance
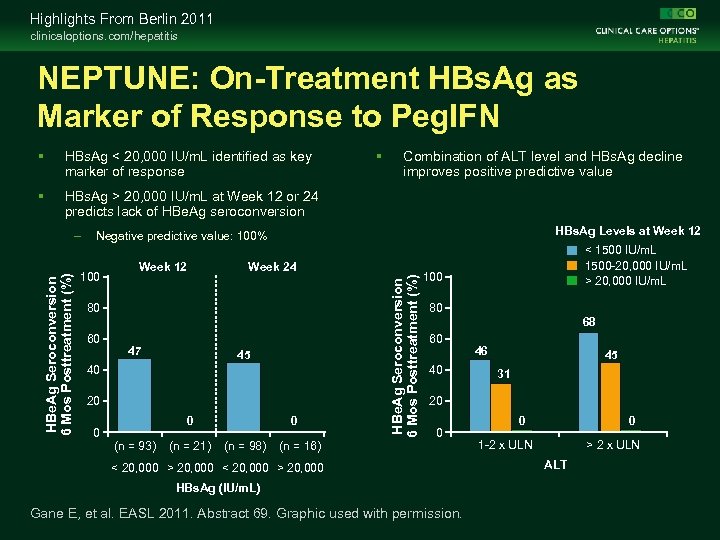
Highlights From Berlin 2011 clinicaloptions. com/hepatitis NEPTUNE: On-Treatment HBs. Ag as Marker of Response to Peg. IFN HBs. Ag < 20, 000 IU/m. L identified as key marker of response HBs. Ag > 20, 000 IU/m. L at Week 12 or 24 predicts lack of HBe. Ag seroconversion Combination of ALT level and HBs. Ag decline improves positive predictive value HBs. Ag Levels at Week 12 Negative predictive value: 100% 100 Week 12 Week 24 80 60 47 45 40 20 0 0 (n = 93) (n = 21) 0 (n = 98) HBe. Ag Seroconversion 6 Mos Posttreatment (%) – < 1500 IU/m. L 1500 -20, 000 IU/m. L > 20, 000 IU/m. L 100 80 68 60 40 46 45 31 20 0 (n = 16) < 20, 000 > 20, 000 HBs. Ag (IU/m. L) Gane E, et al. EASL 2011. Abstract 69. Graphic used with permission. 0 0 1 -2 x ULN > 2 x ULN ALT
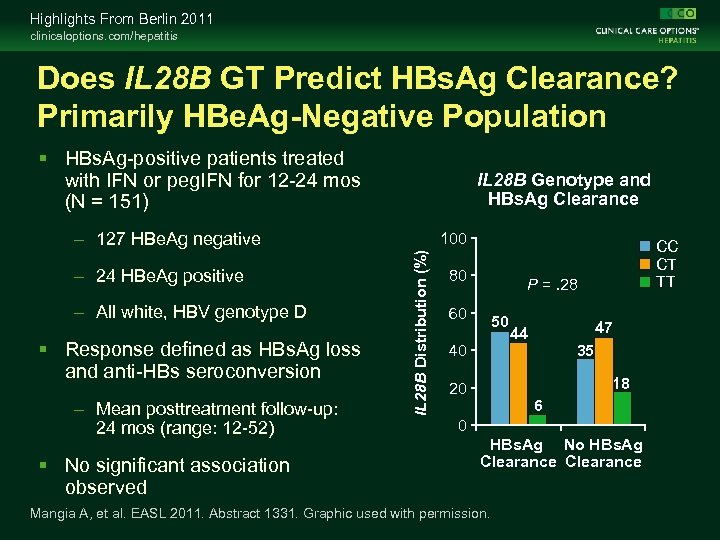
Highlights From Berlin 2011 clinicaloptions. com/hepatitis Does IL 28 B GT Predict HBs. Ag Clearance? Primarily HBe. Ag-Negative Population HBs. Ag-positive patients treated with IFN or peg. IFN for 12 -24 mos (N = 151) IL 28 B Genotype and HBs. Ag Clearance – 127 HBe. Ag negative – All white, HBV genotype D Response defined as HBs. Ag loss and anti-HBs seroconversion – Mean posttreatment follow-up: 24 mos (range: 12 -52) No significant association observed IL 28 B Distribution (%) – 24 HBe. Ag positive 100 80 CC CT TT P =. 28 60 50 40 47 44 35 18 20 6 0 HBs. Ag No HBs. Ag Clearance Mangia A, et al. EASL 2011. Abstract 1331. Graphic used with permission.
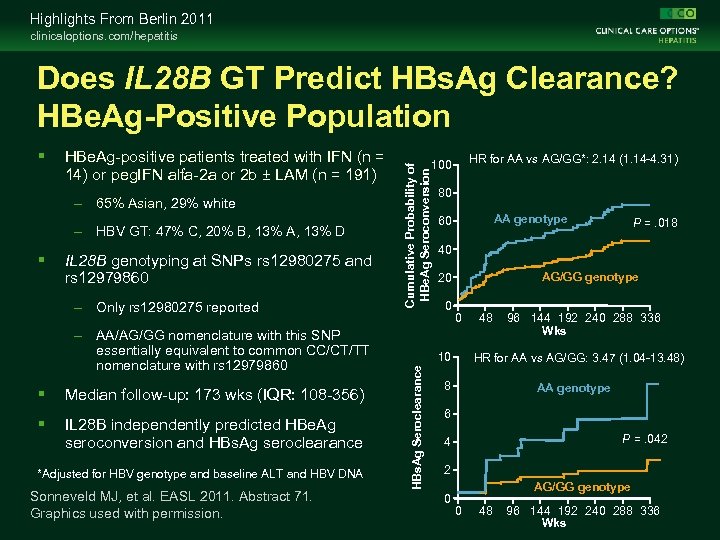
Highlights From Berlin 2011 clinicaloptions. com/hepatitis HBe. Ag-positive patients treated with IFN (n = 14) or peg. IFN alfa-2 a or 2 b ± LAM (n = 191) – 65% Asian, 29% white – HBV GT: 47% C, 20% B, 13% A, 13% D IL 28 B genotyping at SNPs rs 12980275 and rs 12979860 – Only rs 12980275 reported – AA/AG/GG nomenclature with this SNP essentially equivalent to common CC/CT/TT nomenclature with rs 12979860 Median follow-up: 173 wks (IQR: 108 -356) IL 28 B independently predicted HBe. Ag seroconversion and HBs. Ag seroclearance *Adjusted for HBV genotype and baseline ALT and HBV DNA Sonneveld MJ, et al. EASL 2011. Abstract 71. Graphics used with permission. HR for AA vs AG/GG*: 2. 14 (1. 14 -4. 31) 100 80 AA genotype 60 P =. 018 40 AG/GG genotype 20 0 0 10 HBs. Ag Seroclearance Cumulative Probability of HBe. Ag Seroconversion Does IL 28 B GT Predict HBs. Ag Clearance? HBe. Ag-Positive Population 48 96 144 192 240 288 336 Wks HR for AA vs AG/GG: 3. 47 (1. 04 -13. 48) 8 AA genotype 6 P =. 042 4 2 0 AG/GG genotype 0 48 96 144 192 240 288 336 Wks

Advanced Liver Disease
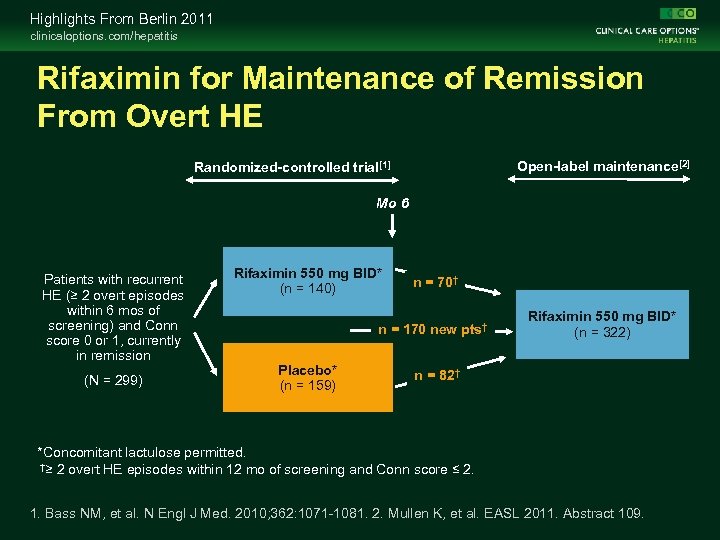
Highlights From Berlin 2011 clinicaloptions. com/hepatitis Rifaximin for Maintenance of Remission From Overt HE Open-label maintenance[2] Randomized-controlled trial[1] Mo 6 Patients with recurrent HE (≥ 2 overt episodes within 6 mos of screening) and Conn score 0 or 1, currently in remission (N = 299) Rifaximin 550 mg BID* (n = 140) n = 70† n = 170 new pts† Placebo* (n = 159) Rifaximin 550 mg BID* (n = 322) n = 82† *Concomitant lactulose permitted. †≥ 2 overt HE episodes within 12 mo of screening and Conn score ≤ 2. 1. Bass NM, et al. N Engl J Med. 2010; 362: 1071 -1081. 2. Mullen K, et al. EASL 2011. Abstract 109.
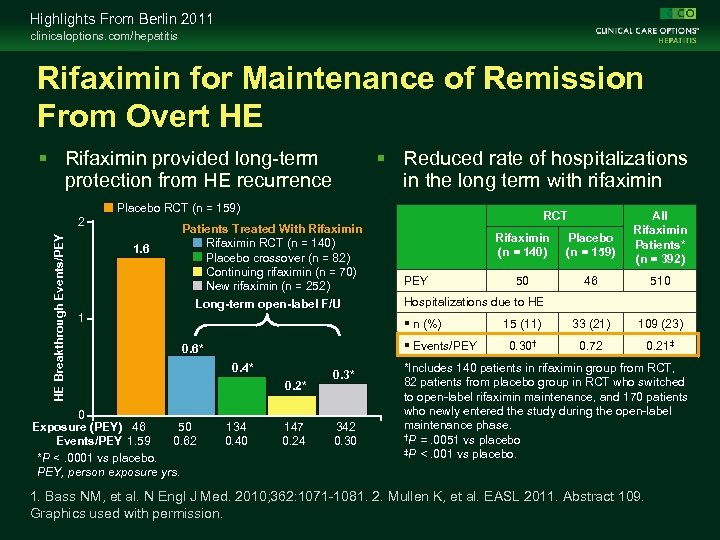
Highlights From Berlin 2011 clinicaloptions. com/hepatitis Rifaximin for Maintenance of Remission From Overt HE Rifaximin provided long-term protection from HE recurrence Reduced rate of hospitalizations in the long term with rifaximin Placebo RCT (n = 159) HE Breakthrough Events/PEY 2 1. 6 RCT Patients Treated With Rifaximin RCT (n = 140) Placebo crossover (n = 82) Continuing rifaximin (n = 70) New rifaximin (n = 252) Long-term open-label F/U 1 Rifaximin (n = 140) PEY Events/PEY 0 Exposure (PEY) 46 50 Events/PEY 1. 59 0. 62 *P <. 0001 vs placebo. PEY, person exposure yrs. 0. 4* 0. 2* 134 0. 40 147 0. 24 0. 3* 342 0. 30 Placebo (n = 159) 50 46 510 15 (11) 33 (21) 109 (23) 0. 30† 0. 72 0. 21‡ Hospitalizations due to HE n (%) 0. 6* All Rifaximin Patients* (n = 392) *Includes 140 patients in rifaximin group from RCT, 82 patients from placebo group in RCT who switched to open-label rifaximin maintenance, and 170 patients who newly entered the study during the open-label maintenance phase. †P =. 0051 vs placebo ‡P <. 001 vs placebo. 1. Bass NM, et al. N Engl J Med. 2010; 362: 1071 -1081. 2. Mullen K, et al. EASL 2011. Abstract 109. Graphics used with permission.
Highlights From Berlin 2011 clinicaloptions. com/hepatitis Impact of Adrenal Dysfunction in Cirrhotic Patients With Ascites Corticotropin stimulation test performed in a series of 85 consecutive cirrhotic patients with ascites – Adrenal dysfunction: change in cortisol < 9 µg/d. L and/or peak cortisol < 18 µg/d. L – Patients followed until liver transplantation or death (median: 198 days) 39% had adrenal dysfunction Survival significantly poorer in pts with adrenal dysfunction (P =. 02) Predictors of mortality (multivariate analysis) – Adrenal dysfunction: HR: 2. 1 (95% CI: 1. 1 -4. 0; P =. 03) – PRA > 4 ng/m. L/hr: HR: 2. 1 (95% CI: 1. 1 -4. 0; P =. 03) – MELD score > 18: HR: 2. 0 (95% CI: 1. 1 -3. 5; P =. 02) Elia C, et al. EASL 2011. Abstract 106.
Highlights From Berlin 2011 clinicaloptions. com/hepatitis Does Moderate Hypothermia Prevent Brain Edema in Acute Liver Failure? * Prospective, randomized, controlled, multicenter trial Patients with acute liver failure and imminent brain edema and planned intracranial pressure monitoring – Standard medical therapy: n = 33 – Median core temperature: 36. 7°C – Standard medical therapy + 3 days moderate hypothermia: n = 21 – Median core temperature: 33. 2°C Similar rates of intracranial hypertension: 57% with MH vs 45% for control (P =. 58) Similar rates of mortality: 48% with MH vs 58% for control (NS) *Slide content based only on abstract data. Larsen, et al. EASL 2011. Abstract 56.
Highlights From Berlin 2011 clinicaloptions. com/hepatitis Radioembolization With 90 Y-Resin Microspheres for Unresectable HCC Multicenter patient series: 82% male, 79% cirrhotic, mean age: 64. 5 yrs HCC etiology: HBV (13%), HCV (44%) Previous procedures: surgical (n = 61), percutaneous ablation (n = 29), intra-arterial (n = 98) Median survival – 1 yr: 52. 8% – 2 yrs: 28. 1% – 3 yrs: 15. 9% Median survival according to BCLC stage (P <. 001) – BCLC stage A (n = 52): 24. 4 mos – BCLC stage B (n = 87): 16. 9 mos – BCLC stage C (n = 183): 10. 0 mos – BCLC stage D (n = 3): 5. 2 mos Sangro B, et al. EASL 2011. Abstract 80.
Highlights From Berlin 2011 clinicaloptions. com/hepatitis Sorafenib + Percutaneous RFA of HCC and PVTT vs Sorafenib Alone Child-Pugh A cirrhotic patients (N = 79) with untreated HCC (single tumor ≤ 6. 5 cm or 3 nodules ≤ 5 cm) and portal vein tumor thrombus (PVTT) Sorafenib 800 mg/day started soon after RFA/diagnosis Combination treatment significantly improved survival (P <. 001) Survival, % Sorafenib + RFA 1 yr 37 60 2 yrs 0 35 3 yrs Not reached 26 Giorgio A, et al. EASL 2011. Abstract 1368.
Highlights From Berlin 2011 clinicaloptions. com/hepatitis Resection of HCC Followed by Salvage Transplantation* 1998 -2008: 114 transplantable patients with good liver function and HCC within Milan criteria underwent resection – Transplantation offered if recurrence of chronic liver disease Recurrence occurred in 80% of resected cases – Only 54% of patients went on to salvage transplantation Risk factors for recurrence beyond Milan criteria (multivariate analysis) – Poor differentiation, presence of vascular invasion, older than 60 yrs of age Risk factors for missing opportunity of LT after recurrence (univariate analysis) – Presence of F 4, tumor size > 3 cm, R 1 surgical margin *Slide content based only on abstract data. Fuks D, et al. EASL 2011. Abstract 57.
Highlights From Berlin 2011 clinicaloptions. com/hepatitis Molecular Signature for Diagnosis, Prognosis of HCC* Whole-genome profiles of frozen samples analyzed (N = 110) – HCC of various etiologies, HCA, FNH, cirrhosis, and normal liver tissues – 96 genes analyzed as potential markers of – Benign liver tumors according to their different pathologic and molecular subtypes, and – HCC according to prognosis 5 -gene predictor associated with overall survival in HCC – HR: 2. 6 (CI: 1. 6 -4. 2; P <. 001) – More significantly associated with overall survival than previous G 1 -G 6 or proliferative-transcriptomic classifications *Slide content based only on abstract data. Nault JC, et al. EASL 2011. Abstract 76.
Highlights From Berlin 2011 clinicaloptions. com/hepatitis IL 28 B as Predictor of Response to HCV Treatment in Recurrence After Transplant* Liver transplantation recipients with HCV recurrence who received ≥ 12 wks of peg. IFN/RBV (N = 132) – IL 28 B SNPs rs 8099917 and rs 12979860 genotyped – Recipients: peripheral lymphocytes or liver explants – Donors: liver biopsies immediately after reperfusion of the graft Patients with favorable donor and recipient IL 28 B genotypes had highest probability of SVR (79%; P =. 001 vs other combinations) Multivariate analysis: CC genotype of rs 12979860 identified as baseline independent predictor of SVR (OR: 4. 2; 95% CI: 1. 4 -12. 3; P =. 01) SVR in 85% of recipients with CC genotype and HCV RNA < 6. 5 log 10 IU/m. L vs 23% in patients with CT/TT genotype and HCV RNA > 6. 5 log 10 IU/m. L (P <. 001) *Slide content based only on abstract data. Crespo G, et al. EASL 2011. Abstract 30.

Nonviral Hepatitis
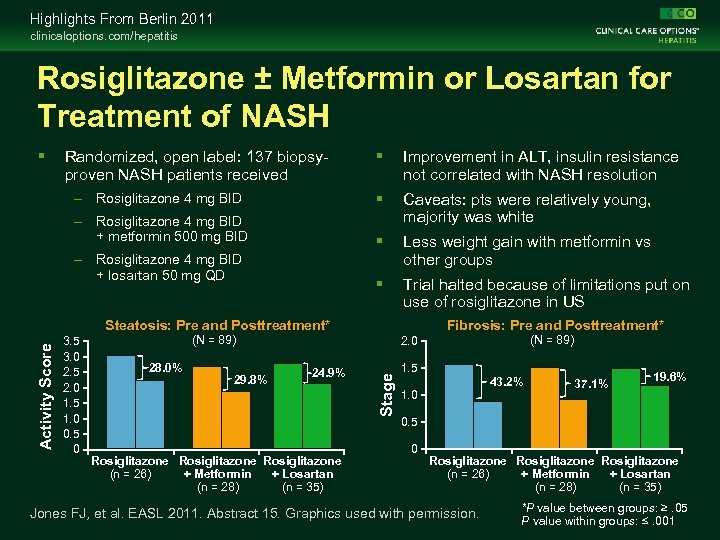
Highlights From Berlin 2011 clinicaloptions. com/hepatitis Rosiglitazone ± Metformin or Losartan for Treatment of NASH Improvement in ALT, insulin resistance not correlated with NASH resolution – Rosiglitazone 4 mg BID + metformin 500 mg BID Caveats: pts were relatively young, majority was white Less weight gain with metformin vs other groups Trial halted because of limitations put on use of rosiglitazone in US Randomized, open label: 137 biopsyproven NASH patients received – Rosiglitazone 4 mg BID + losartan 50 mg QD 3. 5 3. 0 2. 5 2. 0 1. 5 1. 0 0. 5 0 Fibrosis: Pre and Posttreatment* (N = 89) 28. 0% 29. 8% (N = 89) 2. 0 24. 9% Rosiglitazone (n = 26) + Metformin + Losartan (n = 28) (n = 35) Stage Activity Score Steatosis: Pre and Posttreatment* 1. 5 43. 2% 1. 0 37. 1% 19. 6% 0. 5 0 Rosiglitazone (n = 26) + Metformin + Losartan (n = 28) (n = 35) Jones FJ, et al. EASL 2011. Abstract 15. Graphics used with permission. *P value between groups: ≥. 05 P value within groups: ≤. 001
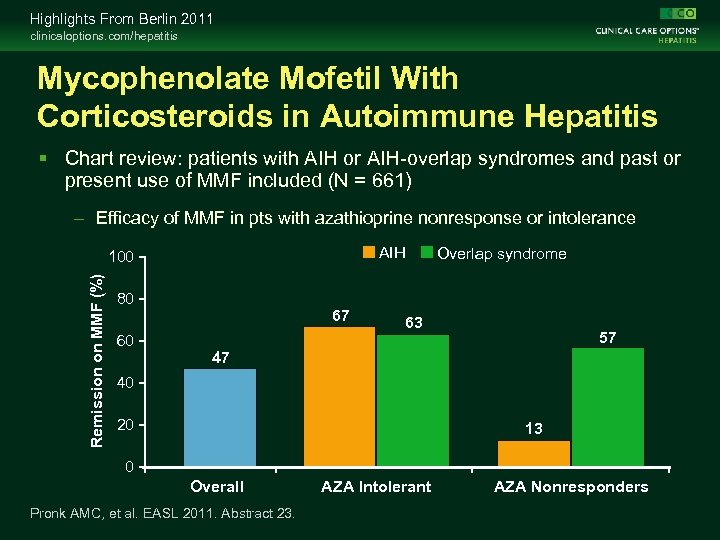
Highlights From Berlin 2011 clinicaloptions. com/hepatitis Mycophenolate Mofetil With Corticosteroids in Autoimmune Hepatitis Chart review: patients with AIH or AIH-overlap syndromes and past or present use of MMF included (N = 661) – Efficacy of MMF in pts with azathioprine nonresponse or intolerance AIH Remission on MMF (%) 100 80 60 67 Overlap syndrome 63 57 47 40 20 13 0 Overall Pronk AMC, et al. EASL 2011. Abstract 23. AZA Intolerant AZA Nonresponders

Go Online for More CCO Coverage of EASL 2011! Capsule Summaries of all the key data Podium to Practice Expert Analysis panel discussion exploring the clinical implications of the most important data from the meeting clinicaloptions. com/Berlin 2011
b063e24de0aa63a7f4ca57f7831a8301.ppt