1866b8781d77d95fc98b491c128ebd67.ppt
- Количество слайдов: 38
 HIGH-ENERGY MECHANOCHEMICAL ACTIVATION OF ACTIVE PRINCIPLES: GENERAL CONCEPTS Mario Grassi (mariog@dicamp. univ. trieste. it) Department of Chemical Engineering (DICAMP) UNIVERSITY OF TRIESTE
HIGH-ENERGY MECHANOCHEMICAL ACTIVATION OF ACTIVE PRINCIPLES: GENERAL CONCEPTS Mario Grassi (mariog@dicamp. univ. trieste. it) Department of Chemical Engineering (DICAMP) UNIVERSITY OF TRIESTE
![1 - INTRODUCTION NON-THERMALLY ACTIVATED CHEMISTRY [1] ELECTROCHEMISTRY MECHANOCHEMISTRY 1 - INTRODUCTION NON-THERMALLY ACTIVATED CHEMISTRY [1] ELECTROCHEMISTRY MECHANOCHEMISTRY](https://present5.com/presentation/1866b8781d77d95fc98b491c128ebd67/image-2.jpg) 1 - INTRODUCTION NON-THERMALLY ACTIVATED CHEMISTRY [1] ELECTROCHEMISTRY MECHANOCHEMISTRY
1 - INTRODUCTION NON-THERMALLY ACTIVATED CHEMISTRY [1] ELECTROCHEMISTRY MECHANOCHEMISTRY
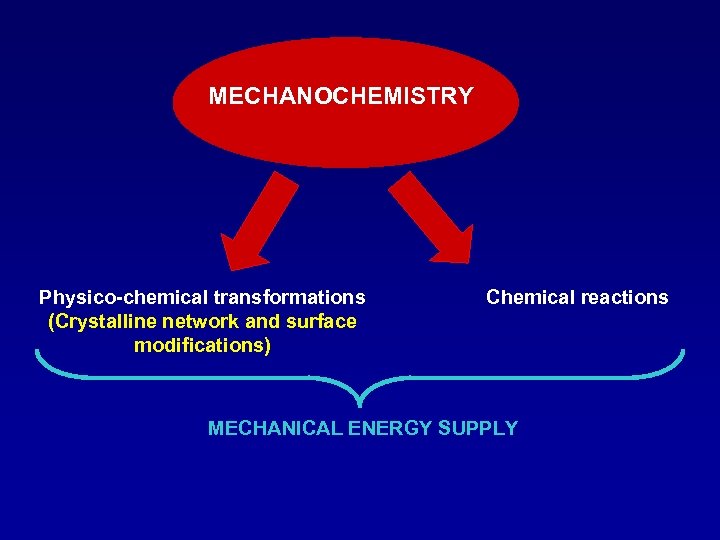 MECHANOCHEMISTRY Physico-chemical transformations (Crystalline network and surface modifications) Chemical reactions MECHANICAL ENERGY SUPPLY
MECHANOCHEMISTRY Physico-chemical transformations (Crystalline network and surface modifications) Chemical reactions MECHANICAL ENERGY SUPPLY
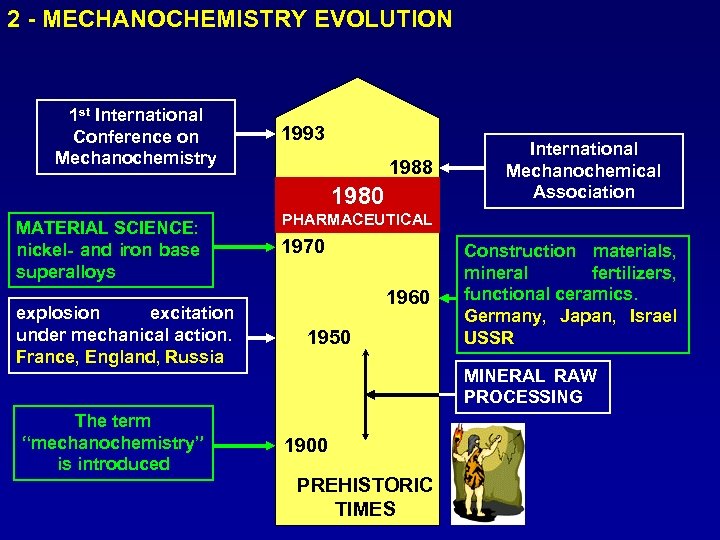 2 - MECHANOCHEMISTRY EVOLUTION 1 st International Conference on Mechanochemistry 1993 1988 1980 MATERIAL SCIENCE: nickel- and iron base superalloys explosion excitation under mechanical action. France, England, Russia The term “mechanochemistry” is introduced International Mechanochemical Association PHARMACEUTICAL 1970 1960 1950 Construction materials, mineral fertilizers, functional ceramics. Germany, Japan, Israel USSR MINERAL RAW PROCESSING 1900 PREHISTORIC TIMES
2 - MECHANOCHEMISTRY EVOLUTION 1 st International Conference on Mechanochemistry 1993 1988 1980 MATERIAL SCIENCE: nickel- and iron base superalloys explosion excitation under mechanical action. France, England, Russia The term “mechanochemistry” is introduced International Mechanochemical Association PHARMACEUTICAL 1970 1960 1950 Construction materials, mineral fertilizers, functional ceramics. Germany, Japan, Israel USSR MINERAL RAW PROCESSING 1900 PREHISTORIC TIMES
 3 - WHY MECHANOCHEMISTRY IN THE PHARMACEUTICAL FIELD? 1 Getting pharmaceutical products avoiding the use of solvents (their elimination can be difficult, expensive and can alter drug activated status) 2 Possibility of increasing the bioavailability of poorly water soluble drugs (class 2 drugs [2])
3 - WHY MECHANOCHEMISTRY IN THE PHARMACEUTICAL FIELD? 1 Getting pharmaceutical products avoiding the use of solvents (their elimination can be difficult, expensive and can alter drug activated status) 2 Possibility of increasing the bioavailability of poorly water soluble drugs (class 2 drugs [2])
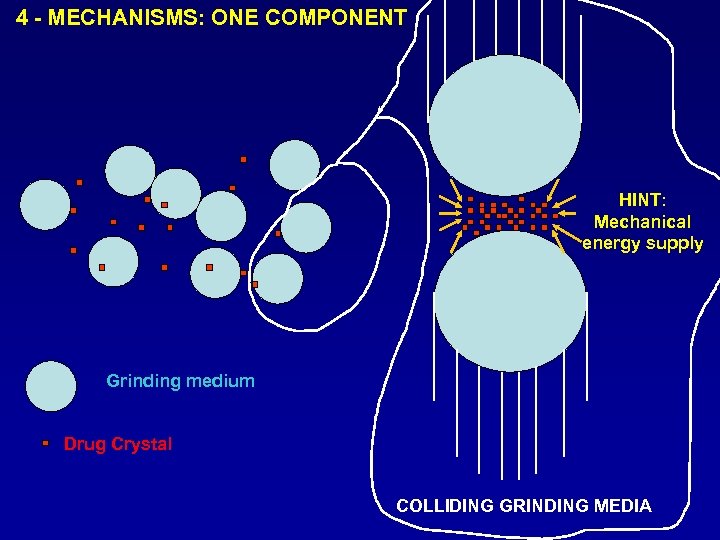 4 - MECHANISMS: ONE COMPONENT HINT: Mechanical energy supply Grinding medium Drug Crystal COLLIDING GRINDING MEDIA
4 - MECHANISMS: ONE COMPONENT HINT: Mechanical energy supply Grinding medium Drug Crystal COLLIDING GRINDING MEDIA
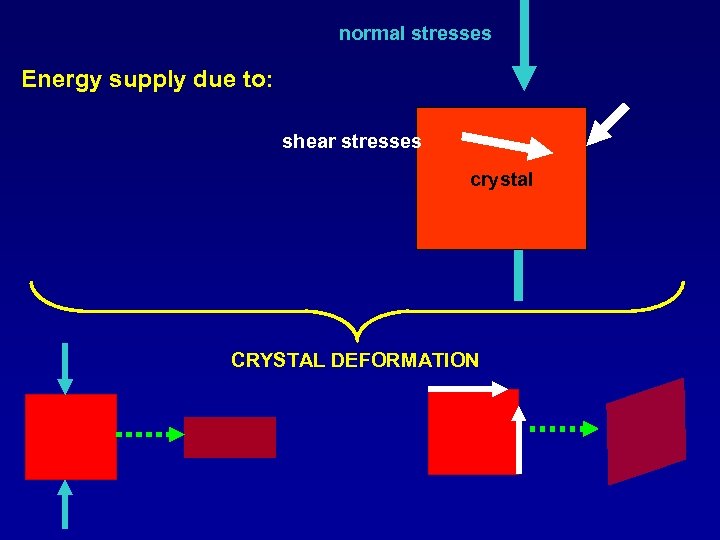 normal stresses Energy supply due to: shear stresses crystal CRYSTAL DEFORMATION
normal stresses Energy supply due to: shear stresses crystal CRYSTAL DEFORMATION
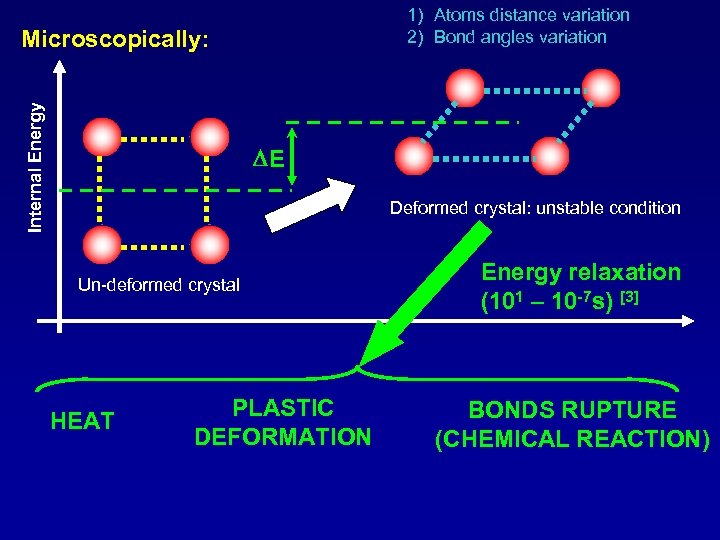 1) Atoms distance variation 2) Bond angles variation Internal Energy Microscopically: DE Deformed crystal: unstable condition Un-deformed crystal HEAT PLASTIC DEFORMATION Energy relaxation (101 – 10 -7 s) [3] BONDS RUPTURE (CHEMICAL REACTION)
1) Atoms distance variation 2) Bond angles variation Internal Energy Microscopically: DE Deformed crystal: unstable condition Un-deformed crystal HEAT PLASTIC DEFORMATION Energy relaxation (101 – 10 -7 s) [3] BONDS RUPTURE (CHEMICAL REACTION)
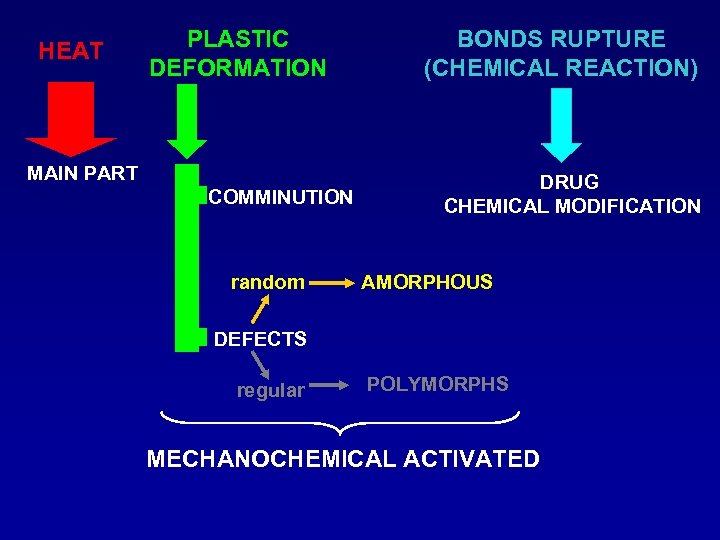 HEAT PLASTIC DEFORMATION MAIN PART COMMINUTION random BONDS RUPTURE (CHEMICAL REACTION) DRUG CHEMICAL MODIFICATION AMORPHOUS DEFECTS regular POLYMORPHS MECHANOCHEMICAL ACTIVATED
HEAT PLASTIC DEFORMATION MAIN PART COMMINUTION random BONDS RUPTURE (CHEMICAL REACTION) DRUG CHEMICAL MODIFICATION AMORPHOUS DEFECTS regular POLYMORPHS MECHANOCHEMICAL ACTIVATED
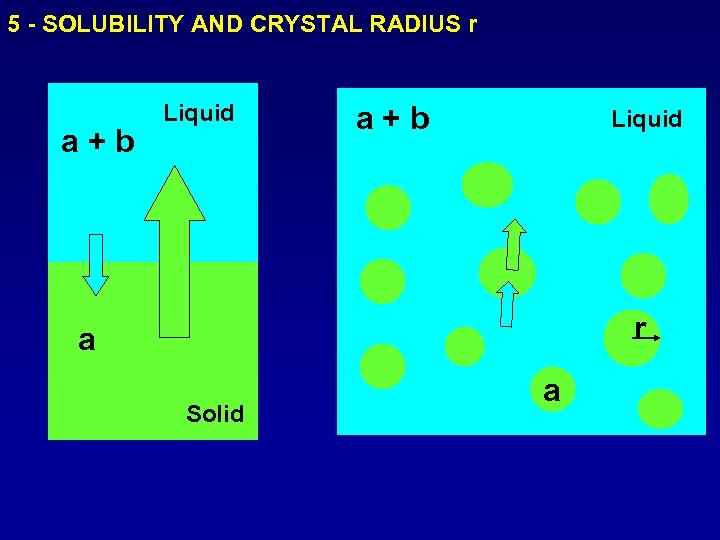 5 - SOLUBILITY AND CRYSTAL RADIUS r a+b Liquid r a Solid a
5 - SOLUBILITY AND CRYSTAL RADIUS r a+b Liquid r a Solid a
![a+b Liquid Kelvin equation[4] r a It holds for an ideal solution gsl = a+b Liquid Kelvin equation[4] r a It holds for an ideal solution gsl =](https://present5.com/presentation/1866b8781d77d95fc98b491c128ebd67/image-11.jpg) a+b Liquid Kelvin equation[4] r a It holds for an ideal solution gsl = solid-liquid surface tension vs = solid solute molar volume R = universal gas constant T = temperature
a+b Liquid Kelvin equation[4] r a It holds for an ideal solution gsl = solid-liquid surface tension vs = solid solute molar volume R = universal gas constant T = temperature
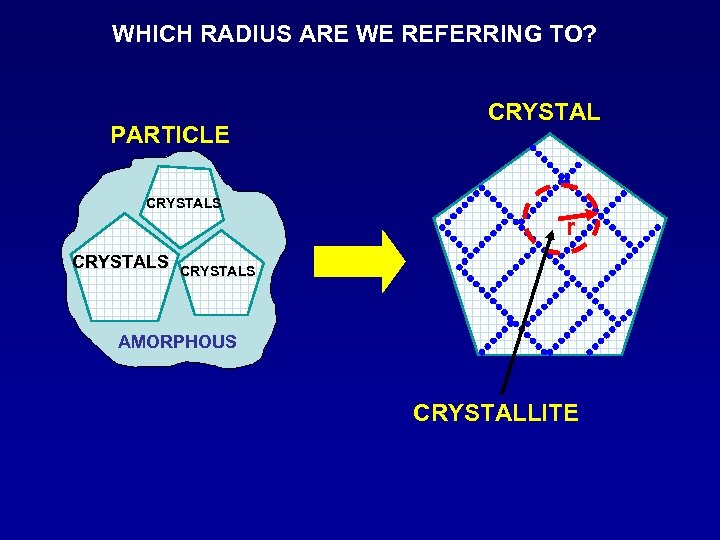 WHICH RADIUS ARE WE REFERRING TO? PARTICLE CRYSTALS r CRYSTALS AMORPHOUS CRYSTALLITE
WHICH RADIUS ARE WE REFERRING TO? PARTICLE CRYSTALS r CRYSTALS AMORPHOUS CRYSTALLITE
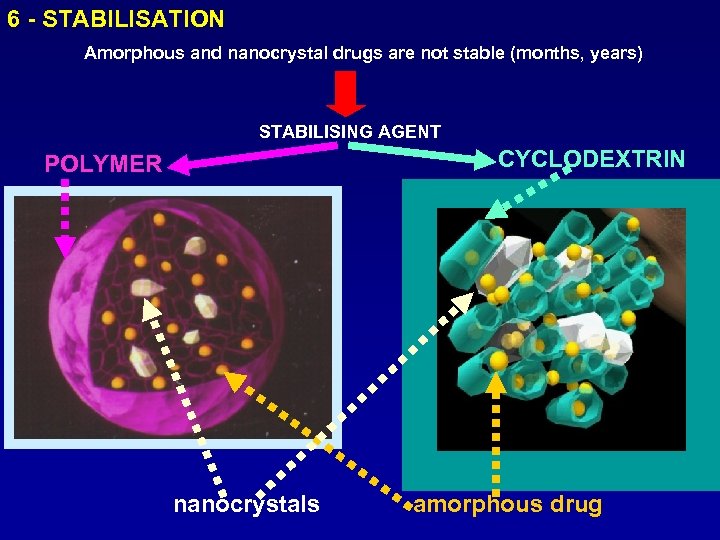 6 - STABILISATION Amorphous and nanocrystal drugs are not stable (months, years) STABILISING AGENT CYCLODEXTRIN POLYMER nanocrystals amorphous drug
6 - STABILISATION Amorphous and nanocrystal drugs are not stable (months, years) STABILISING AGENT CYCLODEXTRIN POLYMER nanocrystals amorphous drug
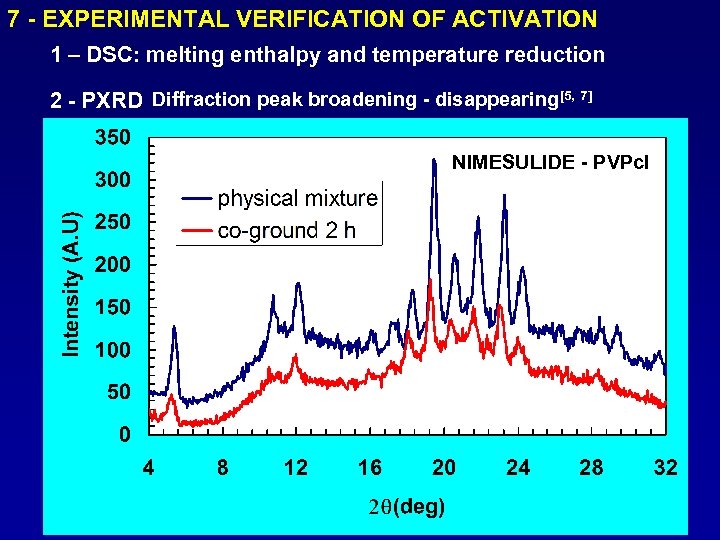 7 - EXPERIMENTAL VERIFICATION OF ACTIVATION 1 – DSC: melting enthalpy and temperature reduction 2 - PXRD Diffraction peak broadening - disappearing[5, 7] NIMESULIDE - PVPcl
7 - EXPERIMENTAL VERIFICATION OF ACTIVATION 1 – DSC: melting enthalpy and temperature reduction 2 - PXRD Diffraction peak broadening - disappearing[5, 7] NIMESULIDE - PVPcl
![3 – IN VITRO Test Increased release kinetics[5] NIMESULIDE - PVPcl WATER 37°C, p. 3 – IN VITRO Test Increased release kinetics[5] NIMESULIDE - PVPcl WATER 37°C, p.](https://present5.com/presentation/1866b8781d77d95fc98b491c128ebd67/image-15.jpg) 3 – IN VITRO Test Increased release kinetics[5] NIMESULIDE - PVPcl WATER 37°C, p. H = 5. 5
3 – IN VITRO Test Increased release kinetics[5] NIMESULIDE - PVPcl WATER 37°C, p. H = 5. 5
![4 – IN VIVO Test Increased Bioavailability NIFEDIPINE – PEG 600 HPMC [8] Blood 4 – IN VIVO Test Increased Bioavailability NIFEDIPINE – PEG 600 HPMC [8] Blood](https://present5.com/presentation/1866b8781d77d95fc98b491c128ebd67/image-16.jpg) 4 – IN VIVO Test Increased Bioavailability NIFEDIPINE – PEG 600 HPMC [8] Blood concentration (Beagle dogs) AUC = 122 ng h/ml Cmax = 89 ng/ml Tmax = 0. 5 h AUC = 47 ng h/ml Cmax = 9 ng/ml Tmax = 1. 4 h
4 – IN VIVO Test Increased Bioavailability NIFEDIPINE – PEG 600 HPMC [8] Blood concentration (Beagle dogs) AUC = 122 ng h/ml Cmax = 89 ng/ml Tmax = 0. 5 h AUC = 47 ng h/ml Cmax = 9 ng/ml Tmax = 1. 4 h
![8 – MILLS TYPES [3] 1 BALLS MILLS (Tumbling mills, Planetary, vibrational, Spex mills 8 – MILLS TYPES [3] 1 BALLS MILLS (Tumbling mills, Planetary, vibrational, Spex mills](https://present5.com/presentation/1866b8781d77d95fc98b491c128ebd67/image-17.jpg) 8 – MILLS TYPES [3] 1 BALLS MILLS (Tumbling mills, Planetary, vibrational, Spex mills and attritors) 2 SHEAR ACTION MILLS (Rollers) 3 SHOCK ACTION MILLS (Jet mills, high peripheral-speed pin mills )
8 – MILLS TYPES [3] 1 BALLS MILLS (Tumbling mills, Planetary, vibrational, Spex mills and attritors) 2 SHEAR ACTION MILLS (Rollers) 3 SHOCK ACTION MILLS (Jet mills, high peripheral-speed pin mills )
![BALLS MILLS [9, 10] Tumbling mill Inco Alloys International BALLS MILLS [9, 10] Tumbling mill Inco Alloys International](https://present5.com/presentation/1866b8781d77d95fc98b491c128ebd67/image-18.jpg) BALLS MILLS [9, 10] Tumbling mill Inco Alloys International
BALLS MILLS [9, 10] Tumbling mill Inco Alloys International
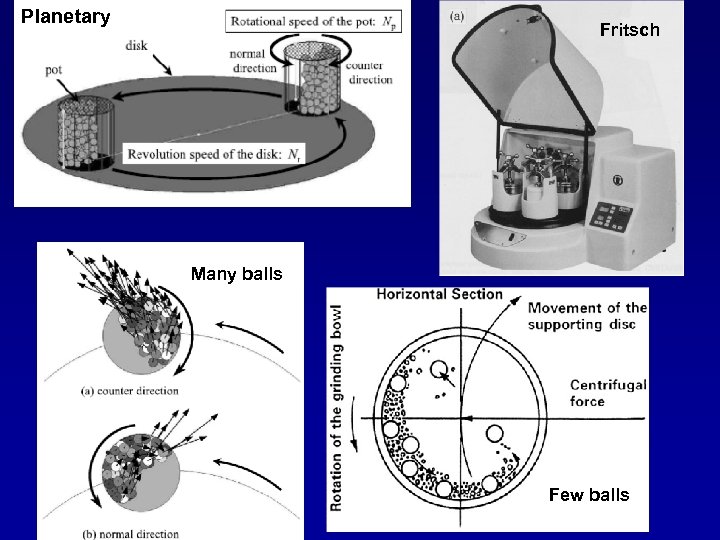 Planetary Fritsch Many balls Few balls
Planetary Fritsch Many balls Few balls
 Vibrational Sweco
Vibrational Sweco
 Spex mills
Spex mills
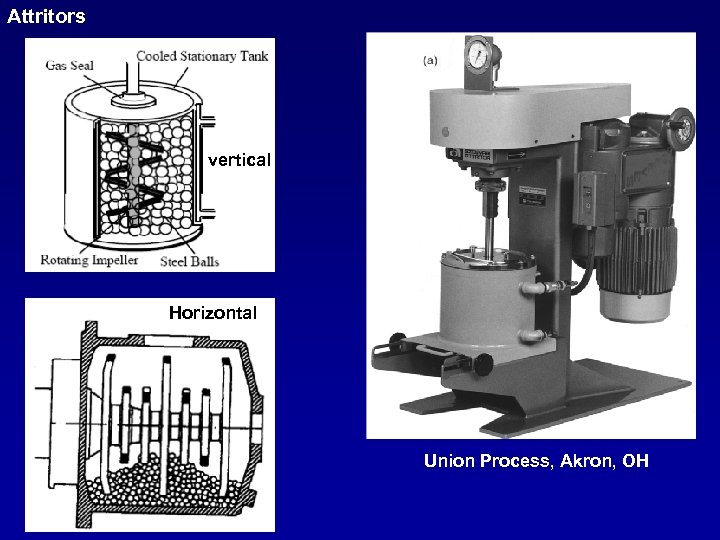 Attritors vertical Horizontal Union Process, Akron, OH
Attritors vertical Horizontal Union Process, Akron, OH
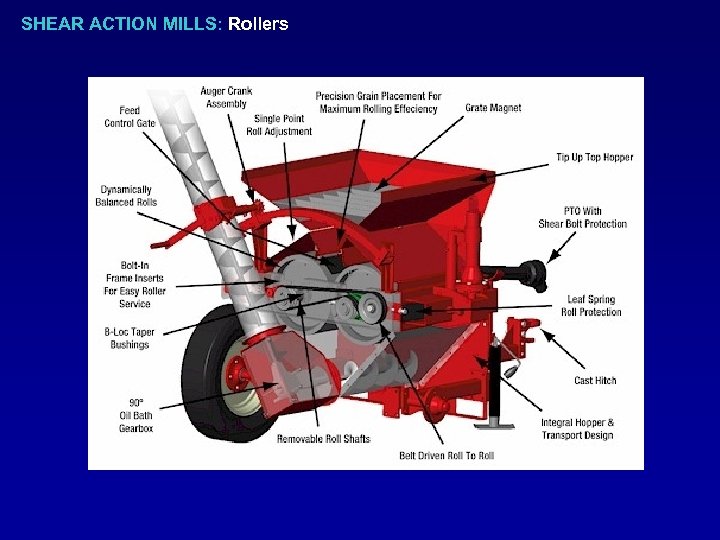 SHEAR ACTION MILLS: Rollers
SHEAR ACTION MILLS: Rollers
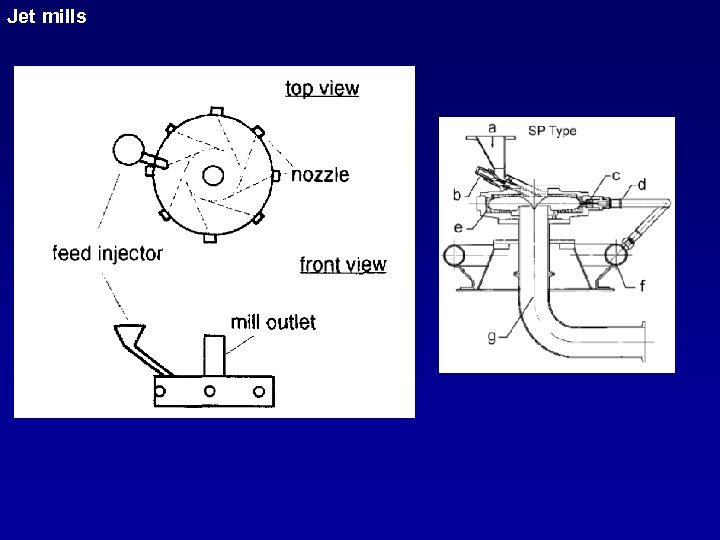 Jet mills
Jet mills
 Pin mills
Pin mills
![MILLS ENERGY [3] MILLS ENERGY [3]](https://present5.com/presentation/1866b8781d77d95fc98b491c128ebd67/image-26.jpg) MILLS ENERGY [3]
MILLS ENERGY [3]
 9 – CENTRAL QUESTION MILL OPERATION CONDITIONS GROUND MATERIAL PROPERTIES
9 – CENTRAL QUESTION MILL OPERATION CONDITIONS GROUND MATERIAL PROPERTIES
 1 TRIALS AND ERROR (small variations of the operating conditions) 2 MATHEMATICAL MODELLING APPROACH (attainment of general principles working for a wide range of operating conditions and different mills)
1 TRIALS AND ERROR (small variations of the operating conditions) 2 MATHEMATICAL MODELLING APPROACH (attainment of general principles working for a wide range of operating conditions and different mills)
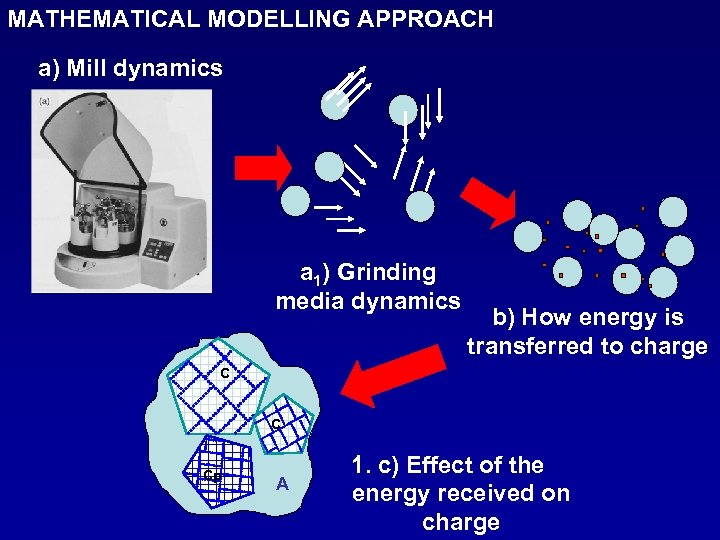 MATHEMATICAL MODELLING APPROACH a) Mill dynamics a 1) Grinding media dynamics b) How energy is transferred to charge C C Cp A 1. c) Effect of the energy received on charge
MATHEMATICAL MODELLING APPROACH a) Mill dynamics a 1) Grinding media dynamics b) How energy is transferred to charge C C Cp A 1. c) Effect of the energy received on charge
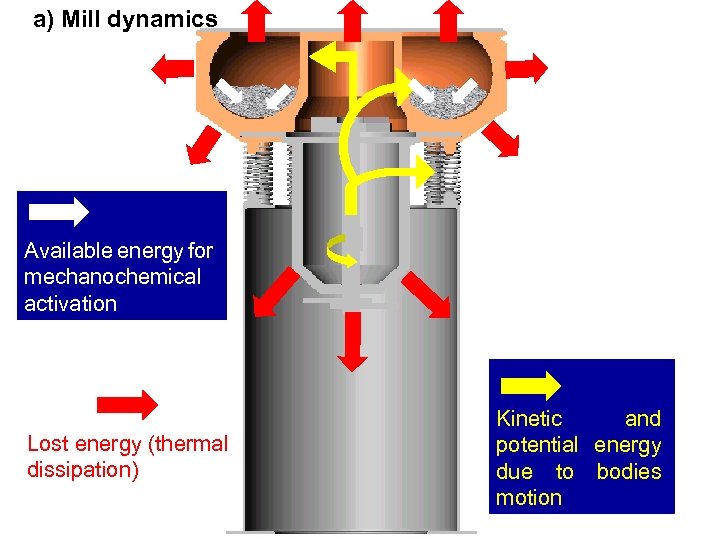 EXAMPLE: VIBRATORY MILL a) Mill dynamics Available energy for mechanochemical activation Lost energy (thermal dissipation) Kinetic and potential energy due to bodies motion
EXAMPLE: VIBRATORY MILL a) Mill dynamics Available energy for mechanochemical activation Lost energy (thermal dissipation) Kinetic and potential energy due to bodies motion
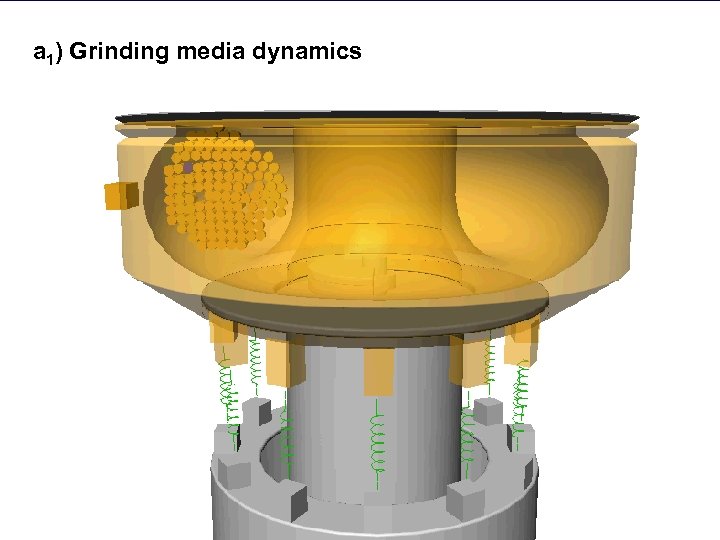 a 1) Grinding media dynamics
a 1) Grinding media dynamics
![b) Energy transfer to charge (uniformity conditions)[9] Charge Grinding medium k = charge fraction b) Energy transfer to charge (uniformity conditions)[9] Charge Grinding medium k = charge fraction](https://present5.com/presentation/1866b8781d77d95fc98b491c128ebd67/image-32.jpg) b) Energy transfer to charge (uniformity conditions)[9] Charge Grinding medium k = charge fraction involved in one hint
b) Energy transfer to charge (uniformity conditions)[9] Charge Grinding medium k = charge fraction involved in one hint
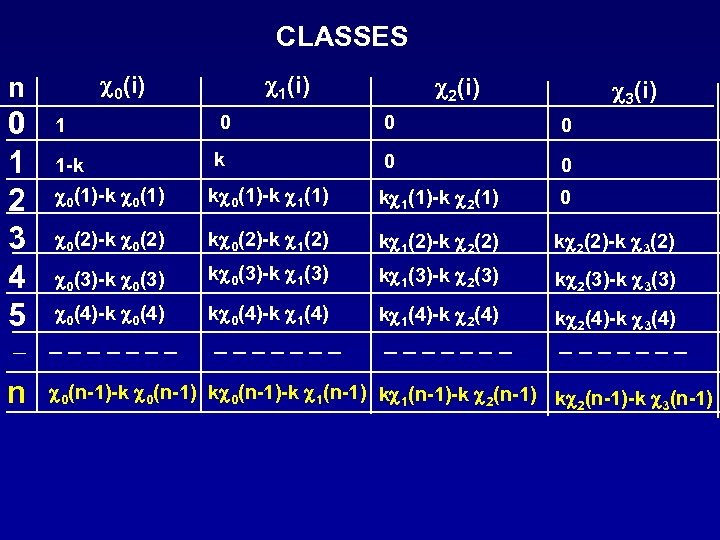 CLASSES c 0(i) n 0 1 2 3 4 5 c 2(i) c 3(i) 1 0 0 0 1 -k k 0 0 c 0(1)-k c 0(1) kc 0(1)-k c 1(1) kc 1(1)-k c 2(1) 0 c 0(2)-k c 0(2) kc 0(2)-k c 1(2) kc 1(2)-k c 2(2) kc 2(2)-k c 3(2) c 0(3)-k c 0(3) kc 0(3)-k c 1(3) kc 1(3)-k c 2(3) kc 2(3)-k c 3(3) c 0(4)-k c 0(4) kc 0(4)-k c 1(4) kc 1(4)-k c 2(4) kc 2(4)-k c 3(4) ------- - ------- n c 1(i) c 0(n-1)-k c 0(n-1) kc 0(n-1)-k c 1(n-1) kc 1(n-1)-k c 2(n-1) kc (n-1)-k c (n-1) 2 3
CLASSES c 0(i) n 0 1 2 3 4 5 c 2(i) c 3(i) 1 0 0 0 1 -k k 0 0 c 0(1)-k c 0(1) kc 0(1)-k c 1(1) kc 1(1)-k c 2(1) 0 c 0(2)-k c 0(2) kc 0(2)-k c 1(2) kc 1(2)-k c 2(2) kc 2(2)-k c 3(2) c 0(3)-k c 0(3) kc 0(3)-k c 1(3) kc 1(3)-k c 2(3) kc 2(3)-k c 3(3) c 0(4)-k c 0(4) kc 0(4)-k c 1(4) kc 1(4)-k c 2(4) kc 2(4)-k c 3(4) ------- - ------- n c 1(i) c 0(n-1)-k c 0(n-1) kc 0(n-1)-k c 1(n-1) kc 1(n-1)-k c 2(n-1) kc (n-1)-k c (n-1) 2 3
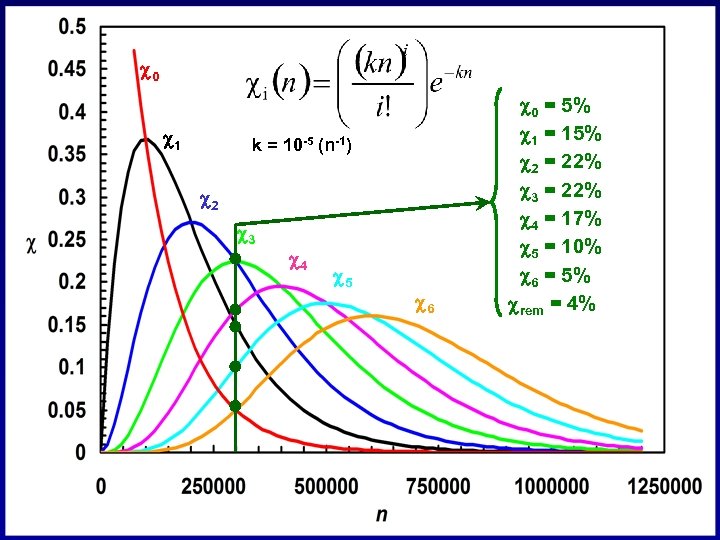 c 0 c 1 k = 10 -5 (n-1) c 2 c 3 c 4 c 5 c 6 c 0 = 5% c 1 = 15% c 2 = 22% c 3 = 22% c 4 = 17% c 5 = 10% c 6 = 5% crem = 4%
c 0 c 1 k = 10 -5 (n-1) c 2 c 3 c 4 c 5 c 6 c 0 = 5% c 1 = 15% c 2 = 22% c 3 = 22% c 4 = 17% c 5 = 10% c 6 = 5% crem = 4%
![c) Effect of the energy received on charge [10] k-1 Crystal Nano Crystal k c) Effect of the energy received on charge [10] k-1 Crystal Nano Crystal k](https://present5.com/presentation/1866b8781d77d95fc98b491c128ebd67/image-35.jpg) c) Effect of the energy received on charge [10] k-1 Crystal Nano Crystal k 1 k 3 k-2 k 2 Amorphous
c) Effect of the energy received on charge [10] k-1 Crystal Nano Crystal k 1 k 3 k-2 k 2 Amorphous
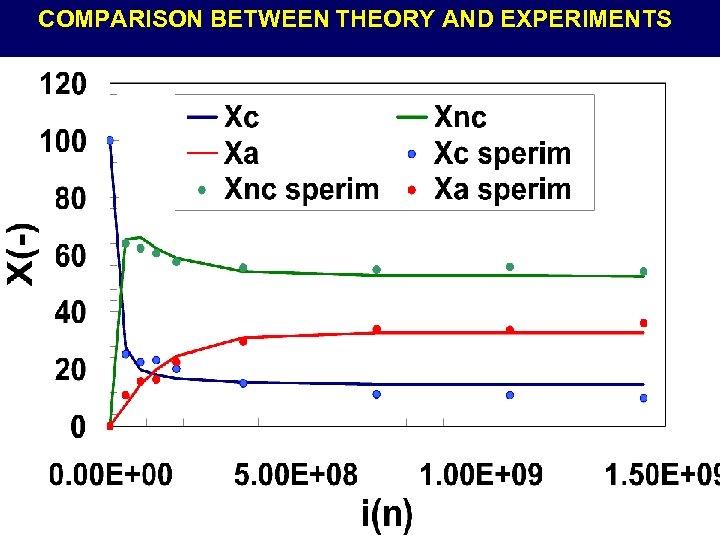 COMPARISON BETWEEN THEORY AND EXPERIMENTS
COMPARISON BETWEEN THEORY AND EXPERIMENTS
 10 REFERENCES 1. Tkacova K. 1993. First international conference on mechanochemistry: an introduction. Proc. First Intl. Conf. on Mechanochemitsry. Cambridge Interscience Publishing. 1: 9 -17. 2. Amidon GL, Lennernäs H, Vinod PS, Crison JR. 1995. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm. Res. , 12: 413 -420. 3. Tkacova K. 1989. Mechanical Activation of Minerals. Amsterdam, New York: Elsevier. 4. Adamson, Gast. Physical Chemistry of Surfaces; Wiley Interscience, New York, Toronto, 1997, chapters II, III and X. 5. Grassi M, Grassi G, Lapasin R, Colombo I. 2007. Understanding drug release and absorption mechanisms: a physical and mathematical approach. Boca Raton: CRC Press 6. Brun, Lallemand, Quinson, Eyraud. J. De Chimie Physique, 70(6) (1973) 979 -989.
10 REFERENCES 1. Tkacova K. 1993. First international conference on mechanochemistry: an introduction. Proc. First Intl. Conf. on Mechanochemitsry. Cambridge Interscience Publishing. 1: 9 -17. 2. Amidon GL, Lennernäs H, Vinod PS, Crison JR. 1995. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm. Res. , 12: 413 -420. 3. Tkacova K. 1989. Mechanical Activation of Minerals. Amsterdam, New York: Elsevier. 4. Adamson, Gast. Physical Chemistry of Surfaces; Wiley Interscience, New York, Toronto, 1997, chapters II, III and X. 5. Grassi M, Grassi G, Lapasin R, Colombo I. 2007. Understanding drug release and absorption mechanisms: a physical and mathematical approach. Boca Raton: CRC Press 6. Brun, Lallemand, Quinson, Eyraud. J. De Chimie Physique, 70(6) (1973) 979 -989.
 7. Bergese P, Colombo I, Gervasoni D, Depero LE. 2003. Assessment of the x-ray diffraction-absorption method for quantitative analysis of largely amorphous pharmaceutical composites. J. Appl. Cryst. 36: 74 -79. 8. Sugimoto M, Okagaki T, Narisawa S, Koida Y, Nakajima K. 1998. Improvement of dissolution characteristics and bioavailabilty of poorly water-soluble drugs by novel cogrinding method using water-soluble polymer. Int. J. Pharm. 160: 11 -19. 9. Delogu F, Cocco G. 2000. Relating Single-Impact Events to Macrokinetic Features in Mechanical Alloying Processes. J. Mat. Synthesis and Processing 8: 271 -277. 10. D. Manca, N. Coceani, L. Magarotto, I. Colombo, M. Grassi. High-Energy Mechanochemical Activation of Active Principles. Convegno GRICU 2004, Nuove Frontiere di Applicazione delle Metodologie dell’Ingegneria Chimica, Porto d’Ischia (Na), 12 -15 Settembre 2004, Volume I, 123 -126.
7. Bergese P, Colombo I, Gervasoni D, Depero LE. 2003. Assessment of the x-ray diffraction-absorption method for quantitative analysis of largely amorphous pharmaceutical composites. J. Appl. Cryst. 36: 74 -79. 8. Sugimoto M, Okagaki T, Narisawa S, Koida Y, Nakajima K. 1998. Improvement of dissolution characteristics and bioavailabilty of poorly water-soluble drugs by novel cogrinding method using water-soluble polymer. Int. J. Pharm. 160: 11 -19. 9. Delogu F, Cocco G. 2000. Relating Single-Impact Events to Macrokinetic Features in Mechanical Alloying Processes. J. Mat. Synthesis and Processing 8: 271 -277. 10. D. Manca, N. Coceani, L. Magarotto, I. Colombo, M. Grassi. High-Energy Mechanochemical Activation of Active Principles. Convegno GRICU 2004, Nuove Frontiere di Applicazione delle Metodologie dell’Ingegneria Chimica, Porto d’Ischia (Na), 12 -15 Settembre 2004, Volume I, 123 -126.


