 Heuristics for Process Synthesis CHEN 4460 – Process Synthesis, Simulation and Optimization Dr. Mario Richard Eden Department of Chemical Engineering Auburn University Lab Lecture No. 1 – Heuristics for Process Synthesis September 4, 2012 Contains Material Developed by Dr. Daniel R. Lewin, Technion, Israel
Heuristics for Process Synthesis CHEN 4460 – Process Synthesis, Simulation and Optimization Dr. Mario Richard Eden Department of Chemical Engineering Auburn University Lab Lecture No. 1 – Heuristics for Process Synthesis September 4, 2012 Contains Material Developed by Dr. Daniel R. Lewin, Technion, Israel
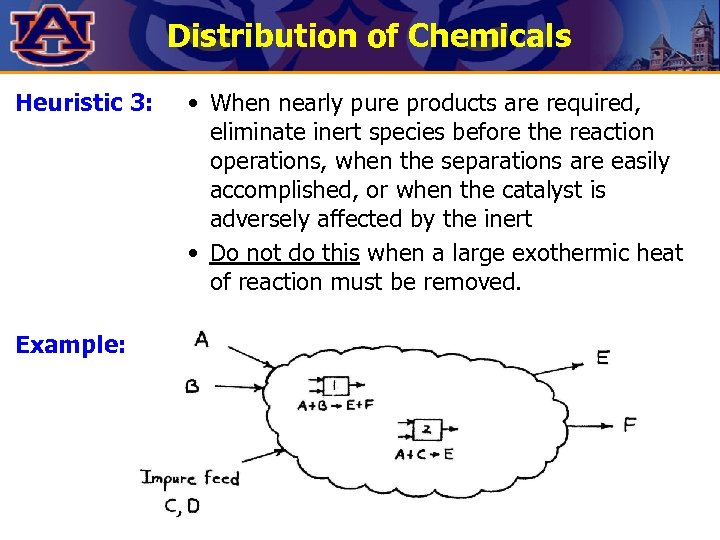 Distribution of Chemicals Heuristic 3: Example: • When nearly pure products are required, eliminate inert species before the reaction operations, when the separations are easily accomplished, or when the catalyst is adversely affected by the inert • Do not do this when a large exothermic heat of reaction must be removed.
Distribution of Chemicals Heuristic 3: Example: • When nearly pure products are required, eliminate inert species before the reaction operations, when the separations are easily accomplished, or when the catalyst is adversely affected by the inert • Do not do this when a large exothermic heat of reaction must be removed.
 Example: MTBE Manufacture To satisfy the Clean Air Act of 1990, gasoline must have a minimum oxygen atom content of 2. 0 mole%. The most common source of this oxygen is methyl-tertiary-butyl-ether (MTBE) which is manufactured by the reaction: CH 3 OH + Iso-butene MTBE
Example: MTBE Manufacture To satisfy the Clean Air Act of 1990, gasoline must have a minimum oxygen atom content of 2. 0 mole%. The most common source of this oxygen is methyl-tertiary-butyl-ether (MTBE) which is manufactured by the reaction: CH 3 OH + Iso-butene MTBE
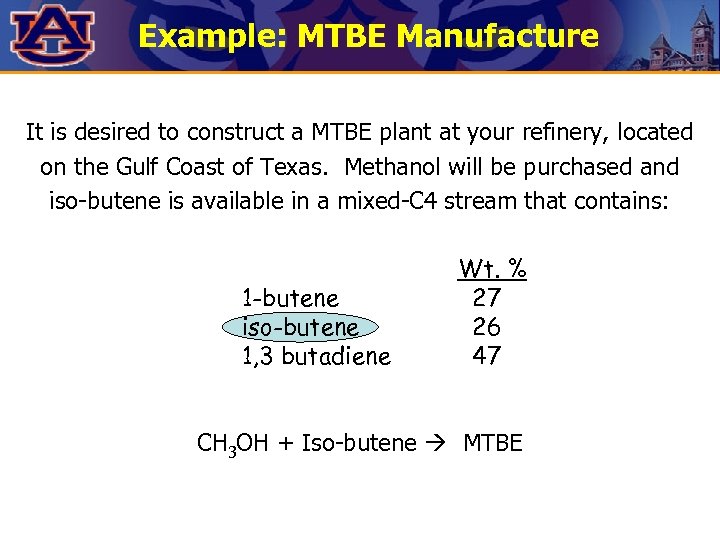 Example: MTBE Manufacture It is desired to construct a MTBE plant at your refinery, located on the Gulf Coast of Texas. Methanol will be purchased and iso-butene is available in a mixed-C 4 stream that contains: 1 -butene iso-butene 1, 3 butadiene Wt. % 27 26 47 CH 3 OH + Iso-butene MTBE
Example: MTBE Manufacture It is desired to construct a MTBE plant at your refinery, located on the Gulf Coast of Texas. Methanol will be purchased and iso-butene is available in a mixed-C 4 stream that contains: 1 -butene iso-butene 1, 3 butadiene Wt. % 27 26 47 CH 3 OH + Iso-butene MTBE
 Example: MTBE Manufacture a 1 -butene 5. 13 1, 3 -butadiene 4. 96 iso-butene 4. 04 1, 3 -butadiene & 1 -butene, can be separated by distillation before or after the reaction operation. Other considerations, such as their impact on the catalyst, the volumes of the reactors and distillation towers, and the temperature levels in the exothermic reactors should be evaluated in making this decision.
Example: MTBE Manufacture a 1 -butene 5. 13 1, 3 -butadiene 4. 96 iso-butene 4. 04 1, 3 -butadiene & 1 -butene, can be separated by distillation before or after the reaction operation. Other considerations, such as their impact on the catalyst, the volumes of the reactors and distillation towers, and the temperature levels in the exothermic reactors should be evaluated in making this decision.
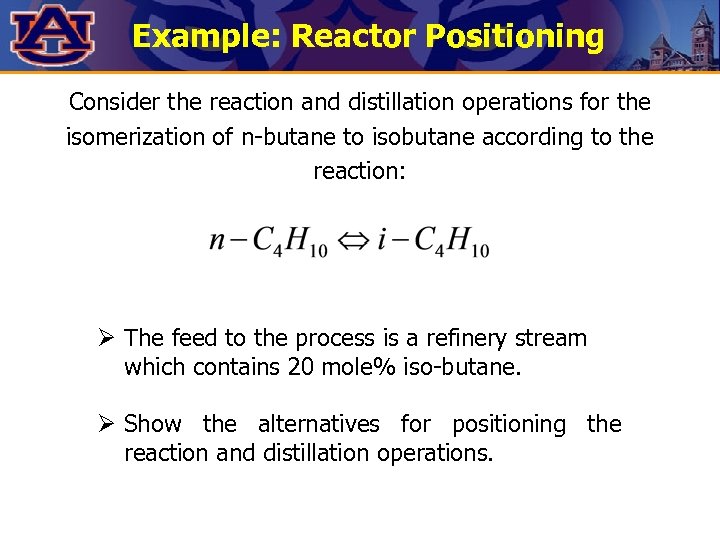 Example: Reactor Positioning Consider the reaction and distillation operations for the isomerization of n-butane to isobutane according to the reaction: Ø The feed to the process is a refinery stream which contains 20 mole% iso-butane. Ø Show the alternatives for positioning the reaction and distillation operations.
Example: Reactor Positioning Consider the reaction and distillation operations for the isomerization of n-butane to isobutane according to the reaction: Ø The feed to the process is a refinery stream which contains 20 mole% iso-butane. Ø Show the alternatives for positioning the reaction and distillation operations.
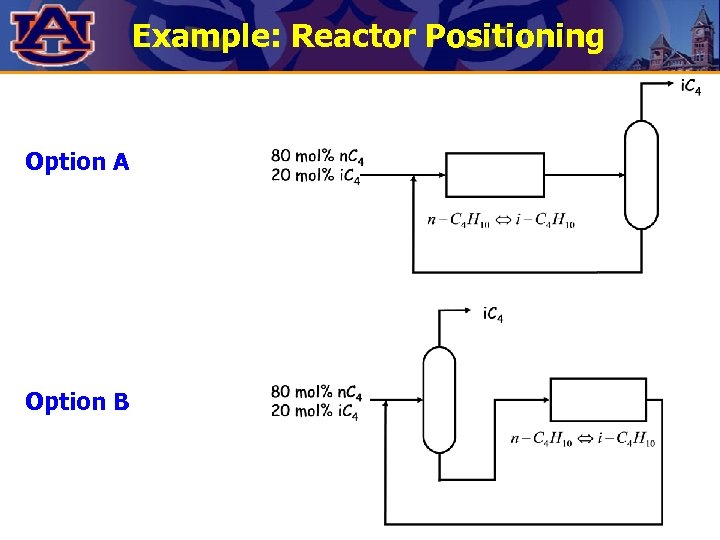 Example: Reactor Positioning Option A Option B
Example: Reactor Positioning Option A Option B
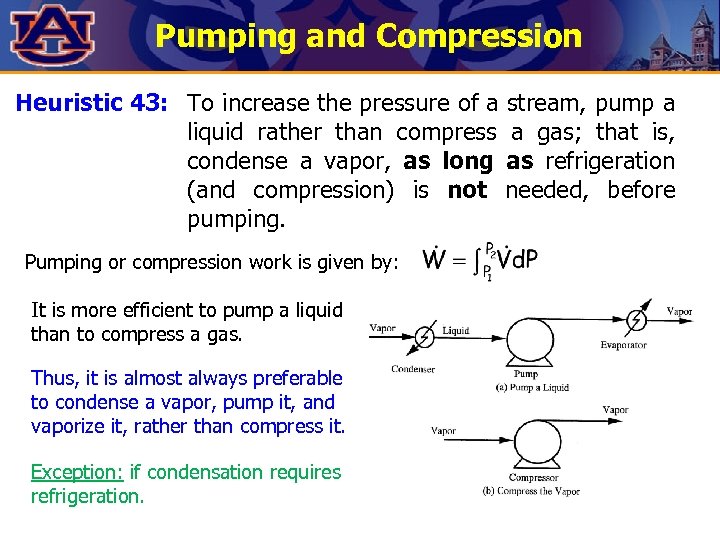 Pumping and Compression Heuristic 43: To increase the pressure of a stream, pump a liquid rather than compress a gas; that is, condense a vapor, as long as refrigeration (and compression) is not needed, before pumping. Pumping or compression work is given by: It is more efficient to pump a liquid than to compress a gas. Thus, it is almost always preferable to condense a vapor, pump it, and vaporize it, rather than compress it. Exception: if condensation requires refrigeration.
Pumping and Compression Heuristic 43: To increase the pressure of a stream, pump a liquid rather than compress a gas; that is, condense a vapor, as long as refrigeration (and compression) is not needed, before pumping. Pumping or compression work is given by: It is more efficient to pump a liquid than to compress a gas. Thus, it is almost always preferable to condense a vapor, pump it, and vaporize it, rather than compress it. Exception: if condensation requires refrigeration.
 Example: Feed Preparation Ethylbenzene is to be taken from storage at 25°C and 1 atm and fed to a styrene reactor at 400°C and 5 atm at 100, 000 lb/h. Ø Show two alternatives for positioning the temperature and pressure-increase operations.
Example: Feed Preparation Ethylbenzene is to be taken from storage at 25°C and 1 atm and fed to a styrene reactor at 400°C and 5 atm at 100, 000 lb/h. Ø Show two alternatives for positioning the temperature and pressure-increase operations.
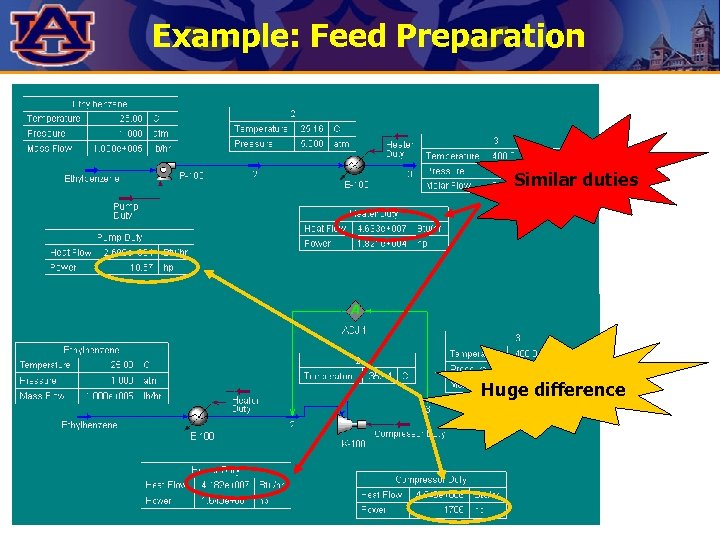 Example: Feed Preparation Similar duties Huge difference
Example: Feed Preparation Similar duties Huge difference
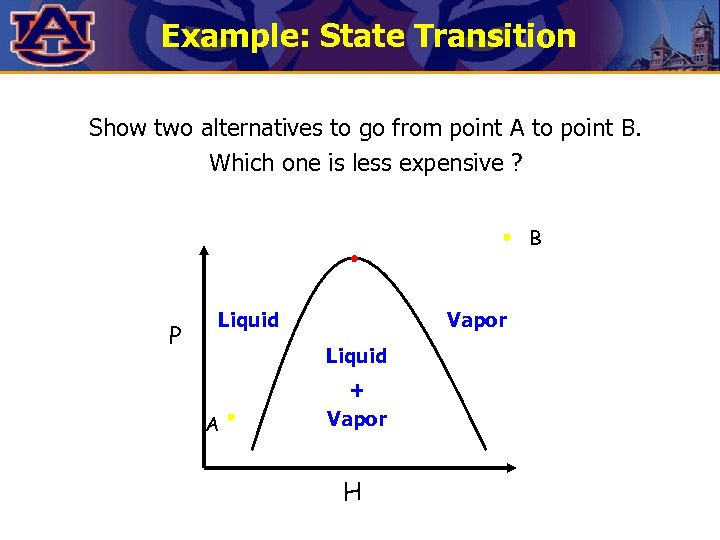 Example: State Transition Show two alternatives to go from point A to point B. Which one is less expensive ? B P Liquid Vapor Liquid A + Vapor H
Example: State Transition Show two alternatives to go from point A to point B. Which one is less expensive ? B P Liquid Vapor Liquid A + Vapor H
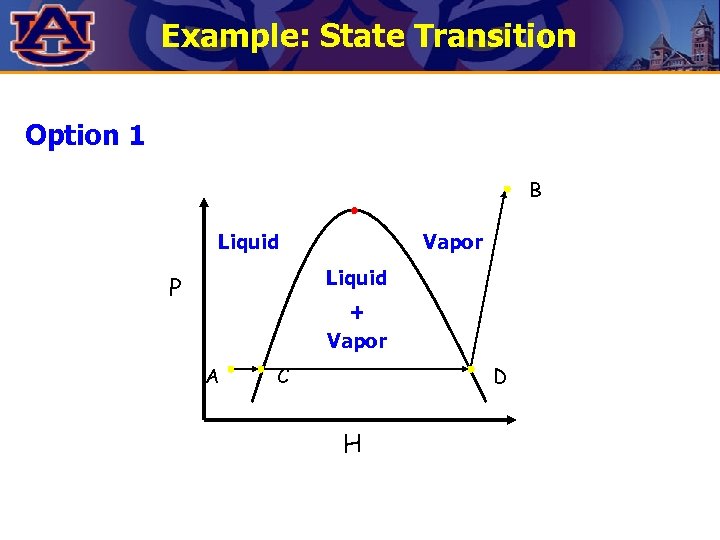 Example: State Transition Option 1 B Liquid Vapor Liquid P + Vapor A C D H
Example: State Transition Option 1 B Liquid Vapor Liquid P + Vapor A C D H
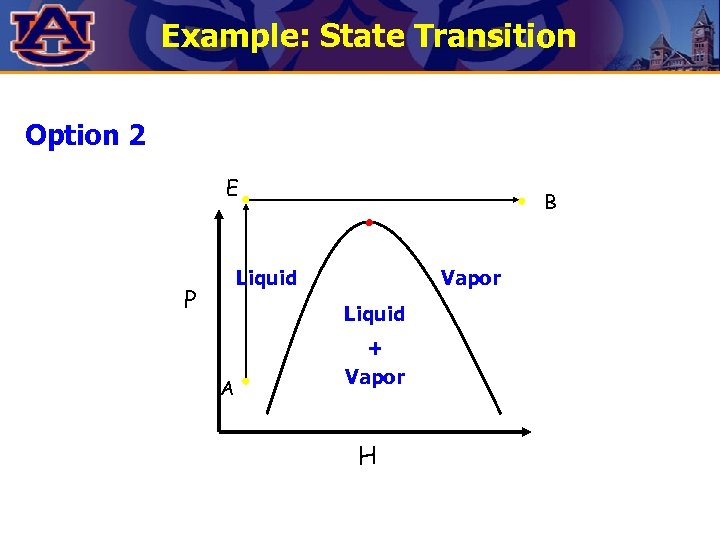 Example: State Transition Option 2 E B Liquid P Vapor Liquid A + Vapor H
Example: State Transition Option 2 E B Liquid P Vapor Liquid A + Vapor H