d320fd74f313cbd54abe4810fad2447c.ppt
- Количество слайдов: 57
 Hereditary Angioedema Market A MARKET WITH A LOT OF COMPETITORS. By Philippe Breton Alexia Dumoulin Hortense Masias
Hereditary Angioedema Market A MARKET WITH A LOT OF COMPETITORS. By Philippe Breton Alexia Dumoulin Hortense Masias
 Presentation of the pathology
Presentation of the pathology
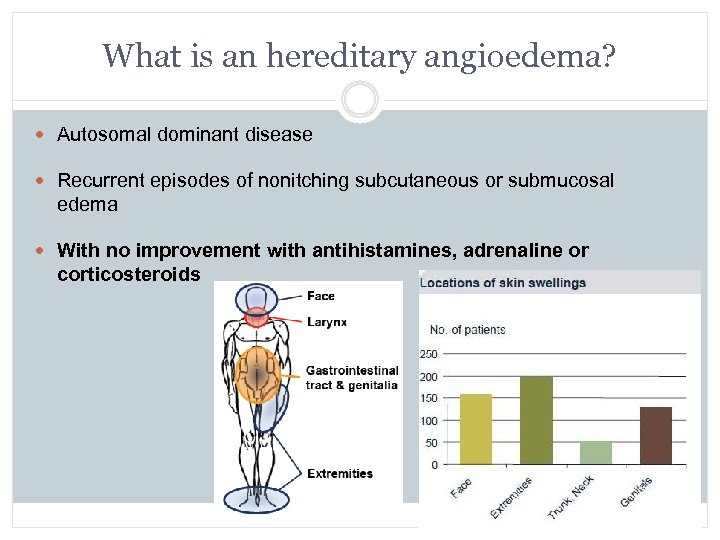 What is an hereditary angioedema? Autosomal dominant disease Recurrent episodes of nonitching subcutaneous or submucosal edema With no improvement with antihistamines, adrenaline or corticosteroids
What is an hereditary angioedema? Autosomal dominant disease Recurrent episodes of nonitching subcutaneous or submucosal edema With no improvement with antihistamines, adrenaline or corticosteroids
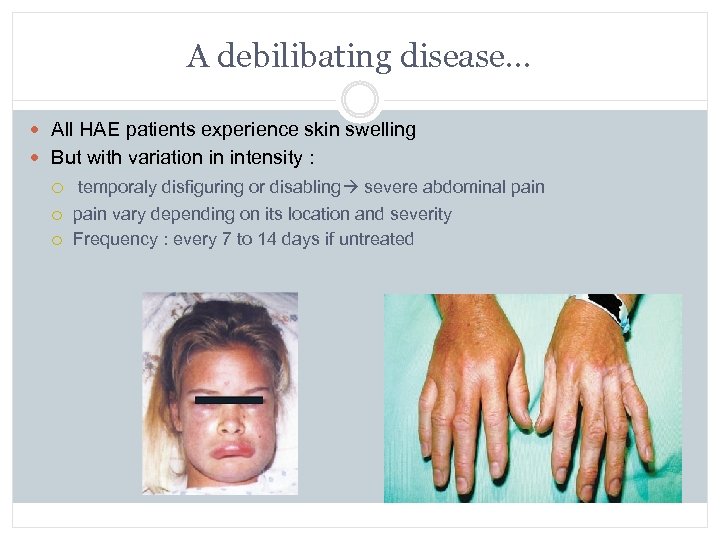 A debilibating disease… All HAE patients experience skin swelling But with variation in intensity : temporaly disfiguring or disabling severe abdominal pain vary depending on its location and severity Frequency : every 7 to 14 days if untreated
A debilibating disease… All HAE patients experience skin swelling But with variation in intensity : temporaly disfiguring or disabling severe abdominal pain vary depending on its location and severity Frequency : every 7 to 14 days if untreated
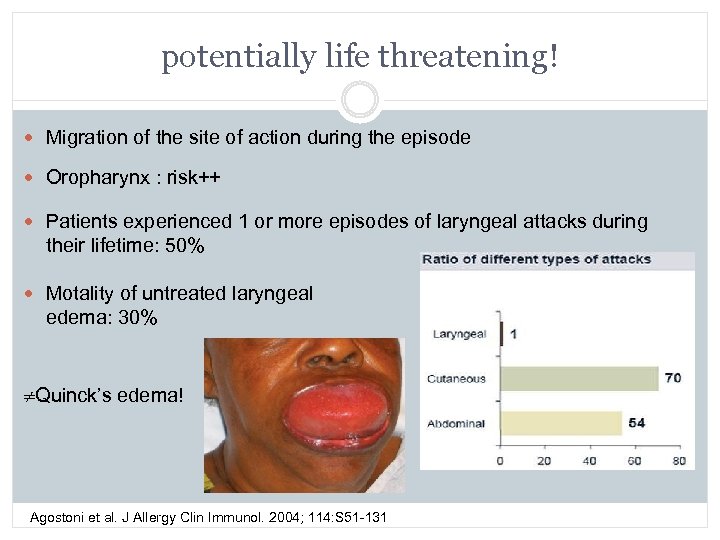 potentially life threatening! Migration of the site of action during the episode Oropharynx : risk++ Patients experienced 1 or more episodes of laryngeal attacks during their lifetime: 50% Motality of untreated laryngeal edema: 30% Quinck’s edema! Agostoni et al. J Allergy Clin Immunol. 2004; 114: S 51 -131
potentially life threatening! Migration of the site of action during the episode Oropharynx : risk++ Patients experienced 1 or more episodes of laryngeal attacks during their lifetime: 50% Motality of untreated laryngeal edema: 30% Quinck’s edema! Agostoni et al. J Allergy Clin Immunol. 2004; 114: S 51 -131
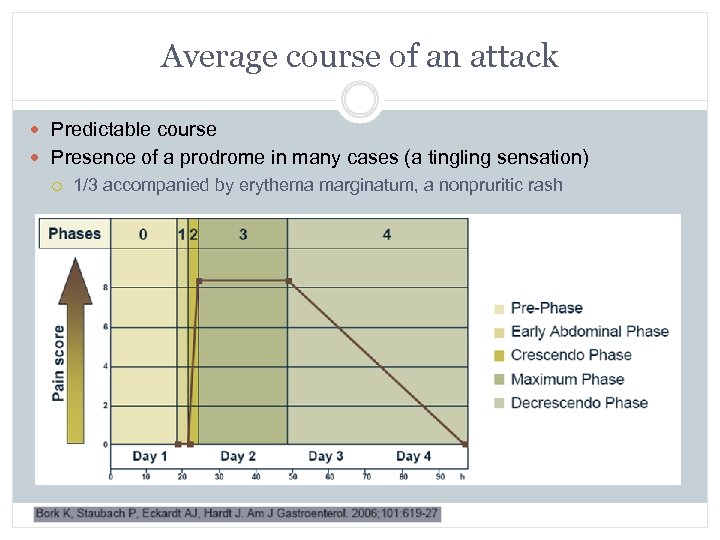 Average course of an attack Predictable course Presence of a prodrome in many cases (a tingling sensation) 1/3 accompanied by erythema marginatum, a nonpruritic rash
Average course of an attack Predictable course Presence of a prodrome in many cases (a tingling sensation) 1/3 accompanied by erythema marginatum, a nonpruritic rash
 Epidemiology Prevalence : 1 -2 cases / 50 000 (Orphan) No differences known among ethnics groups Symptoms typically begin in childhood ( 2 or 3 years of age), worsen around puberty, and persist throughout life, with unpredictable severity. Origin of the attacks : unknown Potential trigger : stress, dental procedures, minor trauma Menstruation, pregnancy, oral contraceptives and hormone replacement therapy worsen
Epidemiology Prevalence : 1 -2 cases / 50 000 (Orphan) No differences known among ethnics groups Symptoms typically begin in childhood ( 2 or 3 years of age), worsen around puberty, and persist throughout life, with unpredictable severity. Origin of the attacks : unknown Potential trigger : stress, dental procedures, minor trauma Menstruation, pregnancy, oral contraceptives and hormone replacement therapy worsen
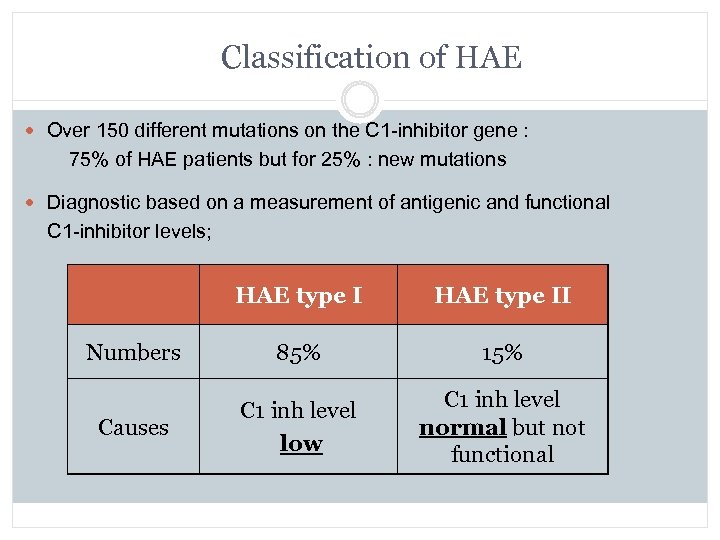 Classification of HAE Over 150 different mutations on the C 1 -inhibitor gene : 75% of HAE patients but for 25% : new mutations Diagnostic based on a measurement of antigenic and functional C 1 -inhibitor levels; HAE type II Numbers 85% 15% Causes C 1 inh level low C 1 inh level normal but not functional
Classification of HAE Over 150 different mutations on the C 1 -inhibitor gene : 75% of HAE patients but for 25% : new mutations Diagnostic based on a measurement of antigenic and functional C 1 -inhibitor levels; HAE type II Numbers 85% 15% Causes C 1 inh level low C 1 inh level normal but not functional
 First difficulty : the diagnostic Antigenic and functional C 1 INH levels Persistently low antigenic C 4 level Often mis or underdiagnosed : ½ in the US many HAE symptoms # allergies, headaches, colic, appendicitis symptoms may be not recurrent or do not recur in the same location various angioedema with similar clinical and laboratory data
First difficulty : the diagnostic Antigenic and functional C 1 INH levels Persistently low antigenic C 4 level Often mis or underdiagnosed : ½ in the US many HAE symptoms # allergies, headaches, colic, appendicitis symptoms may be not recurrent or do not recur in the same location various angioedema with similar clinical and laboratory data
 Current treatments
Current treatments
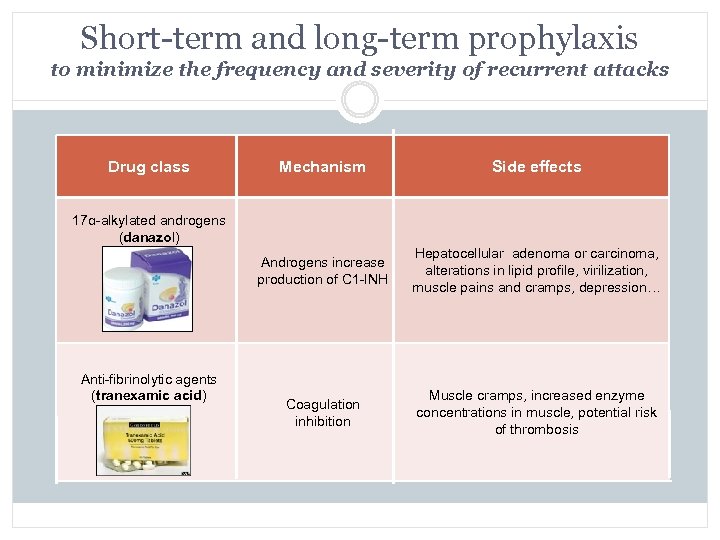 Short-term and long-term prophylaxis to minimize the frequency and severity of recurrent attacks Drug class Mechanism Side effects Androgens increase production of C 1 -INH Hepatocellular adenoma or carcinoma, alterations in lipid profile, virilization, muscle pains and cramps, depression… Coagulation inhibition Muscle cramps, increased enzyme concentrations in muscle, potential risk of thrombosis 17α-alkylated androgens (danazol) Anti-fibrinolytic agents (tranexamic acid)
Short-term and long-term prophylaxis to minimize the frequency and severity of recurrent attacks Drug class Mechanism Side effects Androgens increase production of C 1 -INH Hepatocellular adenoma or carcinoma, alterations in lipid profile, virilization, muscle pains and cramps, depression… Coagulation inhibition Muscle cramps, increased enzyme concentrations in muscle, potential risk of thrombosis 17α-alkylated androgens (danazol) Anti-fibrinolytic agents (tranexamic acid)
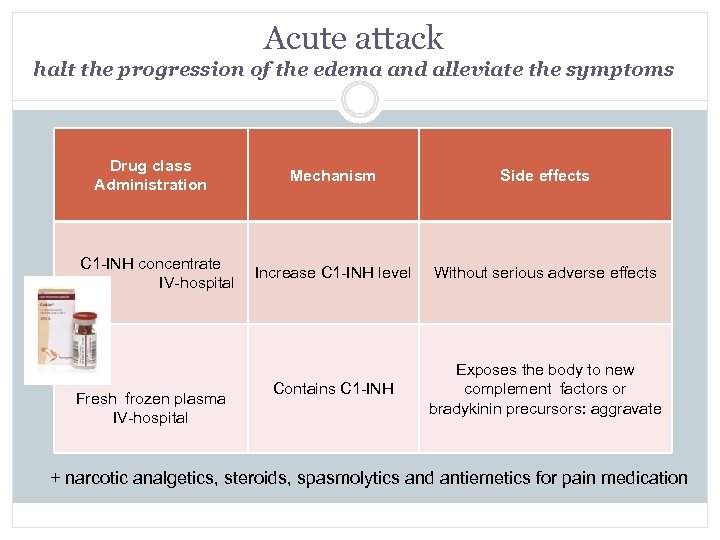 Acute attack halt the progression of the edema and alleviate the symptoms Drug class Administration Mechanism Side effects C 1 -INH concentrate IV-hospital Increase C 1 -INH level Without serious adverse effects Contains C 1 -INH Exposes the body to new complement factors or bradykinin precursors: aggravate Fresh frozen plasma IV-hospital + narcotic analgetics, steroids, spasmolytics and antiemetics for pain medication
Acute attack halt the progression of the edema and alleviate the symptoms Drug class Administration Mechanism Side effects C 1 -INH concentrate IV-hospital Increase C 1 -INH level Without serious adverse effects Contains C 1 -INH Exposes the body to new complement factors or bradykinin precursors: aggravate Fresh frozen plasma IV-hospital + narcotic analgetics, steroids, spasmolytics and antiemetics for pain medication
 HAE pathophysiology : What is the best way to treat ?
HAE pathophysiology : What is the best way to treat ?
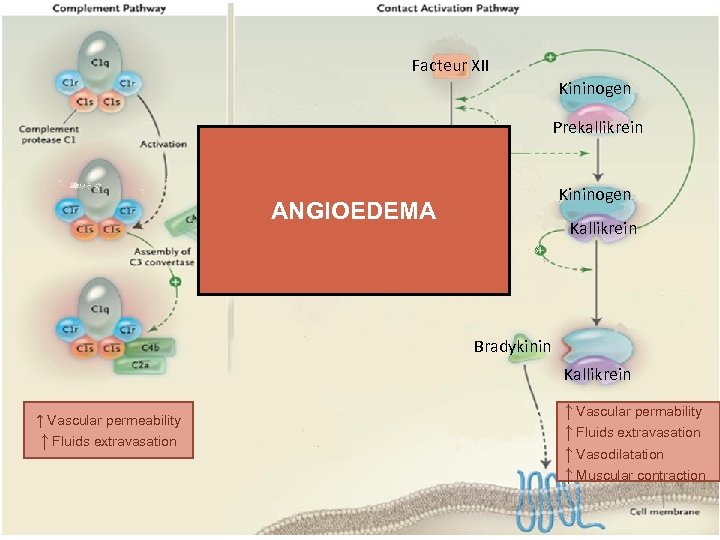 Facteur XII Kininogen Prekallikrein Facteur XIIa Kininogen ANGIOEDEMA Kallikrein Bradykinin Kallikrein ↑ Vascular permeability ↑ Fluids extravasation ↑ Vascular permability ↑ Fluids extravasation ↑ Vasodilatation ↑ Muscular contraction
Facteur XII Kininogen Prekallikrein Facteur XIIa Kininogen ANGIOEDEMA Kallikrein Bradykinin Kallikrein ↑ Vascular permeability ↑ Fluids extravasation ↑ Vascular permability ↑ Fluids extravasation ↑ Vasodilatation ↑ Muscular contraction
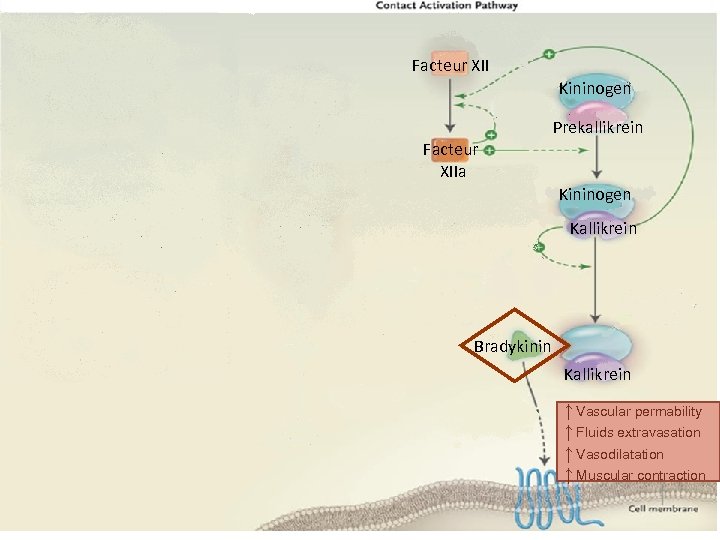 Facteur XII Kininogen Prekallikrein Facteur XIIa Kininogen Kallikrein Bradykinin Kallikrein ↑ Vascular permability ↑ Fluids extravasation ↑ Vasodilatation ↑ Muscular contraction
Facteur XII Kininogen Prekallikrein Facteur XIIa Kininogen Kallikrein Bradykinin Kallikrein ↑ Vascular permability ↑ Fluids extravasation ↑ Vasodilatation ↑ Muscular contraction
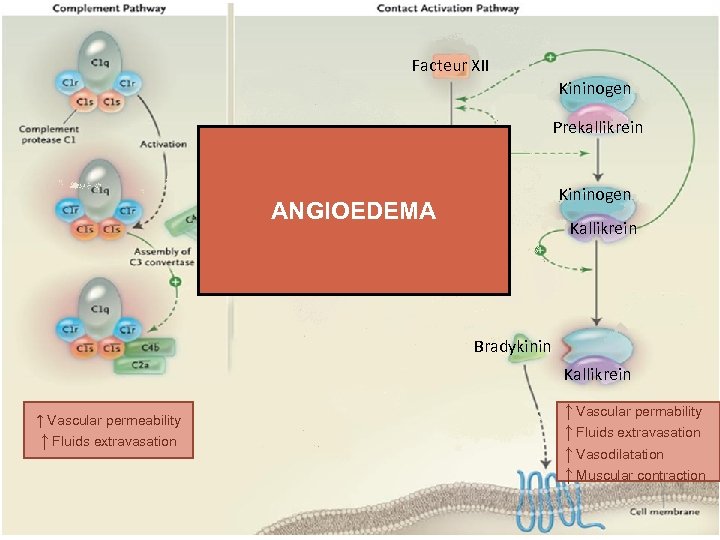 Facteur XII Kininogen Prekallikrein Facteur XIIa Kininogen ANGIOEDEMA Kallikrein Bradykinin Kallikrein ↑ Vascular permeability ↑ Fluids extravasation ↑ Vascular permability ↑ Fluids extravasation ↑ Vasodilatation ↑ Muscular contraction
Facteur XII Kininogen Prekallikrein Facteur XIIa Kininogen ANGIOEDEMA Kallikrein Bradykinin Kallikrein ↑ Vascular permeability ↑ Fluids extravasation ↑ Vascular permability ↑ Fluids extravasation ↑ Vasodilatation ↑ Muscular contraction
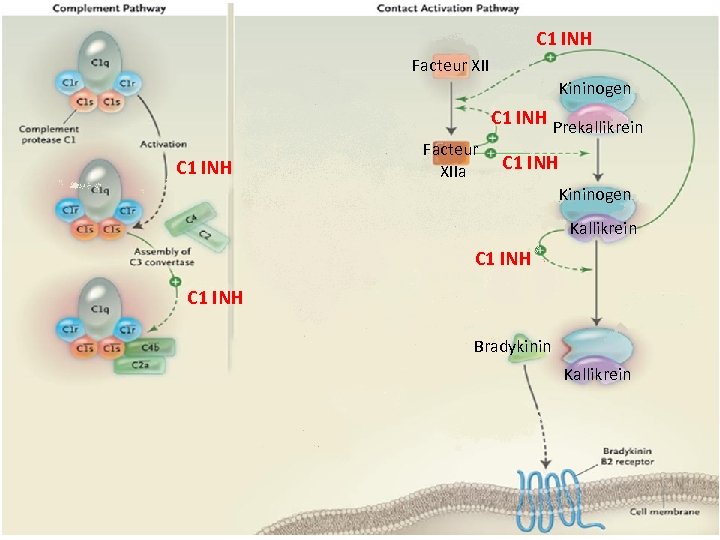 C 1 INH Facteur XII Kininogen C 1 INH Prekallikrein C 1 INH Facteur XIIa C 1 INH Kininogen Kallikrein C 1 INH Bradykinin Kallikrein
C 1 INH Facteur XII Kininogen C 1 INH Prekallikrein C 1 INH Facteur XIIa C 1 INH Kininogen Kallikrein C 1 INH Bradykinin Kallikrein
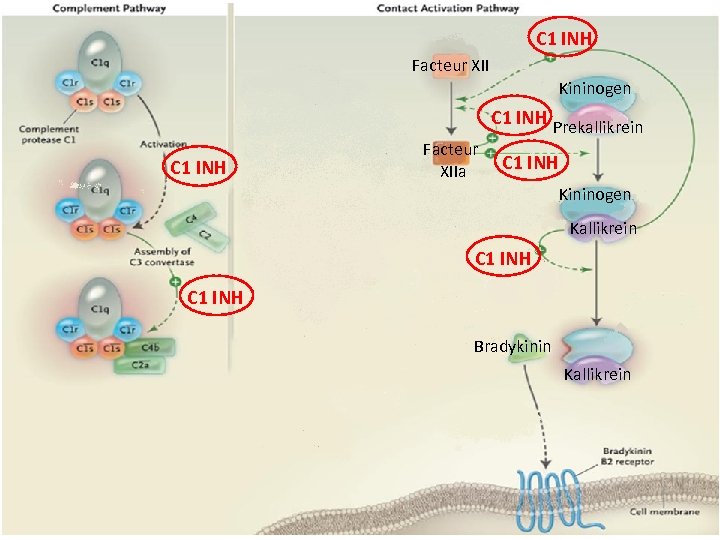 C 1 INH Facteur XII Kininogen C 1 INH Prekallikrein C 1 INH Facteur XIIa C 1 INH Kininogen Kallikrein C 1 INH Bradykinin Kallikrein
C 1 INH Facteur XII Kininogen C 1 INH Prekallikrein C 1 INH Facteur XIIa C 1 INH Kininogen Kallikrein C 1 INH Bradykinin Kallikrein
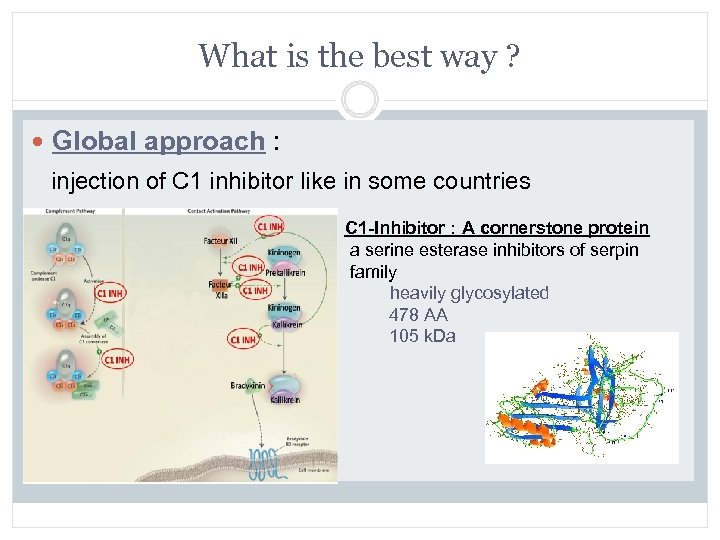 What is the best way ? Global approach : injection of C 1 inhibitor like in some countries C 1 -Inhibitor : A cornerstone protein a serine esterase inhibitors of serpin family heavily glycosylated 478 AA 105 k. Da
What is the best way ? Global approach : injection of C 1 inhibitor like in some countries C 1 -Inhibitor : A cornerstone protein a serine esterase inhibitors of serpin family heavily glycosylated 478 AA 105 k. Da
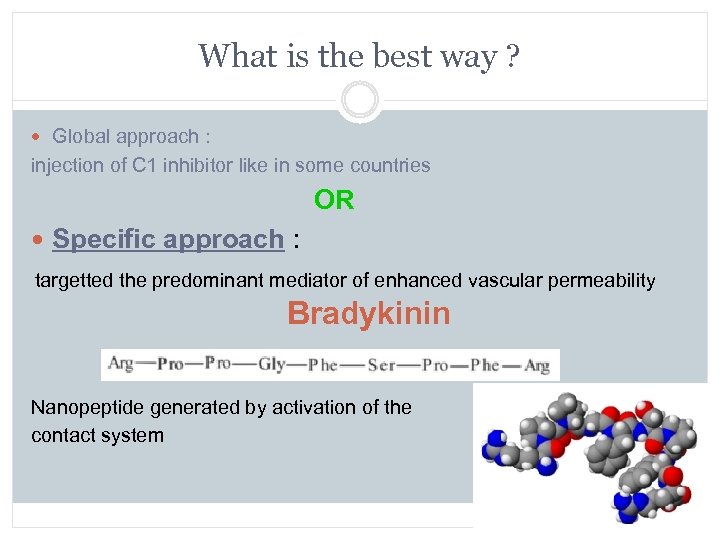 What is the best way ? Global approach : injection of C 1 inhibitor like in some countries OR Specific approach : targetted the predominant mediator of enhanced vascular permeability Bradykinin Nanopeptide generated by activation of the contact system
What is the best way ? Global approach : injection of C 1 inhibitor like in some countries OR Specific approach : targetted the predominant mediator of enhanced vascular permeability Bradykinin Nanopeptide generated by activation of the contact system
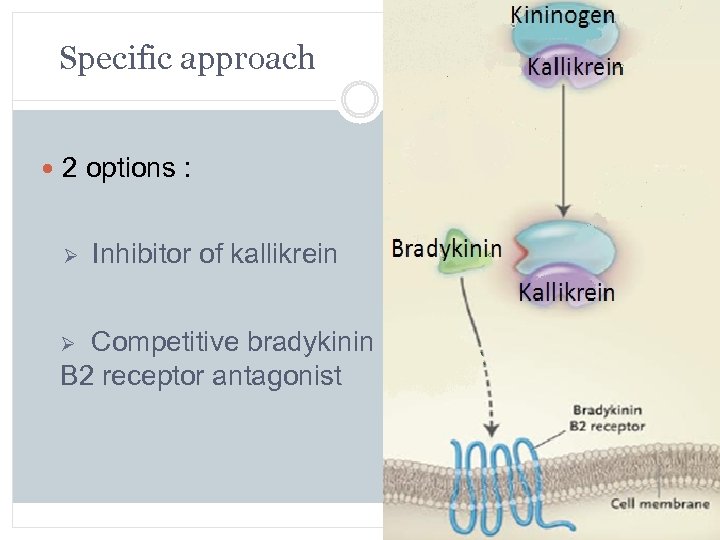 Specific approach 2 options : Ø Inhibitor of kallikrein Ø Competitive bradykinin B 2 receptor antagonist
Specific approach 2 options : Ø Inhibitor of kallikrein Ø Competitive bradykinin B 2 receptor antagonist
 New developments
New developments
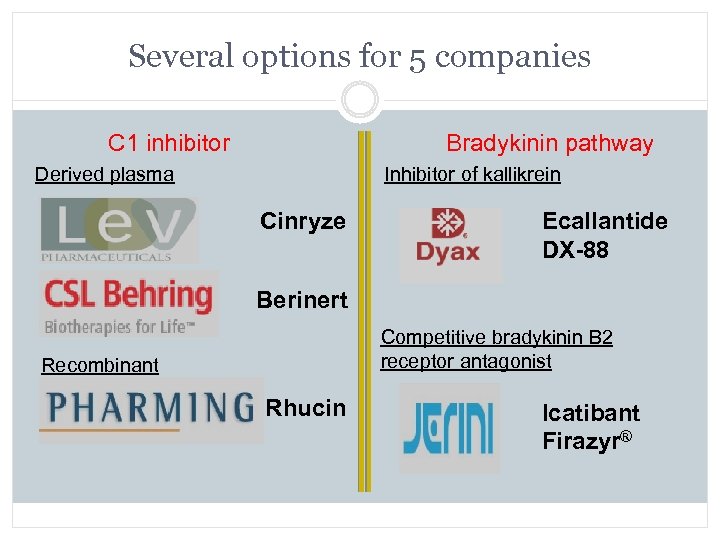 Several options for 5 companies C 1 inhibitor Bradykinin pathway Derived plasma Inhibitor of kallikrein Cinryze Ecallantide DX-88 Berinert Competitive bradykinin B 2 receptor antagonist Recombinant Rhucin Icatibant Firazyr®
Several options for 5 companies C 1 inhibitor Bradykinin pathway Derived plasma Inhibitor of kallikrein Cinryze Ecallantide DX-88 Berinert Competitive bradykinin B 2 receptor antagonist Recombinant Rhucin Icatibant Firazyr®
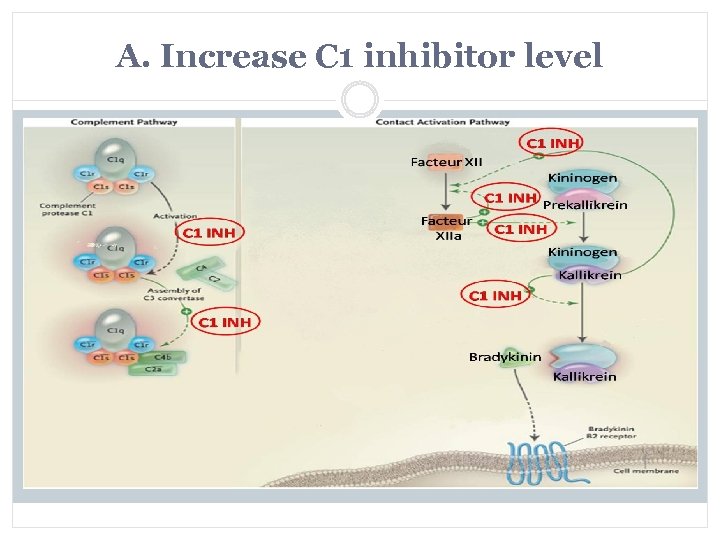 A. Increase C 1 inhibitor level
A. Increase C 1 inhibitor level
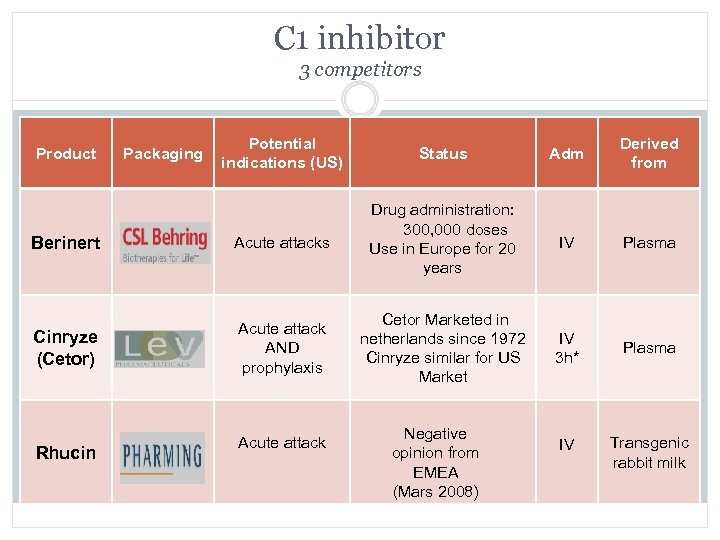 C 1 inhibitor 3 competitors Potential indications (US) Status Adm Derived from Berinert Acute attacks Drug administration: 300, 000 doses Use in Europe for 20 years IV Plasma Cinryze (Cetor) Acute attack AND prophylaxis Cetor Marketed in netherlands since 1972 Cinryze similar for US Market IV 3 h* Plasma IV Transgenic rabbit milk Product Rhucin Packaging Acute attack Negative opinion from EMEA (Mars 2008)
C 1 inhibitor 3 competitors Potential indications (US) Status Adm Derived from Berinert Acute attacks Drug administration: 300, 000 doses Use in Europe for 20 years IV Plasma Cinryze (Cetor) Acute attack AND prophylaxis Cetor Marketed in netherlands since 1972 Cinryze similar for US Market IV 3 h* Plasma IV Transgenic rabbit milk Product Rhucin Packaging Acute attack Negative opinion from EMEA (Mars 2008)
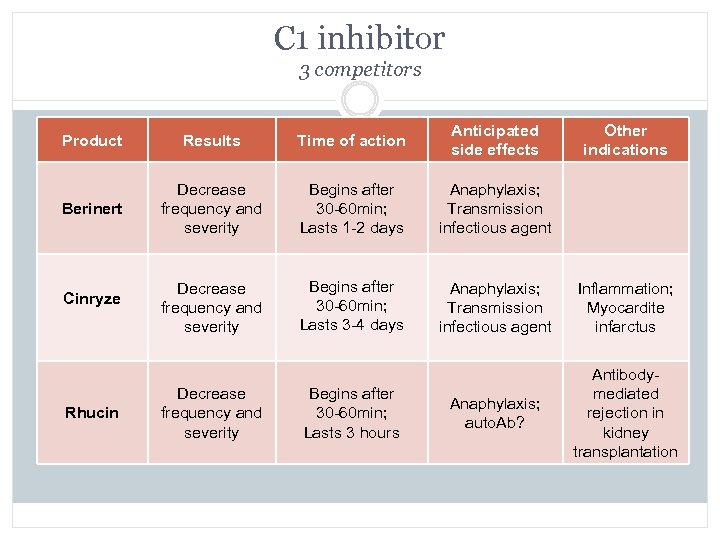 C 1 inhibitor 3 competitors Product Results Time of action Anticipated side effects Berinert Decrease frequency and severity Begins after 30 -60 min; Lasts 1 -2 days Anaphylaxis; Transmission infectious agent Decrease frequency and severity Begins after 30 -60 min; Lasts 3 -4 days Anaphylaxis; Transmission infectious agent Inflammation; Myocardite infarctus Anaphylaxis; auto. Ab? Antibodymediated rejection in kidney transplantation Cinryze Rhucin Decrease frequency and severity Begins after 30 -60 min; Lasts 3 hours Other indications
C 1 inhibitor 3 competitors Product Results Time of action Anticipated side effects Berinert Decrease frequency and severity Begins after 30 -60 min; Lasts 1 -2 days Anaphylaxis; Transmission infectious agent Decrease frequency and severity Begins after 30 -60 min; Lasts 3 -4 days Anaphylaxis; Transmission infectious agent Inflammation; Myocardite infarctus Anaphylaxis; auto. Ab? Antibodymediated rejection in kidney transplantation Cinryze Rhucin Decrease frequency and severity Begins after 30 -60 min; Lasts 3 hours Other indications
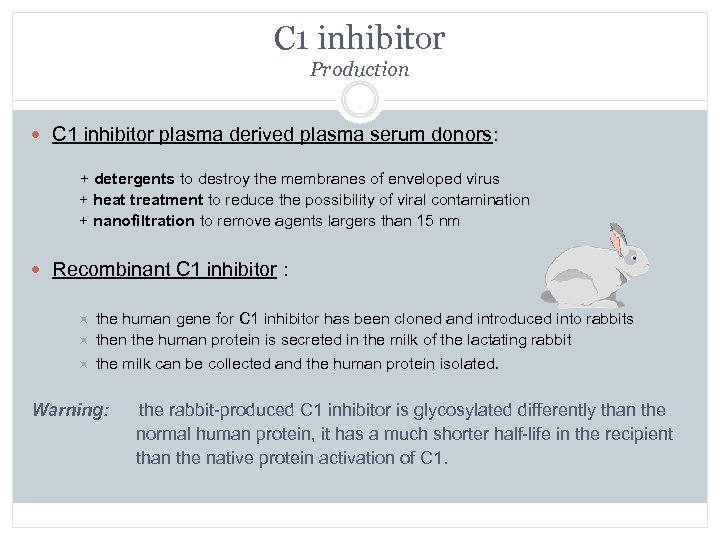 C 1 inhibitor Production C 1 inhibitor plasma derived plasma serum donors: + detergents to destroy the membranes of enveloped virus + heat treatment to reduce the possibility of viral contamination + nanofiltration to remove agents largers than 15 nm Recombinant C 1 inhibitor : the human gene for C 1 inhibitor has been cloned and introduced into rabbits then the human protein is secreted in the milk of the lactating rabbit the milk can be collected and the human protein isolated. Warning: the rabbit-produced C 1 inhibitor is glycosylated differently than the normal human protein, it has a much shorter half-life in the recipient than the native protein activation of C 1.
C 1 inhibitor Production C 1 inhibitor plasma derived plasma serum donors: + detergents to destroy the membranes of enveloped virus + heat treatment to reduce the possibility of viral contamination + nanofiltration to remove agents largers than 15 nm Recombinant C 1 inhibitor : the human gene for C 1 inhibitor has been cloned and introduced into rabbits then the human protein is secreted in the milk of the lactating rabbit the milk can be collected and the human protein isolated. Warning: the rabbit-produced C 1 inhibitor is glycosylated differently than the normal human protein, it has a much shorter half-life in the recipient than the native protein activation of C 1.
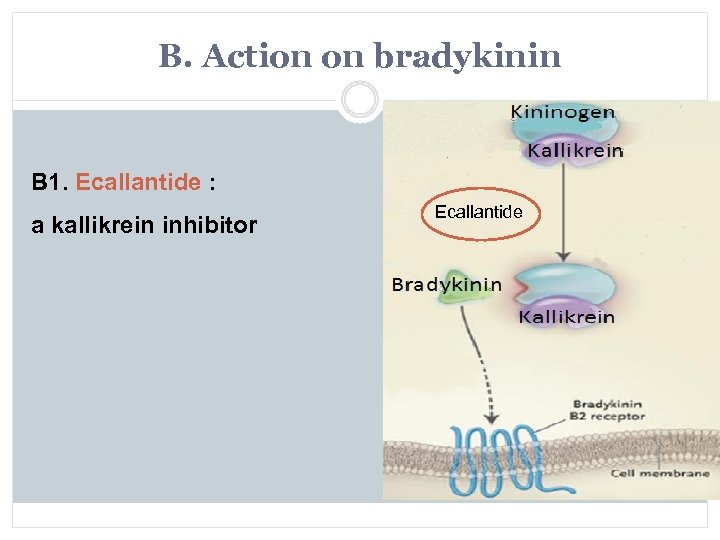 B. Action on bradykinin B 1. Ecallantide : a kallikrein inhibitor Ecallantide
B. Action on bradykinin B 1. Ecallantide : a kallikrein inhibitor Ecallantide
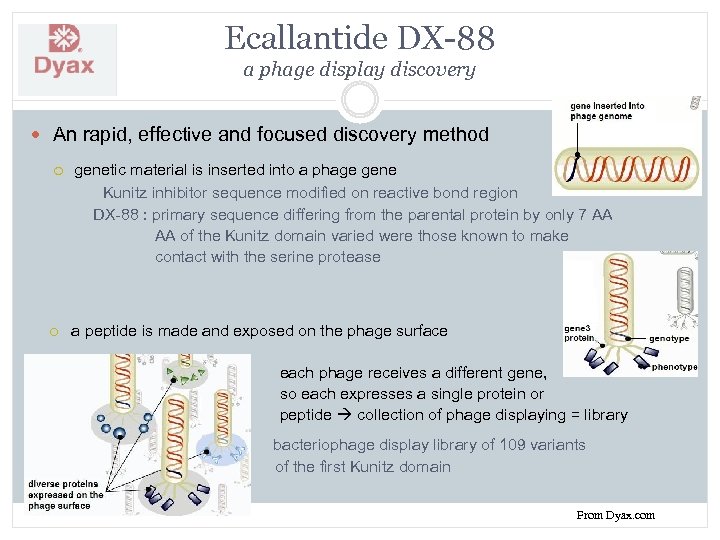 Ecallantide DX-88 a phage display discovery An rapid, effective and focused discovery method genetic material is inserted into a phage gene Kunitz inhibitor sequence modified on reactive bond region DX-88 : primary sequence differing from the parental protein by only 7 AA AA of the Kunitz domain varied were those known to make contact with the serine protease a peptide is made and exposed on the phage surface each phage receives a different gene, so each expresses a single protein or peptide collection of phage displaying = library bacteriophage display library of 109 variants of the first Kunitz domain From Dyax. com
Ecallantide DX-88 a phage display discovery An rapid, effective and focused discovery method genetic material is inserted into a phage gene Kunitz inhibitor sequence modified on reactive bond region DX-88 : primary sequence differing from the parental protein by only 7 AA AA of the Kunitz domain varied were those known to make contact with the serine protease a peptide is made and exposed on the phage surface each phage receives a different gene, so each expresses a single protein or peptide collection of phage displaying = library bacteriophage display library of 109 variants of the first Kunitz domain From Dyax. com
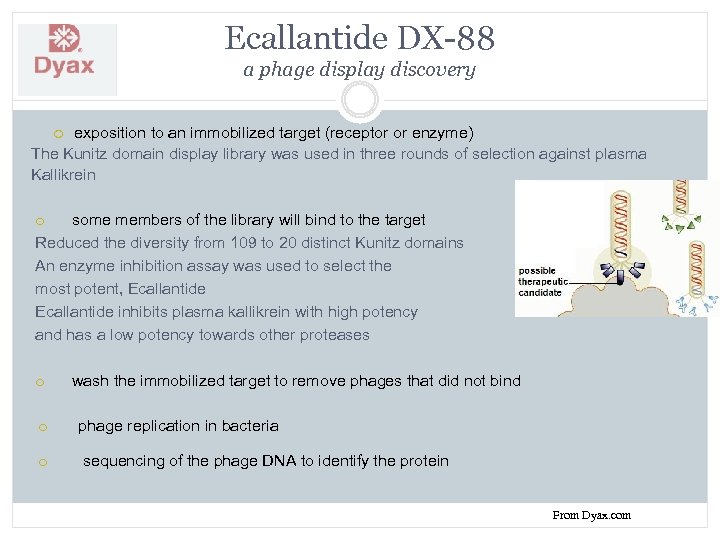 Ecallantide DX-88 a phage display discovery exposition to an immobilized target (receptor or enzyme) The Kunitz domain display library was used in three rounds of selection against plasma Kallikrein o some members of the library will bind to the target Reduced the diversity from 109 to 20 distinct Kunitz domains An enzyme inhibition assay was used to select the most potent, Ecallantide inhibits plasma kallikrein with high potency and has a low potency towards other proteases o wash the immobilized target to remove phages that did not bind o phage replication in bacteria o sequencing of the phage DNA to identify the protein From Dyax. com
Ecallantide DX-88 a phage display discovery exposition to an immobilized target (receptor or enzyme) The Kunitz domain display library was used in three rounds of selection against plasma Kallikrein o some members of the library will bind to the target Reduced the diversity from 109 to 20 distinct Kunitz domains An enzyme inhibition assay was used to select the most potent, Ecallantide inhibits plasma kallikrein with high potency and has a low potency towards other proteases o wash the immobilized target to remove phages that did not bind o phage replication in bacteria o sequencing of the phage DNA to identify the protein From Dyax. com
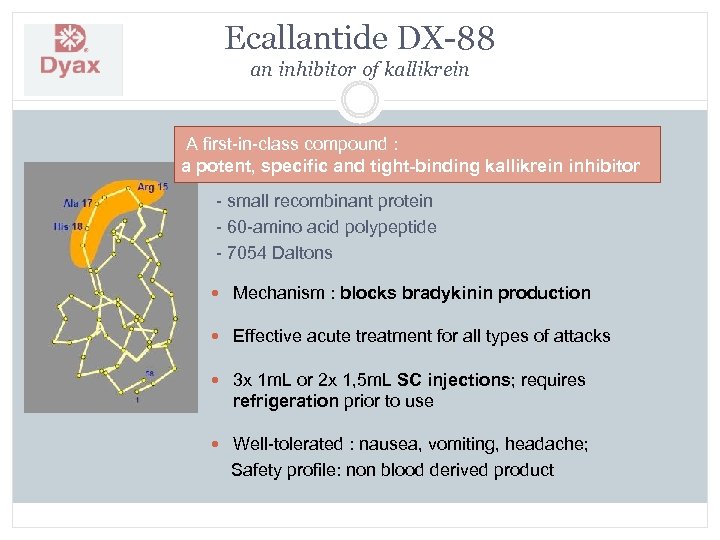 Ecallantide DX-88 an inhibitor of kallikrein A first-in-class compound : a potent, specific and tight-binding kallikrein inhibitor - small recombinant protein - 60 -amino acid polypeptide - 7054 Daltons Mechanism : blocks bradykinin production Effective acute treatment for all types of attacks 3 x 1 m. L or 2 x 1, 5 m. L SC injections; requires refrigeration prior to use Well-tolerated : nausea, vomiting, headache; Safety profile: non blood derived product
Ecallantide DX-88 an inhibitor of kallikrein A first-in-class compound : a potent, specific and tight-binding kallikrein inhibitor - small recombinant protein - 60 -amino acid polypeptide - 7054 Daltons Mechanism : blocks bradykinin production Effective acute treatment for all types of attacks 3 x 1 m. L or 2 x 1, 5 m. L SC injections; requires refrigeration prior to use Well-tolerated : nausea, vomiting, headache; Safety profile: non blood derived product
 Ecallantide DX-88 an inhibitor of kallikrein Phase III trials : EDEMA 3 (completed in November 2006) EDEMA 4 (completed in June 2008 ) primary endpoint : symptom improvement at four hours EDEMA 4 : under a special protocol assessment (SPA) to further support the validity of the patient reported outcome methodology and to confirm efficacy and safety Efficacy determined by : Treatment Outcome Score (TOS) = an overall measure of response to treatment Mean Symptom Complex Severity score=measure symptoms severity at a specific point in time Successful response at 4 hours (P < 0. 001) - Efficacity in 30 mn
Ecallantide DX-88 an inhibitor of kallikrein Phase III trials : EDEMA 3 (completed in November 2006) EDEMA 4 (completed in June 2008 ) primary endpoint : symptom improvement at four hours EDEMA 4 : under a special protocol assessment (SPA) to further support the validity of the patient reported outcome methodology and to confirm efficacy and safety Efficacy determined by : Treatment Outcome Score (TOS) = an overall measure of response to treatment Mean Symptom Complex Severity score=measure symptoms severity at a specific point in time Successful response at 4 hours (P < 0. 001) - Efficacity in 30 mn
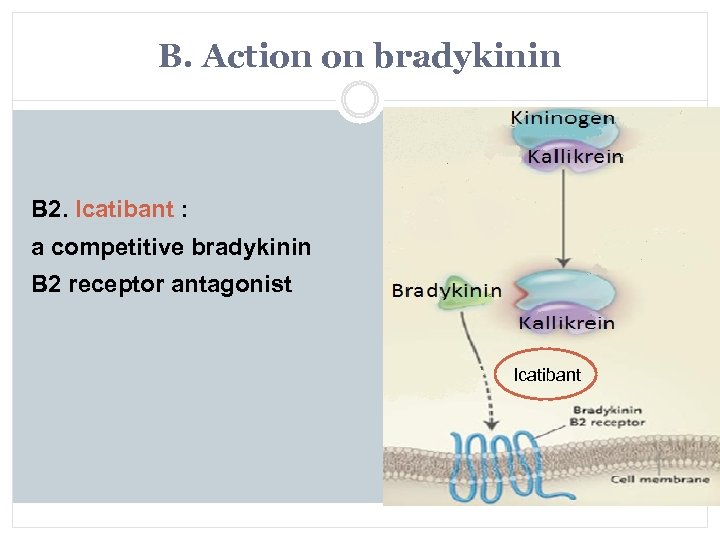 B. Action on bradykinin B 2. Icatibant : a competitive bradykinin B 2 receptor antagonist Icatibant
B. Action on bradykinin B 2. Icatibant : a competitive bradykinin B 2 receptor antagonist Icatibant
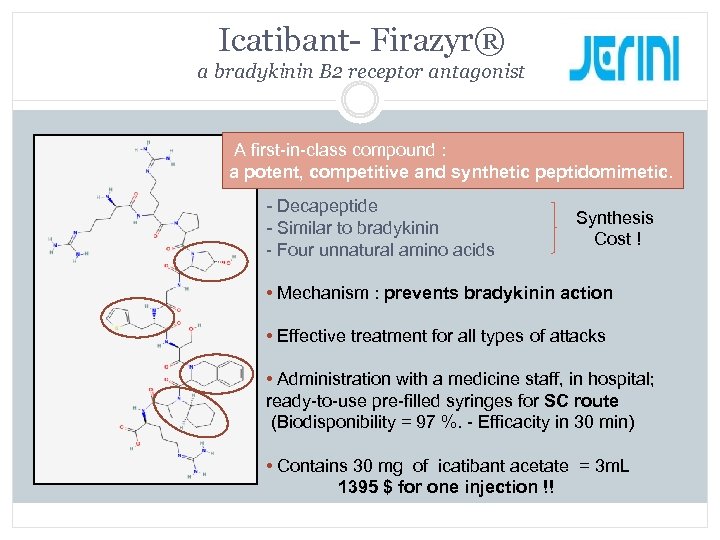 Icatibant- Firazyr® a bradykinin B 2 receptor antagonist A first-in-class compound : a potent, competitive and synthetic peptidomimetic. - Decapeptide - Similar to bradykinin - Four unnatural amino acids Synthesis Cost ! • Mechanism : prevents bradykinin action • Effective treatment for all types of attacks • Administration with a medicine staff, in hospital; ready-to-use pre-filled syringes for SC route (Biodisponibility = 97 %. - Efficacity in 30 min) • Contains 30 mg of icatibant acetate = 3 m. L 1395 $ for one injection !!
Icatibant- Firazyr® a bradykinin B 2 receptor antagonist A first-in-class compound : a potent, competitive and synthetic peptidomimetic. - Decapeptide - Similar to bradykinin - Four unnatural amino acids Synthesis Cost ! • Mechanism : prevents bradykinin action • Effective treatment for all types of attacks • Administration with a medicine staff, in hospital; ready-to-use pre-filled syringes for SC route (Biodisponibility = 97 %. - Efficacity in 30 min) • Contains 30 mg of icatibant acetate = 3 m. L 1395 $ for one injection !!
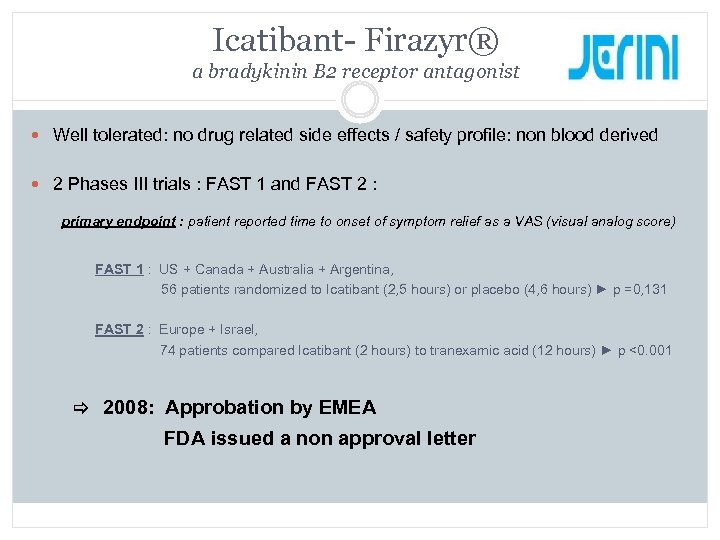 Icatibant- Firazyr® a bradykinin B 2 receptor antagonist Well tolerated: no drug related side effects / safety profile: non blood derived 2 Phases III trials : FAST 1 and FAST 2 : primary endpoint : patient reported time to onset of symptom relief as a VAS (visual analog score) FAST 1 : US + Canada + Australia + Argentina, 56 patients randomized to Icatibant (2, 5 hours) or placebo (4, 6 hours) ► p =0, 131 FAST 2 : Europe + Israel, 74 patients compared Icatibant (2 hours) to tranexamic acid (12 hours) ► p <0. 001 2008: Approbation by EMEA FDA issued a non approval letter
Icatibant- Firazyr® a bradykinin B 2 receptor antagonist Well tolerated: no drug related side effects / safety profile: non blood derived 2 Phases III trials : FAST 1 and FAST 2 : primary endpoint : patient reported time to onset of symptom relief as a VAS (visual analog score) FAST 1 : US + Canada + Australia + Argentina, 56 patients randomized to Icatibant (2, 5 hours) or placebo (4, 6 hours) ► p =0, 131 FAST 2 : Europe + Israel, 74 patients compared Icatibant (2 hours) to tranexamic acid (12 hours) ► p <0. 001 2008: Approbation by EMEA FDA issued a non approval letter
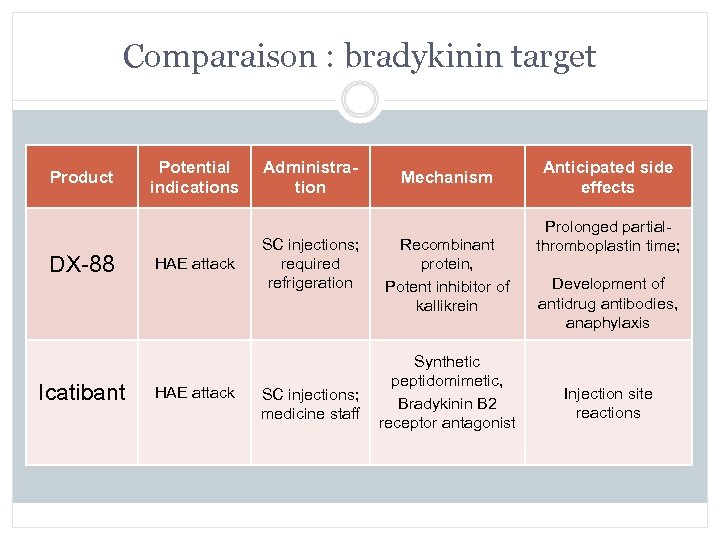 Comparaison : bradykinin target Product DX-88 Icatibant Potential indications HAE attack Administration SC injections; required refrigeration Mechanism Recombinant protein, Potent inhibitor of kallikrein Synthetic peptidomimetic, SC injections; Bradykinin B 2 medicine staff receptor antagonist Anticipated side effects Prolonged partialthromboplastin time; Development of antidrug antibodies, anaphylaxis Injection site reactions
Comparaison : bradykinin target Product DX-88 Icatibant Potential indications HAE attack Administration SC injections; required refrigeration Mechanism Recombinant protein, Potent inhibitor of kallikrein Synthetic peptidomimetic, SC injections; Bradykinin B 2 medicine staff receptor antagonist Anticipated side effects Prolonged partialthromboplastin time; Development of antidrug antibodies, anaphylaxis Injection site reactions
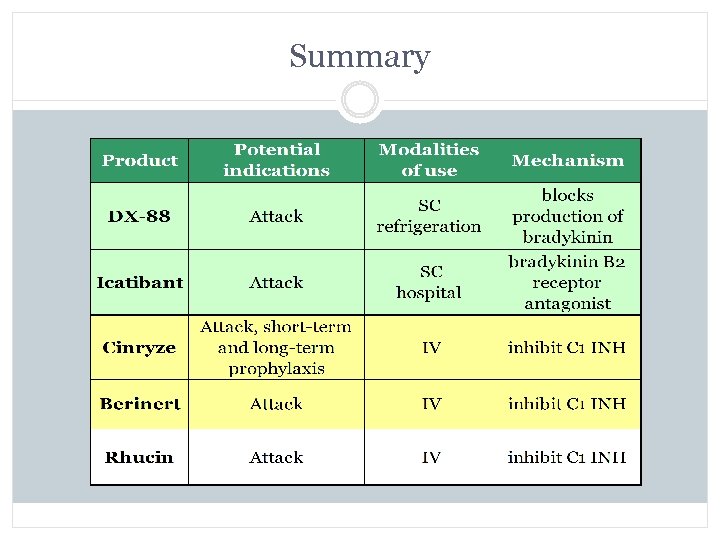 Summary
Summary
 The HAE market A. Why should you focus on ?
The HAE market A. Why should you focus on ?
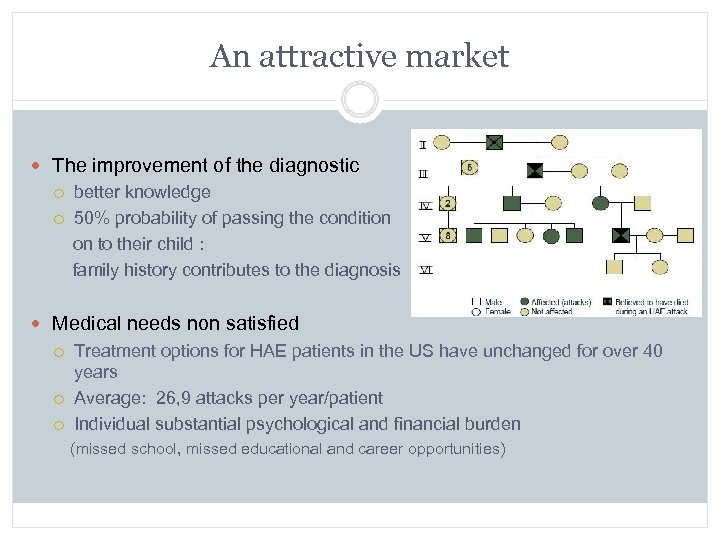 An attractive market The improvement of the diagnostic better knowledge 50% probability of passing the condition on to their child : family history contributes to the diagnosis Medical needs non satisfied Treatment options for HAE patients in the US have unchanged for over 40 years Average: 26, 9 attacks per year/patient Individual substantial psychological and financial burden (missed school, missed educational and career opportunities)
An attractive market The improvement of the diagnostic better knowledge 50% probability of passing the condition on to their child : family history contributes to the diagnosis Medical needs non satisfied Treatment options for HAE patients in the US have unchanged for over 40 years Average: 26, 9 attacks per year/patient Individual substantial psychological and financial burden (missed school, missed educational and career opportunities)
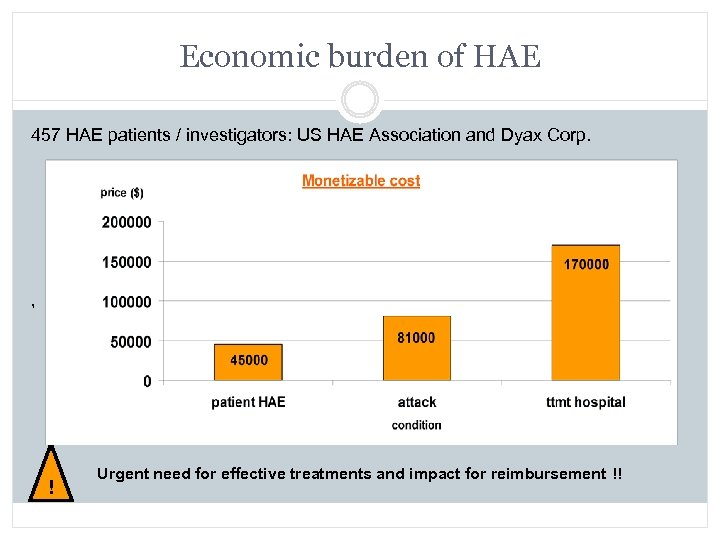 Economic burden of HAE 457 HAE patients / investigators: US HAE Association and Dyax Corp. , Urgent need for effective treatments and impact for reimbursement !! !
Economic burden of HAE 457 HAE patients / investigators: US HAE Association and Dyax Corp. , Urgent need for effective treatments and impact for reimbursement !! !
 The HAE market B. A market in evolution
The HAE market B. A market in evolution
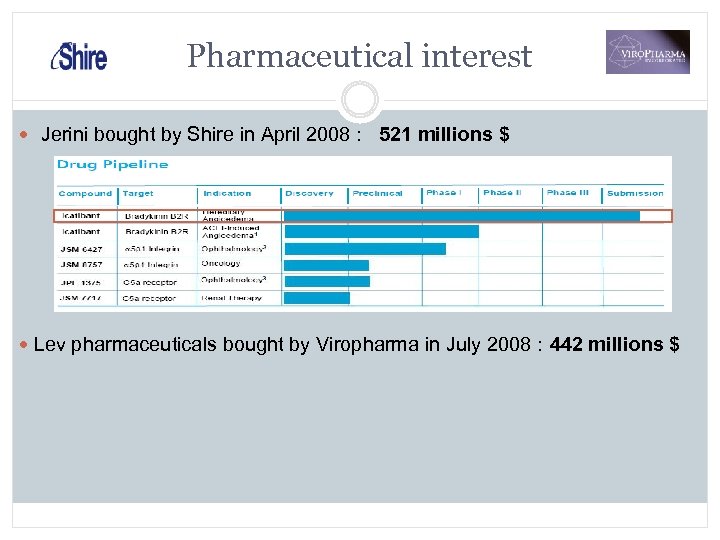 Pharmaceutical interest Jerini bought by Shire in April 2008 : 521 millions $ Lev pharmaceuticals bought by Viropharma in July 2008 : 442 millions $
Pharmaceutical interest Jerini bought by Shire in April 2008 : 521 millions $ Lev pharmaceuticals bought by Viropharma in July 2008 : 442 millions $
 Future market Competition Cinryze/Berinert in terms of Orphan exclusivity Both have the same profile but : Cinryze applied for acute and prophylactic treatment Berinert has not applied for a prevention indication = FDA approval of Cinryze would assure that Lev would have that market to itself Approval from FDA = seven years of market exclusivity in the U. S. for the indication
Future market Competition Cinryze/Berinert in terms of Orphan exclusivity Both have the same profile but : Cinryze applied for acute and prophylactic treatment Berinert has not applied for a prevention indication = FDA approval of Cinryze would assure that Lev would have that market to itself Approval from FDA = seven years of market exclusivity in the U. S. for the indication
 The HAE market C. Which strategy to adopt to conquer the market ?
The HAE market C. Which strategy to adopt to conquer the market ?
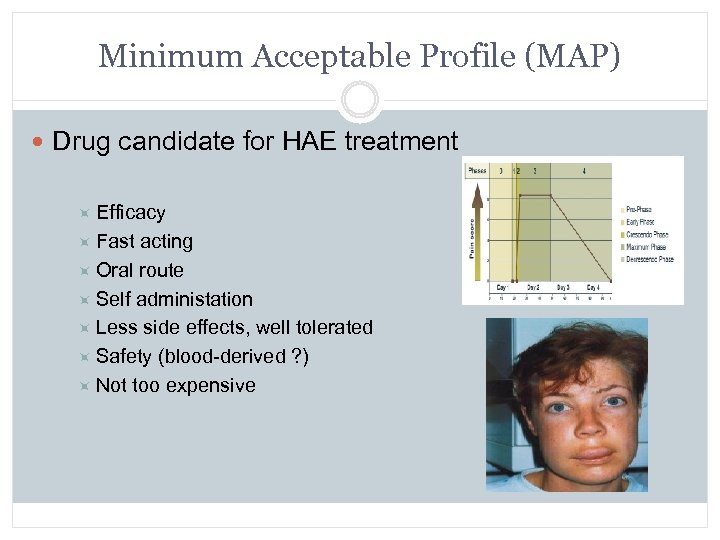 Minimum Acceptable Profile (MAP) Drug candidate for HAE treatment Efficacy Fast acting Oral route Self administation Less side effects, well tolerated Safety (blood-derived ? ) Not too expensive
Minimum Acceptable Profile (MAP) Drug candidate for HAE treatment Efficacy Fast acting Oral route Self administation Less side effects, well tolerated Safety (blood-derived ? ) Not too expensive
 Future market Approval from FDA & EMEA A real work in collaboration with the fundation to launge the good clinical trials Rhucin : numbers of patients Ecallantide : necessity of an other clinical trial To propose products more effective than products already on the market To provide a clinical advantage for patients
Future market Approval from FDA & EMEA A real work in collaboration with the fundation to launge the good clinical trials Rhucin : numbers of patients Ecallantide : necessity of an other clinical trial To propose products more effective than products already on the market To provide a clinical advantage for patients
 HAE Market Opportunity : Marketing Mix environment company Drug Candidate
HAE Market Opportunity : Marketing Mix environment company Drug Candidate
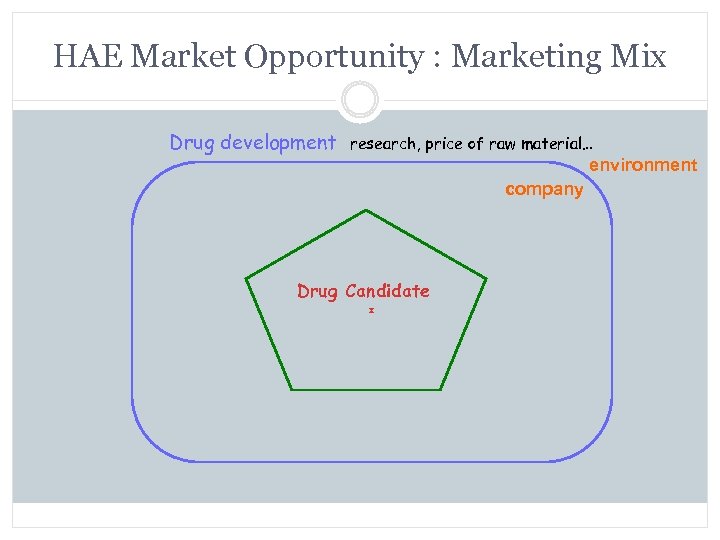 HAE Market Opportunity : Marketing Mix Drug development research, price of raw material… environment company Drug Candidate
HAE Market Opportunity : Marketing Mix Drug development research, price of raw material… environment company Drug Candidate
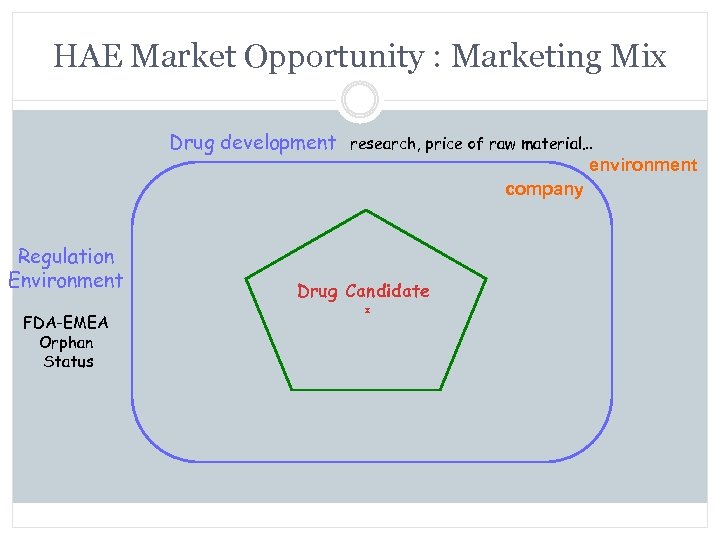 HAE Market Opportunity : Marketing Mix Drug development research, price of raw material… environment company Regulation Environment FDA-EMEA Orphan Status Drug Candidate
HAE Market Opportunity : Marketing Mix Drug development research, price of raw material… environment company Regulation Environment FDA-EMEA Orphan Status Drug Candidate
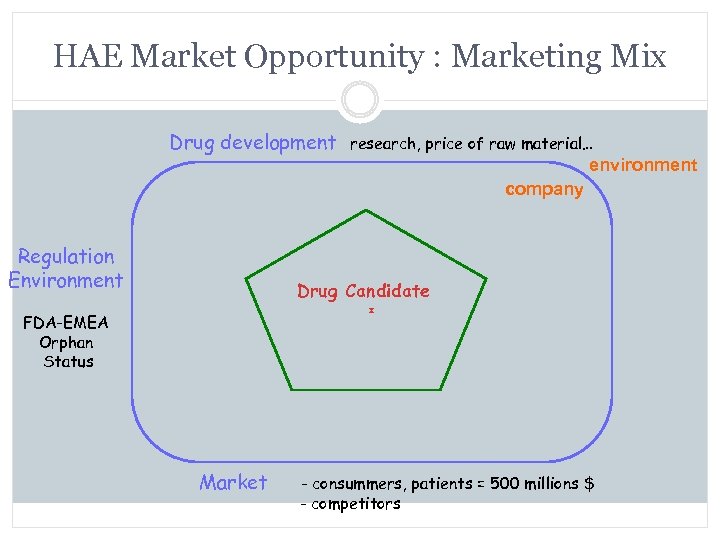 HAE Market Opportunity : Marketing Mix Drug development research, price of raw material… environment company Regulation Environment Drug Candidate FDA-EMEA Orphan Status Market - consummers, patients = 500 millions $ - competitors
HAE Market Opportunity : Marketing Mix Drug development research, price of raw material… environment company Regulation Environment Drug Candidate FDA-EMEA Orphan Status Market - consummers, patients = 500 millions $ - competitors
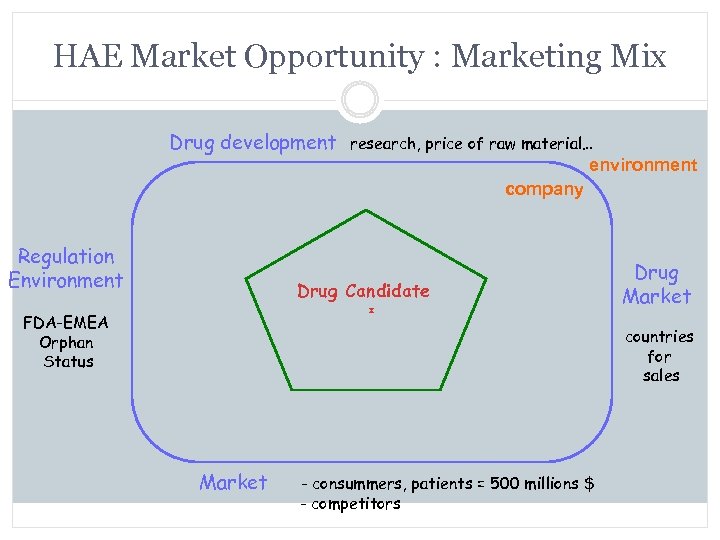 HAE Market Opportunity : Marketing Mix Drug development research, price of raw material… environment company Regulation Environment Drug Candidate FDA-EMEA Orphan Status Drug Market countries for sales Market - consummers, patients = 500 millions $ - competitors
HAE Market Opportunity : Marketing Mix Drug development research, price of raw material… environment company Regulation Environment Drug Candidate FDA-EMEA Orphan Status Drug Market countries for sales Market - consummers, patients = 500 millions $ - competitors
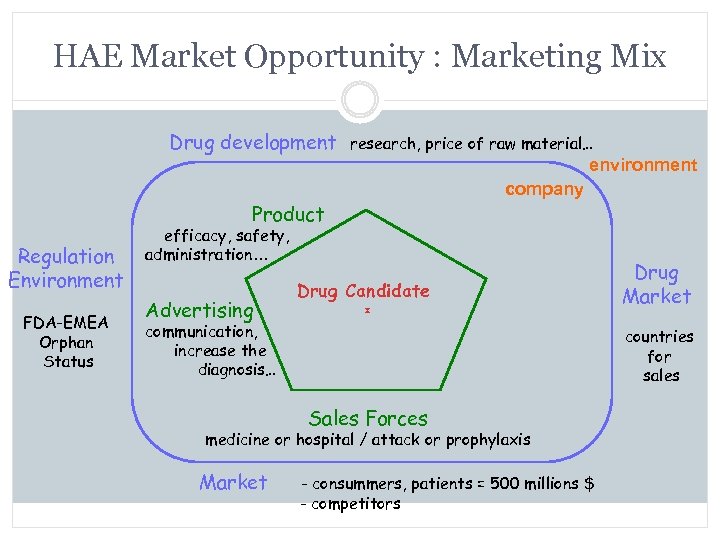
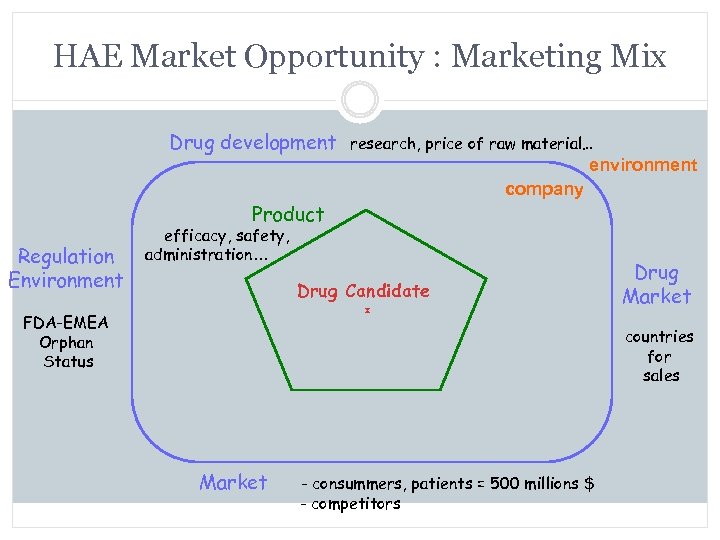 HAE Market Opportunity : Marketing Mix Drug development research, price of raw material… environment Product Regulation Environment company efficacy, safety, administration… Drug Candidate FDA-EMEA Orphan Status Drug Market countries for sales Market - consummers, patients = 500 millions $ - competitors
HAE Market Opportunity : Marketing Mix Drug development research, price of raw material… environment Product Regulation Environment company efficacy, safety, administration… Drug Candidate FDA-EMEA Orphan Status Drug Market countries for sales Market - consummers, patients = 500 millions $ - competitors
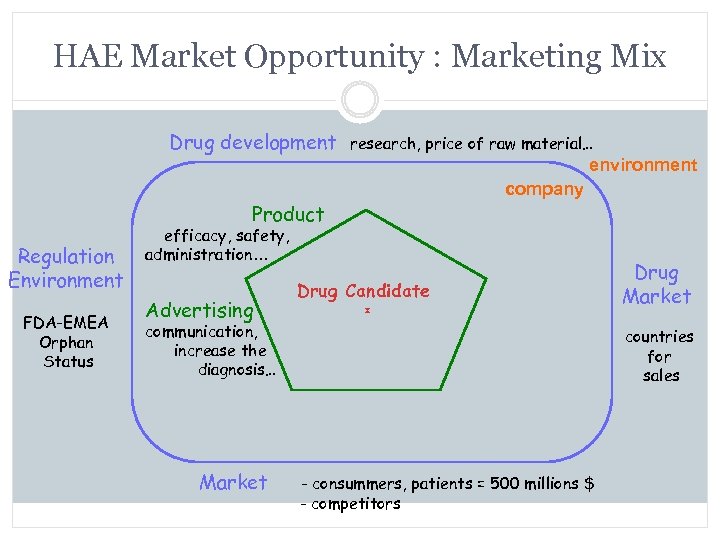 HAE Market Opportunity : Marketing Mix Drug development research, price of raw material… environment Product Regulation Environment FDA-EMEA Orphan Status company efficacy, safety, administration… Advertising communication, increase the diagnosis… Market Drug Candidate Drug Market countries for sales - consummers, patients = 500 millions $ - competitors
HAE Market Opportunity : Marketing Mix Drug development research, price of raw material… environment Product Regulation Environment FDA-EMEA Orphan Status company efficacy, safety, administration… Advertising communication, increase the diagnosis… Market Drug Candidate Drug Market countries for sales - consummers, patients = 500 millions $ - competitors
 HAE Market Opportunity : Marketing Mix Drug development research, price of raw material… environment Product Regulation Environment FDA-EMEA Orphan Status company efficacy, safety, administration… Advertising communication, increase the diagnosis… Drug Candidate countries for sales Sales Forces medicine or hospital / attack or prophylaxis Market Drug Market - consummers, patients = 500 millions $ - competitors
HAE Market Opportunity : Marketing Mix Drug development research, price of raw material… environment Product Regulation Environment FDA-EMEA Orphan Status company efficacy, safety, administration… Advertising communication, increase the diagnosis… Drug Candidate countries for sales Sales Forces medicine or hospital / attack or prophylaxis Market Drug Market - consummers, patients = 500 millions $ - competitors
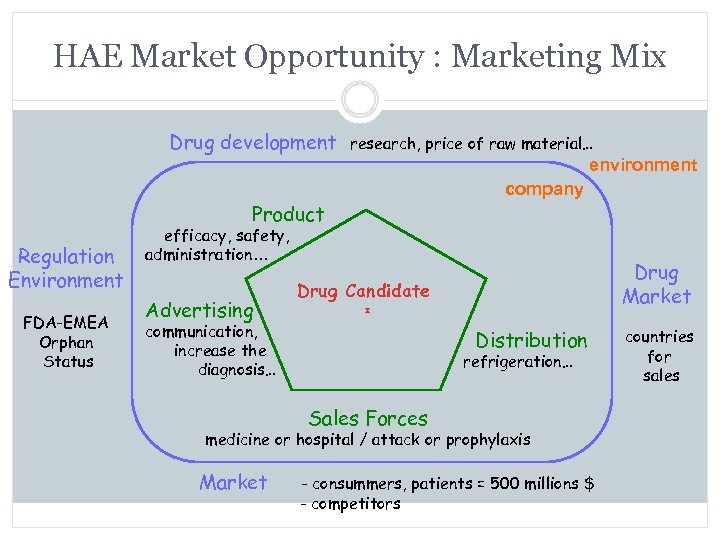 HAE Market Opportunity : Marketing Mix Drug development research, price of raw material… environment Product Regulation Environment FDA-EMEA Orphan Status company efficacy, safety, administration… Advertising communication, increase the diagnosis… Drug Market Drug Candidate Distribution refrigeration… Sales Forces medicine or hospital / attack or prophylaxis Market - consummers, patients = 500 millions $ - competitors countries for sales
HAE Market Opportunity : Marketing Mix Drug development research, price of raw material… environment Product Regulation Environment FDA-EMEA Orphan Status company efficacy, safety, administration… Advertising communication, increase the diagnosis… Drug Market Drug Candidate Distribution refrigeration… Sales Forces medicine or hospital / attack or prophylaxis Market - consummers, patients = 500 millions $ - competitors countries for sales
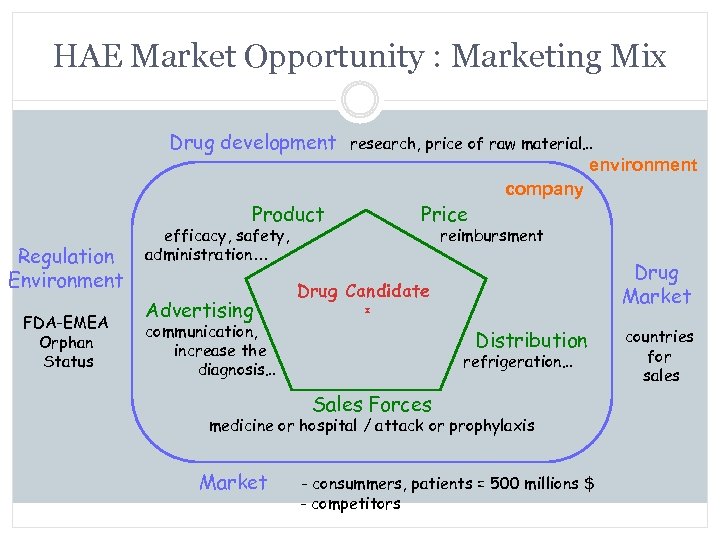 HAE Market Opportunity : Marketing Mix Drug development research, price of raw material… environment Product Regulation Environment FDA-EMEA Orphan Status efficacy, safety, administration… Advertising communication, increase the diagnosis… Price company reimbursment Drug Market Drug Candidate Distribution refrigeration… Sales Forces medicine or hospital / attack or prophylaxis Market - consummers, patients = 500 millions $ - competitors countries for sales
HAE Market Opportunity : Marketing Mix Drug development research, price of raw material… environment Product Regulation Environment FDA-EMEA Orphan Status efficacy, safety, administration… Advertising communication, increase the diagnosis… Price company reimbursment Drug Market Drug Candidate Distribution refrigeration… Sales Forces medicine or hospital / attack or prophylaxis Market - consummers, patients = 500 millions $ - competitors countries for sales
 Thank you for your attention! Any questions ?
Thank you for your attention! Any questions ?


