0a1de87a1294bd0cc15e132919cfabf7.ppt
- Количество слайдов: 70

HER 2 Positive Breast Cancer Charles E. Geyer, Jr. MD, FACP Professor of Medicine Division of Hematology, Oncology and Palliative Care Virginia Commonwealth University Associate Director of Clinical Research Massey Cancer Center Heme/Onc Fellows Lecture Series November 1, 2016
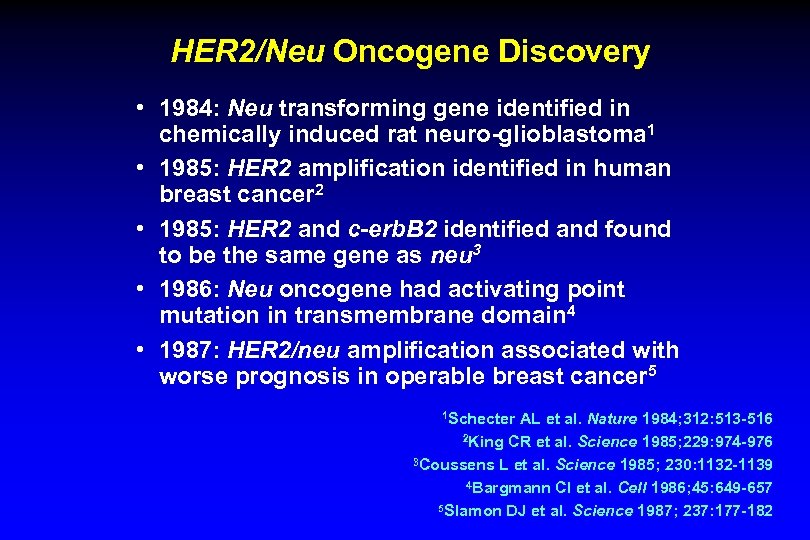
HER 2/Neu Oncogene Discovery • 1984: Neu transforming gene identified in chemically induced rat neuro-glioblastoma 1 • 1985: HER 2 amplification identified in human breast cancer 2 • 1985: HER 2 and c-erb. B 2 identified and found to be the same gene as neu 3 • 1986: Neu oncogene had activating point mutation in transmembrane domain 4 • 1987: HER 2/neu amplification associated with worse prognosis in operable breast cancer 5 1 Schecter AL et al. Nature 1984; 312: 513 -516 2 King CR et al. Science 1985; 229: 974 -976 3 Coussens L et al. Science 1985; 230: 1132 -1139 4 Bargmann CI et al. Cell 1986; 45: 649 -657 5 Slamon DJ et al. Science 1987; 237: 177 -182
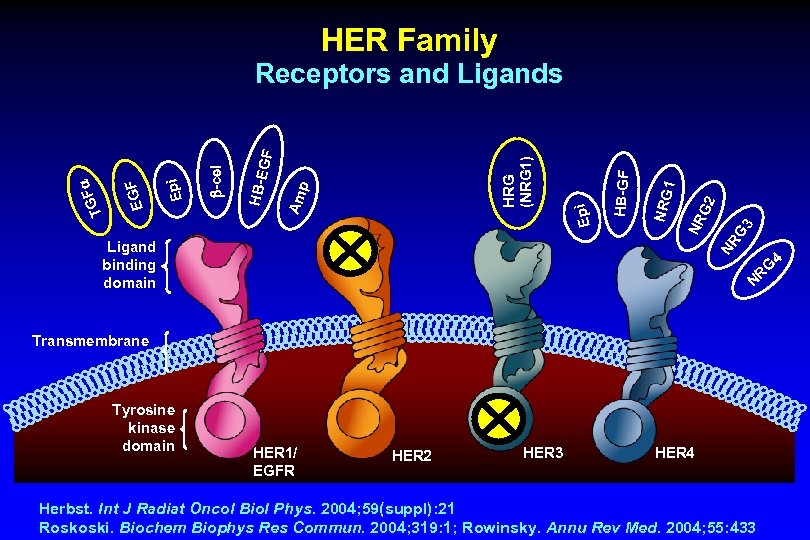
HER Family Ligand binding domain 3 NR G 2 1 NRG HB-GF Epi Am p HRG (NRG 1) GF HB-E -cel Epi EGF TG F Receptors and Ligands G NR Transmembrane Tyrosine kinase domain HER 1/ EGFR HER 2 HER 3 HER 4 Herbst. Int J Radiat Oncol Biol Phys. 2004; 59(suppl): 21 Roskoski. Biochem Biophys Res Commun. 2004; 319: 1; Rowinsky. Annu Rev Med. 2004; 55: 433 4
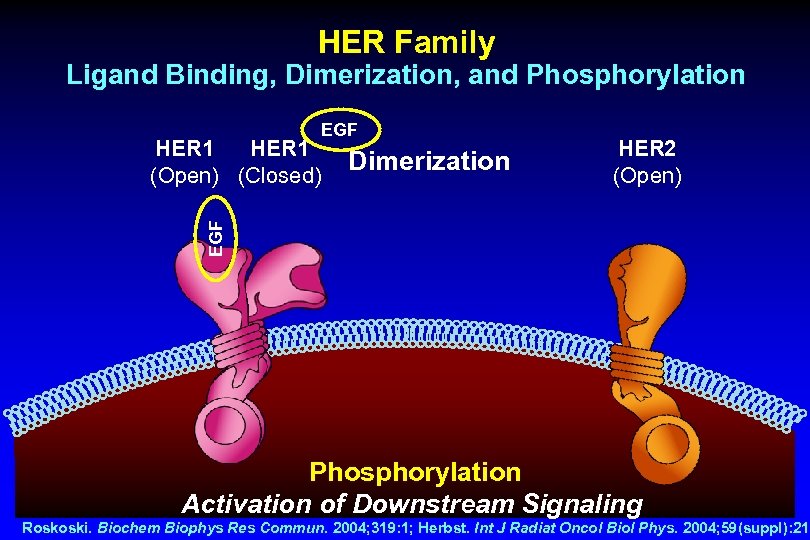
HER Family Ligand Binding, Dimerization, and Phosphorylation EGF Dimerization HER 2 (Open) EGF HER 1 (Open) (Closed) Phosphorylation Activation of Downstream Signaling Roskoski. Biochem Biophys Res Commun. 2004; 319: 1; Herbst. Int J Radiat Oncol Biol Phys. 2004; 59(suppl): 21
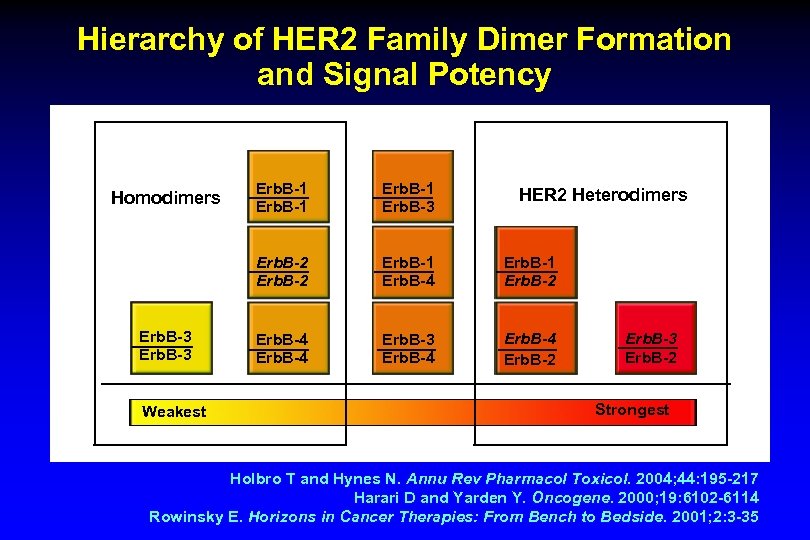
Hierarchy of HER 2 Family Dimer Formation and Signal Potency Erb. B-3 Weakest Erb. B-1 Erb. B-3 Erb. B-2 Homodimers Erb. B-1 Erb. B-4 Erb. B-1 Erb. B-2 Erb. B-4 Erb. B-3 Erb. B-4 Erb. B-2 HER 2 Heterodimers Erb. B-3 Erb. B-2 Strongest Holbro T and Hynes N. Annu Rev Pharmacol Toxicol. 2004; 44: 195 -217 Harari D and Yarden Y. Oncogene. 2000; 19: 6102 -6114 Rowinsky E. Horizons in Cancer Therapies: From Bench to Bedside. 2001; 2: 3 -35
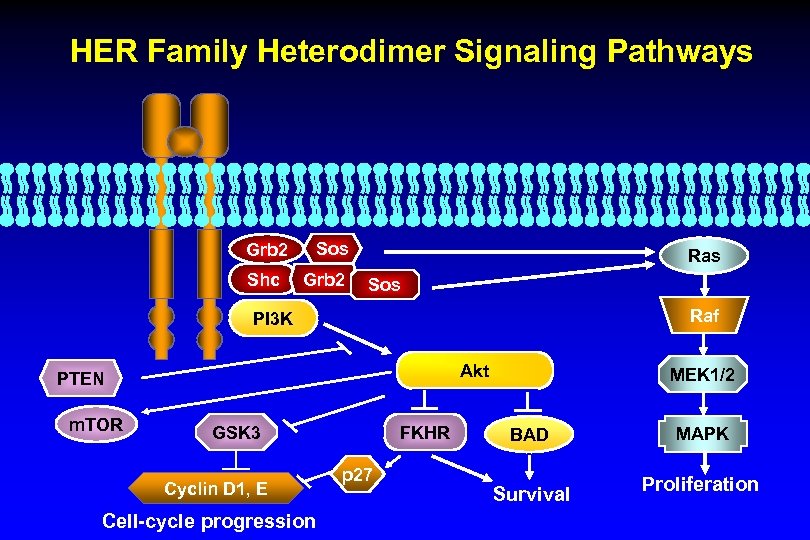
HER Family Heterodimer Signaling Pathways Grb 2 Shc Sos Grb 2 Ras Sos Raf PI 3 K Akt PTEN m. TOR GSK 3 Cyclin D 1, E Cell-cycle progression FKHR p 27 MEK 1/2 BAD Survival MAPK Proliferation
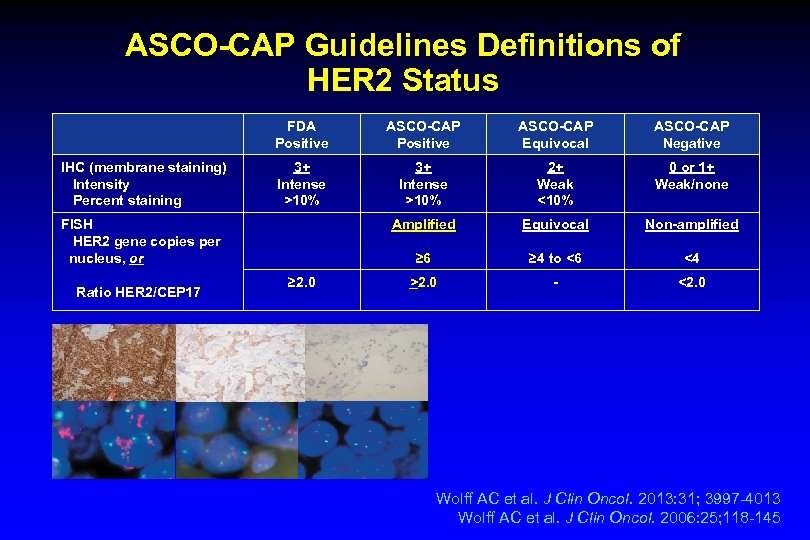
ASCO-CAP Guidelines Definitions of HER 2 Status FDA Positive IHC (membrane staining) Intensity Percent staining ASCO-CAP Positive ASCO-CAP Equivocal ASCO-CAP Negative 3+ Intense >10% 2+ Weak <10% 0 or 1+ Weak/none Amplified Equivocal Non-amplified ≥ 6 ≥ 4 to <6 <4 >2. 0 - <2. 0 FISH HER 2 gene copies per nucleus, or Ratio HER 2/CEP 17 ≥ 2. 0 Wolff AC et al. J Clin Oncol. 2013: 31; 3997 -4013 Wolff AC et al. J Clin Oncol. 2006: 25; 118 -145
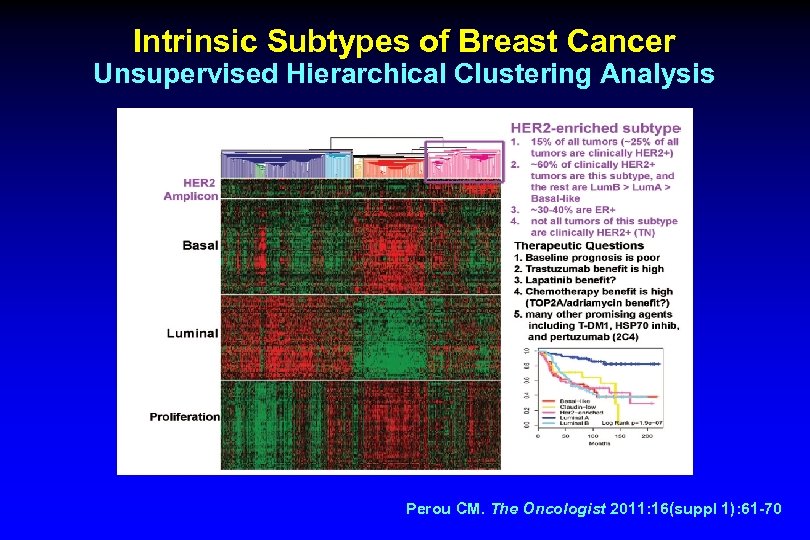
Intrinsic Subtypes of Breast Cancer Unsupervised Hierarchical Clustering Analysis Perou CM. The Oncologist 2011: 16(suppl 1): 61 -70
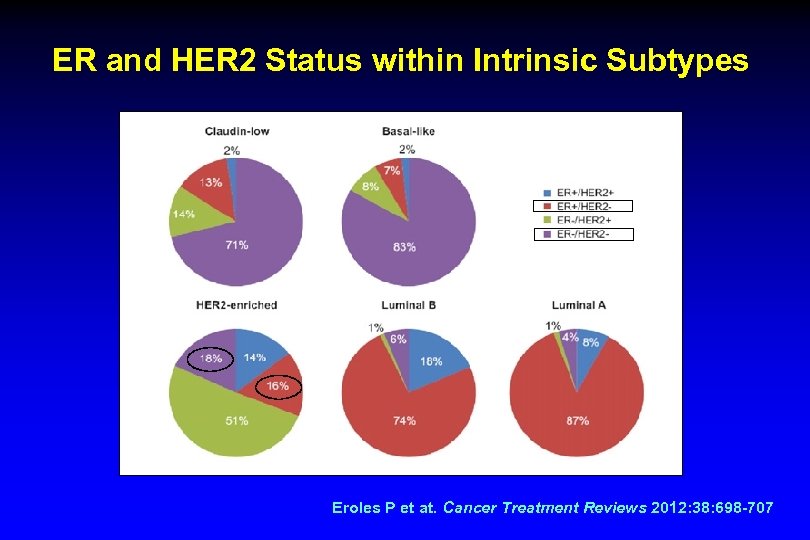
ER and HER 2 Status within Intrinsic Subtypes Eroles P et at. Cancer Treatment Reviews 2012: 38: 698 -707
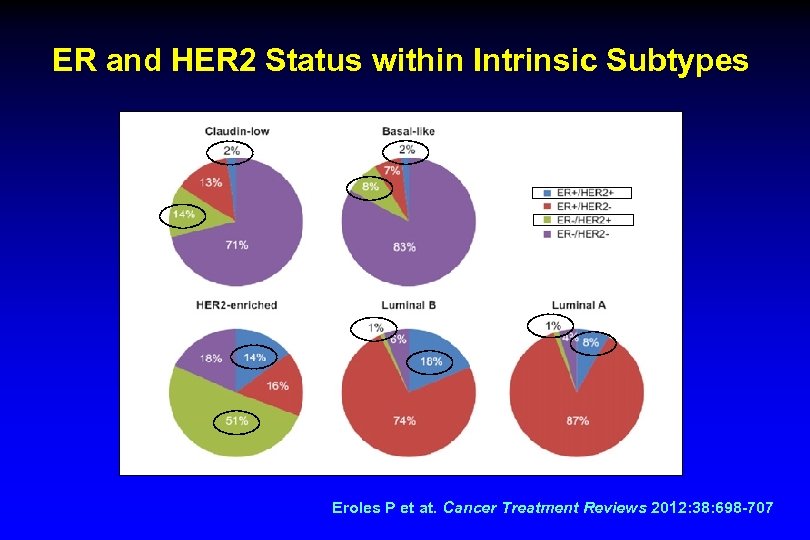
ER and HER 2 Status within Intrinsic Subtypes Eroles P et at. Cancer Treatment Reviews 2012: 38: 698 -707
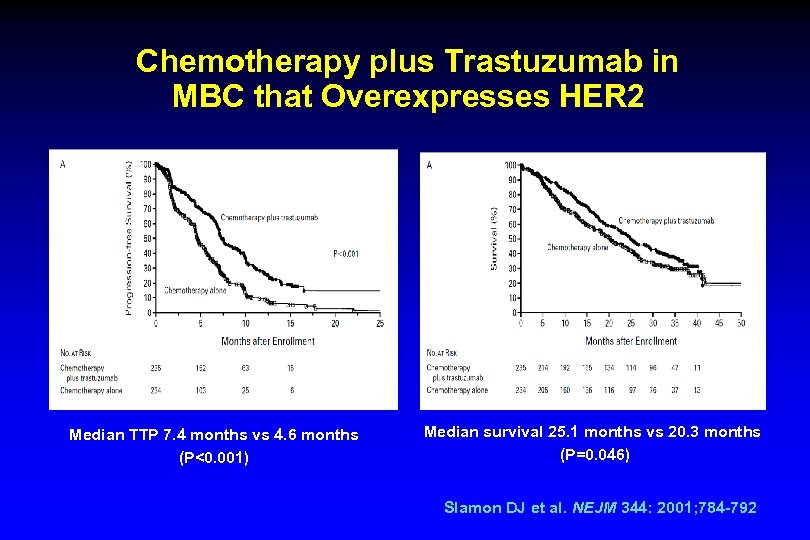
Chemotherapy plus Trastuzumab in MBC that Overexpresses HER 2 Median TTP 7. 4 months vs 4. 6 months (P<0. 001) Median survival 25. 1 months vs 20. 3 months (P=0. 046) Slamon DJ et al. NEJM 344: 2001; 784 -792
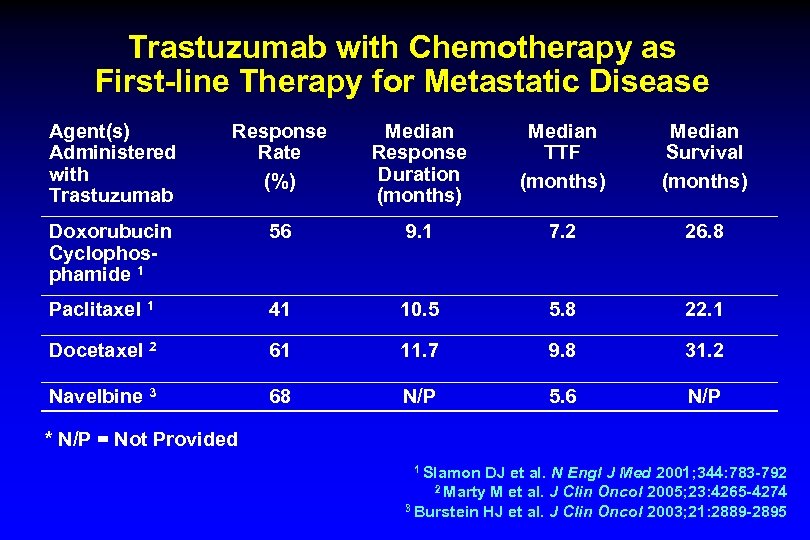
Trastuzumab with Chemotherapy as First-line Therapy for Metastatic Disease Agent(s) Administered with Trastuzumab Response Rate (%) Median Response Duration (months) Median TTF Survival (months) Doxorubucin Cyclophosphamide 1 56 9. 1 7. 2 26. 8 Paclitaxel 1 41 10. 5 5. 8 22. 1 Docetaxel 2 61 11. 7 9. 8 31. 2 Navelbine 3 68 N/P 5. 6 N/P * N/P = Not Provided 1 Slamon DJ et al. N Engl J Med 2001; 344: 783 -792 2 Marty M et al. J Clin Oncol 2005; 23: 4265 -4274 3 Burstein HJ et al. J Clin Oncol 2003; 21: 2889 -2895
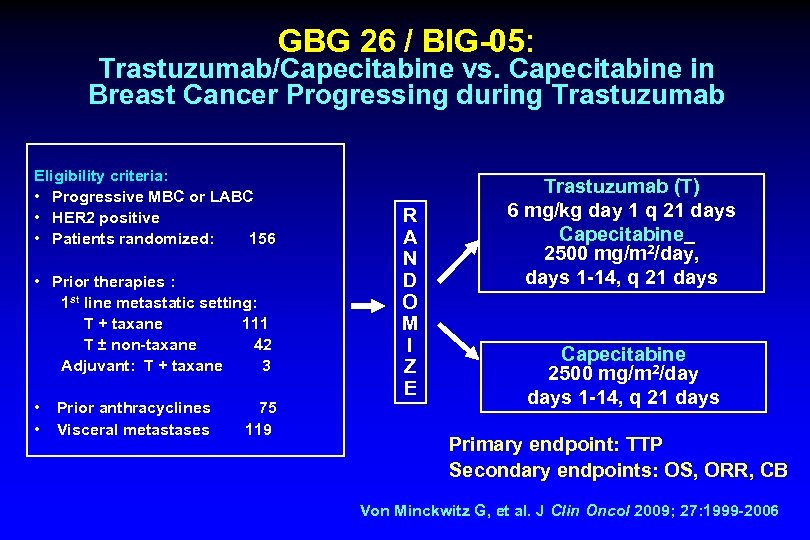
GBG 26 / BIG-05: Trastuzumab/Capecitabine vs. Capecitabine in Breast Cancer Progressing during Trastuzumab Eligibility criteria: • Progressive MBC or LABC • HER 2 positive • Patients randomized: 156 • Prior therapies : 1 st line metastatic setting: T + taxane 111 T ± non-taxane 42 Adjuvant: T + taxane 3 • • Prior anthracyclines 75 Visceral metastases 119 R A N D O M I Z E Trastuzumab (T) 6 mg/kg day 1 q 21 days Capecitabine 2500 mg/m 2/day, days 1 -14, q 21 days Capecitabine 2500 mg/m 2/day days 1 -14, q 21 days Primary endpoint: TTP Secondary endpoints: OS, ORR, CB Von Minckwitz G, et al. J Clin Oncol 2009; 27: 1999 -2006
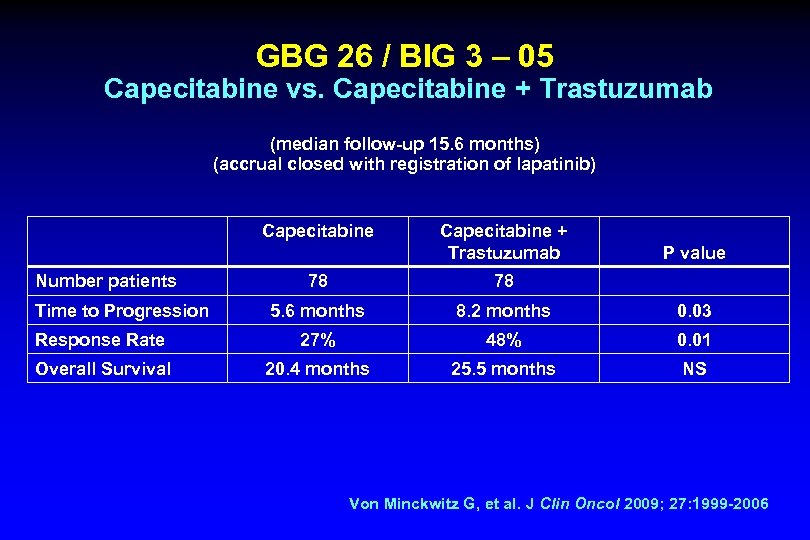
GBG 26 / BIG 3 – 05 Capecitabine vs. Capecitabine + Trastuzumab (median follow-up 15. 6 months) (accrual closed with registration of lapatinib) Capecitabine Number patients Capecitabine + Trastuzumab P value 78 78 5. 6 months 8. 2 months 0. 03 Response Rate 27% 48% 0. 01 Overall Survival 20. 4 months 25. 5 months NS Time to Progression Von Minckwitz G, et al. J Clin Oncol 2009; 27: 1999 -2006
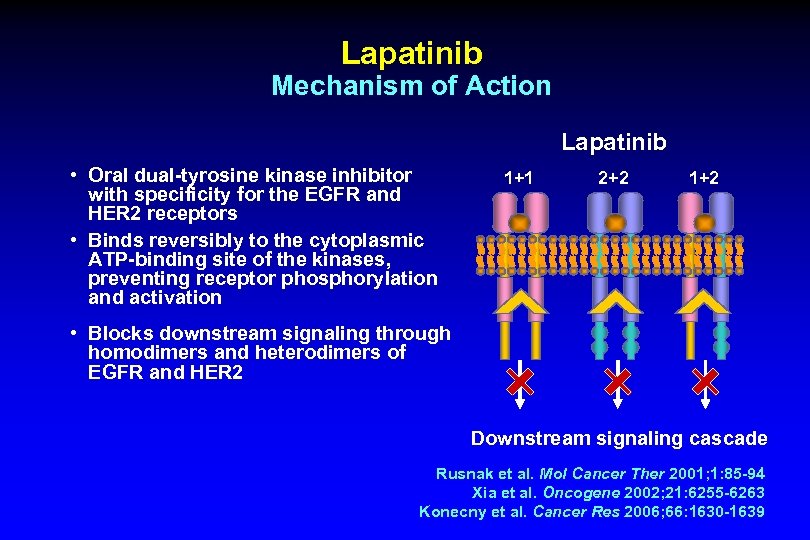
Lapatinib Mechanism of Action Lapatinib • Oral dual-tyrosine kinase inhibitor with specificity for the EGFR and HER 2 receptors • Binds reversibly to the cytoplasmic ATP-binding site of the kinases, preventing receptor phosphorylation and activation 1+1 2+2 1+2 • Blocks downstream signaling through homodimers and heterodimers of EGFR and HER 2 Downstream signaling cascade Rusnak et al. Mol Cancer Ther 2001; 1: 85 -94 Xia et al. Oncogene 2002; 21: 6255 -6263 Konecny et al. Cancer Res 2006; 66: 1630 -1639
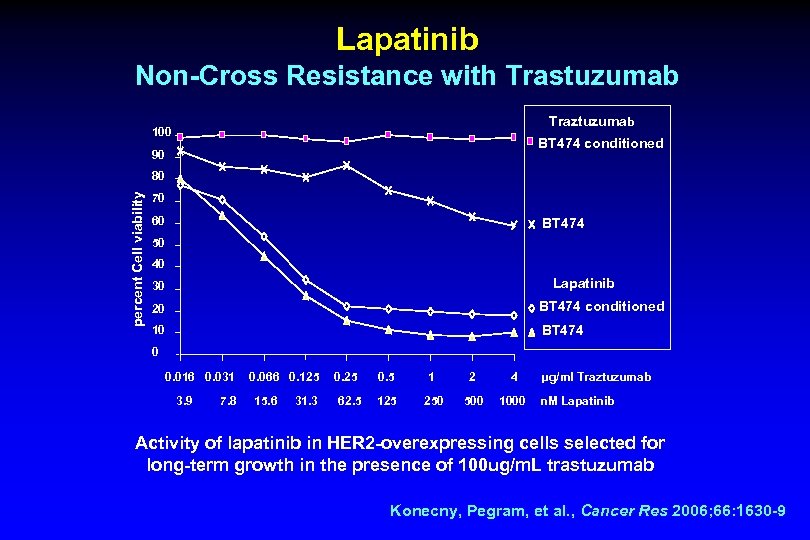
Lapatinib Non-Cross Resistance with Trastuzumab Traztuzumab 100 BT 474 conditioned 90 percent Cell viability 80 70 60 BT 474 50 40 Lapatinib 30 20 BT 474 conditioned 10 BT 474 0 0. 016 0. 031 3. 9 7. 8 0. 066 0. 125 15. 6 31. 3 0. 25 62. 5 0. 5 1 2 4 125 250 500 1000 µg/ml Traztuzumab n. M Lapatinib Activity of lapatinib in HER 2 -overexpressing cells selected for long-term growth in the presence of 100 ug/m. L trastuzumab Konecny, Pegram, et al. , Cancer Res 2006; 66: 1630 -9
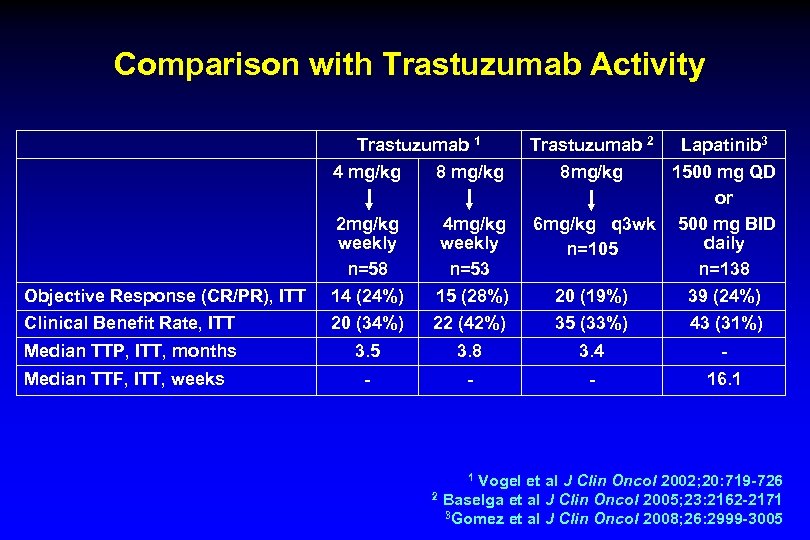
Comparison with Trastuzumab Activity Trastuzumab 1 Trastuzumab 2 Lapatinib 3 4 mg/kg 8 mg/kg 8 mg/kg Objective Response (CR/PR), ITT 1500 mg QD or 2 mg/kg 4 mg/kg 6 mg/kg q 3 wk 500 mg BID weekly daily n=105 n=58 n=53 n=138 14 (24%) 15 (28%) 20 (19%) 39 (24%) Clinical Benefit Rate, ITT 20 (34%) 22 (42%) 35 (33%) 43 (31%) Median TTP, ITT, months 3. 5 3. 8 3. 4 - - 16. 1 Median TTF, ITT, weeks 1 Vogel et al J Clin Oncol 2002; 20: 719 -726 Clin Oncol 2005; 23: 2162 -2171 3 Gomez et al J Clin Oncol 2008; 26: 2999 -3005 2 Baselga et al J
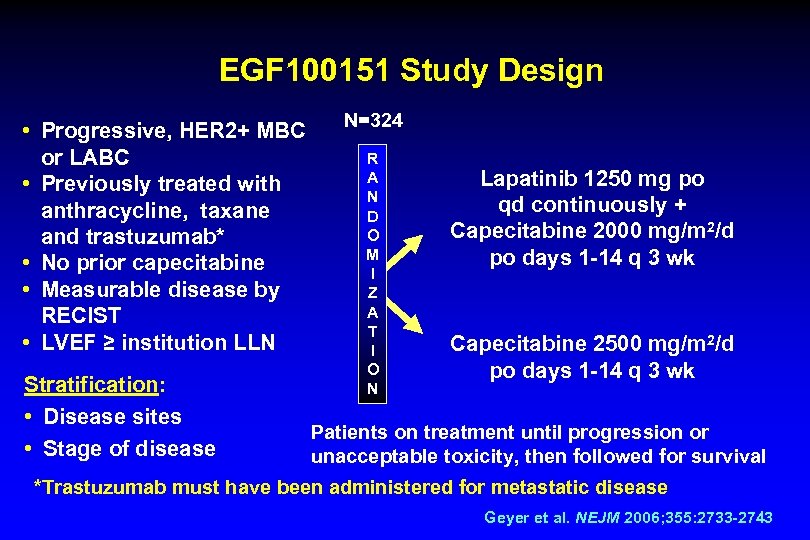
EGF 100151 Study Design • Progressive, HER 2+ MBC or LABC • Previously treated with anthracycline, taxane and trastuzumab* • No prior capecitabine • Measurable disease by RECIST • LVEF ≥ institution LLN Stratification: • Disease sites • Stage of disease N=324 R A N D O M I Z A T I O N Lapatinib 1250 mg po qd continuously + Capecitabine 2000 mg/m 2/d po days 1 -14 q 3 wk Capecitabine 2500 mg/m 2/d po days 1 -14 q 3 wk Patients on treatment until progression or unacceptable toxicity, then followed for survival *Trastuzumab must have been administered for metastatic disease Geyer et al. NEJM 2006; 355: 2733 -2743
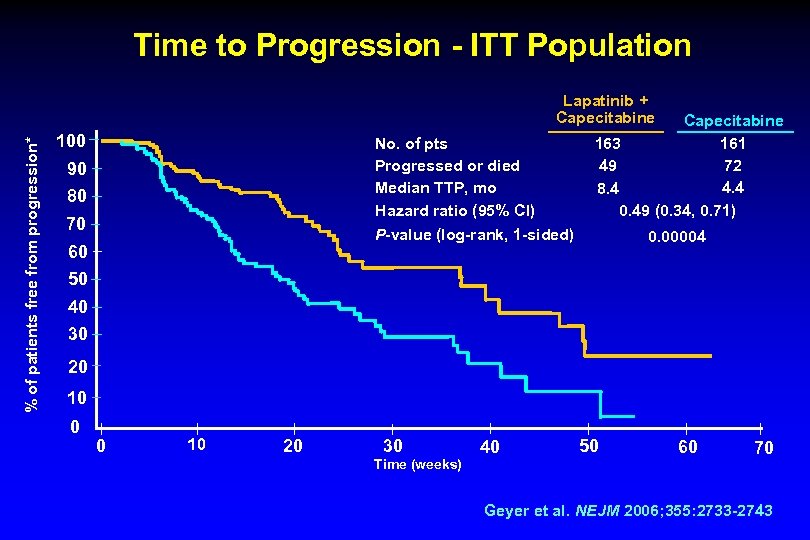
Time to Progression - ITT Population % of patients free from progression* Lapatinib + Capecitabine 100 No. of pts Progressed or died Median TTP, mo Hazard ratio (95% CI) P-value (log-rank, 1 -sided) 90 80 70 60 50 Capecitabine 163 161 49 72 4. 4 8. 4 0. 49 (0. 34, 0. 71) 0. 00004 40 30 20 10 0 0 10 20 30 Time (weeks) 40 50 60 70 Geyer et al. NEJM 2006; 355: 2733 -2743
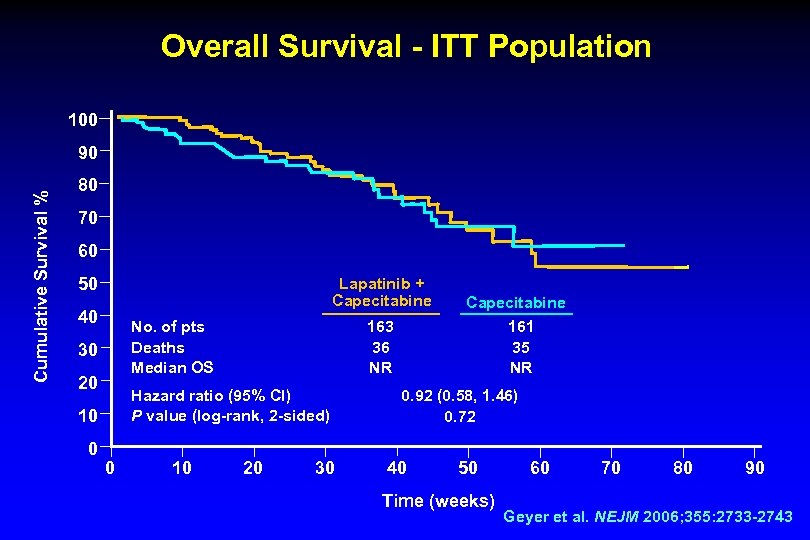
Overall Survival - ITT Population 100 Cumulative Survival % 90 80 70 60 50 Lapatinib + Capecitabine 40 No. of pts Deaths Median OS 30 20 Hazard ratio (95% CI) P value (log-rank, 2 -sided) 10 0 163 36 NR 0 10 20 30 Capecitabine 161 35 NR 0. 92 (0. 58, 1. 46) 0. 72 40 50 Time (weeks) 60 70 80 90 Geyer et al. NEJM 2006; 355: 2733 -2743
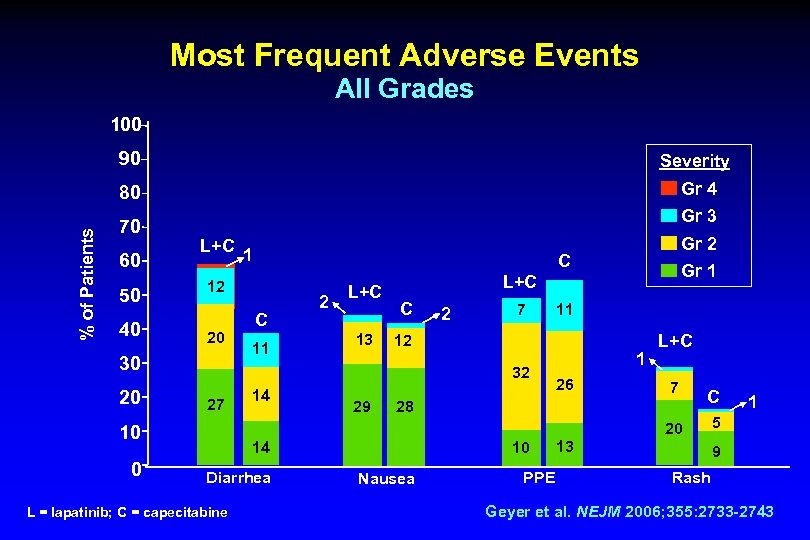
Most Frequent Adverse Events All Grades 100 Severity 80 % of Patients 90 Gr 4 70 60 50 40 Gr 3 L+C 20 11 L+C 13 C 2 7 14 29 11 12 1 26 L+C 7 28 C 20 10 0 C 2 Gr 1 L+C 32 27 Gr 2 C 12 30 20 1 10 14 Diarrhea L = lapatinib; C = capecitabine Nausea PPE 13 1 5 9 Rash Geyer et al. NEJM 2006; 355: 2733 -2743
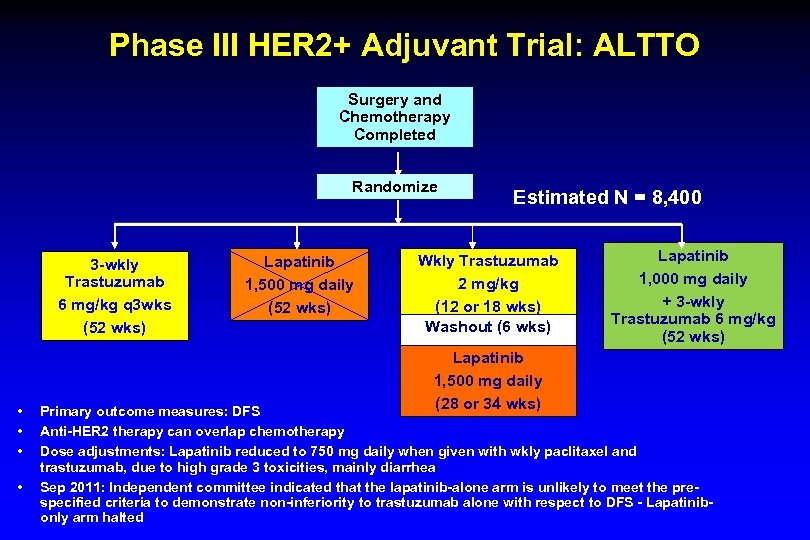
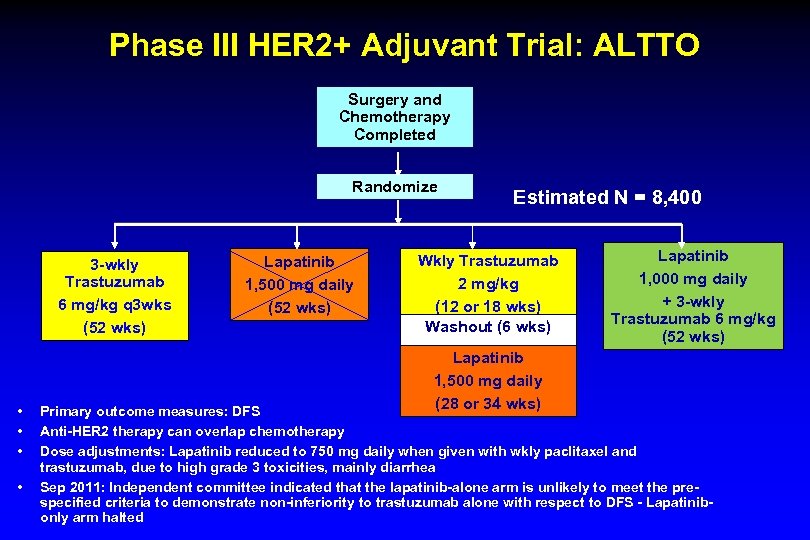
Phase III HER 2+ Adjuvant Trial: ALTTO Surgery and Chemotherapy Completed Randomize 3 -wkly Trastuzumab 6 mg/kg q 3 wks (52 wks) • • Lapatinib 1, 500 mg daily (52 wks) Estimated N = 8, 400 Wkly Trastuzumab 2 mg/kg (12 or 18 wks) Washout (6 wks) Lapatinib 1, 500 mg daily (28 or 34 wks) Lapatinib 1, 000 mg daily + 3 -wkly Trastuzumab 6 mg/kg (52 wks) Primary outcome measures: DFS Anti-HER 2 therapy can overlap chemotherapy Dose adjustments: Lapatinib reduced to 750 mg daily when given with wkly paclitaxel and trastuzumab, due to high grade 3 toxicities, mainly diarrhea Sep 2011: Independent committee indicated that the lapatinib-alone arm is unlikely to meet the prespecified criteria to demonstrate non-inferiority to trastuzumab alone with respect to DFS - Lapatinibonly arm halted
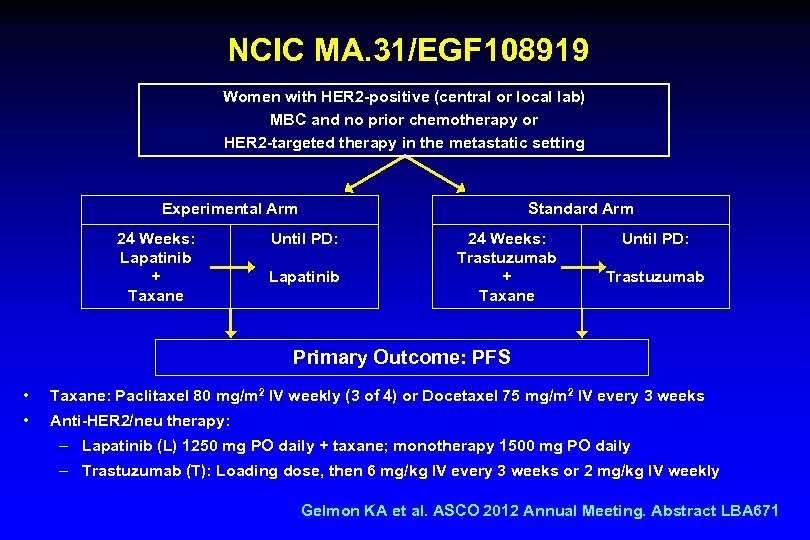
NCIC MA. 31/EGF 108919 Women with HER 2 -positive (central or local lab) MBC and no prior chemotherapy or HER 2 -targeted therapy in the metastatic setting Experimental Arm 24 Weeks: Lapatinib + Taxane Standard Arm Until PD: Lapatinib 24 Weeks: Trastuzumab + Taxane Until PD: Trastuzumab Primary Outcome: PFS • Taxane: Paclitaxel 80 mg/m 2 IV weekly (3 of 4) or Docetaxel 75 mg/m 2 IV every 3 weeks • Anti-HER 2/neu therapy: – Lapatinib (L) 1250 mg PO daily + taxane; monotherapy 1500 mg PO daily – Trastuzumab (T): Loading dose, then 6 mg/kg IV every 3 weeks or 2 mg/kg IV weekly Gelmon KA et al. ASCO 2012 Annual Meeting. Abstract LBA 671
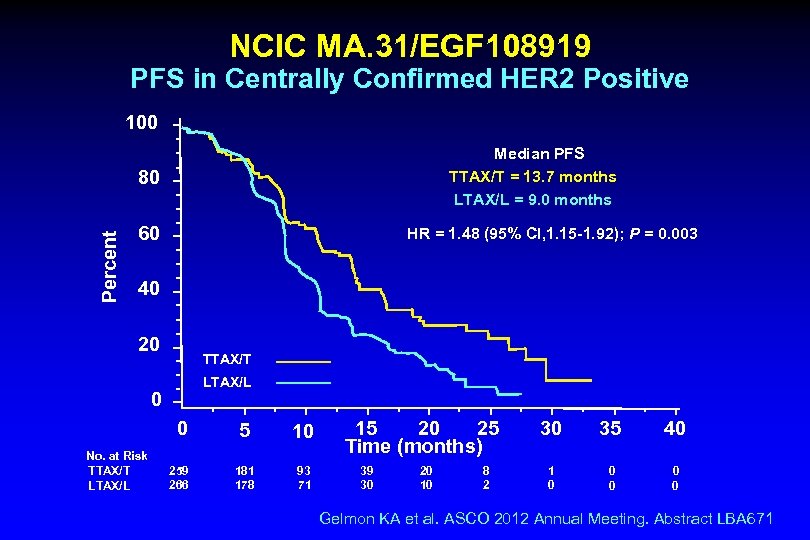
NCIC MA. 31/EGF 108919 PFS in Centrally Confirmed HER 2 Positive 100 Median PFS TTAX/T = 13. 7 months LTAX/L = 9. 0 months Percent 80 60 HR = 1. 48 (95% CI, 1. 15 -1. 92); P = 0. 003 40 20 TTAX/T LTAX/L 0 0 No. at Risk TTAX/T LTAX/L 5 10 259 266 181 178 93 71 20 15 25 Time (months) 39 30 20 10 8 2 30 35 40 1 0 0 0 Gelmon KA et al. ASCO 2012 Annual Meeting. Abstract LBA 671
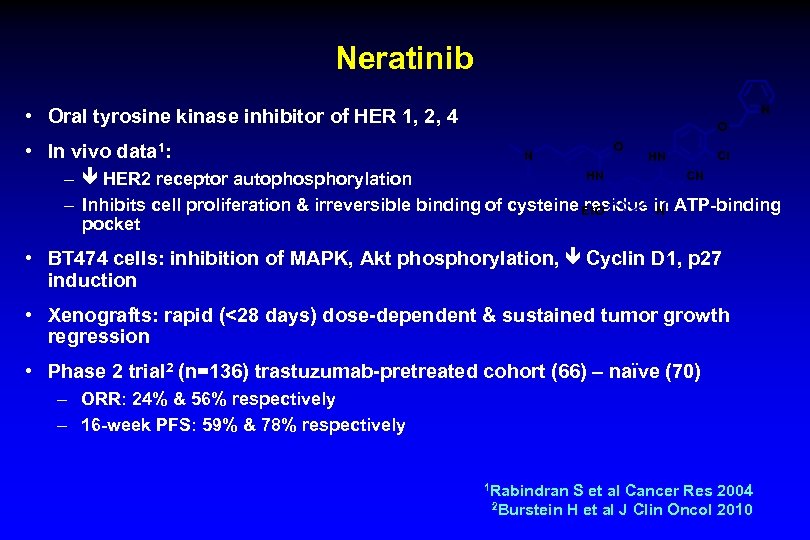
Neratinib N • Oral tyrosine kinase inhibitor of HER 1, 2, 4 • In vivo data 1: O N O HN CI HN CN – HER 2 receptor autophosphorylation – Inhibits cell proliferation & irreversible binding of cysteine residue in ATP-binding N Et. O pocket • BT 474 cells: inhibition of MAPK, Akt phosphorylation, Cyclin D 1, p 27 induction • Xenografts: rapid (<28 days) dose-dependent & sustained tumor growth regression • Phase 2 trial 2 (n=136) trastuzumab-pretreated cohort (66) – naïve (70) – ORR: 24% & 56% respectively – 16 -week PFS: 59% & 78% respectively 1 Rabindran S et al Cancer Res 2004 2 Burstein H et al J Clin Oncol 2010
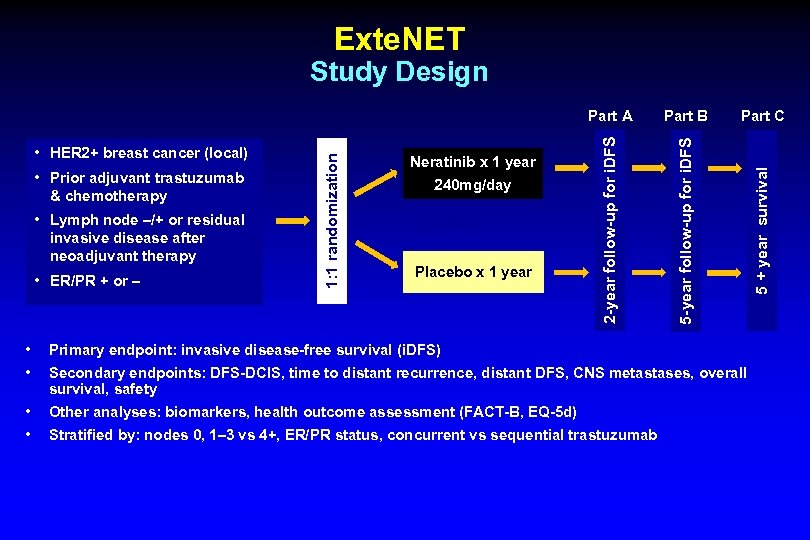
Exte. NET • Lymph node –/+ or residual invasive disease after neoadjuvant therapy • ER/PR + or – 240 mg/day N=2840 Placebo x 1 year • • Primary endpoint: invasive disease-free survival (i. DFS) • • Part C 5 + year survival • Prior adjuvant trastuzumab & chemotherapy Neratinib x 1 year Part B 5 -year follow-up for i. DFS • HER 2+ breast cancer (local) 1: 1 randomization Part A 2 -year follow-up for i. DFS Study Design Other analyses: biomarkers, health outcome assessment (FACT-B, EQ-5 d) Secondary endpoints: DFS-DCIS, time to distant recurrence, distant DFS, CNS metastases, overall survival, safety Stratified by: nodes 0, 1– 3 vs 4+, ER/PR status, concurrent vs sequential trastuzumab

Study Evolution April 2009 Sponsor 1 • • Sample size: 3850 • Stage 1– 3 c • <2 years from trastuzumab 1 o: Invasive DFS Feb 2010 a Sponsor 2 • 1 o: a. ITT i. DFS • Sample size: 3300 • a. ITT population: • Stage 2– 3, LN+ • <1 year trastuzumab October 2011 Sponsors 2 / 3 • Recruitment ceased Nov 2011 • Patients recruited: 2840 • Follow-up truncated at 2 years for all patients I-SPY 2 b: (neoadjuvant study to define agents which meet high January 2014 Sponsor 3 Bayesian predictive probabilities of success) • 1 o endpoint: ITT i. DFS (n=2840) • Restored 5 yr & OS follow-up • p. CR: 39% paclitaxel + neratinib vs. 23% paclitaxel + trastuzumab • 95% probability of superiority vs. standard therapy • CRO providing independent statistical analyses to IDMC was consistent from onset • CRO managing data collection & monitoring was consistent from onset • Sponsor 3 remained blinded to treatment allocation for primary analysis & remains blinded for survival events a December 2009 SABCS NCCTG N 9831 & BCIRG 006 update b December 2013 a. ITT = Amended ITT population
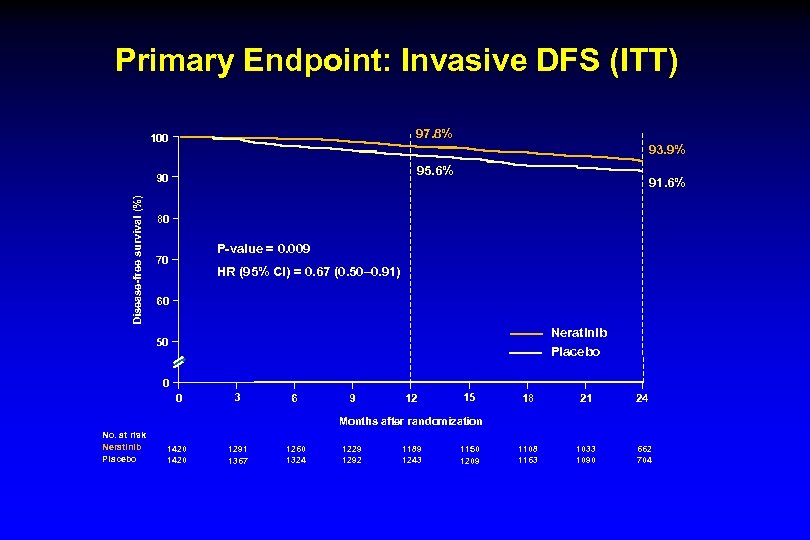
Primary Endpoint: Invasive DFS (ITT) 97. 8% 100 93. 9% 95. 6% Disease-free survival (%) 90 91. 6% 80 P-value = 0. 009 70 HR (95% CI) = 0. 67 (0. 50– 0. 91) 60 Neratinib Placebo 50 0 0 3 6 9 12 15 18 21 24 1108 1163 1033 1090 662 704 Months after randomization No. at risk Neratinib Placebo 1420 1291 1367 1260 1324 1229 1292 1189 1243 1150 1209
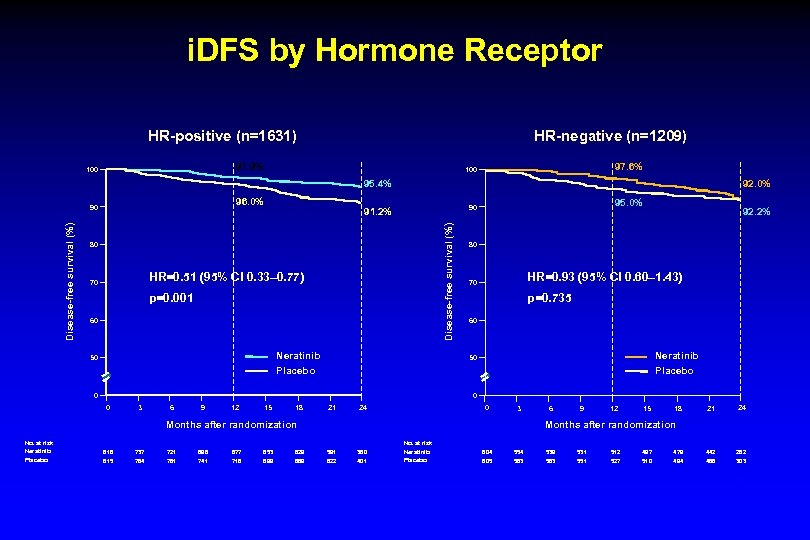
i. DFS by Hormone Receptor HR-positive (n=1631) HR-negative (n=1209) 97. 9% 100 97. 6% 100 95. 4% 96. 0% 80 HR=0. 51 (95% CI 0. 33– 0. 77) 70 p=0. 001 60 Neratinib 50 95. 0% 90 91. 2% Disease-free survival (%) 90 92. 0% 80 HR=0. 93 (95% CI 0. 60– 1. 43) 70 p=0. 735 60 Neratinib 50 Placebo 0 0 0 3 6 9 12 15 18 21 24 0 3 Months after randomization No. at risk Neratinib Placebo 92. 2% 816 815 737 784 721 761 698 741 677 716 653 699 629 669 6 9 12 15 18 21 24 442 468 282 303 Months after randomization 591 622 380 401 No. at risk Neratinib Placebo 604 605 554 583 539 563 531 551 512 527 497 510 479 494
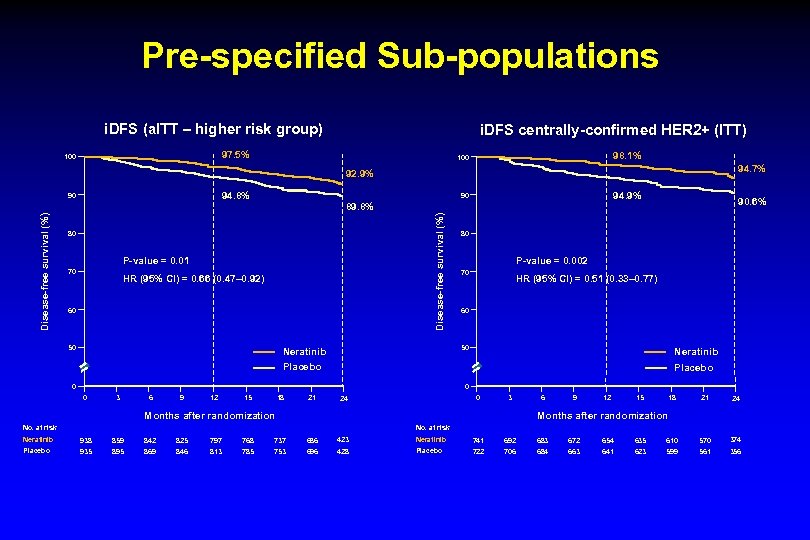
Pre-specified Sub-populations i. DFS (a. ITT – higher risk group) i. DFS centrally-confirmed HER 2+ (ITT) 97. 5% 100 98. 1% 100 94. 7% 92. 9% 94. 8% 80 P-value = 0. 01 70 94. 9% 90 89. 8% Disease-free survival (%) 90 HR (95% CI) = 0. 66 (0. 47– 0. 92) 60 50 90. 6% 80 P-value = 0. 002 70 HR (95% CI) = 0. 51 (0. 33– 0. 77) 60 50 Neratinib Placebo 0 0 0 3 6 9 12 15 18 21 0 24 3 6 9 12 15 18 21 24 Months after randomization No. at risk Neratinib 938 859 842 825 797 768 737 686 423 Neratinib 741 692 683 672 654 635 610 570 374 Placebo 935 895 869 846 813 785 753 696 428 Placebo 722 706 684 663 641 623 599 561 356
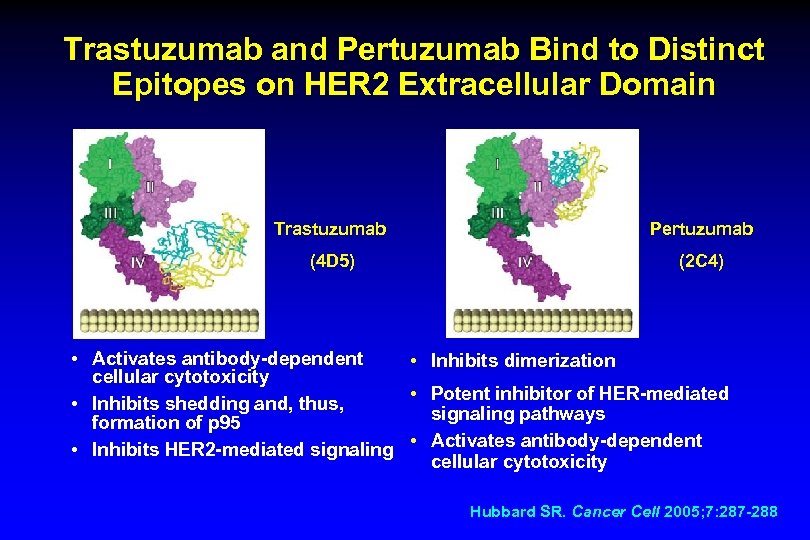
Trastuzumab and Pertuzumab Bind to Distinct Epitopes on HER 2 Extracellular Domain Trastuzumab Pertuzumab (4 D 5) (2 C 4) • Activates antibody-dependent • Inhibits dimerization cellular cytotoxicity • Potent inhibitor of HER-mediated • Inhibits shedding and, thus, signaling pathways formation of p 95 • Inhibits HER 2 -mediated signaling • Activates antibody-dependent cellular cytotoxicity Hubbard SR. Cancer Cell 2005; 7: 287 -288
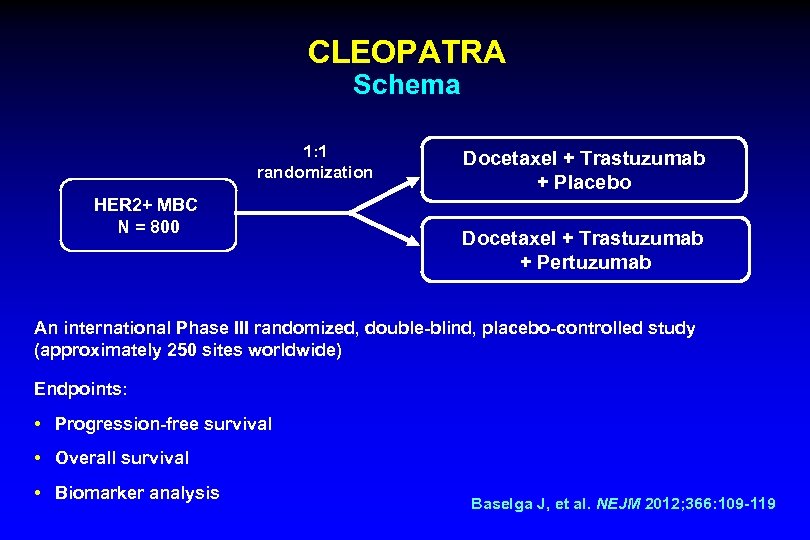
CLEOPATRA Schema 1: 1 randomization HER 2+ MBC N = 800 Docetaxel + Trastuzumab + Placebo Docetaxel + Trastuzumab + Pertuzumab An international Phase III randomized, double-blind, placebo-controlled study (approximately 250 sites worldwide) Endpoints: • Progression-free survival • Overall survival • Biomarker analysis Baselga J, et al. NEJM 2012; 366: 109 -119
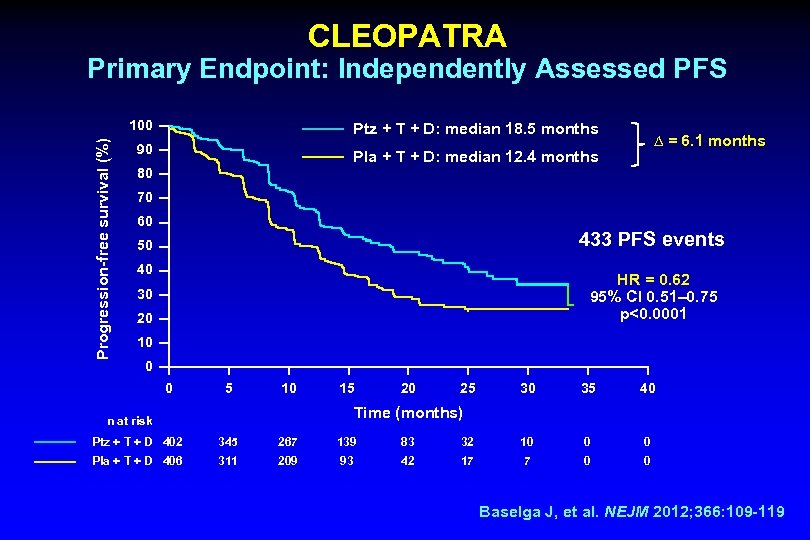
CLEOPATRA Primary Endpoint: Independently Assessed PFS Progression-free survival (%) 100 Ptz + T + D: median 18. 5 months 90 Pla + T + D: median 12. 4 months ∆ = 6. 1 months 80 70 60 433 PFS events 50 40 HR = 0. 62 95% CI 0. 51‒ 0. 75 p<0. 0001 30 20 10 0 0 5 10 15 20 25 30 35 40 Time (months) n at risk Ptz + T + D 402 345 267 139 83 32 10 0 0 Pla + T + D 406 311 209 93 42 17 7 0 0 Baselga J, et al. NEJM 2012; 366: 109 -119
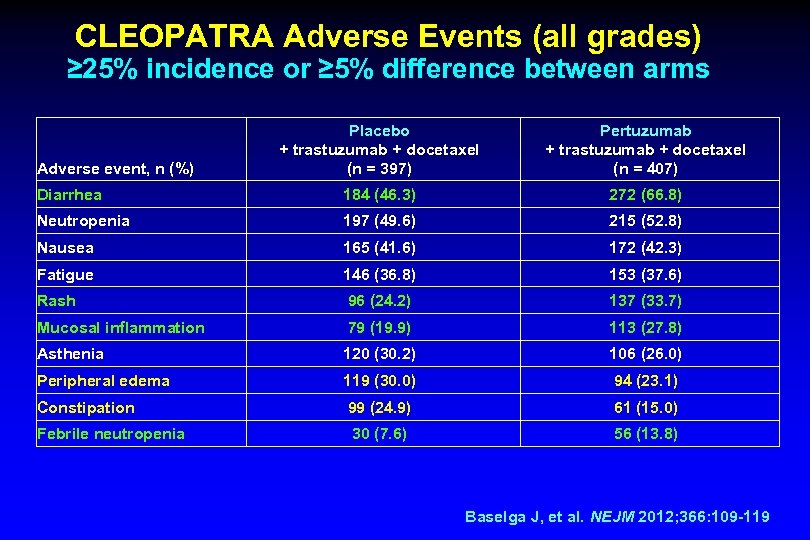
CLEOPATRA Adverse Events (all grades) ≥ 25% incidence or ≥ 5% difference between arms Placebo + trastuzumab + docetaxel (n = 397) Pertuzumab + trastuzumab + docetaxel (n = 407) Diarrhea 184 (46. 3) 272 (66. 8) Neutropenia 197 (49. 6) 215 (52. 8) Nausea 165 (41. 6) 172 (42. 3) Fatigue 146 (36. 8) 153 (37. 6) Rash 96 (24. 2) 137 (33. 7) Mucosal inflammation 79 (19. 9) 113 (27. 8) Asthenia 120 (30. 2) 106 (26. 0) Peripheral edema 119 (30. 0) 94 (23. 1) Constipation 99 (24. 9) 61 (15. 0) Febrile neutropenia 30 (7. 6) 56 (13. 8) Adverse event, n (%) Baselga J, et al. NEJM 2012; 366: 109 -119
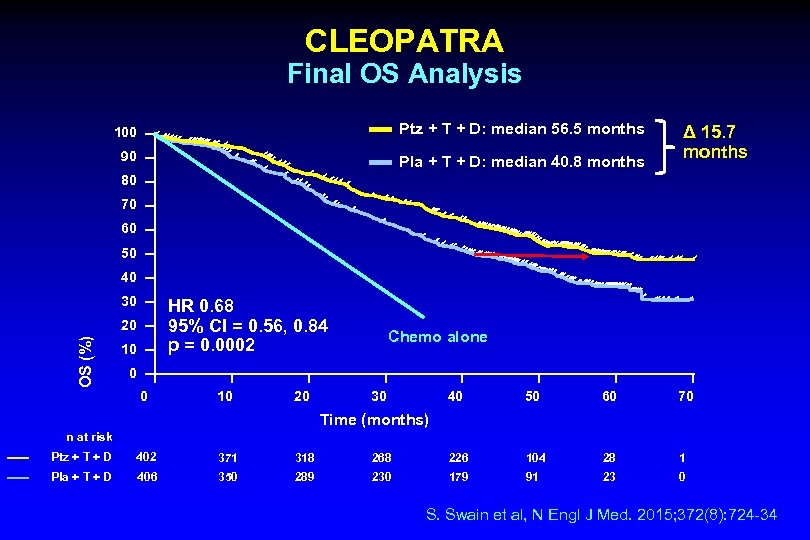
CLEOPATRA Final OS Analysis 100 Ptz + T + D: median 56. 5 months 90 Pla + T + D: median 40. 8 months Δ 15. 7 months 80 70 60 50 40 30 HR 0. 68 95% CI = 0. 56, 0. 84 p = 0. 0002 OS (%) 20 10 Chemo alone 0 0 10 20 30 40 50 60 70 Time (months) n at risk Ptz + T + D 402 371 318 268 226 104 28 1 Pla + T + D 406 350 289 230 179 91 23 0 S. Swain et al, N Engl J Med. 2015; 372(8): 724 -34
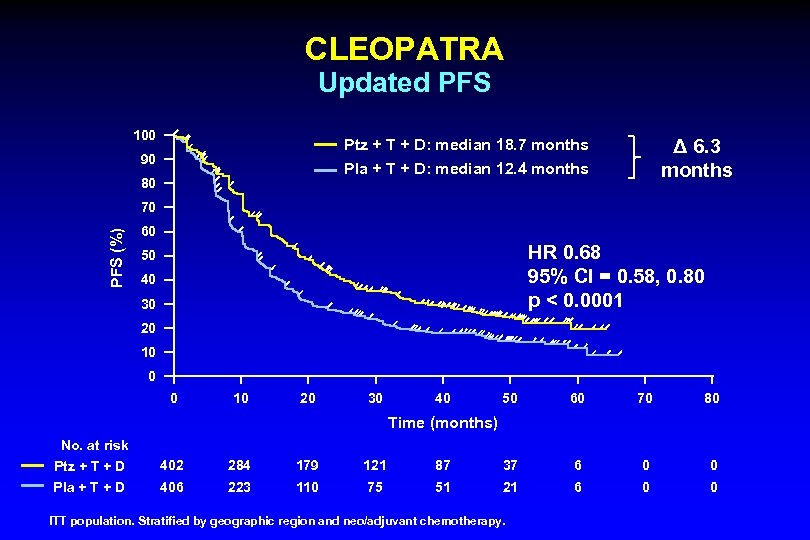
CLEOPATRA Updated PFS 100 Δ 6. 3 months Ptz + T + D: median 18. 7 months 90 Pla + T + D: median 12. 4 months 80 PFS (%) 70 60 HR 0. 68 95% CI = 0. 58, 0. 80 p < 0. 0001 50 40 30 20 10 0 0 10 20 30 40 50 60 70 80 Time (months) No. at risk Ptz + T + D 402 284 179 121 87 37 6 0 0 Pla + T + D 406 223 110 75 51 21 6 0 0 ITT population. Stratified by geographic region and neo/adjuvant chemotherapy.
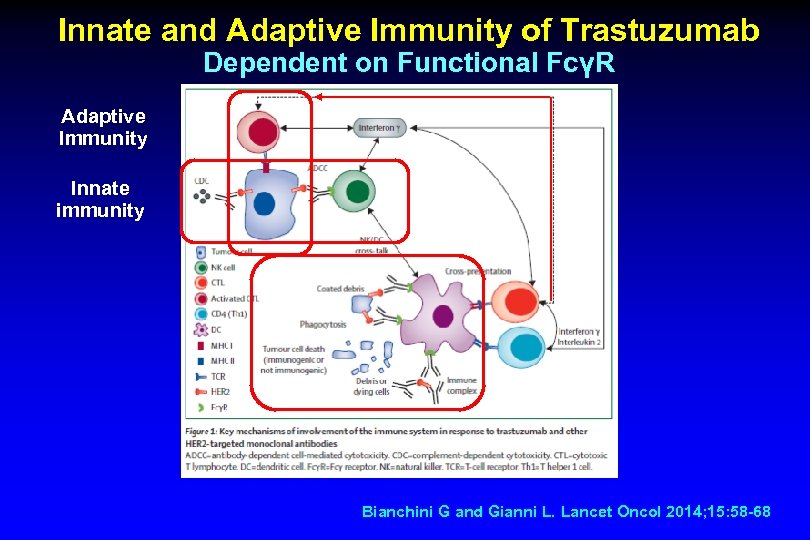
Innate and Adaptive Immunity of Trastuzumab Dependent on Functional FcγR Adaptive Immunity Innate immunity Bianchini G and Gianni L. Lancet Oncol 2014; 15: 58 -68
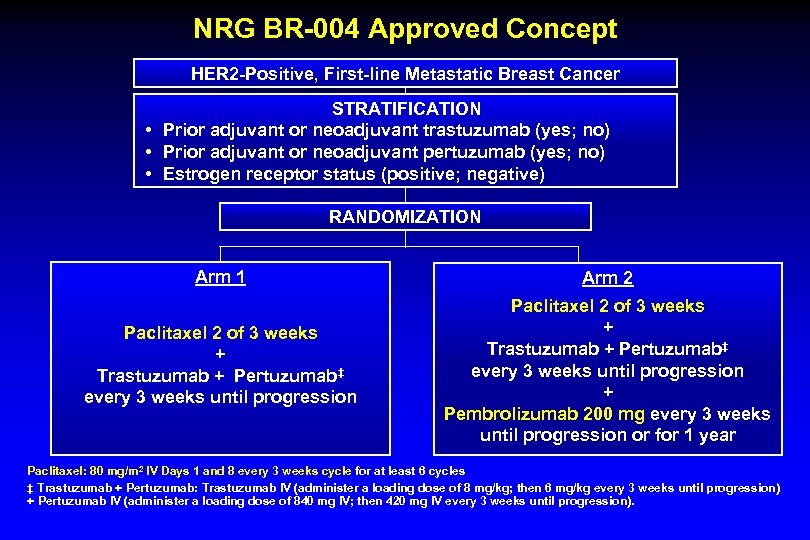
NRG BR-004 Approved Concept HER 2 -Positive, First-line Metastatic Breast Cancer STRATIFICATION • Prior adjuvant or neoadjuvant trastuzumab (yes; no) • Prior adjuvant or neoadjuvant pertuzumab (yes; no) • Estrogen receptor status (positive; negative) RANDOMIZATION Arm 1 Arm 2 Paclitaxel 2 of 3 weeks + Trastuzumab + Pertuzumab‡ every 3 weeks until progression + Pembrolizumab 200 mg every 3 weeks until progression or for 1 year Paclitaxel: 80 mg/m 2 IV Days 1 and 8 every 3 weeks cycle for at least 6 cycles ‡ Trastuzumab + Pertuzumab: Trastuzumab IV (administer a loading dose of 8 mg/kg; then 6 mg/kg every 3 weeks until progression) + Pertuzumab IV (administer a loading dose of 840 mg IV; then 420 mg IV every 3 weeks until progression).
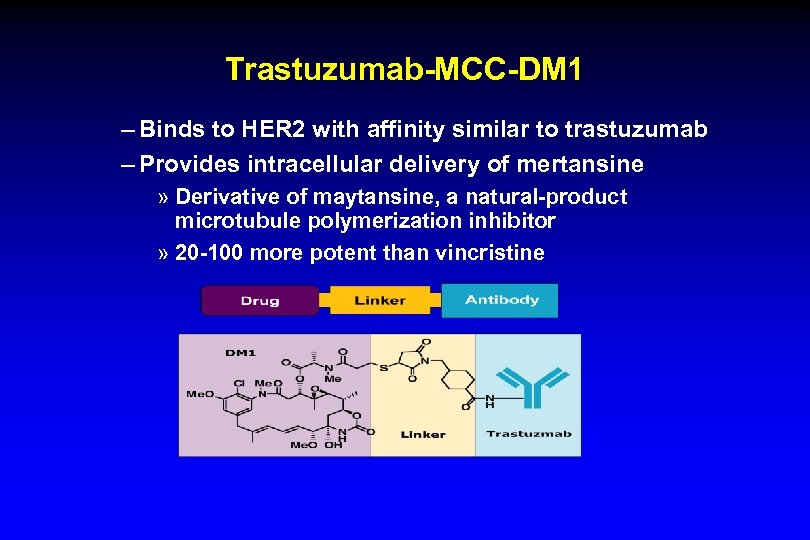
Trastuzumab-MCC-DM 1 – Binds to HER 2 with affinity similar to trastuzumab – Provides intracellular delivery of mertansine » Derivative of maytansine, a natural-product microtubule polymerization inhibitor » 20 -100 more potent than vincristine
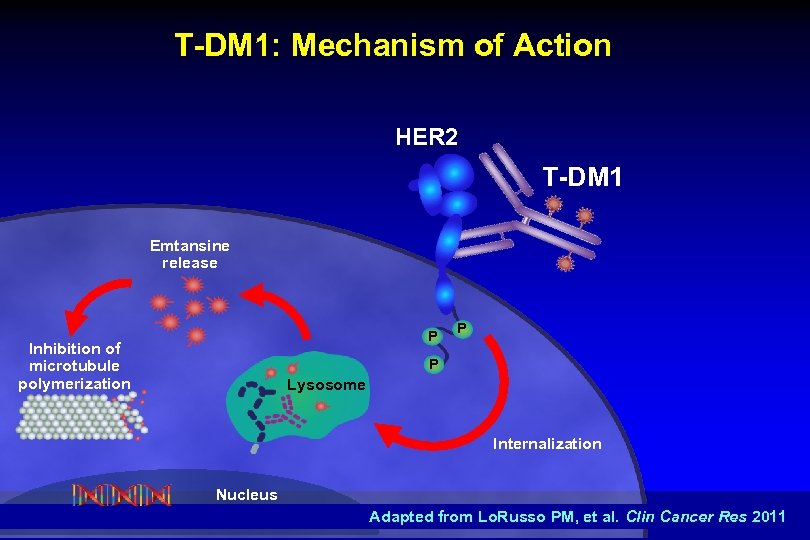
T-DM 1: Mechanism of Action HER 2 T-DM 1 Emtansine release P Inhibition of microtubule polymerization P P Lysosome Internalization Nucleus Adapted from Lo. Russo PM, et al. Clin Cancer Res 2011
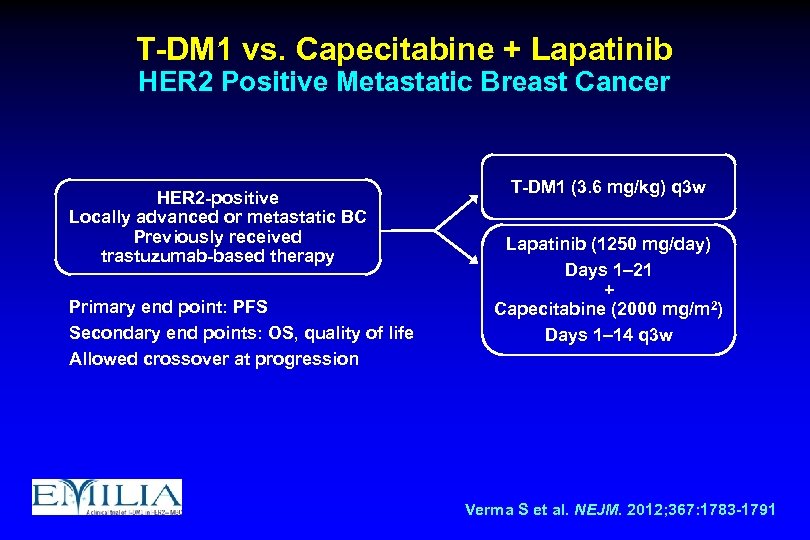
T-DM 1 vs. Capecitabine + Lapatinib HER 2 Positive Metastatic Breast Cancer HER 2 -positive Locally advanced or metastatic BC Previously received trastuzumab-based therapy Primary end point: PFS Secondary end points: OS, quality of life Allowed crossover at progression T-DM 1 (3. 6 mg/kg) q 3 w Lapatinib (1250 mg/day) Days 1– 21 + Capecitabine (2000 mg/m 2) Days 1– 14 q 3 w Verma S et al. NEJM. 2012; 367: 1783 -1791
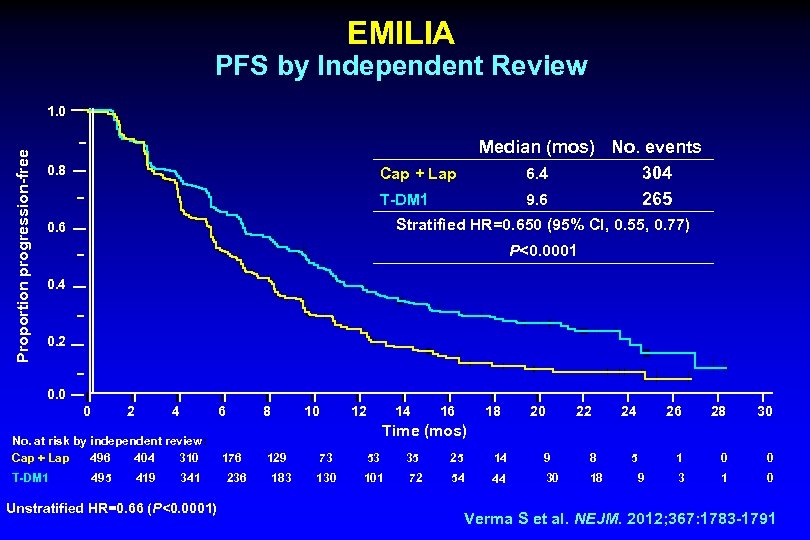
EMILIA PFS by Independent Review Proportion progression-free 1. 0 0. 8 Median (mos) No. events 6. 4 304 9. 6 265 Cap + Lap T-DM 1 Stratified HR=0. 650 (95% CI, 0. 55, 0. 77) 0. 6 P<0. 0001 0. 4 0. 2 0. 0 0 2 4 6 No. at risk by independent review: Cap + Lap 496 404 310 T-DM 1 495 419 341 Unstratified HR=0. 66 (P<0. 0001) 8 10 12 14 16 18 20 22 24 26 28 30 5 1 0 0 3 1 0 Time (mos) 176 236 129 183 73 53 35 25 14 9 8 130 101 72 54 44 30 18 9 Verma S et al. NEJM. 2012; 367: 1783 -1791
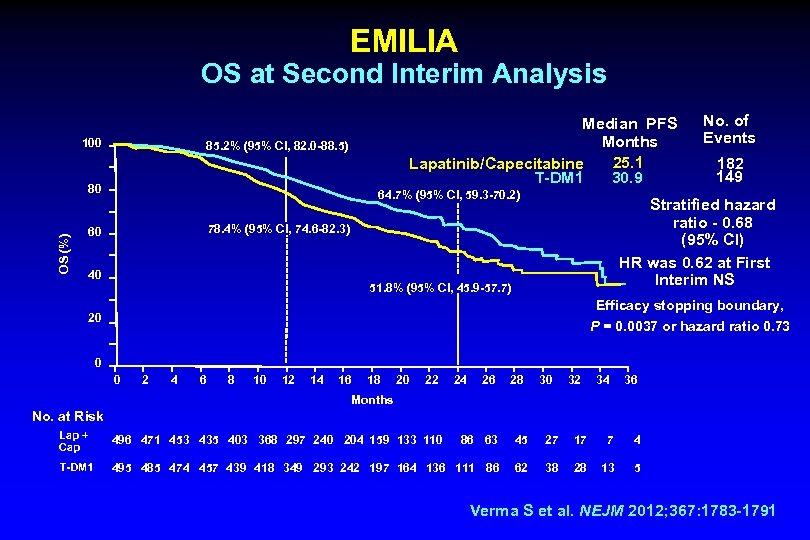
EMILIA OS at Second Interim Analysis 100 Median PFS Months 25. 1 Lapatinib/Capecitabine T-DM 1 30. 9 85. 2% (95% CI, 82. 0 -88. 5) OS (%) 80 64. 7% (95% CI, 59. 3 -70. 2) 182 149 Stratified hazard ratio - 0. 68 (95% CI) HR was 0. 62 at First Interim NS 78. 4% (95% CI, 74. 6 -82. 3) 60 No. of Events 40 51. 8% (95% CI, 45. 9 -57. 7) Efficacy stopping boundary, P = 0. 0037 or hazard ratio 0. 73 20 0 0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30 32 34 36 Months No. at Risk Lap + Cap 496 471 453 435 403 368 297 240 204 159 133 110 86 63 45 27 17 7 4 T-DM 1 495 485 474 457 439 418 349 293 242 197 164 136 111 86 62 38 28 13 5 Verma S et al. NEJM 2012; 367: 1783 -1791
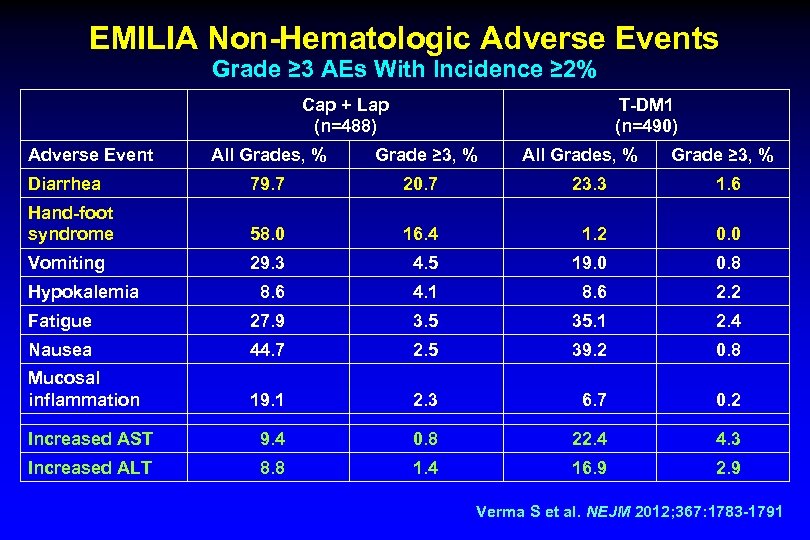
EMILIA Non-Hematologic Adverse Events Grade ≥ 3 AEs With Incidence ≥ 2% Cap + Lap (n=488) Adverse Event All Grades, % T-DM 1 (n=490) Grade ≥ 3, % All Grades, % Grade ≥ 3, % Diarrhea 79. 7 20. 7 23. 3 1. 6 Hand-foot syndrome 58. 0 16. 4 1. 2 0. 0 Vomiting 29. 3 4. 5 19. 0 0. 8 8. 6 4. 1 8. 6 2. 2 Fatigue 27. 9 3. 5 35. 1 2. 4 Nausea 44. 7 2. 5 39. 2 0. 8 Mucosal inflammation 19. 1 2. 3 6. 7 0. 2 Increased AST 9. 4 0. 8 22. 4 4. 3 Increased ALT 8. 8 1. 4 16. 9 2. 9 Hypokalemia Verma S et al. NEJM 2012; 367: 1783 -1791
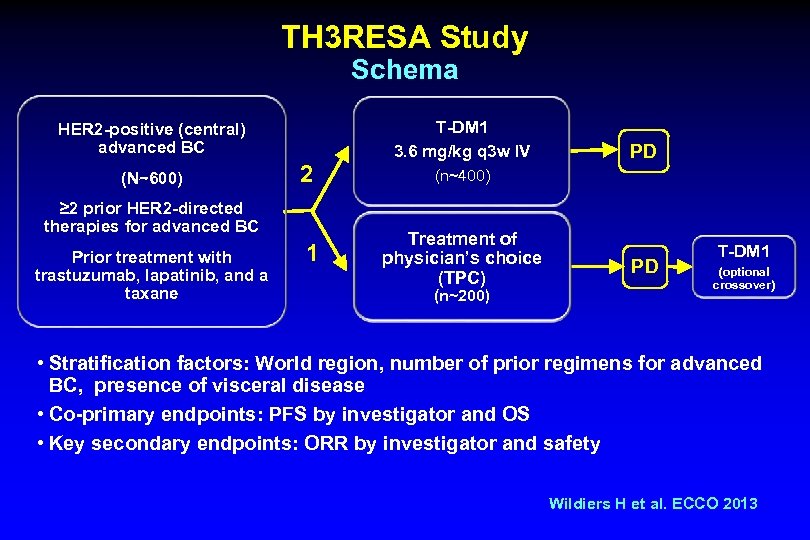
TH 3 RESA Study Schema HER 2 -positive (central) advanced BC (N~600) 2 ≥ 2 prior HER 2 -directed therapies for advanced BC Prior treatment with trastuzumab, lapatinib, and a taxane 1 T-DM 1 3. 6 mg/kg q 3 w IV PD (n~400) Treatment of physician’s choice (TPC) (n~200) PD T-DM 1 (optional crossover) • Stratification factors: World region, number of prior regimens for advanced BC, presence of visceral disease • Co-primary endpoints: PFS by investigator and OS • Key secondary endpoints: ORR by investigator and safety Wildiers H et al. ECCO 2013
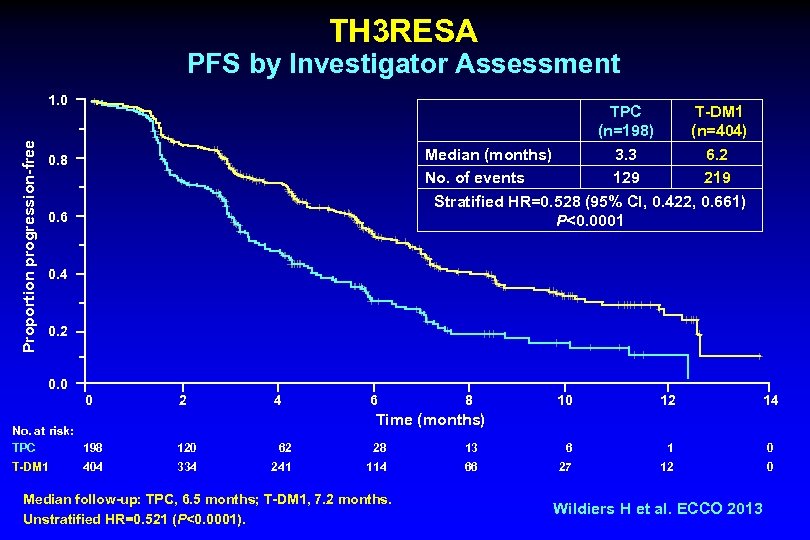
TH 3 RESA PFS by Investigator Assessment Proportion progression-free 1. 0 TPC T-DM 1 (n=198) (n=404) Median (months) 3. 3 6. 2 No. of events 129 219 Stratified HR=0. 528 (95% CI, 0. 422, 0. 661) P<0. 0001 0. 8 0. 6 0. 4 0. 2 0. 0 0 2 4 6 8 10 12 14 Time (months) No. at risk: TPC 198 120 62 28 13 6 1 0 T-DM 1 334 241 114 66 27 12 0 404 Median follow-up: TPC, 6. 5 months; T-DM 1, 7. 2 months. Unstratified HR=0. 521 (P<0. 0001). Wildiers H et al. ECCO 2013
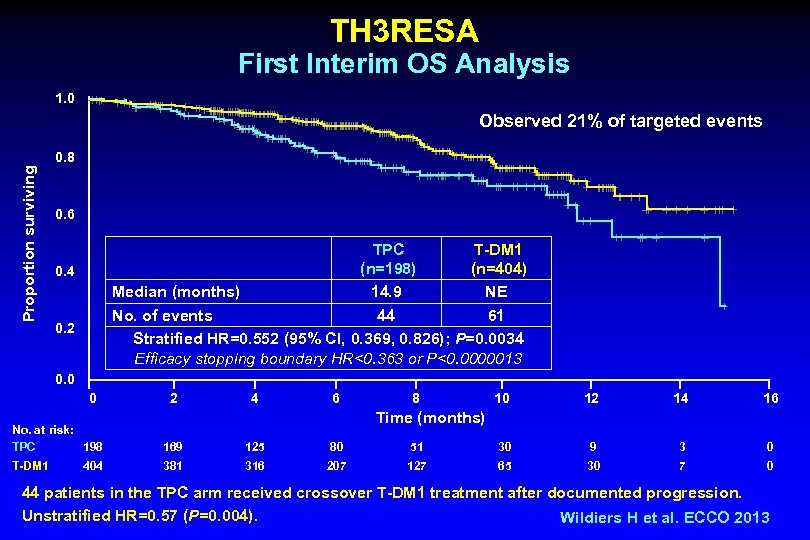
TH 3 RESA First Interim OS Analysis 1. 0 Observed 21% of targeted events Proportion surviving 0. 8 0. 6 TPC T-DM 1 (n=198) (n=404) Median (months) 14. 9 NE No. of events 44 61 Stratified HR=0. 552 (95% CI, 0. 369, 0. 826); P=0. 0034 Efficacy stopping boundary HR<0. 363 or P<0. 0000013 0. 4 0. 2 0. 0 0 2 4 6 8 10 12 14 16 Time (months) No. at risk: TPC 198 169 125 80 51 30 9 3 0 T-DM 1 381 316 207 127 65 30 7 0 404 44 patients in the TPC arm received crossover T-DM 1 treatment after documented progression. Unstratified HR=0. 57 (P=0. 004). Wildiers H et al. ECCO 2013
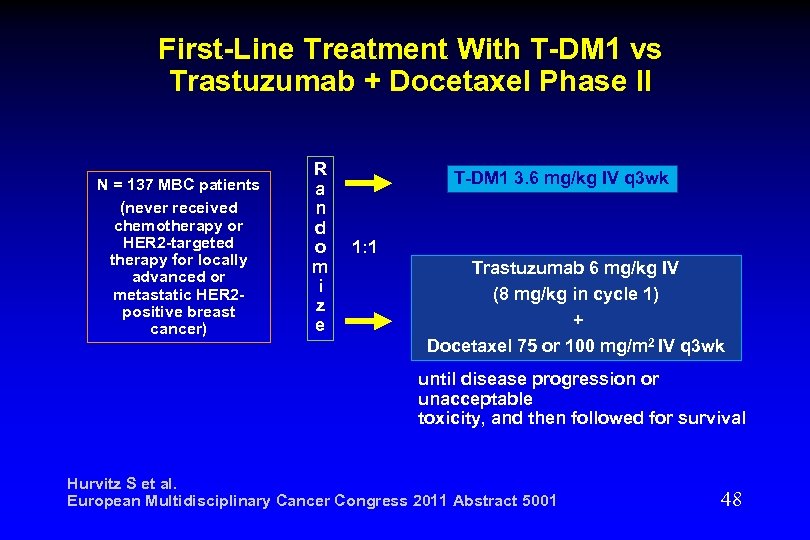
First-Line Treatment With T-DM 1 vs Trastuzumab + Docetaxel Phase II N = 137 MBC patients (never received chemotherapy or HER 2 -targeted therapy for locally advanced or metastatic HER 2 positive breast cancer) R a n d o m i z e T-DM 1 3. 6 mg/kg IV q 3 wk 1: 1 Trastuzumab 6 mg/kg IV (8 mg/kg in cycle 1) + Docetaxel 75 or 100 mg/m 2 IV q 3 wk until disease progression or unacceptable toxicity, and then followed for survival Hurvitz S et al. European Multidisciplinary Cancer Congress 2011 Abstract 5001 48
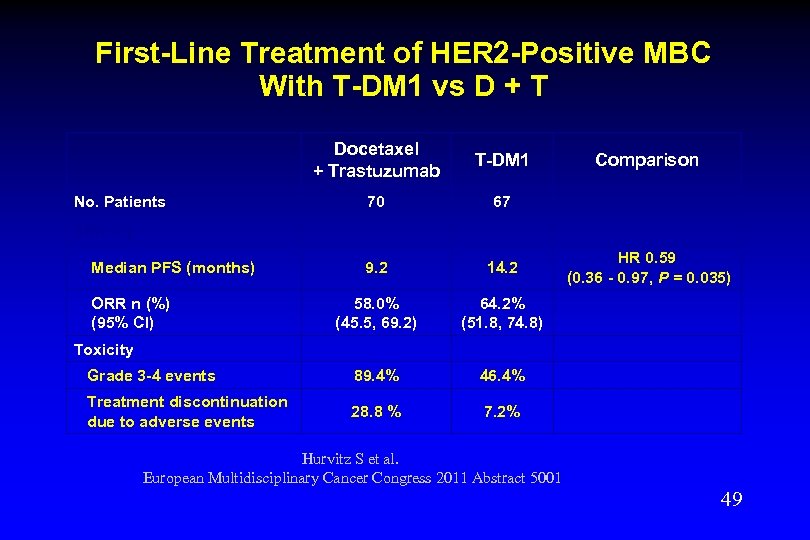
First-Line Treatment of HER 2 -Positive MBC With T-DM 1 vs D + T Docetaxel + Trastuzumab T-DM 1 70 67 9. 2 14. 2 58. 0% (45. 5, 69. 2) 64. 2% (51. 8, 74. 8) Grade 3 -4 events 89. 4% 46. 4% Treatment discontinuation due to adverse events 28. 8 % 7. 2% No. Patients Comparison Efficacy Median PFS (months) ORR n (%) (95% CI) HR 0. 59 (0. 36 - 0. 97, P = 0. 035) Toxicity Hurvitz S et al. European Multidisciplinary Cancer Congress 2011 Abstract 5001 49
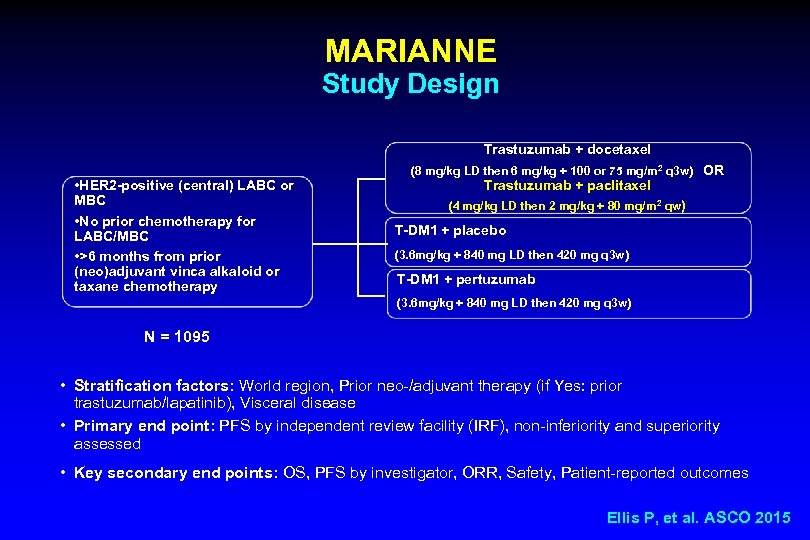
MARIANNE Study Design Trastuzumab + docetaxel • HER 2 -positive (central) LABC or MBC • No prior chemotherapy for LABC/MBC • >6 months from prior (neo)adjuvant vinca alkaloid or taxane chemotherapy (8 mg/kg LD then 6 mg/kg + 100 or 75 mg/m 2 q 3 w) OR Trastuzumab + paclitaxel (4 mg/kg LD then 2 mg/kg + 80 mg/m 2 qw) T-DM 1 + placebo (3. 6 mg/kg + 840 mg LD then 420 mg q 3 w) T-DM 1 + pertuzumab (3. 6 mg/kg + 840 mg LD then 420 mg q 3 w) N = 1095 • Stratification factors: World region, Prior neo-/adjuvant therapy (if Yes: prior trastuzumab/lapatinib), Visceral disease • Primary end point: PFS by independent review facility (IRF), non-inferiority and superiority assessed • Key secondary end points: OS, PFS by investigator, ORR, Safety, Patient-reported outcomes Ellis P, et al. ASCO 2015
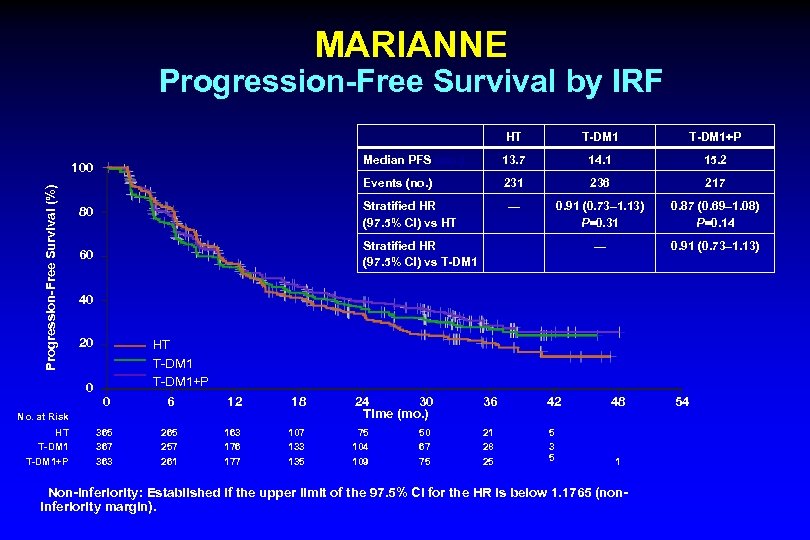
MARIANNE Progression-Free Survival by IRF Progression-Free Survival (%) T-DM 1+P 13. 7 14. 1 15. 2 Events (no. ) 231 236 217 — 0. 91 (0. 73– 1. 13) P=0. 31 0. 87 (0. 69– 1. 08) P=0. 14 — 0. 91 (0. 73– 1. 13) 80 Stratified HR (97. 5% CI) vs HT 60 Stratified HR (97. 5% CI) vs T-DM 1 40 20 0 HT T-DM 1+P 0 6 12 18 365 367 363 265 257 261 163 176 177 107 133 135 No. at Risk HT T-DM 1+P T-DM 1 Median PFS (mo. ) 100 HT 24 30 Time (mo. ) 36 42 48 75 104 109 21 28 25 5 3 5 1 50 67 75 Non-inferiority: Established if the upper limit of the 97. 5% CI for the HR is below 1. 1765 (noninferiority margin). 54
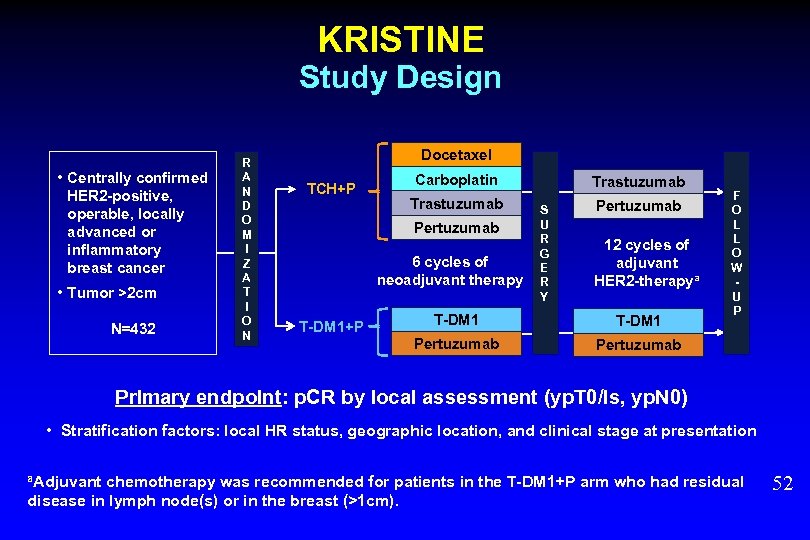
KRISTINE Study Design • Centrally confirmed HER 2 -positive, operable, locally advanced or inflammatory breast cancer • Tumor >2 cm N=432 R A N D O M I Z A T I O N Docetaxel Carboplatin Trastuzumab TCH+P Pertuzumab 6 cycles of neoadjuvant therapy T-DM 1+P S U R G E R Y 12 cycles of adjuvant HER 2 -therapya T-DM 1 Pertuzumab F O L L O W U P Pertuzumab Primary endpoint: p. CR by local assessment (yp. T 0/is, yp. N 0) • Stratification factors: local HR status, geographic location, and clinical stage at presentation a. Adjuvant chemotherapy was recommended for patients in the T-DM 1+P arm who had residual disease in lymph node(s) or in the breast (>1 cm). 52
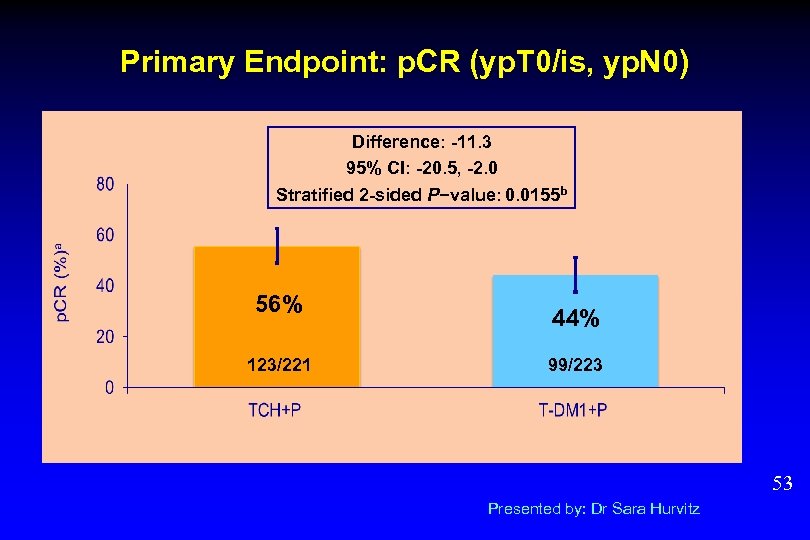
Primary Endpoint: p. CR (yp. T 0/is, yp. N 0) Difference: -11. 3 95% CI: -20. 5, -2. 0 Stratified 2 -sided P−value: 0. 0155 b 56% 123/221 44% 99/223 53 Presented by: Dr Sara Hurvitz
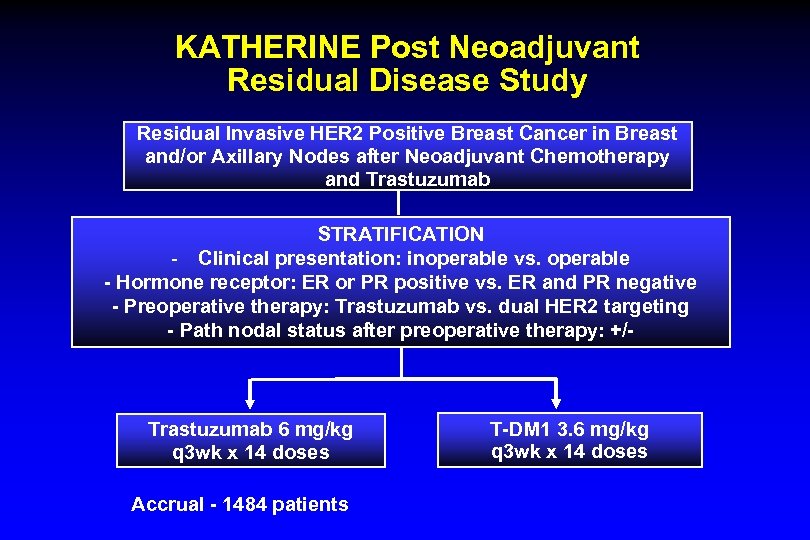
KATHERINE Post Neoadjuvant Residual Disease Study Residual Invasive HER 2 Positive Breast Cancer in Breast and/or Axillary Nodes after Neoadjuvant Chemotherapy and Trastuzumab STRATIFICATION - Clinical presentation: inoperable vs. operable - Hormone receptor: ER or PR positive vs. ER and PR negative - Preoperative therapy: Trastuzumab vs. dual HER 2 targeting - Path nodal status after preoperative therapy: +/- Trastuzumab 6 mg/kg q 3 wk x 14 doses Accrual - 1484 patients T-DM 1 3. 6 mg/kg q 3 wk x 14 doses
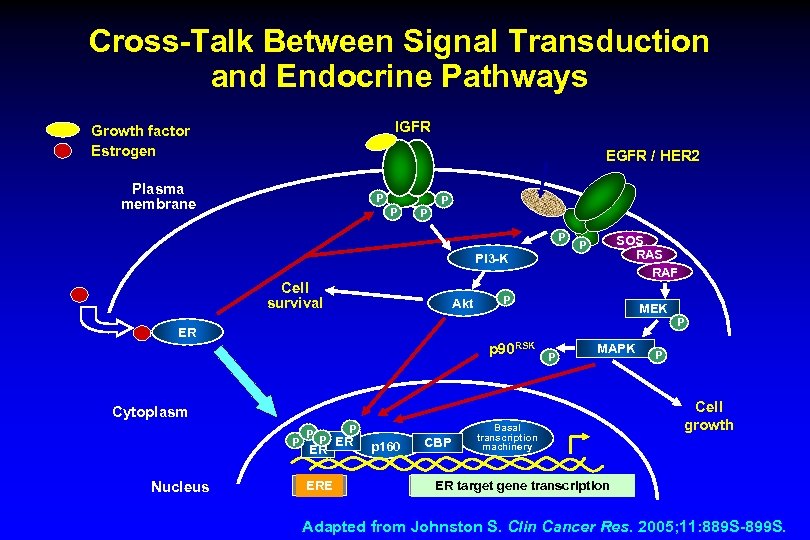
Cross-Talk Between Signal Transduction and Endocrine Pathways IGFR Growth factor Estrogen EGFR / HER 2 Plasma membrane P P P SOS RAF P PI 3 -K Cell survival Akt P MEK P ER p 90 RSK P MAPK Cytoplasm P Nucleus P P P ER ER ERE p 160 CBP Basal transcription machinery P Cell growth ER target gene transcription Adapted from Johnston S. Clin Cancer Res. 2005; 11: 889 S-899 S.
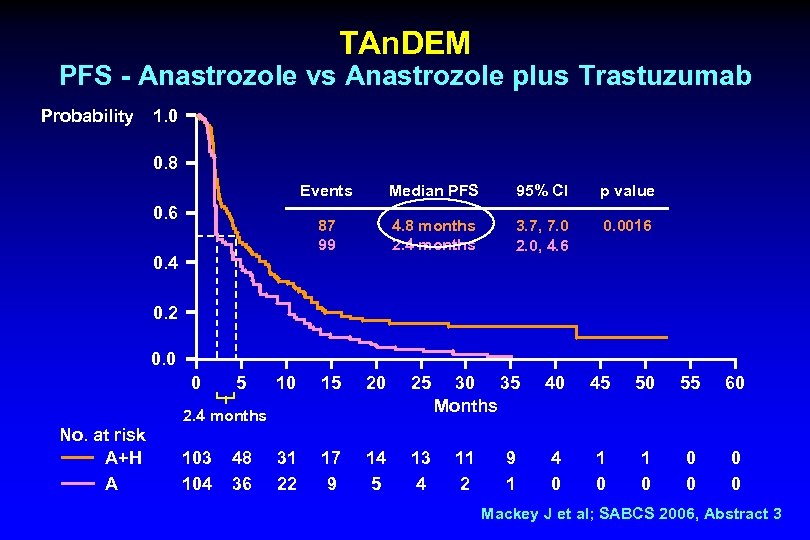
TAn. DEM PFS - Anastrozole vs Anastrozole plus Trastuzumab Probability 1. 0 0. 8 Events 0. 4 95% CI p value 87 99 0. 6 Median PFS 4. 8 months 2. 4 months 3. 7, 7. 0 2. 0, 4. 6 0. 0016 0. 2 0. 0 0 5 10 15 20 25 31 22 17 9 14 5 13 4 2. 4 months No. at risk A+H A 103 104 48 36 30 35 Months 11 2 9 1 40 45 50 55 60 4 0 1 0 0 0 Mackey J et al; SABCS 2006, Abstract 3
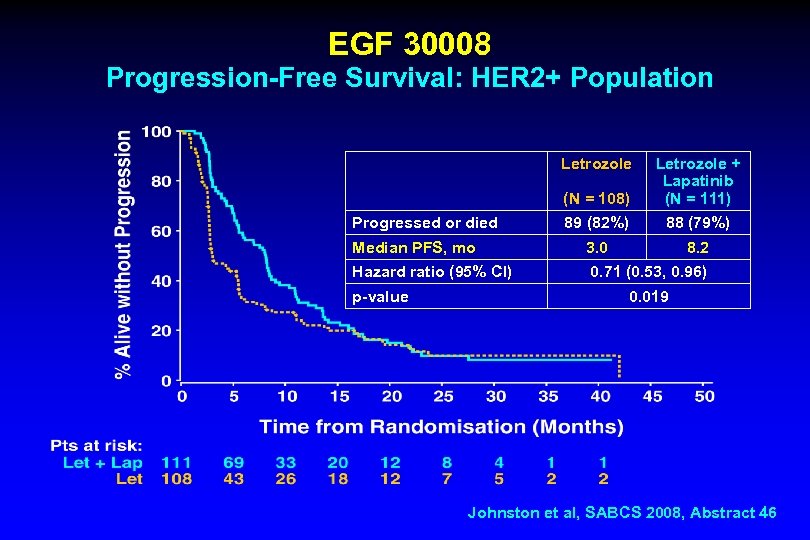
EGF 30008 Progression-Free Survival: HER 2+ Population Letrozole (N = 108) Letrozole + Lapatinib (N = 111) 89 (82%) 88 (79%) Median PFS, mo 3. 0 8. 2 Hazard ratio (95% CI) 0. 71 (0. 53, 0. 96) Progressed or died p-value 0. 019 Johnston et al, SABCS 2008, Abstract 46
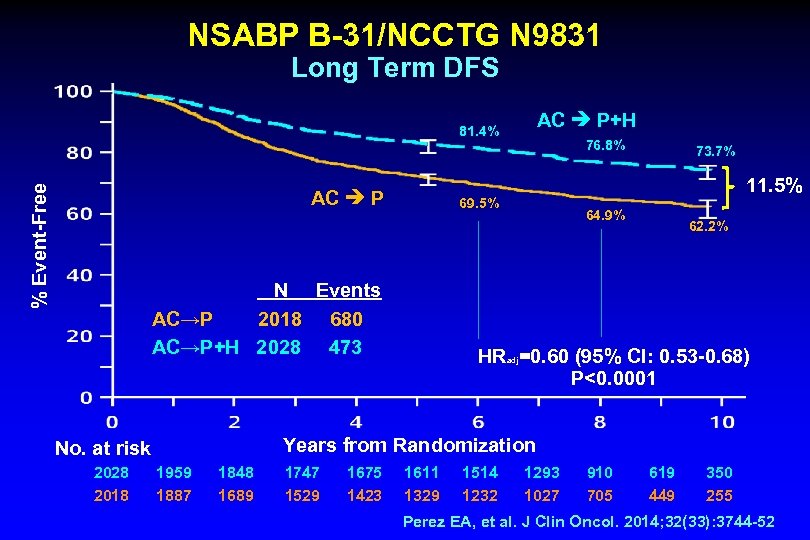
NSABP B-31/NCCTG N 9831 Long Term DFS AC P+H % Event-Free 81. 4% AC P N Events AC→P 2018 680 AC→P+H 2028 473 No. at risk 76. 8% 73. 7% 11. 5% 69. 5% 64. 9% 62. 2% HR =0. 60 (95% CI: 0. 53 -0. 68) P<0. 0001 adj Years from Randomization 2028 1959 1848 1747 1675 1611 1514 1293 910 619 350 2018 1887 1689 1529 1423 1329 1232 1027 705 449 255 Perez EA, et al. J Clin Oncol. 2014; 32(33): 3744 -52
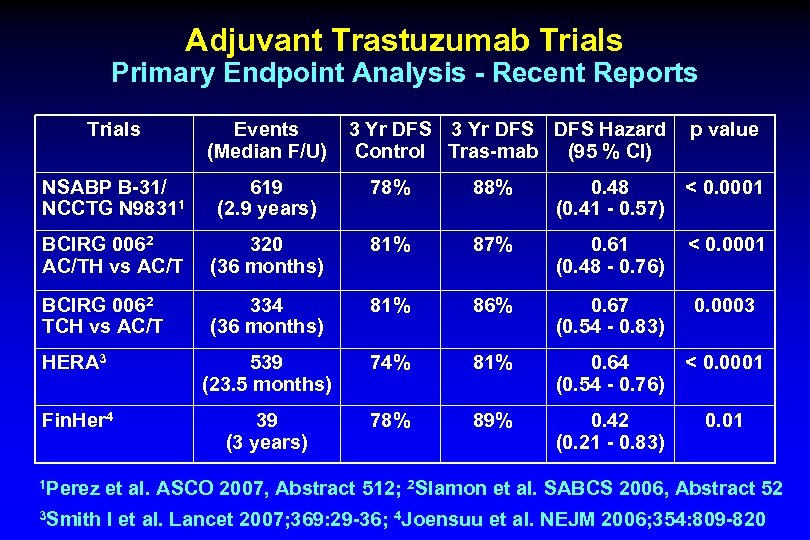
Adjuvant Trastuzumab Trials Primary Endpoint Analysis - Recent Reports Trials Events 3 Yr DFS DFS Hazard p value (Median F/U) Control Tras-mab (95 % CI) NSABP B-31/ NCCTG N 98311 619 78% (2. 9 years) 88% 0. 48 < 0. 0001 (0. 41 - 0. 57) BCIRG 0062 AC/TH vs AC/T 320 81% (36 months) 87% 0. 61 < 0. 0001 (0. 48 - 0. 76) BCIRG 0062 TCH vs AC/T 334 81% (36 months) 86% 0. 67 (0. 54 - 0. 83) 539 74% (23. 5 months) 81% 0. 64 < 0. 0001 (0. 54 - 0. 76) 39 78% (3 years) 89% 0. 42 (0. 21 - 0. 83) HERA 3 Fin. Her 4 0. 0003 0. 01 1 Perez et al. ASCO 2007, Abstract 512; 2 Slamon et al. SABCS 2006, Abstract 52 3 Smith I et al. Lancet 2007; 369: 29 -36; 4 Joensuu et al. NEJM 2006; 354: 809 -820
Phase III HER 2+ Adjuvant Trial: ALTTO Surgery and Chemotherapy Completed Randomize 3 -wkly Trastuzumab 6 mg/kg q 3 wks (52 wks) • • Lapatinib 1, 500 mg daily (52 wks) Estimated N = 8, 400 Wkly Trastuzumab 2 mg/kg (12 or 18 wks) Washout (6 wks) Lapatinib 1, 500 mg daily (28 or 34 wks) Lapatinib 1, 000 mg daily + 3 -wkly Trastuzumab 6 mg/kg (52 wks) Primary outcome measures: DFS Anti-HER 2 therapy can overlap chemotherapy Dose adjustments: Lapatinib reduced to 750 mg daily when given with wkly paclitaxel and trastuzumab, due to high grade 3 toxicities, mainly diarrhea Sep 2011: Independent committee indicated that the lapatinib-alone arm is unlikely to meet the prespecified criteria to demonstrate non-inferiority to trastuzumab alone with respect to DFS - Lapatinibonly arm halted
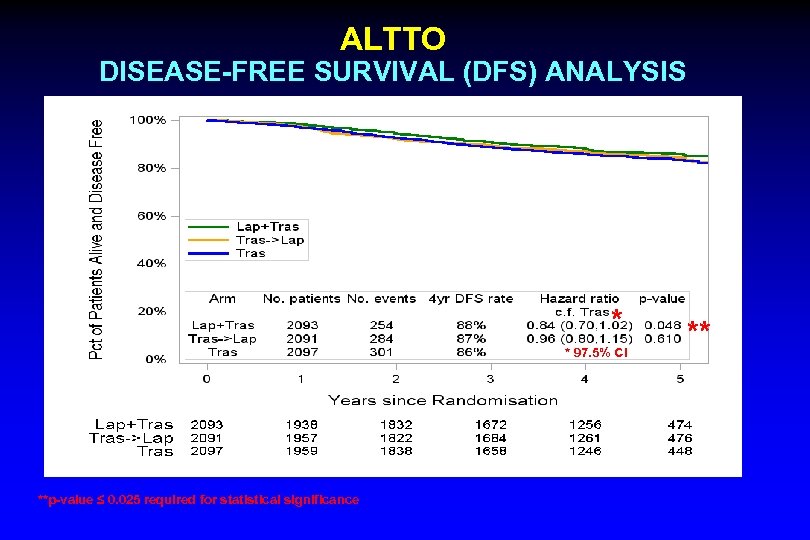
ALTTO DISEASE-FREE SURVIVAL (DFS) ANALYSIS MFU = 4. 5 yrs * * 97. 5% CI **p-value ≤ 0. 025 required for statistical significance **
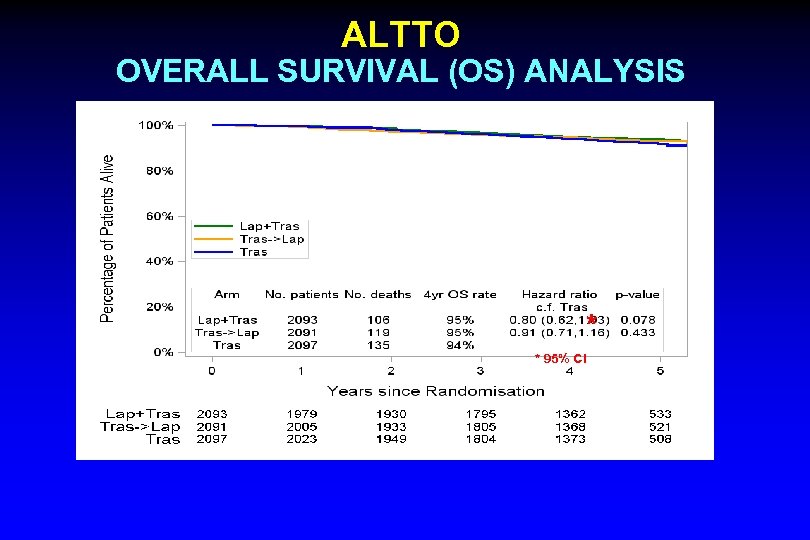
ALTTO OVERALL SURVIVAL (OS) ANALYSIS MFU = 4. 5 yrs * * 95% CI

BETH: A Randomized Phase III Study Evaluating Adjuvant Bevacizumab Added to Trastuzumab/Chemotherapy for Treatment of HER 2+ Early Breast Cancer TRIO -011 / NSABP B-44 -1 / BO 20906 D. Slamon, S. Swain, M. Buyse, M. Martin, C. Geyer, Y-H. Im, T. Pienkowski, S-B. Kim, N. Robert, G. Steger, J. Crown, S. Verma, W. Eiermann, J. Costantino, SA. Im, E. Mamounas, L. Schwartzberg, A. Paterson, J. Mackey, L. Provencher, M. Press, M. Thirlwell, V. Bee-Munteanu, V. Henschel, A. Crepelle-Flechais, N. Wolmark
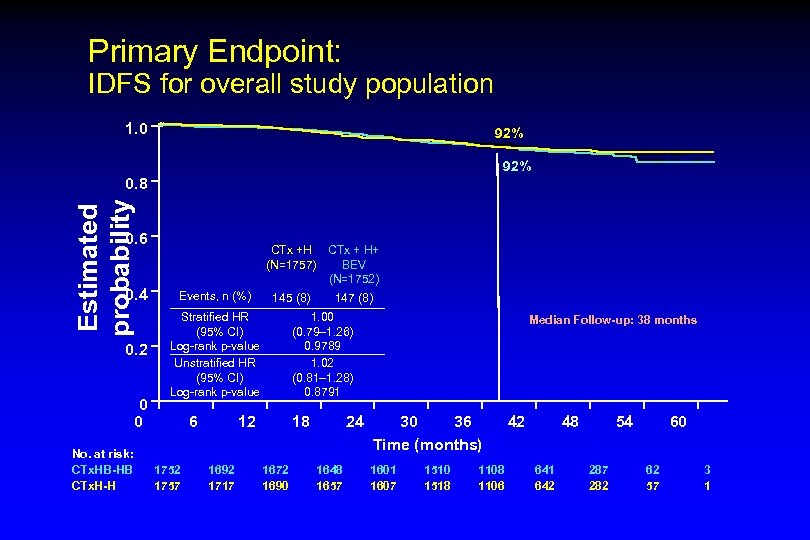
Primary Endpoint: IDFS for overall study population 1. 0 92% Estimated probability 0. 8 0. 6 CTx +H CTx + H+ (N=1757) BEV (N=1752) 0. 4 Events, n (%) 0. 2 Stratified HR (95% CI) Log-rank p-value Unstratified HR (95% CI) Log-rank p-value 0 0 No. at risk: CTx. HB-HB CTx. H-H 6 1752 1757 145 (8) 1. 00 (0. 79– 1. 26) 0. 9789 1. 02 (0. 81– 1. 28) 0. 8791 12 1692 1717 147 (8) 18 1672 1690 24 1648 1657 Median Follow-up: 38 months 30 36 Time (months) 1601 1607 1510 1518 1106 42 48 641 642 54 287 282 60 62 57 3 1
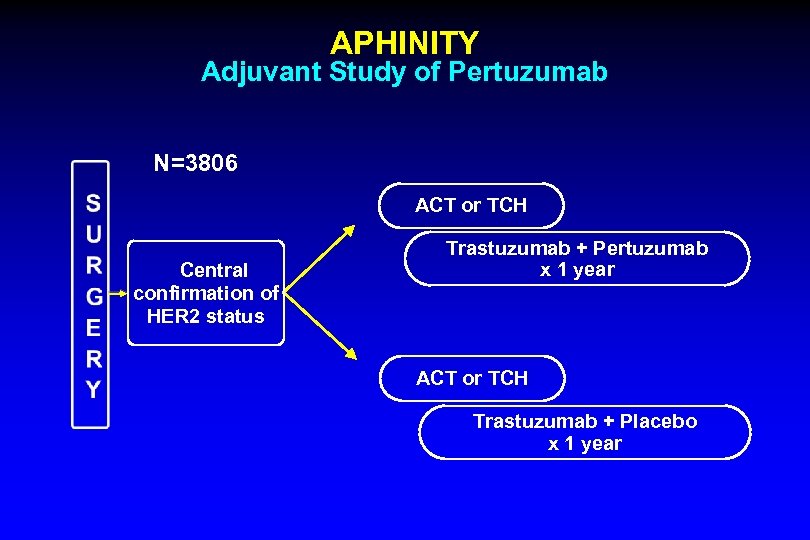
APHINITY Adjuvant Study of Pertuzumab N=3806 ACT or TCH Central confirmation of HER 2 status Trastuzumab + Pertuzumab x 1 year ACT or TCH Trastuzumab + Placebo x 1 year
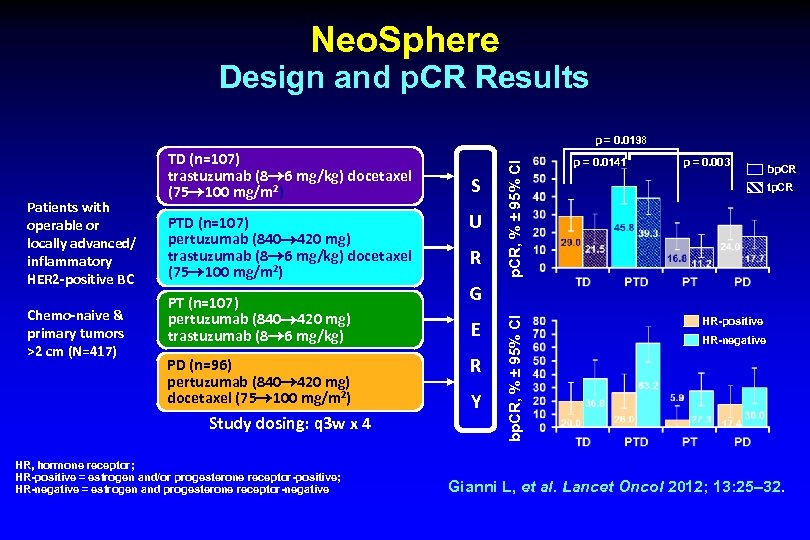
Neo. Sphere Design and p. CR Results Chemo-naive & primary tumors >2 cm (N=417) PTD (n=107) pertuzumab (840 420 mg) trastuzumab (8 6 mg/kg) docetaxel (75 100 mg/m 2) PT (n=107) pertuzumab (840 420 mg) trastuzumab (8 6 mg/kg) PD (n=96) pertuzumab (840 420 mg) docetaxel (75 100 mg/m 2) Study dosing: q 3 w x 4 HR, hormone receptor; HR-positive = estrogen and/or progesterone receptor-positive; HR-negative = estrogen and progesterone receptor-negative S U R p = 0. 0141 p = 0. 003 bp. CR tp. CR G E R Y bp. CR, % ± 95% CI Patients with operable or locally advanced/ inflammatory HER 2 -positive BC TD (n=107) trastuzumab (8 6 mg/kg) docetaxel (75 100 mg/m 2) p. CR, % ± 95% CI p = 0. 0198 HR-positive HR-negative Gianni L, et al. Lancet Oncol 2012; 13: 25– 32.
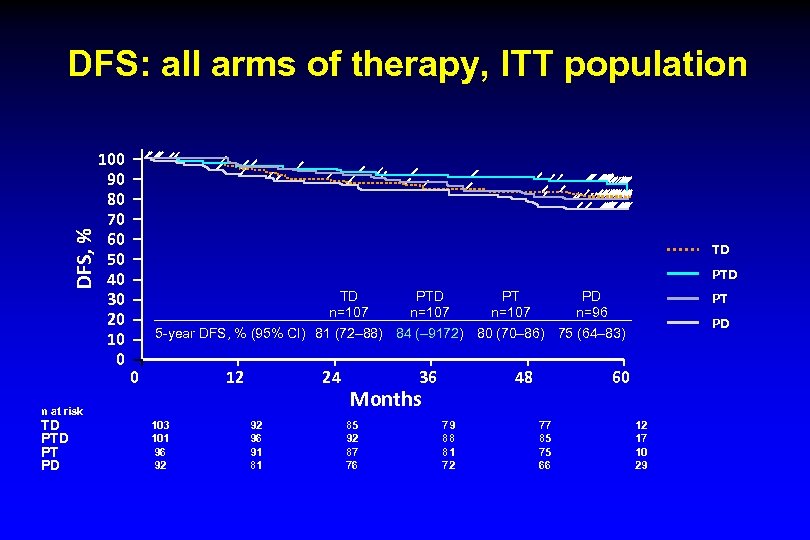
DFS, % DFS: all arms of therapy, ITT population 100 90 80 70 60 50 40 30 20 10 0 TD PTD TD n=107 PT n=107 PD n=96 PT PD 5 -year DFS, % (95% CI) 81 (72– 88) 84 (– 9172) 80 (70– 86) 75 (64– 83) 0 12 24 n at risk TD PT PD PTD n=107 103 101 96 92 92 96 91 81 36 48 Months 85 92 87 76 79 88 81 72 60 77 85 75 66 12 17 10 29
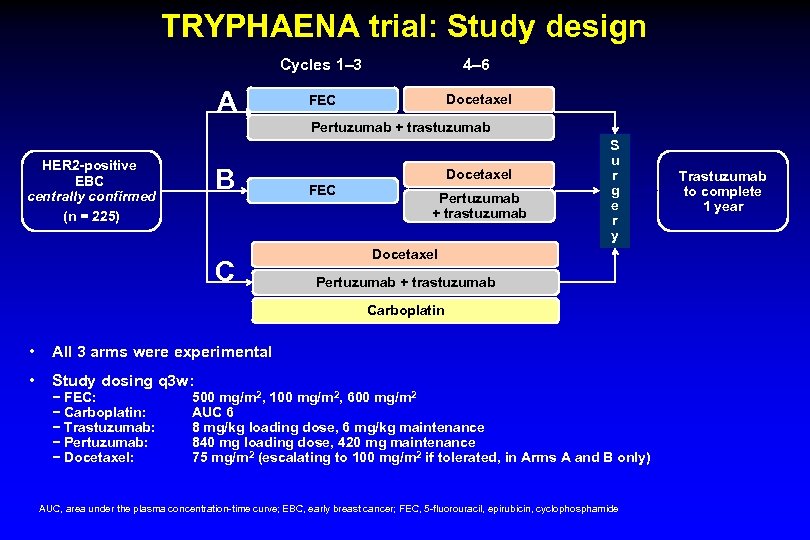
TRYPHAENA trial: Study design Cycles 1‒ 3 A 4‒ 6 FEC Docetaxel Pertuzumab + trastuzumab HER 2 -positive EBC centrally confirmed (n = 225) B C Docetaxel FEC Pertuzumab + trastuzumab S u r g e r y Docetaxel Pertuzumab + trastuzumab Carboplatin • All 3 arms were experimental • Study dosing q 3 w: − FEC: − Carboplatin: − Trastuzumab: − Pertuzumab: − Docetaxel: 500 mg/m 2, 100 mg/m 2, 600 mg/m 2 AUC 6 8 mg/kg loading dose, 6 mg/kg maintenance 840 mg loading dose, 420 mg maintenance 75 mg/m 2 (escalating to 100 mg/m 2 if tolerated, in Arms A and B only) AUC, area under the plasma concentration-time curve; EBC, early breast cancer; FEC, 5 -fluorouracil, epirubicin, cyclophosphamide Trastuzumab to complete 1 year
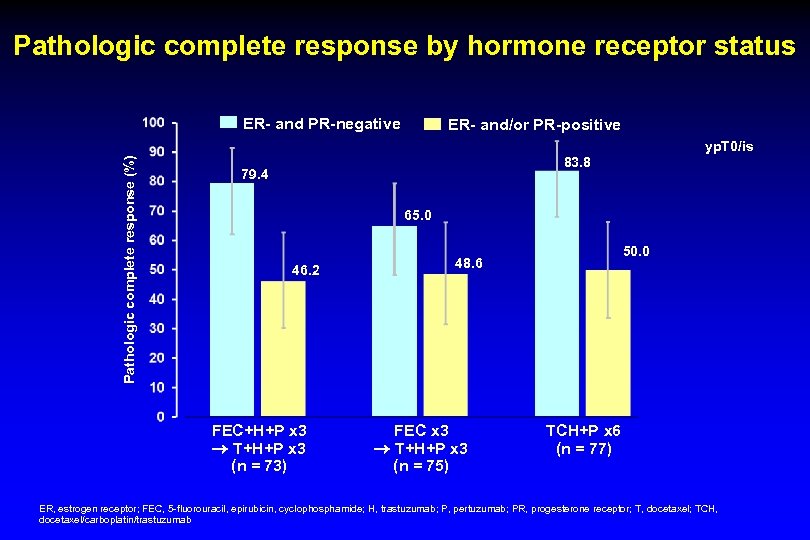
Pathologic complete response by hormone receptor status ER- and PR-negative ER- and/or PR-positive Pathologic complete response (%) yp. T 0/is 83. 8 79. 4 65. 0 46. 2 FEC+H+P x 3 T+H+P x 3 (n = 73) 50. 0 48. 6 FEC x 3 T+H+P x 3 (n = 75) TCH+P x 6 (n = 77) ER, estrogen receptor; FEC, 5 -fluorouracil, epirubicin, cyclophosphamide; H, trastuzumab; P, pertuzumab; PR, progesterone receptor; T, docetaxel; TCH, docetaxel/carboplatin/trastuzumab

Thank you for your attention
0a1de87a1294bd0cc15e132919cfabf7.ppt