edab31fc4ff30234d94a459aae25bc28.ppt
- Количество слайдов: 50

Hepatitis C Virus Casey Mc. Grath BIO 360

Outline • • • Epidemiology Introduction to Hepatitis C Virus Immune response Novel drug therapies Conclusions
Epidemiology Hepatitis C Virus (HCV): • ~170 million people worldwide • Chronic hepatitis, liver cirrhosis, hepatocellular carcinoma (HCC) • Transmitted via blood-transfusions, intravenous drug use
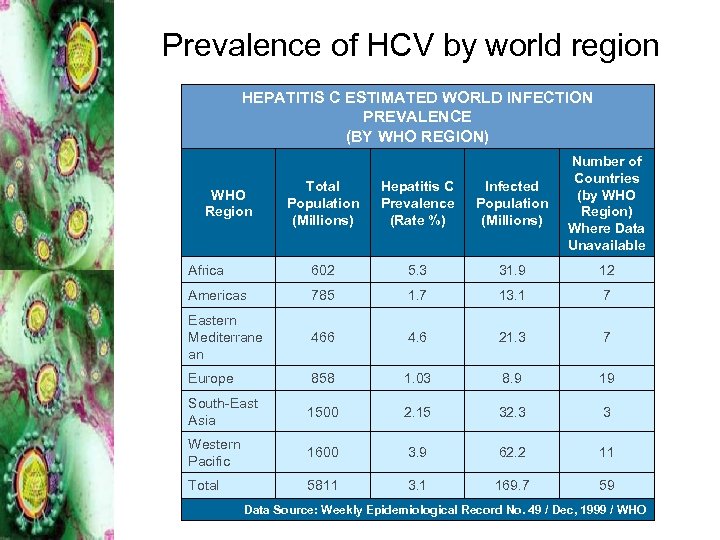
Prevalence of HCV by world region HEPATITIS C ESTIMATED WORLD INFECTION PREVALENCE (BY WHO REGION) Total Population (Millions) Hepatitis C Prevalence (Rate %) Infected Population (Millions) Number of Countries (by WHO Region) Where Data Unavailable Africa 602 5. 3 31. 9 12 Americas 785 1. 7 13. 1 7 Eastern Mediterrane an 466 4. 6 21. 3 7 Europe 858 1. 03 8. 9 19 South-East Asia 1500 2. 15 32. 3 3 Western Pacific 1600 3. 9 62. 2 11 Total 5811 3. 1 169. 7 59 WHO Region Data Source: Weekly Epidemiological Record No. 49 / Dec, 1999 / WHO
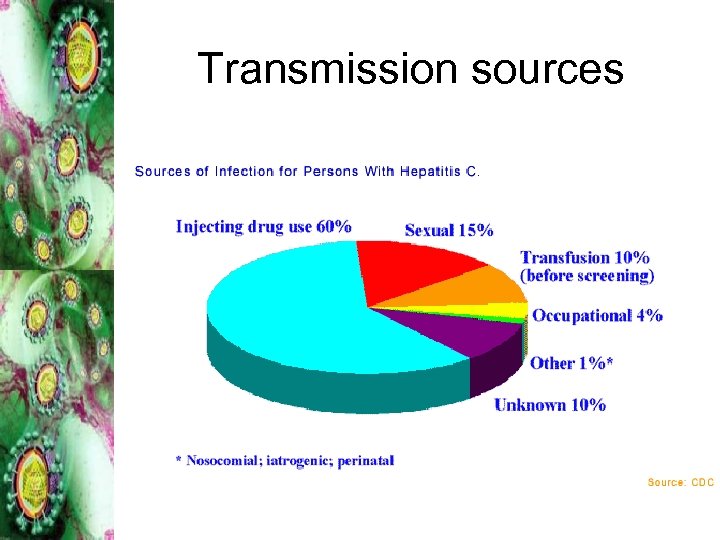
Transmission sources
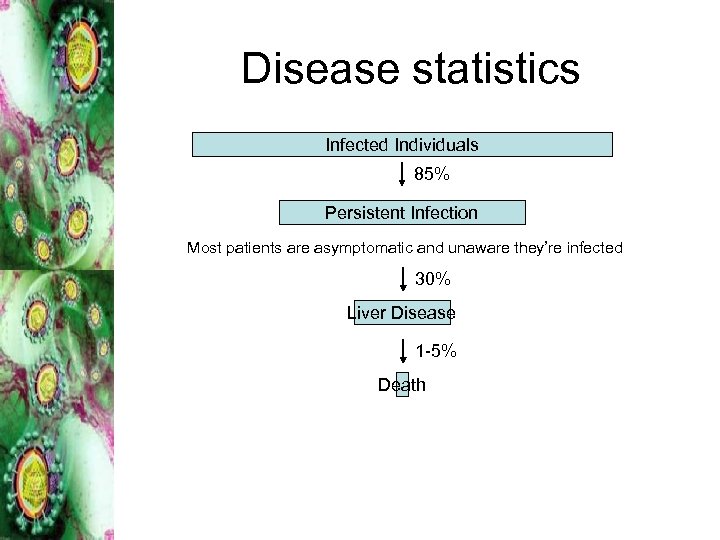
Disease statistics Infected Individuals 85% Persistent Infection Most patients are asymptomatic and unaware they’re infected 30% Liver Disease 1 -5% Death

HCV research • Unknowns • No cell culture system • No small animal model

Outline • • • Epidemiology Introduction to Hepatitis C Virus Immune response Novel drug therapies Conclusions
HCV • Genus Hepacivirus • Family Flaviviridae, with classical flaviviruses and animal pestiviruses • 6 genotypes worldwide, many subtypes and isolates based on nucleotide diversity • Quasispecies within individual
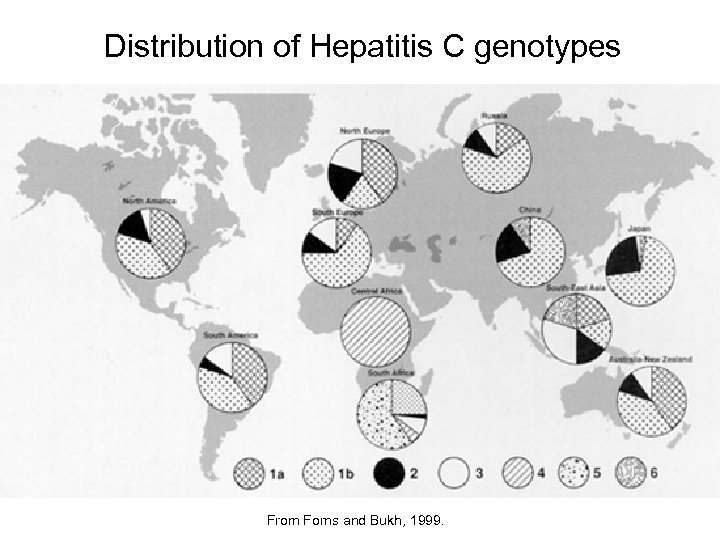
Distribution of Hepatitis C genotypes From Forns and Bukh, 1999.
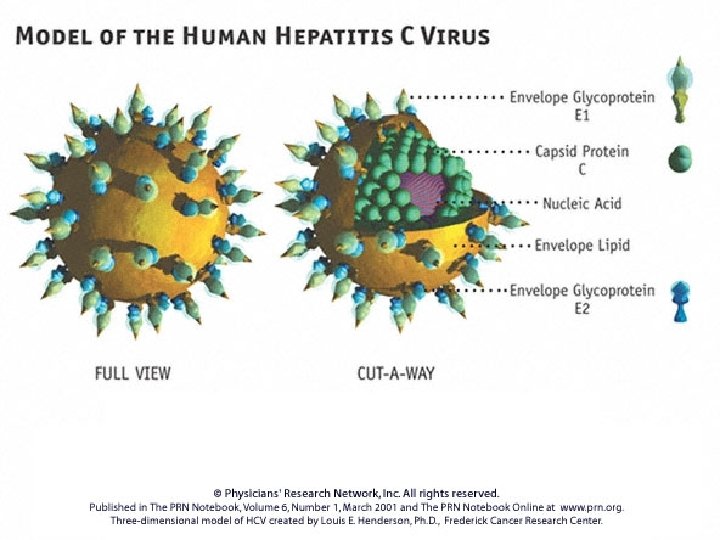
HCV virion structure Hypothesized structure: • Icosahedral lipid membrane with E 1/E 2 glycoproteins • Icosahedral nucleocapsid
HCV Genome • 9. 6 kb positive strand RNA genome • 5’ (with IRES) and 3’ noncoding regions • Open reading frame encoding polyprotein of ~3000 amino acids
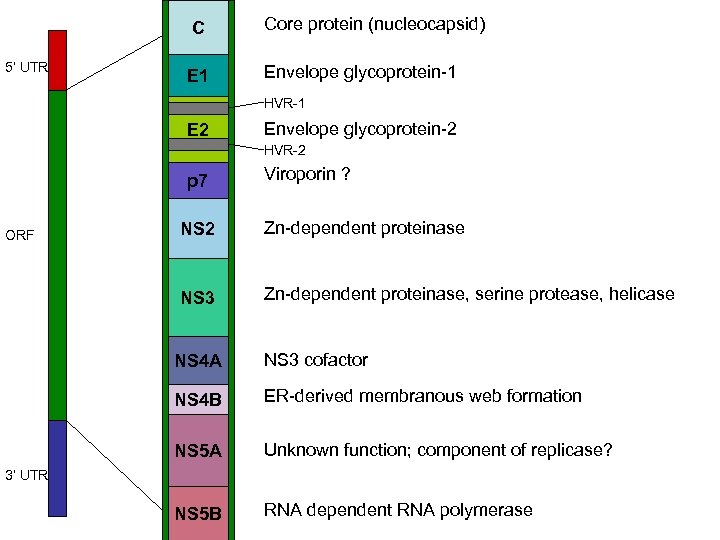
5’ UTR ORF C Core protein (nucleocapsid) E 1 Envelope glycoprotein-1 HVR-1 E 2 Envelope glycoprotein-2 HVR-2 p 7 Viroporin ? NS 2 Zn-dependent proteinase NS 3 Zn-dependent proteinase, serine protease, helicase NS 4 A NS 3 cofactor NS 4 B ER-derived membranous web formation NS 5 A Unknown function; component of replicase? NS 5 B RNA dependent RNA polymerase 3’ UTR
Protein F • Newly discovered protein F • Produced by ribosomal frameshift mutation around codon 11 of Core protein • Infected individuals contain antibodies • Function unknown
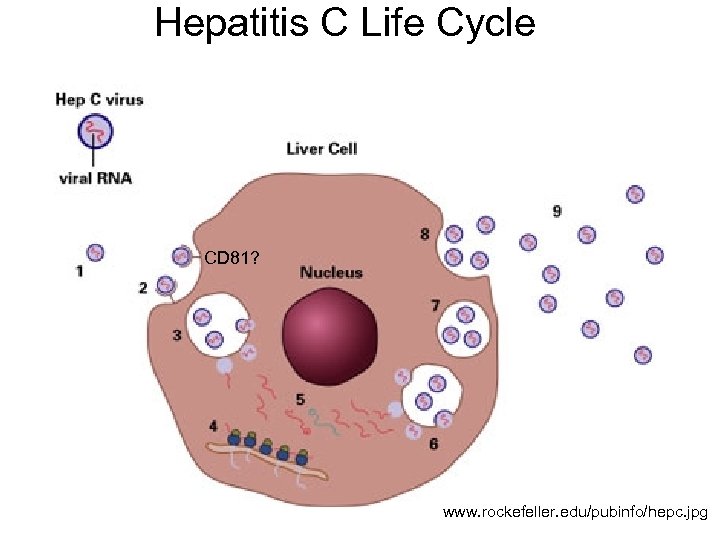
Hepatitis C Life Cycle CD 81? www. rockefeller. edu/pubinfo/hepc. jpg

Outline • • • Epidemiology Introduction to Hepatitis C Virus Immune response Novel drug therapies Conclusions
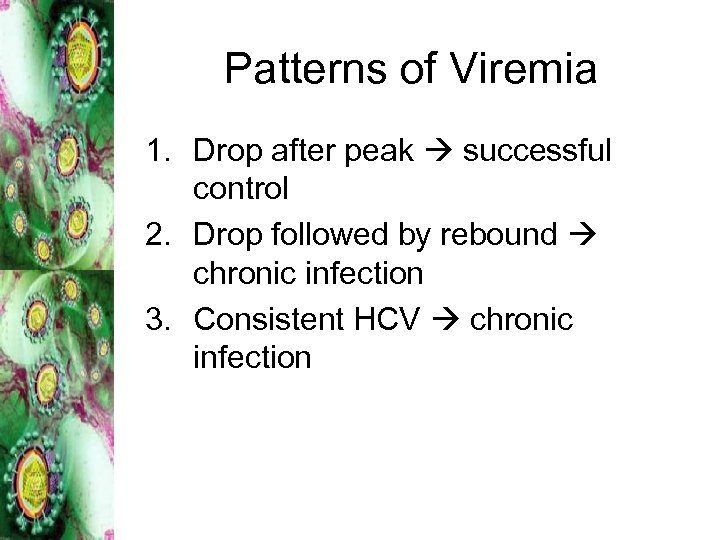
Patterns of Viremia 1. Drop after peak successful control 2. Drop followed by rebound chronic infection 3. Consistent HCV chronic infection
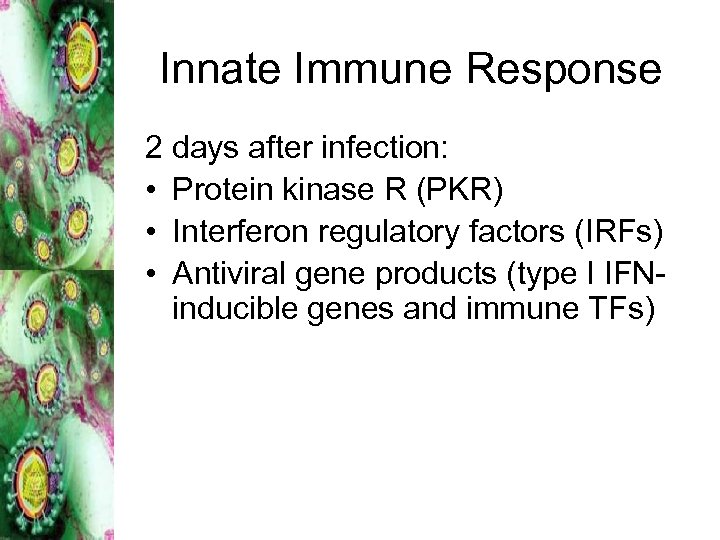
Innate Immune Response 2 days after infection: • Protein kinase R (PKR) • Interferon regulatory factors (IRFs) • Antiviral gene products (type I IFNinducible genes and immune TFs)
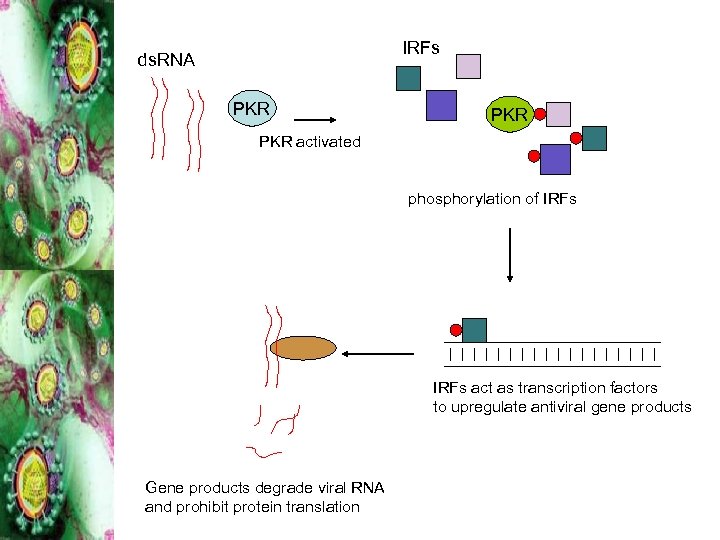
IRFs ds. RNA PKR PKR activated phosphorylation of IRFs act as transcription factors to upregulate antiviral gene products Gene products degrade viral RNA and prohibit protein translation

Innate Immune Response • Regardless of infection outcome • Viral resistance • Targeting by HCV proteins? – NS 5 A and E 2 (PKR) – Core (JAK-STAT pathway) – NS 3/4 A (phosphorylated IRF-3)
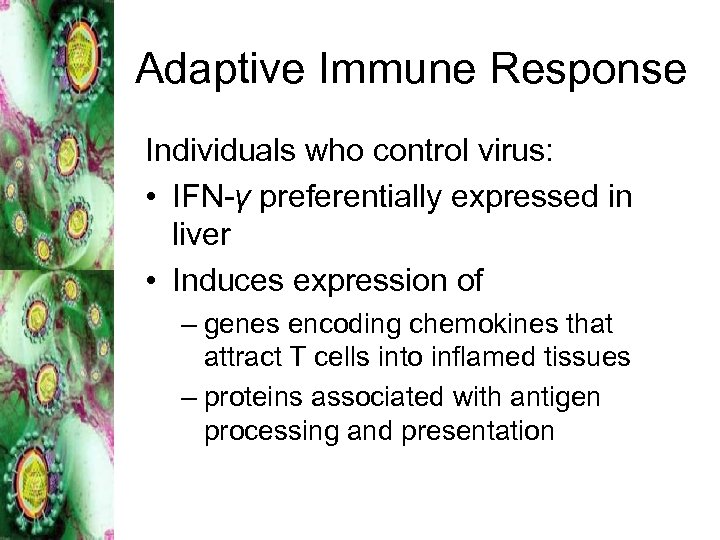
Adaptive Immune Response Individuals who control virus: • IFN-γ preferentially expressed in liver • Induces expression of – genes encoding chemokines that attract T cells into inflamed tissues – proteins associated with antigen processing and presentation
CD 8+ and CD 4+ T cells • More vigorous CD 8+ and CD 4+ T cell responses in all individuals that controlled infection • Chronic infections occur when – unable to mount HCV-specific T cell responses – strong response that results in viral RNA clearance, followed by contraction in CD 8+/CD 4+ and rebound in viremia
Chronic HCV infection • Low frequencies and reduced capacity of HCV-specific CD 8+ cells • Dendritic cells do not mature normally and have impaired stimulatory activity • CD 4+ cells have reduced IL-2 production and proliferation http: //www. lbl. gov/Publications/Currents/Archive/Oct-03 -2003. html
Chronic HCV Infection • Impairment of Natural Killer (NK) cell cytotoxic activity – Reversible in patients responsive to IFN-α drug therapy • Frequency of NKT cells decreased NKT cells (orange) attacking an infected cell (pink) Natural Killer cell http: //www. spectroscopynow. com/ftp_images/killertcells. jpg http: //www. wasatchhealth. com/images/NK-Picture. jpg
Antibodies • Role of antibodies unclear and poorly studied • Virus can be cleared in absence of detectable antibody responses • Neutralizing antibodies target E 2, which is highly variable and able to evade

Immune-mediated liver injury • Mechanisms responsible for liver injury poorly understood • Host immune response and not viral replication • High CD 8+ in liver immunopathogenesis and liver injury
Liver Environment Normal liver: • “Immuno-silent” state • CD 8+ T cells trapped apoptosis • Prevents unnecessary immune response to thousands of antigens liver is exposed to
Liver Environment HCV-infected liver: • Type I IFN production • Release of chemokines that promote infiltration of NK cells • Induced IFN-γ production in NK cells • Expression of chemokines that recruit activated T cells to liver
Liver Environment Depletion of NK cells before hepatotropic viral infection leads to inhibition of virus-specific T cell response and liver injury
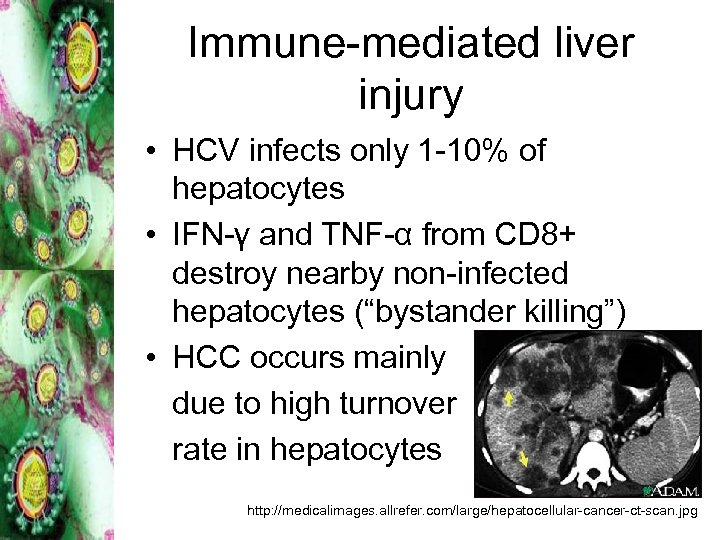
Immune-mediated liver injury • HCV infects only 1 -10% of hepatocytes • IFN-γ and TNF-α from CD 8+ destroy nearby non-infected hepatocytes (“bystander killing”) • HCC occurs mainly due to high turnover rate in hepatocytes http: //medicalimages. allrefer. com/large/hepatocellular-cancer-ct-scan. jpg

Outline • • • Epidemiology Introduction to Hepatitis C Virus Immune response Novel drug therapies Conclusions
Current therapy • Combination pegylated interferon-α and ribavirin (nucleoside analog) • Mechanism poorly understood • Protein synthesis suppression; degradation of plus strand RNA • 50 -80% effective
Current therapy Side effects: • Flu-like symptoms, tiredness, hair loss, trouble with thinking, moodiness, and depression • Hematologic – Anemia – bone marrow suppression by IFN neutropenia, thrombocytopenia – ribavirin directly toxic to red blood cells hemolysis • Worsening of liver disease

Novel drug therapies • • • Non-nucleoside inhibitors (NNIs) Protease inhibitor TGF-β Cyclosporin A Arsenic trioxide RNA therapieis
Non-nucleoside Inhibitors • Target Rd. Rp • Discovery method • Structurally distinct: – Benzothiadiazine – Disubstituted phenylalanine – 2 benzimidazole derivatives • Allosteric inhibition • Distinct binding sites http: //www. replizyme. com/images/rev_rna_hep_c. gif
Protease Inhibitor • • BILN 2061—NS 3 protease inhibitor Peptidomimetic Oral ingestion Clinical trial: – Rapid decline in viral load – Rebound 4 -11 days after treatment http: //web. chemistry. gatech. edu/~williams/b. Course_Information/6521/protein/images/hcvmac 1. gif
Transforming growth factor-β • Naturally occurring cytokine induced by core protein • Direct effect on HCV replication unknown • Decreased viral load • Increased fibrosis and cirrhosis
Cyclosporin A • Immunosuppressive drug • Mechanism unknown • FK 506 does not suppress HCV replication • Cs. A binds to cyclophilins and blocks calcineurin inhibits stimulation of genes essential for T cell activation • Combination with IFN http: //www. alexis-corp. com/files/formula/lkt-c 9611. gif
Arsenic Trioxide • Inhibits HCV replication at submicromolar concentrations • Non-toxic • Combination with IFN • Mechanism unknown
RNA treatments • Treatments that use RNA to halt viral replication • Three treatments in development: – RNA interference (RNAi) to degrade viral RNA – Small RNAs to bind to viral proteins – RNAs to outcompete viral proteins for binding to cellular proteins
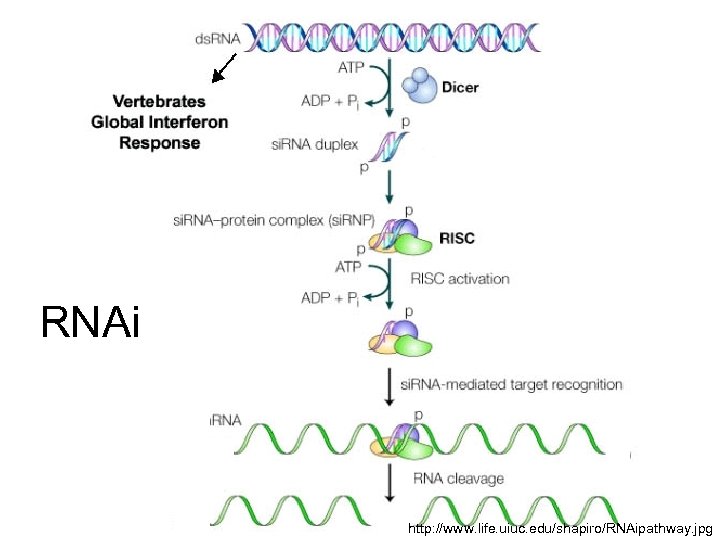
RNAi http: //www. life. uiuc. edu/shapiro/RNAipathway. jpg
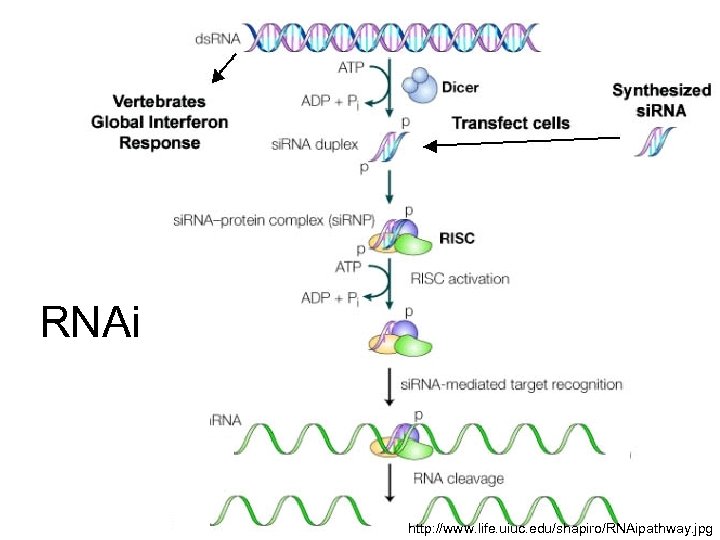
RNAi http: //www. life. uiuc. edu/shapiro/RNAipathway. jpg
RNAi • Inhibits HCV replication • Highly sequence specific (to 1 nt) • Multiple si. RNAs to target different sites of viral genome • Short hairpin RNAs targeting conserved motifs encoded by retroviruses
Small RNAs • Overexpression of viral RNA elements • Bind to viral regulatory proteins and prevent binding of viral RNA inhibits gene expression • RNAs analogous to 5’ UTR inhibited IRES-mediated translation • Combats sequence specificity problem
si. RNAs • si. RNAs targeted to cellular cofactors for HCV – La, PTB, h. VAP-33 • Blocks HCV replication • Combats sequence specificity problem • Adenoviral-mediated expression

Conclusions • HCV is a major worldwide health concern • Much remains unknown about HCV • Current drug therapy is inadequate and insufficient • Novel therapies offer IFN-resistant patients and those with serious side effects hope of elimination of hepatitis C infection http: //www. english. bayerconosur. com/noticias/tema 008 -1. asp

References • • Ahmad, A. and Alvarez, F. (2004). Role of NK and NKT cells in the immunopathogenesis of HCV-induced hepatitis. Journal of Leukocyte Biology 76: 743 -759. CDC FAQ: http: //www. cdc. gov/ncidod/diseases/hepatitis/c/faq. htm Forns, X. and Bukh, J. (1999). The Molecular Biology of Hepatitis C Virus: Genotypes and Quasispecies. Clinics in Liver Disease 3. Guo, J. , Sohn, A. , Zhu, Q. and Seeger, C. (2004). Mechanism of the interferon alpha response against hepatitis C virus replicons. Virology 325: 71 -81. Hwang, D. et al (2004). Inhibition of hepatitis C virus replication by arsenic trioxide. Antimicrobial Agents and Chemotherapy 48: 28762882. Kowdley, K. V. (2005). Hematologic side effects of interferon and ribavirin therapy. Journal of Clinical Gastroenterology 39, Suppl 1: S 3 S 8. Kronke, J. , Kittler, R. , Buchholz, F. , Windisch, M. P. , Pietschmann, T. , Bartenschlager, R. and Fresei, M. (2004). Alternative approaches for efficient inhibition of hepatitis C virus RNA replication by small interfering RNAs. Journal of Virology 78: 3436 -3446. Slide template picture: http: //www. english. bayerconosur. com/noticias/tema 008 -1. asp

References • • • Lamarre, D. et al (2003). An NS 3 protease inhibitor with antiviral effects in humans infected with hepatitis C virus. Nature 426: 186 -189. Liver Foundation: http: //www. liverfoundation. org/db/articles/1028 Mercer D, Schiller D, Elliot J, Douglas DN, Hao C, Rinfret A, Addison WR. (2001) Hepatitis C virus replication in mice with chimeric human livers. Nat Med 7: 927 -933. Moradpour, D. , Cerny, A. , Heim, M. H. and Blum, H. E. (2001). Hepatitis C: an update. Swiss Medical Weekly 131: 231 -298. Moradpour, D. and Blum, H. E. (2004). A primer on the molecular virology of hepatitis C. Liver International 24: 519 -525. Murata, T. , Ohshima, T. , Yamaji, M. , Hosaka, M. , Miyanari, Y. , Hijikata, M. and Shimotohno, K. (2005). Suppression of hepatitis C virus replicon by TGF-β. Virology 331: 407 -417. Nakagawa, M. et al (2004). Specific inhibition of hepatitis C virus replication by cyclosporine A. Biochemical and Biophysical Research Communications 313: 42 -47. Penin, F. , Dubuisson, J. , Rey, F. A. , Moradpour, D. and Pawlotsky, J. (2004). Structural Biology of Hepatitis C Virus. Hepatology 39: 5 -19. Puig, M. , Major, M. E. , Mihallik, K. and Feinstone, S. M. (2004). Immunization of chimpanzees with an envelope protein-based vaccine enhances specific humoral and cellular immune responses that delay hepatitis C virus infection. Vaccine 22: 991 -1000

References • • Ray, P. S. and Das, S. (2004). Inhibition of hepatitis C virus IRESmediated translation by small RNAs analogous to stem-loop structures of the 5’-untranslated region. Nucleic Acids Research 32: 1678 -1687. Sarisky, R. T. (2004). Non-nucleoside inhibitors of the HCV polymerase. Journal of Antimicrobial Chemotherapy 54: 14 -16. Shoukry, N. H. , Cawthon, A. G. and Walker, C. M. (2004). Cell-mediated immunity and the outcome of hepatitis C virus infection. Annual Reviews in Microbiology 58: 391 -424. Sun, J. , Li, K. , Shata, M. T. and Chan, T. (2004). The immunologic basis for hepatitis C infection. Current Opinions in Gastroenterology 20: 598 -602. Trujillo-Murillo, et al. (2004). Experimental models for hepatitis C virus (HCV): New opportunities for combating hepatitis C. Annals of Hepatology 3: 54 -62. World Health Organization (WHO) (1999). Weekly Epidemiological Record No. 49, December. Zhang, J. , Yamada, O. , Sakamoto, T. , Yoshida, H. , Iwai, T. , Matsushita, Y. , Shimamura, H. , Araki, H. and Shimotohno, K. (2004). Down-regulation of viral replication by adenoviral-mediated expression of si. RNA against cellular cofactors for hepatitis C virus. Virology 320: 135 -143.
edab31fc4ff30234d94a459aae25bc28.ppt