ab38e5411a26da7b638a1e56c561821a.ppt
- Количество слайдов: 30
 Hepatitis C in Children
Hepatitis C in Children
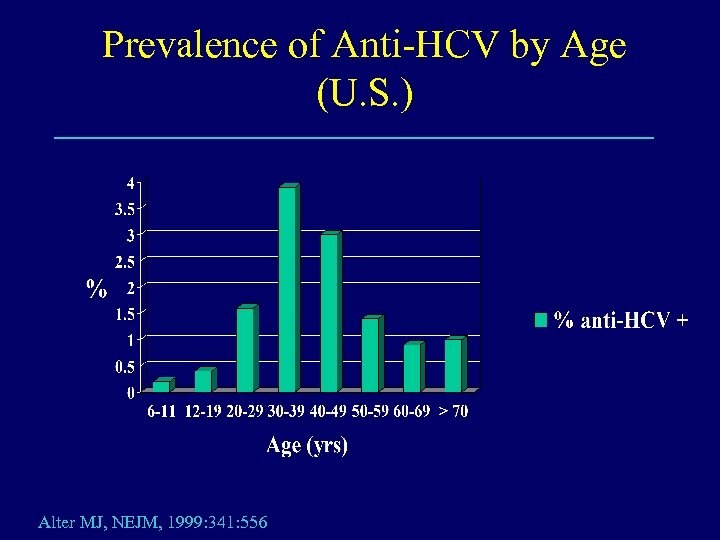 Prevalence of Anti-HCV by Age (U. S. ) Alter MJ, NEJM, 1999: 341: 556
Prevalence of Anti-HCV by Age (U. S. ) Alter MJ, NEJM, 1999: 341: 556
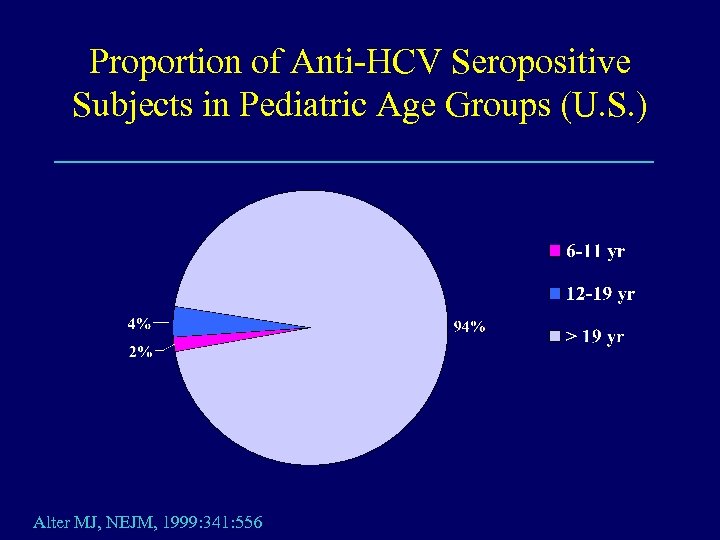 Proportion of Anti-HCV Seropositive Subjects in Pediatric Age Groups (U. S. ) Alter MJ, NEJM, 1999: 341: 556
Proportion of Anti-HCV Seropositive Subjects in Pediatric Age Groups (U. S. ) Alter MJ, NEJM, 1999: 341: 556
 Children at Risk for HCV Infection • Recurrent blood or blood product transfusion – hemophilia (80 -95%), thalassemia (60 -75%) • Blood or blood product transfusion prior to 1992 – ALL (4 -9%), cardiac surgery (4 -5%), orthopedic surgery, NICU care • Adolescents with “high-risk” behaviors • Children born to HCV-infected women
Children at Risk for HCV Infection • Recurrent blood or blood product transfusion – hemophilia (80 -95%), thalassemia (60 -75%) • Blood or blood product transfusion prior to 1992 – ALL (4 -9%), cardiac surgery (4 -5%), orthopedic surgery, NICU care • Adolescents with “high-risk” behaviors • Children born to HCV-infected women
 Low Seroprevalence of Anti-HCV in Urban Adolescents • 869 subjects from Adolescent Clinic or a high-school based clinic • 3. 2% (22/669) repeatedly anti-HBc seropositive • 1 (0. 1%) anti-HCV seropositive Jonas MM, J Pediatr 1997; 131: 314
Low Seroprevalence of Anti-HCV in Urban Adolescents • 869 subjects from Adolescent Clinic or a high-school based clinic • 3. 2% (22/669) repeatedly anti-HBc seropositive • 1 (0. 1%) anti-HCV seropositive Jonas MM, J Pediatr 1997; 131: 314
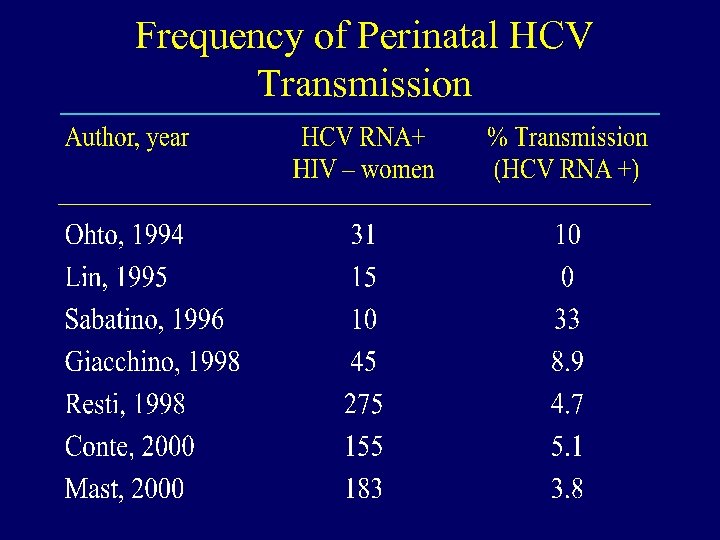 Frequency of Perinatal HCV Transmission
Frequency of Perinatal HCV Transmission
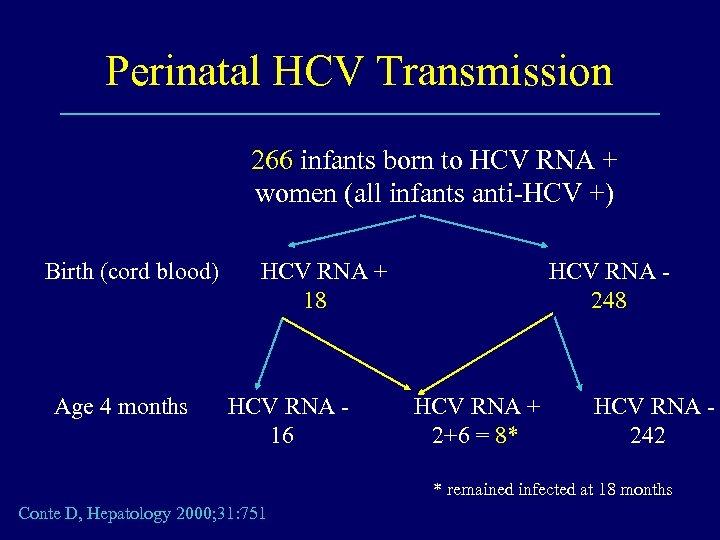 Perinatal HCV Transmission 266 infants born to HCV RNA + women (all infants anti-HCV +) Birth (cord blood) Age 4 months HCV RNA + 18 HCV RNA 16 HCV RNA 248 HCV RNA + 2+6 = 8* HCV RNA 242 * remained infected at 18 months Conte D, Hepatology 2000; 31: 751
Perinatal HCV Transmission 266 infants born to HCV RNA + women (all infants anti-HCV +) Birth (cord blood) Age 4 months HCV RNA + 18 HCV RNA 16 HCV RNA 248 HCV RNA + 2+6 = 8* HCV RNA 242 * remained infected at 18 months Conte D, Hepatology 2000; 31: 751
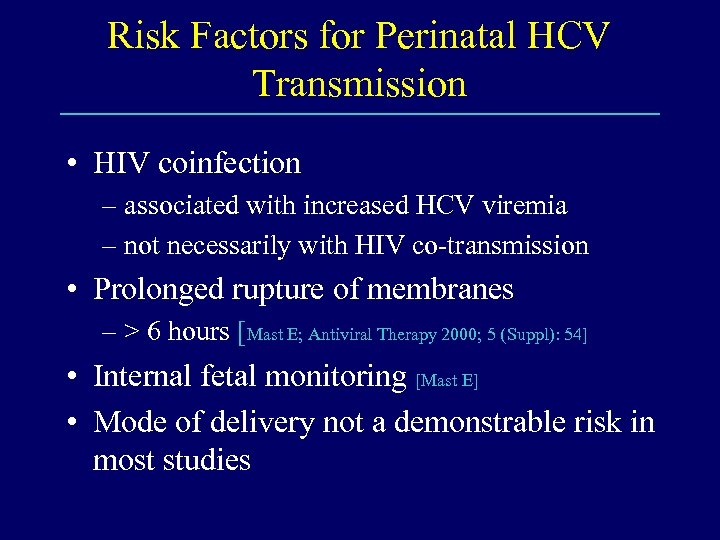 Risk Factors for Perinatal HCV Transmission • HIV coinfection – associated with increased HCV viremia – not necessarily with HIV co-transmission • Prolonged rupture of membranes – > 6 hours [Mast E; Antiviral Therapy 2000; 5 (Suppl): 54] • Internal fetal monitoring [Mast E] • Mode of delivery not a demonstrable risk in most studies
Risk Factors for Perinatal HCV Transmission • HIV coinfection – associated with increased HCV viremia – not necessarily with HIV co-transmission • Prolonged rupture of membranes – > 6 hours [Mast E; Antiviral Therapy 2000; 5 (Suppl): 54] • Internal fetal monitoring [Mast E] • Mode of delivery not a demonstrable risk in most studies
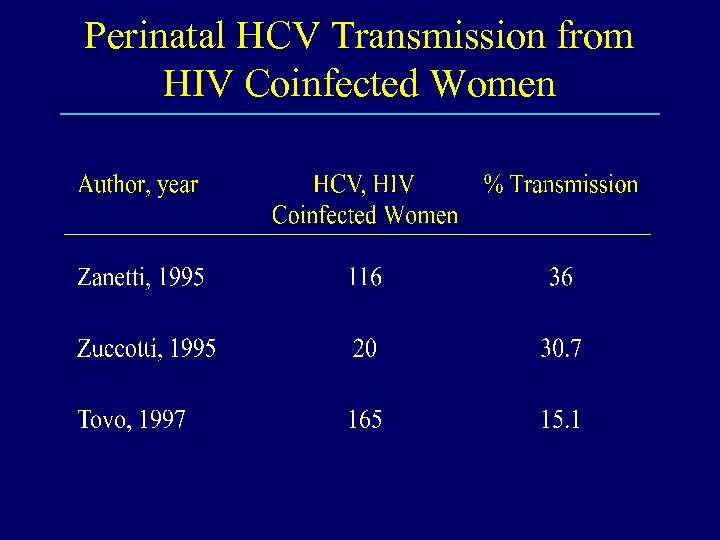 Perinatal HCV Transmission from HIV Coinfected Women
Perinatal HCV Transmission from HIV Coinfected Women
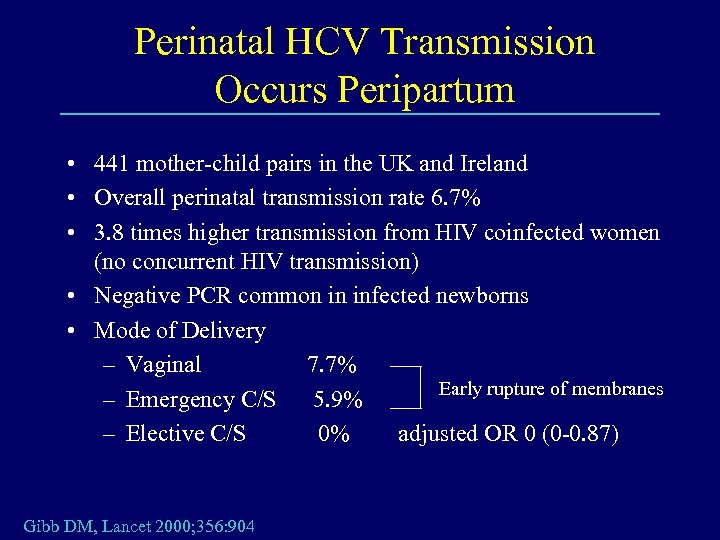 Perinatal HCV Transmission Occurs Peripartum • 441 mother-child pairs in the UK and Ireland • Overall perinatal transmission rate 6. 7% • 3. 8 times higher transmission from HIV coinfected women (no concurrent HIV transmission) • Negative PCR common in infected newborns • Mode of Delivery – Vaginal 7. 7% Early rupture of membranes – Emergency C/S 5. 9% – Elective C/S 0% adjusted OR 0 (0 -0. 87) Gibb DM, Lancet 2000; 356: 904
Perinatal HCV Transmission Occurs Peripartum • 441 mother-child pairs in the UK and Ireland • Overall perinatal transmission rate 6. 7% • 3. 8 times higher transmission from HIV coinfected women (no concurrent HIV transmission) • Negative PCR common in infected newborns • Mode of Delivery – Vaginal 7. 7% Early rupture of membranes – Emergency C/S 5. 9% – Elective C/S 0% adjusted OR 0 (0 -0. 87) Gibb DM, Lancet 2000; 356: 904
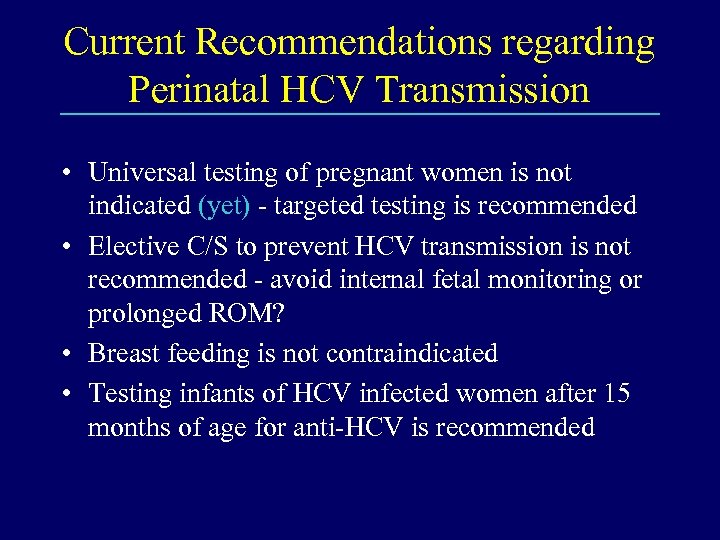 Current Recommendations regarding Perinatal HCV Transmission • Universal testing of pregnant women is not indicated (yet) - targeted testing is recommended • Elective C/S to prevent HCV transmission is not recommended - avoid internal fetal monitoring or prolonged ROM? • Breast feeding is not contraindicated • Testing infants of HCV infected women after 15 months of age for anti-HCV is recommended
Current Recommendations regarding Perinatal HCV Transmission • Universal testing of pregnant women is not indicated (yet) - targeted testing is recommended • Elective C/S to prevent HCV transmission is not recommended - avoid internal fetal monitoring or prolonged ROM? • Breast feeding is not contraindicated • Testing infants of HCV infected women after 15 months of age for anti-HCV is recommended
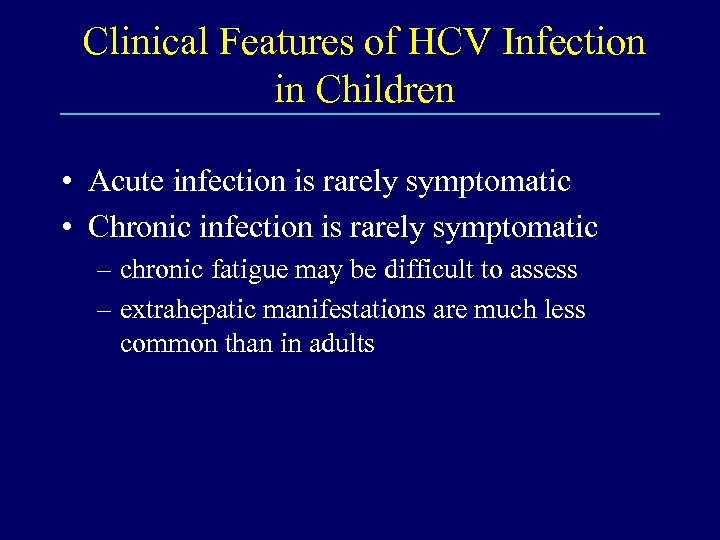 Clinical Features of HCV Infection in Children • Acute infection is rarely symptomatic • Chronic infection is rarely symptomatic – chronic fatigue may be difficult to assess – extrahepatic manifestations are much less common than in adults
Clinical Features of HCV Infection in Children • Acute infection is rarely symptomatic • Chronic infection is rarely symptomatic – chronic fatigue may be difficult to assess – extrahepatic manifestations are much less common than in adults
 Natural History of HCV Infection in Children - Critical Issues • What is the rate of progression of liver disease? – Does fibrosis progress linearly? • What are the risk factors for progression during childhood? • What is the role of underlying disease? Mode of acquisition? • Most natural history studies thus far are crosssectional cohort studies; prospective studies go out only a few years - is this enough?
Natural History of HCV Infection in Children - Critical Issues • What is the rate of progression of liver disease? – Does fibrosis progress linearly? • What are the risk factors for progression during childhood? • What is the role of underlying disease? Mode of acquisition? • Most natural history studies thus far are crosssectional cohort studies; prospective studies go out only a few years - is this enough?
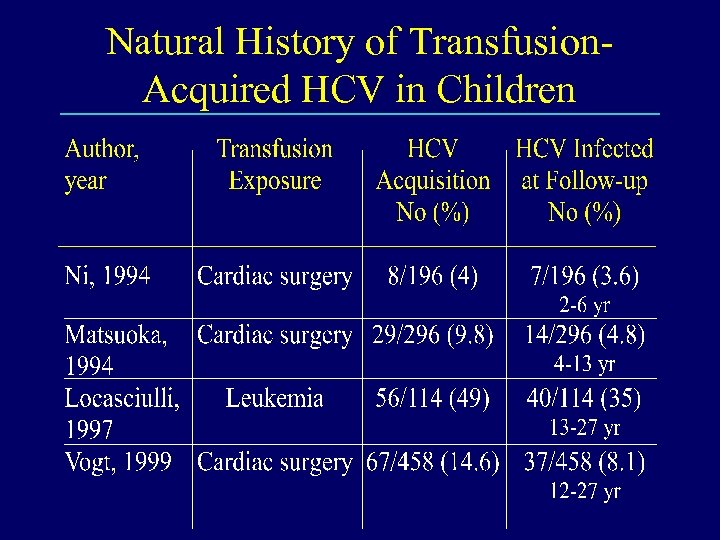 Natural History of Transfusion. Acquired HCV in Children
Natural History of Transfusion. Acquired HCV in Children
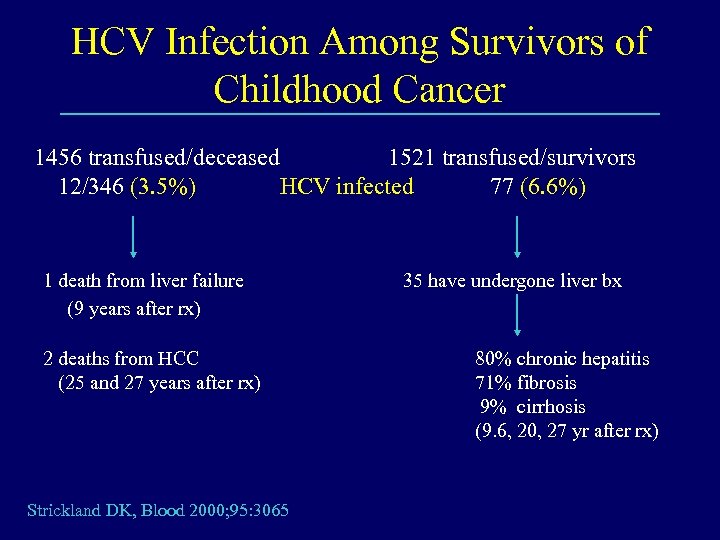 HCV Infection Among Survivors of Childhood Cancer 1456 transfused/deceased 1521 transfused/survivors 12/346 (3. 5%) HCV infected 77 (6. 6%) 1 death from liver failure (9 years after rx) 2 deaths from HCC (25 and 27 years after rx) Strickland DK, Blood 2000; 95: 3065 35 have undergone liver bx 80% chronic hepatitis 71% fibrosis 9% cirrhosis (9. 6, 20, 27 yr after rx)
HCV Infection Among Survivors of Childhood Cancer 1456 transfused/deceased 1521 transfused/survivors 12/346 (3. 5%) HCV infected 77 (6. 6%) 1 death from liver failure (9 years after rx) 2 deaths from HCC (25 and 27 years after rx) Strickland DK, Blood 2000; 95: 3065 35 have undergone liver bx 80% chronic hepatitis 71% fibrosis 9% cirrhosis (9. 6, 20, 27 yr after rx)
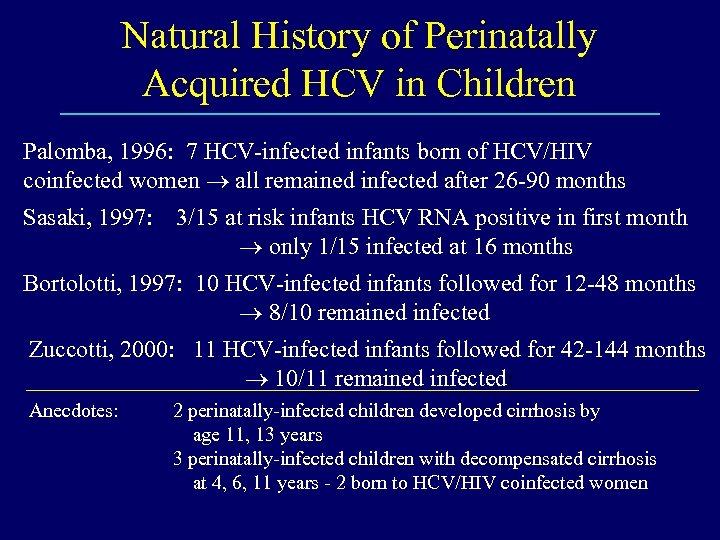 Natural History of Perinatally Acquired HCV in Children Palomba, 1996: 7 HCV-infected infants born of HCV/HIV coinfected women all remained infected after 26 -90 months Sasaki, 1997: 3/15 at risk infants HCV RNA positive in first month only 1/15 infected at 16 months Bortolotti, 1997: 10 HCV-infected infants followed for 12 -48 months 8/10 remained infected Zuccotti, 2000: 11 HCV-infected infants followed for 42 -144 months 10/11 remained infected: Anecdotes: 2 perinatally-infected children developed cirrhosis by age 11, 13 years 3 perinatally-infected children with decompensated cirrhosis at 4, 6, 11 years - 2 born to HCV/HIV coinfected women
Natural History of Perinatally Acquired HCV in Children Palomba, 1996: 7 HCV-infected infants born of HCV/HIV coinfected women all remained infected after 26 -90 months Sasaki, 1997: 3/15 at risk infants HCV RNA positive in first month only 1/15 infected at 16 months Bortolotti, 1997: 10 HCV-infected infants followed for 12 -48 months 8/10 remained infected Zuccotti, 2000: 11 HCV-infected infants followed for 42 -144 months 10/11 remained infected: Anecdotes: 2 perinatally-infected children developed cirrhosis by age 11, 13 years 3 perinatally-infected children with decompensated cirrhosis at 4, 6, 11 years - 2 born to HCV/HIV coinfected women
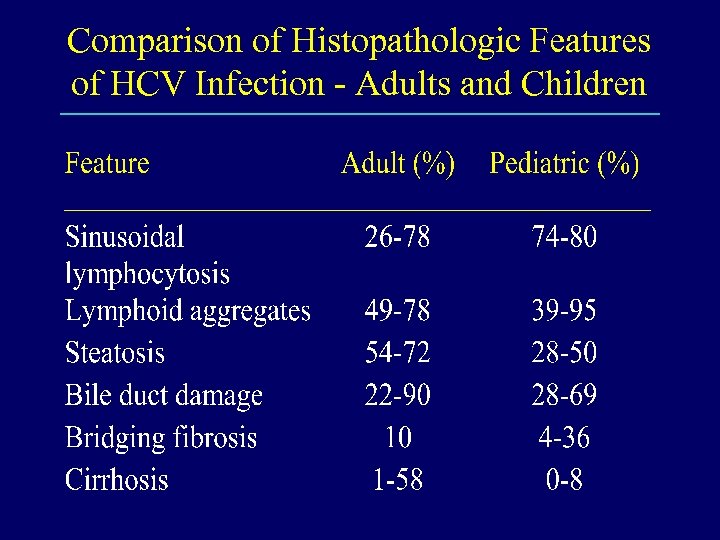 Comparison of Histopathologic Features of HCV Infection - Adults and Children
Comparison of Histopathologic Features of HCV Infection - Adults and Children
 Histopathologic Features of HCV Infection in Children • The features are generally the same as seen in adults - suggesting the same disease and pathogenesis • There is an association between extent of fibrosis and age and duration of infection
Histopathologic Features of HCV Infection in Children • The features are generally the same as seen in adults - suggesting the same disease and pathogenesis • There is an association between extent of fibrosis and age and duration of infection
 Natural History of HCV Infection in Children - Summary • May be different in children infected by transfusion vs those infected perinatally • May be different according to underlying disease for which transfusion was indicated • Benign in the first 20 - 25 years in most instances; a few have aggressive disease • Not known in the 3 rd decade and beyond
Natural History of HCV Infection in Children - Summary • May be different in children infected by transfusion vs those infected perinatally • May be different according to underlying disease for which transfusion was indicated • Benign in the first 20 - 25 years in most instances; a few have aggressive disease • Not known in the 3 rd decade and beyond
 HCV in Childhood Goals of Therapy • • • Sustained normalization of ALT Sustained virologic response Improvement in hepatic histology Decrease risk of cirrhosis Decrease risk of HCC
HCV in Childhood Goals of Therapy • • • Sustained normalization of ALT Sustained virologic response Improvement in hepatic histology Decrease risk of cirrhosis Decrease risk of HCC
 Treatment of HCV Infection in Children Interferon Monotherapy • • No large, randomized, controlled trials Heterogeneous patient groups Different dosages and types of interferon Different lengths of treatment
Treatment of HCV Infection in Children Interferon Monotherapy • • No large, randomized, controlled trials Heterogeneous patient groups Different dosages and types of interferon Different lengths of treatment
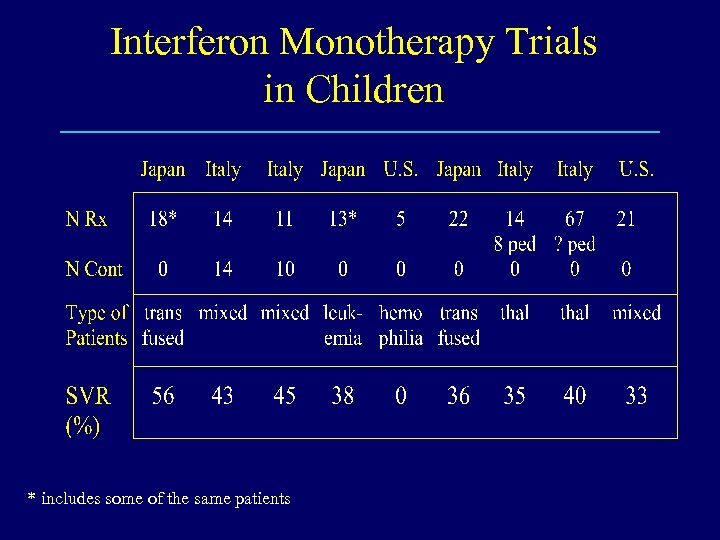 Interferon Monotherapy Trials in Children * includes some of the same patients
Interferon Monotherapy Trials in Children * includes some of the same patients
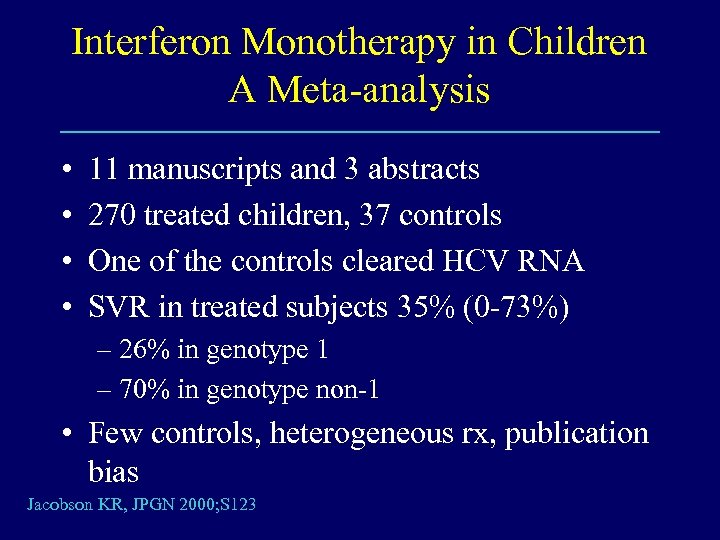 Interferon Monotherapy in Children A Meta-analysis • • 11 manuscripts and 3 abstracts 270 treated children, 37 controls One of the controls cleared HCV RNA SVR in treated subjects 35% (0 -73%) – 26% in genotype 1 – 70% in genotype non-1 • Few controls, heterogeneous rx, publication bias Jacobson KR, JPGN 2000; S 123
Interferon Monotherapy in Children A Meta-analysis • • 11 manuscripts and 3 abstracts 270 treated children, 37 controls One of the controls cleared HCV RNA SVR in treated subjects 35% (0 -73%) – 26% in genotype 1 – 70% in genotype non-1 • Few controls, heterogeneous rx, publication bias Jacobson KR, JPGN 2000; S 123
 Interferon Monotherapy Greater Efficacy in Children? • • • Younger age Earlier stage of liver disease Different modes of acquisition Higher relative interferon dose Lack of co-morbid factors Artifact
Interferon Monotherapy Greater Efficacy in Children? • • • Younger age Earlier stage of liver disease Different modes of acquisition Higher relative interferon dose Lack of co-morbid factors Artifact
 IFN Side Effects in Children • • Flu-like illness Neutropenia Weight loss / failure to gain weight Irritability, poor school performance, behavior disturbance • Seizures, lower seizure threshold • Spastic diplegia (infants) • Long-term?
IFN Side Effects in Children • • Flu-like illness Neutropenia Weight loss / failure to gain weight Irritability, poor school performance, behavior disturbance • Seizures, lower seizure threshold • Spastic diplegia (infants) • Long-term?
 Ribavirin Toxicity • Hemolytic anemia – most common in the first weeks – stabilizes thereafter – usually < 2 g drop in hemoglobin – reversible, dose dependent • Teratogenic, male and female • Mutagenic in animal models
Ribavirin Toxicity • Hemolytic anemia – most common in the first weeks – stabilizes thereafter – usually < 2 g drop in hemoglobin – reversible, dose dependent • Teratogenic, male and female • Mutagenic in animal models
 Rebetron Pediatric Clinical. Trials • Phase I Dose Finding Study (Complete) – 48 weeks treatment, 24 weeks follow up – Intron A 3 MIU/m 2 TIW & Rebetol 8, 12, 15 mg/kg/day • Dose of 15 mg/kg/day selected for phase III – 61 children age 5 to 16 enrolled • 57 naïve/4 relapse • Phase III Open Label Study (Ongoing) – 48 weeks treatment, 24 weeks follow up – Intron A 3 MIU/m 2 TIW & Rebetol 15 mg/kg/day – 105 children age 3 to 16 enrolled • naïve only
Rebetron Pediatric Clinical. Trials • Phase I Dose Finding Study (Complete) – 48 weeks treatment, 24 weeks follow up – Intron A 3 MIU/m 2 TIW & Rebetol 8, 12, 15 mg/kg/day • Dose of 15 mg/kg/day selected for phase III – 61 children age 5 to 16 enrolled • 57 naïve/4 relapse • Phase III Open Label Study (Ongoing) – 48 weeks treatment, 24 weeks follow up – Intron A 3 MIU/m 2 TIW & Rebetol 15 mg/kg/day – 105 children age 3 to 16 enrolled • naïve only
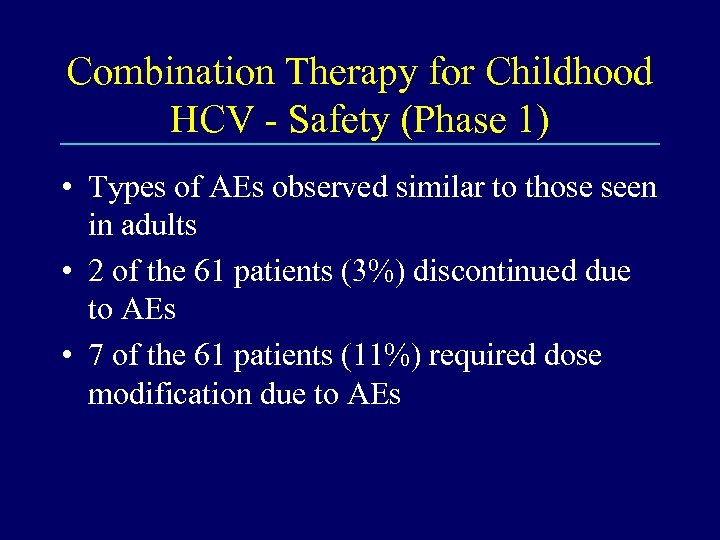 Combination Therapy for Childhood HCV - Safety (Phase 1) • Types of AEs observed similar to those seen in adults • 2 of the 61 patients (3%) discontinued due to AEs • 7 of the 61 patients (11%) required dose modification due to AEs
Combination Therapy for Childhood HCV - Safety (Phase 1) • Types of AEs observed similar to those seen in adults • 2 of the 61 patients (3%) discontinued due to AEs • 7 of the 61 patients (11%) required dose modification due to AEs
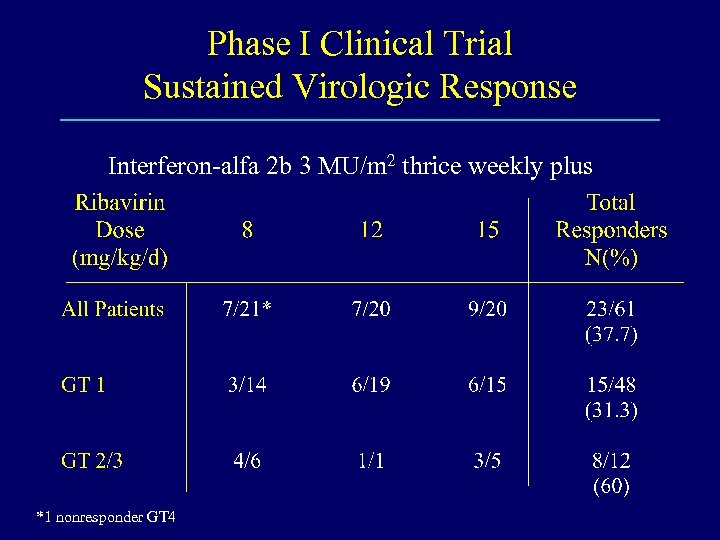 Phase I Clinical Trial Sustained Virologic Response Interferon-alfa 2 b 3 MU/m 2 thrice weekly plus *1 nonresponder GT 4
Phase I Clinical Trial Sustained Virologic Response Interferon-alfa 2 b 3 MU/m 2 thrice weekly plus *1 nonresponder GT 4
 HCV in Childhood Therapeutic Considerations • The long-term natural history is still not completely known - benign in most? Aggressive in some. • Which children should be treated? – Use same criteria as for adults – None - no DBRCT’s – All - short duration, mild liver disease, more likely to respond?
HCV in Childhood Therapeutic Considerations • The long-term natural history is still not completely known - benign in most? Aggressive in some. • Which children should be treated? – Use same criteria as for adults – None - no DBRCT’s – All - short duration, mild liver disease, more likely to respond?


