263a0fff0cc2621c5d19e30e5fdae375.ppt
- Количество слайдов: 69

Hepatitis A, B, C, D, E, G, … diagnostic tools and their use Geert Leroux-Roels Laboratorium voor Klinische Biologie UZ Gent
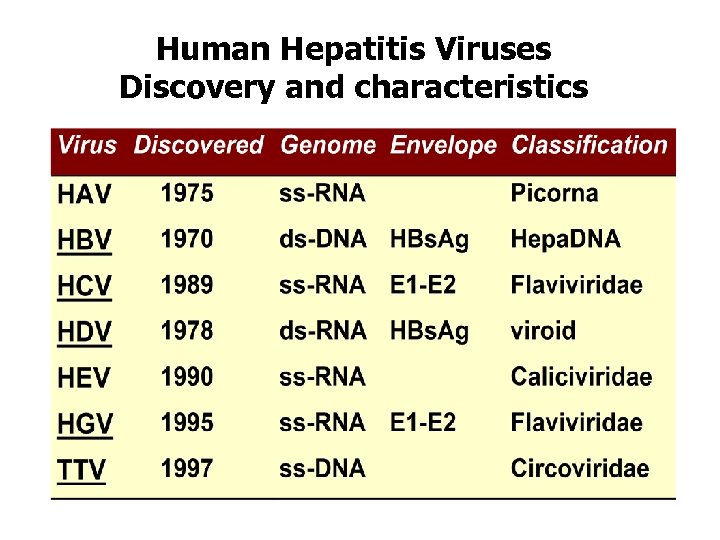
Human Hepatitis Viruses Discovery and characteristics
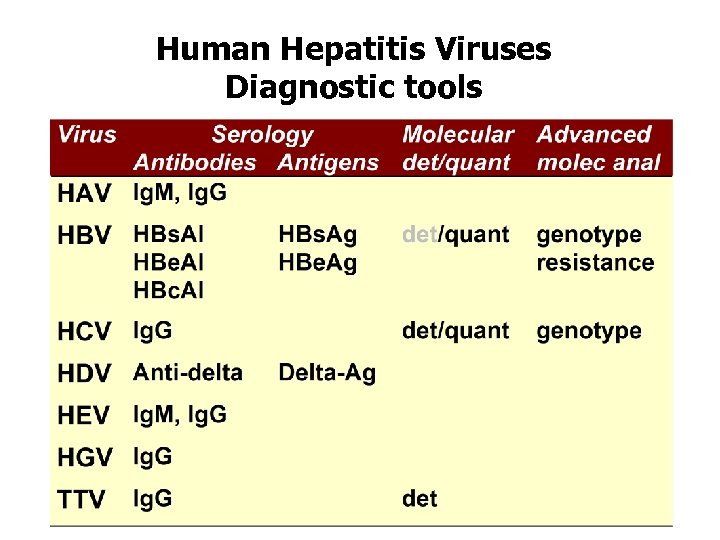
Human Hepatitis Viruses Diagnostic tools
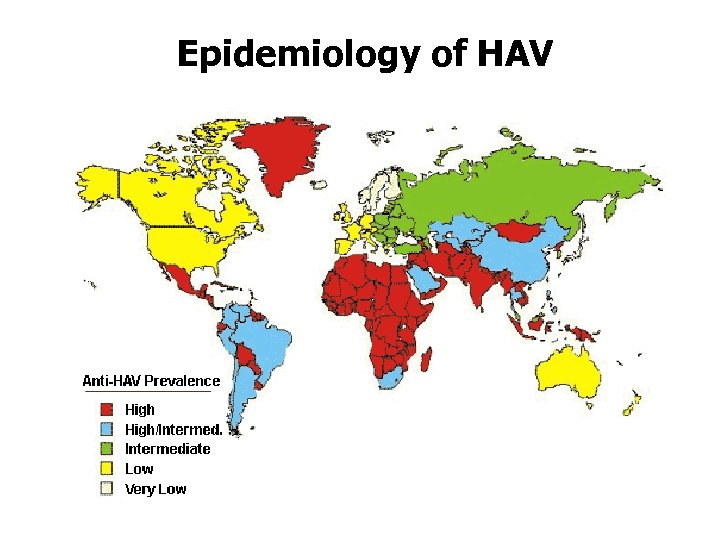
Epidemiology of HAV
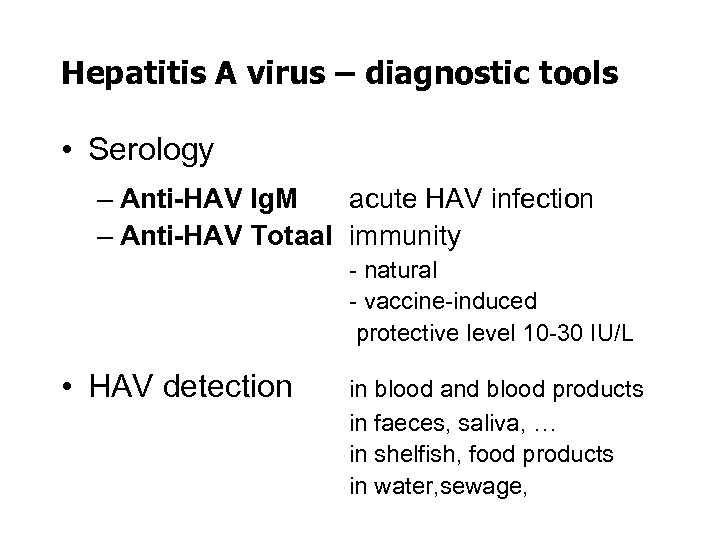
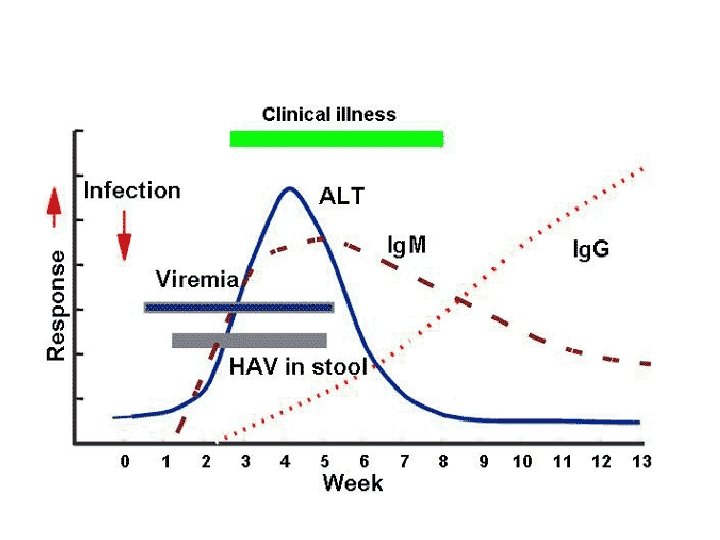
Hepatitis A virus – diagnostic tools • Serology – Anti-HAV Ig. M acute HAV infection – Anti-HAV Totaal immunity - natural - vaccine-induced protective level 10 -30 IU/L • HAV detection in blood and blood products in faeces, saliva, … in shelfish, food products in water, sewage,
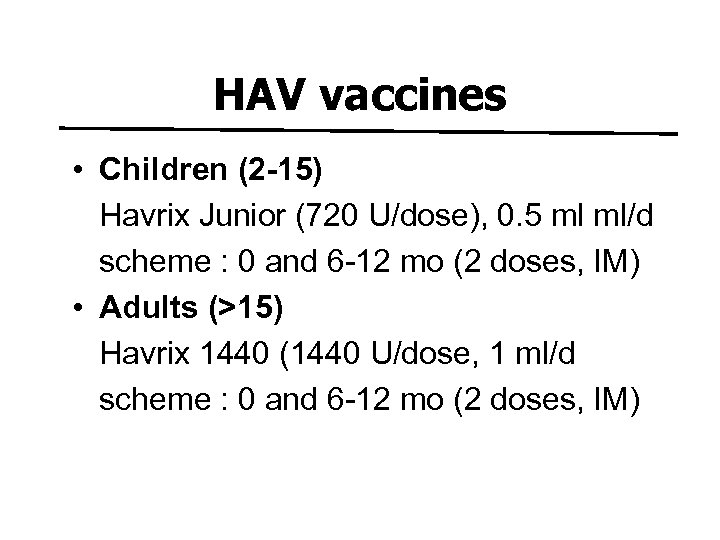
HAV vaccines • Children (2 -15) Havrix Junior (720 U/dose), 0. 5 ml ml/d scheme : 0 and 6 -12 mo (2 doses, IM) • Adults (>15) Havrix 1440 (1440 U/dose, 1 ml/d scheme : 0 and 6 -12 mo (2 doses, IM)
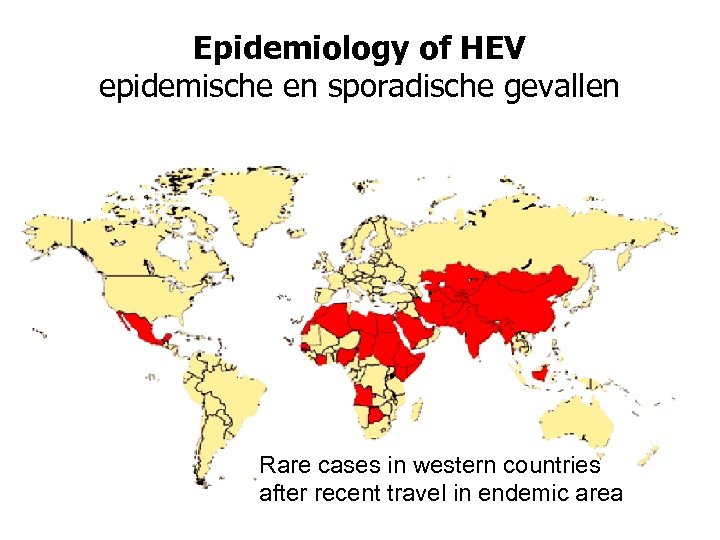
Epidemiology of HEV epidemische en sporadische gevallen Rare cases in western countries after recent travel in endemic area
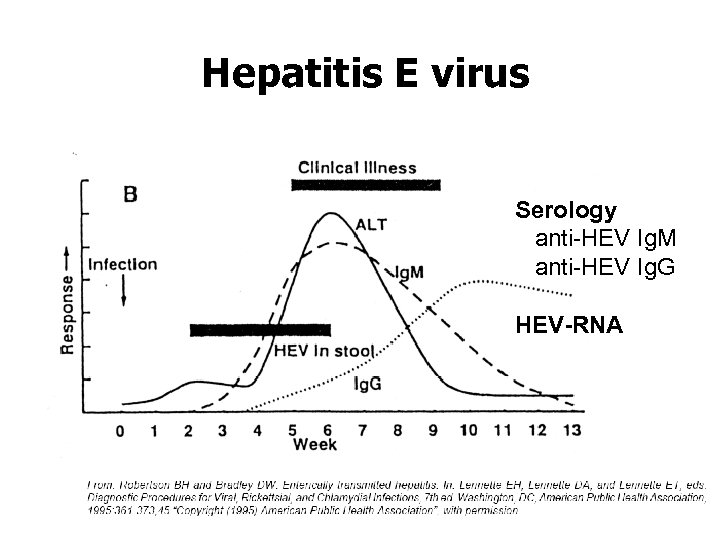
Hepatitis E virus Serology anti-HEV Ig. M anti-HEV Ig. G HEV-RNA

Hepatitis B virus Diagnostic tools and interpretation
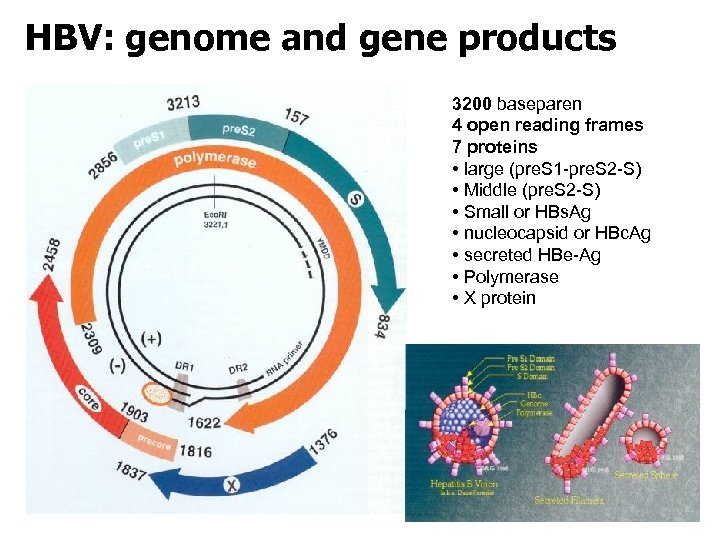
HBV: genome and gene products 3200 baseparen 4 open reading frames 7 proteins • large (pre. S 1 -pre. S 2 -S) • Middle (pre. S 2 -S) • Small or HBs. Ag • nucleocapsid or HBc. Ag • secreted HBe-Ag • Polymerase • X protein
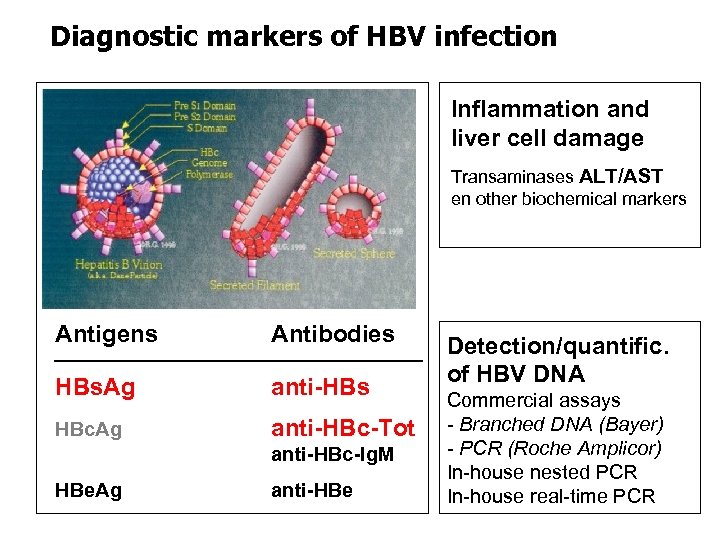
Diagnostic markers of HBV infection Inflammation and liver cell damage Transaminases ALT/AST en other biochemical markers Antigens Antibodies HBs. Ag anti-HBs HBc. Ag anti-HBc-Tot anti-HBc-Ig. M HBe. Ag anti-HBe Detection/quantific. of HBV DNA Commercial assays - Branched DNA (Bayer) - PCR (Roche Amplicor) In-house nested PCR In-house real-time PCR
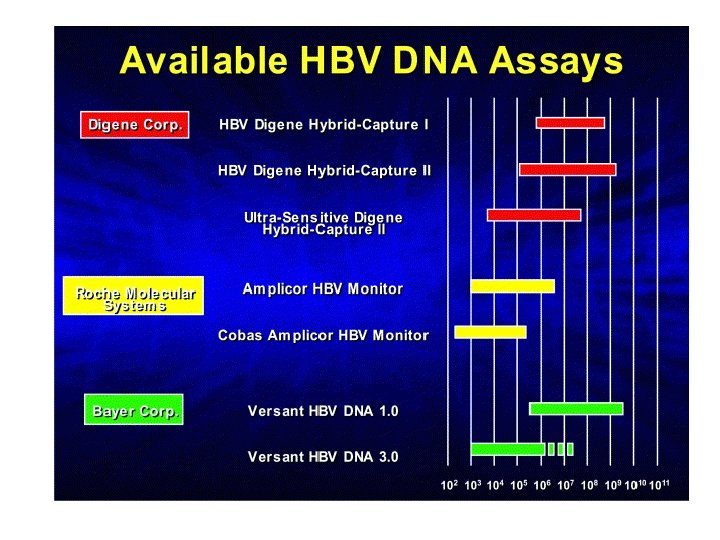
Usefulness of HBV-DNA quantification • Diagnosis – Acute HBV infection : HBV DNA is not useful – Chronic HBV infection – Is HBV replicating ? • HBe. Agpos : not useful • HBe. Agneg/anti-HBepos : useful, “threshold value” ? • Prognosis • Therapy – Decision to treat : ALT, biopsy, HBe. Agpos – Selection treatment – Monitoring : HBV DNA, ALT, HBe. Ag, anti-HBe,
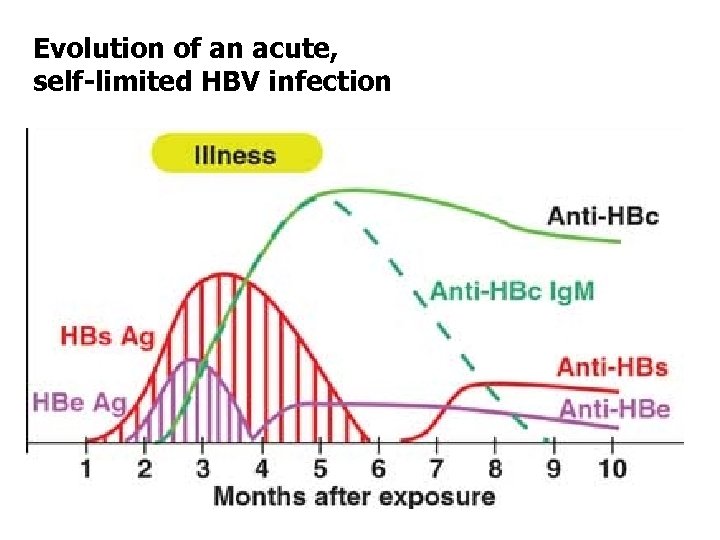
Evolution of an acute, self-limited HBV infection
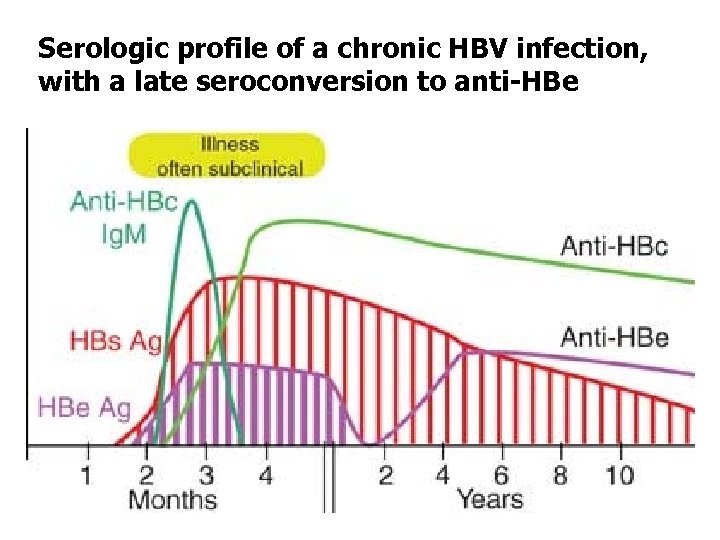
Serologic profile of a chronic HBV infection, with a late seroconversion to anti-HBe

Case 1 • • • 41 years old Afghan male political refugee In training for assistant-cook Medical screening exam for ‘hepatitis’ No symptoms No history of hepatitis
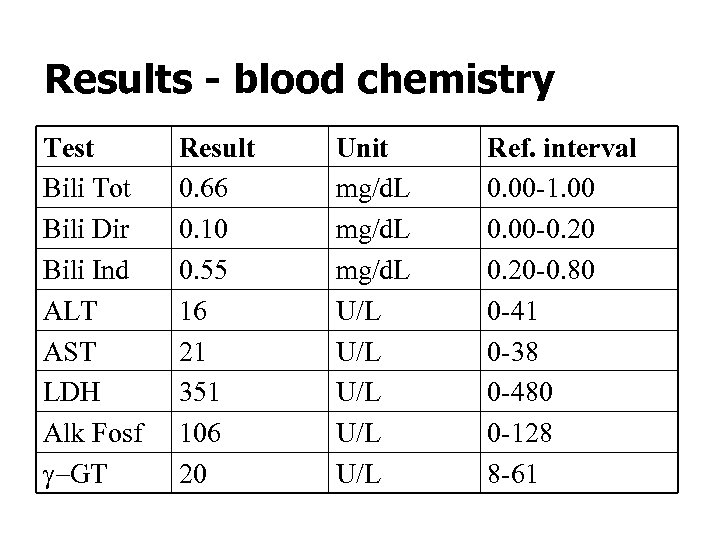
Results - blood chemistry Test Bili Tot Bili Dir Bili Ind ALT AST LDH Alk Fosf g-GT Result 0. 66 0. 10 0. 55 16 21 351 106 20 Unit mg/d. L U/L U/L U/L Ref. interval 0. 00 -1. 00 0. 00 -0. 20 -0. 80 0 -41 0 -38 0 -480 0 -128 8 -61
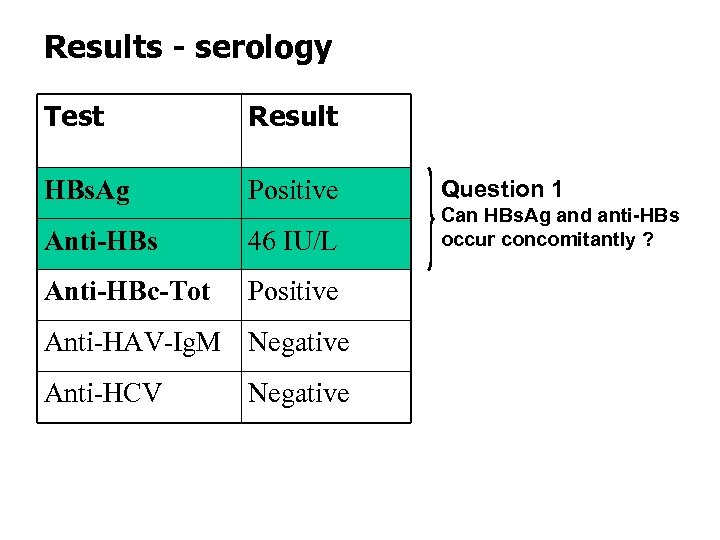
Results - serology Test Result HBs. Ag Positive Anti-HBs 46 IU/L Anti-HBc-Tot Positive Anti-HAV-Ig. M Negative Anti-HCV Negative Question 1 Can HBs. Ag and anti-HBs occur concomitantly ?
![[HBs. Ag-anti-HBs] immune complexes • Are present during the clearance phase of an acute [HBs. Ag-anti-HBs] immune complexes • Are present during the clearance phase of an acute](https://present5.com/presentation/263a0fff0cc2621c5d19e30e5fdae375/image-20.jpg)
[HBs. Ag-anti-HBs] immune complexes • Are present during the clearance phase of an acute HBV infection in the “window phase” and in some chronic HBV patients • Routine tests for HBs. Ag/anti-HBs do not detect immune complexes (IC) • IC dissociatie (ICD) by treatment of serum (100µl) with HCl (50 µl, 0. 5 N, 1 h at 37°C) and neutralisation with Na. OH (50 µl, 0. 5 N) (Rabenau et al. 1996) • Research tests can detect IC’s
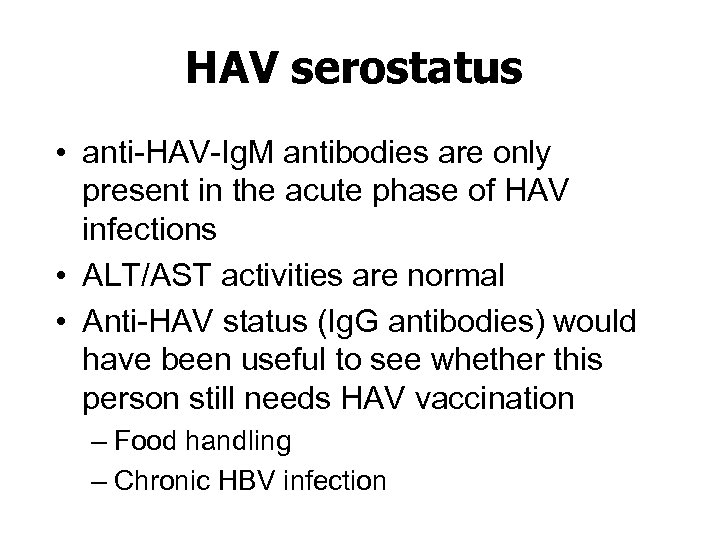
HAV serostatus • anti-HAV-Ig. M antibodies are only present in the acute phase of HAV infections • ALT/AST activities are normal • Anti-HAV status (Ig. G antibodies) would have been useful to see whether this person still needs HAV vaccination – Food handling – Chronic HBV infection
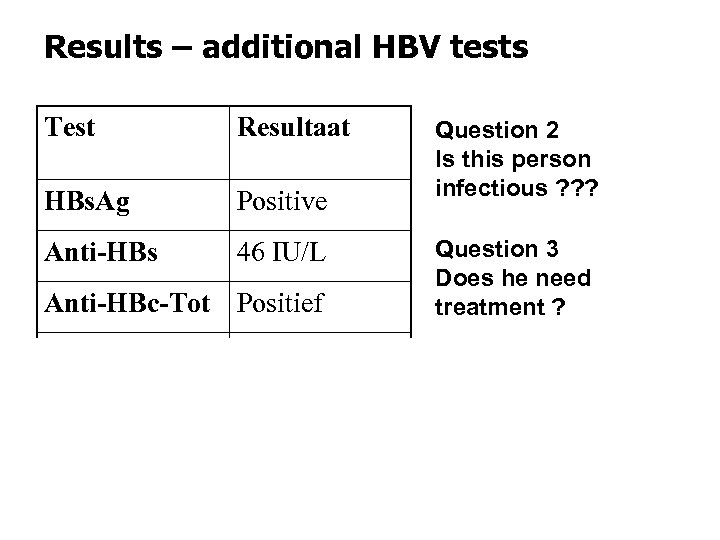
Results – additional HBV tests Test Resultaat HBs. Ag Positive Anti-HBs 46 IU/L Anti-HBc-Tot Positief HBe. Ag Negative Anti-HBe Positive HBV DNA 8 400 g. Eq/ml Question 2 Is this person infectious ? ? ? Question 3 Does he need treatment ?

• Infectivity is LOW – Spouse and daughter show no signs or markers of HBV infection (HBs. Alneg) – Vaccination of household (sexual contact) ! – Twinrix is an alternative for Engerix-B • Therapy is not indicated – Normal transaminases, low DNA – Follow-up : annually ?
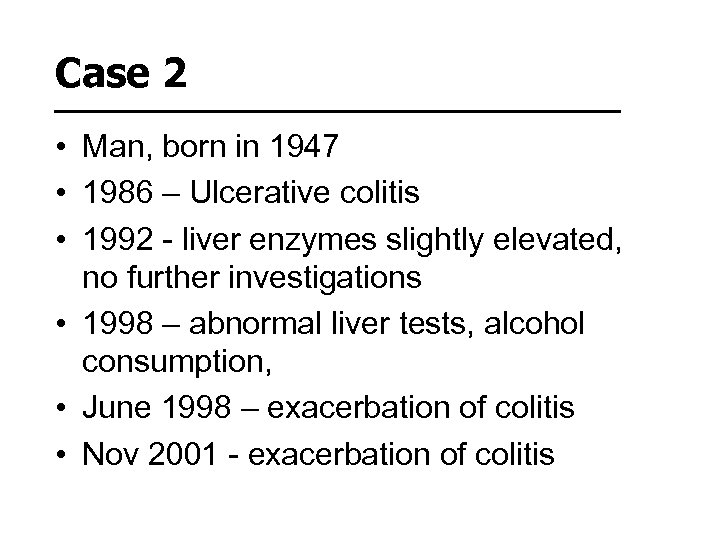
Case 2 • Man, born in 1947 • 1986 – Ulcerative colitis • 1992 - liver enzymes slightly elevated, no further investigations • 1998 – abnormal liver tests, alcohol consumption, • June 1998 – exacerbation of colitis • Nov 2001 - exacerbation of colitis
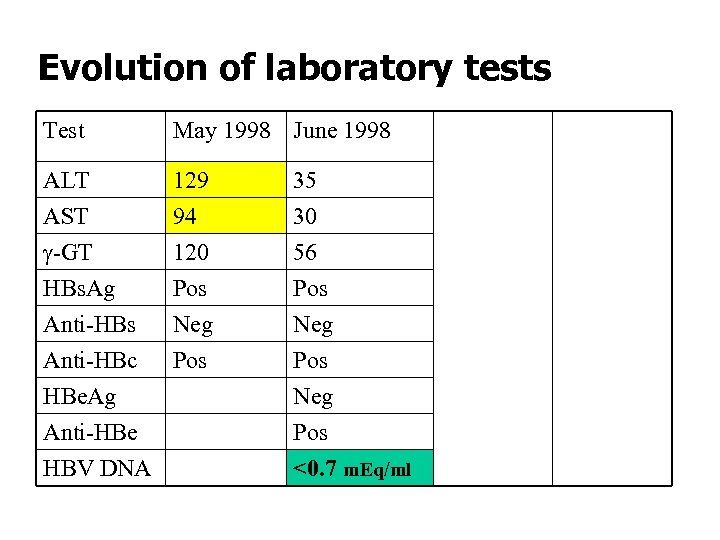
Evolution of laboratory tests Test May 1998 June 1999 Sep 2003 ALT AST g-GT 129 94 120 35 30 56 24 17 28 42 33 58 Pos Neg Pos <0. 7 m. Eq/ml Pos Neg Pos HBs. Ag Pos Anti-HBs Neg Anti-HBc Pos HBe. Ag Anti-HBe HBV DNA
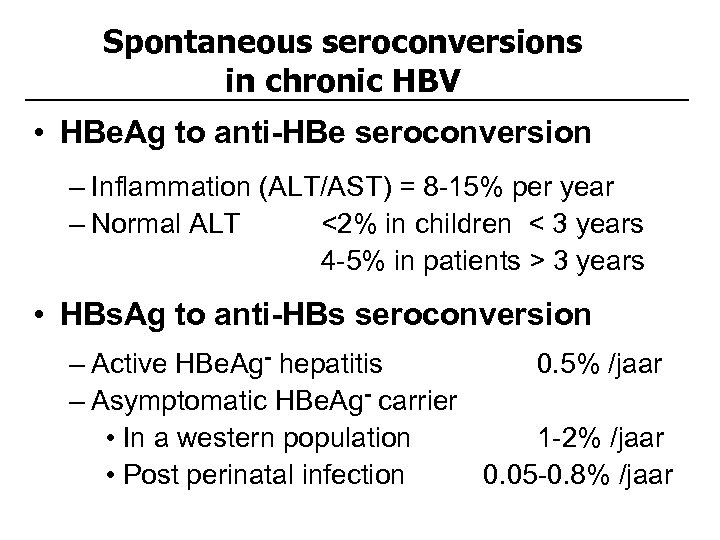
Spontaneous seroconversions in chronic HBV • HBe. Ag to anti-HBe seroconversion – Inflammation (ALT/AST) = 8 -15% per year – Normal ALT <2% in children < 3 years 4 -5% in patients > 3 years • HBs. Ag to anti-HBs seroconversion – Active HBe. Ag- hepatitis 0. 5% /jaar – Asymptomatic HBe. Ag- carrier • In a western population 1 -2% /jaar • Post perinatal infection 0. 05 -0. 8% /jaar
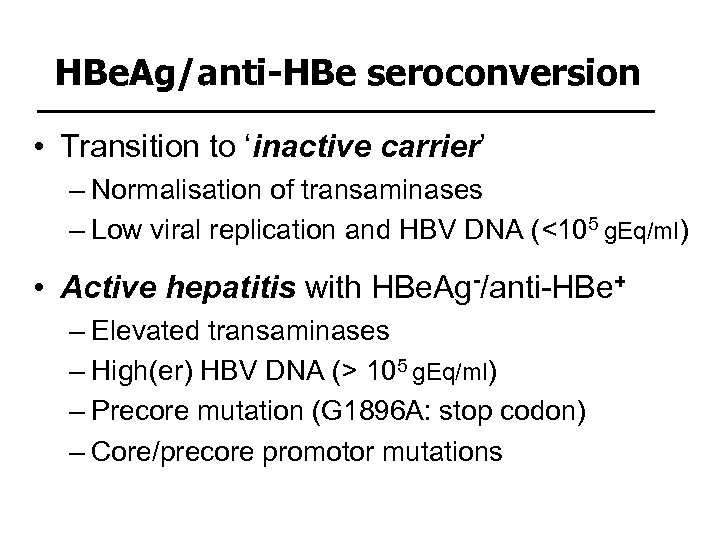
HBe. Ag/anti-HBe seroconversion • Transition to ‘inactive carrier’ – Normalisation of transaminases – Low viral replication and HBV DNA (<105 g. Eq/ml) • Active hepatitis with HBe. Ag-/anti-HBe+ – Elevated transaminases – High(er) HBV DNA (> 105 g. Eq/ml) – Precore mutation (G 1896 A: stop codon) – Core/precore promotor mutations
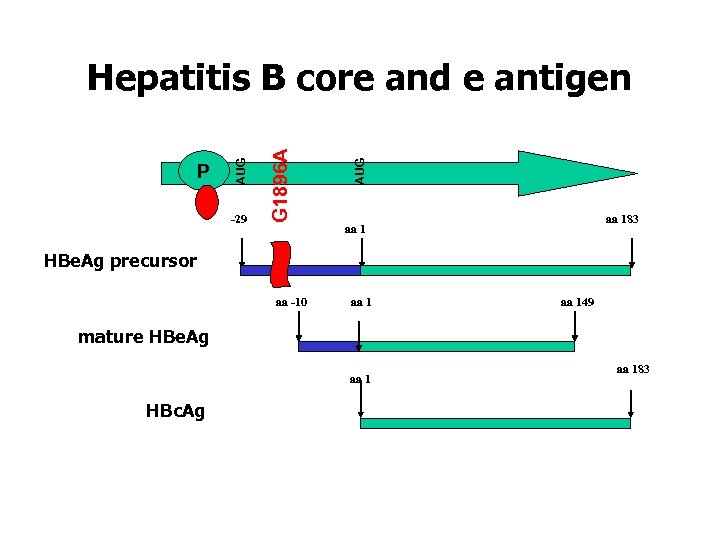
-29 AUG G 1896 A P AUG Hepatitis B core and e antigen aa 183 aa 1 HBe. Ag precursor aa -10 aa 149 mature HBe. Ag aa 1 HBc. Ag aa 183
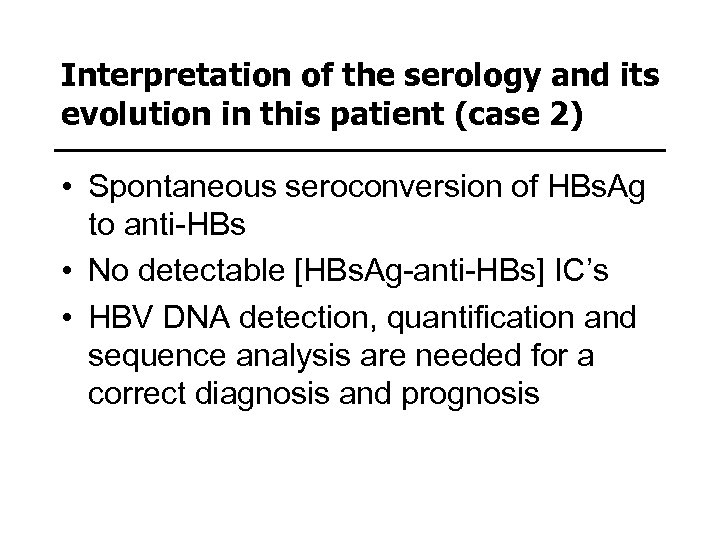
Interpretation of the serology and its evolution in this patient (case 2) • Spontaneous seroconversion of HBs. Ag to anti-HBs • No detectable [HBs. Ag-anti-HBs] IC’s • HBV DNA detection, quantification and sequence analysis are needed for a correct diagnosis and prognosis
Case 3 • • • Man, 45 years Traffic accident => brain death Possible organ donor (liver ? ) Serology : HBs. Agneg, anti-HBsneg, anti-HBcpos, anti. HCVneg, anti-HIVneg, CMVneg • Biochemistry : no abnormalities
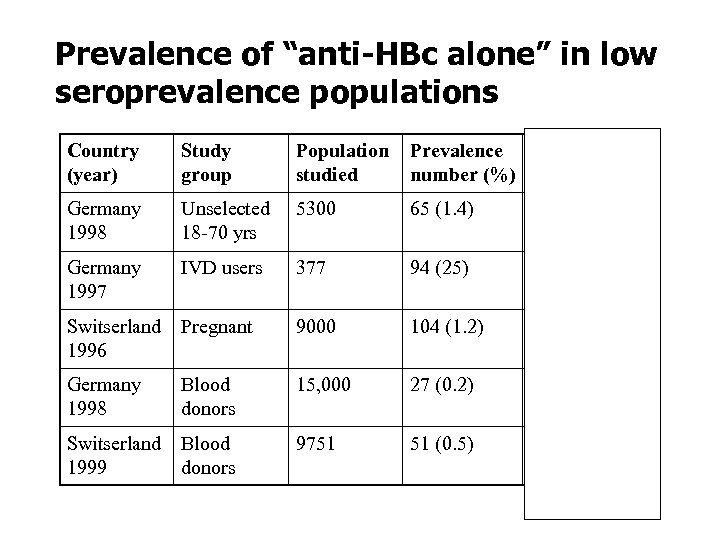
Prevalence of “anti-HBc alone” in low seroprevalence populations Country (year) Study group Population studied Prevalence HBV DNA+ number (%) Germany 1998 Unselected 18 -70 yrs 5300 65 (1. 4) Germany 1997 IVD users 377 94 (25) Switserland 1996 Pregnant 9000 104 (1. 2) 0/104 (0) Germany 1998 Blood donors 15, 000 27 (0. 2) 2/27 (7. 4) Switserland 1999 Blood donors 9751 51 (0. 5) 2/51 (3. 9) 5/65 (7. 7)
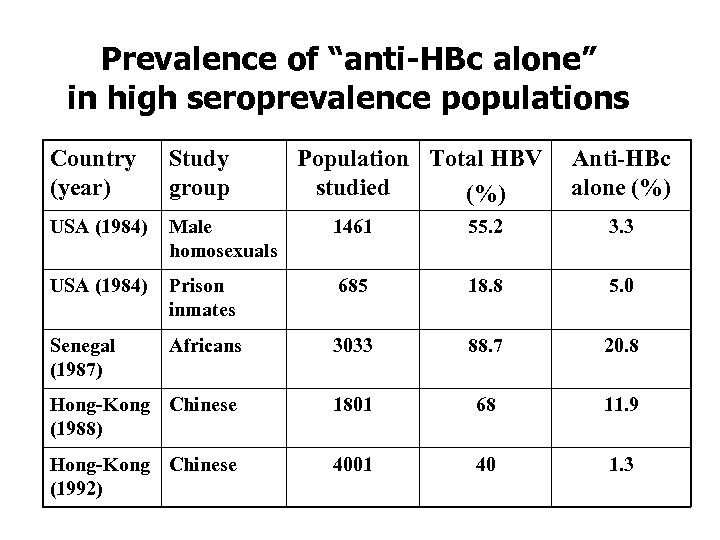
Prevalence of “anti-HBc alone” in high seroprevalence populations Country (year) Study group Population Total HBV studied (%) Anti-HBc alone (%) USA (1984) Male homosexuals 1461 55. 2 3. 3 USA (1984) Prison inmates 685 18. 8 5. 0 Senegal (1987) Africans 3033 88. 7 20. 8 Hong-Kong Chinese (1988) 1801 68 11. 9 Hong-Kong Chinese (1992) 4001 40 1. 3
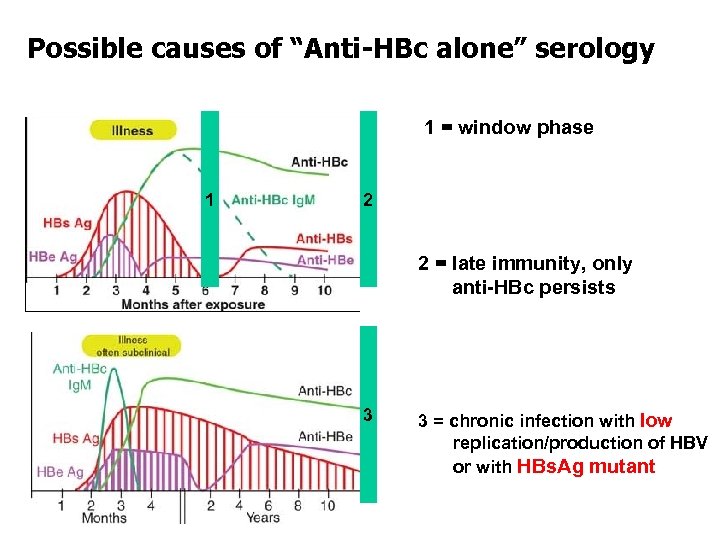
Possible causes of “Anti-HBc alone” serology 1 = window phase 1 2 2 = late immunity, only anti-HBc persists 3 3 = chronic infection with low replication/production of HBV or with HBs. Ag mutant
“Occult hepatitis” • Typical serology HBs. Agneg, anti-HBsneg, anti-HBcpos • HBV DNA < detection limit of routine PCR test (e. g. < 200 g. Eq/m. L) • HBV DNA in the liver
Influence of HCV infection on HBV replication • HCV core protein suppresses HBV replication with a factor 2 to 4 • HCV infection reduces the expression of HBs. Ag in the liver • Treatment of HCV with IFNa also has an effect on HBV

“anti-HBc alone” can be : 1. 2. 3. 4. 5. Window phase Late immunity – only sign of past infection Chronic infection – “occult infection” HBs. Ag mutant “False” positive test result • • really “false positive” – low signal, 2 nd EIA Core-binding antibodies (Ig. M vs Ig. G)
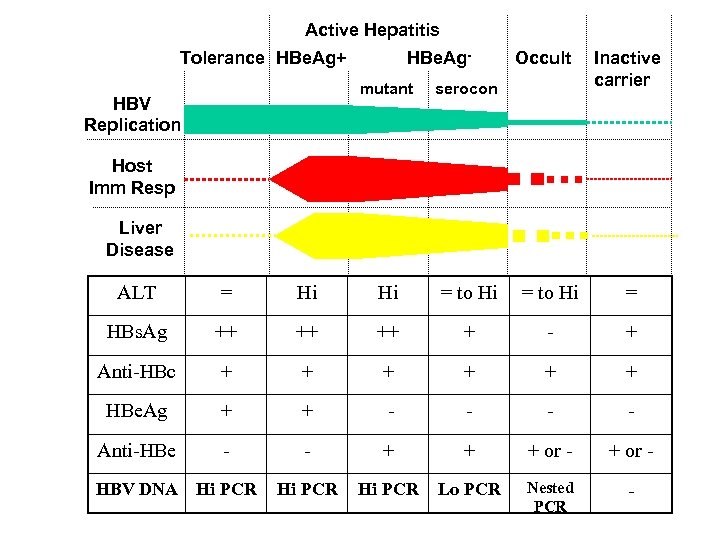
Active Hepatitis Tolerance HBe. Ag+ HBe. Ag- Occult mutant serocon HBV Replication Inactive carrier Host Imm Resp Liver Disease ALT = Hi Hi = to Hi = HBs. Ag ++ ++ ++ + - + Anti-HBc + + + HBe. Ag + + - - Anti-HBe - - + + + or - HBV DNA Hi PCR Lo PCR Nested PCR -
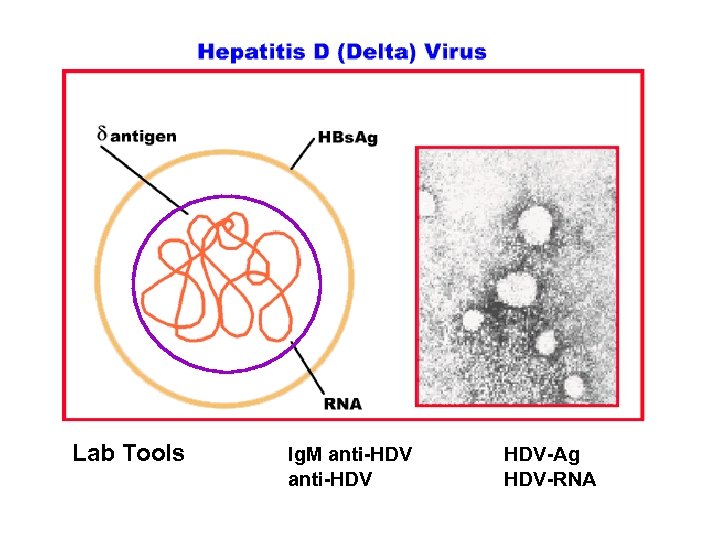
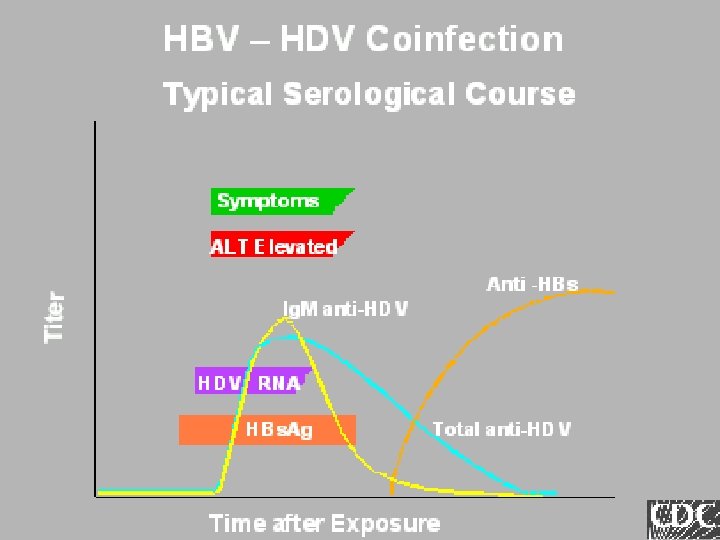
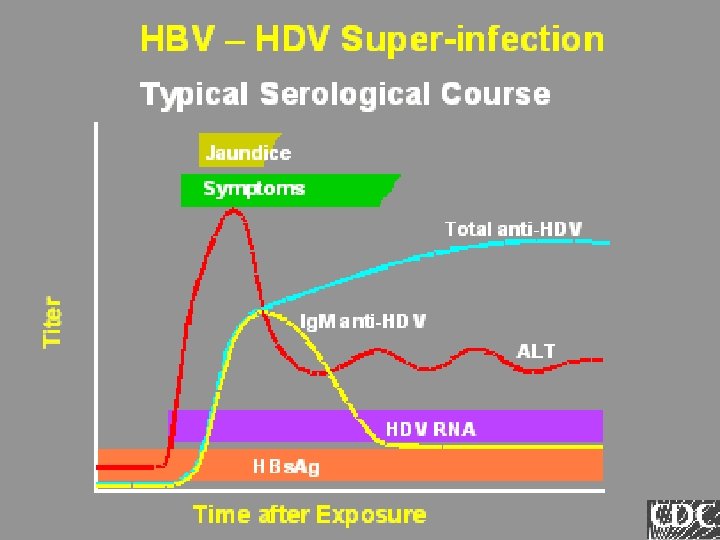
Lab Tools Ig. M anti-HDV HDV-Ag HDV-RNA

Hepatitis C virus Diagnostic tools and interpretation
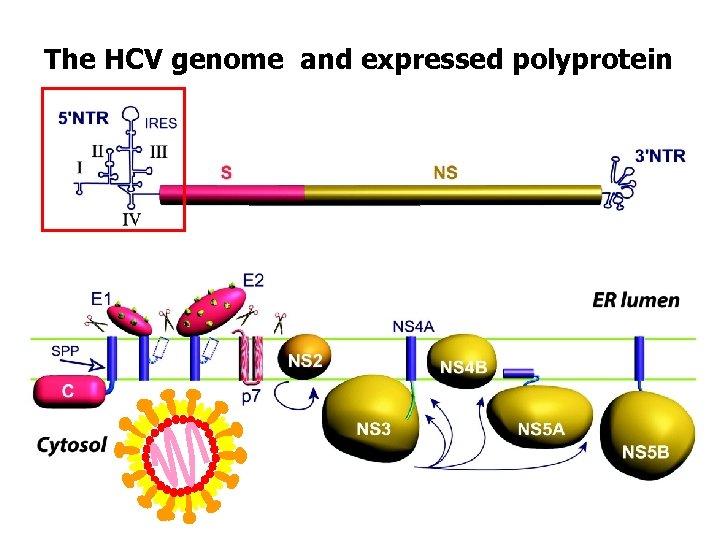
The HCV genome and expressed polyprotein
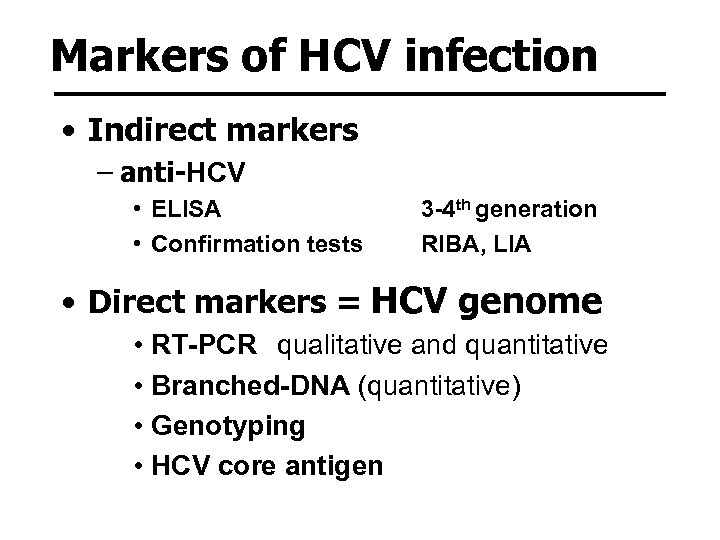
Markers of HCV infection • Indirect markers – anti-HCV • ELISA • Confirmation tests 3 -4 th generation RIBA, LIA • Direct markers = HCV genome • RT-PCR qualitative and quantitative • Branched-DNA (quantitative) • Genotyping • HCV core antigen
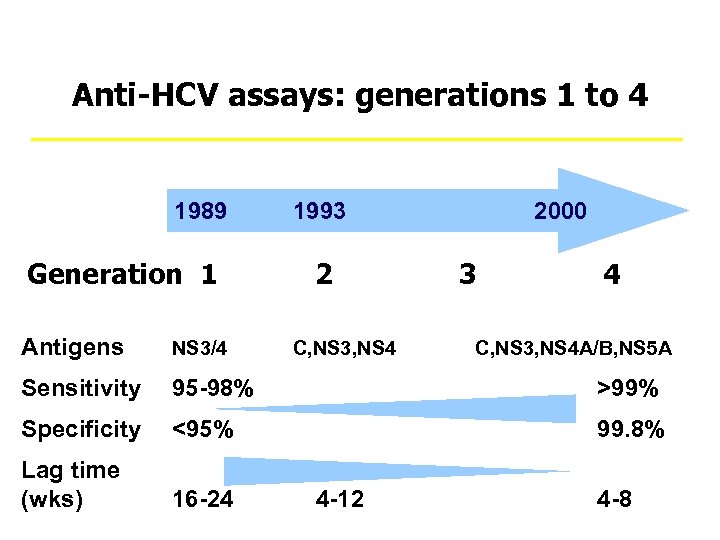
Anti-HCV assays: generations 1 to 4 1989 1993 Generation 1 2 2000 3 4 Antigens NS 3/4 C, NS 3, NS 4 A/B, NS 5 A Sensitivity 95 -98% >99% Specificity <95% 99. 8% Lag time (wks) 16 -24 4 -12 4 -8
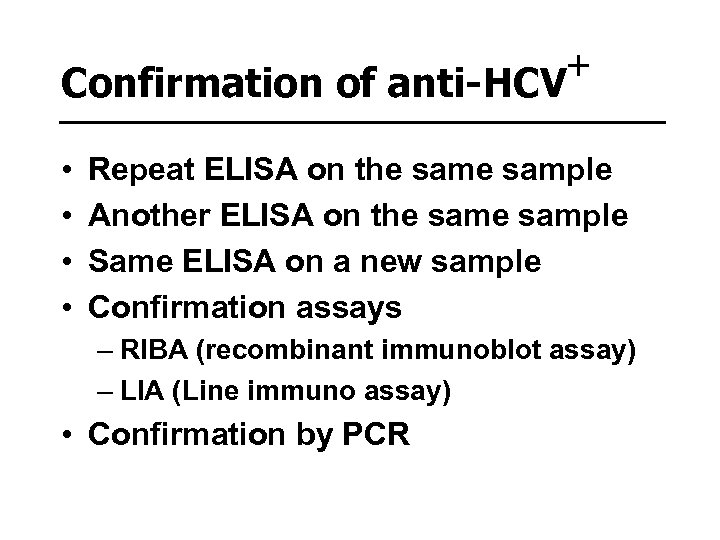
+ Confirmation of anti-HCV • • Repeat ELISA on the sample Another ELISA on the sample Same ELISA on a new sample Confirmation assays – RIBA (recombinant immunoblot assay) – LIA (Line immuno assay) • Confirmation by PCR
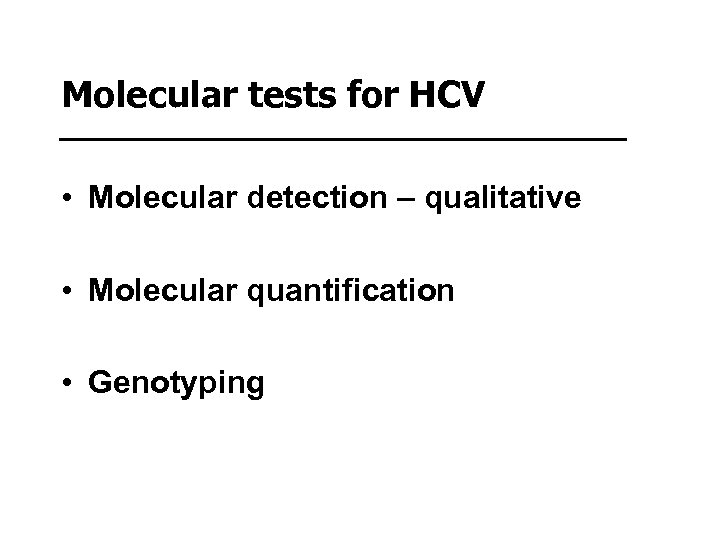
Molecular tests for HCV • Molecular detection – qualitative • Molecular quantification • Genotyping
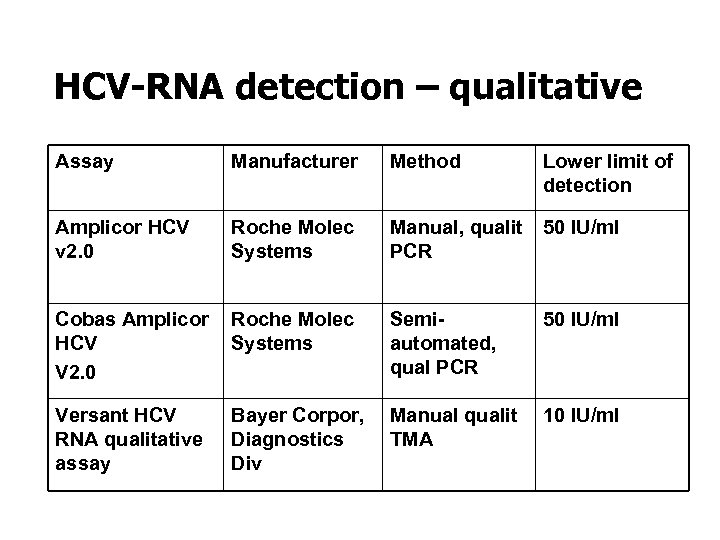
HCV-RNA detection – qualitative Assay Manufacturer Method Amplicor HCV v 2. 0 Roche Molec Systems Manual, qualit 50 IU/ml PCR Cobas Amplicor Roche Molec HCV Systems V 2. 0 Versant HCV RNA qualitative assay Semiautomated, qual PCR Lower limit of detection 50 IU/ml Bayer Corpor, Manual qualit 10 IU/ml Diagnostics TMA Div

Qualitative HCV-RNA tests • Confirms diagnosis of HCV infection • Useful for the early diagnosis of acute hepatitis C • Demonstrates the presence of active infection • « Gold standard » for documenting response to therapy
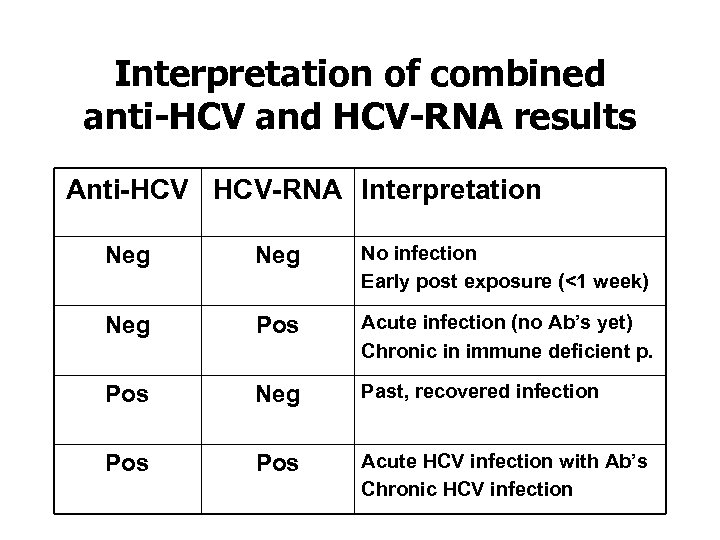
Interpretation of combined anti-HCV and HCV-RNA results Anti-HCV HCV-RNA Interpretation Neg No infection Early post exposure (<1 week) Neg Pos Acute infection (no Ab’s yet) Chronic in immune deficient p. Pos Neg Past, recovered infection Pos Acute HCV infection with Ab’s Chronic HCV infection
Treatment of HCV • Clinical studies evaluating the efficacy of different treatment protocols : drugs, doses, duration, … have revealed the importance of 1) HCV-RNA quantification 2) genotyping
Quantitative HCV RNA tests • Generally less sensitive than qualitative HCV-RNA test => Taqman ! • Positive in >95% of untreated patients with chronic hepatitis C • Useful in predicting response to therapy and determination of early virological response (EVR)
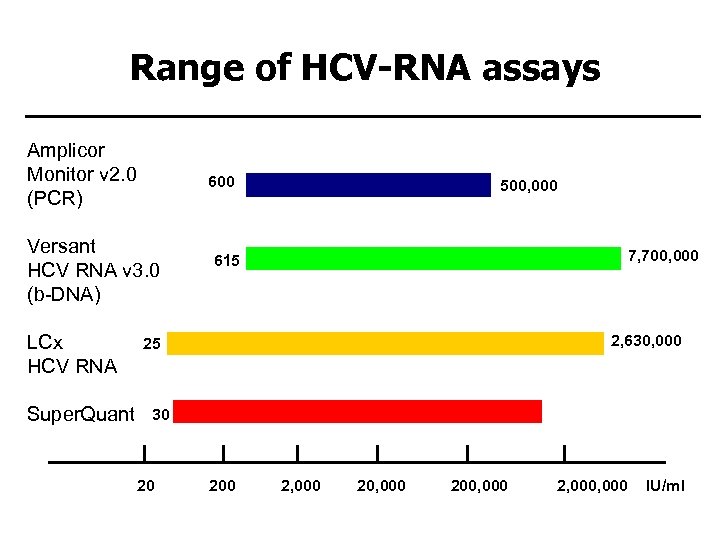
Range of HCV-RNA assays Amplicor Monitor v 2. 0 (PCR) 600 Versant HCV RNA v 3. 0 (b-DNA) LCx HCV RNA Super. Quant 500, 000 7, 700, 000 615 2, 630, 000 25 30 20 1, 470, 000 2, 000 20, 000 200, 000 2, 000 IU/ml
Molecular Genotyping • Direct Sequencing – ‘Home-made’ methods: NS 5 B-, E 1 -based – 5’-noncoding: Trugene - Visible Genetics • ‘Reverse Hybridization’ – Inno Li. PA (Innogenetics)
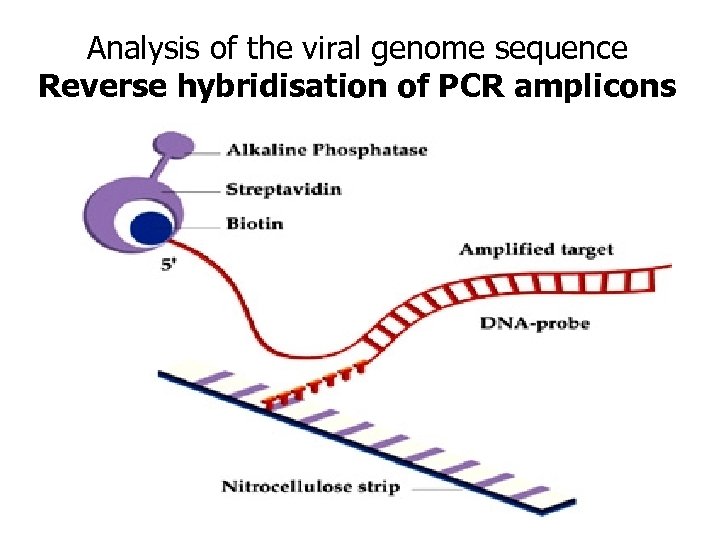
Analysis of the viral genome sequence Reverse hybridisation of PCR amplicons

Treatment of HCV • Interferon-a + Ribavirine • Pegylated IFN-a + Ribavirine
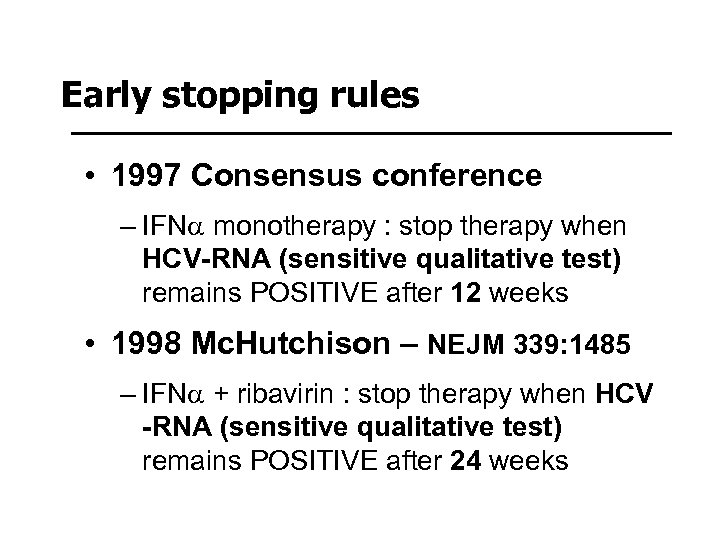
Early stopping rules • 1997 Consensus conference – IFNa monotherapy : stop therapy when HCV-RNA (sensitive qualitative test) remains POSITIVE after 12 weeks • 1998 Mc. Hutchison – NEJM 339: 1485 – IFNa + ribavirin : stop therapy when HCV -RNA (sensitive qualitative test) remains POSITIVE after 24 weeks
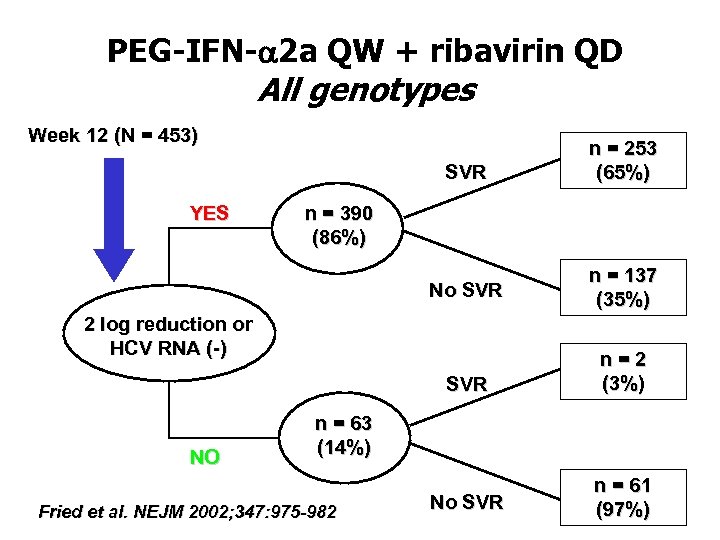
PEG-IFN-a 2 a QW + ribavirin QD All genotypes Week 12 (N = 453) SVR No SVR n = 137 (35%) SVR YES n = 253 (65%) n = 2 (3%) No SVR n = 61 (97%) n = 390 (86%) 2 log reduction or HCV RNA (-) NO n = 63 (14%) Fried et al. NEJM 2002; 347: 975 -982
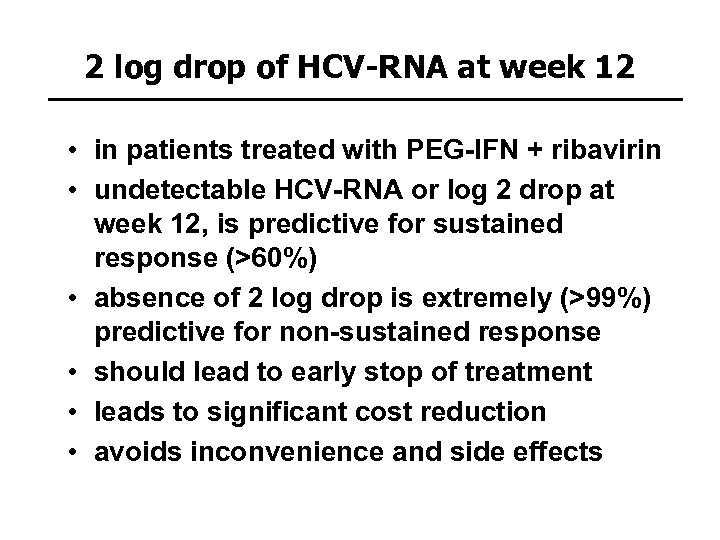
2 log drop of HCV-RNA at week 12 • in patients treated with PEG-IFN + ribavirin • undetectable HCV-RNA or log 2 drop at week 12, is predictive for sustained response (>60%) • absence of 2 log drop is extremely (>99%) predictive for non-sustained response • should lead to early stop of treatment • leads to significant cost reduction • avoids inconvenience and side effects
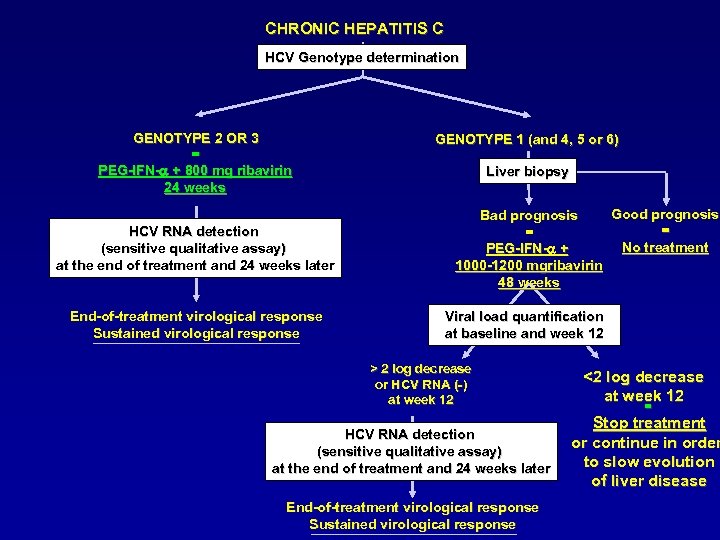
CHRONIC HEPATITIS C HCV Genotype determination GENOTYPE 2 OR 3 = PEG-IFN-a + 800 mg ribavirin 24 weeks HCV RNA detection (sensitive qualitative assay) at the end of treatment and 24 weeks later End-of-treatment virological response Sustained virological response GENOTYPE 1 (and 4, 5 or 6) Liver biopsy Good prognosis Bad prognosis = = No treatment PEG-IFN-a + 1000 -1200 mgribavirin 48 weeks Viral load quantification at baseline and week 12 > 2 log decrease or HCV RNA (-) at week 12 HCV RNA detection (sensitive qualitative assay) at the end of treatment and 24 weeks later End-of-treatment virological response Sustained virological response <2 log decrease at week 12 = Stop treatment or continue in order to slow evolution of liver disease

Cost-benefit of monitoring early viral response (EVR) a simulation for Belgium
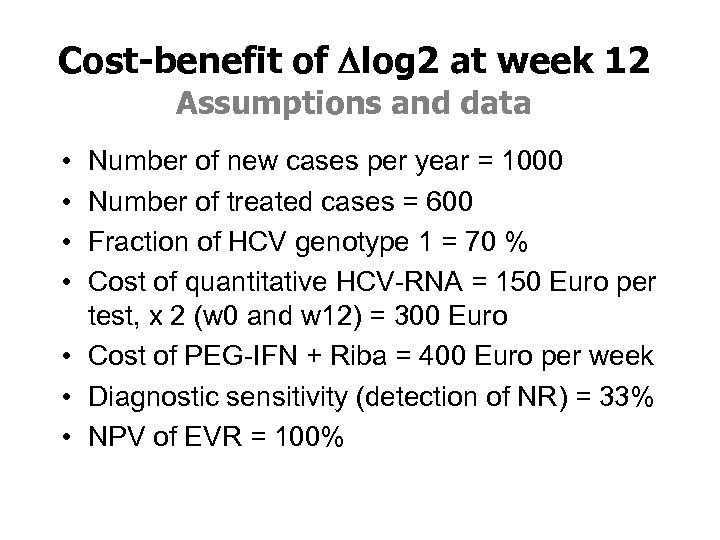
Cost-benefit of Dlog 2 at week 12 Assumptions and data • • Number of new cases per year = 1000 Number of treated cases = 600 Fraction of HCV genotype 1 = 70 % Cost of quantitative HCV-RNA = 150 Euro per test, x 2 (w 0 and w 12) = 300 Euro • Cost of PEG-IFN + Riba = 400 Euro per week • Diagnostic sensitivity (detection of NR) = 33% • NPV of EVR = 100%
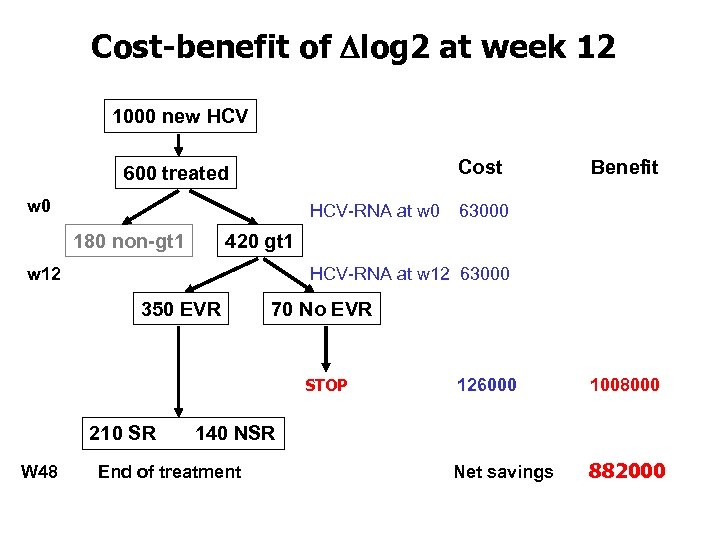
Cost-benefit of Dlog 2 at week 12 1000 new HCV Cost 600 treated w 0 HCV-RNA at w 0 180 non-gt 1 Benefit 63000 420 gt 1 w 12 HCV-RNA at w 12 63000 350 EVR 70 No EVR STOP 126000 1008000 210 SR 140 NSR W 48 End of treatment Net savings 882000
Molecular diagnostic tools for detection and monitoring of HCV infections • Correct diagnosis • Selection of treatment and duration • Decision to “Stop treatment” – Reduce costs (medication, doctor visits, . . ) – Reduce the discomfort and suffering of patients – Reduce the loss of labour time – The impact on costs (direct, indirect) will increase if the diagnostic sensitivity of the EVR algorythm can be improved (>33%)
Additional Hepatitis Agents • 12% o post-transfusion hepatitis unrelated to A-E • 18% of acute hepatitis unrelated to A-E • Up to 40% of fulminant hepatitis no etiology is present • Cases of acute hepatitis followed by aplastic anemia

New viruses not proven to cause human hepatitis • • HGV TTV TLMV TTV-like minivirus Sanban Yonban Sen virus
HGV – GBV-C • Related to HCV - Flaviviridae • Parenteral transmission • Replicates in lymphocytes and not in hepatocytes • HGV infection prolongs survival in HIV • Does not cause hepatitis and not even co-morbidity in (frequent) association with HBV or HCV
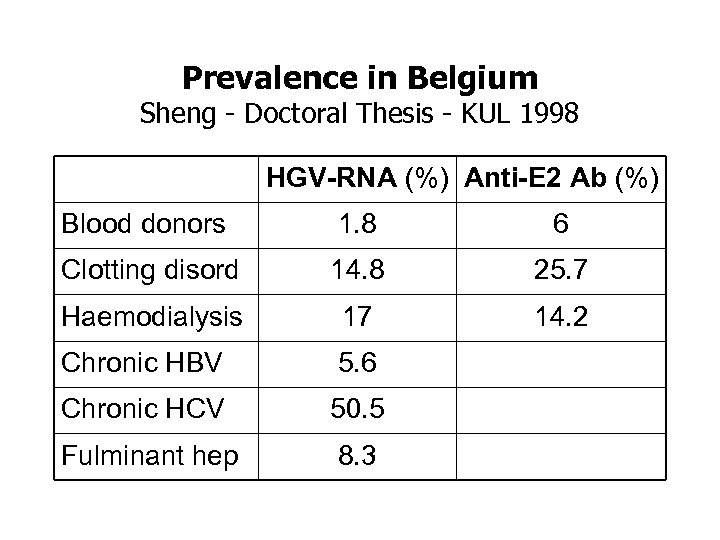
Prevalence in Belgium Sheng - Doctoral Thesis - KUL 1998 HGV-RNA (%) Anti-E 2 Ab (%) Blood donors 1. 8 6 Clotting disord 14. 8 25. 7 Haemodialysis 17 14. 2 Chronic HBV 5. 6 Chronic HCV 50. 5 Fulminant hep 8. 3
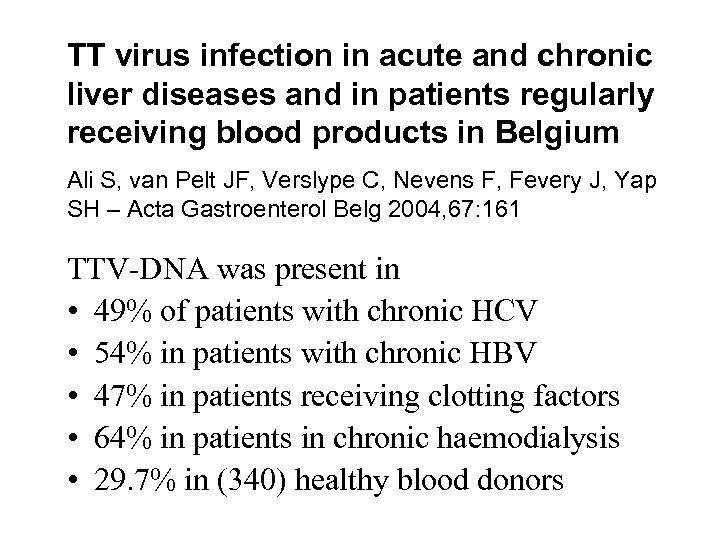
TT virus infection in acute and chronic liver diseases and in patients regularly receiving blood products in Belgium Ali S, van Pelt JF, Verslype C, Nevens F, Fevery J, Yap SH – Acta Gastroenterol Belg 2004, 67: 161 TTV-DNA was present in • 49% of patients with chronic HCV • 54% in patients with chronic HBV • 47% in patients receiving clotting factors • 64% in patients in chronic haemodialysis • 29. 7% in (340) healthy blood donors
Analysis of samples for less common forms of hepatitis Dr. Robert Vranckx Wetenschappelijk Instituut Volksgezondheid Juliette Wytmansstraat 14 1050 Brussel Prof. Dr. Patrick Goubau AIDS Reference Laboratory, UCL Avenue Hippocrate 54/92 B-1200 Brussels HEV-Al HDV-Ag ? HGV-RNA ? HDV-Al
263a0fff0cc2621c5d19e30e5fdae375.ppt