99f7ba149c1d0c300eb6656152e279b0.ppt
- Количество слайдов: 74
Heparin-Induced Thrombocytopenia “Heparin as a Cause of Thrombosis” and “The HIT Syndromes” Amjad Al. Mahameed, MD, MPH Diovision of Cardiology Beth Israel Deaconess Medical Center Boston

Why We Should Not Talk About HIT • • • It is rare and over publicized Not every hospitalized patient get heparin… No unifying clinical picture and the serology is “poor”. . How can we justify anticoagulating a thrombocytopenic patient using medications that do not have antidotes? Most hospitalized patients with thrombocytopenia have multifactorial etiology. . • Just stop haparin and it will “go away”…. • Or start a LMWH and don’t worry about it any more • Or, start warfarin and it “will take care of the problem” • This is DIC, not HIT. . You “must” continue heparin… • It is a disease born in the “industry”…
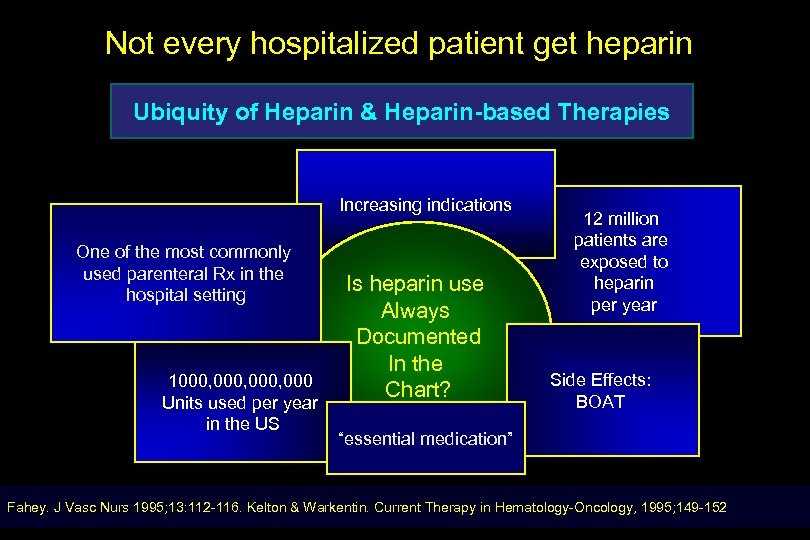
Not every hospitalized patient get heparin Ubiquity of Heparin & Heparin-based Therapies Increasing indications One of the most commonly used parenteral Rx in the hospital setting 1000, 000 Units used per year in the US Is heparin use Always Documented In the Chart? 12 million patients are exposed to heparin per year Side Effects: BOAT “essential medication” Fahey. J Vasc Nurs 1995; 13: 112 -116. Kelton & Warkentin. Current Therapy in Hematology-Oncology, 1995; 149 -152

Is it a rare and over publicized disease LET’S DO THE MATH 12 million patients exposed annually x Up to 5% incidence of HIT = Up to 600, 000 cases every year How many cases are recognized and treated properly? 18, 000 !!!
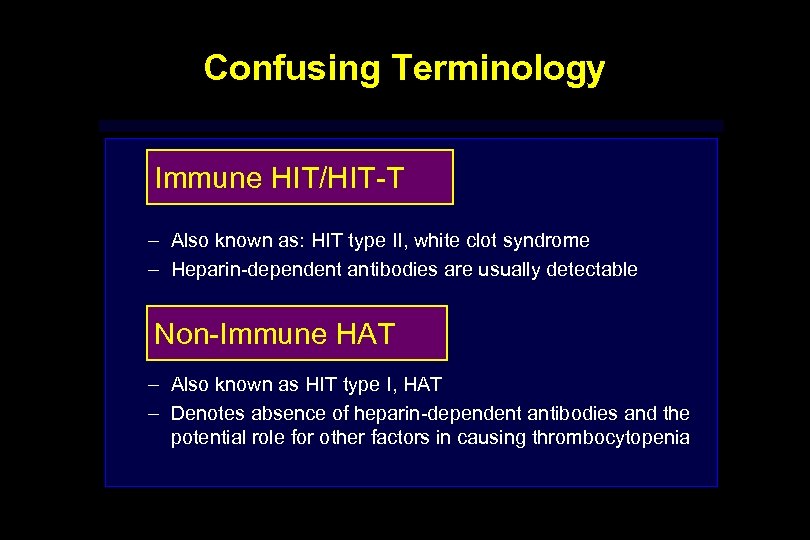
Confusing Terminology Immune HIT/HIT-T – Also known as: HIT type II, white clot syndrome – Heparin-dependent antibodies are usually detectable Non-Immune HAT – Also known as HIT type I, HAT – Denotes absence of heparin-dependent antibodies and the potential role for other factors in causing thrombocytopenia
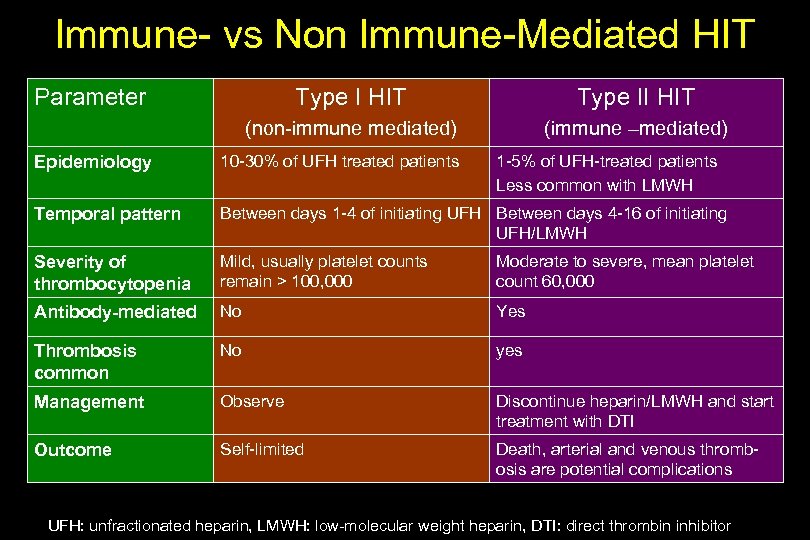
Immune- vs Non Immune-Mediated HIT Parameter Type I HIT Type II HIT (non-immune mediated) (immune –mediated) Epidemiology 10 -30% of UFH treated patients 1 -5% of UFH-treated patients Less common with LMWH Temporal pattern Between days 1 -4 of initiating UFH Between days 4 -16 of initiating UFH/LMWH Severity of thrombocytopenia Mild, usually platelet counts remain > 100, 000 Moderate to severe, mean platelet count 60, 000 Antibody-mediated No Yes Thrombosis common No yes Management Observe Discontinue heparin/LMWH and start treatment with DTI Outcome Self-limited Death, arterial and venous thrombosis are potential complications UFH: unfractionated heparin, LMWH: low-molecular weight heparin, DTI: direct thrombin inhibitor
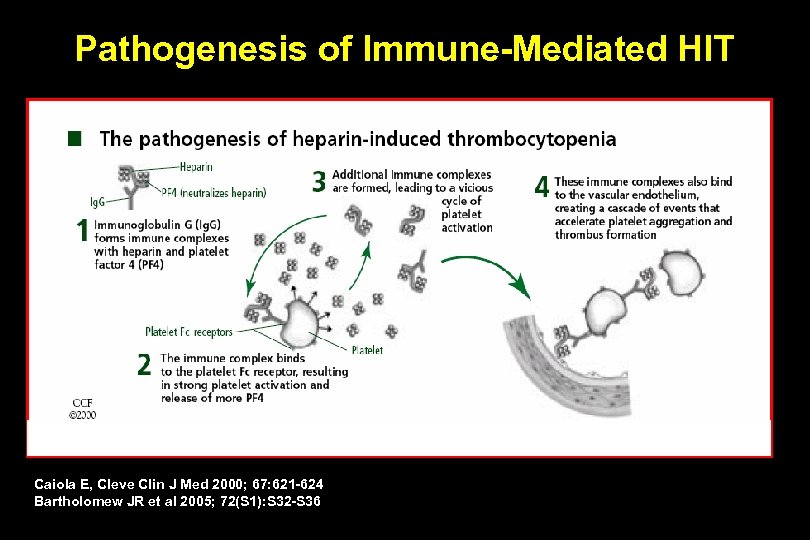
Pathogenesis of Immune-Mediated HIT Caiola E, Cleve Clin J Med 2000; 67: 621 -624 Bartholomew JR et al 2005; 72(S 1): S 32 -S 36
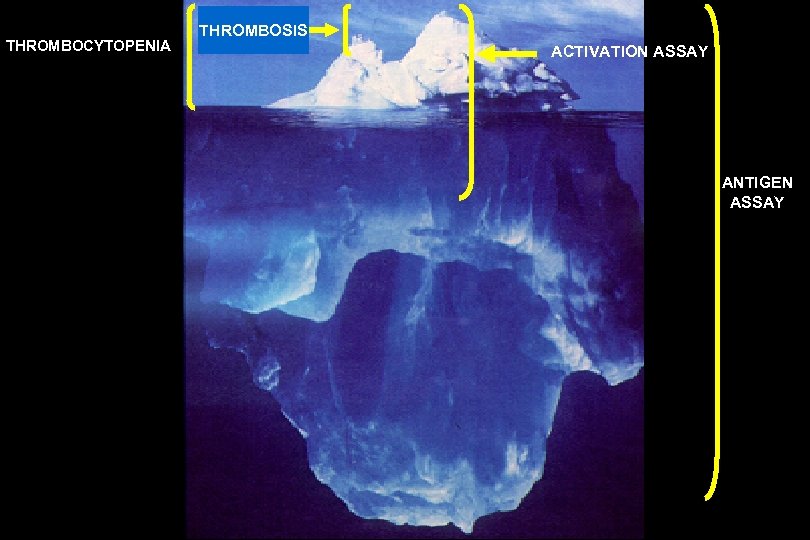
THROMBOCYTOPENIA THROMBOSIS ACTIVATION ASSAY ANTIGEN ASSAY
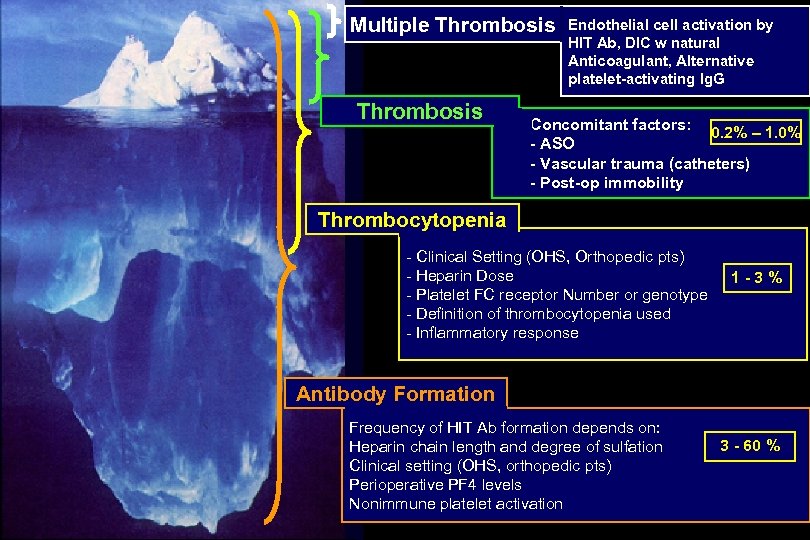
Multiple Thrombosis Endothelial cell activation by HIT Ab, DIC w natural Anticoagulant, Alternative platelet-activating Ig. G Concomitant factors: 0. 2% – 1. 0% - ASO - Vascular trauma (catheters) - Post-op immobility Thrombocytopenia - Clinical Setting (OHS, Orthopedic pts) - Heparin Dose - Platelet FC receptor Number or genotype - Definition of thrombocytopenia used - Inflammatory response 1 -3% Antibody Formation Frequency of HIT Ab formation depends on: Heparin chain length and degree of sulfation Clinical setting (OHS, orthopedic pts) Perioperative PF 4 levels Nonimmune platelet activation 3 - 60 %
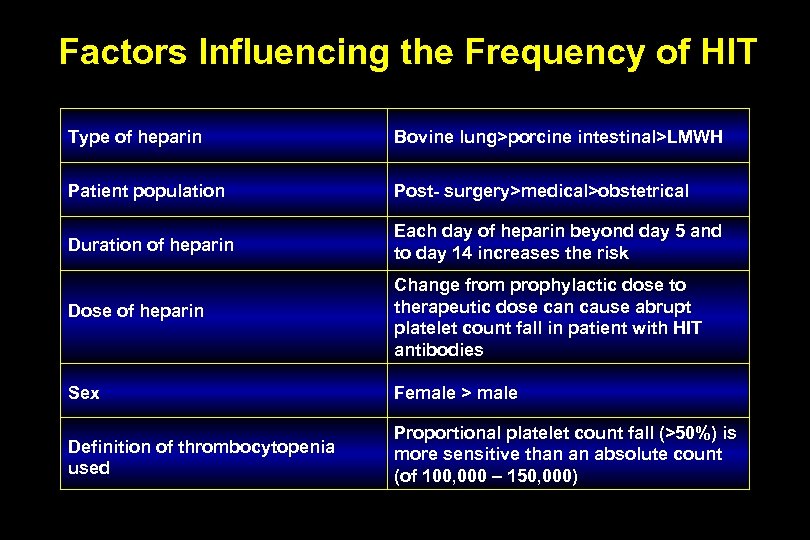
Factors Influencing the Frequency of HIT Type of heparin Bovine lung>porcine intestinal>LMWH Patient population Post- surgery>medical>obstetrical Duration of heparin Each day of heparin beyond day 5 and to day 14 increases the risk Dose of heparin Change from prophylactic dose to therapeutic dose can cause abrupt platelet count fall in patient with HIT antibodies Sex Female > male Definition of thrombocytopenia used Proportional platelet count fall (>50%) is more sensitive than an absolute count (of 100, 000 – 150, 000)

No unifying clinical picture DIFFERENTIATE CLASSICAL HIT FROM ATYPICAL FORMS
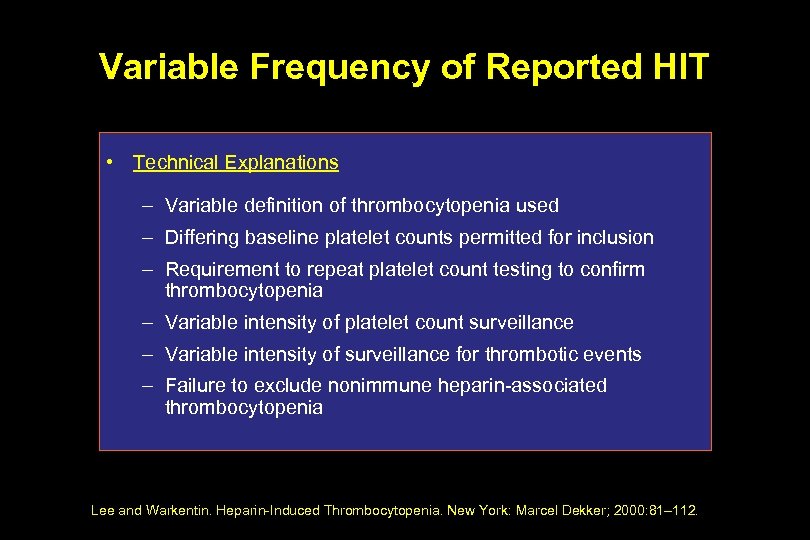
Variable Frequency of Reported HIT • Technical Explanations – Variable definition of thrombocytopenia used – Differing baseline platelet counts permitted for inclusion – Requirement to repeat platelet count testing to confirm thrombocytopenia – Variable intensity of platelet count surveillance – Variable intensity of surveillance for thrombotic events – Failure to exclude nonimmune heparin-associated thrombocytopenia Lee and Warkentin. Heparin-Induced Thrombocytopenia. New York: Marcel Dekker; 2000: 81– 112.
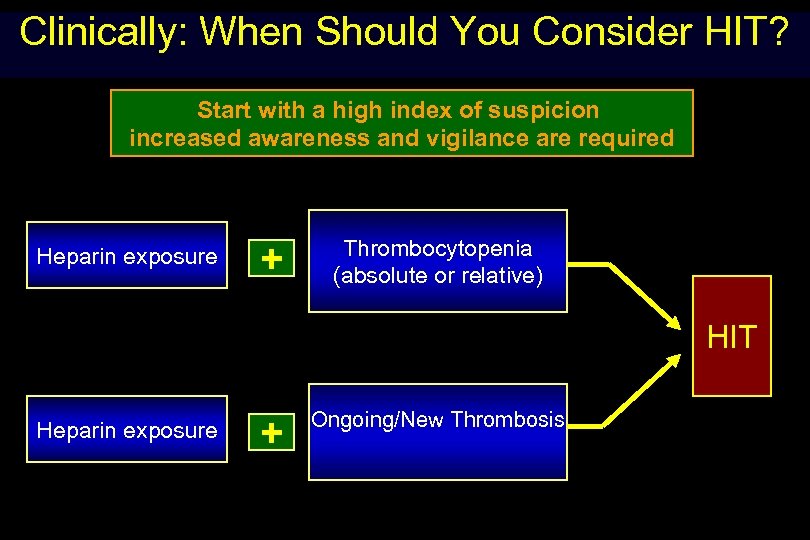
Clinically: When Should You Consider HIT? Start with a high index of suspicion increased awareness and vigilance are required Heparin exposure + Thrombocytopenia (absolute or relative) HIT Heparin exposure + Ongoing/New Thrombosis
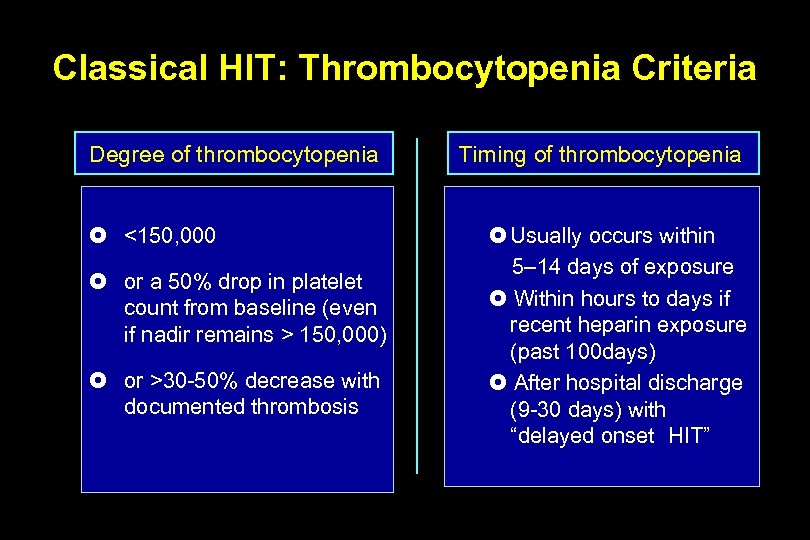
Classical HIT: Thrombocytopenia Criteria Degree of thrombocytopenia <150, 000 or a 50% drop in platelet count from baseline (even if nadir remains > 150, 000) or >30 -50% decrease with documented thrombosis Timing of thrombocytopenia Usually occurs within 5– 14 days of exposure Within hours to days if recent heparin exposure (past 100 days) After hospital discharge (9 -30 days) with “delayed onset HIT”
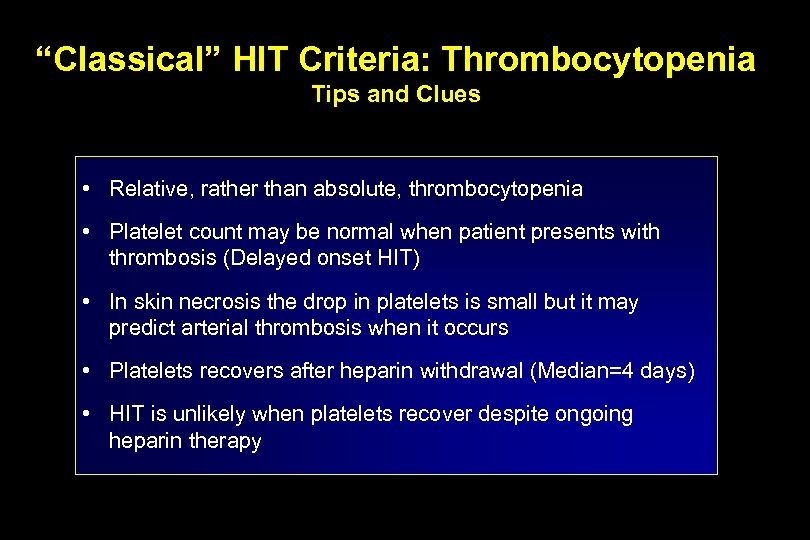
“Classical” HIT Criteria: Thrombocytopenia Tips and Clues • Relative, rather than absolute, thrombocytopenia • Platelet count may be normal when patient presents with thrombosis (Delayed onset HIT) • In skin necrosis the drop in platelets is small but it may predict arterial thrombosis when it occurs • Platelets recovers after heparin withdrawal (Median=4 days) • HIT is unlikely when platelets recover despite ongoing heparin therapy
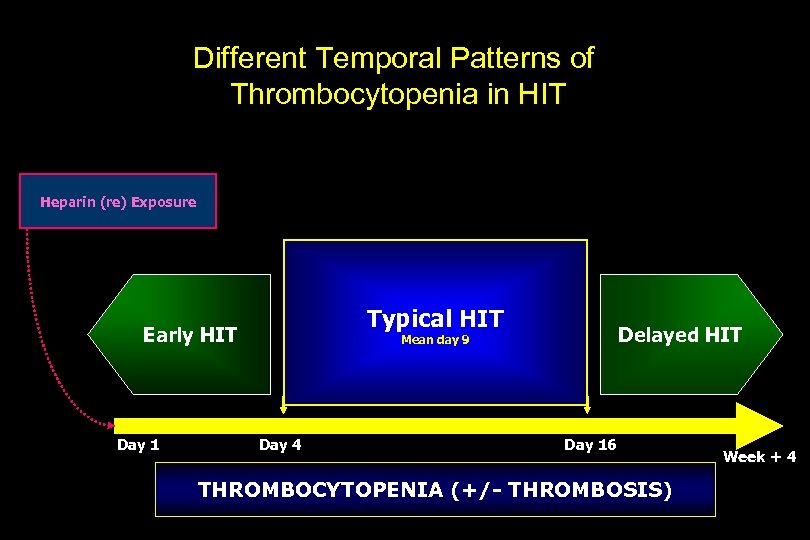
Different Temporal Patterns of Thrombocytopenia in HIT Heparin (re) Exposure Typical HIT Early HIT Day 1 Delayed HIT Mean day 9 Day 4 Day 16 THROMBOCYTOPENIA (+/- THROMBOSIS) Week + 4
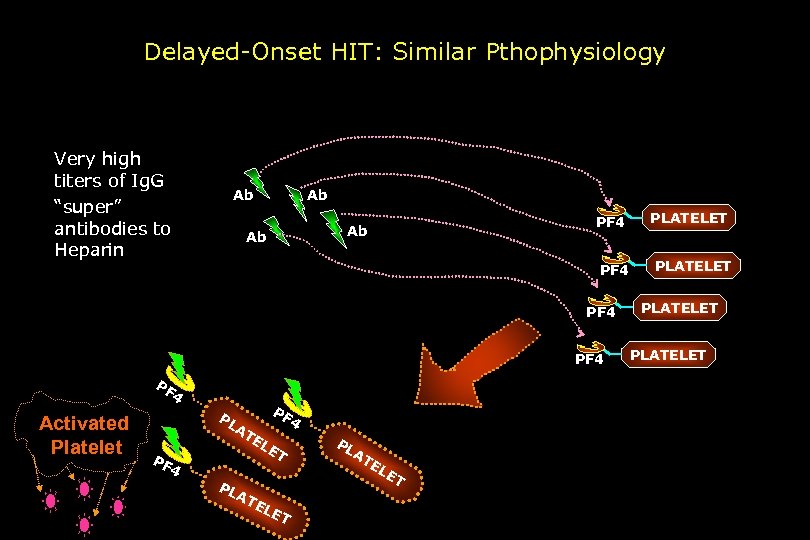
Delayed-Onset HIT: Similar Pthophysiology Very high titers of Ig. G “super” antibodies to Heparin Ab Ab PF 4 PF 4 Activated Platelet PL AT PF PF 4 EL E T 4 PL AT EL ET PL A TE LE T PLATELET
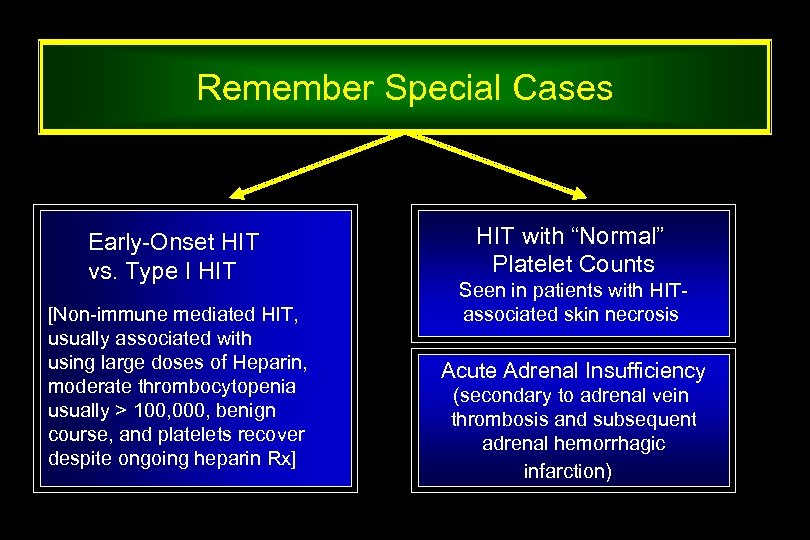
Remember Special Cases Early-Onset HIT vs. Type I HIT [Non-immune mediated HIT, usually associated with using large doses of Heparin, moderate thrombocytopenia usually > 100, 000, benign course, and platelets recover despite ongoing heparin Rx] HIT with “Normal” Platelet Counts Seen in patients with HITassociated skin necrosis Acute Adrenal Insufficiency (secondary to adrenal vein thrombosis and subsequent adrenal hemorrhagic infarction)
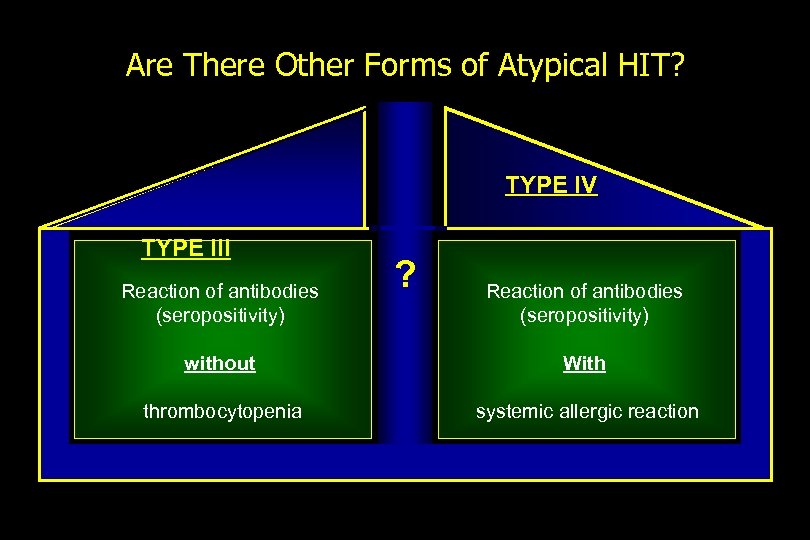
Are There Other Forms of Atypical HIT? TYPE IV TYPE III Reaction of antibodies (seropositivity) ? Reaction of antibodies (seropositivity) without With thrombocytopenia systemic allergic reaction

Thrombocytopenia in most hospitalized patients is multifactorial in etiology
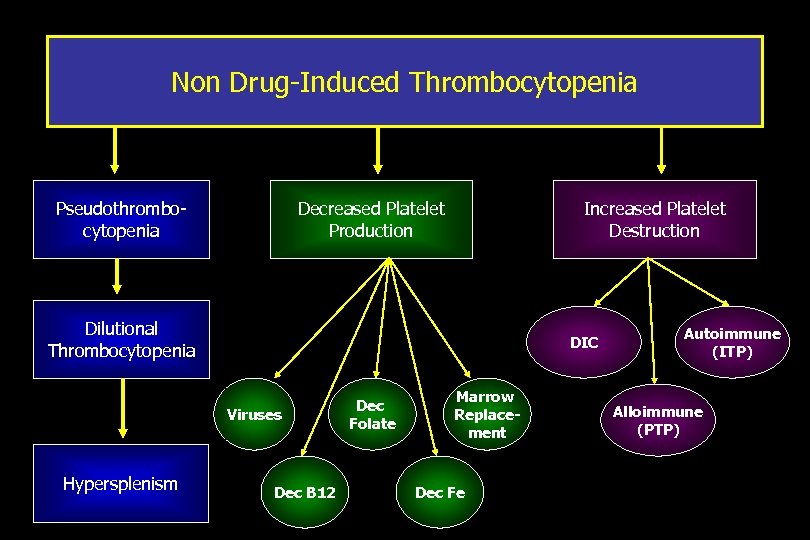
Non Drug-Induced Thrombocytopenia Pseudothrombocytopenia Decreased Platelet Production Increased Platelet Destruction Dilutional Thrombocytopenia DIC Viruses Hypersplenism Dec B 12 Dec Folate Marrow Replacement Dec Fe Autoimmune (ITP) Alloimmune (PTP)

Just hold Heparin and It will Go Away! How can we justify anticoagulating thrombocytopenic patients with medications that do not have antidotes?

HIT is a Thrombotic Storm! Thrombosis Begets Thrombosis !
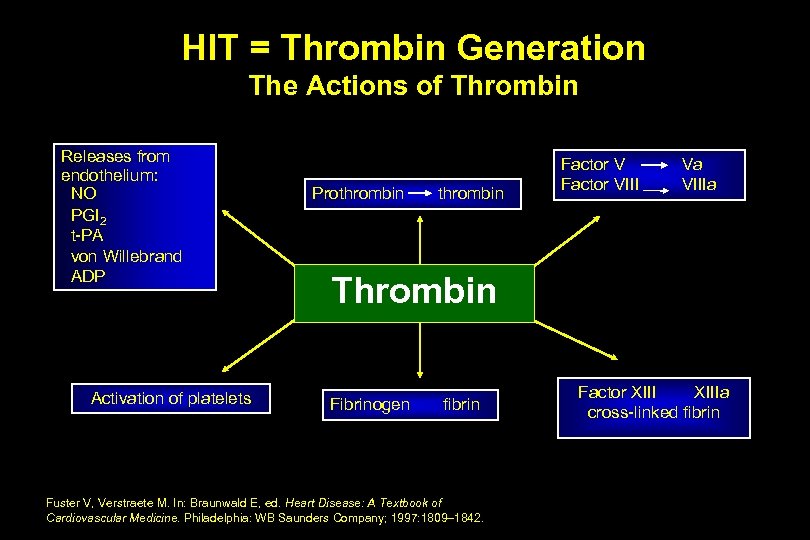
HIT = Thrombin Generation The Actions of Thrombin Releases from endothelium: NO PGI 2 t-PA von Willebrand ADP Activation of platelets Prothrombin Factor VIII Va VIIIa Thrombin Fibrinogen fibrin Fuster V, Verstraete M. In: Braunwald E, ed. Heart Disease: A Textbook of Cardiovascular Medicine. Philadelphia: WB Saunders Company; 1997: 1809– 1842. Factor XIIIa cross-linked fibrin
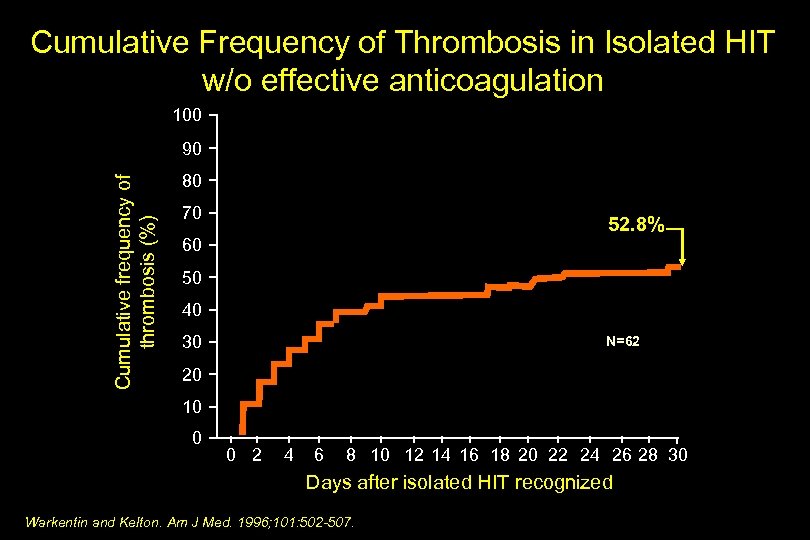
Cumulative Frequency of Thrombosis in Isolated HIT w/o effective anticoagulation 100 Cumulative frequency of thrombosis (%) 90 80 70 52. 8% 60 50 40 30 N=62 20 10 0 0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30 Days after isolated HIT recognized Warkentin and Kelton. Am J Med. 1996; 101: 502 -507.

Just hold Heparin and It will Go Away! How can we justify anticoagulating thrombocytopenic patients with medications that do not have antidotes? • A transient increase in risks of new thrombosis is observed once heparin is stopped w/o an alternative AC (Greinacher Blood 2000) • Patients with the lowest platelet counts are most likely to experience devastating thrombosis and are in greatest need of alternative anticoagulation (Rice, Arch Intern Med 2004) • 10 of HIT pts have platelets 10, 000 -20, 000. Bleeding is rare even when fully anticoagulated. (Rice, Arch Intern Med 2004) • 6% per day incidence of new thrombosis while awaiting serologic confirmation w/o DTI Rx. This was decreased to 1. 3% per day after Refludan Rx (Greinacher et al Blood 2000) • Therapeutic dose Argatroban lowered new thrombosis from 23% to 6 -8% (P < 0. 001) and lowered the frequency of composite end point of new thrombosis, all cause mortality, and limb amputation from 39% to 26 -28% (P = 0. 04) (Lewis BE. Circulation 2001 and Arch Intern Med 2003)
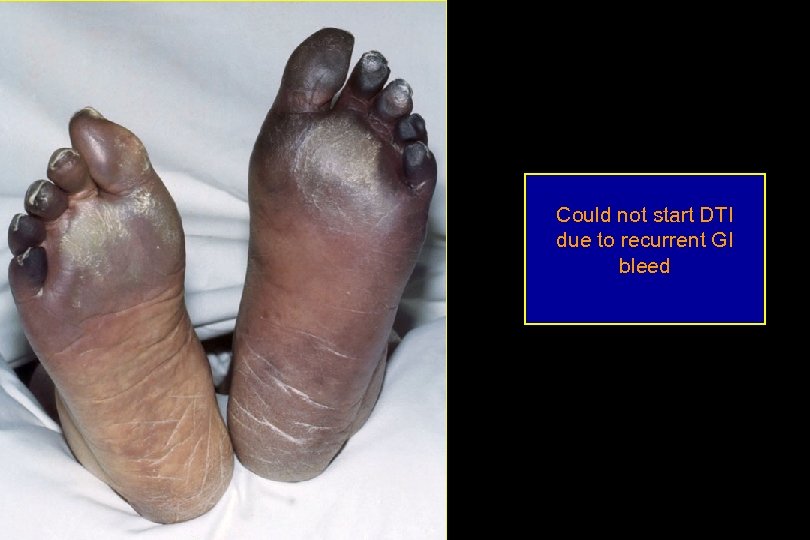
Could not start DTI due to recurrent GI bleed
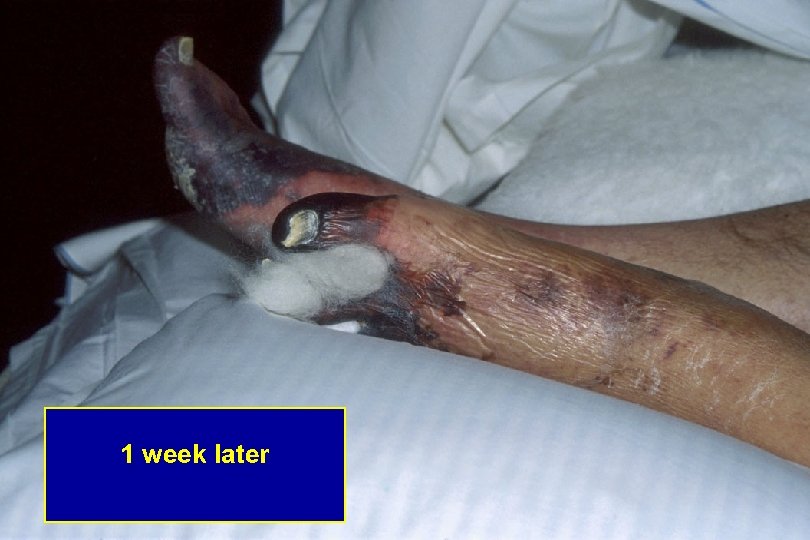
1 week later
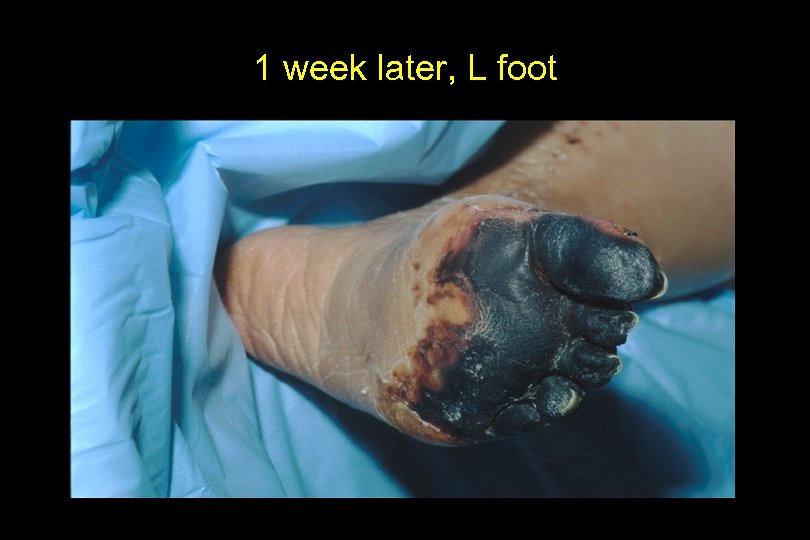
1 week later, L foot
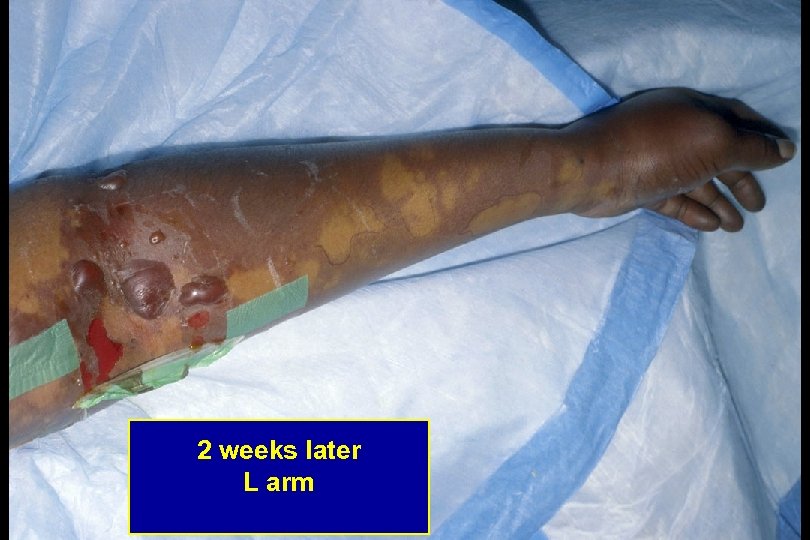
2 weeks later L arm
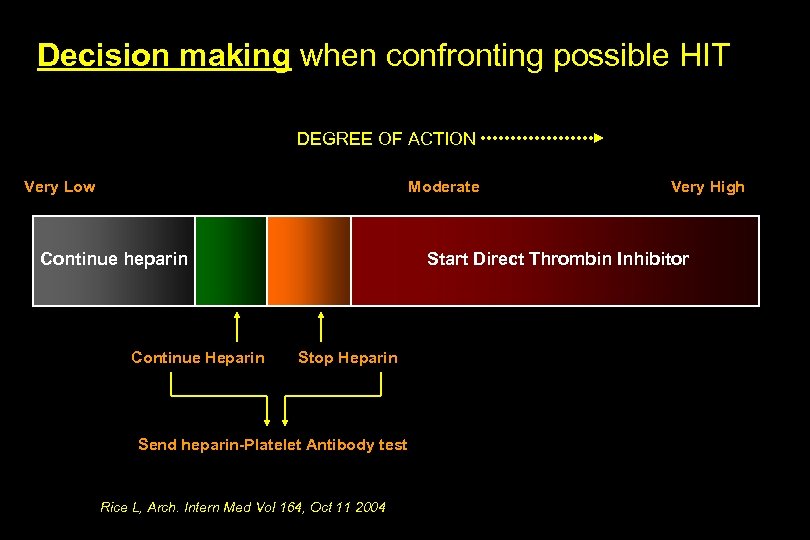
Decision making when confronting possible HIT DEGREE OF ACTION Very Low Moderate Continue heparin Continue Heparin Very High Start Direct Thrombin Inhibitor Stop Heparin Send heparin-Platelet Antibody test Rice L, Arch. Intern Med Vol 164, Oct 11 2004
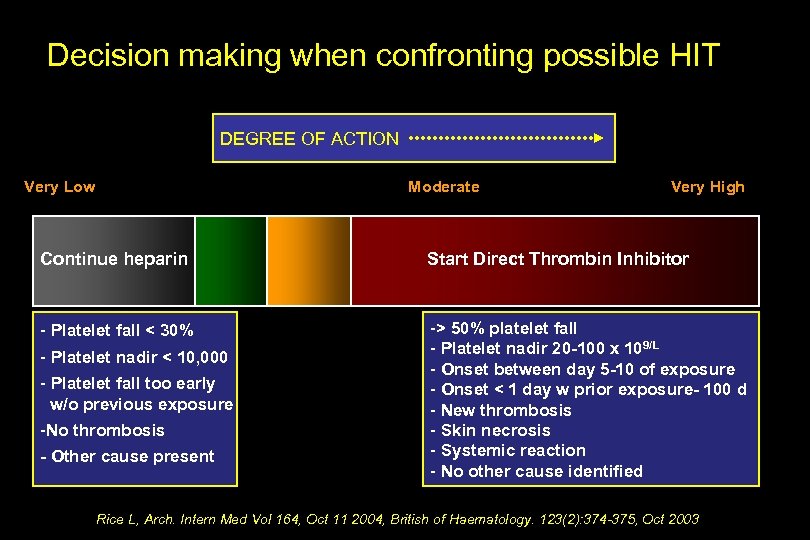
Decision making when confronting possible HIT DEGREE OF ACTION Very Low Moderate Very High Continue heparin Start Direct Thrombin Inhibitor - Platelet fall < 30% -> 50% platelet fall - Platelet nadir 20 -100 x 109/L - Onset between day 5 -10 of exposure - Onset < 1 day w prior exposure- 100 d - New thrombosis - Skin necrosis - Systemic reaction - No other cause identified - Platelet nadir < 10, 000 - Platelet fall too early w/o previous exposure -No thrombosis - Other cause present Rice L, Arch. Intern Med Vol 164, Oct 11 2004, British of Haematology. 123(2): 374 -375, Oct 2003
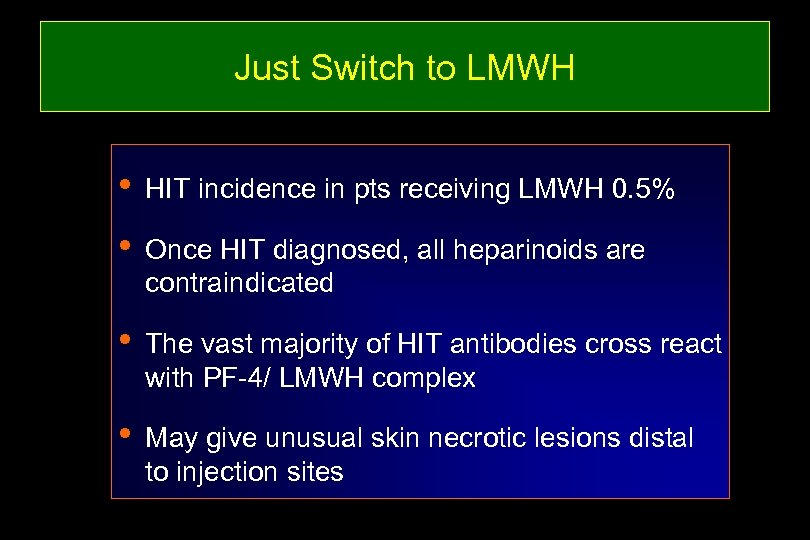
Just Switch to LMWH • HIT incidence in pts receiving LMWH 0. 5% • Once HIT diagnosed, all heparinoids are contraindicated • The vast majority of HIT antibodies cross react with PF-4/ LMWH complex • May give unusual skin necrotic lesions distal to injection sites
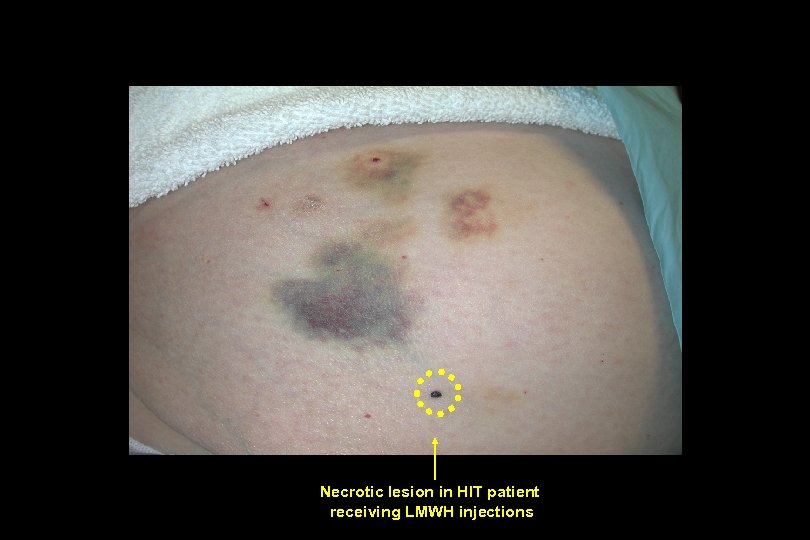
Necrotic lesion in HIT patient receiving LMWH injections
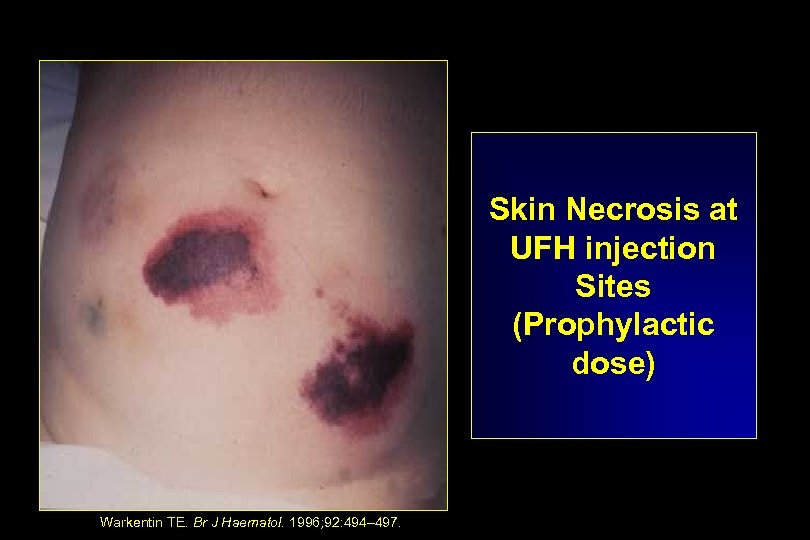
Skin Necrosis at UFH injection Sites (Prophylactic dose) Warkentin TE. Br J Haematol. 1996; 92: 494– 497.
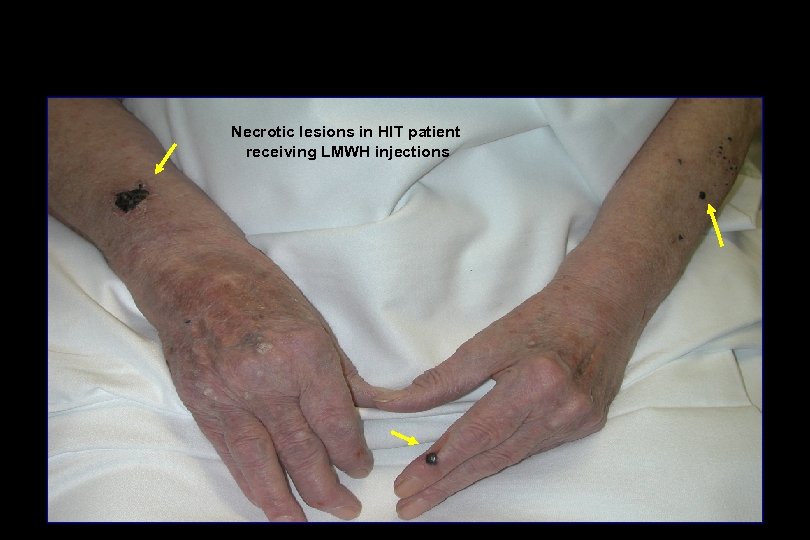
Necrotic lesions in HIT patient receiving LMWH injections
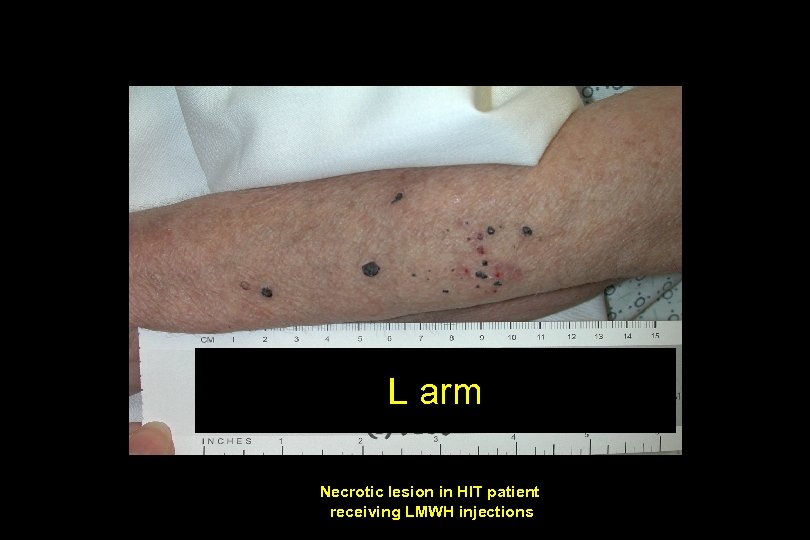
L arm Necrotic lesion in HIT patient receiving LMWH injections

This can’t be HIT because the platelet count is not low, (or too low)
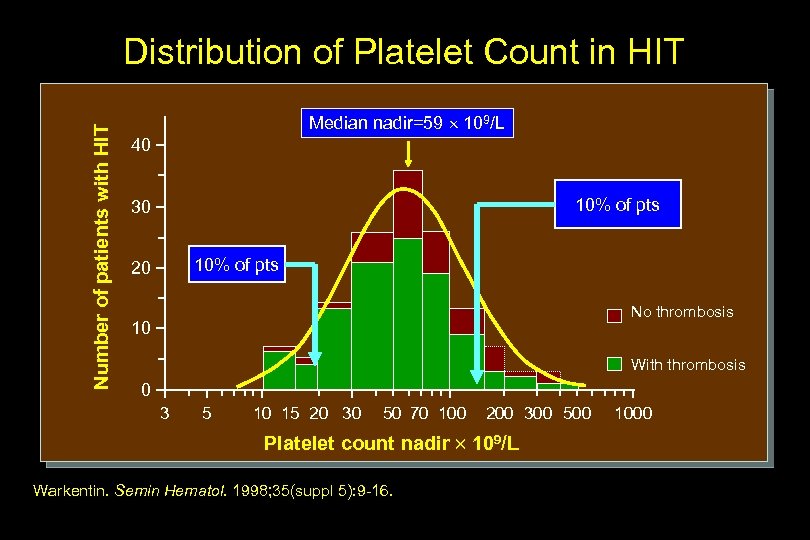
Number of patients with HIT Distribution of Platelet Count in HIT Median nadir=59 109/L 40 10% of pts 30 10% of pts 20 No thrombosis 10 With thrombosis 0 3 5 10 15 20 30 50 70 100 200 300 500 Platelet count nadir 109/L Warkentin. Semin Hematol. 1998; 35(suppl 5): 9 -16. 1000
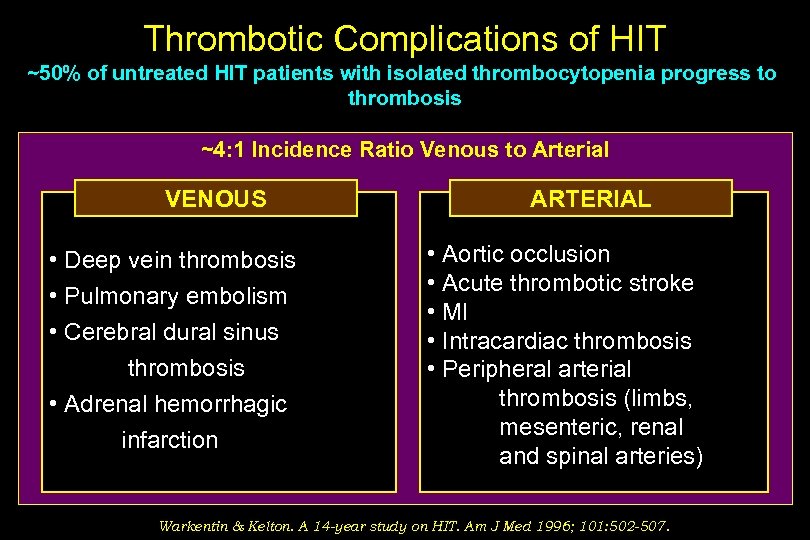
Thrombotic Complications of HIT ~50% of untreated HIT patients with isolated thrombocytopenia progress to thrombosis ~4: 1 Incidence Ratio Venous to Arterial VENOUS • Deep vein thrombosis • Pulmonary embolism • Cerebral dural sinus thrombosis • Adrenal hemorrhagic infarction ARTERIAL • Aortic occlusion • Acute thrombotic stroke • MI • Intracardiac thrombosis • Peripheral arterial thrombosis (limbs, mesenteric, renal and spinal arteries) Warkentin & Kelton. A 14 -year study on HIT. Am J Med 1996; 101: 502 -507.

Well, what about “good ol’ Coumadin”? Let’s get a therapeutic INR, quickly. .

Serologic Tests: A Quick Overview
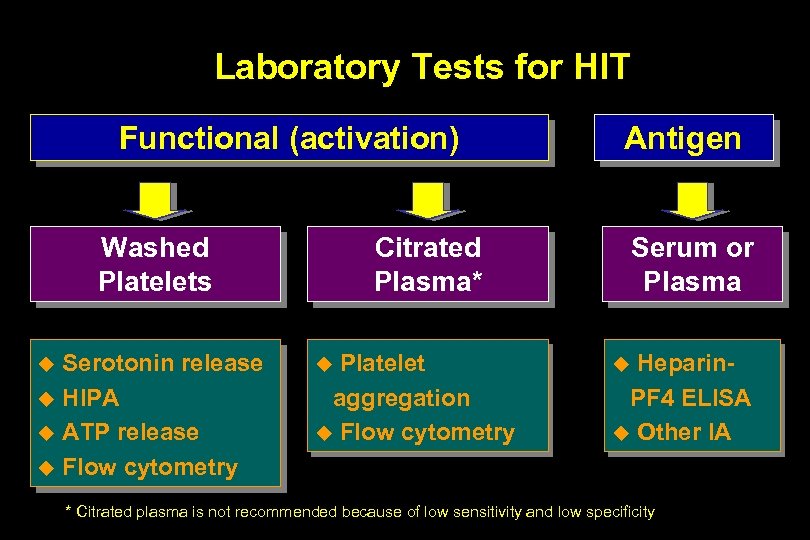
Laboratory Tests for HIT Functional (activation) Washed Platelets Serotonin release u HIPA u ATP release u Flow cytometry u Antigen Citrated Plasma* Platelet aggregation u Flow cytometry u Serum or Plasma Heparin. PF 4 ELISA u Other IA u * Citrated plasma is not recommended because of low sensitivity and low specificity
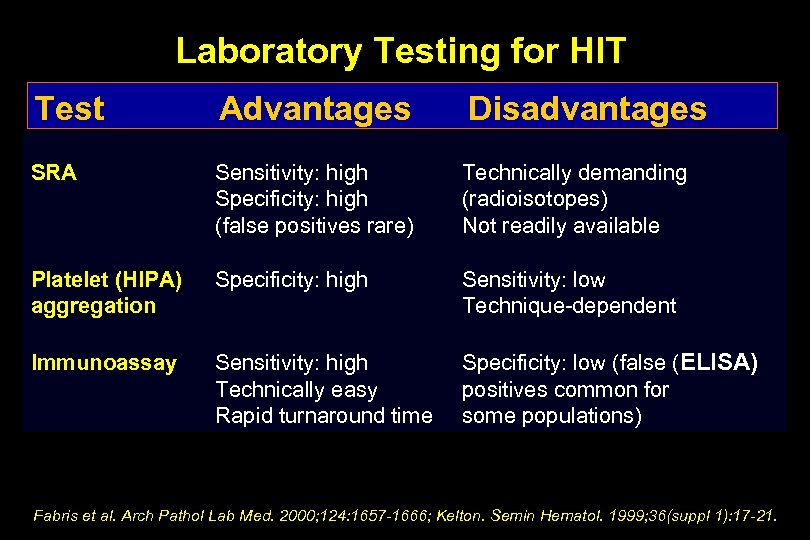
Laboratory Testing for HIT Test Advantages Disadvantages SRA Sensitivity: high Specificity: high (false positives rare) Technically demanding (radioisotopes) Not readily available Platelet (HIPA) aggregation Specificity: high Sensitivity: low Technique-dependent Immunoassay Sensitivity: high Technically easy Rapid turnaround time Specificity: low (false (ELISA) positives common for some populations) Fabris et al. Arch Pathol Lab Med. 2000; 124: 1657 -1666; Kelton. Semin Hematol. 1999; 36(suppl 1): 17 -21.

Now…… Which Serologic Markers are Required to Establish the Diagnosis of HIT?

HIT is a CLINICAL DIAGNOSIS HIT is a CLINICAL DIAGNOSIS HIT is a CLINICAL DIAGNOSIS HIT is a CLINICAL DIAGNOSIS

Serologic Markers and the Diagnosis of HIT • Serologic markers (SRA, HIPA, PF 4) are helpful, BUT: • They must be interpreted within the CLINICAL context, AND: • They are NOT required as part of the diagnostic criteria

Principles of Management of HIT

Treatment of Suspected HIT • Discontinue ALL heparin immediately • Initiate alternative anticoagulation • Monitor carefully for thrombosis • Avoid prophylactic platelet transfusions • Document HIT in medical records • Laboratory evaluation • Monitor platelet counts recovery • Medi-alert bracelets, heparin “allergy” education

Discontinue ALL Heparin Exposure • Heparin gtt, SQ, line flushes • LMWH SQ therapeutic and prophylactic doses • Heparin-coated catheters • Hemodialysis • TPN preparations • Other sources of potential; heparin exposure include: ECMO, CPB, Cardiac cath/endovascular intervention procedures
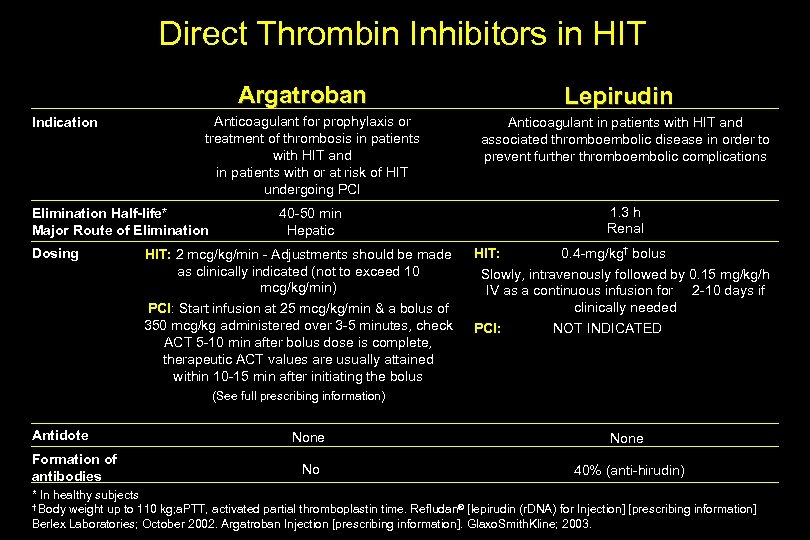
Direct Thrombin Inhibitors in HIT Argatroban Indication Anticoagulant for prophylaxis or treatment of thrombosis in patients with HIT and in patients with or at risk of HIT undergoing PCI Elimination Half-life* Major Route of Elimination Dosing Lepirudin Anticoagulant in patients with HIT and associated thromboembolic disease in order to prevent further thromboembolic complications 1. 3 h Renal 40 -50 min Hepatic HIT: 2 mcg/kg/min - Adjustments should be made as clinically indicated (not to exceed 10 mcg/kg/min) PCI: Start infusion at 25 mcg/kg/min & a bolus of 350 mcg/kg administered over 3 -5 minutes, check ACT 5 -10 min after bolus dose is complete, therapeutic ACT values are usually attained within 10 -15 min after initiating the bolus HIT: 0. 4 -mg/kg† bolus Slowly, intravenously followed by 0. 15 mg/kg/h IV as a continuous infusion for 2 -10 days if clinically needed PCI: NOT INDICATED (See full prescribing information) Antidote Formation of antibodies None No 40% (anti-hirudin) * In healthy subjects † Body weight up to 110 kg; a. PTT, activated partial thromboplastin time. Refludan® [lepirudin (r. DNA) for Injection] [prescribing information] Berlex Laboratories; October 2002. Argatroban Injection [prescribing information]. Glaxo. Smith. Kline; 2003.
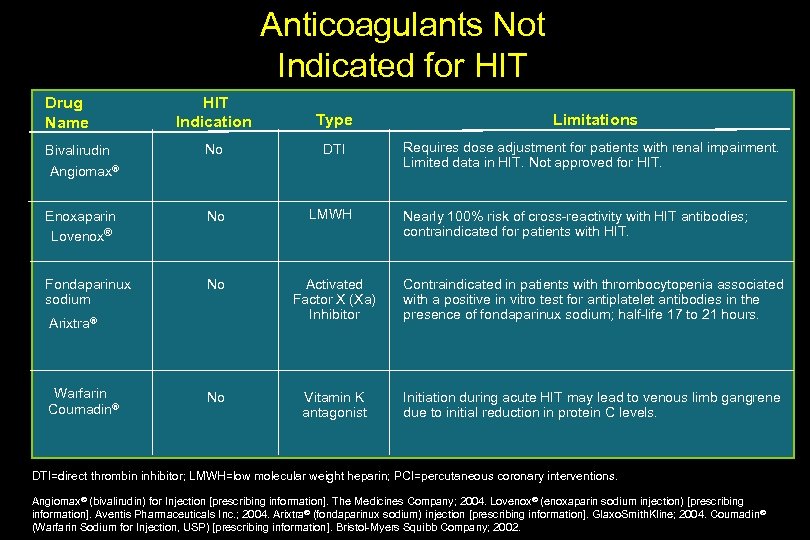
Anticoagulants Not Indicated for HIT Indication Type Limitations No DTI Requires dose adjustment for patients with renal impairment. Limited data in HIT. Not approved for HIT. Enoxaparin Lovenox® No LMWH Fondaparinux sodium No Activated Factor X (Xa) Inhibitor Contraindicated in patients with thrombocytopenia associated with a positive in vitro test for antiplatelet antibodies in the presence of fondaparinux sodium; half-life 17 to 21 hours. No Vitamin K antagonist Initiation during acute HIT may lead to venous limb gangrene due to initial reduction in protein C levels. Drug Name Bivalirudin Angiomax® Arixtra® Warfarin Coumadin® Nearly 100% risk of cross-reactivity with HIT antibodies; contraindicated for patients with HIT. DTI=direct thrombin inhibitor; LMWH=low molecular weight heparin; PCI=percutaneous coronary interventions. Angiomax® (bivalirudin) for Injection [prescribing information]. The Medicines Company; 2004. Lovenox® (enoxaparin sodium injection) [prescribing information]. Aventis Pharmaceuticals Inc. ; 2004. Arixtra® (fondaparinux sodium) injection [prescribing information]. Glaxo. Smith. Kline; 2004. Coumadin® (Warfarin Sodium for Injection, USP) [prescribing information]. Bristol-Myers Squibb Company; 2002.
Heparin as a Cause of Thrombosis “Heparin-Induced Thrombocytopenia” and “The HIT Syndromes” Amjad Al. Mahameed, M. D. Associate Staff Section of Vascular Medicine Department of Cardiovascular Medicine Cleveland Clinic Foundation
Lepirudin 63 60 65 COO– 10 Tyr 3 C 0 14 ys 6 Cys Leu 1 NH 3+ 50 47 L ys Lepirudin (recombinant herudin) is indicated for anticoagulation in patients with HIT and associated thromboembolic disease in order to prevent further thromboembolic complications 2 C 1 C 8 ys 6 ys Cys 2 2 20 39 C ys 40 Val
Argatroban is indicated as an anticoagulant for prophylaxis or treatment of thrombosis in patients with heparin-induced thrombocytopenia
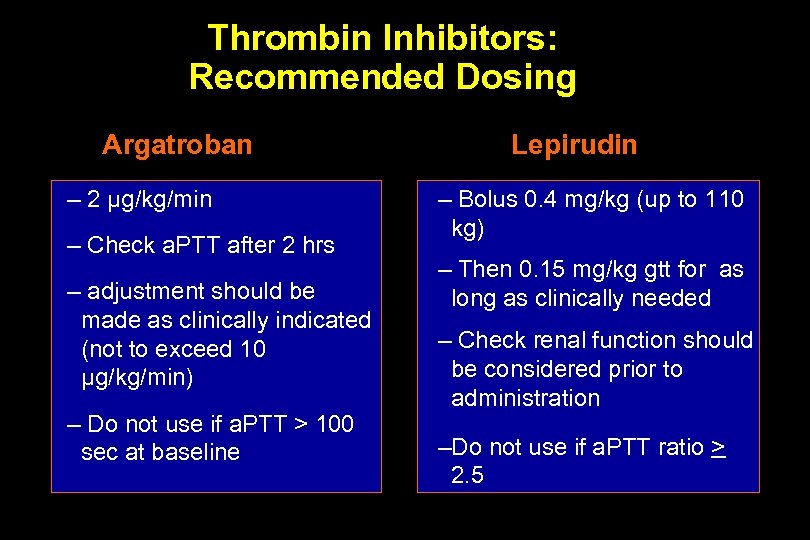
Thrombin Inhibitors: Recommended Dosing Argatroban – 2 µg/kg/min – Check a. PTT after 2 hrs – adjustment should be made as clinically indicated (not to exceed 10 µg/kg/min) – Do not use if a. PTT > 100 sec at baseline Lepirudin – Bolus 0. 4 mg/kg (up to 110 kg) – Then 0. 15 mg/kg gtt for as long as clinically needed – Check renal function should be considered prior to administration –Do not use if a. PTT ratio > 2. 5

Clinical Studies of Argatroban The safety and efficacy of Argatroban as anticoagulant therapy were demonstrated in two (ARG-911 and ARG-915) multicenter, prospective, open-label clinical trials in 568 patients with HIT
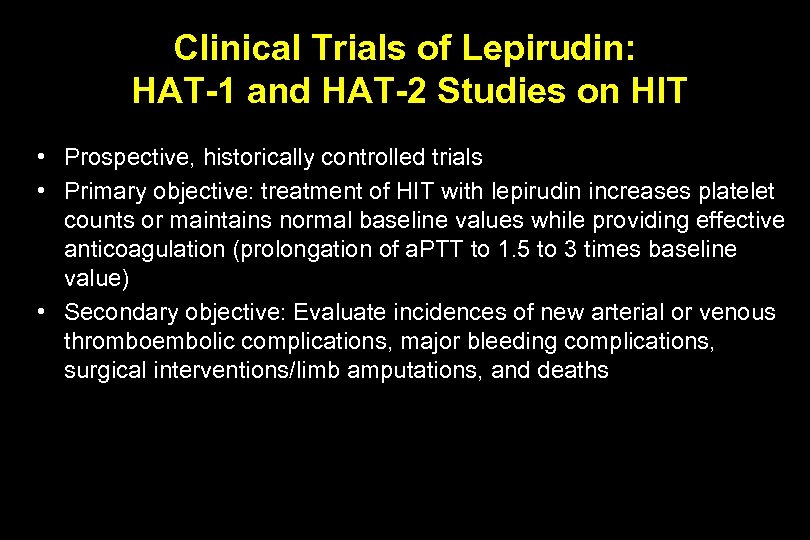
Clinical Trials of Lepirudin: HAT-1 and HAT-2 Studies on HIT • Prospective, historically controlled trials • Primary objective: treatment of HIT with lepirudin increases platelet counts or maintains normal baseline values while providing effective anticoagulation (prolongation of a. PTT to 1. 5 to 3 times baseline value) • Secondary objective: Evaluate incidences of new arterial or venous thromboembolic complications, major bleeding complications, surgical interventions/limb amputations, and deaths
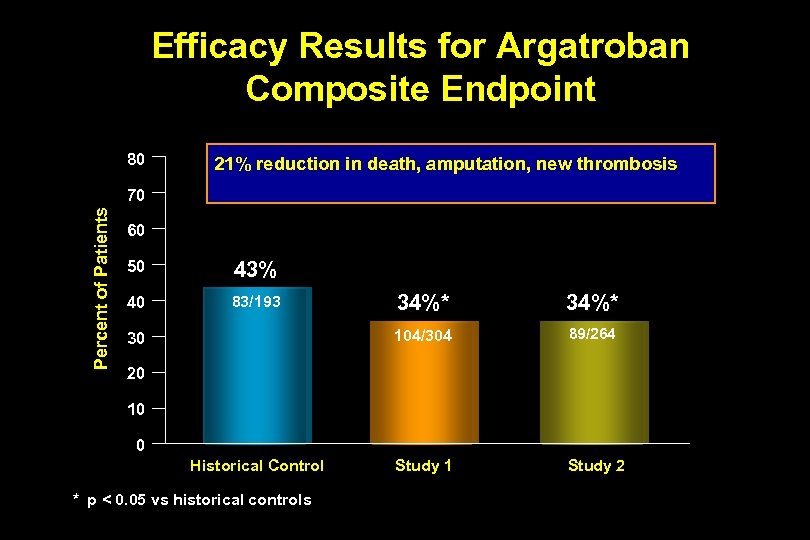
Efficacy Results for Argatroban Composite Endpoint 80 21% reduction in death, amputation, new thrombosis Percent of Patients 70 60 50 43% 40 83/193 34%* 104/304 30 34%* 89/264 Study 1 Study 2 20 10 0 Historical Control * p < 0. 05 vs historical controls
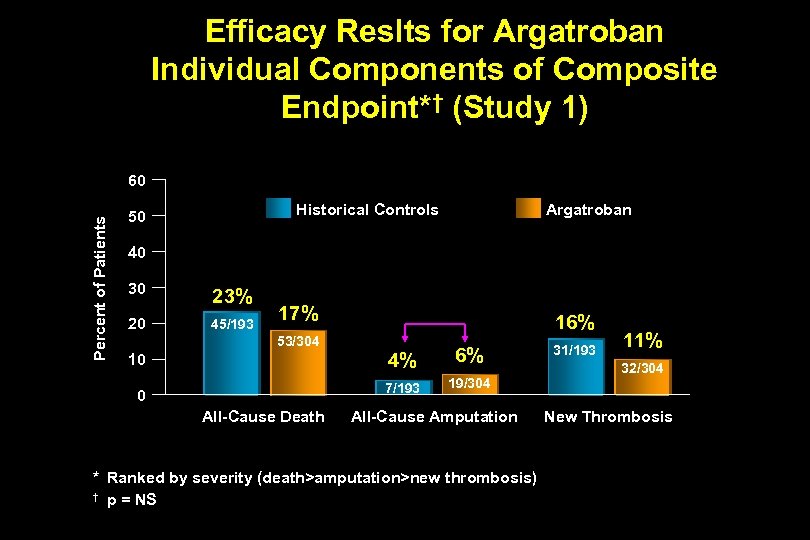
Efficacy Reslts for Argatroban Individual Components of Composite Endpoint*† (Study 1) Percent of Patients 60 Historical Controls 50 Argatroban 40 30 23% 20 45/193 17% 16% 53/304 4% 0 All-Cause Death 6% 7/193 10 19/304 All-Cause Amputation * Ranked by severity (death>amputation>new thrombosis) † p = NS 31/193 11% 32/304 New Thrombosis
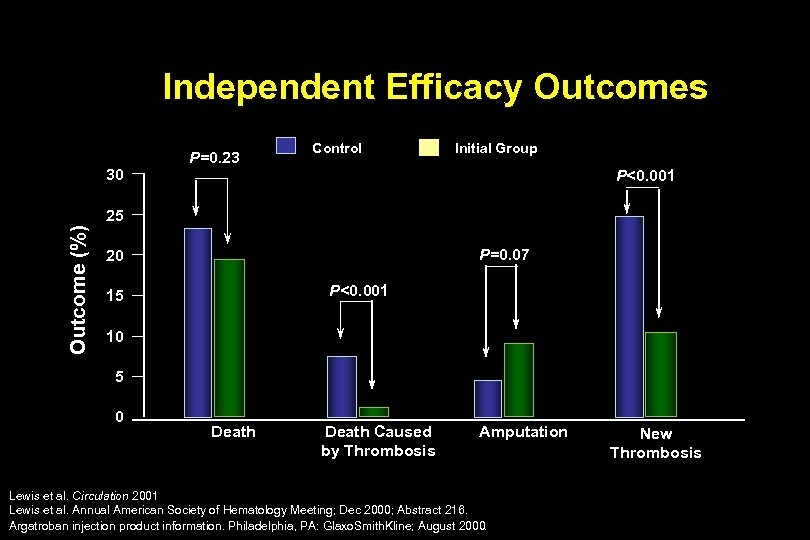
Independent Efficacy Outcomes 30 P=0. 23 Control Initial Group P<0. 001 Outcome (%) 25 P=0. 07 20 P<0. 001 15 10 5 0 Death Caused by Thrombosis Amputation Lewis et al. Circulation 2001 Lewis et al. Annual American Society of Hematology Meeting; Dec 2000; Abstract 216. Argatroban injection product information. Philadelphia, PA: Glaxo. Smith. Kline; August 2000. New Thrombosis
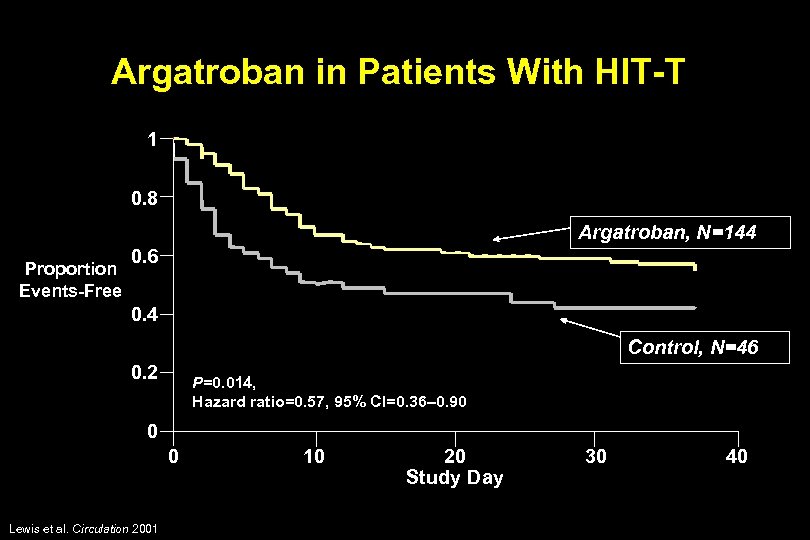
Argatroban in Patients With HIT-T 1 0. 8 Argatroban, N=144 Proportion Events-Free 0. 6 0. 4 Control, N=46 0. 2 P=0. 014, Hazard ratio=0. 57, 95% CI=0. 36– 0. 90 0 0 Lewis et al. Circulation 2001 10 20 Study Day 30 40
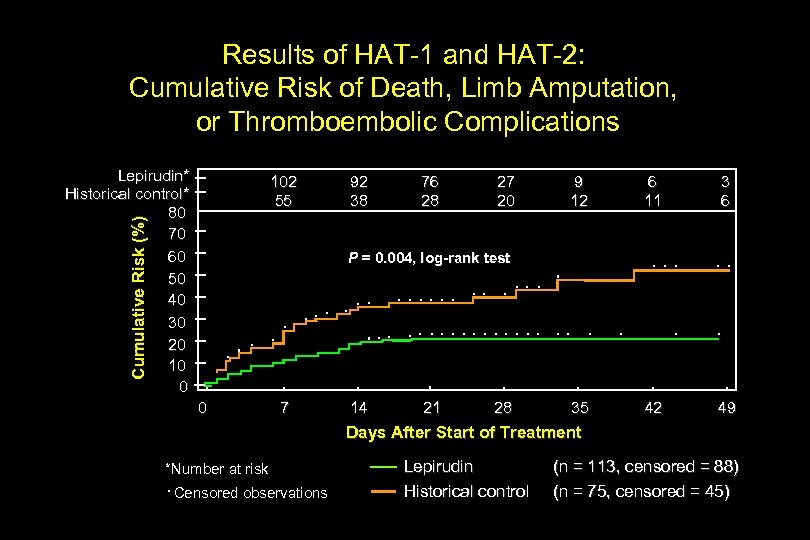
Results of HAT-1 and HAT-2: Cumulative Risk of Death, Limb Amputation, or Thromboembolic Complications Lepirudin* Historical control* 80 70 60 50 40 30 20 10 0 Cumulative Risk (%) 102 55 92 38 76 28 27 20 9 12 6 11 3 6 35 42 49 P = 0. 004, log-rank test 0 7 14 21 28 Days After Start of Treatment *Number at risk Censored observations Lepirudin Historical control (n = 113, censored = 88) (n = 75, censored = 45)
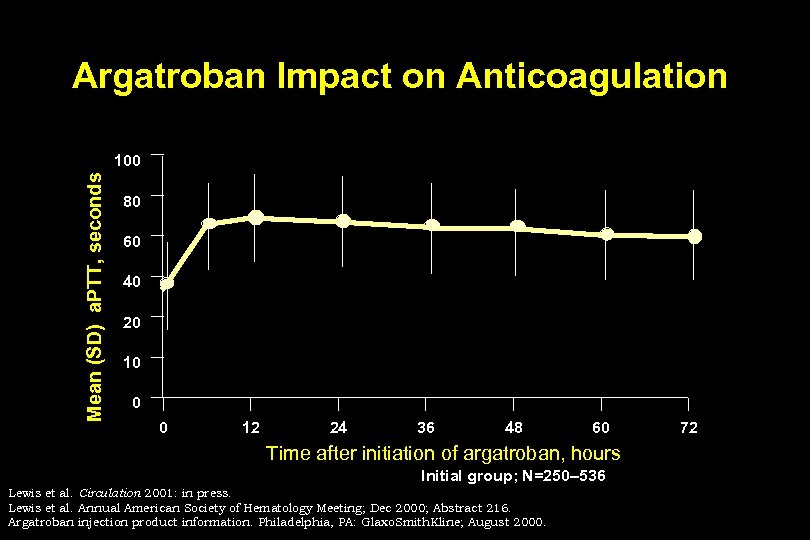
Argatroban Impact on Anticoagulation Mean (SD) a. PTT, seconds 100 80 60 40 20 10 0 0 12 24 36 48 60 Time after initiation of argatroban, hours Initial group; N=250– 536 Lewis et al. Circulation 2001: in press. Lewis et al. Annual American Society of Hematology Meeting; Dec 2000; Abstract 216. Argatroban injection product information. Philadelphia, PA: Glaxo. Smith. Kline; August 2000. 72
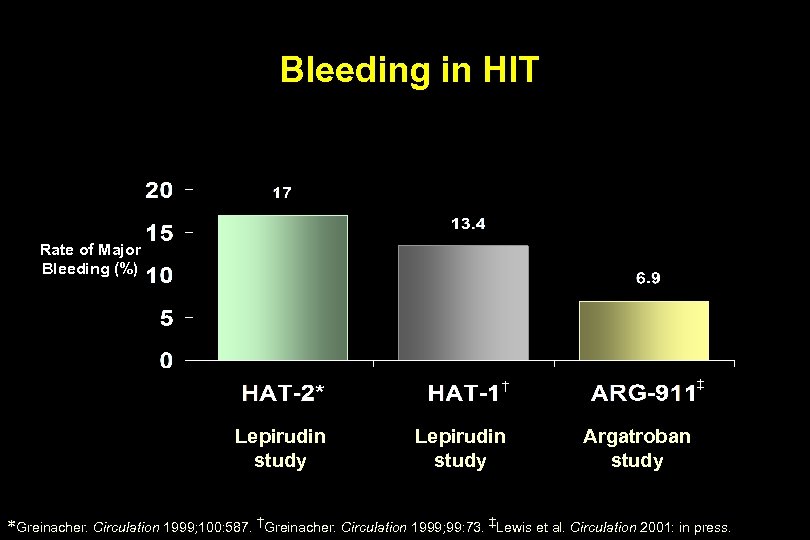
Bleeding in HIT Rate of Major Bleeding (%) ‡ † Lepirudin study Argatroban study *Greinacher. Circulation 1999; 100: 587. †Greinacher. Circulation 1999; 99: 73. ‡Lewis et al. Circulation 2001: in press.
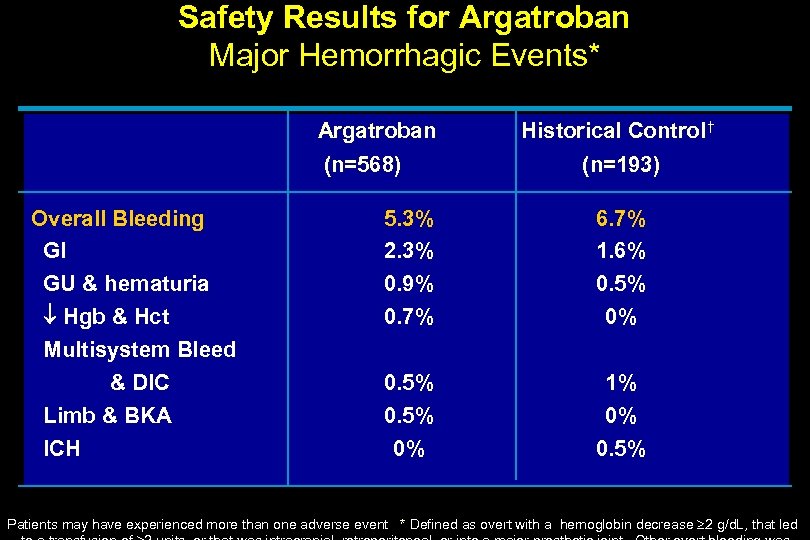
Safety Results for Argatroban Major Hemorrhagic Events* Argatroban (n=568) Overall Bleeding GI GU & hematuria Hgb & Hct Multisystem Bleed & DIC Limb & BKA ICH Historical Control † (n=193) 5. 3% 2. 3% 0. 9% 0. 7% 6. 7% 1. 6% 0. 5% 0% 1% 0% 0. 5% Patients may have experienced more than one adverse event * Defined as overt with a hemoglobin decrease 2 g/d. L, that led
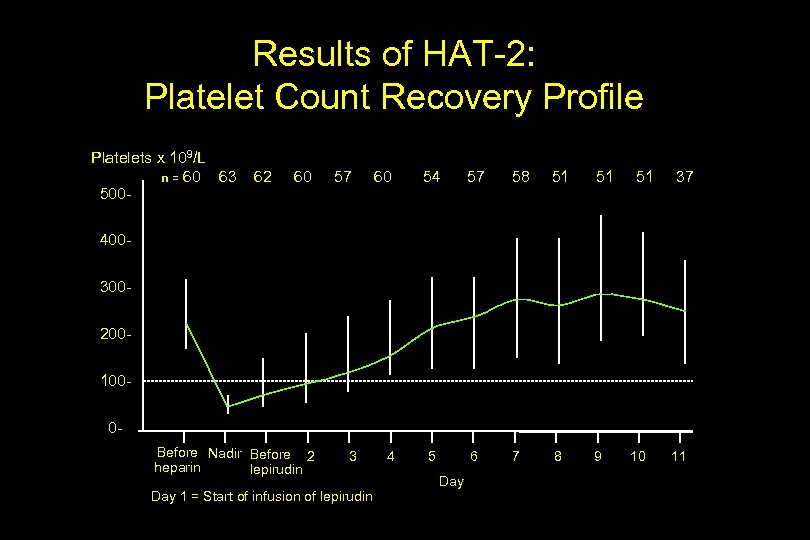
Results of HAT-2: Platelet Count Recovery Profile Platelets x 109/L n = 60 63 500 - 62 60 57 60 54 57 58 51 51 5 6 7 8 9 51 37 4003002001000 Before Nadir Before 2 heparin lepirudin 3 Day 1 = Start of infusion of lepirudin 4 Day 10 11
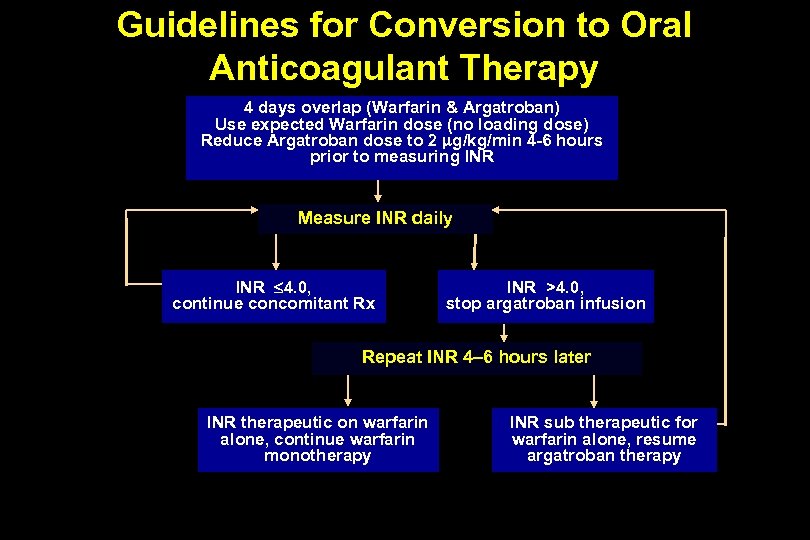
Guidelines for Conversion to Oral Anticoagulant Therapy 4 days overlap (Warfarin & Argatroban) Use expected Warfarin dose (no loading dose) Reduce Argatroban dose to 2 g/kg/min 4 -6 hours prior to measuring INR Measure INR daily INR 4. 0, continue concomitant Rx INR >4. 0, stop argatroban infusion Repeat INR 4– 6 hours later INR therapeutic on warfarin alone, continue warfarin monotherapy INR sub therapeutic for warfarin alone, resume argatroban therapy
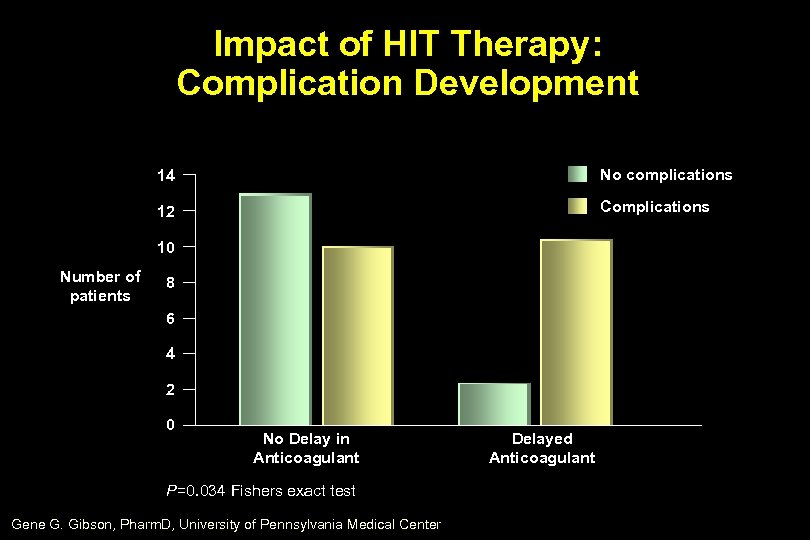
Impact of HIT Therapy: Complication Development 14 No complications 12 Complications 10 Number of patients 8 6 4 2 0 No Delay in Anticoagulant P=0. 034 Fishers exact test Gene G. Gibson, Pharm. D, University of Pennsylvania Medical Center Delayed Anticoagulant
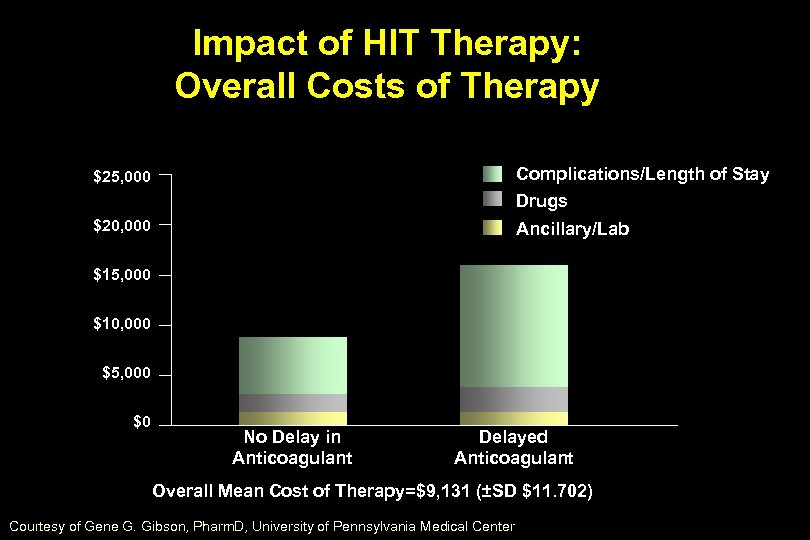
Impact of HIT Therapy: Overall Costs of Therapy $25, 000 Complications/Length of Stay Drugs $20, 000 Ancillary/Lab $15, 000 $10, 000 $5, 000 $0 No Delay in Anticoagulant Delayed Anticoagulant Overall Mean Cost of Therapy=$9, 131 (±SD $11. 702) Courtesy of Gene G. Gibson, Pharm. D, University of Pennsylvania Medical Center
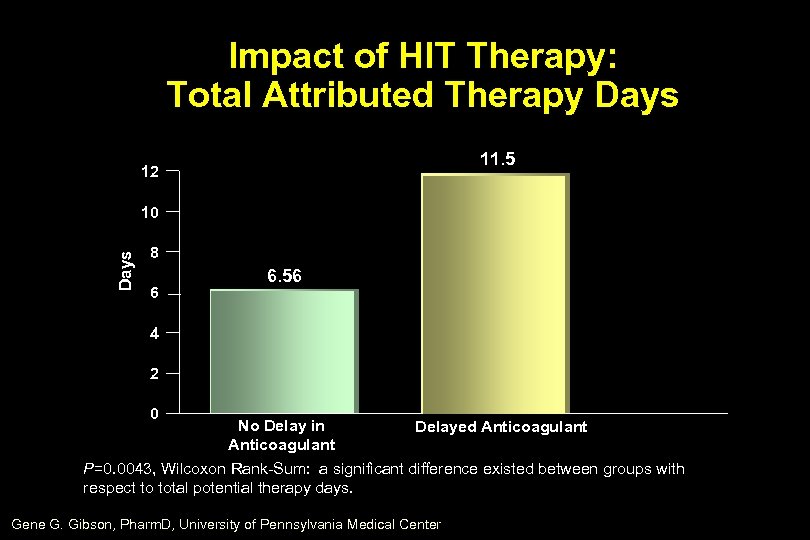
Impact of HIT Therapy: Total Attributed Therapy Days 11. 5 12 Days 10 8 6 6. 56 4 2 0 No Delay in Delayed Anticoagulant P=0. 0043, Wilcoxon Rank-Sum: a significant difference existed between groups with respect to total potential therapy days. Gene G. Gibson, Pharm. D, University of Pennsylvania Medical Center

Conclusions • Heparin, although an important anticoagulant, has several drawbacks, most notably its ability to cause HIT • HIT can lead to severe and even life-threatening thromboembolic disorders • Treatment of HIT should be initiated before laboratory confirmation • A new generation of drugs such as the thrombin inhibitors, including the hirudins, may provide important new options for the treatment and possible prevention of HIT
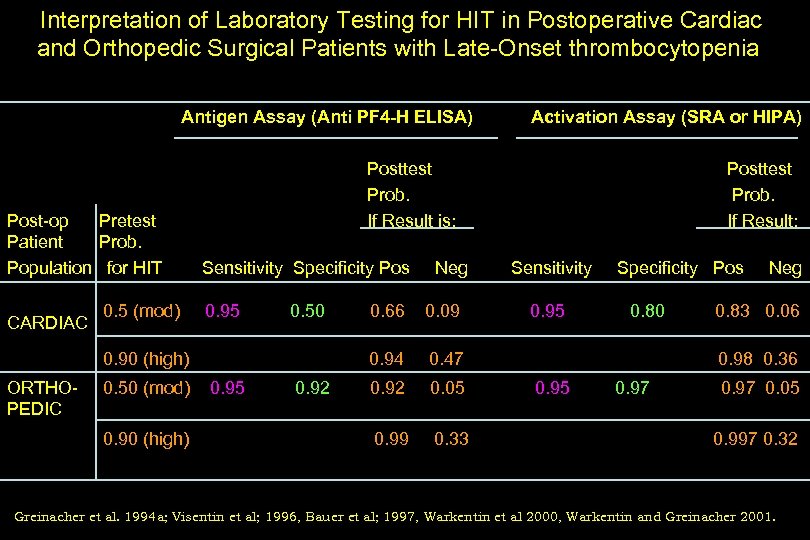
Interpretation of Laboratory Testing for HIT in Postoperative Cardiac and Orthopedic Surgical Patients with Late-Onset thrombocytopenia Antigen Assay (Anti PF 4 -H ELISA) Post-op Pretest Patient Prob. Population for HIT CARDIAC 0. 5 (mod) Posttest Prob. If Result is: Sensitivity Specificity Pos 0. 95 0. 50 (mod) 0. 90 (high) Neg 0. 95 0. 92 0. 66 0. 09 0. 94 0. 90 (high) ORTHOPEDIC Activation Assay (SRA or HIPA) 0. 05 0. 99 0. 33 Sensitivity 0. 95 Specificity Pos 0. 47 0. 92 Posttest Prob. If Result: 0. 80 Neg 0. 83 0. 06 0. 98 0. 36 0. 95 0. 97 0. 05 0. 997 0. 32 Greinacher et al. 1994 a; Visentin et al; 1996, Bauer et al; 1997, Warkentin et al 2000, Warkentin and Greinacher 2001.
99f7ba149c1d0c300eb6656152e279b0.ppt