7924e072aa0e61fc798e004e54e0bc66.ppt
- Количество слайдов: 62
 Hemostasis and TEG® Technology www. haemoscope. com Copyright © 2001 -2003 Haemoscope Corporation 1
Hemostasis and TEG® Technology www. haemoscope. com Copyright © 2001 -2003 Haemoscope Corporation 1
 Hemostasis Issues Facing Clinicians • Before surgery – Is there a coagulopathy present and how should it be treated – Prophylactic treatment / Autologous platelet plasmapheresis • During surgery – What coagulopathy is developing • After surgery – If the patient is bleeding, is it due to • Surgical • Excess of heparin • Coagulopathy and how it should be treated 2 Ü
Hemostasis Issues Facing Clinicians • Before surgery – Is there a coagulopathy present and how should it be treated – Prophylactic treatment / Autologous platelet plasmapheresis • During surgery – What coagulopathy is developing • After surgery – If the patient is bleeding, is it due to • Surgical • Excess of heparin • Coagulopathy and how it should be treated 2 Ü
 Normal Hemostasis… … is controlled activation of clot formation and clot lysis that stops hemorrhage without permitting inappropriate clotting (thrombosis). Ü 3 Laposata et al. University of Pennsylvania Medical School/Mass. General Hospital
Normal Hemostasis… … is controlled activation of clot formation and clot lysis that stops hemorrhage without permitting inappropriate clotting (thrombosis). Ü 3 Laposata et al. University of Pennsylvania Medical School/Mass. General Hospital
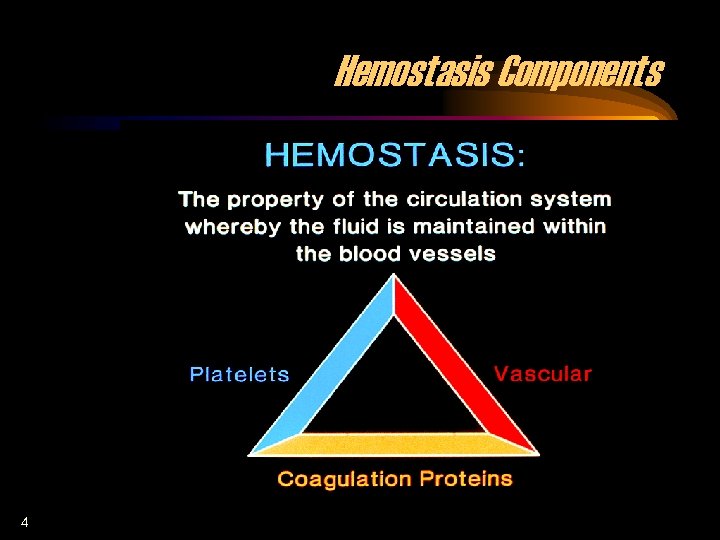 Hemostasis Components 4
Hemostasis Components 4
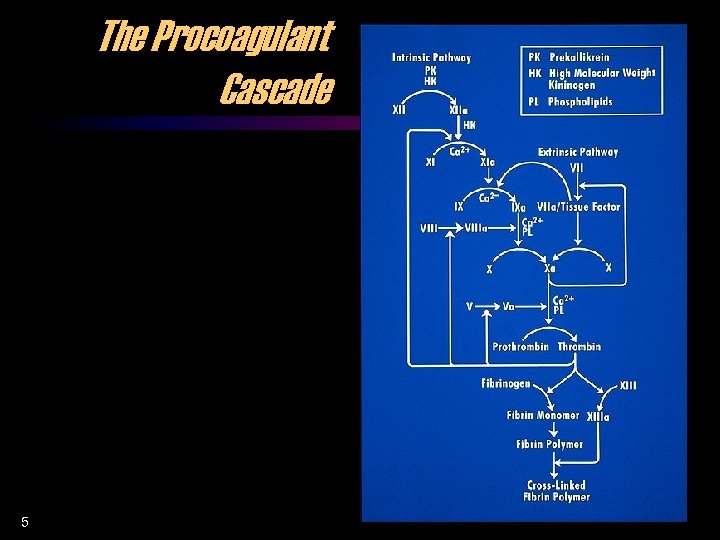 The Procoagulant Cascade 5
The Procoagulant Cascade 5
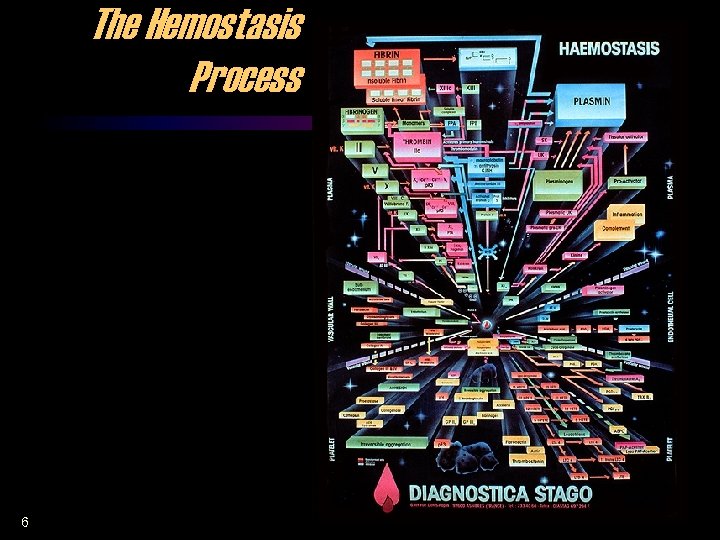 The Hemostasis Process 6
The Hemostasis Process 6
 The Clot 7 • The only end result of the activated coagulation protein is the fibrin strand which, together with activated platelets, forms fibrin-platelet bonding to produce the final clot. • The strength and stability of the clot, that is its physical properties, determine its ability to do the work of hemostasis, which is to mechanically impede hemorrhage. • The clot is in essence a damage control device, a temporary stopper, which gradually dissolves during vascular recovery. Ü
The Clot 7 • The only end result of the activated coagulation protein is the fibrin strand which, together with activated platelets, forms fibrin-platelet bonding to produce the final clot. • The strength and stability of the clot, that is its physical properties, determine its ability to do the work of hemostasis, which is to mechanically impede hemorrhage. • The clot is in essence a damage control device, a temporary stopper, which gradually dissolves during vascular recovery. Ü
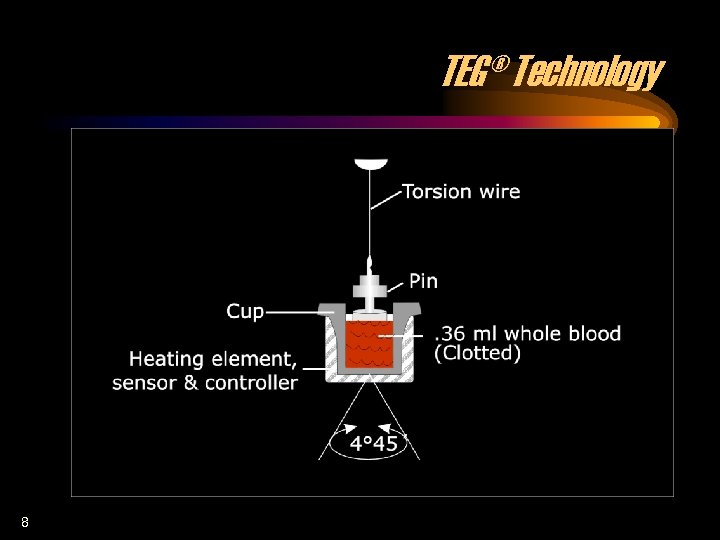 TEG® Technology 8
TEG® Technology 8
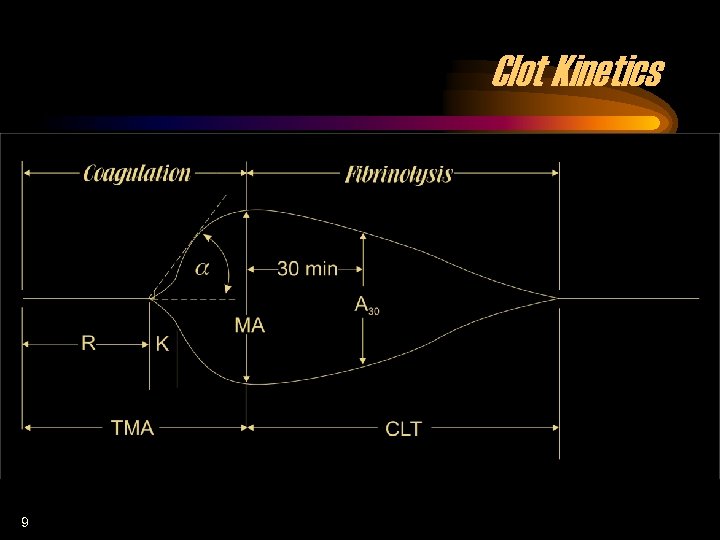 Clot Kinetics 9
Clot Kinetics 9
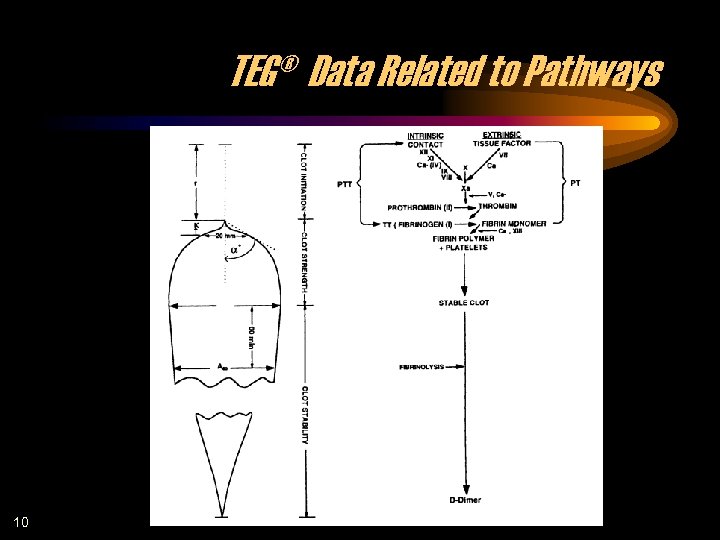 TEG® Data Related to Pathways 10
TEG® Data Related to Pathways 10
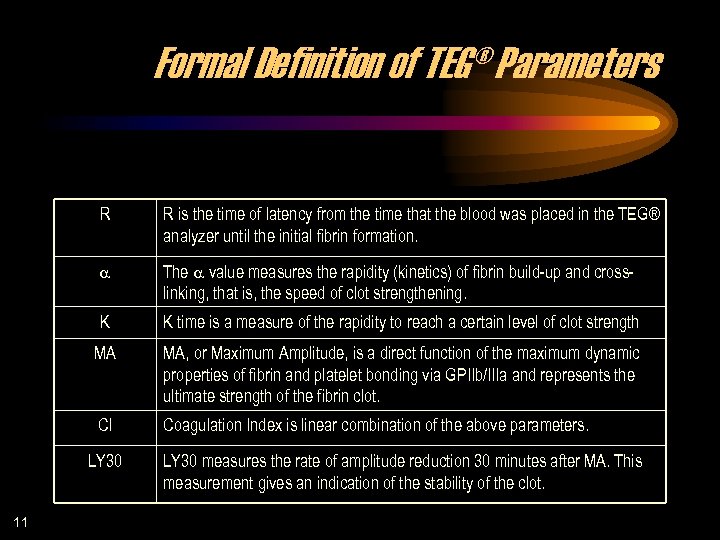 Formal Definition of TEG® Parameters R R is the time of latency from the time that the blood was placed in the TEG® analyzer until the initial fibrin formation. a The a value measures the rapidity (kinetics) of fibrin build-up and crosslinking, that is, the speed of clot strengthening. K K time is a measure of the rapidity to reach a certain level of clot strength MA MA, or Maximum Amplitude, is a direct function of the maximum dynamic properties of fibrin and platelet bonding via GPIIb/IIIa and represents the ultimate strength of the fibrin clot. CI Coagulation Index is linear combination of the above parameters. LY 30 11 LY 30 measures the rate of amplitude reduction 30 minutes after MA. This measurement gives an indication of the stability of the clot.
Formal Definition of TEG® Parameters R R is the time of latency from the time that the blood was placed in the TEG® analyzer until the initial fibrin formation. a The a value measures the rapidity (kinetics) of fibrin build-up and crosslinking, that is, the speed of clot strengthening. K K time is a measure of the rapidity to reach a certain level of clot strength MA MA, or Maximum Amplitude, is a direct function of the maximum dynamic properties of fibrin and platelet bonding via GPIIb/IIIa and represents the ultimate strength of the fibrin clot. CI Coagulation Index is linear combination of the above parameters. LY 30 11 LY 30 measures the rate of amplitude reduction 30 minutes after MA. This measurement gives an indication of the stability of the clot.
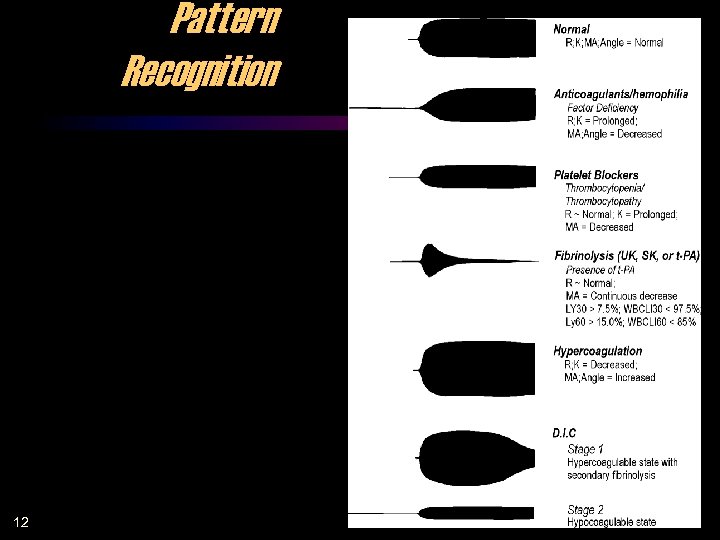 Pattern Recognition 12
Pattern Recognition 12
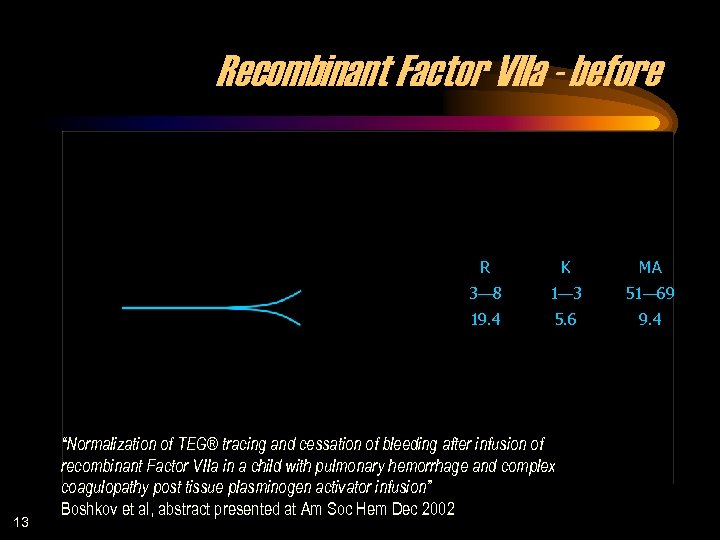 Recombinant Factor VIIa - before R MA 3— 8 1— 3 51— 69 19. 4 13 K 5. 6 9. 4 “Normalization of TEG® tracing and cessation of bleeding after infusion of recombinant Factor VIIa in a child with pulmonary hemorrhage and complex coagulopathy post tissue plasminogen activator infusion” Boshkov et al, abstract presented at Am Soc Hem Dec 2002
Recombinant Factor VIIa - before R MA 3— 8 1— 3 51— 69 19. 4 13 K 5. 6 9. 4 “Normalization of TEG® tracing and cessation of bleeding after infusion of recombinant Factor VIIa in a child with pulmonary hemorrhage and complex coagulopathy post tissue plasminogen activator infusion” Boshkov et al, abstract presented at Am Soc Hem Dec 2002
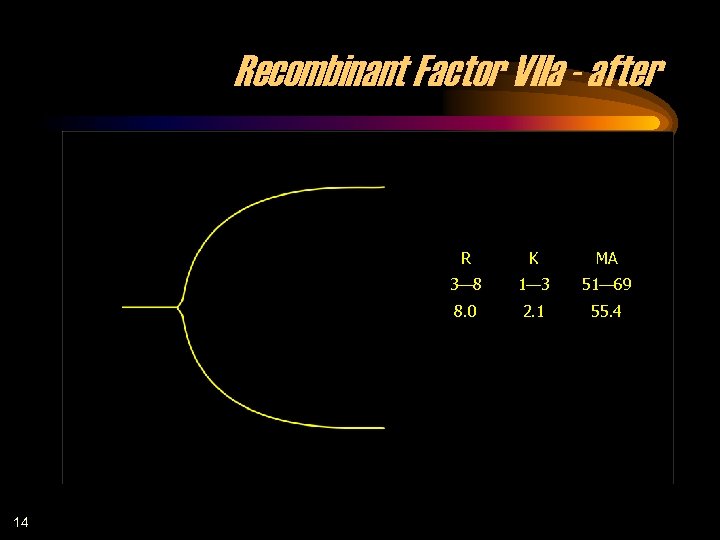 Recombinant Factor VIIa - after R MA 3— 8 1— 3 51— 69 8. 0 14 K 2. 1 55. 4
Recombinant Factor VIIa - after R MA 3— 8 1— 3 51— 69 8. 0 14 K 2. 1 55. 4
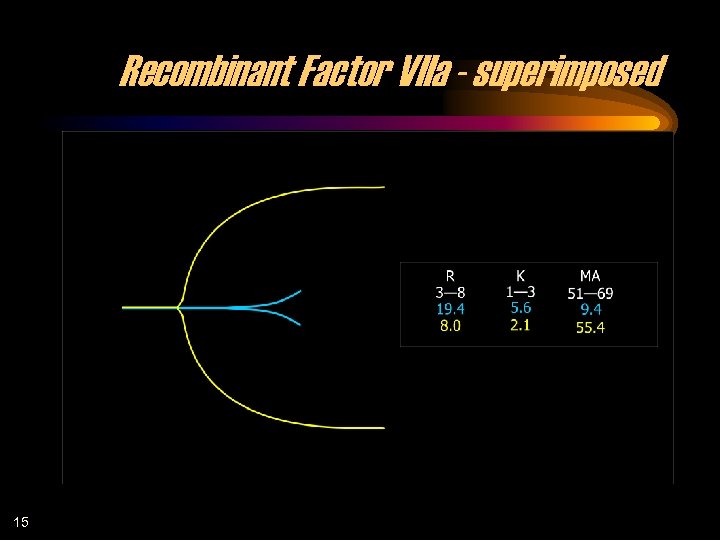 Recombinant Factor VIIa - superimposed 15
Recombinant Factor VIIa - superimposed 15
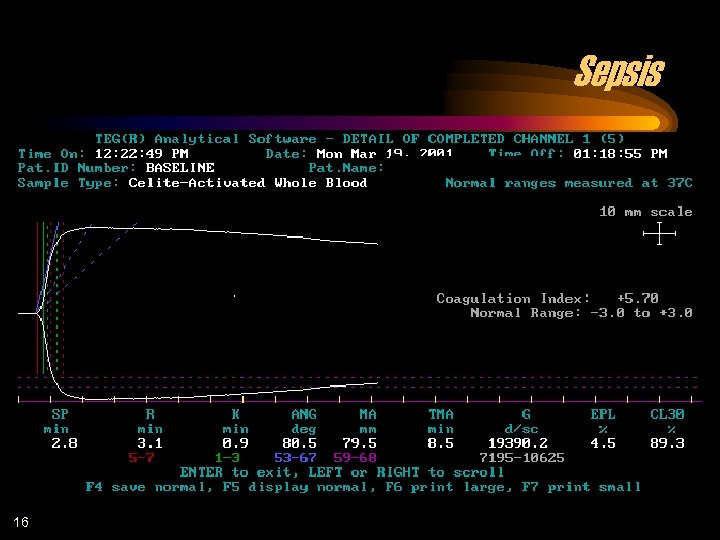 Sepsis 16
Sepsis 16
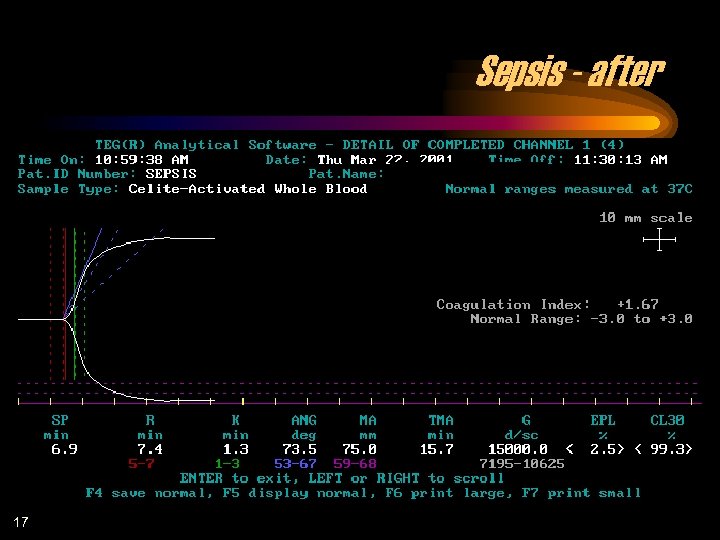 Sepsis - after 17
Sepsis - after 17
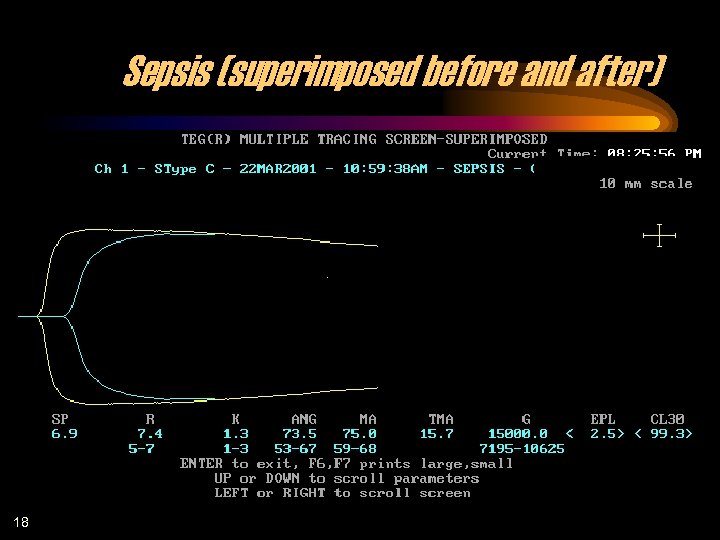 Sepsis (superimposed before and after) 18
Sepsis (superimposed before and after) 18
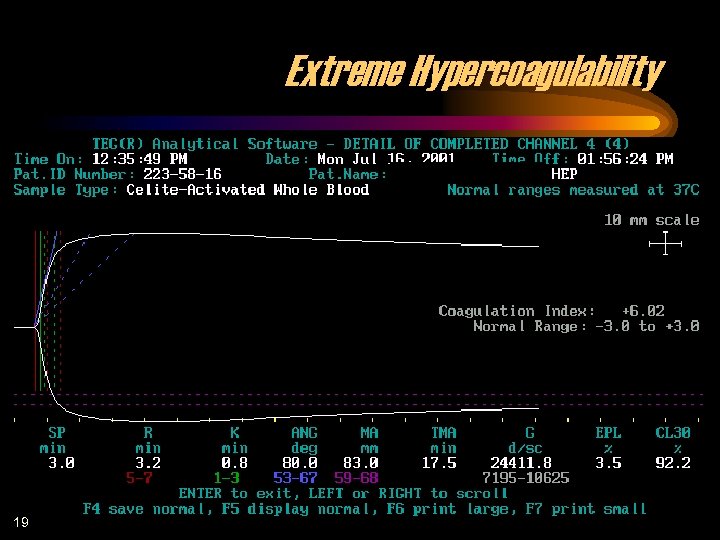 Extreme Hypercoagulability 19
Extreme Hypercoagulability 19
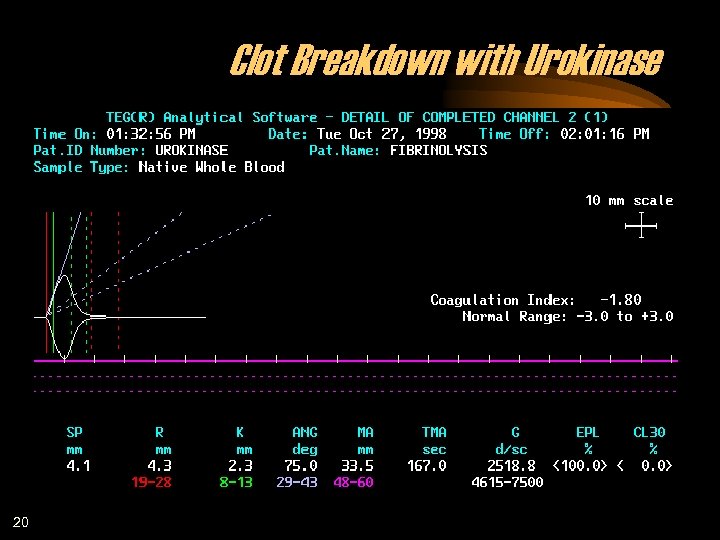 Clot Breakdown with Urokinase 20
Clot Breakdown with Urokinase 20
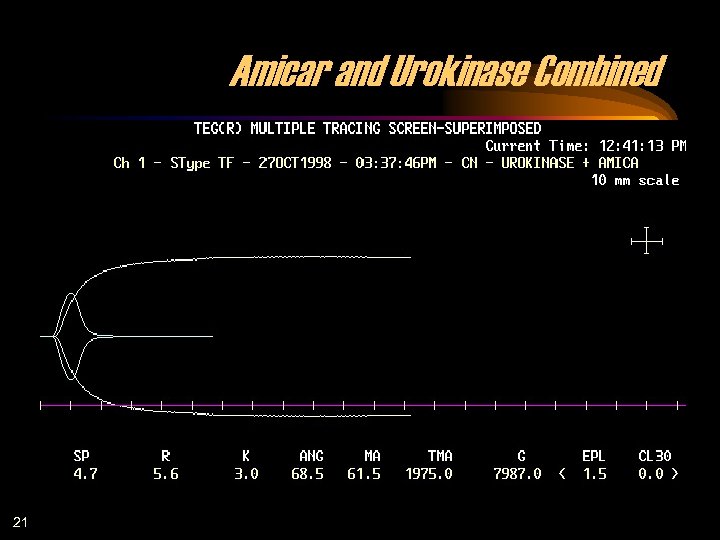 Amicar and Urokinase Combined 21
Amicar and Urokinase Combined 21
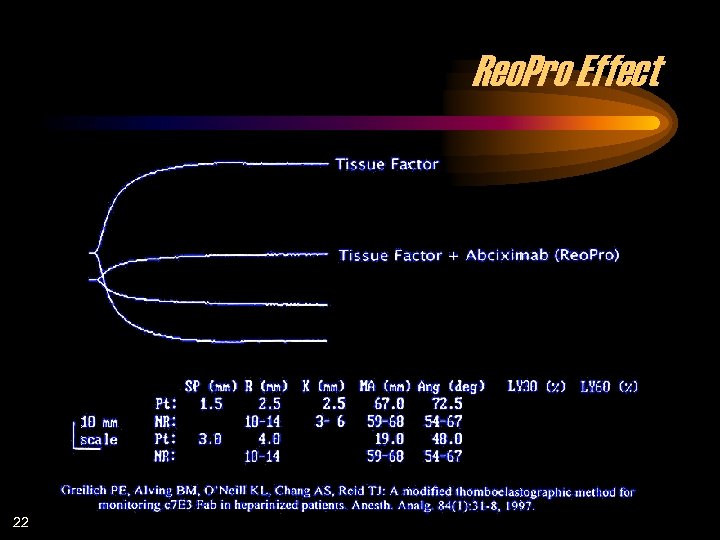 Reo. Pro Effect 22
Reo. Pro Effect 22
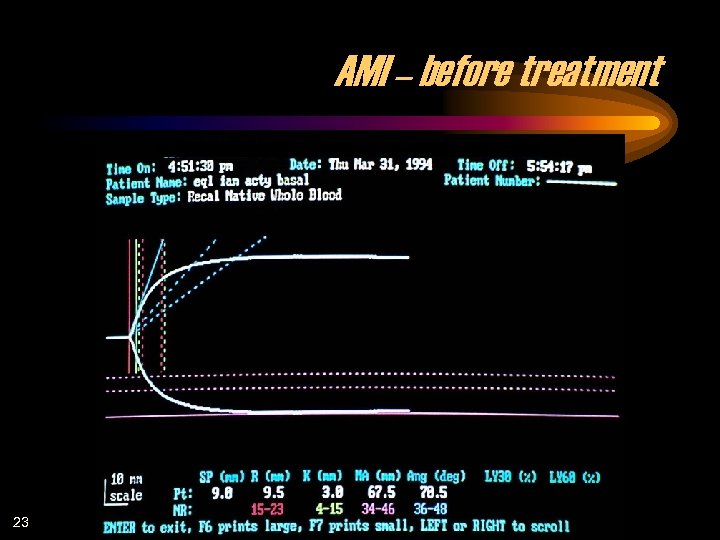 AMI – before treatment 23
AMI – before treatment 23
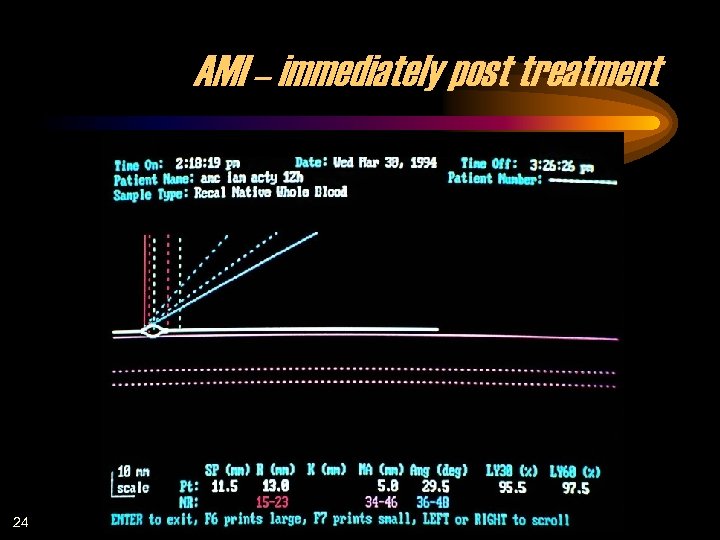 AMI – immediately post treatment 24
AMI – immediately post treatment 24
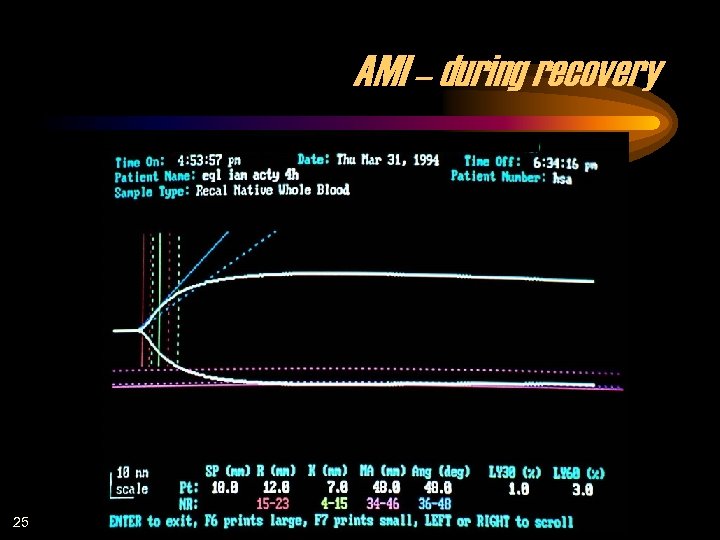 AMI – during recovery 25
AMI – during recovery 25
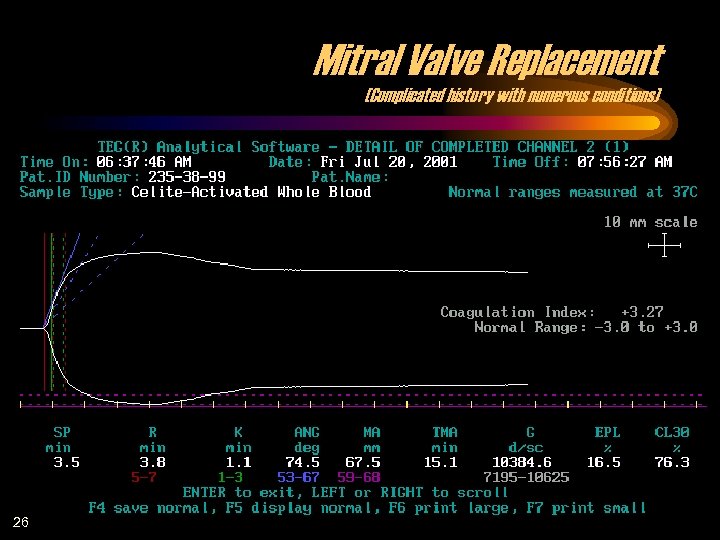 Mitral Valve Replacement (Complicated history with numerous conditions) 26
Mitral Valve Replacement (Complicated history with numerous conditions) 26
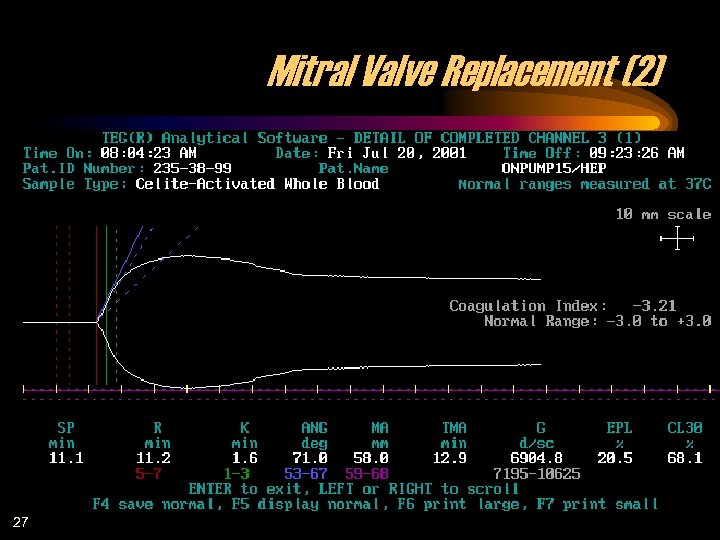 Mitral Valve Replacement (2) 27
Mitral Valve Replacement (2) 27
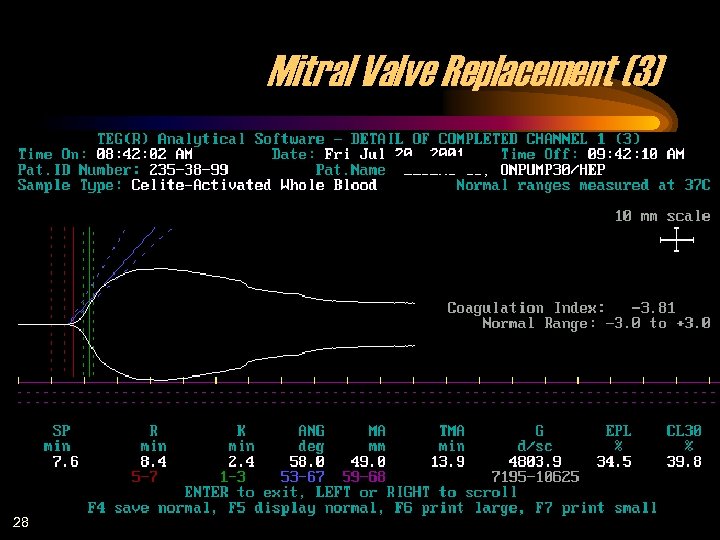 Mitral Valve Replacement (3) 28
Mitral Valve Replacement (3) 28
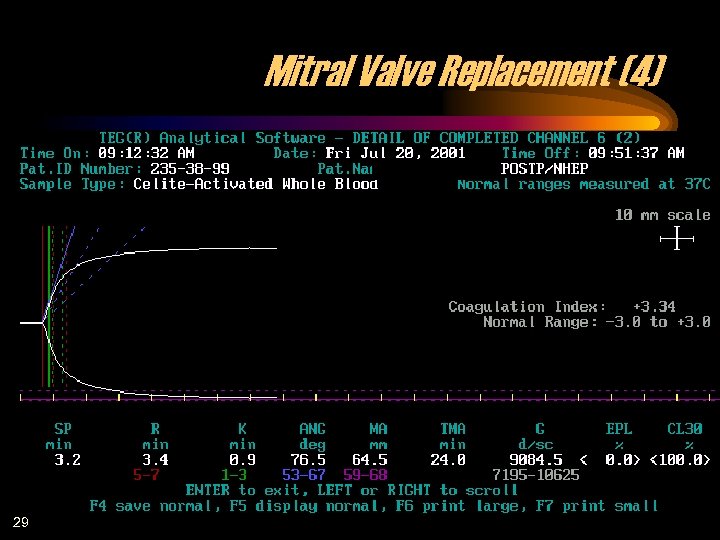 Mitral Valve Replacement (4) 29
Mitral Valve Replacement (4) 29
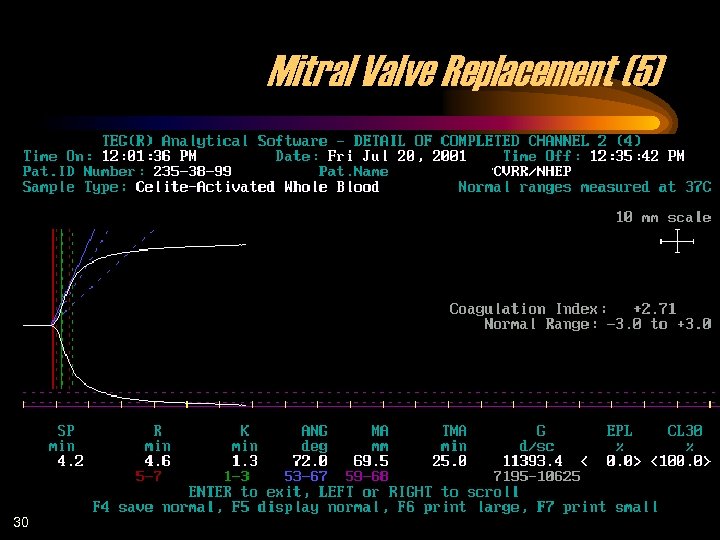 Mitral Valve Replacement (5) 30
Mitral Valve Replacement (5) 30
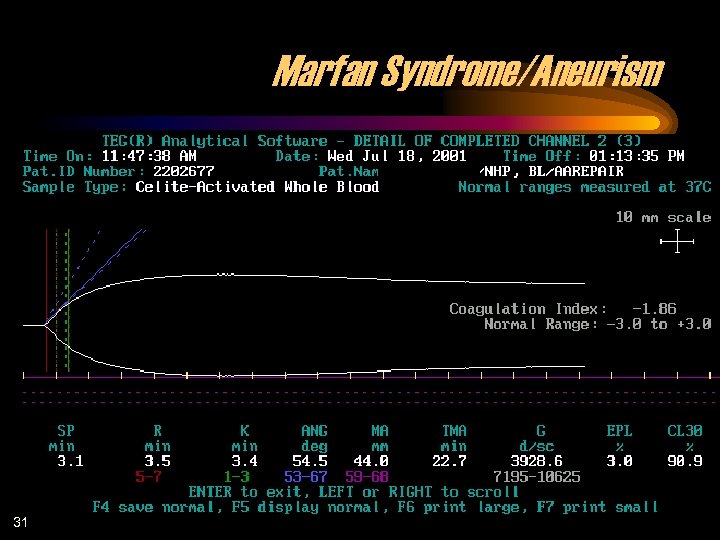 Marfan Syndrome/Aneurism 31
Marfan Syndrome/Aneurism 31
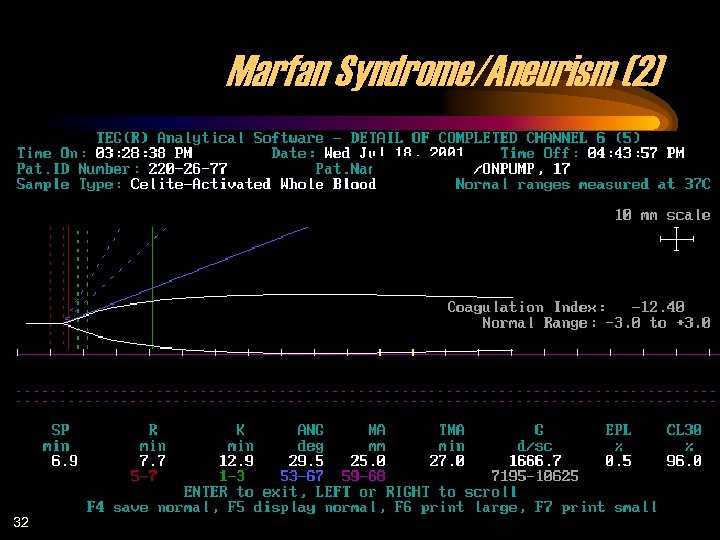 Marfan Syndrome/Aneurism (2) 32
Marfan Syndrome/Aneurism (2) 32
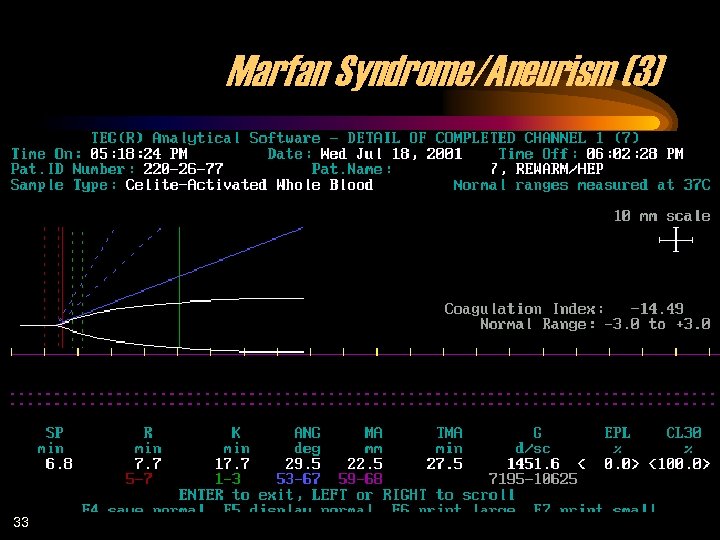 Marfan Syndrome/Aneurism (3) 33
Marfan Syndrome/Aneurism (3) 33
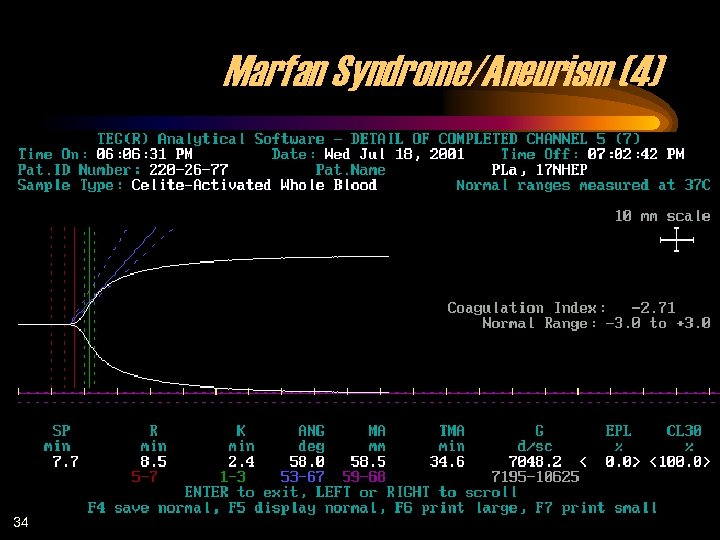 Marfan Syndrome/Aneurism (4) 34
Marfan Syndrome/Aneurism (4) 34
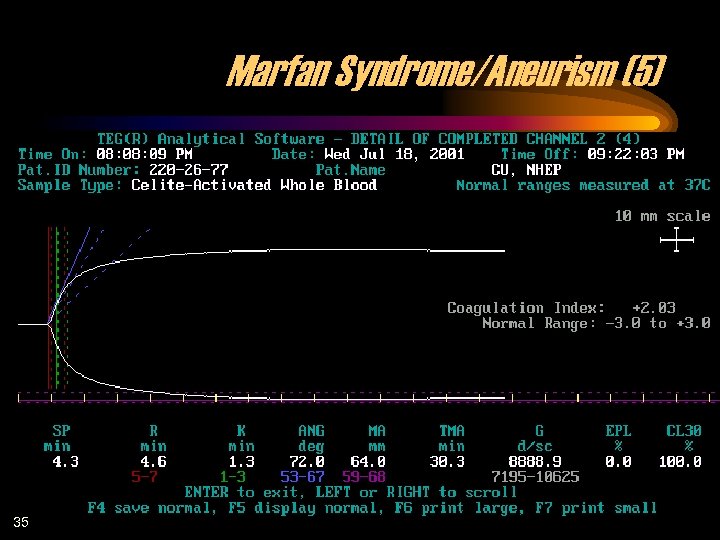 Marfan Syndrome/Aneurism (5) 35
Marfan Syndrome/Aneurism (5) 35
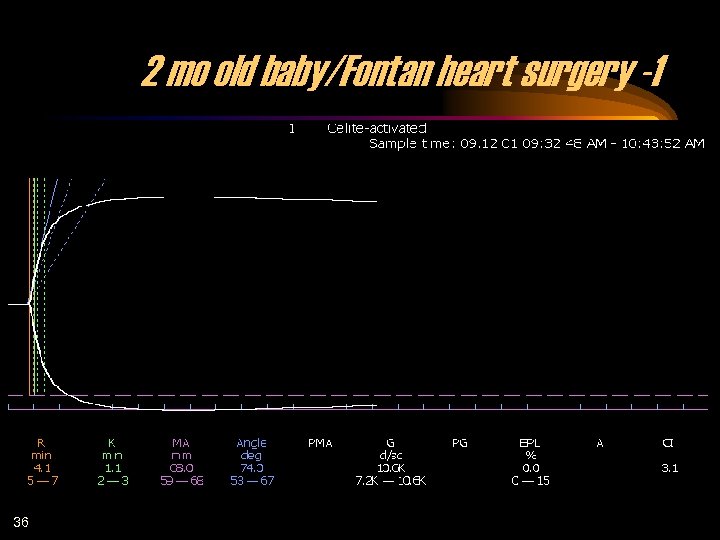 2 mo old baby/Fontan heart surgery -1 36
2 mo old baby/Fontan heart surgery -1 36
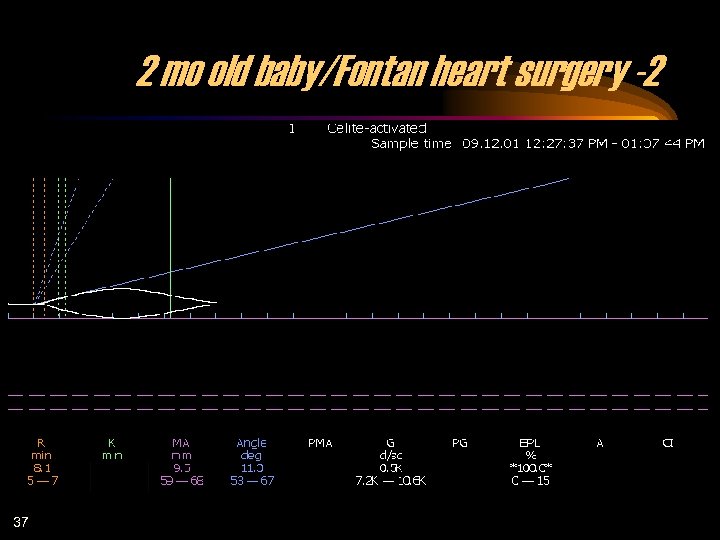 2 mo old baby/Fontan heart surgery -2 37
2 mo old baby/Fontan heart surgery -2 37
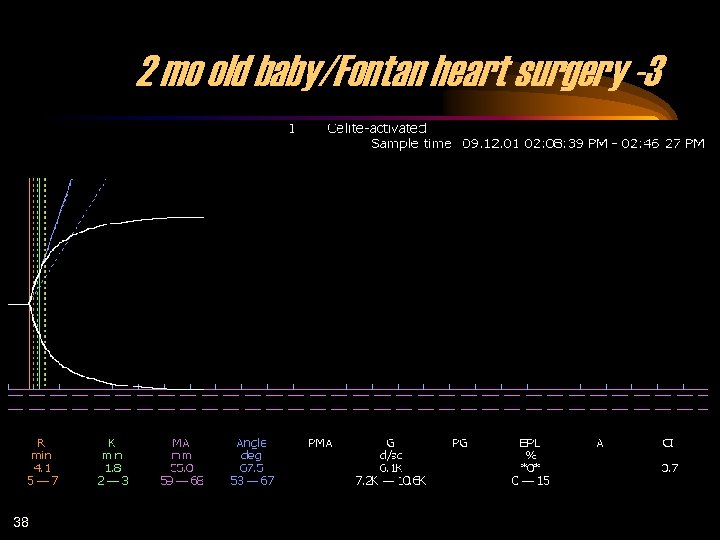 2 mo old baby/Fontan heart surgery -3 38
2 mo old baby/Fontan heart surgery -3 38
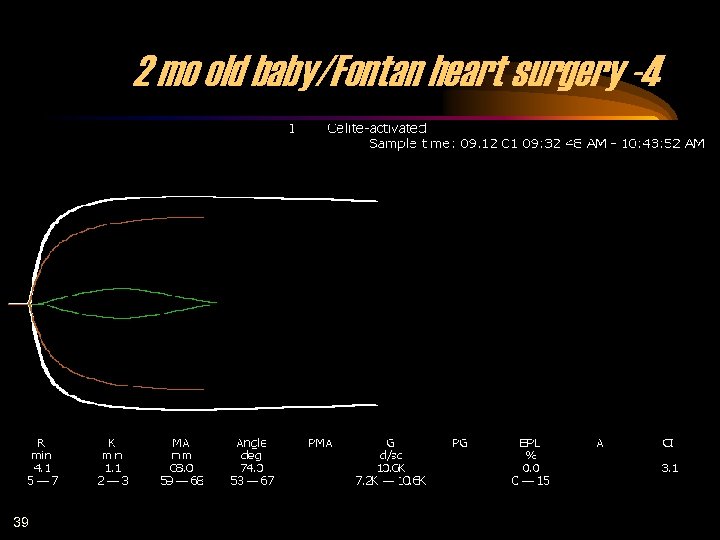 2 mo old baby/Fontan heart surgery -4 39
2 mo old baby/Fontan heart surgery -4 39
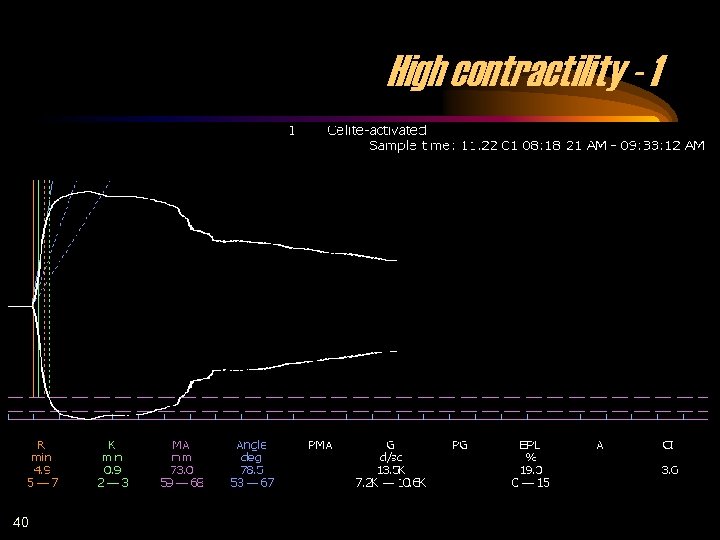 High contractility - 1 40
High contractility - 1 40
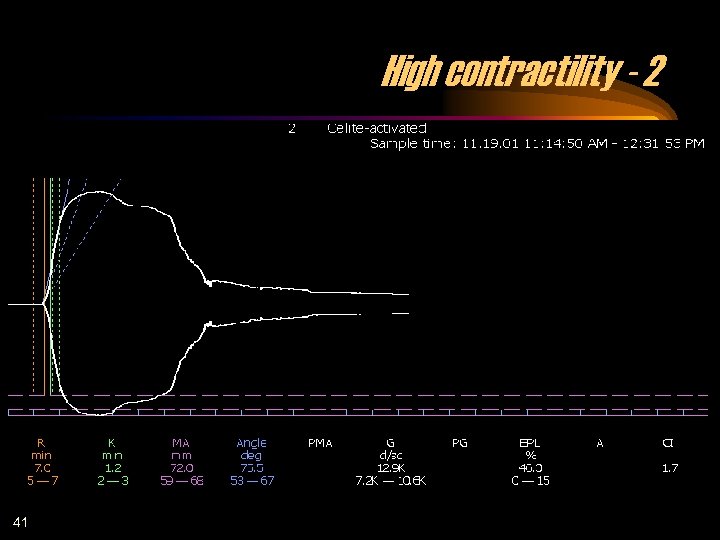 High contractility - 2 41
High contractility - 2 41
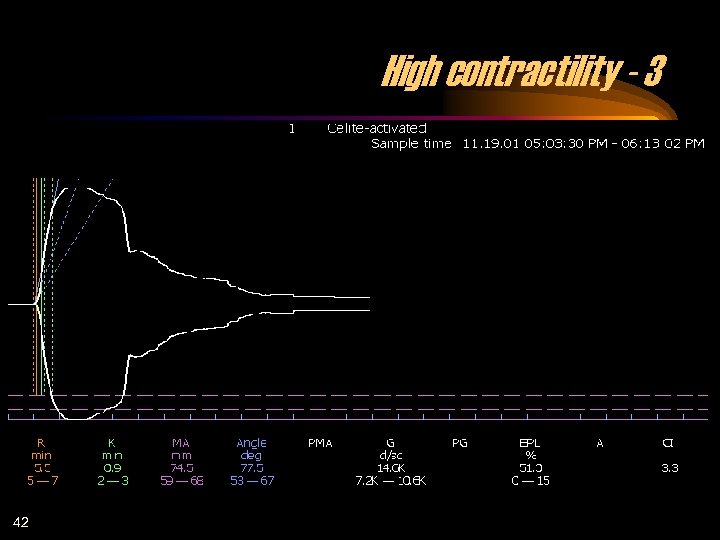 High contractility - 3 42
High contractility - 3 42
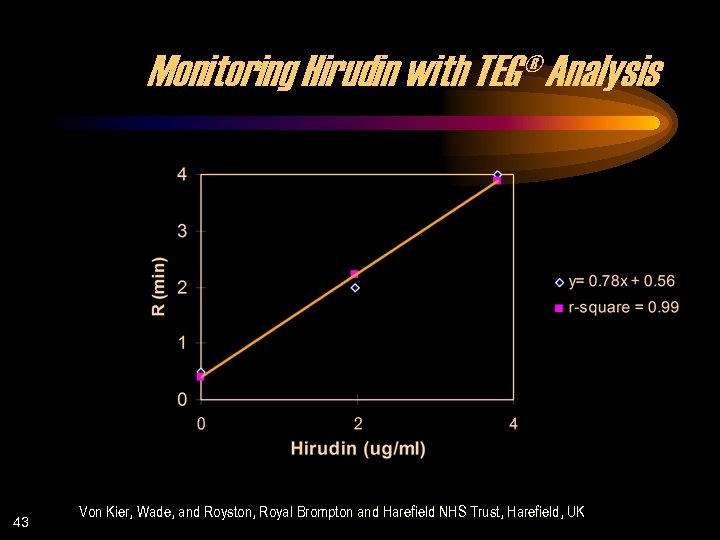 Monitoring Hirudin with TEG® Analysis 43 Von Kier, Wade, and Royston, Royal Brompton and Harefield NHS Trust, Harefield, UK
Monitoring Hirudin with TEG® Analysis 43 Von Kier, Wade, and Royston, Royal Brompton and Harefield NHS Trust, Harefield, UK
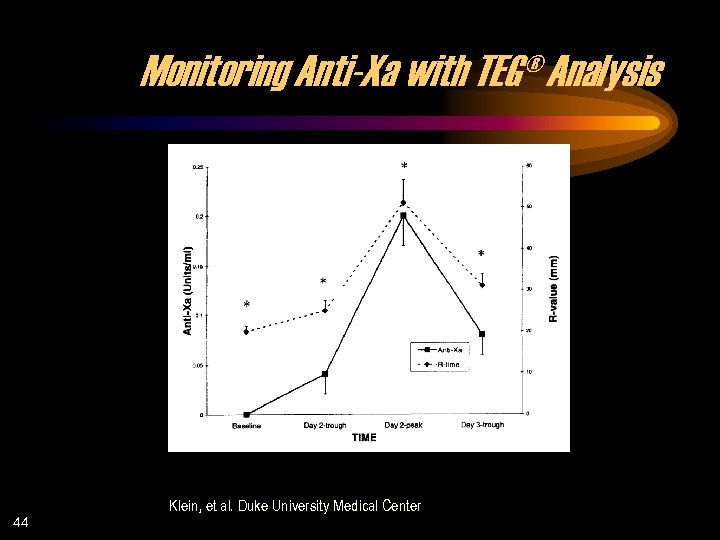 Monitoring Anti-Xa with TEG® Analysis 44 Klein, et al. Duke University Medical Center
Monitoring Anti-Xa with TEG® Analysis 44 Klein, et al. Duke University Medical Center
 TEG® Tracing Schematic 45
TEG® Tracing Schematic 45
 Standard Protocol for Cardiovascular Applications • Baseline tracing on induction – 1 sample with kaolin and heparinase (heparinase in case of heparin presence or contamination) • At rewarming (approx 36°) on CPB – 1 sample with kaolin and heparinase • *10 min post protamine, 2 TEG® columns needed – 1 sample with kaolin and heparinase – 1 sample with kaolin only Ü 46
Standard Protocol for Cardiovascular Applications • Baseline tracing on induction – 1 sample with kaolin and heparinase (heparinase in case of heparin presence or contamination) • At rewarming (approx 36°) on CPB – 1 sample with kaolin and heparinase • *10 min post protamine, 2 TEG® columns needed – 1 sample with kaolin and heparinase – 1 sample with kaolin only Ü 46
 Post Protamine 47 • Looking at only the R parameter, if the samples with and without heparinase are the same, the patient has received enough protamine to reverse heparin. • If both tracings are normal and the patient is bleeding, the reason is surgical. • If the R without heparinase is elongated and the heparinase tracing is normal and the patient is bleeding, the bleeding is due to excess of heparin. • If the tracing with heparinase shows a coagulopathy, the patient is treated accordingly. Most likely coagulopathies will be consistent with those observed during monitoring while the patient is on the pump. Ü
Post Protamine 47 • Looking at only the R parameter, if the samples with and without heparinase are the same, the patient has received enough protamine to reverse heparin. • If both tracings are normal and the patient is bleeding, the reason is surgical. • If the R without heparinase is elongated and the heparinase tracing is normal and the patient is bleeding, the bleeding is due to excess of heparin. • If the tracing with heparinase shows a coagulopathy, the patient is treated accordingly. Most likely coagulopathies will be consistent with those observed during monitoring while the patient is on the pump. Ü
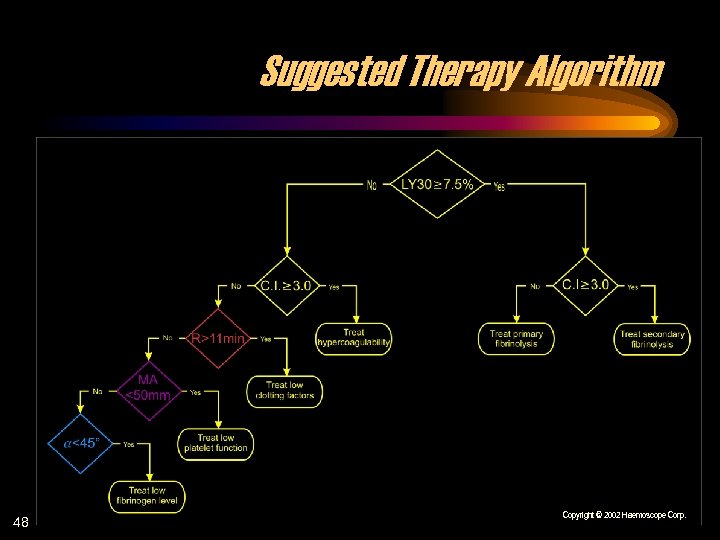 Suggested Therapy Algorithm 48 Copyright © 2002 Haemoscope Corp.
Suggested Therapy Algorithm 48 Copyright © 2002 Haemoscope Corp.
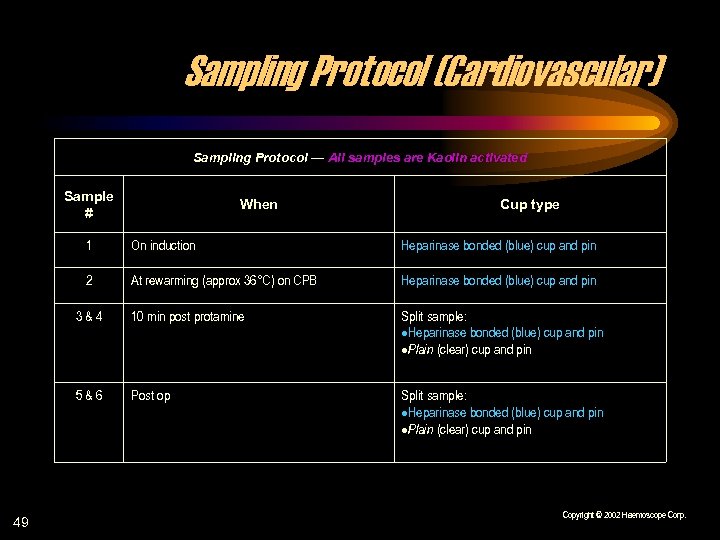 Sampling Protocol (Cardiovascular) Sampling Protocol — All samples are Kaolin activated Sample # When Cup type 1 Heparinase bonded (blue) cup and pin 2 At rewarming (approx 36°C) on CPB Heparinase bonded (blue) cup and pin 3&4 10 min post protamine Split sample: Heparinase bonded (blue) cup and pin Plain (clear) cup and pin 5&6 49 On induction Post op Split sample: Heparinase bonded (blue) cup and pin Plain (clear) cup and pin Copyright © 2002 Haemoscope Corp.
Sampling Protocol (Cardiovascular) Sampling Protocol — All samples are Kaolin activated Sample # When Cup type 1 Heparinase bonded (blue) cup and pin 2 At rewarming (approx 36°C) on CPB Heparinase bonded (blue) cup and pin 3&4 10 min post protamine Split sample: Heparinase bonded (blue) cup and pin Plain (clear) cup and pin 5&6 49 On induction Post op Split sample: Heparinase bonded (blue) cup and pin Plain (clear) cup and pin Copyright © 2002 Haemoscope Corp.
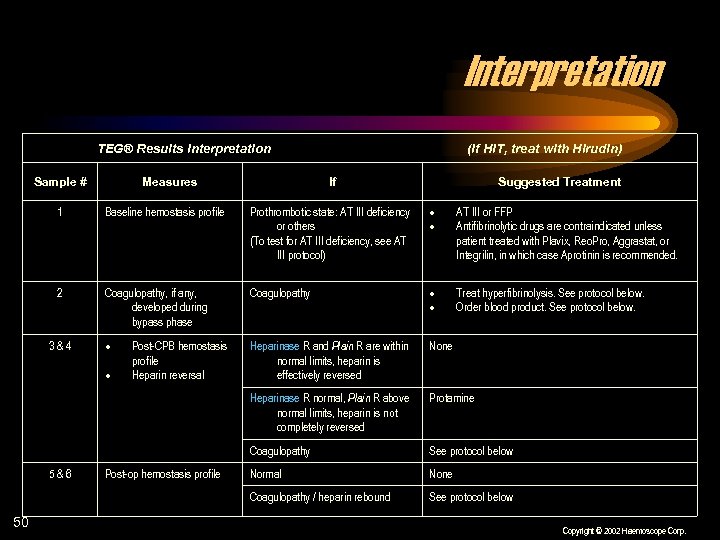 Interpretation TEG® Results Interpretation Sample # Measures (If HIT, treat with Hirudin) If Suggested Treatment 1 Baseline hemostasis profile Prothrombotic state: AT III deficiency or others (To test for AT III deficiency, see AT III protocol) AT III or FFP Antifibrinolytic drugs are contraindicated unless patient treated with Plavix, Reo. Pro, Aggrastat, or Integrilin, in which case Aprotinin is recommended. 2 Coagulopathy, if any, developed during bypass phase Coagulopathy Treat hyperfibrinolysis. See protocol below. Order blood product. See protocol below. Heparinase R and Plain R are within normal limits, heparin is effectively reversed None Heparinase R normal, Plain R above normal limits, heparin is not completely reversed Protamine Coagulopathy See protocol below Normal None Coagulopathy / heparin rebound See protocol below 3&4 5&6 50 Post-CPB hemostasis profile Heparin reversal Post-op hemostasis profile Copyright © 2002 Haemoscope Corp.
Interpretation TEG® Results Interpretation Sample # Measures (If HIT, treat with Hirudin) If Suggested Treatment 1 Baseline hemostasis profile Prothrombotic state: AT III deficiency or others (To test for AT III deficiency, see AT III protocol) AT III or FFP Antifibrinolytic drugs are contraindicated unless patient treated with Plavix, Reo. Pro, Aggrastat, or Integrilin, in which case Aprotinin is recommended. 2 Coagulopathy, if any, developed during bypass phase Coagulopathy Treat hyperfibrinolysis. See protocol below. Order blood product. See protocol below. Heparinase R and Plain R are within normal limits, heparin is effectively reversed None Heparinase R normal, Plain R above normal limits, heparin is not completely reversed Protamine Coagulopathy See protocol below Normal None Coagulopathy / heparin rebound See protocol below 3&4 5&6 50 Post-CPB hemostasis profile Heparin reversal Post-op hemostasis profile Copyright © 2002 Haemoscope Corp.
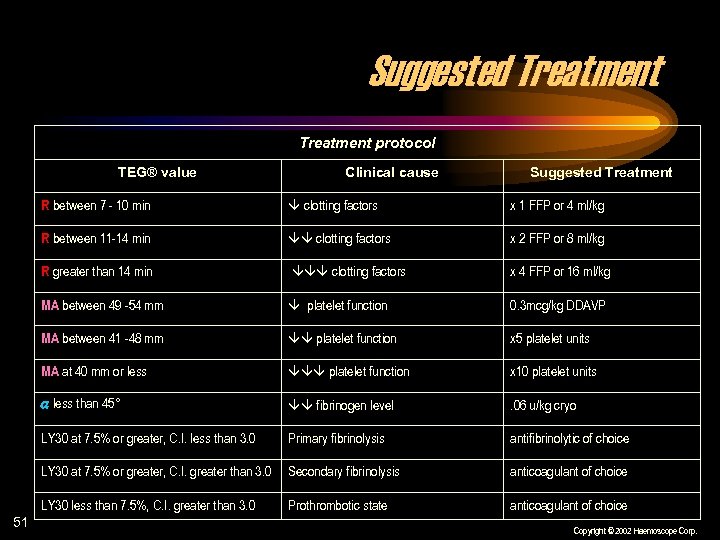 Suggested Treatment protocol TEG® value Clinical cause Suggested Treatment R between 7 - 10 min x 1 FFP or 4 ml/kg R between 11 -14 min clotting factors x 2 FFP or 8 ml/kg R greater than 14 min clotting factors x 4 FFP or 16 ml/kg MA between 49 -54 mm platelet function 0. 3 mcg/kg DDAVP MA between 41 -48 mm platelet function x 5 platelet units MA at 40 mm or less platelet function x 10 platelet units less than 45° fibrinogen level . 06 u/kg cryo LY 30 at 7. 5% or greater, C. I. less than 3. 0 Primary fibrinolysis antifibrinolytic of choice LY 30 at 7. 5% or greater, C. I. greater than 3. 0 Secondary fibrinolysis anticoagulant of choice LY 30 less than 7. 5%, C. I. greater than 3. 0 51 clotting factors Prothrombotic state anticoagulant of choice Copyright © 2002 Haemoscope Corp.
Suggested Treatment protocol TEG® value Clinical cause Suggested Treatment R between 7 - 10 min x 1 FFP or 4 ml/kg R between 11 -14 min clotting factors x 2 FFP or 8 ml/kg R greater than 14 min clotting factors x 4 FFP or 16 ml/kg MA between 49 -54 mm platelet function 0. 3 mcg/kg DDAVP MA between 41 -48 mm platelet function x 5 platelet units MA at 40 mm or less platelet function x 10 platelet units less than 45° fibrinogen level . 06 u/kg cryo LY 30 at 7. 5% or greater, C. I. less than 3. 0 Primary fibrinolysis antifibrinolytic of choice LY 30 at 7. 5% or greater, C. I. greater than 3. 0 Secondary fibrinolysis anticoagulant of choice LY 30 less than 7. 5%, C. I. greater than 3. 0 51 clotting factors Prothrombotic state anticoagulant of choice Copyright © 2002 Haemoscope Corp.
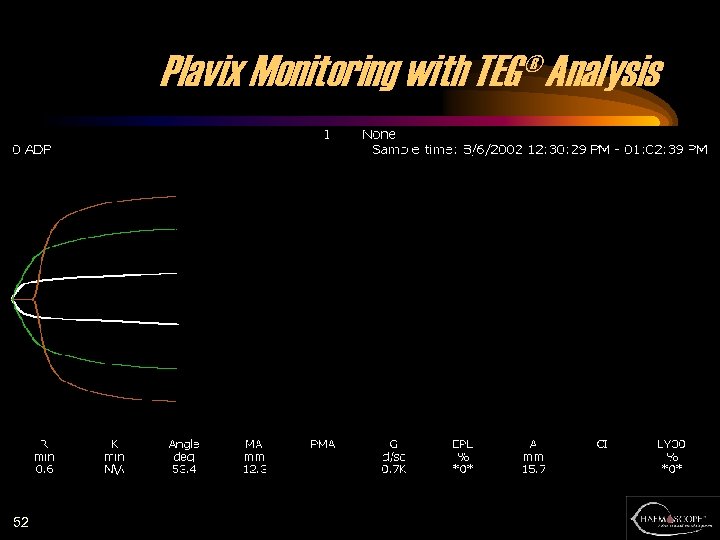 Plavix Monitoring with TEG® Analysis 52
Plavix Monitoring with TEG® Analysis 52
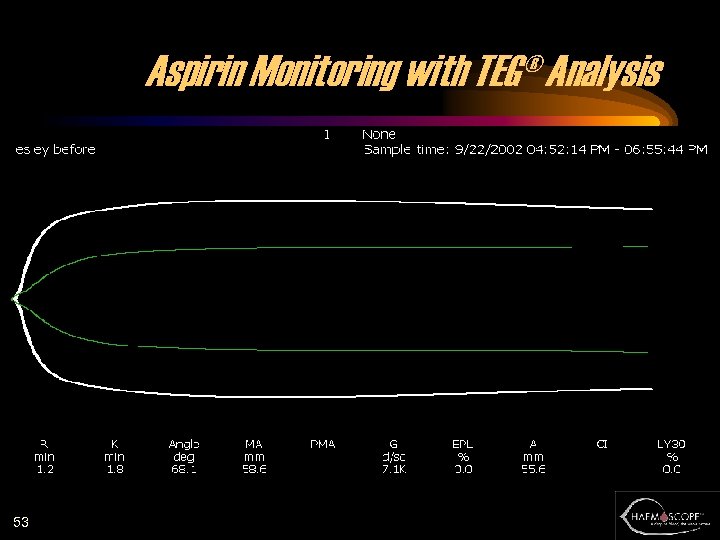 Aspirin Monitoring with TEG® Analysis 53
Aspirin Monitoring with TEG® Analysis 53
![Reduced Hemostatic Factor Transfusion Using Heparinasemodified [TEG® Analysis] During Cardiopulmonary Bypass (CPB) Stephen von Reduced Hemostatic Factor Transfusion Using Heparinasemodified [TEG® Analysis] During Cardiopulmonary Bypass (CPB) Stephen von](https://present5.com/presentation/7924e072aa0e61fc798e004e54e0bc66/image-54.jpg) Reduced Hemostatic Factor Transfusion Using Heparinasemodified [TEG® Analysis] During Cardiopulmonary Bypass (CPB) Stephen von Kier and David Royston Actual Group Predicted C (n=30) 10 DT (n=30) 5 C (TEG®) 2 DT (lab) 12 FFP 16 5 1 22 Platelets 9 1 1 8 Pts transfused 54
Reduced Hemostatic Factor Transfusion Using Heparinasemodified [TEG® Analysis] During Cardiopulmonary Bypass (CPB) Stephen von Kier and David Royston Actual Group Predicted C (n=30) 10 DT (n=30) 5 C (TEG®) 2 DT (lab) 12 FFP 16 5 1 22 Platelets 9 1 1 8 Pts transfused 54
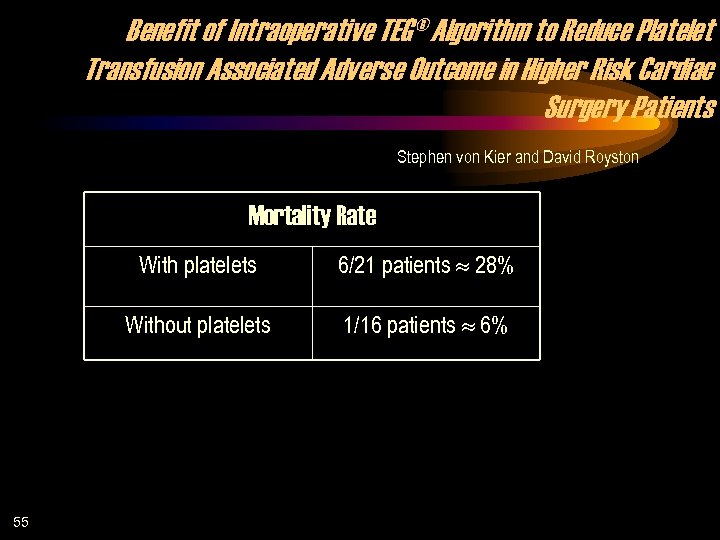 Benefit of Intraoperative TEG® Algorithm to Reduce Platelet Transfusion Associated Adverse Outcome in Higher Risk Cardiac Surgery Patients Stephen von Kier and David Royston Mortality Rate With platelets Without platelets 55 6/21 patients 28% 1/16 patients 6%
Benefit of Intraoperative TEG® Algorithm to Reduce Platelet Transfusion Associated Adverse Outcome in Higher Risk Cardiac Surgery Patients Stephen von Kier and David Royston Mortality Rate With platelets Without platelets 55 6/21 patients 28% 1/16 patients 6%
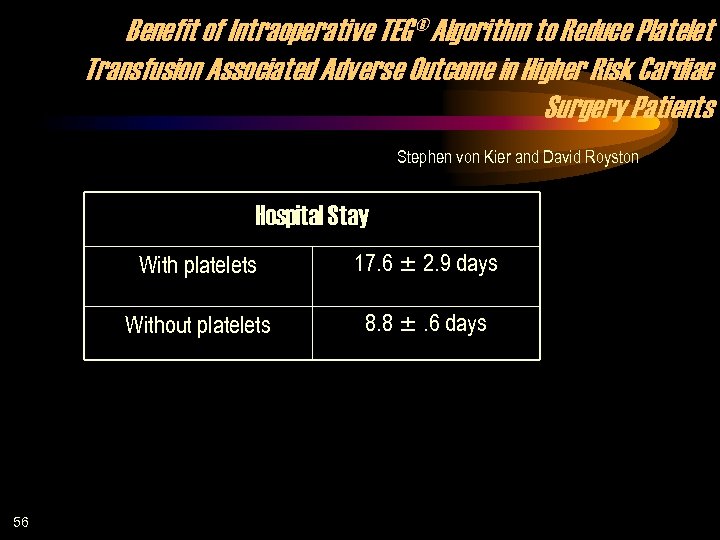 Benefit of Intraoperative TEG® Algorithm to Reduce Platelet Transfusion Associated Adverse Outcome in Higher Risk Cardiac Surgery Patients Stephen von Kier and David Royston Hospital Stay With platelets Without platelets 56 17. 6 2. 9 days 8. 8 . 6 days
Benefit of Intraoperative TEG® Algorithm to Reduce Platelet Transfusion Associated Adverse Outcome in Higher Risk Cardiac Surgery Patients Stephen von Kier and David Royston Hospital Stay With platelets Without platelets 56 17. 6 2. 9 days 8. 8 . 6 days
![[TEG® Analysis] Decreases Transfusion Requirement After Cardiac Surgery Linda Shore-Lesserson MD et al RBC [TEG® Analysis] Decreases Transfusion Requirement After Cardiac Surgery Linda Shore-Lesserson MD et al RBC](https://present5.com/presentation/7924e072aa0e61fc798e004e54e0bc66/image-57.jpg) [TEG® Analysis] Decreases Transfusion Requirement After Cardiac Surgery Linda Shore-Lesserson MD et al RBC intra TEG® analysis Control RBC post Non. RBC intra Non. RBC post CTD (ml) 17/53 10/53 5/53 3/53 577 ± 412 23/52 12/52 8/52 13/52** 659 ± 429 ** p < 0. 006 TEG® sample vs control 57
[TEG® Analysis] Decreases Transfusion Requirement After Cardiac Surgery Linda Shore-Lesserson MD et al RBC intra TEG® analysis Control RBC post Non. RBC intra Non. RBC post CTD (ml) 17/53 10/53 5/53 3/53 577 ± 412 23/52 12/52 8/52 13/52** 659 ± 429 ** p < 0. 006 TEG® sample vs control 57
 TEG® Applications • • 58 Liver transplantation Cardiovascular surgery Heart assist device Percutaneous Transluminal Coronary Angioplasty (PTCA) Trauma Obstetrics ICU Orthopedics Ü
TEG® Applications • • 58 Liver transplantation Cardiovascular surgery Heart assist device Percutaneous Transluminal Coronary Angioplasty (PTCA) Trauma Obstetrics ICU Orthopedics Ü
 TEG® System TEG® Analytical Software • • • 59 Software-assisted diagnosis Early projected values Full report capability Automated QC management Additional data entry Full peer-to-peer network support Standard Windows interface Touch screen and barcode Smoothing algorithm
TEG® System TEG® Analytical Software • • • 59 Software-assisted diagnosis Early projected values Full report capability Automated QC management Additional data entry Full peer-to-peer network support Standard Windows interface Touch screen and barcode Smoothing algorithm
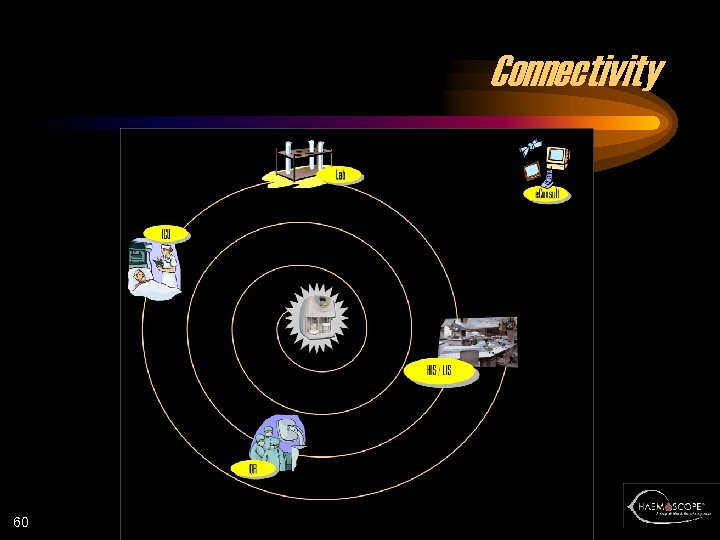 Connectivity 60
Connectivity 60
 TEG® Analyzer 5000 Series 61
TEG® Analyzer 5000 Series 61
 62
62


