dbb8b49f50ae95246eab7093900ae3b1.ppt
- Количество слайдов: 41
 Heat Transfer Conduction, Convection and Radiation
Heat Transfer Conduction, Convection and Radiation
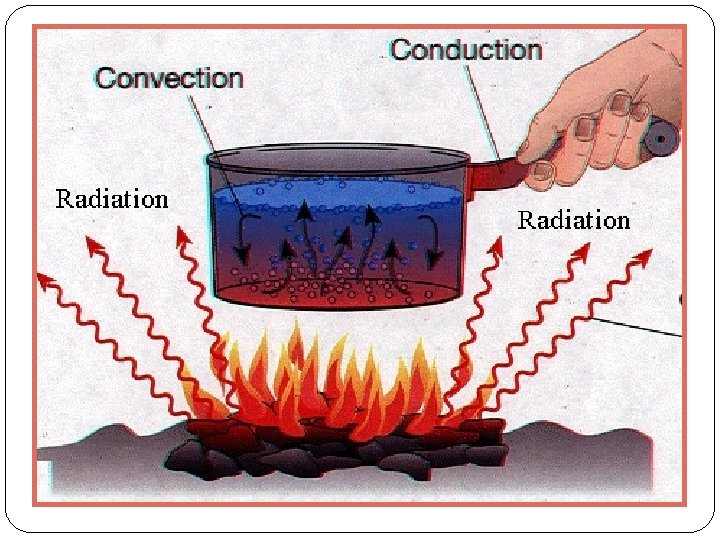
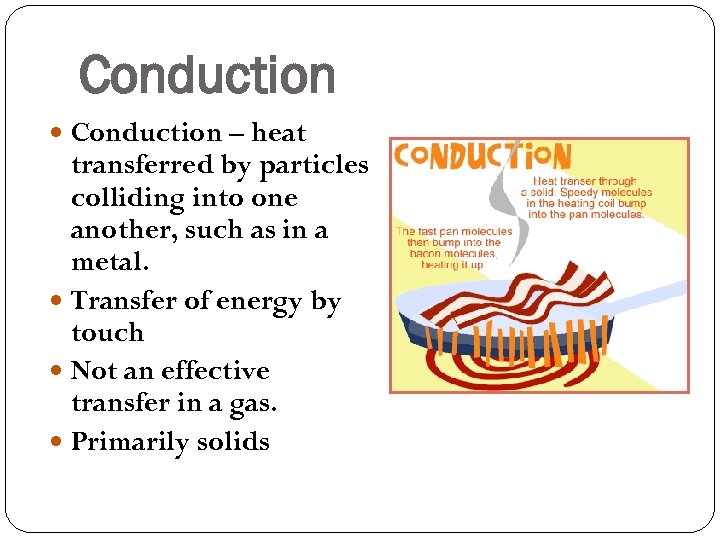 Conduction – heat transferred by particles colliding into one another, such as in a metal. Transfer of energy by touch Not an effective transfer in a gas. Primarily solids
Conduction – heat transferred by particles colliding into one another, such as in a metal. Transfer of energy by touch Not an effective transfer in a gas. Primarily solids
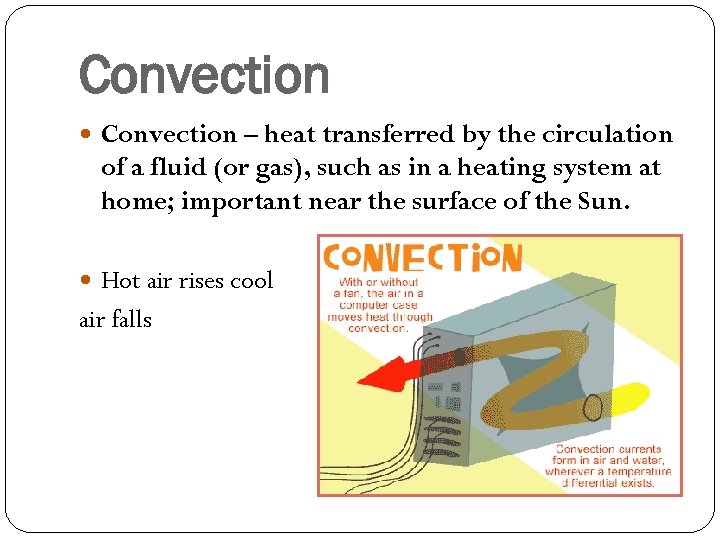 Convection – heat transferred by the circulation of a fluid (or gas), such as in a heating system at home; important near the surface of the Sun. Hot air rises cool air falls
Convection – heat transferred by the circulation of a fluid (or gas), such as in a heating system at home; important near the surface of the Sun. Hot air rises cool air falls
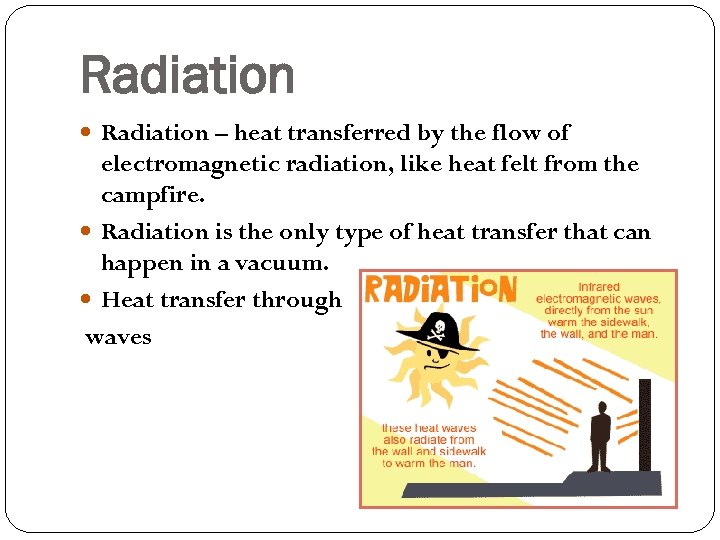 Radiation – heat transferred by the flow of electromagnetic radiation, like heat felt from the campfire. Radiation is the only type of heat transfer that can happen in a vacuum. Heat transfer through waves
Radiation – heat transferred by the flow of electromagnetic radiation, like heat felt from the campfire. Radiation is the only type of heat transfer that can happen in a vacuum. Heat transfer through waves
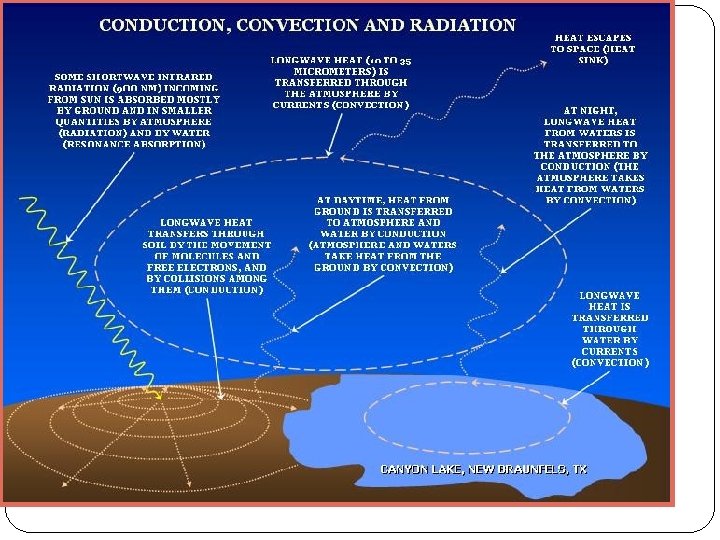
 Heat Transfer CONDUCTION: the transfer of energy through matter by direct contact of particles. This can happen in solids, liquids and gases. CONVECTION: the transfer of energy because of the movement of bulk masses of particles. This can happen only in liquids and gases - not in solids. RADIATION: the transfer of energy by electromagnetic waves. Energy can move by radiation in air like the heat from your electric stove top, or in the vacuum of space the way the Sun heats the Earth. In radiation, the energy does not have to transfer through mass (particles).
Heat Transfer CONDUCTION: the transfer of energy through matter by direct contact of particles. This can happen in solids, liquids and gases. CONVECTION: the transfer of energy because of the movement of bulk masses of particles. This can happen only in liquids and gases - not in solids. RADIATION: the transfer of energy by electromagnetic waves. Energy can move by radiation in air like the heat from your electric stove top, or in the vacuum of space the way the Sun heats the Earth. In radiation, the energy does not have to transfer through mass (particles).
 Heat Vent
Heat Vent
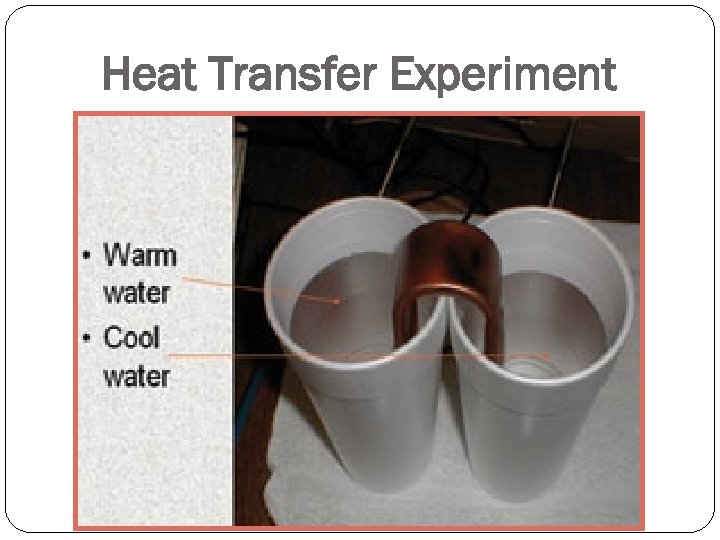 Heat Transfer Experiment
Heat Transfer Experiment
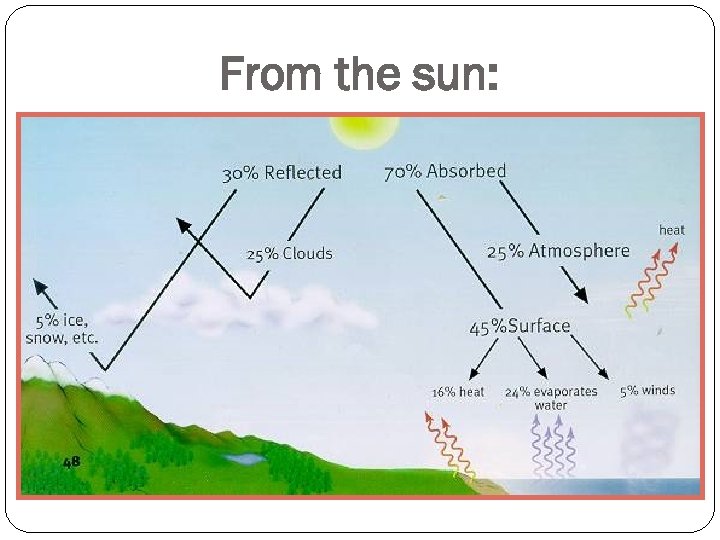 From the sun:
From the sun:
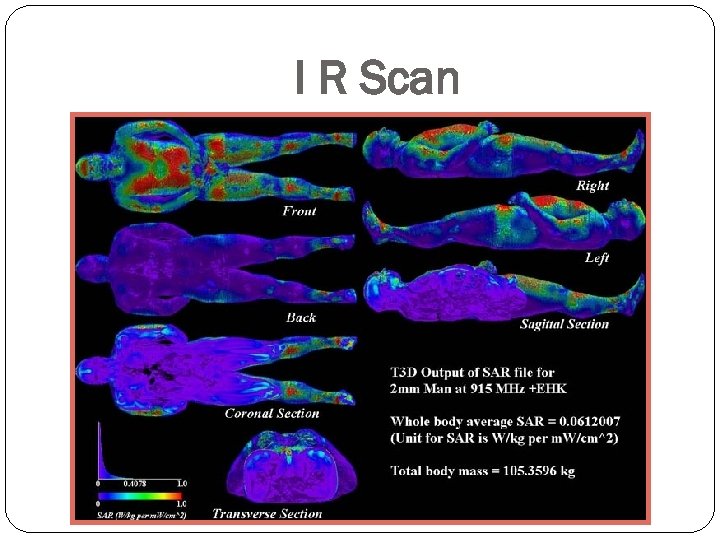 I R Scan
I R Scan
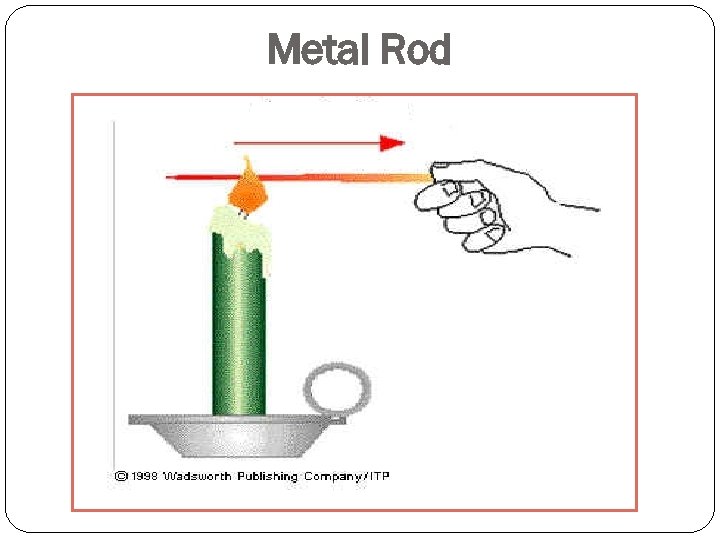 Metal Rod
Metal Rod
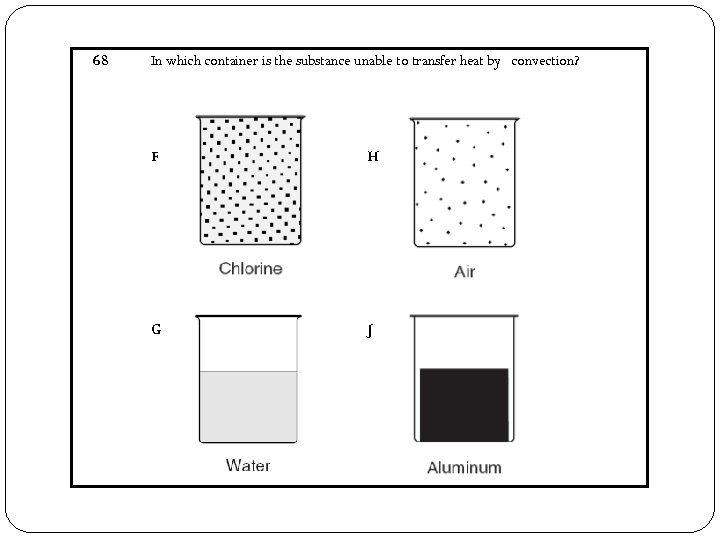 68 In which container is the substance unable to transfer heat by convection? F H G J
68 In which container is the substance unable to transfer heat by convection? F H G J
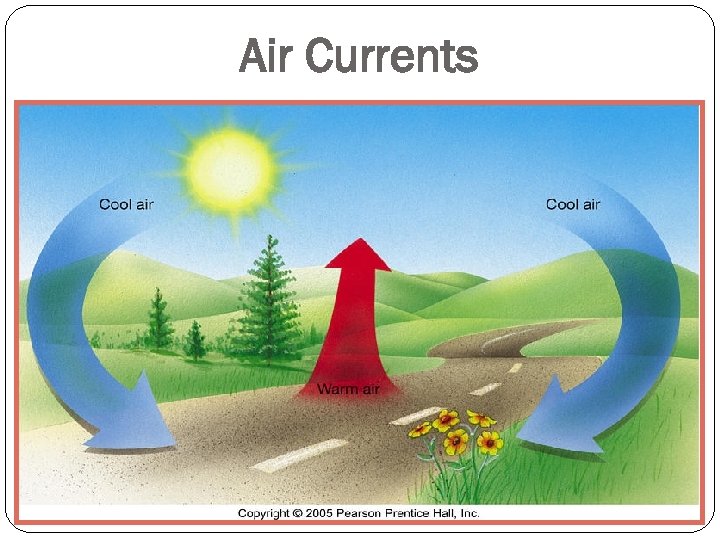 Air Currents
Air Currents
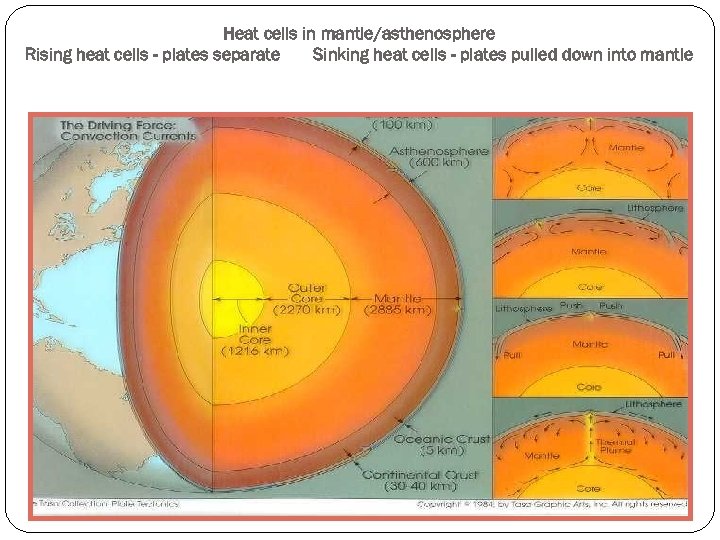 Heat cells in mantle/asthenosphere Rising heat cells - plates separate Sinking heat cells - plates pulled down into mantle
Heat cells in mantle/asthenosphere Rising heat cells - plates separate Sinking heat cells - plates pulled down into mantle
 The Sun
The Sun
 heat transfer : Problem 10 Heat convection occurs in gases and liquids. Heat convection does not occur in solids because solids are unable to — A B C D absorb heat by vibrating transfer heat by fluid motion emit radiation by reflecting light exchange heat by direct contact
heat transfer : Problem 10 Heat convection occurs in gases and liquids. Heat convection does not occur in solids because solids are unable to — A B C D absorb heat by vibrating transfer heat by fluid motion emit radiation by reflecting light exchange heat by direct contact
 Each finger is a different metal rod with glass marbles affixed using wax. The marbles are placed at equal distances on each of the rods and the rods are heated. The heat is moved through the metal to the wax adhesive. As the wax warms up, it looses its stickiness and the marbles subsequently fall off.
Each finger is a different metal rod with glass marbles affixed using wax. The marbles are placed at equal distances on each of the rods and the rods are heated. The heat is moved through the metal to the wax adhesive. As the wax warms up, it looses its stickiness and the marbles subsequently fall off.
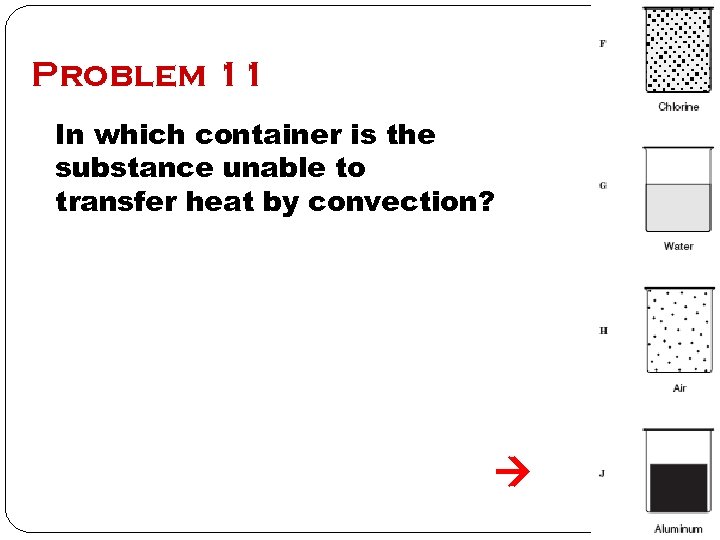 Problem 11 In which container is the substance unable to transfer heat by convection?
Problem 11 In which container is the substance unable to transfer heat by convection?
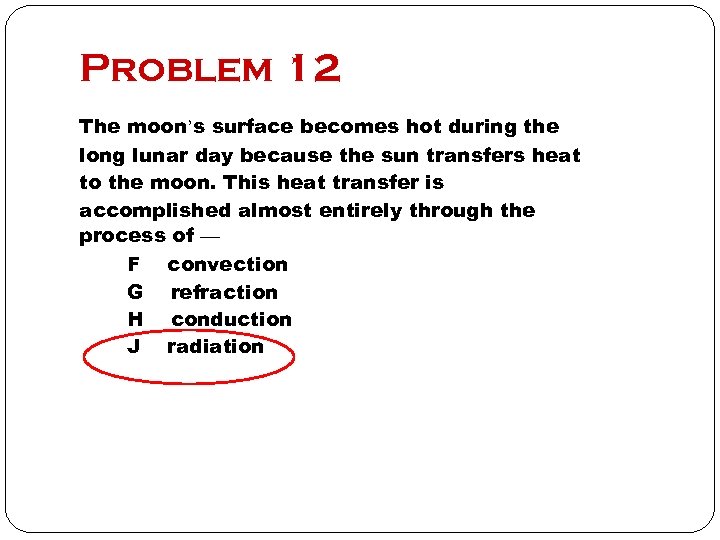 Problem 12 The moon’s surface becomes hot during the long lunar day because the sun transfers heat to the moon. This heat transfer is accomplished almost entirely through the process of — F convection G refraction H conduction J radiation
Problem 12 The moon’s surface becomes hot during the long lunar day because the sun transfers heat to the moon. This heat transfer is accomplished almost entirely through the process of — F convection G refraction H conduction J radiation
 Microwave
Microwave
 Problem 13 A man who was sleeping wakes up because he hears the smoke alarm go off in his house. Before opening the bedroom door, the man feels the door to see whether it is warm. He is assuming that heat would be transferred through the door by — A conduction B convection C radiation D compression
Problem 13 A man who was sleeping wakes up because he hears the smoke alarm go off in his house. Before opening the bedroom door, the man feels the door to see whether it is warm. He is assuming that heat would be transferred through the door by — A conduction B convection C radiation D compression
 Problem 14 The transfer of heat by the movement of air currents in Earth’s atmosphere is an example of — A B C D conduction convection radiation fusion
Problem 14 The transfer of heat by the movement of air currents in Earth’s atmosphere is an example of — A B C D conduction convection radiation fusion
 Heat Lamps
Heat Lamps
 Problem 15 A solar heater uses energy from the sun to heat water. The heater’s panel is painted black to — F improve emission of infrared radiation G reduce the heat loss by convection currents H improve absorption of infrared radiation J reduce the heater’s conducting properties
Problem 15 A solar heater uses energy from the sun to heat water. The heater’s panel is painted black to — F improve emission of infrared radiation G reduce the heat loss by convection currents H improve absorption of infrared radiation J reduce the heater’s conducting properties
 65 Heat convection occurs in gases and liquids. Heat convection does not occur in solids because solids are unable to — A B C D absorb heat by vibrating transfer heat by fluid motion emit radiation by reflecting light exchange heat by direct contact
65 Heat convection occurs in gases and liquids. Heat convection does not occur in solids because solids are unable to — A B C D absorb heat by vibrating transfer heat by fluid motion emit radiation by reflecting light exchange heat by direct contact
 Hot pool near Red Cone Geyser; William S Keller; 1964
Hot pool near Red Cone Geyser; William S Keller; 1964
 66 A solar heater uses energy from the sun to heat water. The heater’s panel is painted black to — F G H J improve emission of infrared radiation reduce the heat loss by convection currents improve absorption of infrared radiation reduce the heater’s conducting properties
66 A solar heater uses energy from the sun to heat water. The heater’s panel is painted black to — F G H J improve emission of infrared radiation reduce the heat loss by convection currents improve absorption of infrared radiation reduce the heater’s conducting properties
 56 Which of the following is an example of solar energy being converted into chemical energy? F G H J Plants producing sugar during the day Water evaporating and condensing in the water cycle The sun unevenly heating Earth’s surface Lava erupting from volcanoes for many days
56 Which of the following is an example of solar energy being converted into chemical energy? F G H J Plants producing sugar during the day Water evaporating and condensing in the water cycle The sun unevenly heating Earth’s surface Lava erupting from volcanoes for many days
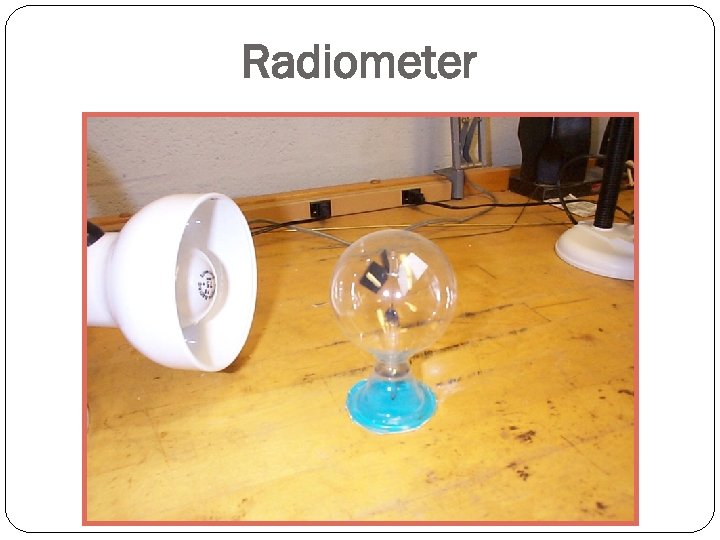 Radiometer
Radiometer
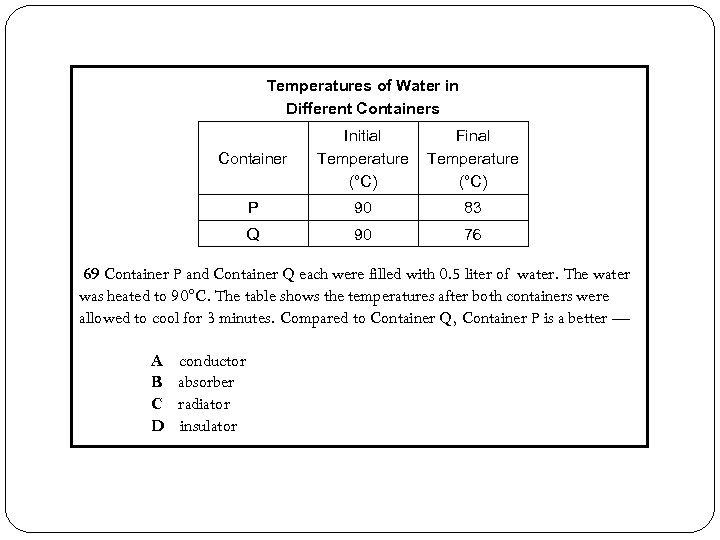 Temperatures of Water in Different Containers Container Initial Temperature (°C) Final Temperature (°C) P 90 83 Q 90 76 69 Container P and Container Q each were filled with 0. 5 liter of water. The water was heated to 90°C. The table shows the temperatures after both containers were allowed to cool for 3 minutes. Compared to Container Q, Container P is a better — A B C D conductor absorber radiator insulator
Temperatures of Water in Different Containers Container Initial Temperature (°C) Final Temperature (°C) P 90 83 Q 90 76 69 Container P and Container Q each were filled with 0. 5 liter of water. The water was heated to 90°C. The table shows the temperatures after both containers were allowed to cool for 3 minutes. Compared to Container Q, Container P is a better — A B C D conductor absorber radiator insulator
 54 Which process best shows the conversion of solar energy to chemical energy? F G H J Prevailing winds causing windmills to spin Green plants making their own food Uranium producing heat to make steam Tides generating electricity
54 Which process best shows the conversion of solar energy to chemical energy? F G H J Prevailing winds causing windmills to spin Green plants making their own food Uranium producing heat to make steam Tides generating electricity
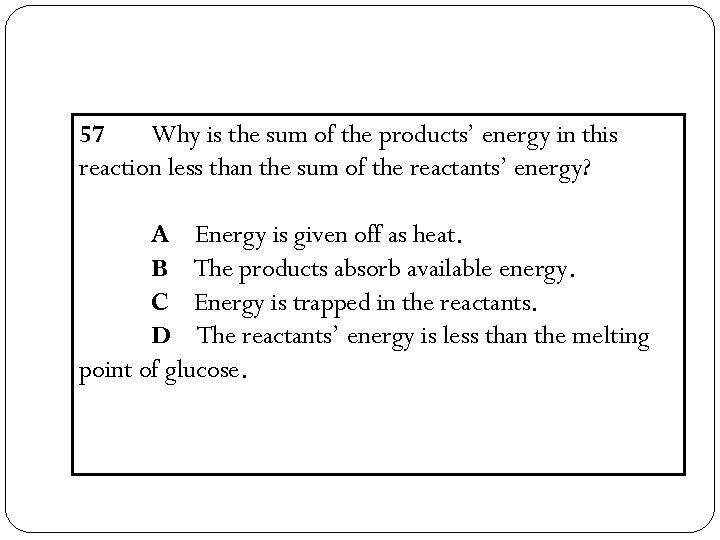 57 Why is the sum of the products’ energy in this reaction less than the sum of the reactants’ energy? A Energy is given off as heat. B The products absorb available energy. C Energy is trapped in the reactants. D The reactants’ energy is less than the melting point of glucose.
57 Why is the sum of the products’ energy in this reaction less than the sum of the reactants’ energy? A Energy is given off as heat. B The products absorb available energy. C Energy is trapped in the reactants. D The reactants’ energy is less than the melting point of glucose.
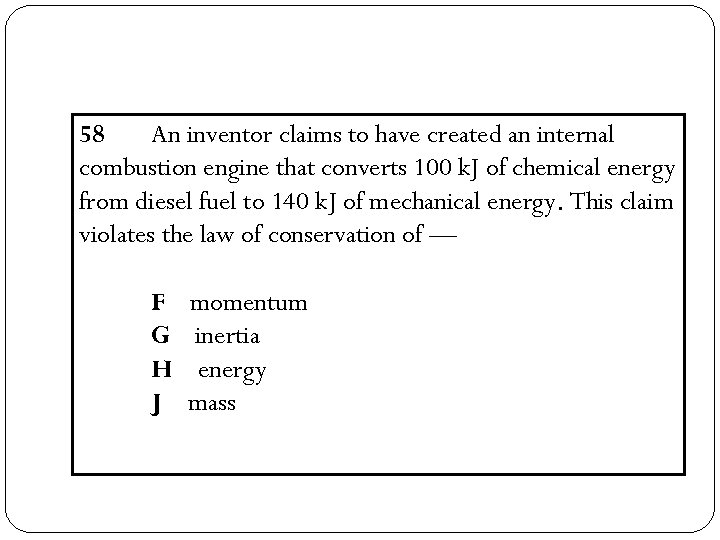 58 An inventor claims to have created an internal combustion engine that converts 100 k. J of chemical energy from diesel fuel to 140 k. J of mechanical energy. This claim violates the law of conservation of — F G H J momentum inertia energy mass
58 An inventor claims to have created an internal combustion engine that converts 100 k. J of chemical energy from diesel fuel to 140 k. J of mechanical energy. This claim violates the law of conservation of — F G H J momentum inertia energy mass
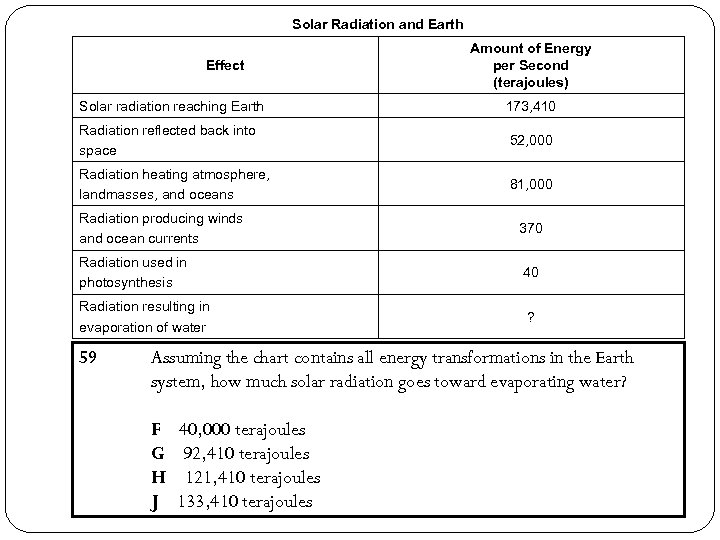 Solar Radiation and Earth Effect Amount of Energy per Second (terajoules) Solar radiation reaching Earth 173, 410 Radiation reflected back into space 52, 000 Radiation heating atmosphere, landmasses, and oceans 81, 000 Radiation producing winds and ocean currents 370 Radiation used in photosynthesis 40 Radiation resulting in evaporation of water ? 59 Assuming the chart contains all energy transformations in the Earth system, how much solar radiation goes toward evaporating water? F G H J 40, 000 terajoules 92, 410 terajoules 121, 410 terajoules 133, 410 terajoules
Solar Radiation and Earth Effect Amount of Energy per Second (terajoules) Solar radiation reaching Earth 173, 410 Radiation reflected back into space 52, 000 Radiation heating atmosphere, landmasses, and oceans 81, 000 Radiation producing winds and ocean currents 370 Radiation used in photosynthesis 40 Radiation resulting in evaporation of water ? 59 Assuming the chart contains all energy transformations in the Earth system, how much solar radiation goes toward evaporating water? F G H J 40, 000 terajoules 92, 410 terajoules 121, 410 terajoules 133, 410 terajoules
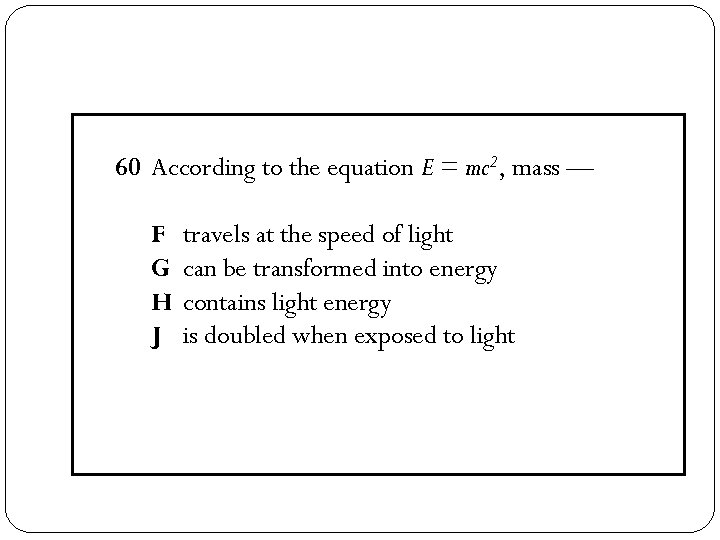 60 According to the equation E = mc 2, mass — F G H J travels at the speed of light can be transformed into energy contains light energy is doubled when exposed to light
60 According to the equation E = mc 2, mass — F G H J travels at the speed of light can be transformed into energy contains light energy is doubled when exposed to light
 63 Because ancient Greeks lived close to water, they may have enjoyed a more constant climate than if they had lived inland. Water warms up and cools down more slowly than land. This is because of water’s — F G H J boiling point specific heat melting point specific gravity
63 Because ancient Greeks lived close to water, they may have enjoyed a more constant climate than if they had lived inland. Water warms up and cools down more slowly than land. This is because of water’s — F G H J boiling point specific heat melting point specific gravity
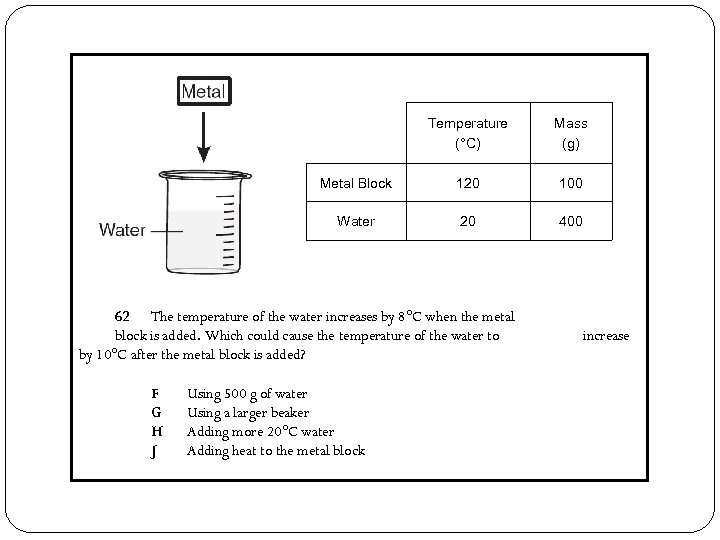 Temperature (°C) Mass (g) Metal Block 120 100 Water 20 400 62 The temperature of the water increases by 8°C when the metal block is added. Which could cause the temperature of the water to by 10°C after the metal block is added? F G H J Using 500 g of water Using a larger beaker Adding more 20°C water Adding heat to the metal block increase
Temperature (°C) Mass (g) Metal Block 120 100 Water 20 400 62 The temperature of the water increases by 8°C when the metal block is added. Which could cause the temperature of the water to by 10°C after the metal block is added? F G H J Using 500 g of water Using a larger beaker Adding more 20°C water Adding heat to the metal block increase
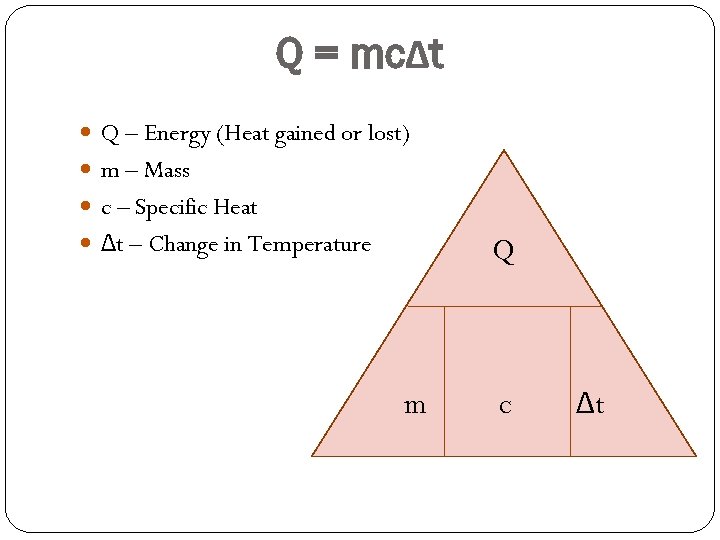 Q = mcΔt Q – Energy (Heat gained or lost) m – Mass c – Specific Heat Δt – Change in Temperature Q m c Δt
Q = mcΔt Q – Energy (Heat gained or lost) m – Mass c – Specific Heat Δt – Change in Temperature Q m c Δt
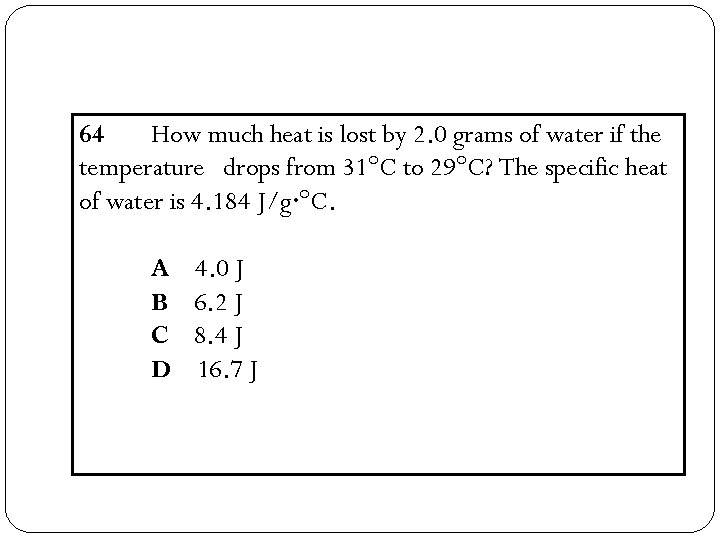 64 How much heat is lost by 2. 0 grams of water if the temperature drops from 31°C to 29°C? The specific heat of water is 4. 184 J/g·°C. A B C D 4. 0 J 6. 2 J 8. 4 J 16. 7 J
64 How much heat is lost by 2. 0 grams of water if the temperature drops from 31°C to 29°C? The specific heat of water is 4. 184 J/g·°C. A B C D 4. 0 J 6. 2 J 8. 4 J 16. 7 J


