6a945e41b8adcbf7c91b9323de8cd9c3.ppt
- Количество слайдов: 28
 Heart Care Centers of Illinois
Heart Care Centers of Illinois
 Design – Single arm, open label, 13 U. S. centers – Patients with ST-segment elevation myocardial infarction (STEMI) • Less than 12 hours duration • No prior thrombolytic therapy • Primary PCI required – Patient population matched to CADILLAC by inclusion and exclusion criteria – Comparable efficacy endpoints
Design – Single arm, open label, 13 U. S. centers – Patients with ST-segment elevation myocardial infarction (STEMI) • Less than 12 hours duration • No prior thrombolytic therapy • Primary PCI required – Patient population matched to CADILLAC by inclusion and exclusion criteria – Comparable efficacy endpoints
 Design (cont. ) – Treatment: Bivalirudin 0. 75 mg/kg bolus, 1. 75 mg/kg/hr infusion for duration of procedure – Optional post-procedure infusion: 0. 25 mg/kg/hr – Abciximab if TIMI flow <3 at end of procedure – Primary endpoints of safety and efficacy evaluated at 7 days/hospital discharge, 30 days and 6 months
Design (cont. ) – Treatment: Bivalirudin 0. 75 mg/kg bolus, 1. 75 mg/kg/hr infusion for duration of procedure – Optional post-procedure infusion: 0. 25 mg/kg/hr – Abciximab if TIMI flow <3 at end of procedure – Primary endpoints of safety and efficacy evaluated at 7 days/hospital discharge, 30 days and 6 months
 Inclusion Criteria – Symptoms of STEMI for at least 30 min within previous 12 hours AND • ST-segment elevation in at least 2 contiguous leads or new LBBB or existing LBBB with positive troponin • Residual high grade stenosis and associated abnormalities in regional wall motion. – Planned primary PCI in native coronary vessel
Inclusion Criteria – Symptoms of STEMI for at least 30 min within previous 12 hours AND • ST-segment elevation in at least 2 contiguous leads or new LBBB or existing LBBB with positive troponin • Residual high grade stenosis and associated abnormalities in regional wall motion. – Planned primary PCI in native coronary vessel
 Exclusion Criteria – Prior LMWH, Thrombolytics, GP IIb/IIIa inhibitors – Cardiogenic shock (SBP <80 for >30 min or a need for intravenous pressors) – Stroke or neurosurgery within 3 months – Severe hypertension not adequately controlled by antihypertensive therapy at the time of study entry (BP >180/110 mm Hg) – Life expectancy <1 year – Heparin only therapy allowed prior to PCI • 30 minute washout, or • ACT <250
Exclusion Criteria – Prior LMWH, Thrombolytics, GP IIb/IIIa inhibitors – Cardiogenic shock (SBP <80 for >30 min or a need for intravenous pressors) – Stroke or neurosurgery within 3 months – Severe hypertension not adequately controlled by antihypertensive therapy at the time of study entry (BP >180/110 mm Hg) – Life expectancy <1 year – Heparin only therapy allowed prior to PCI • 30 minute washout, or • ACT <250
 Primary Endpoints – Efficacy Evaluated at 7 days/hospital discharge, at 30 days, and 6 months composite and individual components of: • Death (cardiac and unknown cause) • Reinfarction • Repeat intervention/revascularization of target lesion as a result of ischemia • Disabling stroke • Rate of subacute thrombosis at 7 days/hospital discharge and at 30 days
Primary Endpoints – Efficacy Evaluated at 7 days/hospital discharge, at 30 days, and 6 months composite and individual components of: • Death (cardiac and unknown cause) • Reinfarction • Repeat intervention/revascularization of target lesion as a result of ischemia • Disabling stroke • Rate of subacute thrombosis at 7 days/hospital discharge and at 30 days
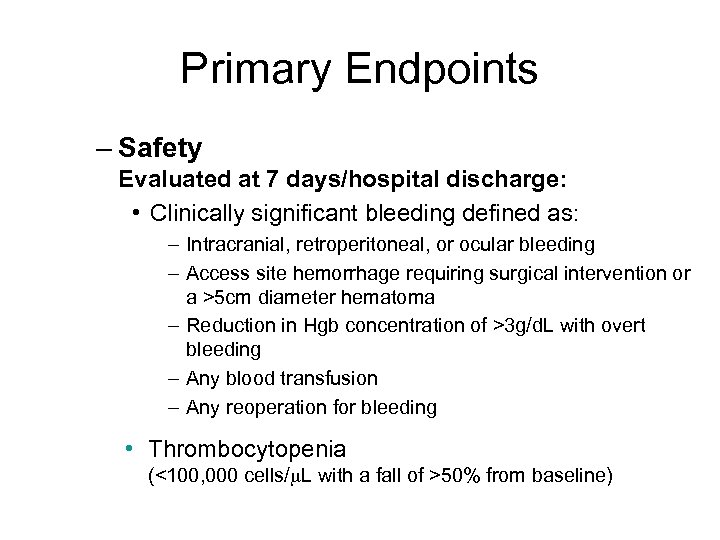 Primary Endpoints – Safety Evaluated at 7 days/hospital discharge: • Clinically significant bleeding defined as: – Intracranial, retroperitoneal, or ocular bleeding – Access site hemorrhage requiring surgical intervention or a >5 cm diameter hematoma – Reduction in Hgb concentration of >3 g/d. L with overt bleeding – Any blood transfusion – Any reoperation for bleeding • Thrombocytopenia (<100, 000 cells/ L with a fall of >50% from baseline)
Primary Endpoints – Safety Evaluated at 7 days/hospital discharge: • Clinically significant bleeding defined as: – Intracranial, retroperitoneal, or ocular bleeding – Access site hemorrhage requiring surgical intervention or a >5 cm diameter hematoma – Reduction in Hgb concentration of >3 g/d. L with overt bleeding – Any blood transfusion – Any reoperation for bleeding • Thrombocytopenia (<100, 000 cells/ L with a fall of >50% from baseline)
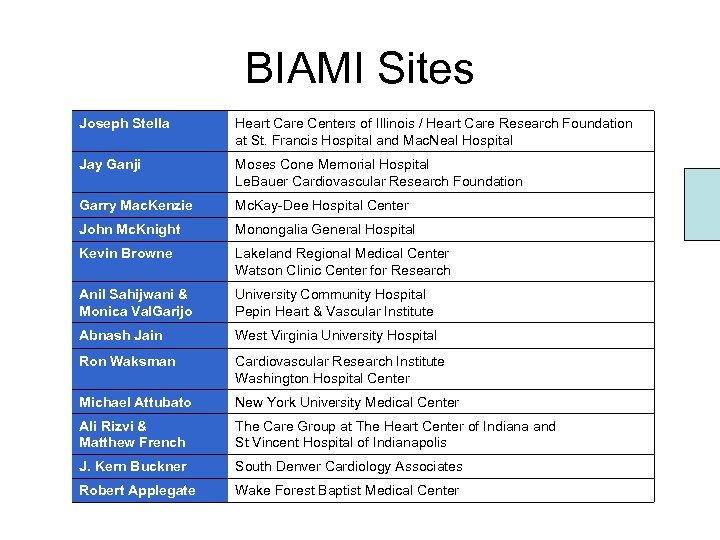 BIAMI Sites Joseph Stella Heart Care Centers of Illinois / Heart Care Research Foundation at St. Francis Hospital and Mac. Neal Hospital Jay Ganji Moses Cone Memorial Hospital Le. Bauer Cardiovascular Research Foundation Garry Mac. Kenzie Mc. Kay-Dee Hospital Center John Mc. Knight Monongalia General Hospital Kevin Browne Lakeland Regional Medical Center Watson Clinic Center for Research Anil Sahijwani & Monica Val. Garijo University Community Hospital Pepin Heart & Vascular Institute Abnash Jain West Virginia University Hospital Ron Waksman Cardiovascular Research Institute Washington Hospital Center Michael Attubato New York University Medical Center Ali Rizvi & Matthew French The Care Group at The Heart Center of Indiana and St Vincent Hospital of Indianapolis J. Kern Buckner South Denver Cardiology Associates Robert Applegate Wake Forest Baptist Medical Center
BIAMI Sites Joseph Stella Heart Care Centers of Illinois / Heart Care Research Foundation at St. Francis Hospital and Mac. Neal Hospital Jay Ganji Moses Cone Memorial Hospital Le. Bauer Cardiovascular Research Foundation Garry Mac. Kenzie Mc. Kay-Dee Hospital Center John Mc. Knight Monongalia General Hospital Kevin Browne Lakeland Regional Medical Center Watson Clinic Center for Research Anil Sahijwani & Monica Val. Garijo University Community Hospital Pepin Heart & Vascular Institute Abnash Jain West Virginia University Hospital Ron Waksman Cardiovascular Research Institute Washington Hospital Center Michael Attubato New York University Medical Center Ali Rizvi & Matthew French The Care Group at The Heart Center of Indiana and St Vincent Hospital of Indianapolis J. Kern Buckner South Denver Cardiology Associates Robert Applegate Wake Forest Baptist Medical Center
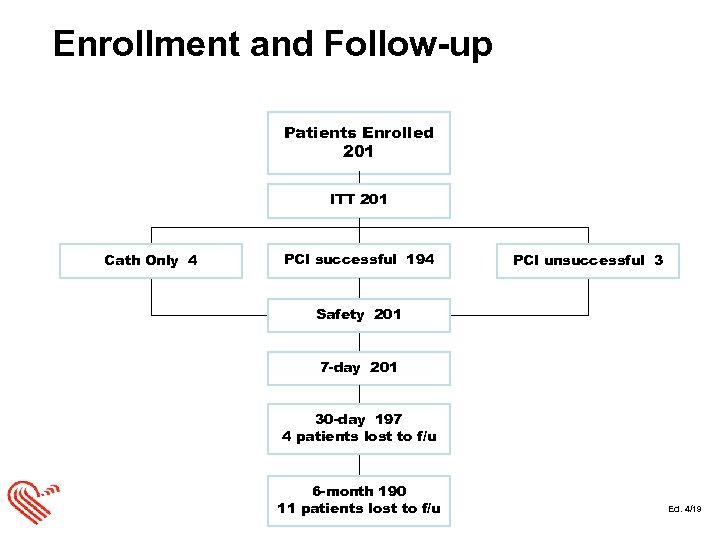 Enrollment and Follow-up Patients Enrolled 201 ITT 201 Cath Only 4 PCI successful 194 PCI unsuccessful 3 Safety 201 7 -day 201 30 -day 197 4 patients lost to f/u 6 -month 190 11 patients lost to f/u Ed. 4/19
Enrollment and Follow-up Patients Enrolled 201 ITT 201 Cath Only 4 PCI successful 194 PCI unsuccessful 3 Safety 201 7 -day 201 30 -day 197 4 patients lost to f/u 6 -month 190 11 patients lost to f/u Ed. 4/19
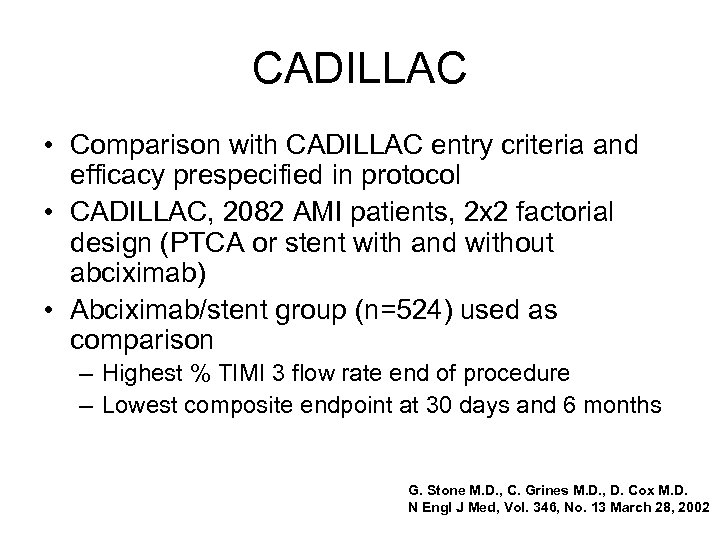 CADILLAC • Comparison with CADILLAC entry criteria and efficacy prespecified in protocol • CADILLAC, 2082 AMI patients, 2 x 2 factorial design (PTCA or stent with and without abciximab) • Abciximab/stent group (n=524) used as comparison – Highest % TIMI 3 flow rate end of procedure – Lowest composite endpoint at 30 days and 6 months G. Stone M. D. , C. Grines M. D. , D. Cox M. D. N Engl J Med, Vol. 346, No. 13 March 28, 2002
CADILLAC • Comparison with CADILLAC entry criteria and efficacy prespecified in protocol • CADILLAC, 2082 AMI patients, 2 x 2 factorial design (PTCA or stent with and without abciximab) • Abciximab/stent group (n=524) used as comparison – Highest % TIMI 3 flow rate end of procedure – Lowest composite endpoint at 30 days and 6 months G. Stone M. D. , C. Grines M. D. , D. Cox M. D. N Engl J Med, Vol. 346, No. 13 March 28, 2002
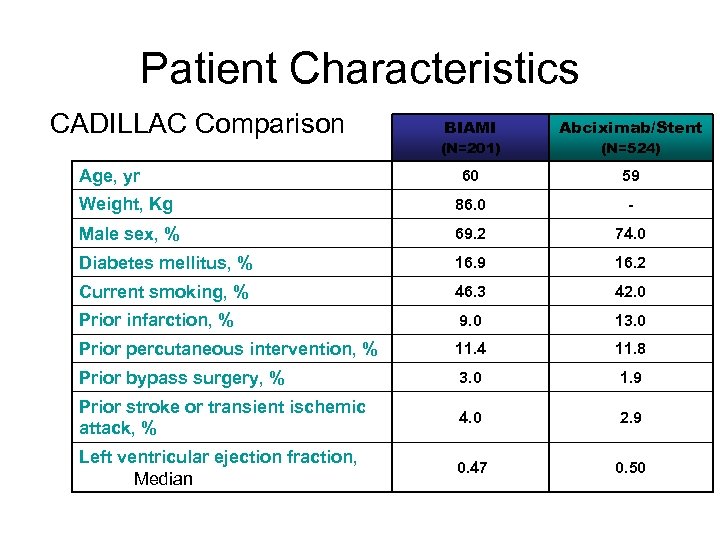 Patient Characteristics CADILLAC Comparison BIAMI Abciximab/Stent (N=201) (N=524) 60 59 Weight, Kg 86. 0 - Male sex, % 69. 2 74. 0 Diabetes mellitus, % 16. 9 16. 2 Current smoking, % 46. 3 42. 0 Prior infarction, % 9. 0 13. 0 Prior percutaneous intervention, % 11. 4 11. 8 Prior bypass surgery, % 3. 0 1. 9 Prior stroke or transient ischemic attack, % 4. 0 2. 9 Left ventricular ejection fraction, Median 0. 47 0. 50 Age, yr
Patient Characteristics CADILLAC Comparison BIAMI Abciximab/Stent (N=201) (N=524) 60 59 Weight, Kg 86. 0 - Male sex, % 69. 2 74. 0 Diabetes mellitus, % 16. 9 16. 2 Current smoking, % 46. 3 42. 0 Prior infarction, % 9. 0 13. 0 Prior percutaneous intervention, % 11. 4 11. 8 Prior bypass surgery, % 3. 0 1. 9 Prior stroke or transient ischemic attack, % 4. 0 2. 9 Left ventricular ejection fraction, Median 0. 47 0. 50 Age, yr
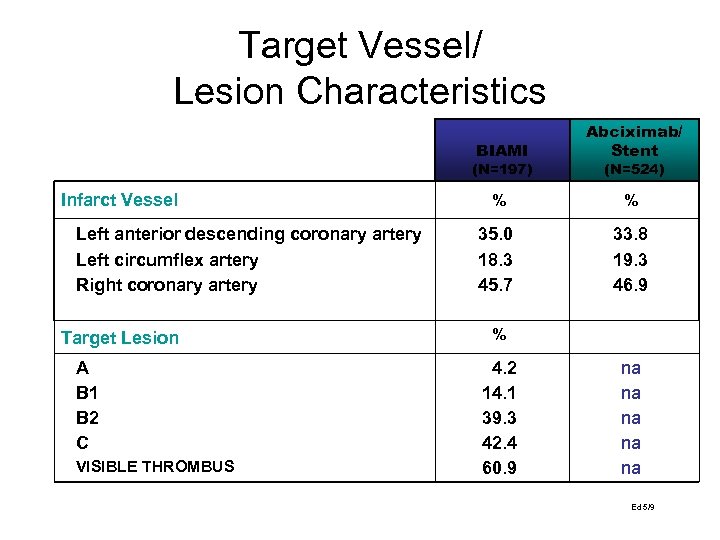 Target Vessel/ Lesion Characteristics BIAMI Abciximab/ Stent % % 35. 0 18. 3 45. 7 33. 8 19. 3 46. 9 (N=197) Infarct Vessel Left anterior descending coronary artery Left circumflex artery Right coronary artery Target Lesion A B 1 B 2 C VISIBLE THROMBUS (N=524) % 4. 2 14. 1 39. 3 42. 4 60. 9 na na na Ed 5/9
Target Vessel/ Lesion Characteristics BIAMI Abciximab/ Stent % % 35. 0 18. 3 45. 7 33. 8 19. 3 46. 9 (N=197) Infarct Vessel Left anterior descending coronary artery Left circumflex artery Right coronary artery Target Lesion A B 1 B 2 C VISIBLE THROMBUS (N=524) % 4. 2 14. 1 39. 3 42. 4 60. 9 na na na Ed 5/9
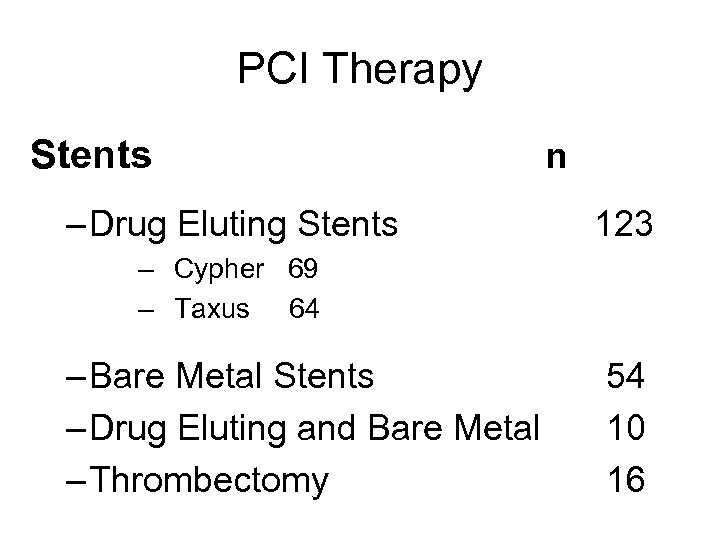 PCI Therapy Stents – Drug Eluting Stents n 123 – Cypher 69 – Taxus 64 – Bare Metal Stents – Drug Eluting and Bare Metal – Thrombectomy 54 10 16
PCI Therapy Stents – Drug Eluting Stents n 123 – Cypher 69 – Taxus 64 – Bare Metal Stents – Drug Eluting and Bare Metal – Thrombectomy 54 10 16
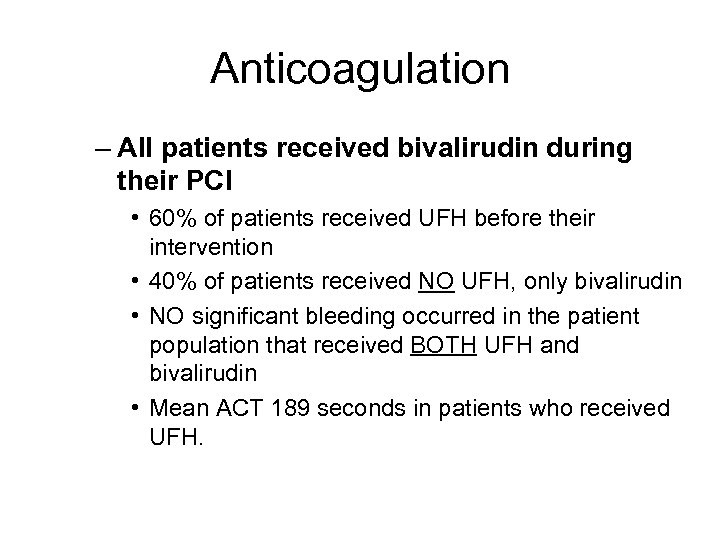 Anticoagulation – All patients received bivalirudin during their PCI • 60% of patients received UFH before their intervention • 40% of patients received NO UFH, only bivalirudin • NO significant bleeding occurred in the patient population that received BOTH UFH and bivalirudin • Mean ACT 189 seconds in patients who received UFH.
Anticoagulation – All patients received bivalirudin during their PCI • 60% of patients received UFH before their intervention • 40% of patients received NO UFH, only bivalirudin • NO significant bleeding occurred in the patient population that received BOTH UFH and bivalirudin • Mean ACT 189 seconds in patients who received UFH.
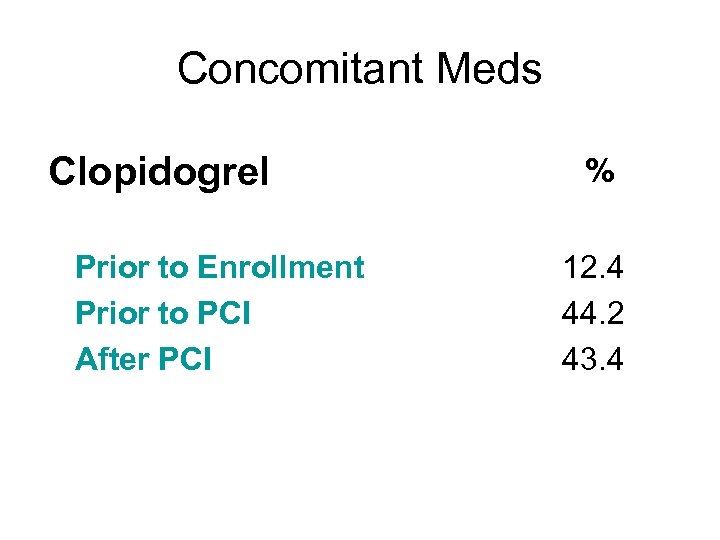 Concomitant Meds Clopidogrel Prior to Enrollment Prior to PCI After PCI % 12. 4 44. 2 43. 4
Concomitant Meds Clopidogrel Prior to Enrollment Prior to PCI After PCI % 12. 4 44. 2 43. 4
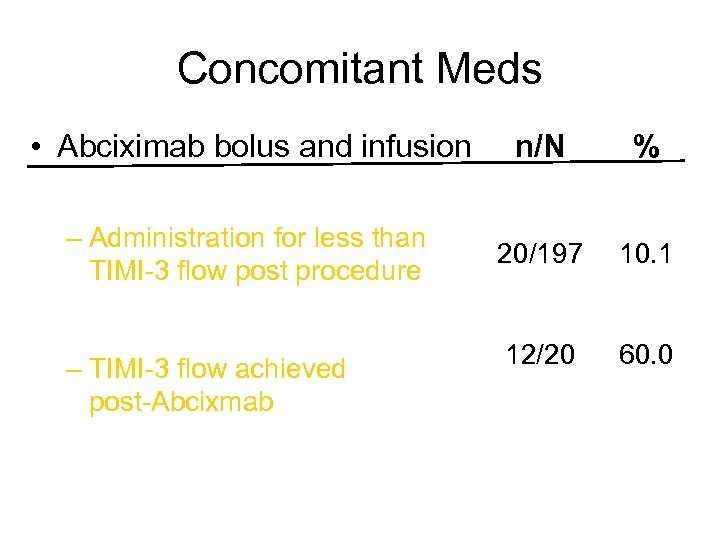 Concomitant Meds • Abciximab bolus and infusion n/N % – Administration for less than TIMI-3 flow post procedure 20/197 10. 1 12/20 60. 0 – TIMI-3 flow achieved post-Abcixmab
Concomitant Meds • Abciximab bolus and infusion n/N % – Administration for less than TIMI-3 flow post procedure 20/197 10. 1 12/20 60. 0 – TIMI-3 flow achieved post-Abcixmab
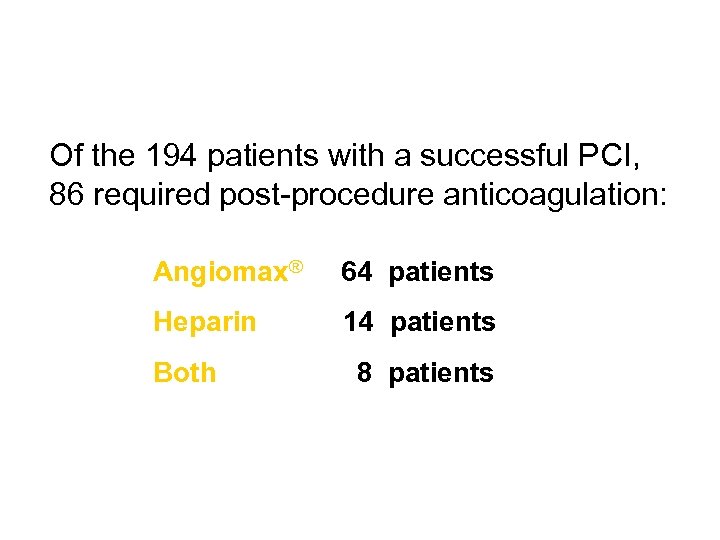 Of the 194 patients with a successful PCI, 86 required post-procedure anticoagulation: Angiomax® 64 patients Heparin 14 patients Both 8 patients
Of the 194 patients with a successful PCI, 86 required post-procedure anticoagulation: Angiomax® 64 patients Heparin 14 patients Both 8 patients
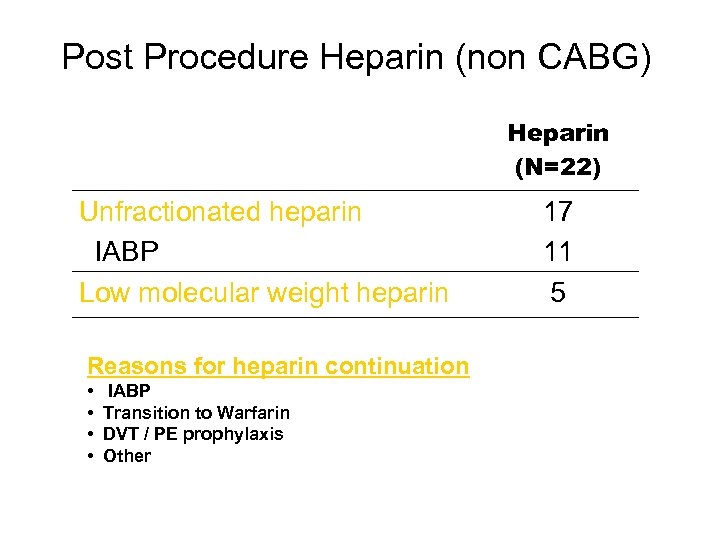 Post Procedure Heparin (non CABG) Heparin (N=22) Unfractionated heparin IABP Low molecular weight heparin Reasons for heparin continuation • • IABP Transition to Warfarin DVT / PE prophylaxis Other 17 11 5
Post Procedure Heparin (non CABG) Heparin (N=22) Unfractionated heparin IABP Low molecular weight heparin Reasons for heparin continuation • • IABP Transition to Warfarin DVT / PE prophylaxis Other 17 11 5
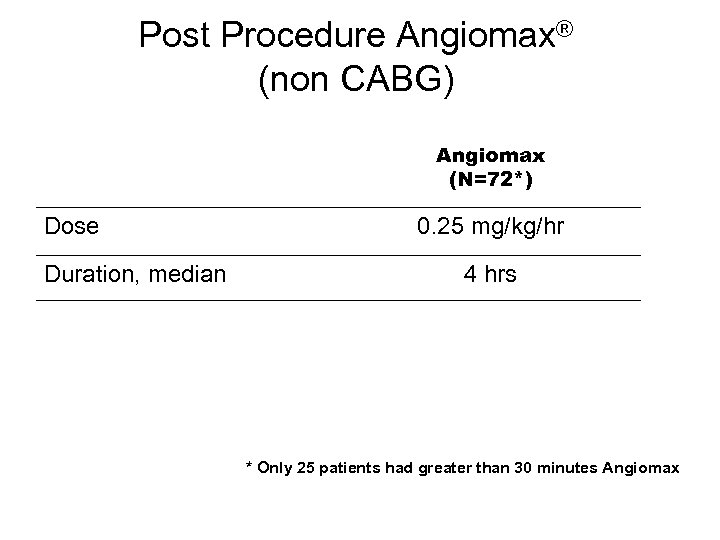 Post Procedure Angiomax® (non CABG) Angiomax (N=72*) Dose Duration, median 0. 25 mg/kg/hr 4 hrs * Only 25 patients had greater than 30 minutes Angiomax
Post Procedure Angiomax® (non CABG) Angiomax (N=72*) Dose Duration, median 0. 25 mg/kg/hr 4 hrs * Only 25 patients had greater than 30 minutes Angiomax
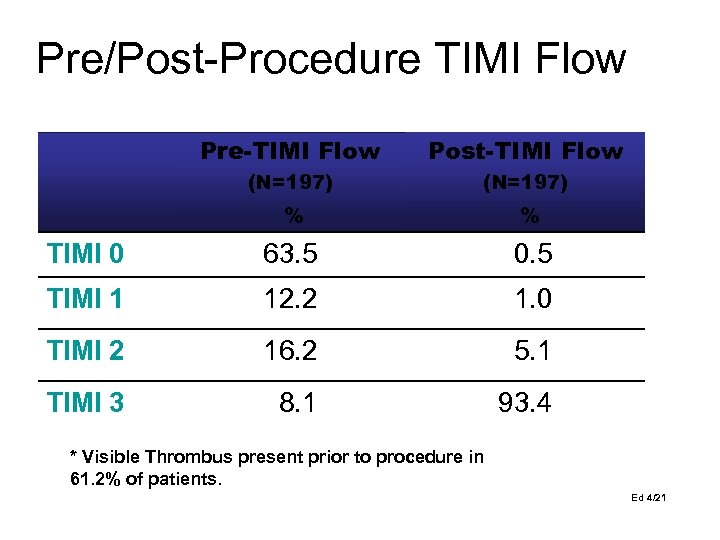 Pre/Post-Procedure TIMI Flow Pre-TIMI Flow Post-TIMI Flow (N=197) % % TIMI 0 63. 5 0. 5 TIMI 1 12. 2 1. 0 TIMI 2 16. 2 5. 1 TIMI 3 8. 1 93. 4 * Visible Thrombus present prior to procedure in 61. 2% of patients. Ed 4/21
Pre/Post-Procedure TIMI Flow Pre-TIMI Flow Post-TIMI Flow (N=197) % % TIMI 0 63. 5 0. 5 TIMI 1 12. 2 1. 0 TIMI 2 16. 2 5. 1 TIMI 3 8. 1 93. 4 * Visible Thrombus present prior to procedure in 61. 2% of patients. Ed 4/21
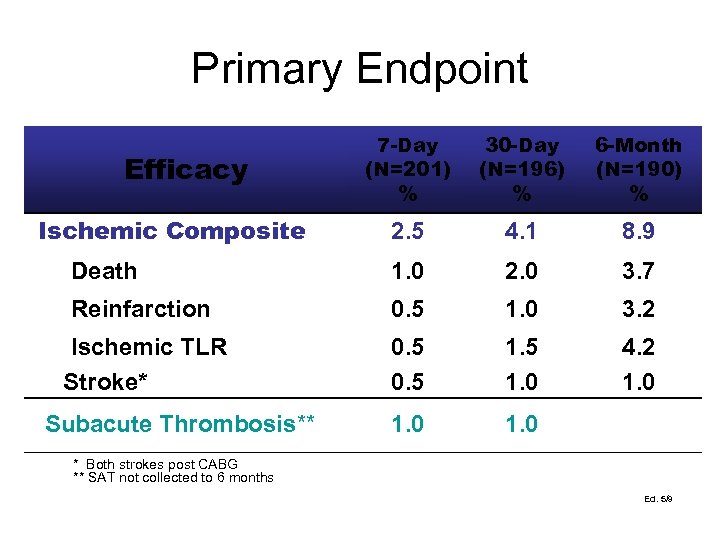 Primary Endpoint 7 -Day (N=201) % 30 -Day (N=196) % 6 -Month (N=190) % 2. 5 4. 1 8. 9 Death 1. 0 2. 0 3. 7 Reinfarction 0. 5 1. 0 3. 2 Ischemic TLR Stroke* 0. 5 1. 0 4. 2 1. 0 Efficacy Ischemic Composite Subacute Thrombosis** * Both strokes post CABG ** SAT not collected to 6 months Ed. 5/9
Primary Endpoint 7 -Day (N=201) % 30 -Day (N=196) % 6 -Month (N=190) % 2. 5 4. 1 8. 9 Death 1. 0 2. 0 3. 7 Reinfarction 0. 5 1. 0 3. 2 Ischemic TLR Stroke* 0. 5 1. 0 4. 2 1. 0 Efficacy Ischemic Composite Subacute Thrombosis** * Both strokes post CABG ** SAT not collected to 6 months Ed. 5/9
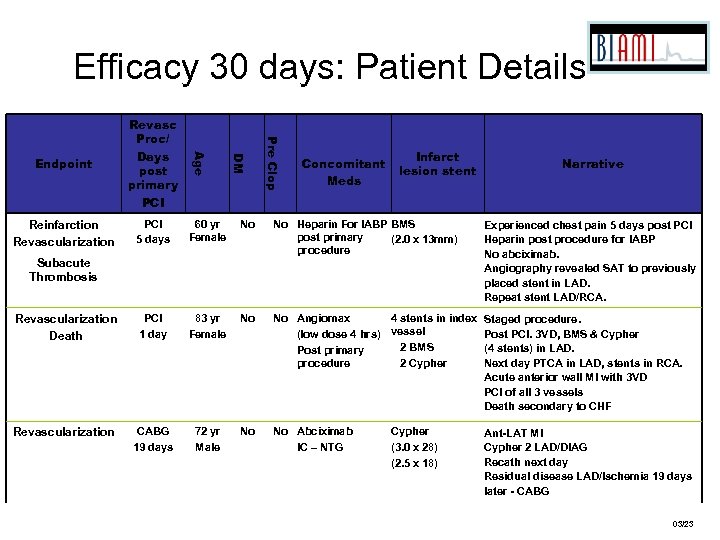 Efficacy 30 days: Patient Details 60 yr Female No No Heparin For IABP BMS post primary (2. 0 x 13 mm) procedure Experienced chest pain 5 days post PCI Heparin post procedure for IABP No abciximab. Angiography revealed SAT to previously placed stent in LAD. Repeat stent LAD/RCA. Revascularization Death #14004 PCI 1 day 83 yr Female No No Angiomax 4 stents in index (low dose 4 hrs) vessel 2 BMS Post primary procedure 2 Cypher Staged procedure. Post PCI. 3 VD, BMS & Cypher (4 stents) in LAD. Next day PTCA in LAD, stents in RCA. Acute anterior wall MI with 3 VD PCI of all 3 vessels Death secondary to CHF Revascularization #07004 CABG 19 days 72 yr Male No No Abciximab IC – NTG Ant-LAT MI Cypher 2 LAD/DIAG Recath next day Residual disease LAD/Ischemia 19 days later - CABG Subacute Thrombosis #01005 Pre Clop PCI 5 days DM Reinfarction Revascularization Age Endpoint Revasc Proc/ Days post primary PCI Concomitant Meds Infarct lesion stent Cypher (3. 0 x 28) (2. 5 x 18) Narrative 03/23
Efficacy 30 days: Patient Details 60 yr Female No No Heparin For IABP BMS post primary (2. 0 x 13 mm) procedure Experienced chest pain 5 days post PCI Heparin post procedure for IABP No abciximab. Angiography revealed SAT to previously placed stent in LAD. Repeat stent LAD/RCA. Revascularization Death #14004 PCI 1 day 83 yr Female No No Angiomax 4 stents in index (low dose 4 hrs) vessel 2 BMS Post primary procedure 2 Cypher Staged procedure. Post PCI. 3 VD, BMS & Cypher (4 stents) in LAD. Next day PTCA in LAD, stents in RCA. Acute anterior wall MI with 3 VD PCI of all 3 vessels Death secondary to CHF Revascularization #07004 CABG 19 days 72 yr Male No No Abciximab IC – NTG Ant-LAT MI Cypher 2 LAD/DIAG Recath next day Residual disease LAD/Ischemia 19 days later - CABG Subacute Thrombosis #01005 Pre Clop PCI 5 days DM Reinfarction Revascularization Age Endpoint Revasc Proc/ Days post primary PCI Concomitant Meds Infarct lesion stent Cypher (3. 0 x 28) (2. 5 x 18) Narrative 03/23
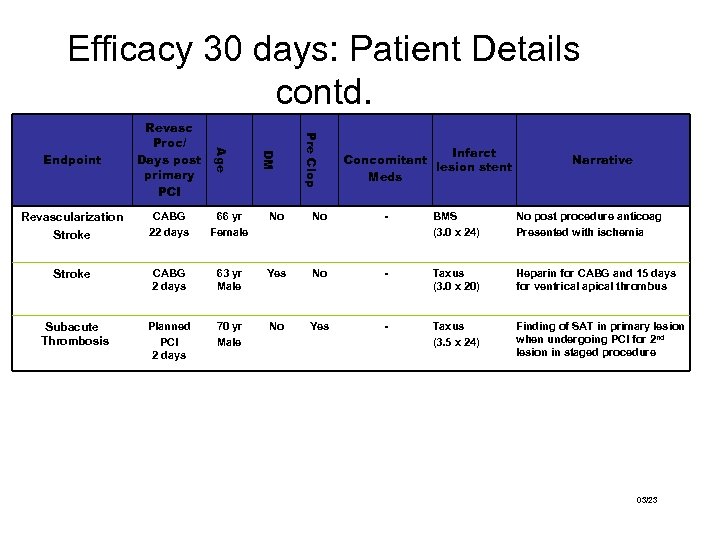 Efficacy 30 days: Patient Details contd. Pre Clop DM Age Endpoint Revasc Proc/ Days post primary PCI Infarct Concomitant lesion stent Meds Narrative Revascularization Stroke #1011 CABG 22 days 66 yr Female No No - BMS (3. 0 x 24) No post procedure anticoag Presented with ischemia Stroke #10003 CABG 2 days 63 yr Male Yes No - Taxus (3. 0 x 20) Heparin for CABG and 15 days for ventrical apical thrombus Subacute Thrombosis #2013 Planned PCI 2 days 70 yr Male No Yes - Taxus (3. 5 x 24) Finding of SAT in primary lesion when undergoing PCI for 2 nd lesion in staged procedure 03/23
Efficacy 30 days: Patient Details contd. Pre Clop DM Age Endpoint Revasc Proc/ Days post primary PCI Infarct Concomitant lesion stent Meds Narrative Revascularization Stroke #1011 CABG 22 days 66 yr Female No No - BMS (3. 0 x 24) No post procedure anticoag Presented with ischemia Stroke #10003 CABG 2 days 63 yr Male Yes No - Taxus (3. 0 x 20) Heparin for CABG and 15 days for ventrical apical thrombus Subacute Thrombosis #2013 Planned PCI 2 days 70 yr Male No Yes - Taxus (3. 5 x 24) Finding of SAT in primary lesion when undergoing PCI for 2 nd lesion in staged procedure 03/23
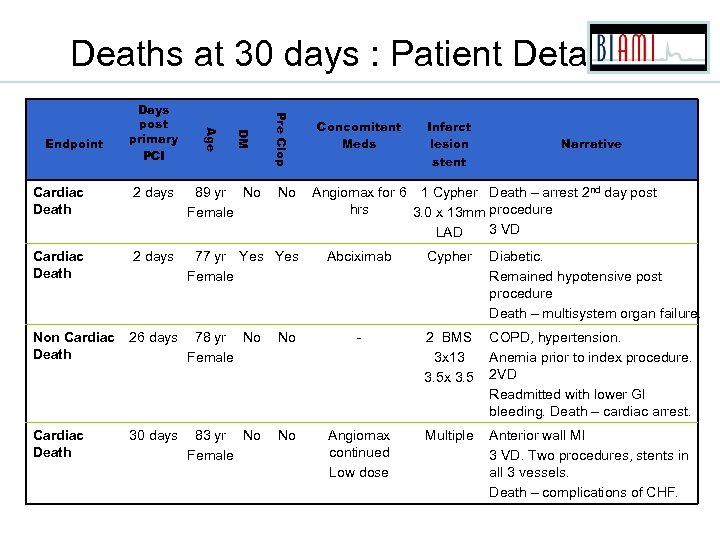 Deaths at 30 days : Patient Details Pre Clop DM Age Endpoint Days post primary PCI Cardiac Death 2 days 89 yr No Female No Cardiac Death 2 days 77 yr Yes Female Non Cardiac Death 26 days 78 yr No Female Cardiac Death 30 days 83 yr No Female Concomitant Meds Infarct lesion stent Narrative Angiomax for 6 1 Cypher Death – arrest 2 nd day post hrs 3. 0 x 13 mm procedure 3 VD LAD Abciximab Cypher Diabetic. Remained hypotensive post procedure Death – multisystem organ failure. No - 2 BMS 3 x 13 3. 5 x 3. 5 COPD, hypertension. Anemia prior to index procedure. 2 VD Readmitted with lower GI bleeding. Death – cardiac arrest. No Angiomax continued Low dose Multiple Anterior wall MI 3 VD. Two procedures, stents in all 3 vessels. Death – complications of CHF.
Deaths at 30 days : Patient Details Pre Clop DM Age Endpoint Days post primary PCI Cardiac Death 2 days 89 yr No Female No Cardiac Death 2 days 77 yr Yes Female Non Cardiac Death 26 days 78 yr No Female Cardiac Death 30 days 83 yr No Female Concomitant Meds Infarct lesion stent Narrative Angiomax for 6 1 Cypher Death – arrest 2 nd day post hrs 3. 0 x 13 mm procedure 3 VD LAD Abciximab Cypher Diabetic. Remained hypotensive post procedure Death – multisystem organ failure. No - 2 BMS 3 x 13 3. 5 x 3. 5 COPD, hypertension. Anemia prior to index procedure. 2 VD Readmitted with lower GI bleeding. Death – cardiac arrest. No Angiomax continued Low dose Multiple Anterior wall MI 3 VD. Two procedures, stents in all 3 vessels. Death – complications of CHF.
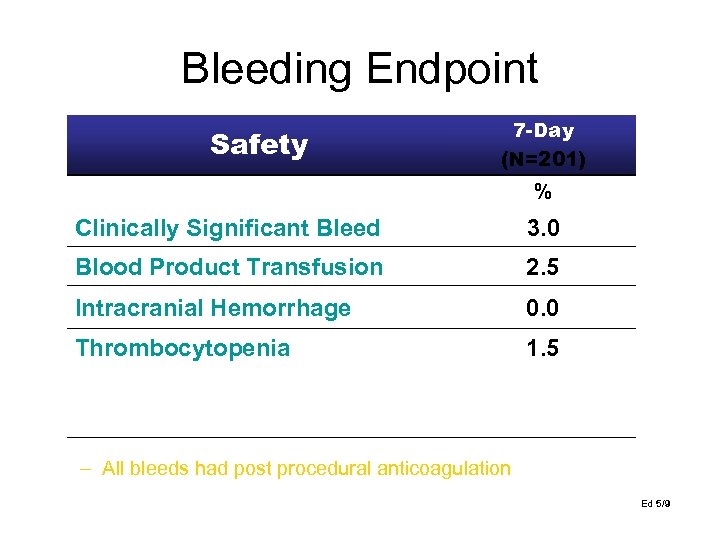 Bleeding Endpoint Safety 7 -Day (N=201) % Clinically Significant Bleed 3. 0 Blood Product Transfusion 2. 5 Intracranial Hemorrhage 0. 0 Thrombocytopenia 1. 5 i. Abciximab & IABP ii. IABP Bleed ii. No PCI / on table arrest #9002 #12005 #6003 – All bleeds had post procedural anticoagulation Ed 5/9
Bleeding Endpoint Safety 7 -Day (N=201) % Clinically Significant Bleed 3. 0 Blood Product Transfusion 2. 5 Intracranial Hemorrhage 0. 0 Thrombocytopenia 1. 5 i. Abciximab & IABP ii. IABP Bleed ii. No PCI / on table arrest #9002 #12005 #6003 – All bleeds had post procedural anticoagulation Ed 5/9
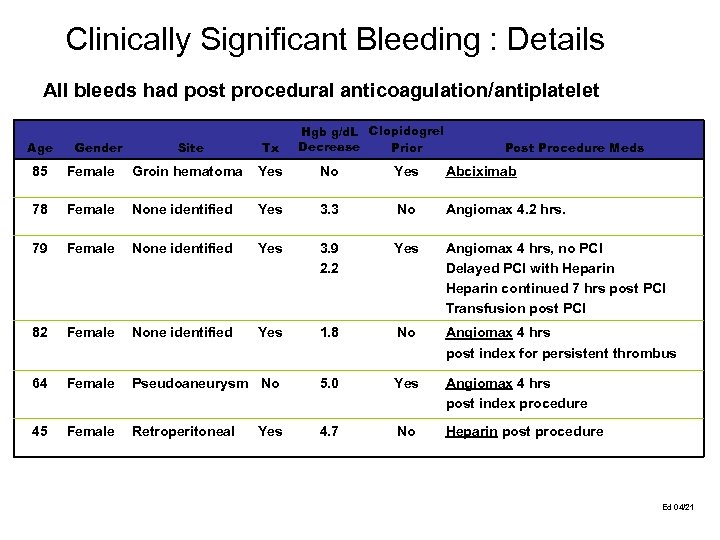 Clinically Significant Bleeding : Details All bleeds had post procedural anticoagulation/antiplatelet Age 85 Gender Hgb g/d. L Clopidogrel Decrease Prior Site Tx Post Procedure Meds Female Groin hematoma Yes No Yes Abciximab Female None identified Yes 3. 3 No Angiomax 4. 2 hrs. Female None identified Yes 3. 9 2. 2 Yes Angiomax 4 hrs, no PCI Delayed PCI with Heparin continued 7 hrs post PCI Transfusion post PCI Female None identified Yes 1. 8 No Angiomax 4 hrs post index for persistent thrombus Female Pseudoaneurysm No 5. 0 Yes Angiomax 4 hrs post index procedure Female Retroperitoneal 4. 7 No Heparin post procedure #07011 78 #14013 79 #14012 82 #07002 64 #14018 45 Yes #1046 Ed 04/21
Clinically Significant Bleeding : Details All bleeds had post procedural anticoagulation/antiplatelet Age 85 Gender Hgb g/d. L Clopidogrel Decrease Prior Site Tx Post Procedure Meds Female Groin hematoma Yes No Yes Abciximab Female None identified Yes 3. 3 No Angiomax 4. 2 hrs. Female None identified Yes 3. 9 2. 2 Yes Angiomax 4 hrs, no PCI Delayed PCI with Heparin continued 7 hrs post PCI Transfusion post PCI Female None identified Yes 1. 8 No Angiomax 4 hrs post index for persistent thrombus Female Pseudoaneurysm No 5. 0 Yes Angiomax 4 hrs post index procedure Female Retroperitoneal 4. 7 No Heparin post procedure #07011 78 #14013 79 #14012 82 #07002 64 #14018 45 Yes #1046 Ed 04/21
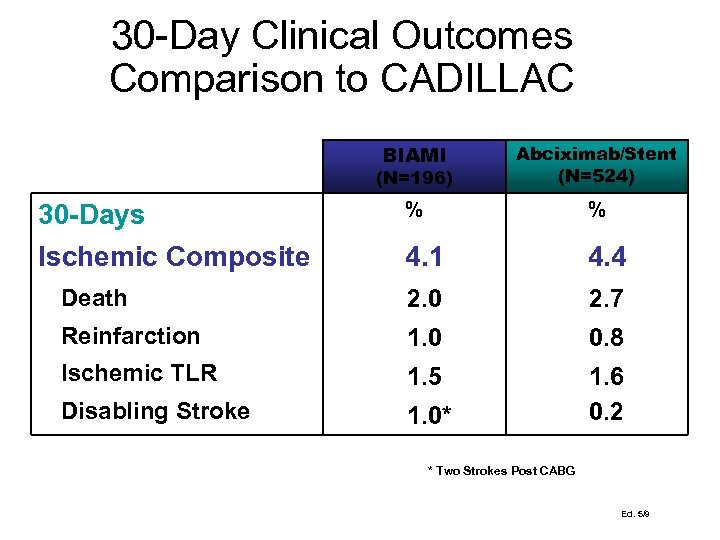 30 -Day Clinical Outcomes Comparison to CADILLAC BIAMI (N=196) Abciximab/Stent (N=524) % % 4. 1 4. 4 Death 2. 0 2. 7 Reinfarction 1. 0 0. 8 Ischemic TLR 1. 5 Disabling Stroke 1. 0* 1. 6 0. 2 30 -Days Ischemic Composite * Two Strokes Post CABG Ed. 5/9
30 -Day Clinical Outcomes Comparison to CADILLAC BIAMI (N=196) Abciximab/Stent (N=524) % % 4. 1 4. 4 Death 2. 0 2. 7 Reinfarction 1. 0 0. 8 Ischemic TLR 1. 5 Disabling Stroke 1. 0* 1. 6 0. 2 30 -Days Ischemic Composite * Two Strokes Post CABG Ed. 5/9
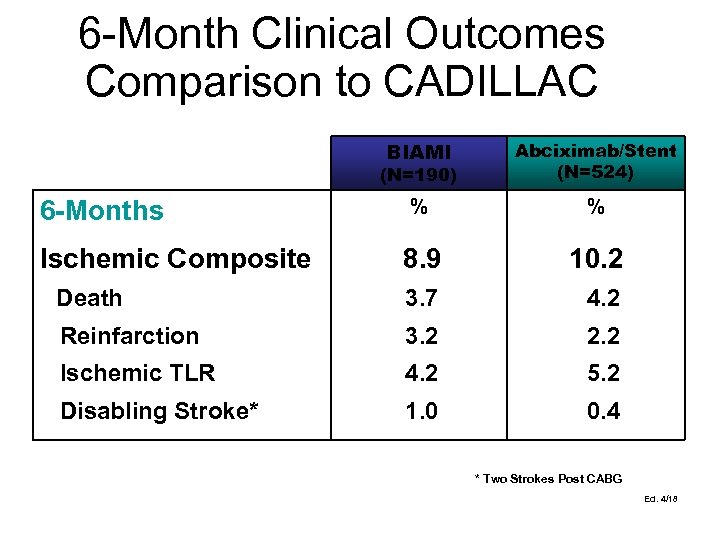 6 -Month Clinical Outcomes Comparison to CADILLAC BIAMI (N=190) Abciximab/Stent (N=524) % % 8. 9 10. 2 Death 3. 7 4. 2 Reinfarction 3. 2 2. 2 Ischemic TLR 4. 2 5. 2 Disabling Stroke* 1. 0 0. 4 6 -Months Ischemic Composite * Two Strokes Post CABG Ed. 4/18
6 -Month Clinical Outcomes Comparison to CADILLAC BIAMI (N=190) Abciximab/Stent (N=524) % % 8. 9 10. 2 Death 3. 7 4. 2 Reinfarction 3. 2 2. 2 Ischemic TLR 4. 2 5. 2 Disabling Stroke* 1. 0 0. 4 6 -Months Ischemic Composite * Two Strokes Post CABG Ed. 4/18


