9dd39614494f48bf5493b49b4a5ad5d7.ppt
- Количество слайдов: 16
 Health Information Technology Standards Panel HITSP Technical Committee and Approval of its Interoperability Specifications 4 Charles Parisot, GE Healthcare August 2006
Health Information Technology Standards Panel HITSP Technical Committee and Approval of its Interoperability Specifications 4 Charles Parisot, GE Healthcare August 2006
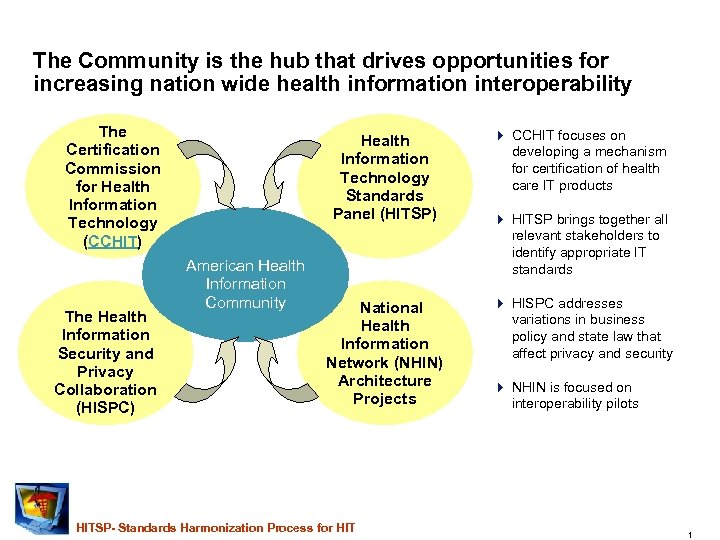 The Community is the hub that drives opportunities for increasing nation wide health information interoperability The Certification Commission for Health Information Technology (CCHIT) The Health Information Security and Privacy Collaboration (HISPC) Health Information Technology Standards Panel (HITSP) American Health Information Community 4 CCHIT focuses on developing a mechanism for certification of health care IT products National Health Information Network (NHIN) Architecture Projects 4 HISPC addresses variations in business policy and state law that affect privacy and security HITSP- Standards Harmonization Process for HIT 4 HITSP brings together all relevant stakeholders to identify appropriate IT standards 4 NHIN is focused on interoperability pilots 1
The Community is the hub that drives opportunities for increasing nation wide health information interoperability The Certification Commission for Health Information Technology (CCHIT) The Health Information Security and Privacy Collaboration (HISPC) Health Information Technology Standards Panel (HITSP) American Health Information Community 4 CCHIT focuses on developing a mechanism for certification of health care IT products National Health Information Network (NHIN) Architecture Projects 4 HISPC addresses variations in business policy and state law that affect privacy and security HITSP- Standards Harmonization Process for HIT 4 HITSP brings together all relevant stakeholders to identify appropriate IT standards 4 NHIN is focused on interoperability pilots 1
 HITSP was formed to prototype a process used to harmonize industry-wide HIT standards. . . 4 HITSP formed under the sponsorship of the American National Standards Institute (ANSI), coordinator of the U. S. voluntary standardization system 4 Brings together a wide range of stakeholders into a formal “panel” to identify, select, and harmonize standards for communicating data throughout the healthcare spectrum 4 Formation of the Panel was endorsed by a number of industry groups and has the oversight and backing ONCHIT 4 John D. Halamka, MD, MS, CIO of the Harvard School of Medicine chairs the Panel 4 A total of 200 organizations participate in HITSP representing consumer, SDO, non. SDOs, and government interests 4 Non SDO make up 67% of the panel and include clinicians, providers, safety net providers, vendors, purchasers, payers, public health professionals, and researchers HITSP- Standards Harmonization Process for HIT 2
HITSP was formed to prototype a process used to harmonize industry-wide HIT standards. . . 4 HITSP formed under the sponsorship of the American National Standards Institute (ANSI), coordinator of the U. S. voluntary standardization system 4 Brings together a wide range of stakeholders into a formal “panel” to identify, select, and harmonize standards for communicating data throughout the healthcare spectrum 4 Formation of the Panel was endorsed by a number of industry groups and has the oversight and backing ONCHIT 4 John D. Halamka, MD, MS, CIO of the Harvard School of Medicine chairs the Panel 4 A total of 200 organizations participate in HITSP representing consumer, SDO, non. SDOs, and government interests 4 Non SDO make up 67% of the panel and include clinicians, providers, safety net providers, vendors, purchasers, payers, public health professionals, and researchers HITSP- Standards Harmonization Process for HIT 2
 . . . The process is repeatable and fully integrated with CCHIT and AHIC 1. For each AHIC Use Case, HITSP Technical Committees identify candidate standards which are harmonized into a final list of standards 4 They also identify overlaps and highlight gaps. Gaps are forwarded to Standards Development Organizations for their guidance as to emerging candidate standards or new standards requirements. 2. The final standards chosen by the Technical Committees are discussed and ratified by the HITSP panel. 3. These standards are available for public comment and feedback. 4. Technical Committees work with SDOs and other groups to produce detailed specifications, an unambiguous “cookbook”, for the implementation of chosen standards. HITSP provides a convening and facilitation function for this activity. 5. HITSP work products are delivered to AHIC for their endorsement. 6. CCHIT will include functional criteria for interoperability based on HITSP specifications in its certification work HITSP- Standards Harmonization Process for HIT 3
. . . The process is repeatable and fully integrated with CCHIT and AHIC 1. For each AHIC Use Case, HITSP Technical Committees identify candidate standards which are harmonized into a final list of standards 4 They also identify overlaps and highlight gaps. Gaps are forwarded to Standards Development Organizations for their guidance as to emerging candidate standards or new standards requirements. 2. The final standards chosen by the Technical Committees are discussed and ratified by the HITSP panel. 3. These standards are available for public comment and feedback. 4. Technical Committees work with SDOs and other groups to produce detailed specifications, an unambiguous “cookbook”, for the implementation of chosen standards. HITSP provides a convening and facilitation function for this activity. 5. HITSP work products are delivered to AHIC for their endorsement. 6. CCHIT will include functional criteria for interoperability based on HITSP specifications in its certification work HITSP- Standards Harmonization Process for HIT 3
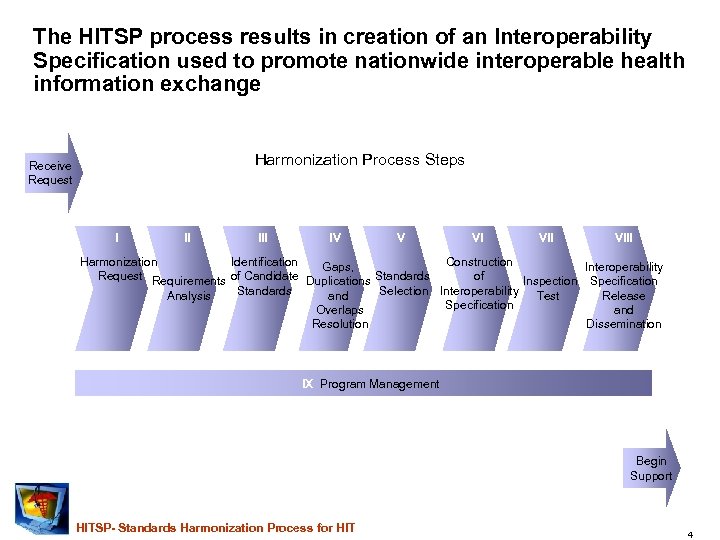 The HITSP process results in creation of an Interoperability Specification used to promote nationwide interoperable health information exchange Harmonization Process Steps Receive Request I II IV V VI VIII Harmonization Identification Construction Gaps, Interoperability Request Requirements of Candidate Duplications Standards of Inspection Specification Standards Selection Interoperability Test Analysis and Release Specification Overlaps and Resolution Dissemination IX Program Management Begin Support HITSP- Standards Harmonization Process for HIT 4
The HITSP process results in creation of an Interoperability Specification used to promote nationwide interoperable health information exchange Harmonization Process Steps Receive Request I II IV V VI VIII Harmonization Identification Construction Gaps, Interoperability Request Requirements of Candidate Duplications Standards of Inspection Specification Standards Selection Interoperability Test Analysis and Release Specification Overlaps and Resolution Dissemination IX Program Management Begin Support HITSP- Standards Harmonization Process for HIT 4
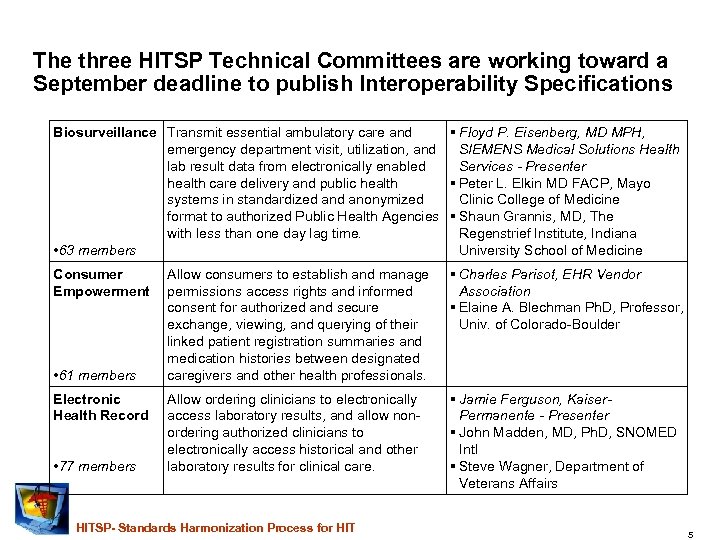 The three HITSP Technical Committees are working toward a September deadline to publish Interoperability Specifications Biosurveillance Transmit essential ambulatory care and Floyd P. Eisenberg, MD MPH, emergency department visit, utilization, and SIEMENS Medical Solutions Health lab result data from electronically enabled Services - Presenter health care delivery and public health Peter L. Elkin MD FACP, Mayo systems in standardized anonymized Clinic College of Medicine format to authorized Public Health Agencies Shaun Grannis, MD, The with less than one day lag time. Regenstrief Institute, Indiana • 63 members University School of Medicine Consumer Allow consumers to establish and manage Empowerment permissions access rights and informed consent for authorized and secure exchange, viewing, and querying of their linked patient registration summaries and medication histories between designated • 61 members caregivers and other health professionals. Charles Parisot, EHR Vendor Association Elaine A. Blechman Ph. D, Professor, Univ. of Colorado-Boulder Electronic Health Record Jamie Ferguson, Kaiser. Permanente - Presenter John Madden, MD, Ph. D, SNOMED Intl Steve Wagner, Department of Veterans Affairs • 77 members Allow ordering clinicians to electronically access laboratory results, and allow nonordering authorized clinicians to electronically access historical and other laboratory results for clinical care. HITSP- Standards Harmonization Process for HIT 5
The three HITSP Technical Committees are working toward a September deadline to publish Interoperability Specifications Biosurveillance Transmit essential ambulatory care and Floyd P. Eisenberg, MD MPH, emergency department visit, utilization, and SIEMENS Medical Solutions Health lab result data from electronically enabled Services - Presenter health care delivery and public health Peter L. Elkin MD FACP, Mayo systems in standardized anonymized Clinic College of Medicine format to authorized Public Health Agencies Shaun Grannis, MD, The with less than one day lag time. Regenstrief Institute, Indiana • 63 members University School of Medicine Consumer Allow consumers to establish and manage Empowerment permissions access rights and informed consent for authorized and secure exchange, viewing, and querying of their linked patient registration summaries and medication histories between designated • 61 members caregivers and other health professionals. Charles Parisot, EHR Vendor Association Elaine A. Blechman Ph. D, Professor, Univ. of Colorado-Boulder Electronic Health Record Jamie Ferguson, Kaiser. Permanente - Presenter John Madden, MD, Ph. D, SNOMED Intl Steve Wagner, Department of Veterans Affairs • 77 members Allow ordering clinicians to electronically access laboratory results, and allow nonordering authorized clinicians to electronically access historical and other laboratory results for clinical care. HITSP- Standards Harmonization Process for HIT 5
 Progress to date has positioned each committee to provide NCVHS with relevant insights into NHIN requirements 4 In June of 2006, HITSP reduced 570 candidate standards to 90 appropriate standards for secure exchange of medication, lab, allergy and demographic data 4 By September 29, 2006, HITSP will deliver unambiguous interoperability specifications which will enable vendors, hospitals and government to create software components for clinical data exchange 4 Beyond 2006, HITSP will develop harmonized standards and unambiguous implementation guides which provide precise instructions for data sharing for all future requests for harmonization 4 Also, it will standardize the interoperability specifications for technology products, while permitting differentiation and competitive advantage in the marketplace. HITSP hopes to empower patients and care providers with Electronic Health Records (EHR) that facilitate easy access to critical health data that is accurate, private and secure. 4 HITSP is a key component of the Health and Human Services vision to create an interoperable healthcare system, and we look forward to our work products empowering patients, providers and government stakeholders in 2006 and beyond HITSP- Standards Harmonization Process for HIT 6
Progress to date has positioned each committee to provide NCVHS with relevant insights into NHIN requirements 4 In June of 2006, HITSP reduced 570 candidate standards to 90 appropriate standards for secure exchange of medication, lab, allergy and demographic data 4 By September 29, 2006, HITSP will deliver unambiguous interoperability specifications which will enable vendors, hospitals and government to create software components for clinical data exchange 4 Beyond 2006, HITSP will develop harmonized standards and unambiguous implementation guides which provide precise instructions for data sharing for all future requests for harmonization 4 Also, it will standardize the interoperability specifications for technology products, while permitting differentiation and competitive advantage in the marketplace. HITSP hopes to empower patients and care providers with Electronic Health Records (EHR) that facilitate easy access to critical health data that is accurate, private and secure. 4 HITSP is a key component of the Health and Human Services vision to create an interoperable healthcare system, and we look forward to our work products empowering patients, providers and government stakeholders in 2006 and beyond HITSP- Standards Harmonization Process for HIT 6
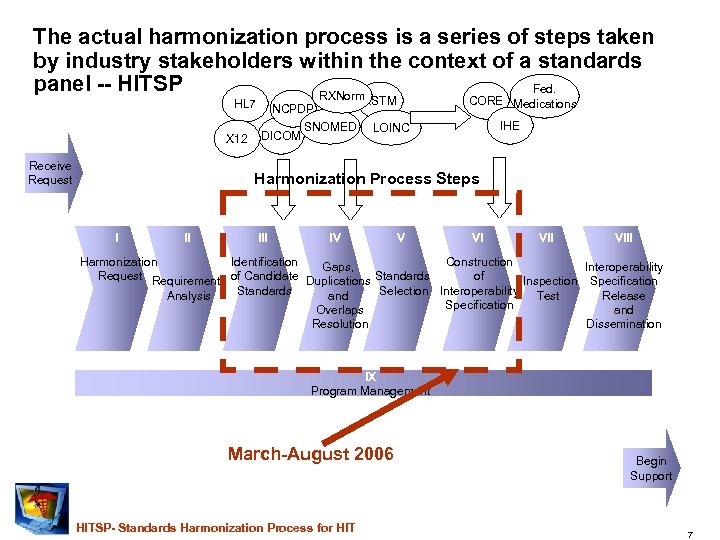 The actual harmonization process is a series of steps taken by industry stakeholders within the context of a standards panel -- HITSP Fed. RXNorm HL 7 X 12 Receive Request ASTM NCPDP DICOM SNOMED CORE Medications IHE LOINC Harmonization Process Steps I II IV V VI VIII Harmonization Identification Construction Gaps, Interoperability Request Requirements of Candidate Duplications Standards of Inspection Specification Standards Selection Interoperability Test Analysis and Release Specification Overlaps and Resolution Dissemination IX Program Management March-August 2006 HITSP- Standards Harmonization Process for HIT Begin Support 7
The actual harmonization process is a series of steps taken by industry stakeholders within the context of a standards panel -- HITSP Fed. RXNorm HL 7 X 12 Receive Request ASTM NCPDP DICOM SNOMED CORE Medications IHE LOINC Harmonization Process Steps I II IV V VI VIII Harmonization Identification Construction Gaps, Interoperability Request Requirements of Candidate Duplications Standards of Inspection Specification Standards Selection Interoperability Test Analysis and Release Specification Overlaps and Resolution Dissemination IX Program Management March-August 2006 HITSP- Standards Harmonization Process for HIT Begin Support 7
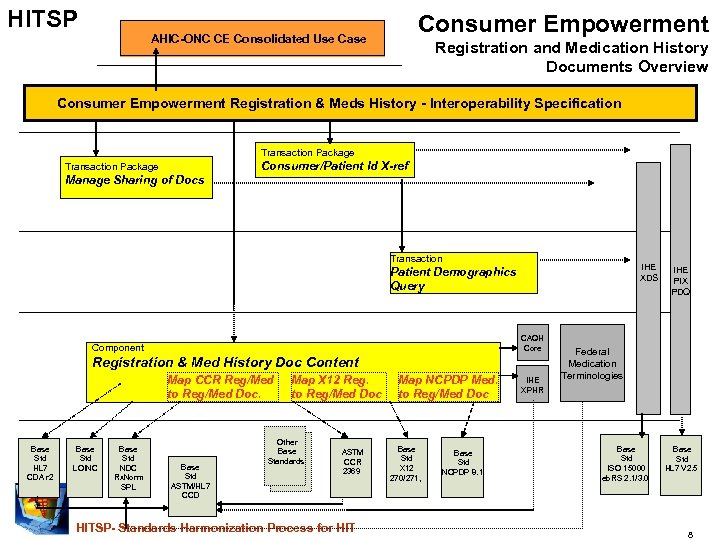 HITSP Consumer Empowerment AHIC-ONC CE Consolidated Use Case Registration and Medication History Documents Overview Consumer Empowerment Registration & Meds History - Interoperability Specification Transaction Package Consumer/Patient Id X-ref Transaction Package Manage Sharing of Docs Transaction IHE XDS Patient Demographics Query CAQH Core Component Registration & Med History Doc Content Map CCR Reg/Med to Reg/Med Doc. Base Std HL 7 CDA r 2 Base Std LOINC Base Std NDC Rx. Norm SPL Base Std ASTM/HL 7 CCD Map X 12 Reg. to Reg/Med Doc Other Base Standards ASTM CCR 2369 HITSP- Standards Harmonization Process for HIT Map NCPDP Med. to Reg/Med Doc Base Std X 12 270/271, Base Std NCPDP 8. 1 IHE XPHR IHE PIX PDQ Federal Medication Terminologies Base Std ISO 15000 eb. RS 2. 1/3. 0 Base Std HL 7 V 2. 5 8
HITSP Consumer Empowerment AHIC-ONC CE Consolidated Use Case Registration and Medication History Documents Overview Consumer Empowerment Registration & Meds History - Interoperability Specification Transaction Package Consumer/Patient Id X-ref Transaction Package Manage Sharing of Docs Transaction IHE XDS Patient Demographics Query CAQH Core Component Registration & Med History Doc Content Map CCR Reg/Med to Reg/Med Doc. Base Std HL 7 CDA r 2 Base Std LOINC Base Std NDC Rx. Norm SPL Base Std ASTM/HL 7 CCD Map X 12 Reg. to Reg/Med Doc Other Base Standards ASTM CCR 2369 HITSP- Standards Harmonization Process for HIT Map NCPDP Med. to Reg/Med Doc Base Std X 12 270/271, Base Std NCPDP 8. 1 IHE XPHR IHE PIX PDQ Federal Medication Terminologies Base Std ISO 15000 eb. RS 2. 1/3. 0 Base Std HL 7 V 2. 5 8
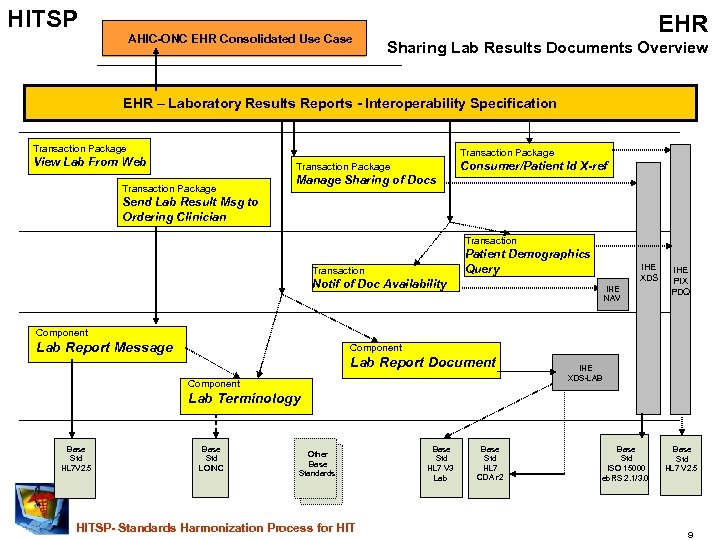 HITSP AHIC-ONC EHR Consolidated Use Case EHR Sharing Lab Results Documents Overview EHR – Laboratory Results Reports - Interoperability Specification Transaction Package View Lab From Web Consumer/Patient Id X-ref Transaction Package Manage Sharing of Docs Send Lab Result Msg to Ordering Clinician Transaction Patient Demographics Query Transaction IHE XDS Notif of Doc Availability IHE NAV IHE PIX PDQ Component Lab Report Message Component Lab Report Document Component IHE XDS-LAB Lab Terminology Base Std HL 7 V 2. 5 Base Std LOINC Other Base Standards HITSP- Standards Harmonization Process for HIT Base Std HL 7 V 3 Lab Base Std HL 7 CDA r 2 Base Std ISO 15000 eb. RS 2. 1/3. 0 Base Std HL 7 V 2. 5 9
HITSP AHIC-ONC EHR Consolidated Use Case EHR Sharing Lab Results Documents Overview EHR – Laboratory Results Reports - Interoperability Specification Transaction Package View Lab From Web Consumer/Patient Id X-ref Transaction Package Manage Sharing of Docs Send Lab Result Msg to Ordering Clinician Transaction Patient Demographics Query Transaction IHE XDS Notif of Doc Availability IHE NAV IHE PIX PDQ Component Lab Report Message Component Lab Report Document Component IHE XDS-LAB Lab Terminology Base Std HL 7 V 2. 5 Base Std LOINC Other Base Standards HITSP- Standards Harmonization Process for HIT Base Std HL 7 V 3 Lab Base Std HL 7 CDA r 2 Base Std ISO 15000 eb. RS 2. 1/3. 0 Base Std HL 7 V 2. 5 9
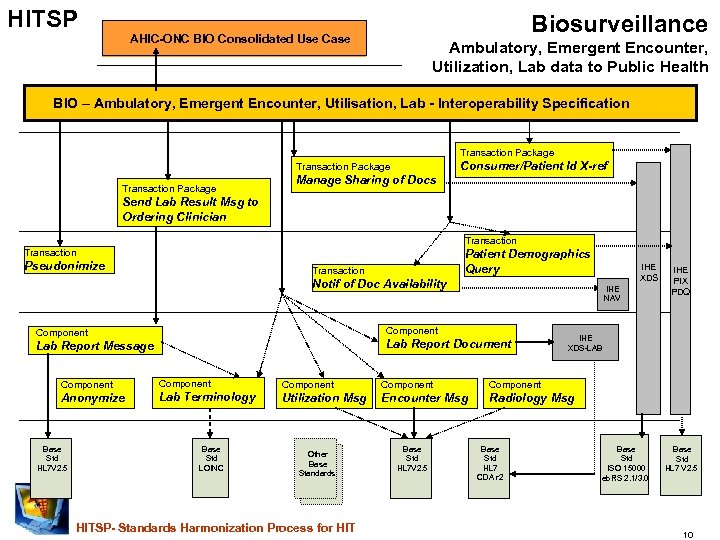 HITSP Biosurveillance AHIC-ONC BIO Consolidated Use Case Ambulatory, Emergent Encounter, Utilization, Lab data to Public Health BIO – Ambulatory, Emergent Encounter, Utilisation, Lab - Interoperability Specification Transaction Package Consumer/Patient Id X-ref Transaction Package Manage Sharing of Docs Send Lab Result Msg to Ordering Clinician Transaction Pseudonimize Patient Demographics Query Transaction IHE XDS Notif of Doc Availability IHE NAV Component Lab Report Document Lab Report Message IHE PIX PDQ IHE XDS-LAB Component Component Anonymize Lab Terminology Utilization Msg Encounter Msg Radiology Msg Base Std HL 7 V 2. 5 Base Std LOINC Other Base Standards HITSP- Standards Harmonization Process for HIT Base Std HL 7 V 2. 5 Base Std HL 7 CDA r 2 Base Std ISO 15000 eb. RS 2. 1/3. 0 Base Std HL 7 V 2. 5 10
HITSP Biosurveillance AHIC-ONC BIO Consolidated Use Case Ambulatory, Emergent Encounter, Utilization, Lab data to Public Health BIO – Ambulatory, Emergent Encounter, Utilisation, Lab - Interoperability Specification Transaction Package Consumer/Patient Id X-ref Transaction Package Manage Sharing of Docs Send Lab Result Msg to Ordering Clinician Transaction Pseudonimize Patient Demographics Query Transaction IHE XDS Notif of Doc Availability IHE NAV Component Lab Report Document Lab Report Message IHE PIX PDQ IHE XDS-LAB Component Component Anonymize Lab Terminology Utilization Msg Encounter Msg Radiology Msg Base Std HL 7 V 2. 5 Base Std LOINC Other Base Standards HITSP- Standards Harmonization Process for HIT Base Std HL 7 V 2. 5 Base Std HL 7 CDA r 2 Base Std ISO 15000 eb. RS 2. 1/3. 0 Base Std HL 7 V 2. 5 10
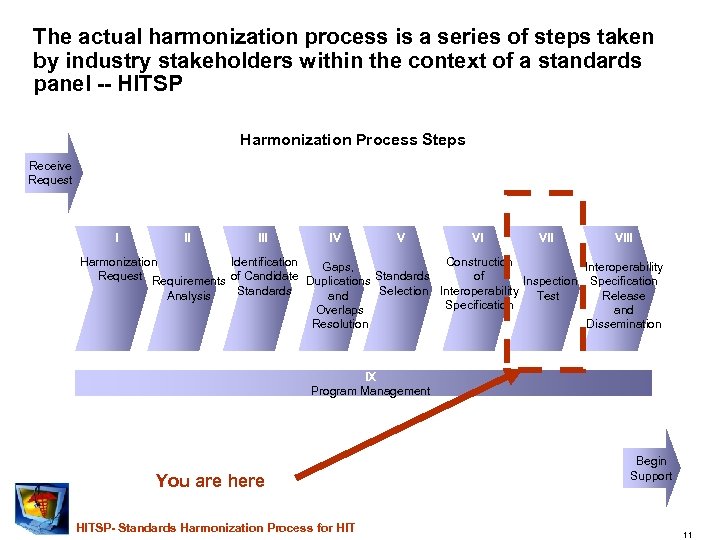 The actual harmonization process is a series of steps taken by industry stakeholders within the context of a standards panel -- HITSP Harmonization Process Steps Receive Request I II IV V VI VIII Harmonization Identification Construction Gaps, Interoperability Request Requirements of Candidate Duplications Standards of Inspection Specification Standards Selection Interoperability Test Analysis and Release Specification Overlaps and Resolution Dissemination IX Program Management You are here HITSP- Standards Harmonization Process for HIT Begin Support 11
The actual harmonization process is a series of steps taken by industry stakeholders within the context of a standards panel -- HITSP Harmonization Process Steps Receive Request I II IV V VI VIII Harmonization Identification Construction Gaps, Interoperability Request Requirements of Candidate Duplications Standards of Inspection Specification Standards Selection Interoperability Test Analysis and Release Specification Overlaps and Resolution Dissemination IX Program Management You are here HITSP- Standards Harmonization Process for HIT Begin Support 11
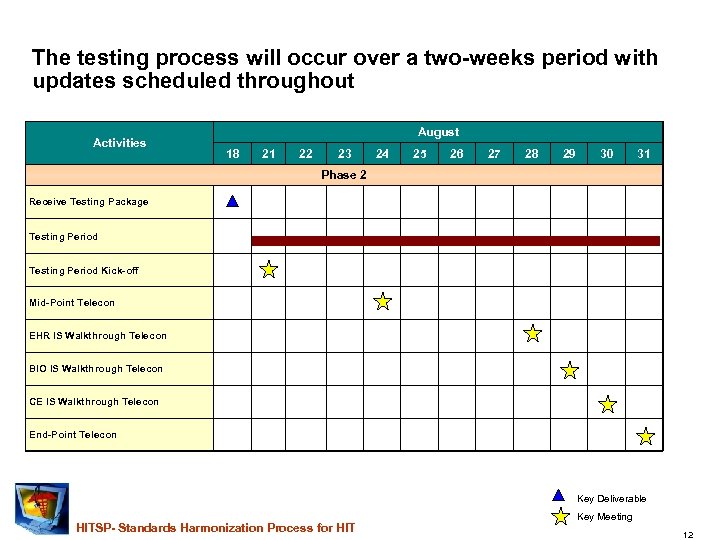 The testing process will occur over a two-weeks period with updates scheduled throughout Activities August 18 21 22 23 24 25 26 27 28 29 30 31 Phase 2 Receive Testing Package Testing Period Kick-off Mid-Point Telecon EHR IS Walkthrough Telecon BIO IS Walkthrough Telecon CE IS Walkthrough Telecon End-Point Telecon Key Deliverable HITSP- Standards Harmonization Process for HIT Key Meeting 12
The testing process will occur over a two-weeks period with updates scheduled throughout Activities August 18 21 22 23 24 25 26 27 28 29 30 31 Phase 2 Receive Testing Package Testing Period Kick-off Mid-Point Telecon EHR IS Walkthrough Telecon BIO IS Walkthrough Telecon CE IS Walkthrough Telecon End-Point Telecon Key Deliverable HITSP- Standards Harmonization Process for HIT Key Meeting 12
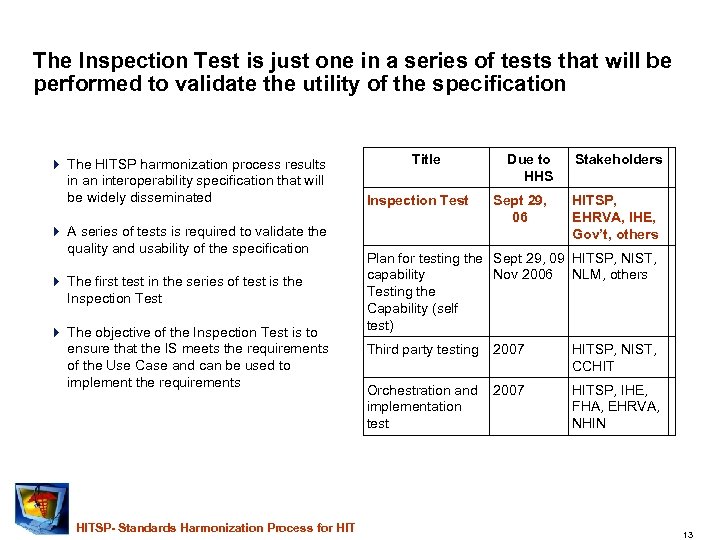 The Inspection Test is just one in a series of tests that will be performed to validate the utility of the specification 4 The HITSP harmonization process results in an interoperability specification that will be widely disseminated 4 A series of tests is required to validate the quality and usability of the specification 4 The first test in the series of test is the Inspection Test 4 The objective of the Inspection Test is to ensure that the IS meets the requirements of the Use Case and can be used to implement the requirements HITSP- Standards Harmonization Process for HIT Title Inspection Test Due to HHS Sept 29, 06 Stakeholders HITSP, EHRVA, IHE, Gov’t, others Plan for testing the Sept 29, 09 HITSP, NIST, capability Nov 2006 NLM, others Testing the Capability (self test) Third party testing 2007 HITSP, NIST, CCHIT Orchestration and 2007 implementation test HITSP, IHE, FHA, EHRVA, NHIN 13
The Inspection Test is just one in a series of tests that will be performed to validate the utility of the specification 4 The HITSP harmonization process results in an interoperability specification that will be widely disseminated 4 A series of tests is required to validate the quality and usability of the specification 4 The first test in the series of test is the Inspection Test 4 The objective of the Inspection Test is to ensure that the IS meets the requirements of the Use Case and can be used to implement the requirements HITSP- Standards Harmonization Process for HIT Title Inspection Test Due to HHS Sept 29, 06 Stakeholders HITSP, EHRVA, IHE, Gov’t, others Plan for testing the Sept 29, 09 HITSP, NIST, capability Nov 2006 NLM, others Testing the Capability (self test) Third party testing 2007 HITSP, NIST, CCHIT Orchestration and 2007 implementation test HITSP, IHE, FHA, EHRVA, NHIN 13
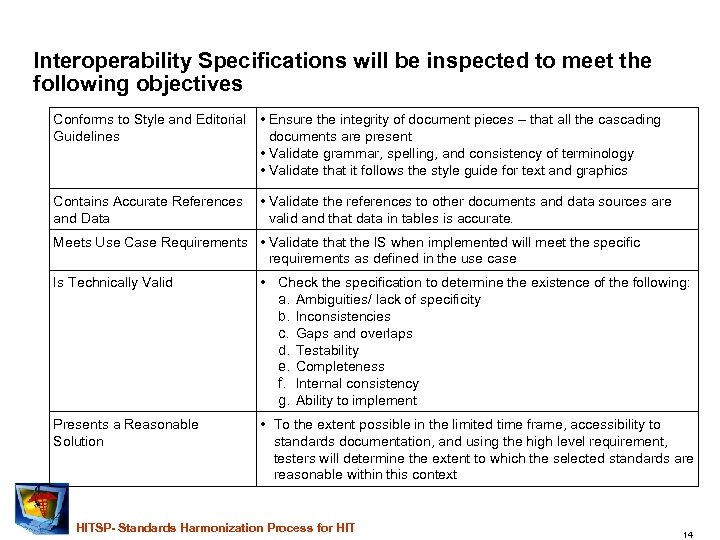 Interoperability Specifications will be inspected to meet the following objectives Conforms to Style and Editorial • Ensure the integrity of document pieces – that all the cascading Guidelines documents are present • Validate grammar, spelling, and consistency of terminology • Validate that it follows the style guide for text and graphics Contains Accurate References • Validate the references to other documents and data sources are and Data valid and that data in tables is accurate. Meets Use Case Requirements • Validate that the IS when implemented will meet the specific requirements as defined in the use case Is Technically Valid • Check the specification to determine the existence of the following: a. Ambiguities/ lack of specificity b. Inconsistencies c. Gaps and overlaps d. Testability e. Completeness f. Internal consistency g. Ability to implement Presents a Reasonable Solution • To the extent possible in the limited time frame, accessibility to standards documentation, and using the high level requirement, testers will determine the extent to which the selected standards are reasonable within this context HITSP- Standards Harmonization Process for HIT 14
Interoperability Specifications will be inspected to meet the following objectives Conforms to Style and Editorial • Ensure the integrity of document pieces – that all the cascading Guidelines documents are present • Validate grammar, spelling, and consistency of terminology • Validate that it follows the style guide for text and graphics Contains Accurate References • Validate the references to other documents and data sources are and Data valid and that data in tables is accurate. Meets Use Case Requirements • Validate that the IS when implemented will meet the specific requirements as defined in the use case Is Technically Valid • Check the specification to determine the existence of the following: a. Ambiguities/ lack of specificity b. Inconsistencies c. Gaps and overlaps d. Testability e. Completeness f. Internal consistency g. Ability to implement Presents a Reasonable Solution • To the extent possible in the limited time frame, accessibility to standards documentation, and using the high level requirement, testers will determine the extent to which the selected standards are reasonable within this context HITSP- Standards Harmonization Process for HIT 14
 The inspection testing process steps. . . 1. Identify and engage key volunteer test resources – assign testers to IS documents 2. Develop the tools and procedures for gathering and responding to test findings 3. Instruct testers about the general process and schedule 4. Distribute Interoperability Specifications to the assigned testers – conduct a test kick-off conference call ~ Aug 21 5. Post IS at HITSP. org and send message to list serve to invite informal review- Aug 18 6. Collect interim test results on conference call ~ Aug 25 7. Collect final results and conduct conference call ~ August 31 8. Analyze test results and disposition them through the formal Change Control Board 9. Provide approved change requests to the IS writers 10. Implement IS changes 11. Validate IS changes HITSP- Standards Harmonization Process for HIT 15
The inspection testing process steps. . . 1. Identify and engage key volunteer test resources – assign testers to IS documents 2. Develop the tools and procedures for gathering and responding to test findings 3. Instruct testers about the general process and schedule 4. Distribute Interoperability Specifications to the assigned testers – conduct a test kick-off conference call ~ Aug 21 5. Post IS at HITSP. org and send message to list serve to invite informal review- Aug 18 6. Collect interim test results on conference call ~ Aug 25 7. Collect final results and conduct conference call ~ August 31 8. Analyze test results and disposition them through the formal Change Control Board 9. Provide approved change requests to the IS writers 10. Implement IS changes 11. Validate IS changes HITSP- Standards Harmonization Process for HIT 15


