Treat_Now_or_Wait.pptx
- Количество слайдов: 31
 HCV Case Study Treat Now or Wait for New Therapies
HCV Case Study Treat Now or Wait for New Therapies
 Program Disclosure • This activity has been planned and implemented in accordance with the Essential Areas and Policies of the Accreditation Council for Continuing Medical Education (ACCME) through the sponsorship of Annenberg Center for Health Sciences at Eisenhower and the Chronic Liver Disease Foundation. Annenberg Center for Health Sciences at Eisenhower is accredited by the ACCME to provide continuing medical education for physicians. • This program is supported by educational grants from Kadmon and Merck Pharmaceuticals.
Program Disclosure • This activity has been planned and implemented in accordance with the Essential Areas and Policies of the Accreditation Council for Continuing Medical Education (ACCME) through the sponsorship of Annenberg Center for Health Sciences at Eisenhower and the Chronic Liver Disease Foundation. Annenberg Center for Health Sciences at Eisenhower is accredited by the ACCME to provide continuing medical education for physicians. • This program is supported by educational grants from Kadmon and Merck Pharmaceuticals.
 Learning Objectives • Describe current data on approved and experimental DAA’s used in combination with Pegylated Interferon and Ribavirin • Define the benefits and risks of treating now versus delaying therapy for different patient populations
Learning Objectives • Describe current data on approved and experimental DAA’s used in combination with Pegylated Interferon and Ribavirin • Define the benefits and risks of treating now versus delaying therapy for different patient populations
 Glenn: Patient Characteristics • 55 year old male • Shift worker • History/risk factors – BMI=34 – Hypertension and dyslipidemia – Moderate drinker/cigarette smoker • Concomitant medications – Simvastatin 20 mg/day – Lisinopril 10 mg/day
Glenn: Patient Characteristics • 55 year old male • Shift worker • History/risk factors – BMI=34 – Hypertension and dyslipidemia – Moderate drinker/cigarette smoker • Concomitant medications – Simvastatin 20 mg/day – Lisinopril 10 mg/day
 Glenn: Baseline Labs • • • Hemoglobin Neutrophils Platelets AST/ALT Albumin Bilirubin 15. 6 g/d. L 1400 cells/mm 3 210, 000 cells/mm 3 55/75 IU/L 4. 1 g/d. L 0. 7 mg/d. L
Glenn: Baseline Labs • • • Hemoglobin Neutrophils Platelets AST/ALT Albumin Bilirubin 15. 6 g/d. L 1400 cells/mm 3 210, 000 cells/mm 3 55/75 IU/L 4. 1 g/d. L 0. 7 mg/d. L
 Glenn: Disease Characteristics • • • Treatment naïve Genotype IL 28 B METAVIR BL viral load 1 a CC F 3 1, 300, 000 IU/m. L
Glenn: Disease Characteristics • • • Treatment naïve Genotype IL 28 B METAVIR BL viral load 1 a CC F 3 1, 300, 000 IU/m. L
 Clinical Decision 1 • How would you manage this patient? 1. Continue to monitor patient but do not start treatment 2. Start patient on first generation protease inhibitor/PEG-IFN/RBV
Clinical Decision 1 • How would you manage this patient? 1. Continue to monitor patient but do not start treatment 2. Start patient on first generation protease inhibitor/PEG-IFN/RBV
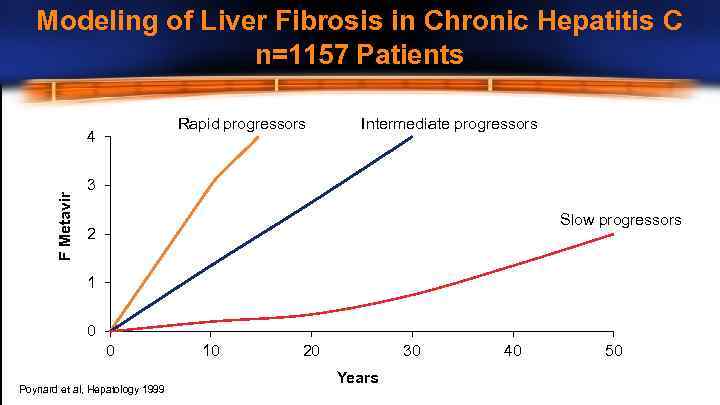 Modeling of Liver Fibrosis in Chronic Hepatitis C n=1157 Patients Rapid progressors F Metavir 4 Intermediate progressors 3 Slow progressors 2 1 0 0 Poynard et al, Hepatology 1999 10 20 30 Years 40 50
Modeling of Liver Fibrosis in Chronic Hepatitis C n=1157 Patients Rapid progressors F Metavir 4 Intermediate progressors 3 Slow progressors 2 1 0 0 Poynard et al, Hepatology 1999 10 20 30 Years 40 50
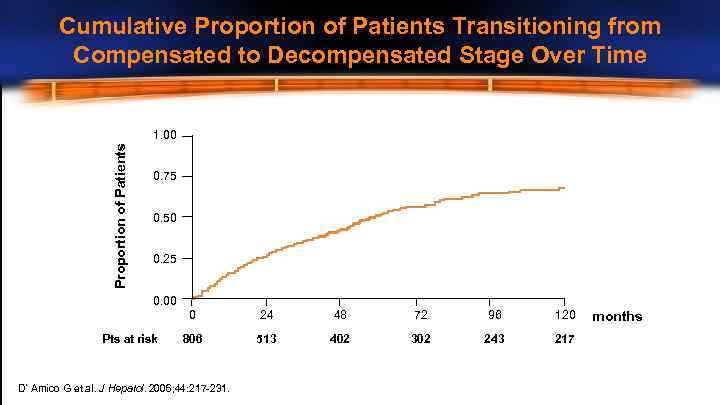 Cumulative Proportion of Patients Transitioning from Compensated to Decompensated Stage Over Time Proportion of Patients 1. 00 0. 75 0. 50 0. 25 0. 00 0 Pts at risk 24 48 72 96 120 806 513 402 302 243 217 D’Amico G et al. J Hepatol. 2006; 44: 217 -231. months
Cumulative Proportion of Patients Transitioning from Compensated to Decompensated Stage Over Time Proportion of Patients 1. 00 0. 75 0. 50 0. 25 0. 00 0 Pts at risk 24 48 72 96 120 806 513 402 302 243 217 D’Amico G et al. J Hepatol. 2006; 44: 217 -231. months
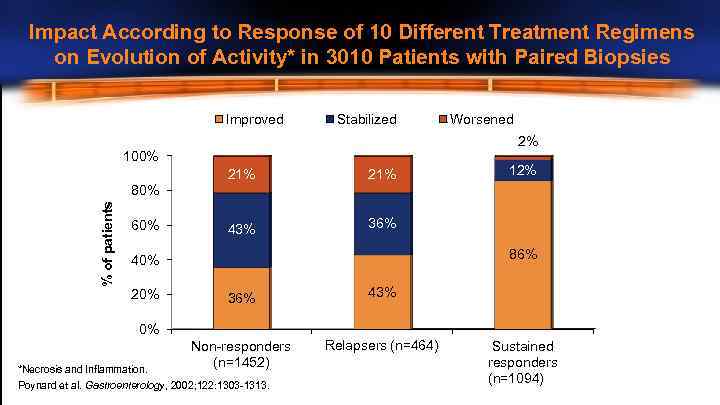 Impact According to Response of 10 Different Treatment Regimens on Evolution of Activity* in 3010 Patients with Paired Biopsies Improved Stabilized Worsened 2% 100% 21% 43% 12% 36% % of patients 80% 60% 86% 40% 20% 36% 43% Non-responders (n=1452) Relapsers (n=464) 0% *Necrosis and Inflammation. Poynard et al. Gastroenterology, 2002; 122: 1303 -1313. Sustained responders (n=1094)
Impact According to Response of 10 Different Treatment Regimens on Evolution of Activity* in 3010 Patients with Paired Biopsies Improved Stabilized Worsened 2% 100% 21% 43% 12% 36% % of patients 80% 60% 86% 40% 20% 36% 43% Non-responders (n=1452) Relapsers (n=464) 0% *Necrosis and Inflammation. Poynard et al. Gastroenterology, 2002; 122: 1303 -1313. Sustained responders (n=1094)
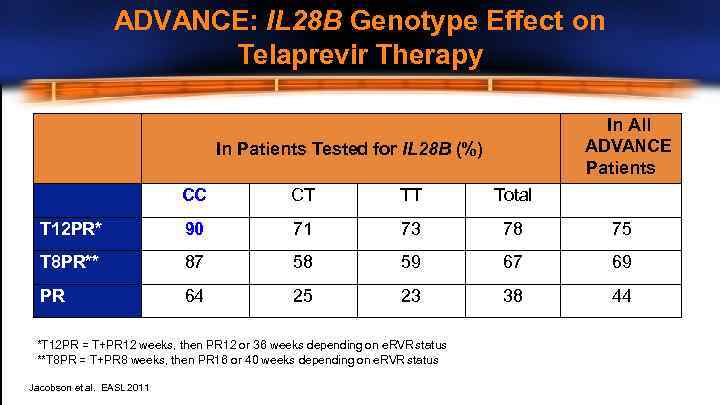 ADVANCE: IL 28 B Genotype Effect on Telaprevir Therapy In All ADVANCE Patients In Patients Tested for IL 28 B (%) CC CT TT Total T 12 PR* 90 71 73 78 75 T 8 PR** 87 58 59 67 69 PR 64 25 23 38 44 *T 12 PR = T+PR 12 weeks, then PR 12 or 36 weeks depending on e. RVR status **T 8 PR = T+PR 8 weeks, then PR 16 or 40 weeks depending on e. RVR status Jacobson et al. EASL 2011
ADVANCE: IL 28 B Genotype Effect on Telaprevir Therapy In All ADVANCE Patients In Patients Tested for IL 28 B (%) CC CT TT Total T 12 PR* 90 71 73 78 75 T 8 PR** 87 58 59 67 69 PR 64 25 23 38 44 *T 12 PR = T+PR 12 weeks, then PR 12 or 36 weeks depending on e. RVR status **T 8 PR = T+PR 8 weeks, then PR 16 or 40 weeks depending on e. RVR status Jacobson et al. EASL 2011
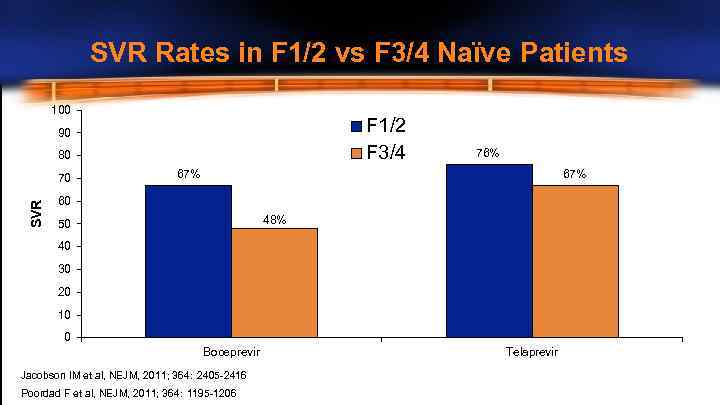 SVR Rates in F 1/2 vs F 3/4 Naïve Patients 100 F 1/2 F 3/4 90 80 SVR 70 76% 67% 60 48% 50 40 30 20 10 0 Boceprevir Jacobson IM et al, NEJM, 2011; 364: 2405 -2416 Poordad F et al, NEJM, 2011; 364: 1195 -1206 Telaprevir
SVR Rates in F 1/2 vs F 3/4 Naïve Patients 100 F 1/2 F 3/4 90 80 SVR 70 76% 67% 60 48% 50 40 30 20 10 0 Boceprevir Jacobson IM et al, NEJM, 2011; 364: 2405 -2416 Poordad F et al, NEJM, 2011; 364: 1195 -1206 Telaprevir
 OPTIMIZE Trial: Telaprevir BID vs TID • • • PR + TVR 1125 mg BID versus 750 mg TID Response-guided therapy 740 patients 29% bridging fibrosis or cirrhosis 57% G 1 a, IL 28 B CC 29% Buti M et al, Abstract LB-8, AASLD 2012
OPTIMIZE Trial: Telaprevir BID vs TID • • • PR + TVR 1125 mg BID versus 750 mg TID Response-guided therapy 740 patients 29% bridging fibrosis or cirrhosis 57% G 1 a, IL 28 B CC 29% Buti M et al, Abstract LB-8, AASLD 2012
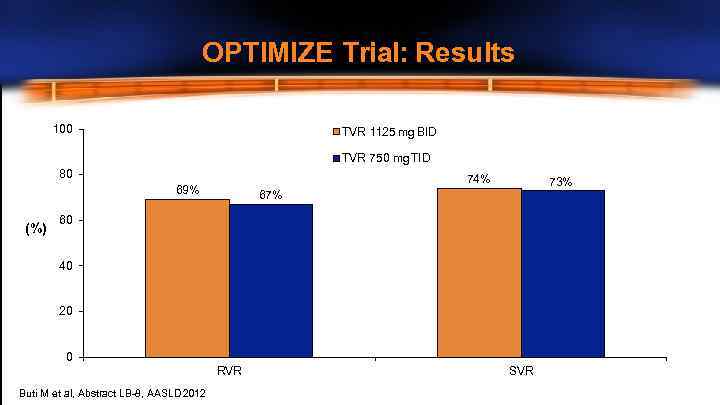 OPTIMIZE Trial: Results 100 TVR 1125 mg BID TVR 750 mg TID 80 74% 69% (%) 73% 67% 60 40 20 0 RVR Buti M et al, Abstract LB-8, AASLD 2012 SVR
OPTIMIZE Trial: Results 100 TVR 1125 mg BID TVR 750 mg TID 80 74% 69% (%) 73% 67% 60 40 20 0 RVR Buti M et al, Abstract LB-8, AASLD 2012 SVR
 Should Glenn Be Treated Now? • F 3 disease – risk of progression with waiting • IL 28 B CC • Potential BID option is attractive
Should Glenn Be Treated Now? • F 3 disease – risk of progression with waiting • IL 28 B CC • Potential BID option is attractive
 The Case for Waiting • Multiple issues with current therapy – – Compliance – pill burden Co-morbidities Adverse effects New treatments on the horizon
The Case for Waiting • Multiple issues with current therapy – – Compliance – pill burden Co-morbidities Adverse effects New treatments on the horizon
 Compliance Pill Burden BOC = 18/d RBV 4 -7/d TVR = 12/d RBV 4 -7/d Food Requirement
Compliance Pill Burden BOC = 18/d RBV 4 -7/d TVR = 12/d RBV 4 -7/d Food Requirement
 Co-Morbidities • Cardiac Risk Factors – Hypertension, hyperlipidemia, smoker • Pre Treatment – DDI – Statin with TVR/BOC likely just stop it • On Treatment – Anemia management consider pre-treatment cardiac testing
Co-Morbidities • Cardiac Risk Factors – Hypertension, hyperlipidemia, smoker • Pre Treatment – DDI – Statin with TVR/BOC likely just stop it • On Treatment – Anemia management consider pre-treatment cardiac testing
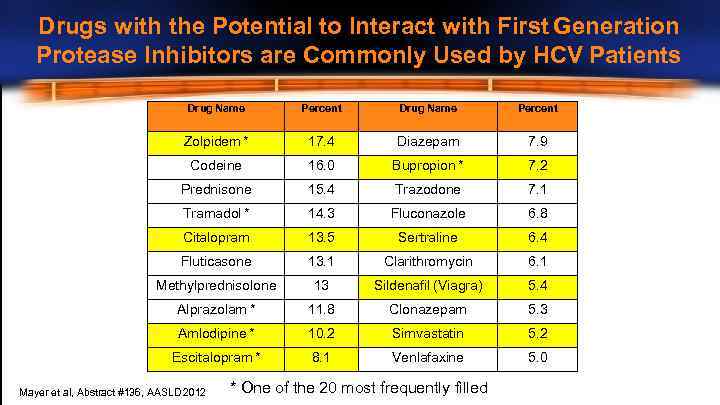 Drugs with the Potential to Interact with First Generation Protease Inhibitors are Commonly Used by HCV Patients Drug Name Percent Zolpidem * 17. 4 Diazepam 7. 9 Codeine 16. 0 Bupropion * 7. 2 Prednisone 15. 4 Trazodone 7. 1 Tramadol * 14. 3 Fluconazole 6. 8 Citalopram 13. 5 Sertraline 6. 4 Fluticasone 13. 1 Clarithromycin 6. 1 Methylprednisolone 13 Sildenafil (Viagra) 5. 4 Alprazolam * 11. 8 Clonazepam 5. 3 Amlodipine * 10. 2 Simvastatin 5. 2 Escitalopram * 8. 1 Venlafaxine 5. 0 Mayer et al, Abstract #136, AASLD 2012 * One of the 20 most frequently filled
Drugs with the Potential to Interact with First Generation Protease Inhibitors are Commonly Used by HCV Patients Drug Name Percent Zolpidem * 17. 4 Diazepam 7. 9 Codeine 16. 0 Bupropion * 7. 2 Prednisone 15. 4 Trazodone 7. 1 Tramadol * 14. 3 Fluconazole 6. 8 Citalopram 13. 5 Sertraline 6. 4 Fluticasone 13. 1 Clarithromycin 6. 1 Methylprednisolone 13 Sildenafil (Viagra) 5. 4 Alprazolam * 11. 8 Clonazepam 5. 3 Amlodipine * 10. 2 Simvastatin 5. 2 Escitalopram * 8. 1 Venlafaxine 5. 0 Mayer et al, Abstract #136, AASLD 2012 * One of the 20 most frequently filled
 New Drug-Drug Interaction Data at AASLD 2012: HCV Protease Inhibitors • No clinically significant interactions – Boceprevir • Prednisone (abstract #1896) • Omeprazole (abstract #1808) • Ethinyl estrodiol/norethidrone (abstract #1901) – Simeprevir (TMC-435) • Cyclosporine/tacrolimus (abstract #80) • Ethinyl estrodiol/norethidrone (abstract #773)
New Drug-Drug Interaction Data at AASLD 2012: HCV Protease Inhibitors • No clinically significant interactions – Boceprevir • Prednisone (abstract #1896) • Omeprazole (abstract #1808) • Ethinyl estrodiol/norethidrone (abstract #1901) – Simeprevir (TMC-435) • Cyclosporine/tacrolimus (abstract #80) • Ethinyl estrodiol/norethidrone (abstract #773)
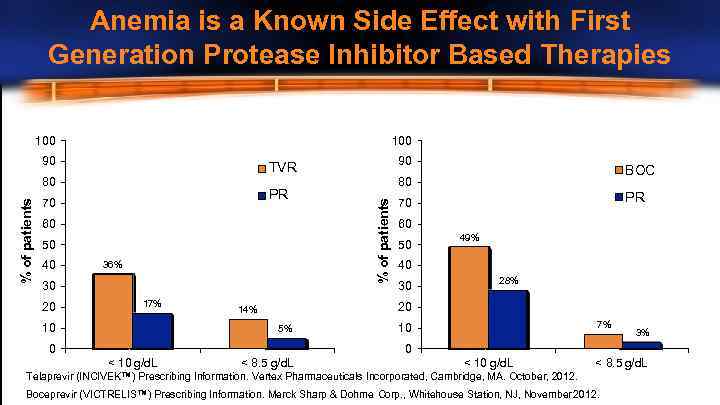 Anemia is a Known Side Effect with First Generation Protease Inhibitor Based Therapies 100 90 PR 70 60 50 36% 30 20 17% 10 BOC 80 % of patients 80 40 90 TVR PR 70 60 50 49% 40 30 28% 20 14% 5% 0 7% 10 3% 0 < 10 g/d. L < 8. 5 g/d. L Telaprevir (INCIVEK™) Prescribing Information. Vertex Pharmaceuticals Incorporated, Cambridge, MA. October, 2012. Boceprevir (VICTRELIS™) Prescribing Information. Merck Sharp & Dohme Corp. , Whitehouse Station, NJ, November 2012.
Anemia is a Known Side Effect with First Generation Protease Inhibitor Based Therapies 100 90 PR 70 60 50 36% 30 20 17% 10 BOC 80 % of patients 80 40 90 TVR PR 70 60 50 49% 40 30 28% 20 14% 5% 0 7% 10 3% 0 < 10 g/d. L < 8. 5 g/d. L Telaprevir (INCIVEK™) Prescribing Information. Vertex Pharmaceuticals Incorporated, Cambridge, MA. October, 2012. Boceprevir (VICTRELIS™) Prescribing Information. Merck Sharp & Dohme Corp. , Whitehouse Station, NJ, November 2012.
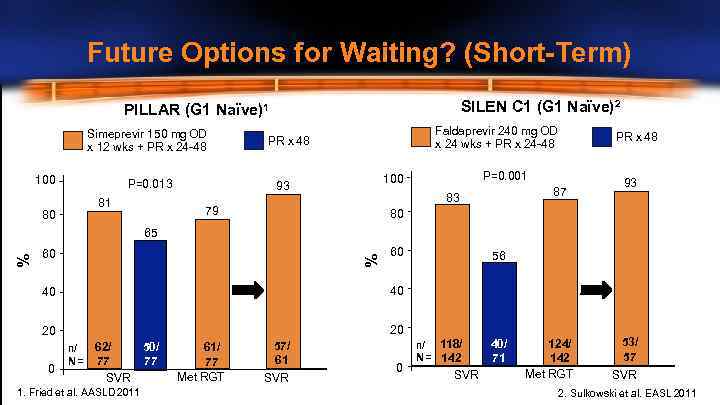 Future Options for Waiting? (Short-Term) SILEN C 1 (G 1 Naïve)2 PILLAR (G 1 Naïve)1 Simeprevir 150 mg OD x 12 wks + PR x 24 -48 100 P=0. 013 81 80 Faldaprevir 240 mg OD x 24 wks + PR x 24 -48 PR x 48 P=0. 001 100 93 87 83 79 PR x 48 93 80 60 % % 65 60 40 40 20 56 20 0 n/ N= 62/ 50/ 77 77 SVR 1. Fried et al. AASLD 2011 61/ 77 Met RGT 57/ 61 SVR 0 n/ 118/ N = 142 SVR 40/ 71 124/ 142 Met RGT 53/ 57 SVR 2. Sulkowski et al. EASL 2011
Future Options for Waiting? (Short-Term) SILEN C 1 (G 1 Naïve)2 PILLAR (G 1 Naïve)1 Simeprevir 150 mg OD x 12 wks + PR x 24 -48 100 P=0. 013 81 80 Faldaprevir 240 mg OD x 24 wks + PR x 24 -48 PR x 48 P=0. 001 100 93 87 83 79 PR x 48 93 80 60 % % 65 60 40 40 20 56 20 0 n/ N= 62/ 50/ 77 77 SVR 1. Fried et al. AASLD 2011 61/ 77 Met RGT 57/ 61 SVR 0 n/ 118/ N = 142 SVR 40/ 71 124/ 142 Met RGT 53/ 57 SVR 2. Sulkowski et al. EASL 2011
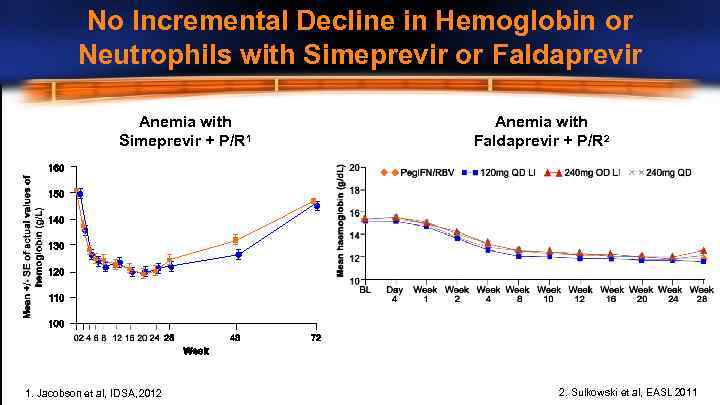 No Incremental Decline in Hemoglobin or Neutrophils with Simeprevir or Faldaprevir Anemia with Simeprevir + P/R 1 1. Jacobson et al, IDSA, 2012 Anemia with Faldaprevir + P/R 2 2. Sulkowski et al, EASL 2011
No Incremental Decline in Hemoglobin or Neutrophils with Simeprevir or Faldaprevir Anemia with Simeprevir + P/R 1 1. Jacobson et al, IDSA, 2012 Anemia with Faldaprevir + P/R 2 2. Sulkowski et al, EASL 2011
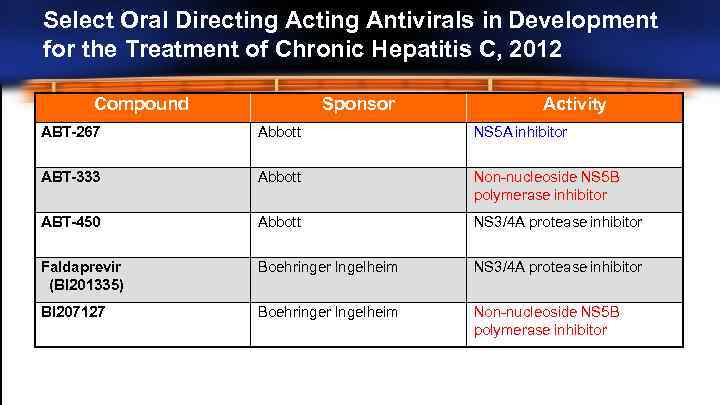 Select Oral Directing Antivirals in Development for the Treatment of Chronic Hepatitis C, 2012 Compound Sponsor Activity ABT-267 Abbott NS 5 A inhibitor ABT-333 Abbott Non-nucleoside NS 5 B polymerase inhibitor ABT-450 Abbott NS 3/4 A protease inhibitor Faldaprevir (BI 201335) Boehringer Ingelheim NS 3/4 A protease inhibitor BI 207127 Boehringer Ingelheim Non-nucleoside NS 5 B polymerase inhibitor
Select Oral Directing Antivirals in Development for the Treatment of Chronic Hepatitis C, 2012 Compound Sponsor Activity ABT-267 Abbott NS 5 A inhibitor ABT-333 Abbott Non-nucleoside NS 5 B polymerase inhibitor ABT-450 Abbott NS 3/4 A protease inhibitor Faldaprevir (BI 201335) Boehringer Ingelheim NS 3/4 A protease inhibitor BI 207127 Boehringer Ingelheim Non-nucleoside NS 5 B polymerase inhibitor
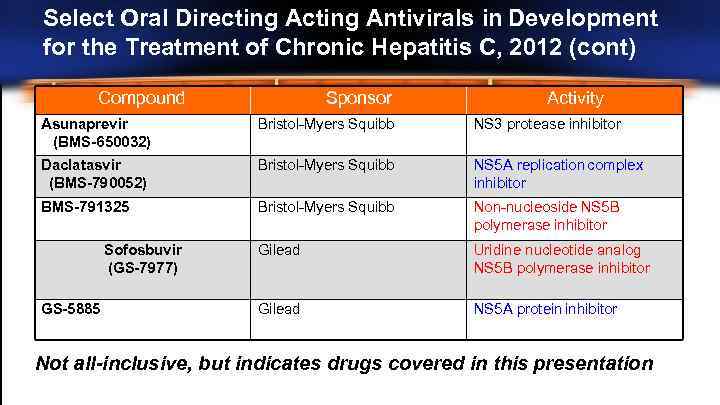 Select Oral Directing Antivirals in Development for the Treatment of Chronic Hepatitis C, 2012 (cont) Compound Sponsor Activity Asunaprevir (BMS-650032) Bristol-Myers Squibb NS 3 protease inhibitor Daclatasvir (BMS-790052) Bristol-Myers Squibb NS 5 A replication complex inhibitor BMS-791325 Bristol-Myers Squibb Non-nucleoside NS 5 B polymerase inhibitor Gilead Uridine nucleotide analog NS 5 B polymerase inhibitor Gilead NS 5 A protein inhibitor Sofosbuvir (GS-7977) GS-5885 Not all-inclusive, but indicates drugs covered in this presentation
Select Oral Directing Antivirals in Development for the Treatment of Chronic Hepatitis C, 2012 (cont) Compound Sponsor Activity Asunaprevir (BMS-650032) Bristol-Myers Squibb NS 3 protease inhibitor Daclatasvir (BMS-790052) Bristol-Myers Squibb NS 5 A replication complex inhibitor BMS-791325 Bristol-Myers Squibb Non-nucleoside NS 5 B polymerase inhibitor Gilead Uridine nucleotide analog NS 5 B polymerase inhibitor Gilead NS 5 A protein inhibitor Sofosbuvir (GS-7977) GS-5885 Not all-inclusive, but indicates drugs covered in this presentation
 Should Glenn Delay Treatment? • IL 28 B CC ~80% chance of shortened therapy - 80 -90% chance of SVR • F 3 disease – risk of progression with waiting • No clear issues with IFN • Seems anxious and willing to be treated now • I would suggest treatment
Should Glenn Delay Treatment? • IL 28 B CC ~80% chance of shortened therapy - 80 -90% chance of SVR • F 3 disease – risk of progression with waiting • No clear issues with IFN • Seems anxious and willing to be treated now • I would suggest treatment
 Glenn: On Treatment Response • Glenn was started on TVR/PEG/RBV • TW 4 and TW 12 – HCV RNA undetectable
Glenn: On Treatment Response • Glenn was started on TVR/PEG/RBV • TW 4 and TW 12 – HCV RNA undetectable
 Clinical Decision 2 • Which regimen should Glenn receive? 1. 12 weeks TVR/PEG/RBV 2. 12 weeks TVR/PEG/RBV + 12 weeks PEG/RBV 3. 12 weeks TVR/PEG/RBV + 24 weeks PEG/RBV 4. 12 weeks TVR/PEG/RBV + 36 weeks PEG/RBV 5. 24 weeks TVR/PEG/RBV
Clinical Decision 2 • Which regimen should Glenn receive? 1. 12 weeks TVR/PEG/RBV 2. 12 weeks TVR/PEG/RBV + 12 weeks PEG/RBV 3. 12 weeks TVR/PEG/RBV + 24 weeks PEG/RBV 4. 12 weeks TVR/PEG/RBV + 36 weeks PEG/RBV 5. 24 weeks TVR/PEG/RBV
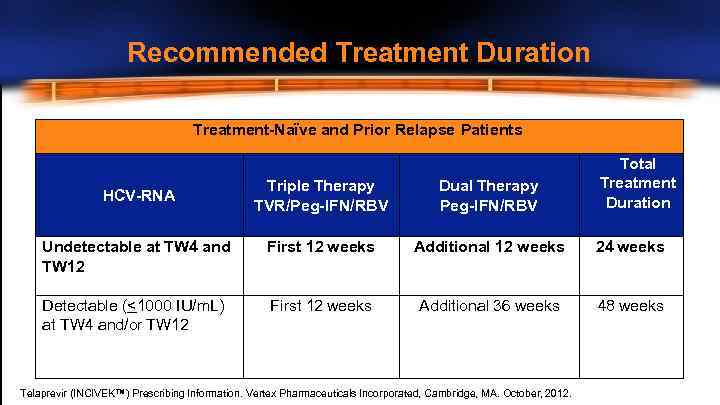 Recommended Treatment Duration Treatment-Naïve and Prior Relapse Patients Total Treatment Duration Triple Therapy TVR/Peg-IFN/RBV Dual Therapy Peg-IFN/RBV Undetectable at TW 4 and TW 12 First 12 weeks Additional 12 weeks 24 weeks Detectable (<1000 IU/m. L) at TW 4 and/or TW 12 First 12 weeks Additional 36 weeks 48 weeks HCV-RNA Telaprevir (INCIVEK™) Prescribing Information. Vertex Pharmaceuticals Incorporated, Cambridge, MA. October, 2012.
Recommended Treatment Duration Treatment-Naïve and Prior Relapse Patients Total Treatment Duration Triple Therapy TVR/Peg-IFN/RBV Dual Therapy Peg-IFN/RBV Undetectable at TW 4 and TW 12 First 12 weeks Additional 12 weeks 24 weeks Detectable (<1000 IU/m. L) at TW 4 and/or TW 12 First 12 weeks Additional 36 weeks 48 weeks HCV-RNA Telaprevir (INCIVEK™) Prescribing Information. Vertex Pharmaceuticals Incorporated, Cambridge, MA. October, 2012.
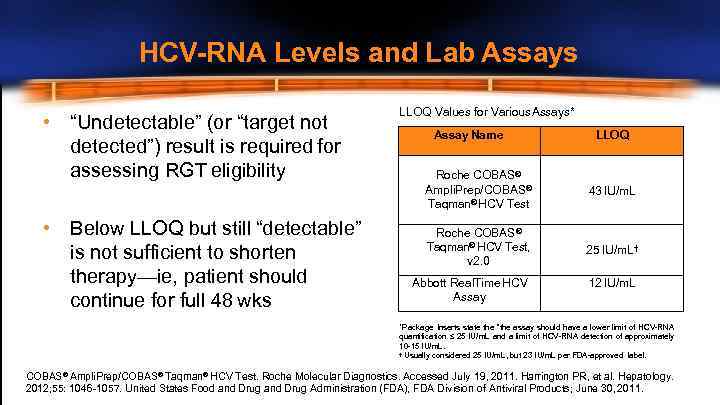 HCV-RNA Levels and Lab Assays • “Undetectable” (or “target not detected”) result is required for assessing RGT eligibility • Below LLOQ but still “detectable” is not sufficient to shorten therapy—ie, patient should continue for full 48 wks LLOQ Values for Various Assays* Assay Name LLOQ Roche COBAS® Ampli. Prep/COBAS® Taqman® HCV Test 43 IU/m. L Roche COBAS® Taqman® HCV Test, v 2. 0 25 IU/m. L† Abbott Real. Time HCV Assay 12 IU/m. L *Package Inserts state the “the assay should have a lower limit of HCV-RNA quantification ≤ 25 IU/m. L and a limit of HCV-RNA detection of approximately 10 -15 IU/m. L. † Usually considered 25 IU/m. L, but 23 IU/m. L per FDA-approved label. COBAS® Ampli. Prep/COBAS® Taqman® HCV Test. Roche Molecular Diagnostics. Accessed July 19, 2011. Harrington PR, et al. Hepatology. 2012; 55: 1046 -1057. United States Food and Drug Administration (FDA), FDA Division of Antiviral Products; June 30, 2011.
HCV-RNA Levels and Lab Assays • “Undetectable” (or “target not detected”) result is required for assessing RGT eligibility • Below LLOQ but still “detectable” is not sufficient to shorten therapy—ie, patient should continue for full 48 wks LLOQ Values for Various Assays* Assay Name LLOQ Roche COBAS® Ampli. Prep/COBAS® Taqman® HCV Test 43 IU/m. L Roche COBAS® Taqman® HCV Test, v 2. 0 25 IU/m. L† Abbott Real. Time HCV Assay 12 IU/m. L *Package Inserts state the “the assay should have a lower limit of HCV-RNA quantification ≤ 25 IU/m. L and a limit of HCV-RNA detection of approximately 10 -15 IU/m. L. † Usually considered 25 IU/m. L, but 23 IU/m. L per FDA-approved label. COBAS® Ampli. Prep/COBAS® Taqman® HCV Test. Roche Molecular Diagnostics. Accessed July 19, 2011. Harrington PR, et al. Hepatology. 2012; 55: 1046 -1057. United States Food and Drug Administration (FDA), FDA Division of Antiviral Products; June 30, 2011.
 Conclusions • Many chronic hepatitis C patients are good candidates for treatment today • The HCV pipeline is promising with potential new treatment modalities in the near future • Physicians should carefully consider individual patient characteristics when deciding whether to initiate or delay treatment
Conclusions • Many chronic hepatitis C patients are good candidates for treatment today • The HCV pipeline is promising with potential new treatment modalities in the near future • Physicians should carefully consider individual patient characteristics when deciding whether to initiate or delay treatment


