4832ce351066d6222376b652c290dc23.ppt
- Количество слайдов: 22

HCLSIG Bio. RDF Sub-group 10 Mar 2008 Clinical Data Interchange Standards Consortium Overview Stephen J Ruberg, Ph. D Senior Research Fellow Eli Lilly & Company

Background • Who is Steve Ruberg? • How does pharma generate and utilize clinical data? • What is CDISC? • What has CDISC done, what are they doing, and what are they considering? • Where does CDISC need help?

Steve Ruberg • Statistician – Not IT, though much learning over last decade – Creator, recipient, analyzer, archiver, submitter of clinical data • Helped launch CDISC as not-for-profit in 2000 – First Chairman; just finished my term on the Board in Dec, 2007

Pharma Clinical Data Company executed trial • Getting data from investigators – Paper and electronic • Getting data from labs – Central vs local • Getting data from other sources – Growing variety of electronic data capture

Electronic Data Capture at an Investigator Site

Pharma Clinical Data Other considerations • Working with a CRO – Defining data specs – Receiving datasets • Submissions to the FDA – Observed/raw data and derived/analysis data – Analysis programs, reports
Pharma Clinical Data Value of Standards • Operational efficiency – Reduce programming and re-mapping of data – Increase automation; better tools • Scientific effectiveness – INTEGRATION OF DATA – Better analysis, interpretation, understanding • FDA remit: Promote and protect health

What is CDISC • CDISC is a global, open, multidisciplinary, non-profit organization that has established standards to support the acquisition, exchange, submission and archive of clinical research data and metadata. The CDISC mission is to develop and support global, platformindependent data standards that enable information system interoperability to improve medical research and related areas of healthcare. CDISC standards are vendor-neutral, platform-independent and freely available via the CDISC website. • Established as a non-profit in 200 • Most members from pharma or companies (CROs, software vendors) that support pharma clinical research

What is CDISC Doing CDISC models – SDTM – Submission Data Tabulation Model (used for FDA submissions; can also be used for CRO submissions to pharma sponsor) • Defines structure (vertical) and some categorization/data domains and minimal terminology – ODM – Operational Data Model • XML messaging standard – LAB – Clinical laboratory data model

What is CDISC Doing CDISC models – SEND – Standard for Exchange of Non-clinical Data • Non-clinical version of SDTM • Used for FDA submissions; can also be used for CRO submissions to pharma sponsor • Defines structure (vertical) and some categorization/data domains and minimal terminology – ADa. M – Analysis Data Model • Suggested structures for FDA submission of analysis data – Protocol Representation Group • Terminology/data definitions to describe a protocol/study

What is CDISC Doing CDISC models – CDASH – Clinical Data Acquisition Standards Harmonization • Non-clinical version of SDTM • Used for FDA submissions; can also be used for CRO submissions to pharma sponsor • Defines structure (vertical) and some categorization/data domains and minimal terminology – ADa. M – Analysis Data Model • Suggested formats for FDA submission of analysis datasets – Terminology • Creating more detailed data element specifications for use in SDTM

What is CDISC Doing • CDISC works in virtual teams – Conference calls, meetings – Tends to focus on pieces (data domains like adverse events, demography, vitals signs, etc. ) of the clinical business • Progress, but slow and limited

What is CDISC Considering • CDISC models have some utility but cannot meet data integration needs – Not enough unambiguous data element definitions (i. e. terminology) – Caution: terminology means different things to different people • Concept • Valid value set

Proposal • WHAT • Build a comprehensive, industry-wide electronically accessible and consumable data element dictionary that contains the “clinical words” used in clinical research studies

What Is a Data Element Definition? • All the pieces of information (i. e. attributes) needed to unambiguously describe a concept • English dictionary analogy – – Word – desk Phonetic spelling – dĕsk Part of speech – noun Definition – a piece of furniture with a flat top for writing [could also be thought of as the concept] – Source – Latin, discus – etc.

The English Dictionary as Attributes Word desk Phonetic Part of spelling Speech dĕsk Noun run rən Verb Noun Definition Source 1. A table, frame or Latin, case with horizontal or discus slightly sloping surface for writing or reading 2. A division of an organization specializing in a particular activity 1. To go faster than a Old walk by springing the English, feet so that only one is iernan touching the ground at a time Something that flows in the course of an operation Year Old Englis h Other Forms None Synonym / Example counter e. g. the Russian desk at the State Department Old run across Sprint, jog, scuttle, Englis run a fever dart, scamper, dash h Operate, conduct

Clinical Data Element for DRAFT Pharma DRAFT • Variable name – – – – – Label / concept Valid values of the variable itself Data type (num, char, date, …) Units Key words (e. g. biomarker, osteoporosis, …) – facilitate searches Source / reference (as needed) SDTM data domain Regulatory requirement [A team needs to define what are the essential attributes that are parsimonious – enough to eliminate ambiguity, but few enough to be useful, consumable, understandable, burdenless. ]
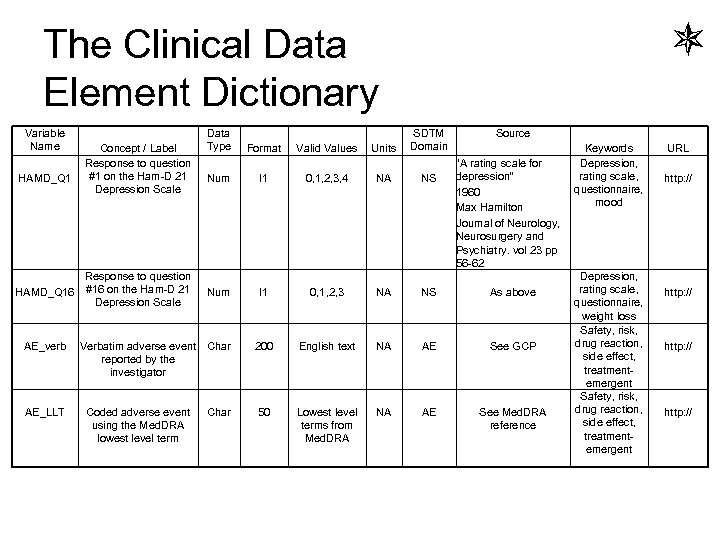
The Clinical Data Element Dictionary Variable Name HAMD_Q 1 Concept / Label Response to question #1 on the Ham-D 21 Depression Scale Response to question HAMD_Q 16 #16 on the Ham-D 21 Depression Scale AE_verb AE_LLT Data Type Format Units Num I 1 0, 1, 2, 3, 4 NA NS Num I 1 0, 1, 2, 3 NA NS 200 English text NA AE 50 Lowest level terms from Med. DRA NA AE Verbatim adverse event Char reported by the investigator Coded adverse event using the Med. DRA lowest level term Valid Values SDTM Domain Char Source Keywords “A rating scale for Depression, depression” rating scale, questionnaire, 1960 mood Max Hamilton Journal of Neurology, Neurosurgery and Psychiatry. vol 23 pp 56 -62 Depression, rating scale, As above questionnaire, weight loss Safety, risk, drug reaction, See GCP side effect, treatmentemergent Safety, risk, drug reaction, See Med. DRA side effect, reference treatmentemergent URL http: //

Proposal • HOW - Business model • An open, electronic, peer production environment with appropriate governance (e. g. a wiki) • • • Like Med. DRA, but open and free Like Wikipedia, but more governance Like LINUX, but more granular and dynamic • We must adopt a more flexible and rapid development process to get this in place
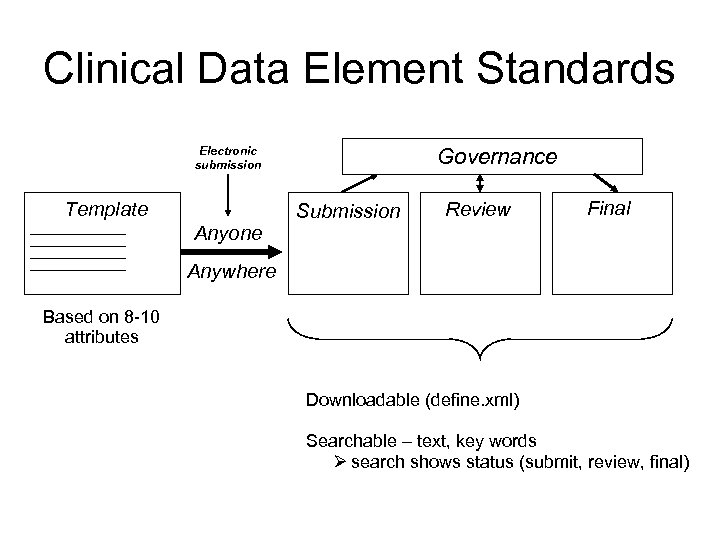
Clinical Data Element Standards Electronic submission Template Anyone Governance Submission Review Final Anywhere Based on 8 -10 attributes Downloadable (define. xml) Searchable – text, key words Ø search shows status (submit, review, final)
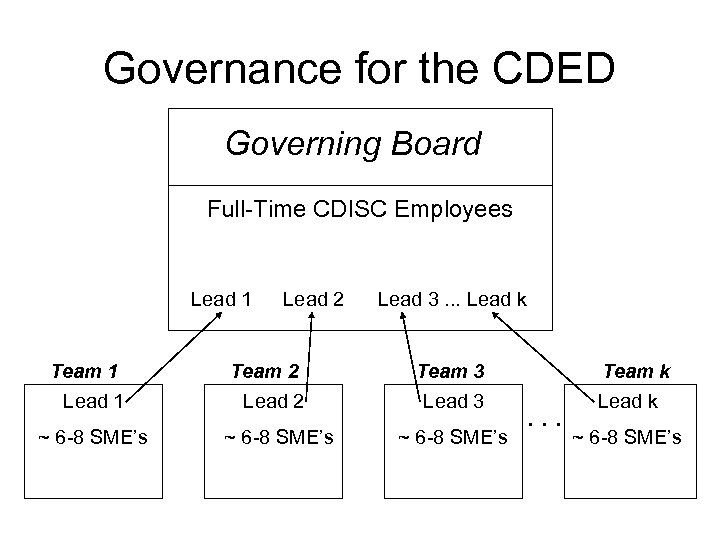
Governance for the CDED Governing Board Full-Time CDISC Employees Lead 1 Team 1 Lead 1 ~ 6 -8 SME’s Lead 2 Team 2 Lead 2 ~ 6 -8 SME’s Lead 3. . . Lead k Team 3 Lead 3 ~ 6 -8 SME’s . . . Team k Lead k ~ 6 -8 SME’s

Next Steps • CDISC is considering this need (data element dictionary) and seems to be moving in this direction – Make your voice heard to CDISC if you see the value – Create a sense of urgency as you see fit • CDISC is considering the wiki approach – Let me and/or CDISC know of your willingness to contribute to a wiki – We could build this together much faster – Requires a new business model / approach • Can W 3 C give advice, input or help?
4832ce351066d6222376b652c290dc23.ppt