c2e528dd83e0a28f9ba7cd2a427db166.ppt
- Количество слайдов: 29
H-Cube Mini: A compact hydrogenation flow reactor for educational purposes
What is Hydrogenation? • Addition of hydrogen to a functional group • Produces cis predominantly over trans (hydrogens attack same side of double bond) • Utilizes a catalyst, which is either homogeneous (soluble) or heterogeneous (insoluble and solid supported) • Key reaction in pharmaceutical, fine chemical, agrochemical, and petrochemical industries, as well as academia • Hydrogenation accounts for 5 -10% of all reactions in the chemistry industries!
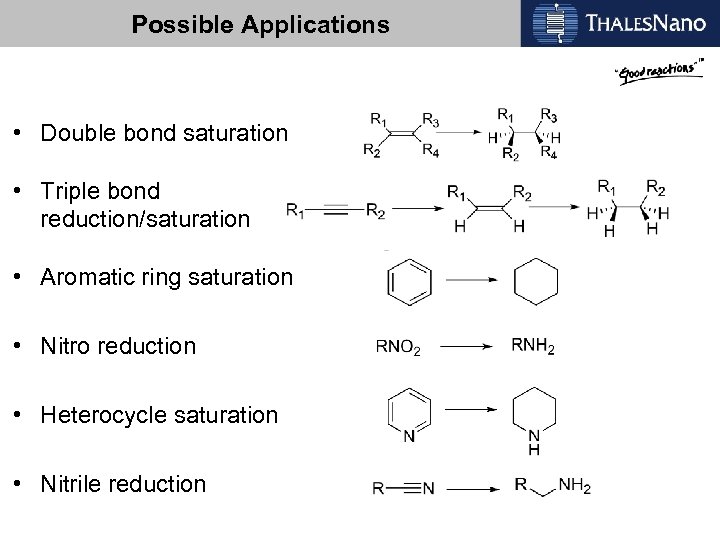
Possible Applications • Double bond saturation • Triple bond reduction/saturation • Aromatic ring saturation • Nitro reduction • Heterocycle saturation • Nitrile reduction
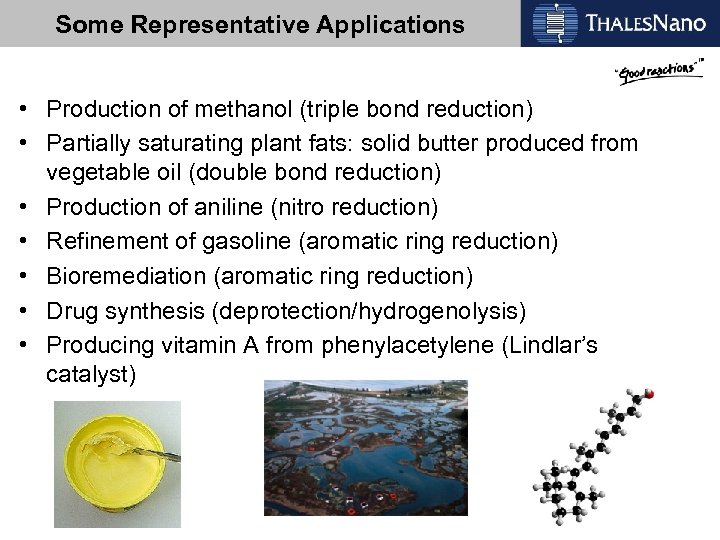
Some Representative Applications • Production of methanol (triple bond reduction) • Partially saturating plant fats: solid butter produced from vegetable oil (double bond reduction) • Production of aniline (nitro reduction) • Refinement of gasoline (aromatic ring reduction) • Bioremediation (aromatic ring reduction) • Drug synthesis (deprotection/hydrogenolysis) • Producing vitamin A from phenylacetylene (Lindlar’s catalyst)

Typical Protocols Hydrogenations usually takes place in a pressure vessel (batch processes) • Parr reactor • Autoclave • Balloon • The Parr Shaker • 78 years old • Industry standard • Homo- and heterogeneous hydrogenations • Pressures up to 2 atm. • Temperatures up to 40 o. C • Batch process

Disadvantages of Batch Processes • • • Need hydrogen cylinder - tough safety regulations Separate laboratory needed Time consuming and difficult to set up Analytical samples difficult to obtain Different reaction scales require different reactors Mixing of 3 phases is inefficient, resulting in poor reaction rates and consequently, long reaction times • Notoriously dangerous § hydrogen gas is flammable § most catalysts are pyrophoric § Catalytic residue can dry out sitting in the waste container and eventually ignite

H-Cube Mini

H-Cube Mini Specifications • Flow rate: 0, 3 -3 m. L/min • Temperature: 25°C to 100°C • Pressure: 1 -100 bar • 30 -70 mm Cat. Carts used • 1 hydrogen cell • Reusable drying Cat. Cart applied • Hydrogen production: 0 or 22 m. L/min • Foldable screen • Small footprint, compact design • Territory specific languages • Updated graphical user interface • Main screen • Graph options • Service screen
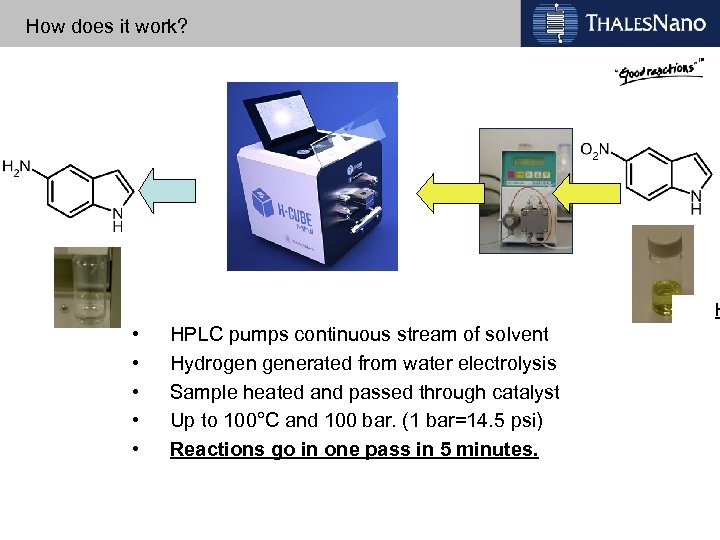
How does it work? H • • • HPLC pumps continuous stream of solvent Hydrogen generated from water electrolysis Sample heated and passed through catalyst Up to 100°C and 100 bar. (1 bar=14. 5 psi) Reactions go in one pass in 5 minutes.

• • • No H 2 cylinders They are a severe safety hazard H-Cube doesn’t use gas cylinders Only water Clean No transportation costs High pressure Low energy Safe Just 2 m. L H 2 @ 1 bar
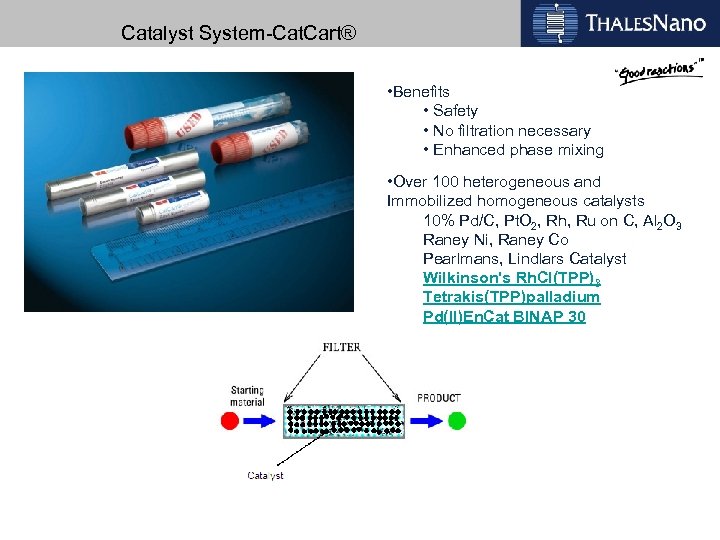
Catalyst System-Cat. Cart® • Benefits • Safety • No filtration necessary • Enhanced phase mixing • Over 100 heterogeneous and Immobilized homogeneous catalysts 10% Pd/C, Pt. O 2, Rh, Ru on C, Al 2 O 3 Raney Ni, Raney Co Pearlmans, Lindlars Catalyst Wilkinson's Rh. Cl(TPP)3 Tetrakis(TPP)palladium Pd(II)En. Cat BINAP 30
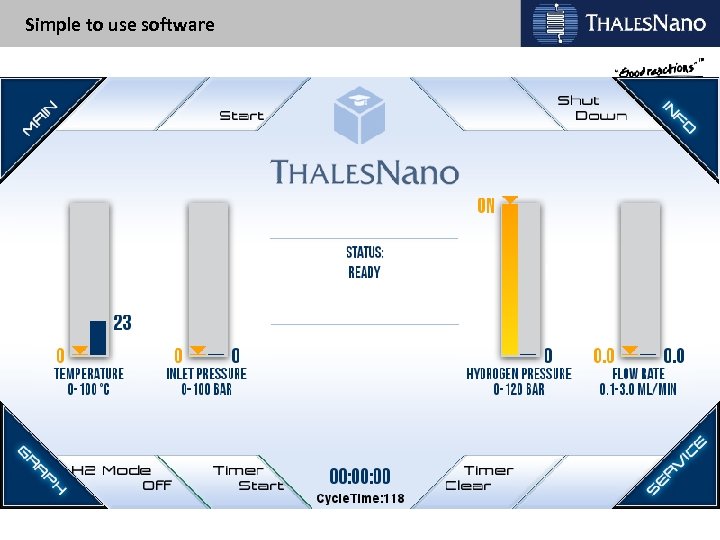
Simple to use software
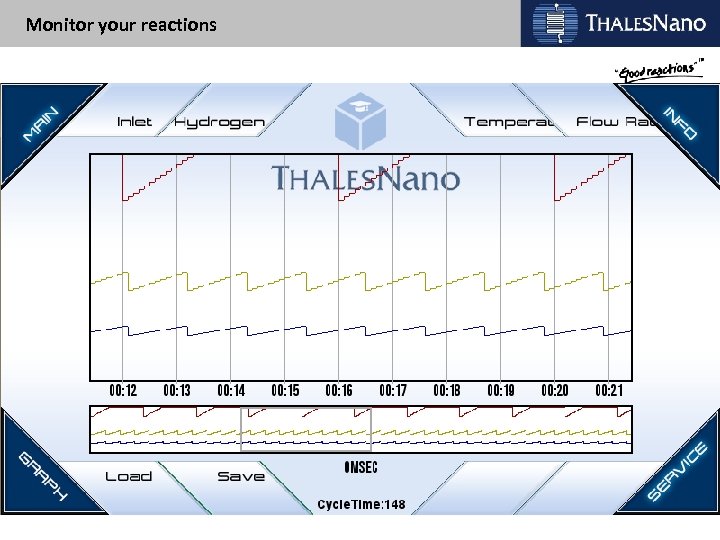
Monitor your reactions

The Dry. Cart • For water sensitive reactions • Filled with molecular sieves to completely dry hydrogen gas production. • Resealable Cat. Carts with specific, PTFE coated elements • Works up to 24 hours reaction time • Easy to regenerate: heat up to 120°C for 1 hour using nitrogen gas flow
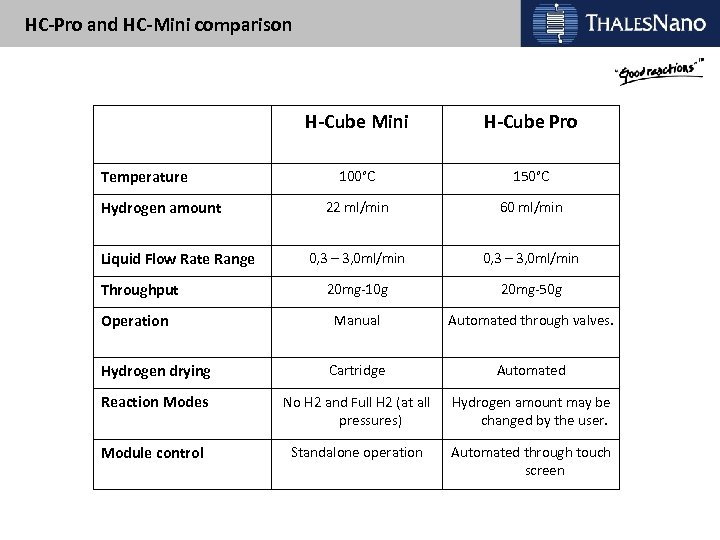
HC-Pro and HC-Mini comparison H-Cube Mini H-Cube Pro 100°C 150°C 22 ml/min 60 ml/min 0, 3 – 3, 0 ml/min 20 mg-10 g 20 mg-50 g Manual Automated through valves. Hydrogen drying Cartridge Automated Reaction Modes No H 2 and Full H 2 (at all pressures) Hydrogen amount may be changed by the user. Module control Standalone operation Automated through touch screen Temperature Hydrogen amount Liquid Flow Rate Range Throughput Operation

Chemistry examples All reactions go to completion in one pass
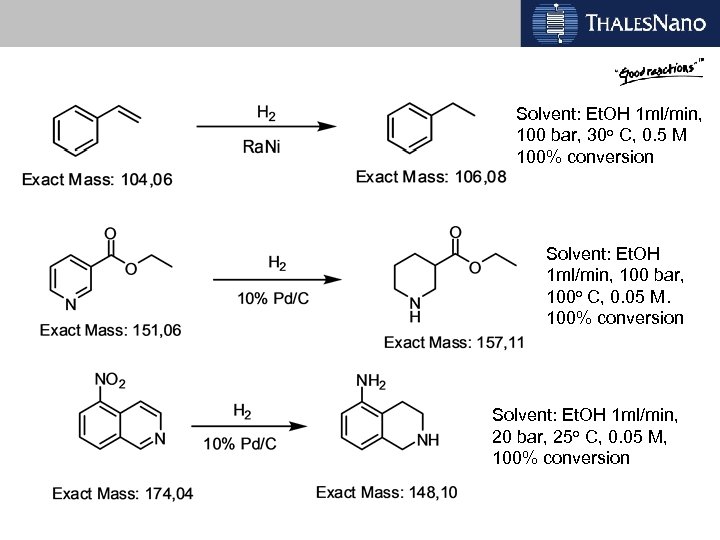
Solvent: Et. OH 1 ml/min, 100 bar, 30 o C, 0. 5 M 100% conversion Solvent: Et. OH 1 ml/min, 100 bar, 100 o C, 0. 05 M. 100% conversion Solvent: Et. OH 1 ml/min, 20 bar, 25 o C, 0. 05 M, 100% conversion
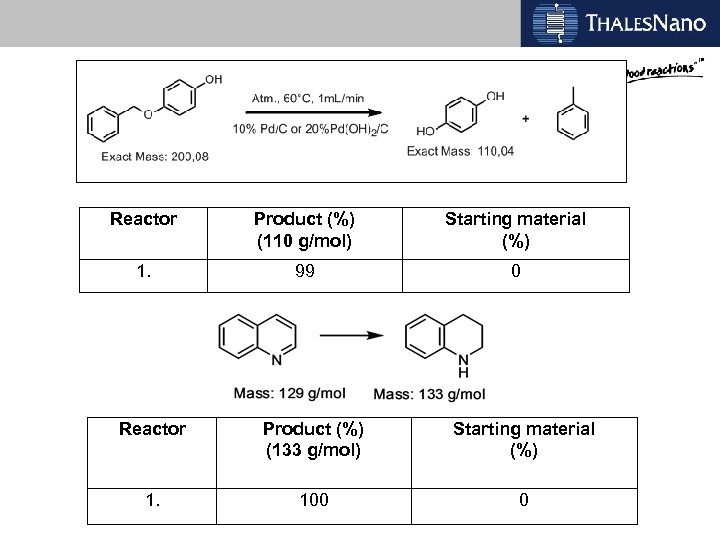
Reactor Product (%) (110 g/mol) Starting material (%) 1. 99 0 Reactor Product (%) (133 g/mol) Starting material (%) 1. 100 0
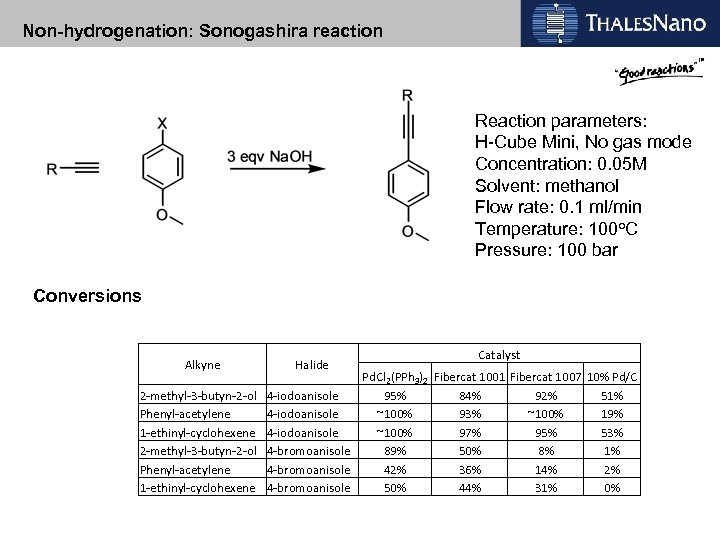
Non-hydrogenation: Sonogashira reaction Reaction parameters: H-Cube Mini, No gas mode Concentration: 0. 05 M Solvent: methanol Flow rate: 0. 1 ml/min Temperature: 100 o. C Pressure: 100 bar Conversions Alkyne Halide Catalyst Pd. Cl 2(PPh 3)2 Fibercat 1001 Fibercat 1007 10% Pd/C 2 -methyl-3 -butyn-2 -ol 4 -iodoanisole 95% 84% 92% 51% Phenyl-acetylene 4 -iodoanisole ~100% 93% ~100% 19% 1 -ethinyl-cyclohexene 4 -iodoanisole ~100% 97% 95% 53% 2 -methyl-3 -butyn-2 -ol 4 -bromoanisole 89% 50% 8% 1% Phenyl-acetylene 4 -bromoanisole 42% 36% 14% 2% 1 -ethinyl-cyclohexene 4 -bromoanisole 50% 44% 31% 0%
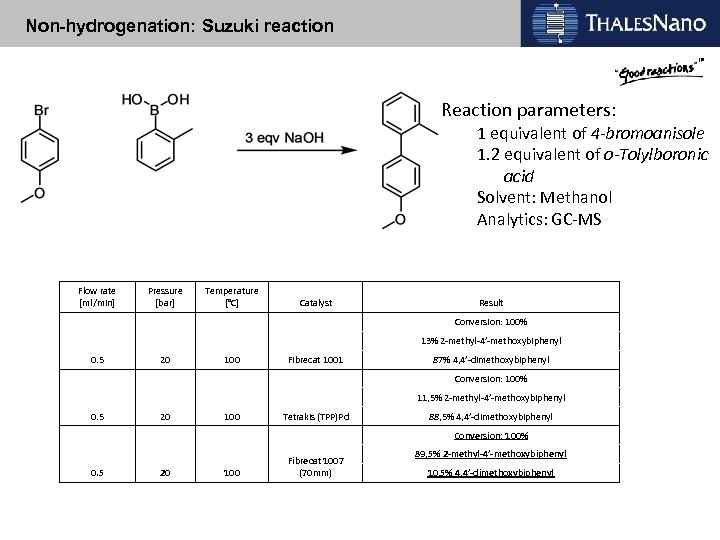
Non-hydrogenation: Suzuki reaction Reaction parameters: 1 equivalent of 4 -bromoanisole 1. 2 equivalent of o-Tolylboronic acid Solvent: Methanol Analytics: GC-MS Flow rate [ml/min] Pressure [bar] Temperature [°C] Catalyst Result Conversion: 100% 13% 2 -methyl-4’-methoxybiphenyl 0. 5 20 100 Fibrecat 1001 87% 4, 4’-dimethoxybiphenyl Conversion: 100% 11, 5% 2 -methyl-4’-methoxybiphenyl 0. 5 20 100 Tetrakis (TPP)Pd 88, 5% 4, 4’-dimethoxybiphenyl Conversion: 100% 0. 5 20 100 Fibrecat 1007 (70 mm) 89, 5% 2 -methyl-4’-methoxybiphenyl 10, 5% 4, 4’-dimethoxybiphenyl

Good For Research Too
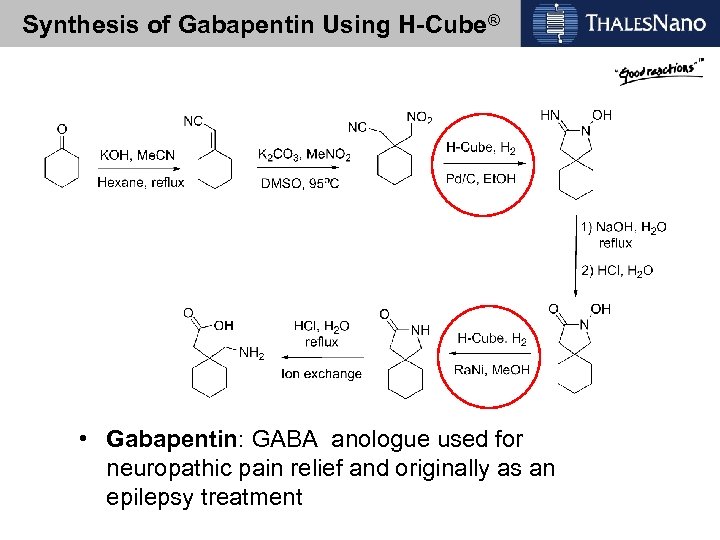
Synthesis of Gabapentin Using H-Cube® • Gabapentin: GABA anologue used for neuropathic pain relief and originally as an epilepsy treatment
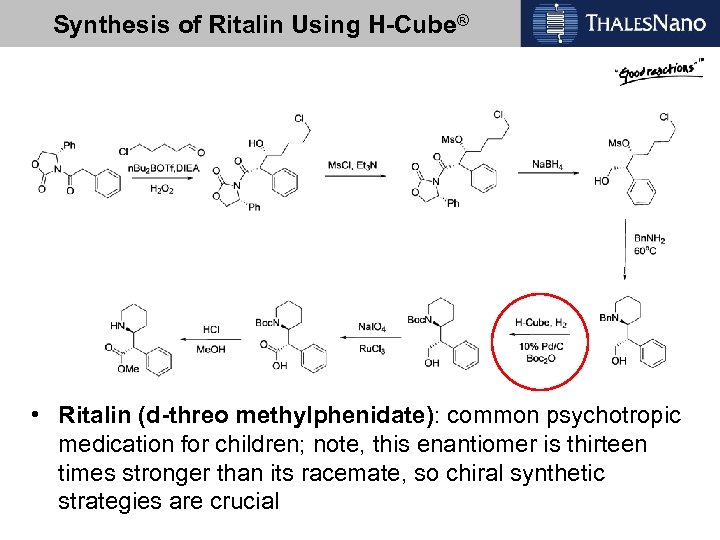
Synthesis of Ritalin Using H-Cube® • Ritalin (d-threo methylphenidate): common psychotropic medication for children; note, this enantiomer is thirteen times stronger than its racemate, so chiral synthetic strategies are crucial
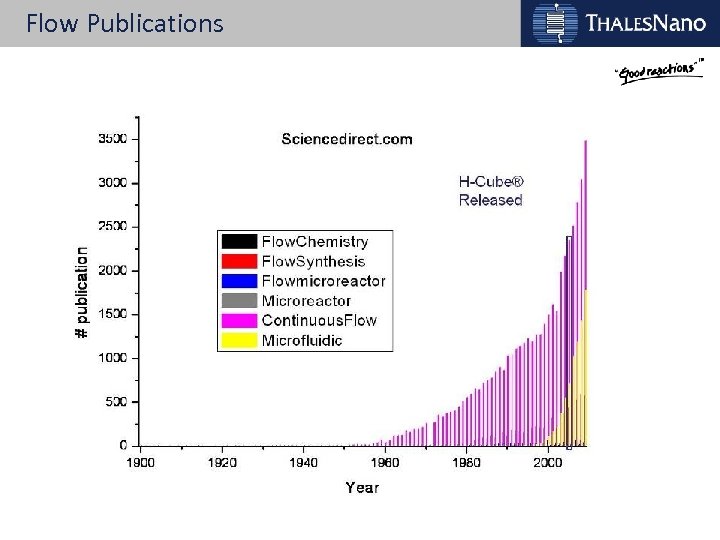
Flow Publications

How to apply to practical lab session?

Practical lab material to help you Hydrogenation Powerpoint presentation Practical lab manual with different sessions Developed in conjunction With University Texas Arlington Video
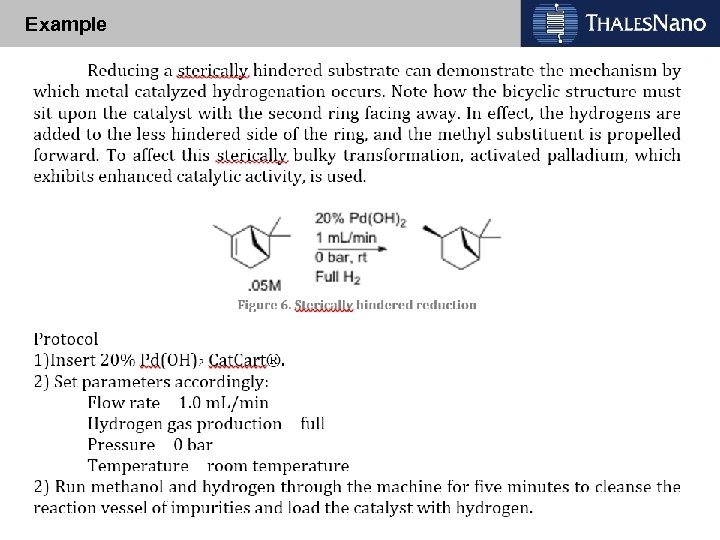
Example

Academic Interest • Over 100 Universities: • Oxford, Cambridge, Imperial • MIT, Scripps, Boston • Marie Curie, Paris • Max Planck, Technical University Vienna • Budapest Technical University • Technical University Prague • Yaroslavl University Russia Over 250 Publications!
THANK YOU FOR YOUR ATTENTION!! ANY QUESTIONS?
c2e528dd83e0a28f9ba7cd2a427db166.ppt