65f69b46189d214514687c498dcb9086.ppt
- Количество слайдов: 28
 Gynecologic Cancer ASCO/ONS Highlights 2011 Association of Northern California Oncologists Lee-may Chen, MD Professor of Clinical Obstetrics, Gynecology, & Reproductive Sciences Division of Gynecologic Oncology UCSF Helen Diller Family Comprehensive Cancer Center
Gynecologic Cancer ASCO/ONS Highlights 2011 Association of Northern California Oncologists Lee-may Chen, MD Professor of Clinical Obstetrics, Gynecology, & Reproductive Sciences Division of Gynecologic Oncology UCSF Helen Diller Family Comprehensive Cancer Center
 Objectives Review, summarize, and interpret new advances and implement changes in the treatment of gynecologic malignancies presented at the 2011 ASCO Annual Meeting
Objectives Review, summarize, and interpret new advances and implement changes in the treatment of gynecologic malignancies presented at the 2011 ASCO Annual Meeting
 New Advances in Gyn Malignancies Biological targets Angiogenesis: Bevacizumab, Cabozantinib, Sorafenib, Aflibercept, Temsirolimus DNA damage repair: Olaparib, Iniparib Folate receptor: Farletuzemab Trabecditin
New Advances in Gyn Malignancies Biological targets Angiogenesis: Bevacizumab, Cabozantinib, Sorafenib, Aflibercept, Temsirolimus DNA damage repair: Olaparib, Iniparib Folate receptor: Farletuzemab Trabecditin
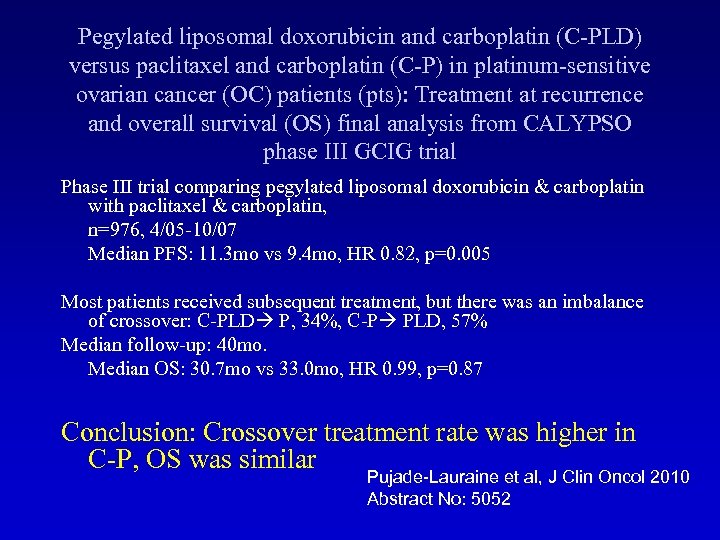 Pegylated liposomal doxorubicin and carboplatin (C-PLD) versus paclitaxel and carboplatin (C-P) in platinum-sensitive ovarian cancer (OC) patients (pts): Treatment at recurrence and overall survival (OS) final analysis from CALYPSO phase III GCIG trial Phase III trial comparing pegylated liposomal doxorubicin & carboplatin with paclitaxel & carboplatin, n=976, 4/05 -10/07 Median PFS: 11. 3 mo vs 9. 4 mo, HR 0. 82, p=0. 005 Most patients received subsequent treatment, but there was an imbalance of crossover: C-PLD P, 34%, C-P PLD, 57% Median follow-up: 40 mo. Median OS: 30. 7 mo vs 33. 0 mo, HR 0. 99, p=0. 87 Conclusion: Crossover treatment rate was higher in C-P, OS was similar Pujade-Lauraine et al, J Clin Oncol 2010 Abstract No: 5052
Pegylated liposomal doxorubicin and carboplatin (C-PLD) versus paclitaxel and carboplatin (C-P) in platinum-sensitive ovarian cancer (OC) patients (pts): Treatment at recurrence and overall survival (OS) final analysis from CALYPSO phase III GCIG trial Phase III trial comparing pegylated liposomal doxorubicin & carboplatin with paclitaxel & carboplatin, n=976, 4/05 -10/07 Median PFS: 11. 3 mo vs 9. 4 mo, HR 0. 82, p=0. 005 Most patients received subsequent treatment, but there was an imbalance of crossover: C-PLD P, 34%, C-P PLD, 57% Median follow-up: 40 mo. Median OS: 30. 7 mo vs 33. 0 mo, HR 0. 99, p=0. 87 Conclusion: Crossover treatment rate was higher in C-P, OS was similar Pujade-Lauraine et al, J Clin Oncol 2010 Abstract No: 5052
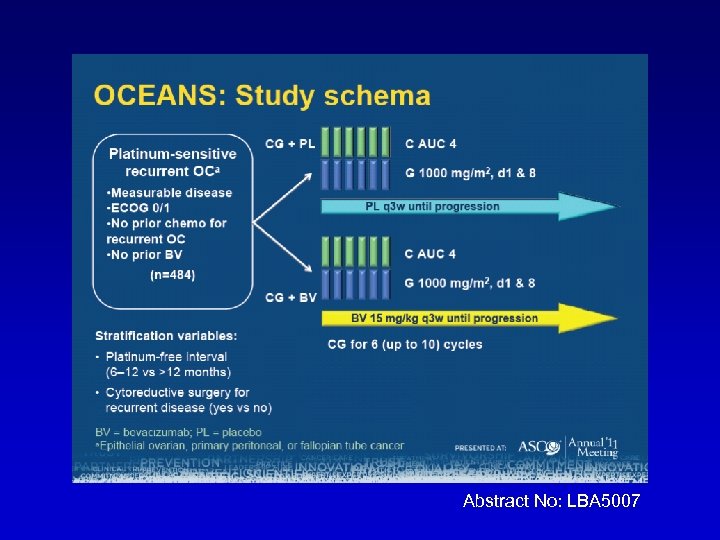
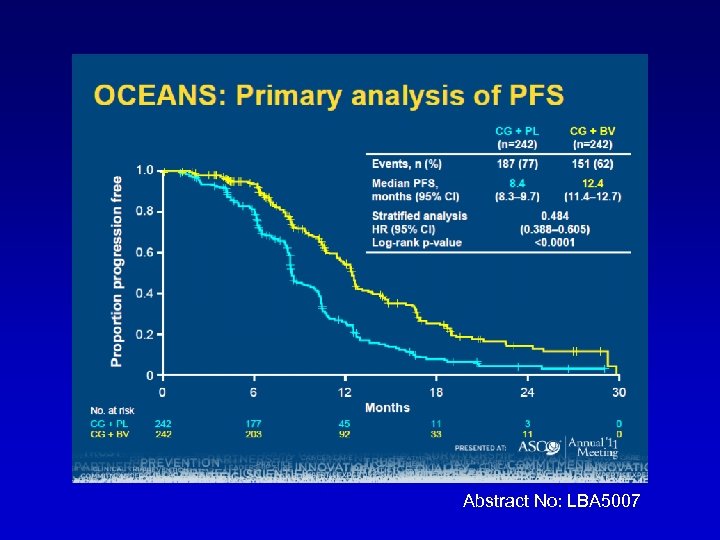
 OCEANS: A randomized, double-blinded, placebocontrolled phase III trial of chemotherapy with or without bevacizumab (BEV) in patients with platinum-sensitive recurrent epithelial ovarian (EOC), primary peritoneal (PPC), or fallopian tube cancer (FTC). C Aghajanian, NJ Finkler, T Rutherford, DA Smith, J Yi, Parmar, LR Nycum, MA Sovak H Memorial Sloan-Kettering Cancer Center, New York, NY; Florida Hospital Gynecologic Oncology, Florida Hospital Cancer Institute, Orlando, FL; Yale University School of Medicine, New Haven, CT; Northwest Cancer Specialists, Vancouver, WA; Genentech Inc. , South San Francisco, CA; Forsyth Regional Cancer Center, Winston-Salem, NC Abstract No: LBA 5007
OCEANS: A randomized, double-blinded, placebocontrolled phase III trial of chemotherapy with or without bevacizumab (BEV) in patients with platinum-sensitive recurrent epithelial ovarian (EOC), primary peritoneal (PPC), or fallopian tube cancer (FTC). C Aghajanian, NJ Finkler, T Rutherford, DA Smith, J Yi, Parmar, LR Nycum, MA Sovak H Memorial Sloan-Kettering Cancer Center, New York, NY; Florida Hospital Gynecologic Oncology, Florida Hospital Cancer Institute, Orlando, FL; Yale University School of Medicine, New Haven, CT; Northwest Cancer Specialists, Vancouver, WA; Genentech Inc. , South San Francisco, CA; Forsyth Regional Cancer Center, Winston-Salem, NC Abstract No: LBA 5007
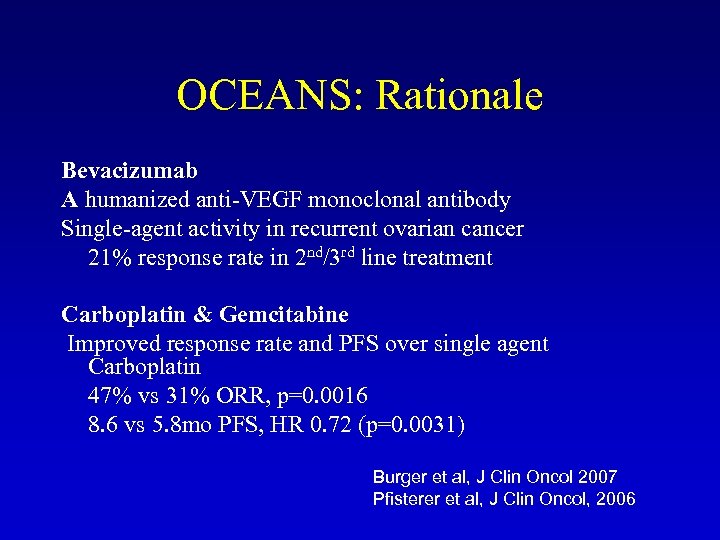 OCEANS: Rationale Bevacizumab A humanized anti-VEGF monoclonal antibody Single-agent activity in recurrent ovarian cancer 21% response rate in 2 nd/3 rd line treatment Carboplatin & Gemcitabine Improved response rate and PFS over single agent Carboplatin 47% vs 31% ORR, p=0. 0016 8. 6 vs 5. 8 mo PFS, HR 0. 72 (p=0. 0031) Burger et al, J Clin Oncol 2007 Pfisterer et al, J Clin Oncol, 2006
OCEANS: Rationale Bevacizumab A humanized anti-VEGF monoclonal antibody Single-agent activity in recurrent ovarian cancer 21% response rate in 2 nd/3 rd line treatment Carboplatin & Gemcitabine Improved response rate and PFS over single agent Carboplatin 47% vs 31% ORR, p=0. 0016 8. 6 vs 5. 8 mo PFS, HR 0. 72 (p=0. 0031) Burger et al, J Clin Oncol 2007 Pfisterer et al, J Clin Oncol, 2006
 Abstract No: LBA 5007
Abstract No: LBA 5007
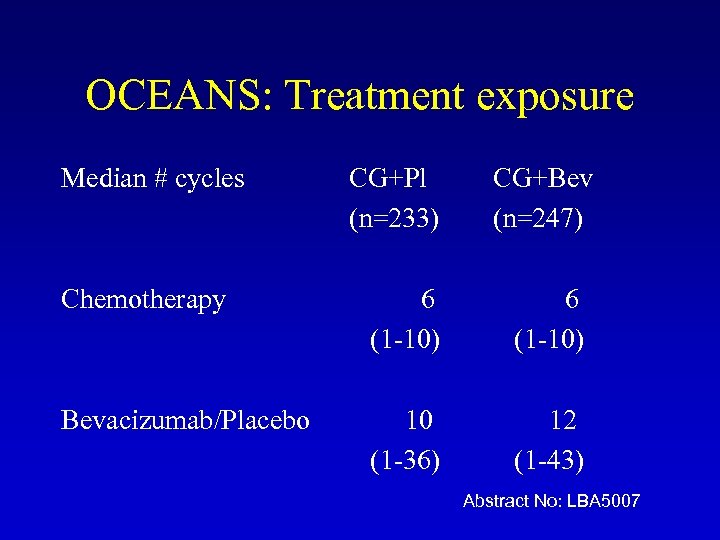 OCEANS: Treatment exposure Median # cycles CG+Pl (n=233) CG+Bev (n=247) Chemotherapy 6 (1 -10) Bevacizumab/Placebo 10 (1 -36) 12 (1 -43) Abstract No: LBA 5007
OCEANS: Treatment exposure Median # cycles CG+Pl (n=233) CG+Bev (n=247) Chemotherapy 6 (1 -10) Bevacizumab/Placebo 10 (1 -36) 12 (1 -43) Abstract No: LBA 5007
 Abstract No: LBA 5007
Abstract No: LBA 5007
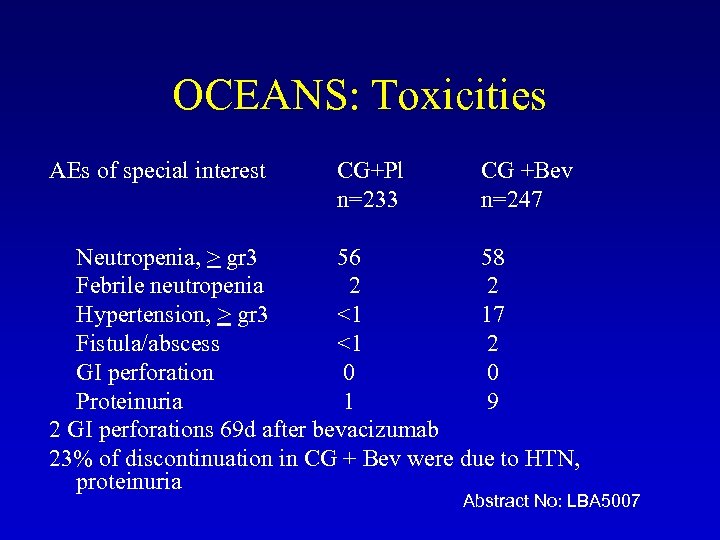 OCEANS: Toxicities AEs of special interest CG+Pl n=233 CG +Bev n=247 Neutropenia, > gr 3 56 58 Febrile neutropenia 2 2 Hypertension, > gr 3 <1 17 Fistula/abscess <1 2 GI perforation 0 0 Proteinuria 1 9 2 GI perforations 69 d after bevacizumab 23% of discontinuation in CG + Bev were due to HTN, proteinuria Abstract No: LBA 5007
OCEANS: Toxicities AEs of special interest CG+Pl n=233 CG +Bev n=247 Neutropenia, > gr 3 56 58 Febrile neutropenia 2 2 Hypertension, > gr 3 <1 17 Fistula/abscess <1 2 GI perforation 0 0 Proteinuria 1 9 2 GI perforations 69 d after bevacizumab 23% of discontinuation in CG + Bev were due to HTN, proteinuria Abstract No: LBA 5007
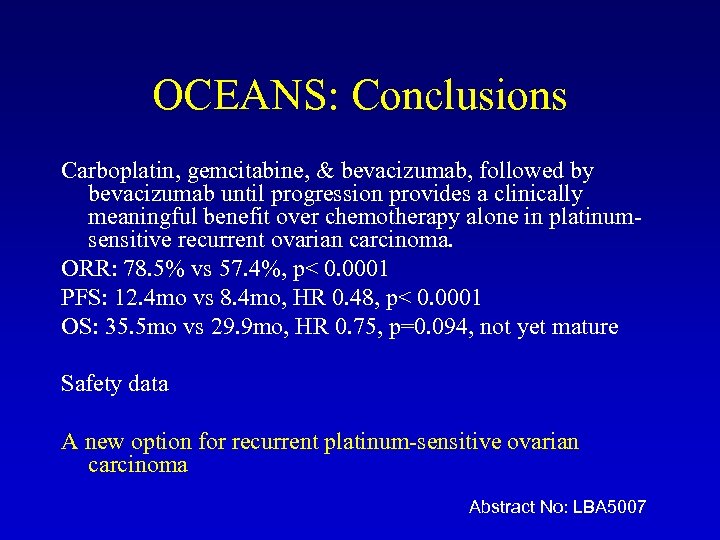 OCEANS: Conclusions Carboplatin, gemcitabine, & bevacizumab, followed by bevacizumab until progression provides a clinically meaningful benefit over chemotherapy alone in platinumsensitive recurrent ovarian carcinoma. ORR: 78. 5% vs 57. 4%, p< 0. 0001 PFS: 12. 4 mo vs 8. 4 mo, HR 0. 48, p< 0. 0001 OS: 35. 5 mo vs 29. 9 mo, HR 0. 75, p=0. 094, not yet mature Safety data A new option for recurrent platinum-sensitive ovarian carcinoma Abstract No: LBA 5007
OCEANS: Conclusions Carboplatin, gemcitabine, & bevacizumab, followed by bevacizumab until progression provides a clinically meaningful benefit over chemotherapy alone in platinumsensitive recurrent ovarian carcinoma. ORR: 78. 5% vs 57. 4%, p< 0. 0001 PFS: 12. 4 mo vs 8. 4 mo, HR 0. 48, p< 0. 0001 OS: 35. 5 mo vs 29. 9 mo, HR 0. 75, p=0. 094, not yet mature Safety data A new option for recurrent platinum-sensitive ovarian carcinoma Abstract No: LBA 5007
 Result of interim analysis of overall survival in the GCIG ICON 7 phase III randomized trial of bevacizumab in women with newly diagnosed ovarian cancer ICON 7: High risk and advanced ovarian cancer treated with debulking surgery, then: Taxol/Carboplatin x 6 cycles, followed by bevacizumab through 18 cycles versus no further treatment N=1528, 12/06 -2/09 PFS: 19. 0 mo vs 17. 3 mo, HR 0. 81, p=0. 0041 Median follow-up: 28 mo. Overall HR 0. 84, p=0. 099 Suboptimal Stage III + Stage IV OS: 36. 6 mo vs 28. 8 mo, HR 0. 64, p=0. 0022 Conclusion: Overall trend for improvement by adding Bev Abstract No: LBA 5006
Result of interim analysis of overall survival in the GCIG ICON 7 phase III randomized trial of bevacizumab in women with newly diagnosed ovarian cancer ICON 7: High risk and advanced ovarian cancer treated with debulking surgery, then: Taxol/Carboplatin x 6 cycles, followed by bevacizumab through 18 cycles versus no further treatment N=1528, 12/06 -2/09 PFS: 19. 0 mo vs 17. 3 mo, HR 0. 81, p=0. 0041 Median follow-up: 28 mo. Overall HR 0. 84, p=0. 099 Suboptimal Stage III + Stage IV OS: 36. 6 mo vs 28. 8 mo, HR 0. 64, p=0. 0022 Conclusion: Overall trend for improvement by adding Bev Abstract No: LBA 5006
 Phase II randomized placebo-controlled study of olaparib (AZD 2281) in patients with platinum-sensitive relapsed serous ovarian cancer (PSR SOC) JA Ledermann, P Harter, C Gourley, M Friedlander, IB Vergote, GJS Rustin, C Scott, W Meier, R Shapira. Frommer, T Safra, D Matei, E Macpherson, C Watkins, J Carmichael, U Matulonis UCL Cancer Institute and UCL Hospitals, London, United Kingdom; Kliniken Essen Mitte, Essen, Germany; Edinburgh Cancer Research UK Centre, Edinburgh, United Kingdom; Department of Medical Oncology, Prince of Wales Hospital and Prince of Wales Clinical School, UNSW, Sydney, Australia; University Hospital Leuven, Belgium; Mount Vernon Cancer Centre, Middlesex, United Kingdom; The Walter and Eliza Hall Institute of Medical Research, Royal Melbourne Hospital, Victoria, Australia; Evangelical Hospital, Düsseldorf, Germany; Oncology Institute, Chaim Sheba Medical Center, Ramat-Gan, Israel; Department of Oncology, Tel Aviv Sourasky Medical Center, Sackler School of Medicine, Tel-Aviv University, Tel Aviv, Israel; Indiana University Simon Cancer Center, Indianapolis, IN; Astra. Zeneca, Macclesfield, United Kingdom; Dana-Farber Cancer Institute, Boston, MA Abstract No: 5003
Phase II randomized placebo-controlled study of olaparib (AZD 2281) in patients with platinum-sensitive relapsed serous ovarian cancer (PSR SOC) JA Ledermann, P Harter, C Gourley, M Friedlander, IB Vergote, GJS Rustin, C Scott, W Meier, R Shapira. Frommer, T Safra, D Matei, E Macpherson, C Watkins, J Carmichael, U Matulonis UCL Cancer Institute and UCL Hospitals, London, United Kingdom; Kliniken Essen Mitte, Essen, Germany; Edinburgh Cancer Research UK Centre, Edinburgh, United Kingdom; Department of Medical Oncology, Prince of Wales Hospital and Prince of Wales Clinical School, UNSW, Sydney, Australia; University Hospital Leuven, Belgium; Mount Vernon Cancer Centre, Middlesex, United Kingdom; The Walter and Eliza Hall Institute of Medical Research, Royal Melbourne Hospital, Victoria, Australia; Evangelical Hospital, Düsseldorf, Germany; Oncology Institute, Chaim Sheba Medical Center, Ramat-Gan, Israel; Department of Oncology, Tel Aviv Sourasky Medical Center, Sackler School of Medicine, Tel-Aviv University, Tel Aviv, Israel; Indiana University Simon Cancer Center, Indianapolis, IN; Astra. Zeneca, Macclesfield, United Kingdom; Dana-Farber Cancer Institute, Boston, MA Abstract No: 5003
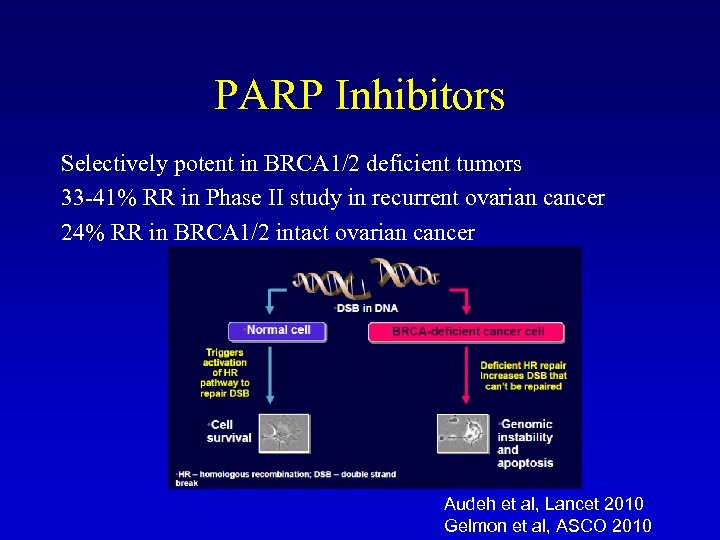 PARP Inhibitors Selectively potent in BRCA 1/2 deficient tumors 33 -41% RR in Phase II study in recurrent ovarian cancer 24% RR in BRCA 1/2 intact ovarian cancer Audeh et al, Lancet 2010 Gelmon et al, ASCO 2010
PARP Inhibitors Selectively potent in BRCA 1/2 deficient tumors 33 -41% RR in Phase II study in recurrent ovarian cancer 24% RR in BRCA 1/2 intact ovarian cancer Audeh et al, Lancet 2010 Gelmon et al, ASCO 2010
 Olaparib Maintenance Platinum sensitive recurrent ovarian carcinoma with stable complete/partial response 2 or more prior platinum-containing regimens Stratified by time to progression and response in last platinum regimen, Jewish descent BRCA testing not required Olaparib: 400 mg PO BID vs Placebo Evaluation for progression by RECIST criteria Primary objective: PFS Abstract No: 5003
Olaparib Maintenance Platinum sensitive recurrent ovarian carcinoma with stable complete/partial response 2 or more prior platinum-containing regimens Stratified by time to progression and response in last platinum regimen, Jewish descent BRCA testing not required Olaparib: 400 mg PO BID vs Placebo Evaluation for progression by RECIST criteria Primary objective: PFS Abstract No: 5003
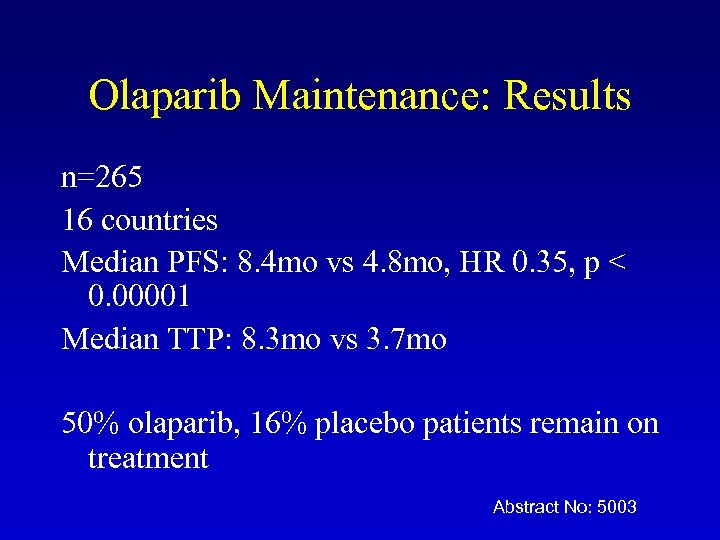 Olaparib Maintenance: Results n=265 16 countries Median PFS: 8. 4 mo vs 4. 8 mo, HR 0. 35, p < 0. 00001 Median TTP: 8. 3 mo vs 3. 7 mo 50% olaparib, 16% placebo patients remain on treatment Abstract No: 5003
Olaparib Maintenance: Results n=265 16 countries Median PFS: 8. 4 mo vs 4. 8 mo, HR 0. 35, p < 0. 00001 Median TTP: 8. 3 mo vs 3. 7 mo 50% olaparib, 16% placebo patients remain on treatment Abstract No: 5003
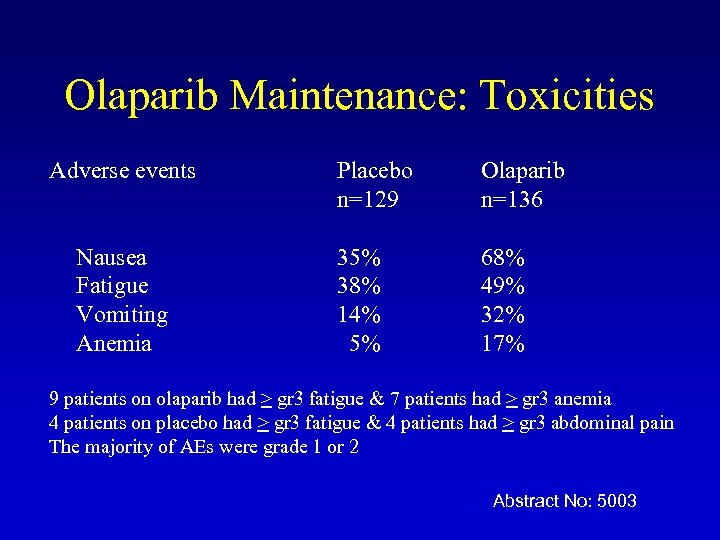 Olaparib Maintenance: Toxicities Adverse events Nausea Fatigue Vomiting Anemia Placebo n=129 Olaparib n=136 35% 38% 14% 5% 68% 49% 32% 17% 9 patients on olaparib had > gr 3 fatigue & 7 patients had > gr 3 anemia 4 patients on placebo had > gr 3 fatigue & 4 patients had > gr 3 abdominal pain The majority of AEs were grade 1 or 2 Abstract No: 5003
Olaparib Maintenance: Toxicities Adverse events Nausea Fatigue Vomiting Anemia Placebo n=129 Olaparib n=136 35% 38% 14% 5% 68% 49% 32% 17% 9 patients on olaparib had > gr 3 fatigue & 7 patients had > gr 3 anemia 4 patients on placebo had > gr 3 fatigue & 4 patients had > gr 3 abdominal pain The majority of AEs were grade 1 or 2 Abstract No: 5003
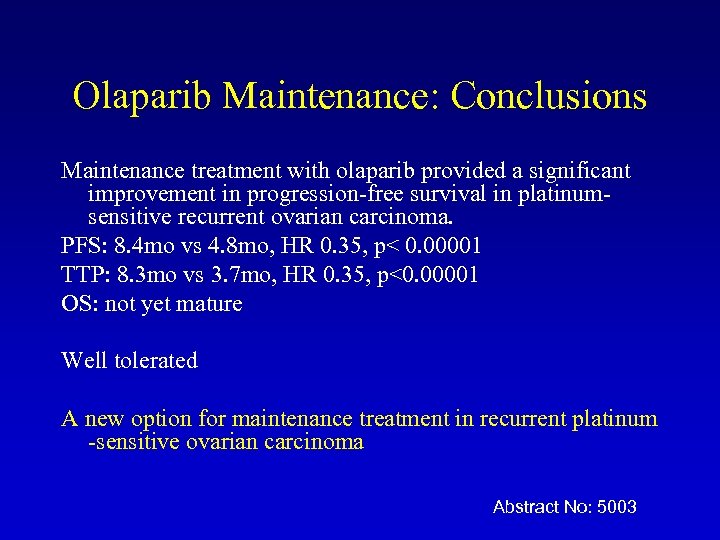 Olaparib Maintenance: Conclusions Maintenance treatment with olaparib provided a significant improvement in progression-free survival in platinumsensitive recurrent ovarian carcinoma. PFS: 8. 4 mo vs 4. 8 mo, HR 0. 35, p< 0. 00001 TTP: 8. 3 mo vs 3. 7 mo, HR 0. 35, p<0. 00001 OS: not yet mature Well tolerated A new option for maintenance treatment in recurrent platinum -sensitive ovarian carcinoma Abstract No: 5003
Olaparib Maintenance: Conclusions Maintenance treatment with olaparib provided a significant improvement in progression-free survival in platinumsensitive recurrent ovarian carcinoma. PFS: 8. 4 mo vs 4. 8 mo, HR 0. 35, p< 0. 00001 TTP: 8. 3 mo vs 3. 7 mo, HR 0. 35, p<0. 00001 OS: not yet mature Well tolerated A new option for maintenance treatment in recurrent platinum -sensitive ovarian carcinoma Abstract No: 5003
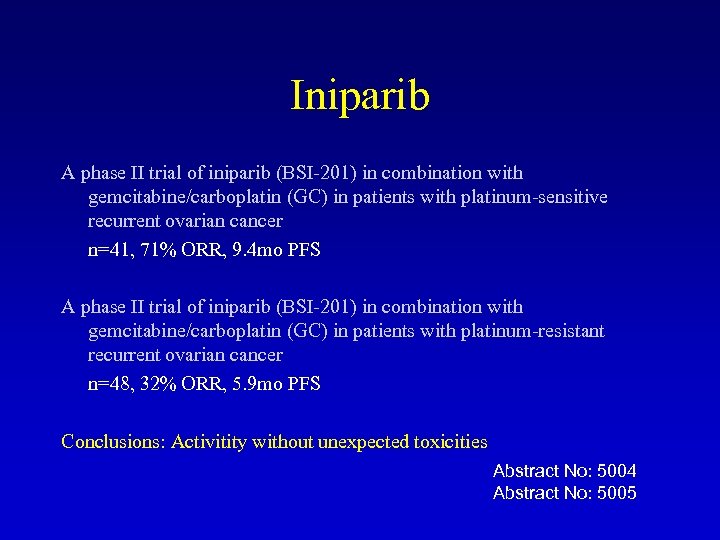 Iniparib A phase II trial of iniparib (BSI-201) in combination with gemcitabine/carboplatin (GC) in patients with platinum-sensitive recurrent ovarian cancer n=41, 71% ORR, 9. 4 mo PFS A phase II trial of iniparib (BSI-201) in combination with gemcitabine/carboplatin (GC) in patients with platinum-resistant recurrent ovarian cancer n=48, 32% ORR, 5. 9 mo PFS Conclusions: Activitity without unexpected toxicities Abstract No: 5004 Abstract No: 5005
Iniparib A phase II trial of iniparib (BSI-201) in combination with gemcitabine/carboplatin (GC) in patients with platinum-sensitive recurrent ovarian cancer n=41, 71% ORR, 9. 4 mo PFS A phase II trial of iniparib (BSI-201) in combination with gemcitabine/carboplatin (GC) in patients with platinum-resistant recurrent ovarian cancer n=48, 32% ORR, 5. 9 mo PFS Conclusions: Activitity without unexpected toxicities Abstract No: 5004 Abstract No: 5005
 Effect of screening on ovarian cancer mortality in the Prostate, Lung, Colorectal, and Ovarian (PLCO) cancer randomized screening trial SS Buys, E Partridge, A Black, C Johnson, L Lamerato, C Isaacs, D Reding, R Greenlee, B Kessel, M Fouad, D Chia, L Ragard, J Rathmell, P Hartge, P Pinsky, G Izmirlian, J Xu, P Prorok, CD Berg Huntsman Cancer Institute at the University of Utah, Salt Lake City, UT; University of Alabama at Birmingham, AL; National Cancer Institute, Bethesda, MD; Henry Ford Health System, Detroit, MI; Henry Ford Hospital, Detroit, MI; Lombardi Comprehensive Cancer Center, Washington, DC; Marshfield Clinic Research Foundation, Marshfield, WI; Marshfield Medical Center, Marshfield, WI; Pacific Health Research Education Institute, Honolulu, HI; Minority Health and Health Disparities Research Center, Birmingham, AL; Department of Pathology and Laboratory Medicine, Los Angeles, CA; Westat, Inc. , Rockville, MD; Information Management Services, Inc. , Bethesda, MD; Division of Cancer Prevention, NCI, Bethesda, MD Abstract No: 5001
Effect of screening on ovarian cancer mortality in the Prostate, Lung, Colorectal, and Ovarian (PLCO) cancer randomized screening trial SS Buys, E Partridge, A Black, C Johnson, L Lamerato, C Isaacs, D Reding, R Greenlee, B Kessel, M Fouad, D Chia, L Ragard, J Rathmell, P Hartge, P Pinsky, G Izmirlian, J Xu, P Prorok, CD Berg Huntsman Cancer Institute at the University of Utah, Salt Lake City, UT; University of Alabama at Birmingham, AL; National Cancer Institute, Bethesda, MD; Henry Ford Health System, Detroit, MI; Henry Ford Hospital, Detroit, MI; Lombardi Comprehensive Cancer Center, Washington, DC; Marshfield Clinic Research Foundation, Marshfield, WI; Marshfield Medical Center, Marshfield, WI; Pacific Health Research Education Institute, Honolulu, HI; Minority Health and Health Disparities Research Center, Birmingham, AL; Department of Pathology and Laboratory Medicine, Los Angeles, CA; Westat, Inc. , Rockville, MD; Information Management Services, Inc. , Bethesda, MD; Division of Cancer Prevention, NCI, Bethesda, MD Abstract No: 5001
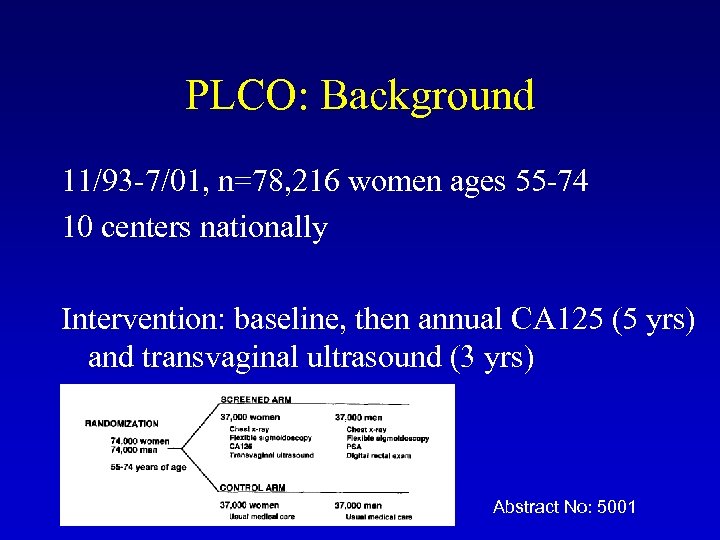 PLCO: Background 11/93 -7/01, n=78, 216 women ages 55 -74 10 centers nationally Intervention: baseline, then annual CA 125 (5 yrs) and transvaginal ultrasound (3 yrs) Abstract No: 5001
PLCO: Background 11/93 -7/01, n=78, 216 women ages 55 -74 10 centers nationally Intervention: baseline, then annual CA 125 (5 yrs) and transvaginal ultrasound (3 yrs) Abstract No: 5001
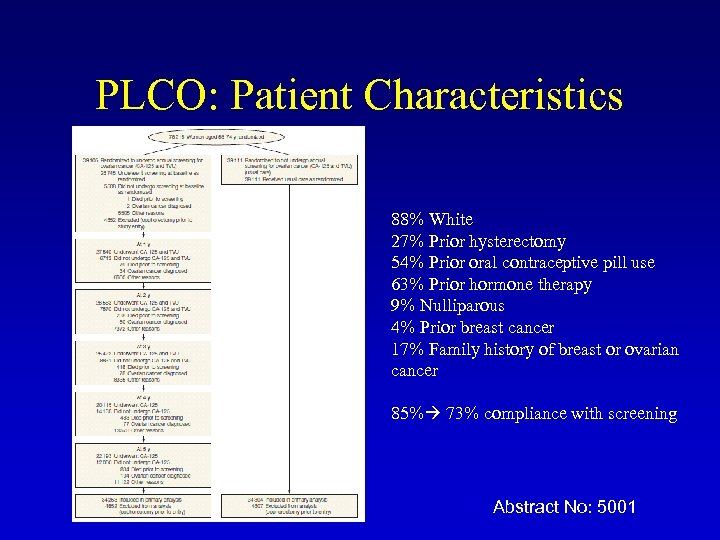 PLCO: Patient Characteristics 88% White 27% Prior hysterectomy 54% Prior oral contraceptive pill use 63% Prior hormone therapy 9% Nulliparous 4% Prior breast cancer 17% Family history of breast or ovarian cancer 85% 73% compliance with screening Abstract No: 5001
PLCO: Patient Characteristics 88% White 27% Prior hysterectomy 54% Prior oral contraceptive pill use 63% Prior hormone therapy 9% Nulliparous 4% Prior breast cancer 17% Family history of breast or ovarian cancer 85% 73% compliance with screening Abstract No: 5001
 PLCO: Results CA 125: 1. 4 -1. 8% positive screen Transvaginal ultrasound: 2. 9 -4. 6% positive screen Ovarian cancers diagnosed 212 cases in screening arm (5. 7 per 10, 000 person years), 77% Stage III/IV 176 in usual care arm (4. 7 per 10, 000 person years), 78% Stage III/IV RR 1. 21, 95% CI 0. 99 -1. 48 Abstract No: 5001
PLCO: Results CA 125: 1. 4 -1. 8% positive screen Transvaginal ultrasound: 2. 9 -4. 6% positive screen Ovarian cancers diagnosed 212 cases in screening arm (5. 7 per 10, 000 person years), 77% Stage III/IV 176 in usual care arm (4. 7 per 10, 000 person years), 78% Stage III/IV RR 1. 21, 95% CI 0. 99 -1. 48 Abstract No: 5001
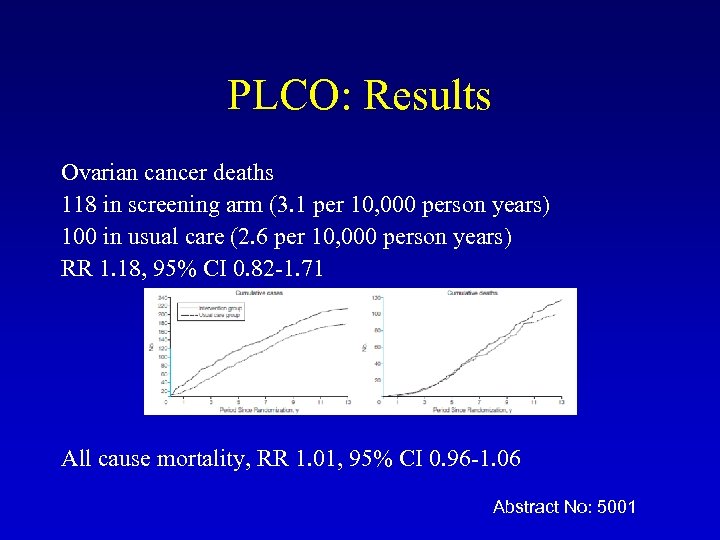 PLCO: Results Ovarian cancer deaths 118 in screening arm (3. 1 per 10, 000 person years) 100 in usual care (2. 6 per 10, 000 person years) RR 1. 18, 95% CI 0. 82 -1. 71 All cause mortality, RR 1. 01, 95% CI 0. 96 -1. 06 Abstract No: 5001
PLCO: Results Ovarian cancer deaths 118 in screening arm (3. 1 per 10, 000 person years) 100 in usual care (2. 6 per 10, 000 person years) RR 1. 18, 95% CI 0. 82 -1. 71 All cause mortality, RR 1. 01, 95% CI 0. 96 -1. 06 Abstract No: 5001
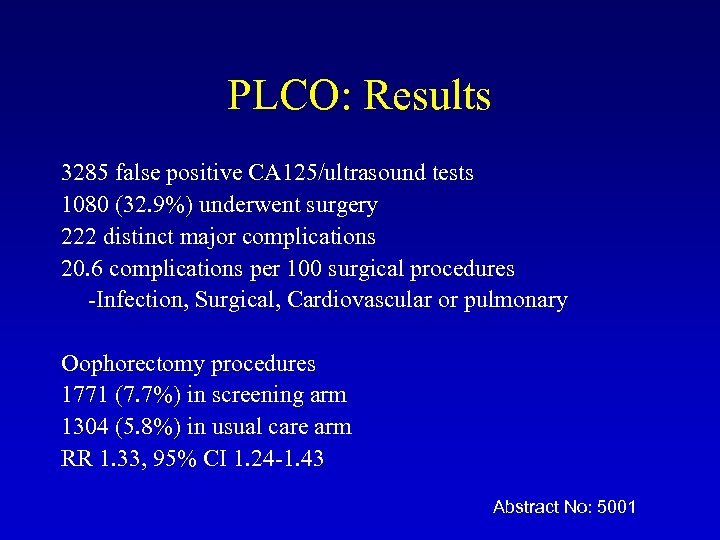 PLCO: Results 3285 false positive CA 125/ultrasound tests 1080 (32. 9%) underwent surgery 222 distinct major complications 20. 6 complications per 100 surgical procedures -Infection, Surgical, Cardiovascular or pulmonary Oophorectomy procedures 1771 (7. 7%) in screening arm 1304 (5. 8%) in usual care arm RR 1. 33, 95% CI 1. 24 -1. 43 Abstract No: 5001
PLCO: Results 3285 false positive CA 125/ultrasound tests 1080 (32. 9%) underwent surgery 222 distinct major complications 20. 6 complications per 100 surgical procedures -Infection, Surgical, Cardiovascular or pulmonary Oophorectomy procedures 1771 (7. 7%) in screening arm 1304 (5. 8%) in usual care arm RR 1. 33, 95% CI 1. 24 -1. 43 Abstract No: 5001
 PLCO: Conclusions Annual CA 125 and transvaginal ultrasound screening does not reduce disease-specific mortality in average risk postmenopausal women. Screening does increase invasive medical procedures, with associated harms and complications. Abstract No: 5001
PLCO: Conclusions Annual CA 125 and transvaginal ultrasound screening does not reduce disease-specific mortality in average risk postmenopausal women. Screening does increase invasive medical procedures, with associated harms and complications. Abstract No: 5001
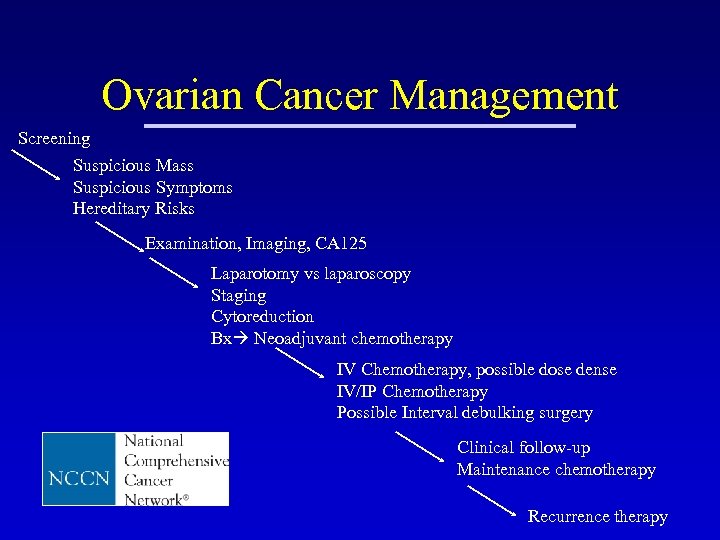 Ovarian Cancer Management Screening Suspicious Mass Suspicious Symptoms Hereditary Risks Examination, Imaging, CA 125 Laparotomy vs laparoscopy Staging Cytoreduction Bx Neoadjuvant chemotherapy IV Chemotherapy, possible dose dense IV/IP Chemotherapy Possible Interval debulking surgery Clinical follow-up Maintenance chemotherapy Recurrence therapy
Ovarian Cancer Management Screening Suspicious Mass Suspicious Symptoms Hereditary Risks Examination, Imaging, CA 125 Laparotomy vs laparoscopy Staging Cytoreduction Bx Neoadjuvant chemotherapy IV Chemotherapy, possible dose dense IV/IP Chemotherapy Possible Interval debulking surgery Clinical follow-up Maintenance chemotherapy Recurrence therapy
 ASCO 2011: Gyn Takeaway OCEANS: Consider bevacizumab in recurrent ovarian cancer PARP inhibitors: Consider olaparib for maintenance in ovarian cancer Screening: Annual CA 125 & USN ineffective for detecting ovarian cancer in low risk women
ASCO 2011: Gyn Takeaway OCEANS: Consider bevacizumab in recurrent ovarian cancer PARP inhibitors: Consider olaparib for maintenance in ovarian cancer Screening: Annual CA 125 & USN ineffective for detecting ovarian cancer in low risk women


