Group 14.pptx
- Количество слайдов: 23

Group 14/4 elements Lesson outcomes You will be able to understand explain trends in oxidation states down the group. You will be able to describe and explain the properties of the oxides of oxidation states II and IV.
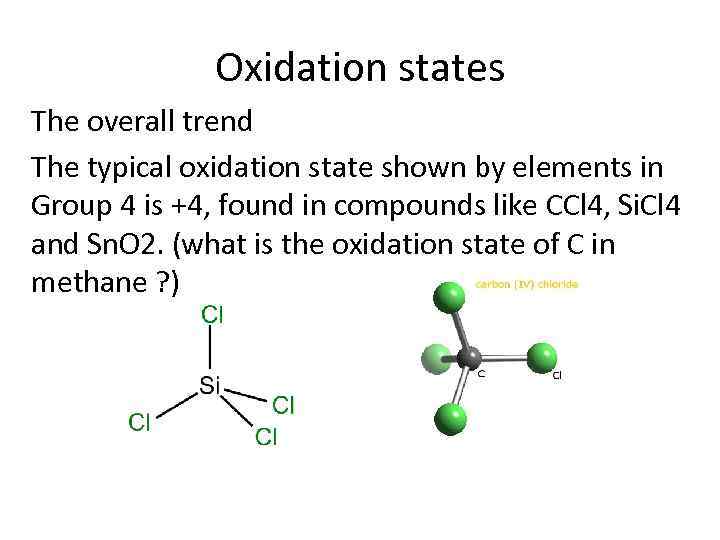
Oxidation states The overall trend The typical oxidation state shown by elements in Group 4 is +4, found in compounds like CCl 4, Si. Cl 4 and Sn. O 2. (what is the oxidation state of C in methane ? )

However, as you go down the Group, there are more and more examples where the oxidation state is +2, such as Sn. Cl 2, Pb. O, and Pb 2+. With tin, the +4 state is still more stable than the +2, but by the time you get to lead, the +2 state is the more stable - and dominates the chemistry of lead.

Carbon with oxidation state of 2 The only common example of the +2 oxidation state in carbon chemistry occurs in carbon monoxide, CO. Carbon monoxide is a strong reducing agent because it is easily oxidised to carbon dioxide where the oxidation state is the more thermodynamically stable +4. For example, carbon monoxide reduces many hot metal oxides to the metal - a reaction which is used, for example, in the extraction of iron in a blast furnace.

Explanation of oxidation states. All of the elements in the group have the outer electronic structure ns 2 npx 1 npy 1, where n varies from 2 (for carbon) to 6 (for lead). The oxidation state of +4 is where all these outer electrons are directly involved in the bonding. As you get closer to the bottom of the Group, there is an increasing tendency for the s 2 pair not to be used in the bonding. This is often known as the inert pair effect - and is dominant in lead chemistry.

Inert pair effect in the formation of covalent bonds If the elements in Group 4 form 2+ ions, they will lose the p electrons, leaving the s 2 pair unused. For example, to form a lead(II) ion, lead will lose the two 6 p electrons, but the 6 s electrons will be left unchanged - an "inert pair". You would normally expect ionisation energies to fall as you go down a Group as the electrons get further from the nucleus. That doesn't quite happen in Group 4.
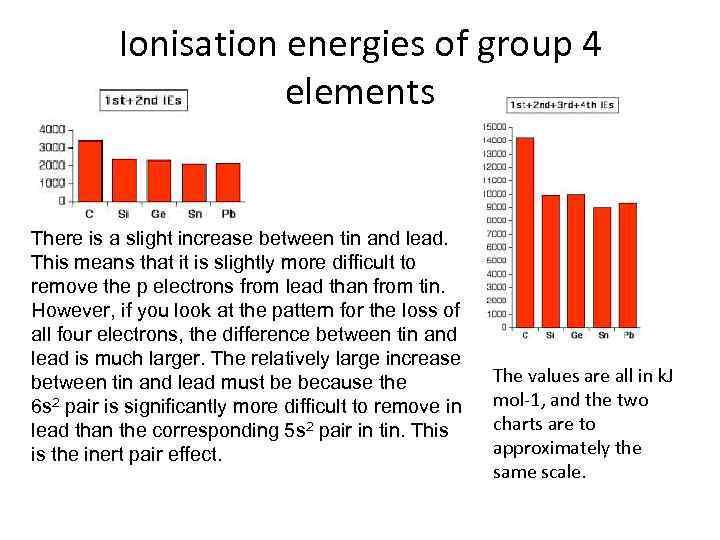
Ionisation energies of group 4 elements There is a slight increase between tin and lead. This means that it is slightly more difficult to remove the p electrons from lead than from tin. However, if you look at the pattern for the loss of all four electrons, the difference between tin and lead is much larger. The relatively large increase between tin and lead must be because the 6 s 2 pair is significantly more difficult to remove in lead than the corresponding 5 s 2 pair in tin. This is the inert pair effect. The values are all in k. J mol-1, and the two charts are to approximately the same scale.
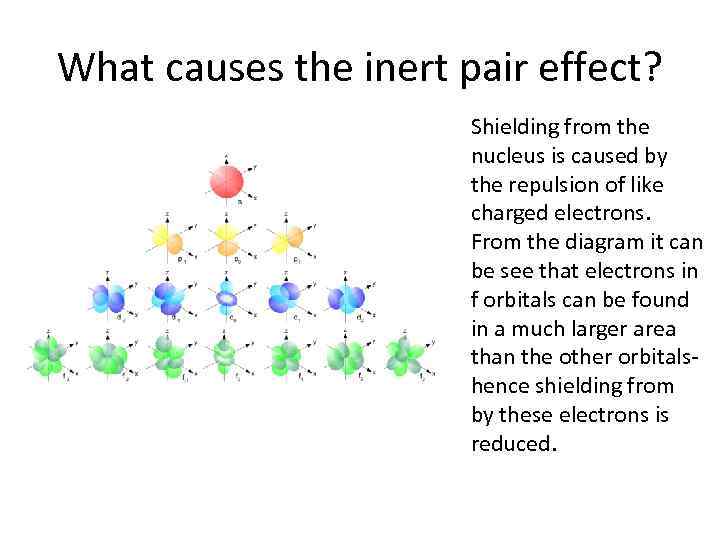
What causes the inert pair effect? Shielding from the nucleus is caused by the repulsion of like charged electrons. From the diagram it can be see that electrons in f orbitals can be found in a much larger area than the other orbitalshence shielding from by these electrons is reduced.
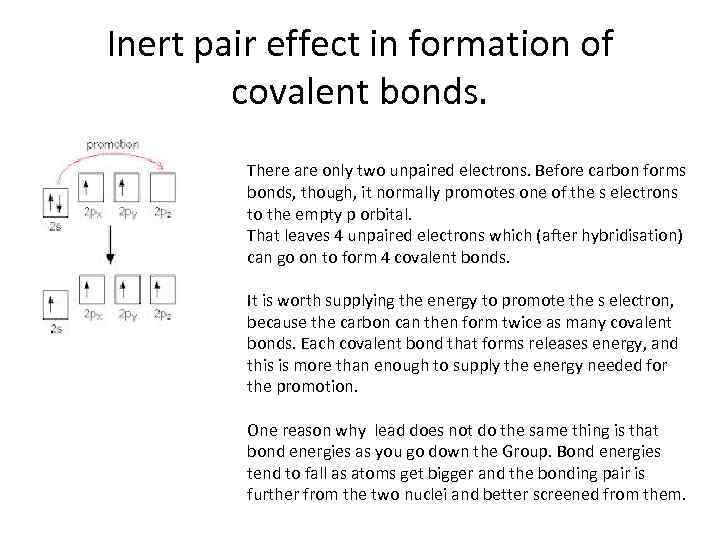
Inert pair effect in formation of covalent bonds. There are only two unpaired electrons. Before carbon forms bonds, though, it normally promotes one of the s electrons to the empty p orbital. That leaves 4 unpaired electrons which (after hybridisation) can go on to form 4 covalent bonds. It is worth supplying the energy to promote the s electron, because the carbon can then form twice as many covalent bonds. Each covalent bond that forms releases energy, and this is more than enough to supply the energy needed for the promotion. One reason why lead does not do the same thing is that bond energies as you go down the Group. Bond energies tend to fall as atoms get bigger and the bonding pair is further from the two nuclei and better screened from them.

Properties of group 4 oxides. Carbon dioxide is a gas whereas silicon dioxide is a hard high-melting solid. The other dioxides in Group 4 are also solids. Carbon can form simple molecules with oxygen because it can form double bonds with it. None of the other elements in Group 4 form double bonds with oxygen, and different structures are formed.

Silicon dioxide Silicon atoms are bigger than carbon. That means that silicon-oxygen bonds will be longer than carbon-oxygen bonds. With the longer silicon-oxygen bonds, the p orbitals on the silicon and the oxygen aren't quite close enough together to allow enough sideways overlap to give a stable pi bond.
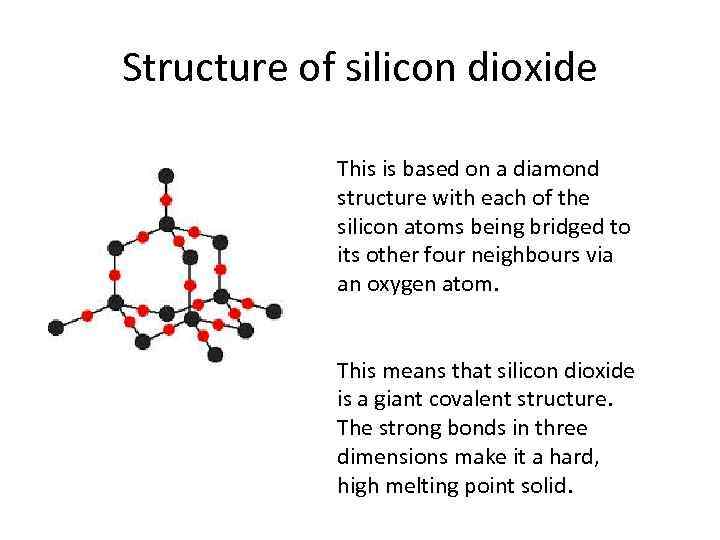
Structure of silicon dioxide This is based on a diamond structure with each of the silicon atoms being bridged to its other four neighbours via an oxygen atom. This means that silicon dioxide is a giant covalent structure. The strong bonds in three dimensions make it a hard, high melting point solid.

Acid /base behaviour The oxides of the elements at the top of Group 4 are acidic, but acidity of the oxides falls as you go down the Group. Towards the bottom of the Group, the oxides become more basic. An oxide which can show both acidic and basic properties is said to be amphoteric. The trend isacidic oxides at the top of the Group moving towards amphoteric ones at the bottom.

Carbon and silicon oxides Carbon monoxide is usually treated as if it was a neutral oxide, but in fact it is very, very slightly acidic. It doesn't react with water, but it will react with hot concentrated sodium hydroxide solution to give a solution of sodium methanoate. CO + Na. OH CHOONa

Carbon and silicon dioxides These are both weakly acidic. With water. Silicon dioxide doesn't react with water, because of the difficulty of breaking up the giant covalent structure. Carbon dioxide does react with water to a slight extent to produce hydrogen ions (strictly, hydroxonium ions) and hydrogencarbonate ions.
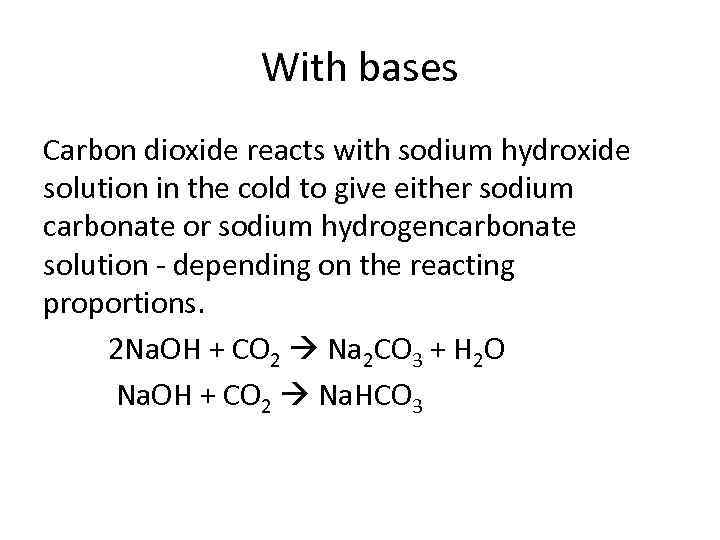
With bases Carbon dioxide reacts with sodium hydroxide solution in the cold to give either sodium carbonate or sodium hydrogencarbonate solution - depending on the reacting proportions. 2 Na. OH + CO 2 Na 2 CO 3 + H 2 O Na. OH + CO 2 Na. HCO 3
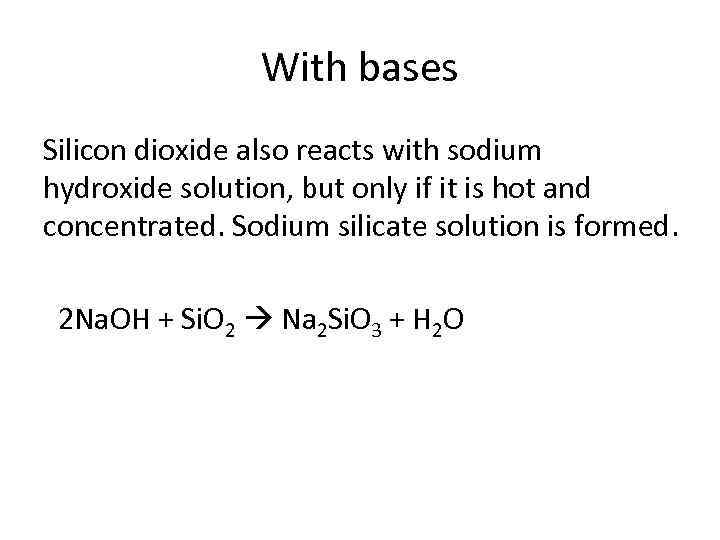
With bases Silicon dioxide also reacts with sodium hydroxide solution, but only if it is hot and concentrated. Sodium silicate solution is formed. 2 Na. OH + Si. O 2 Na 2 Si. O 3 + H 2 O

Germanium, tin and lead oxides All of these oxides are amphoteric - they show both basic and acidic properties. The basic nature of the oxides: These oxides all react with acids to form salts. For example, Ge and Sn react with concentrated hydrochloric acid. This can be summarised as: XO + 2 HCl XCl 2 + H 2 O

Lead(II) chloride is fairly insoluble in water and, instead of getting a solution, it would form an insoluble layer over the lead(II) oxide if you were to use dilute hydrochloric acid - stopping the reaction from going on. Pb. O + 2 HCl Pb. Cl 2 + H 2 O The large excess of chloride ions in the concentrated acid react with the lead(II) chloride to produce soluble complexes such as Pb. Cl 4 2 -. These ionic complexes are soluble in water and so the problem disappears

The acidic nature of oxides All of these oxides also react with bases like sodium hydroxide solution. This time we can generalise without exception: XO + 2 OH- XO 22 - + H 2 O Where X is Ge, Sn or Pb
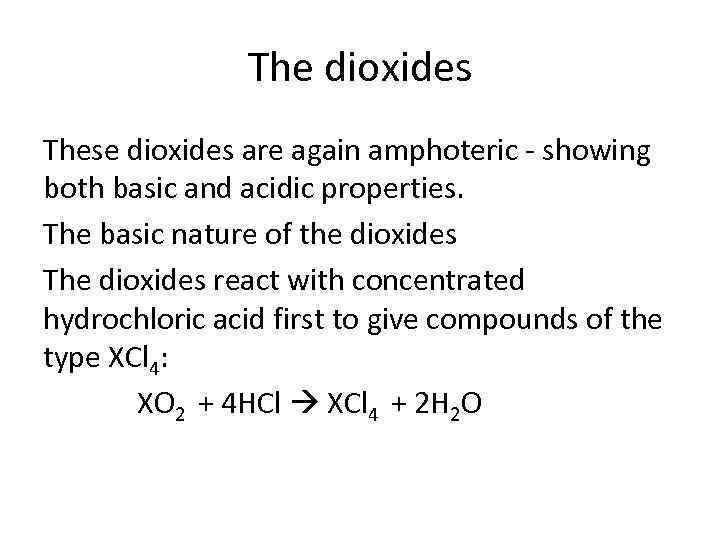
The dioxides These dioxides are again amphoteric - showing both basic and acidic properties. The basic nature of the dioxides The dioxides react with concentrated hydrochloric acid first to give compounds of the type XCl 4: XO 2 + 4 HCl XCl 4 + 2 H 2 O

These will react with excess chloride ions in the hydrochloric acid to give complexes such as XCl 62 -. XCl 4 + 2 Cl- XCl 62 In the case of lead(IV) oxide, the reaction has to be done with ice-cold hydrochloric acid. If the reaction is done any warmer, the lead(IV) chloride decomposes to give lead(II) chloride and chlorine gas. This is an effect of the preferred oxidation state of lead being +2 rather than +4.

The acidic nature of the dioxides The dioxides will react with hot concentrated sodium hydroxide solution to give soluble complexes of the form [X(OH)6]2 -. Complexes are formed when lone pairs from groups bonding with metals form bonds with the metal.
Group 14.pptx