dcfa999d17bd7d9119a02276bb42dec4.ppt
- Количество слайдов: 22
 Green Chemistry Across the Curriculum At St. Olaf College Bob Hanson BCCE 20, Indiana University, July 28, 2008 A Project Supported By the W. M. Keck Foundation 1
Green Chemistry Across the Curriculum At St. Olaf College Bob Hanson BCCE 20, Indiana University, July 28, 2008 A Project Supported By the W. M. Keck Foundation 1
 Goals of Green Chemistry at St. Olaf • Alter the chemistry curriculum ü 1 st year, 2 nd year, 3 rd year • Design a science facility that reflects this effort ü LEED Gold Building www. stolaf. edu/sciencecomplex/ 2
Goals of Green Chemistry at St. Olaf • Alter the chemistry curriculum ü 1 st year, 2 nd year, 3 rd year • Design a science facility that reflects this effort ü LEED Gold Building www. stolaf. edu/sciencecomplex/ 2
 Web App: Green Chemistry Assistant http: //fusion. stolaf. edu/gca A collaborative project between St. Olaf College and US EPA - an extension of the EPA Green Chemistry Expert System SMART module 3
Web App: Green Chemistry Assistant http: //fusion. stolaf. edu/gca A collaborative project between St. Olaf College and US EPA - an extension of the EPA Green Chemistry Expert System SMART module 3
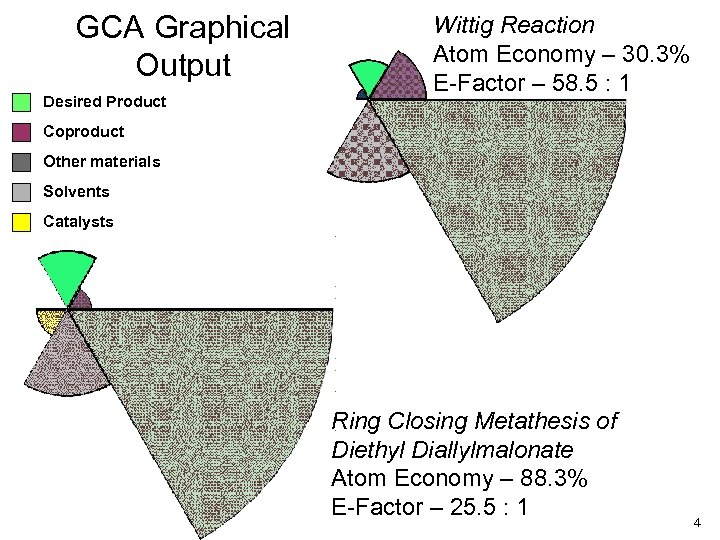 GCA Graphical Output Desired Product Wittig Reaction Atom Economy – 30. 3% E-Factor – 58. 5 : 1 Coproduct Other materials Solvents Catalysts Ring Closing Metathesis of Diethyl Diallylmalonate Atom Economy – 88. 3% E-Factor – 25. 5 : 1 4
GCA Graphical Output Desired Product Wittig Reaction Atom Economy – 30. 3% E-Factor – 58. 5 : 1 Coproduct Other materials Solvents Catalysts Ring Closing Metathesis of Diethyl Diallylmalonate Atom Economy – 88. 3% E-Factor – 25. 5 : 1 4
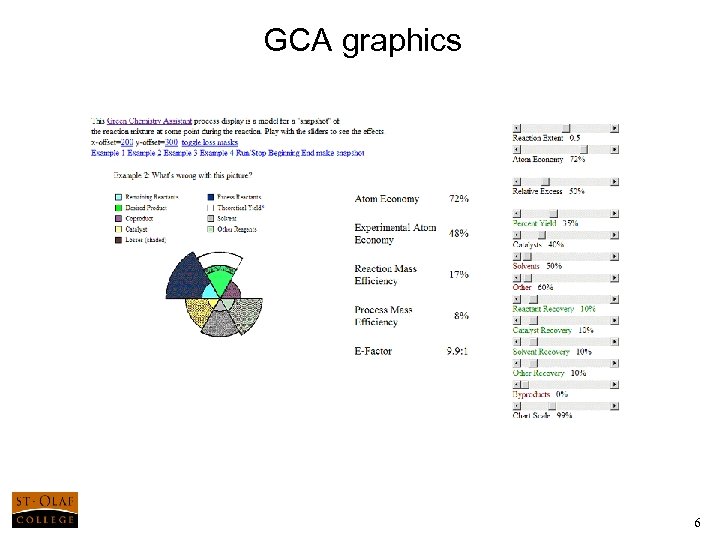
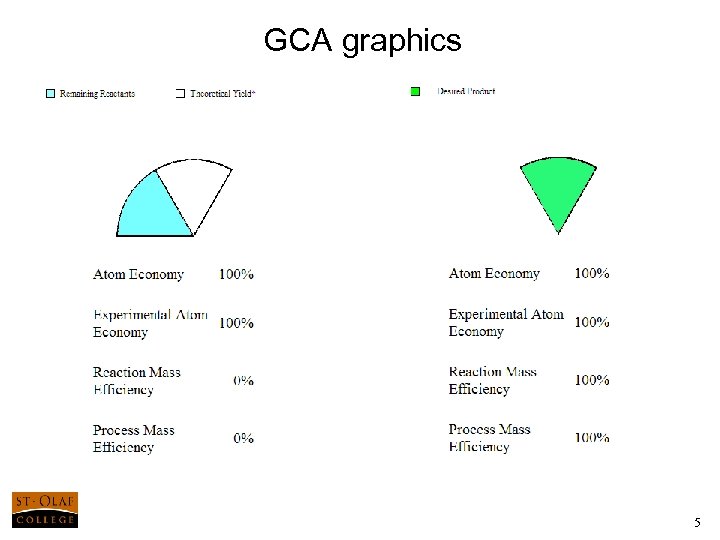 GCA graphics 5
GCA graphics 5
 GCA graphics 6
GCA graphics 6
 1 st Year: Periodic Trends & Solubility • Lab Manual includes “Green Connections” and new pre-/post-lab questions • Replace heptane with ethyl acetate Cl 2 ü Volatile but with lesser hazards ü Option as a renewable resources ü Potentially less harmful degradation products Br 2 • Observed color differences ü Chlorine: colorless, Bromine: orange, and Iodine: yellow • Replace chromate anion with thiosulfate anion and eliminate barium cation I 2 Heptane Et. OAc 7
1 st Year: Periodic Trends & Solubility • Lab Manual includes “Green Connections” and new pre-/post-lab questions • Replace heptane with ethyl acetate Cl 2 ü Volatile but with lesser hazards ü Option as a renewable resources ü Potentially less harmful degradation products Br 2 • Observed color differences ü Chlorine: colorless, Bromine: orange, and Iodine: yellow • Replace chromate anion with thiosulfate anion and eliminate barium cation I 2 Heptane Et. OAc 7
 1 st Year Experimental Changes Ethanol oxidation: kinetics study • Eliminate the chromate oxidation process. • Uses household bleach (6% sodium hypochlorite solution) • Eliminates concentrated hydrochloric acid Mystery Product Reactions • Replace permanganate with iodine redox system • Eliminates phosphoric and hydrochloric acids • 70% waste reduction (30 L annually) 7 experiments revised & changes implemented 8
1 st Year Experimental Changes Ethanol oxidation: kinetics study • Eliminate the chromate oxidation process. • Uses household bleach (6% sodium hypochlorite solution) • Eliminates concentrated hydrochloric acid Mystery Product Reactions • Replace permanganate with iodine redox system • Eliminates phosphoric and hydrochloric acids • 70% waste reduction (30 L annually) 7 experiments revised & changes implemented 8
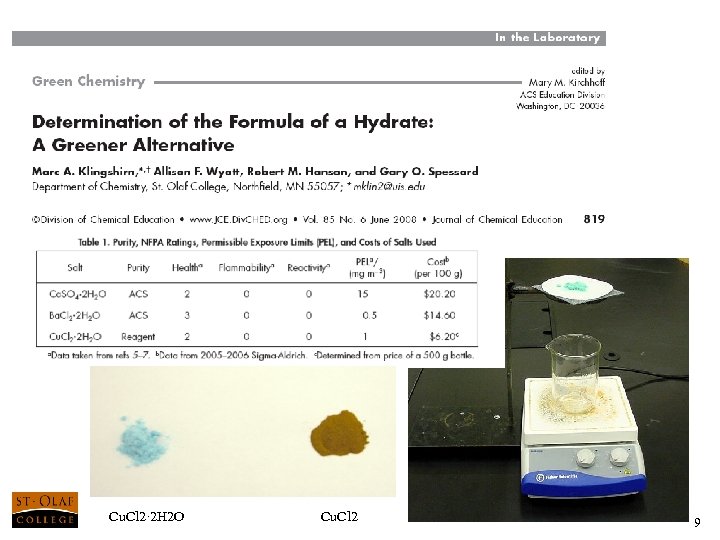 Cu. Cl 2· 2 H 2 O Cu. Cl 2 9
Cu. Cl 2· 2 H 2 O Cu. Cl 2 9
 1 st Year Waste Management Introduction Efforts: Results (unquantified): • Students typically work in groups of two or three. • Students are far more aware of waste issues. • Each group is required to • Students like taking some appoint one student who will responsibility in this regard. take responsible for accounting for group waste and filling out • Faculty become more aware of “waste manifests” for their group. waste issues as well. • Safety discussions and awareness arise spontaneously. 10
1 st Year Waste Management Introduction Efforts: Results (unquantified): • Students typically work in groups of two or three. • Students are far more aware of waste issues. • Each group is required to • Students like taking some appoint one student who will responsibility in this regard. take responsible for accounting for group waste and filling out • Faculty become more aware of “waste manifests” for their group. waste issues as well. • Safety discussions and awareness arise spontaneously. 10
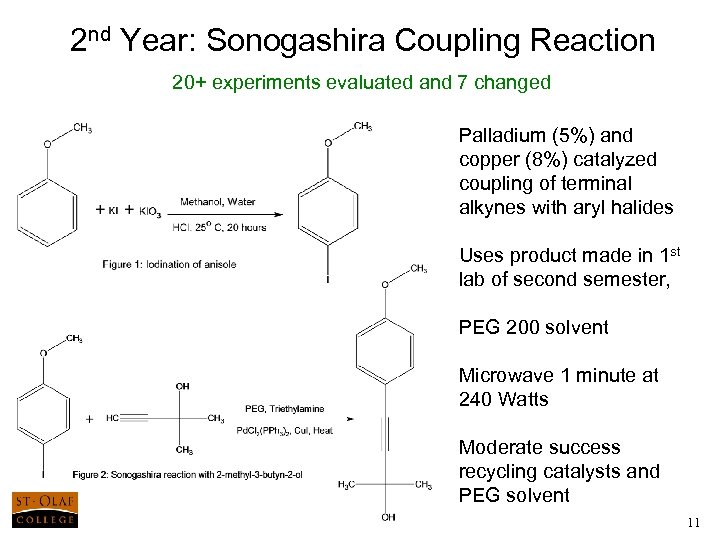 2 nd Year: Sonogashira Coupling Reaction 20+ experiments evaluated and 7 changed Palladium (5%) and copper (8%) catalyzed coupling of terminal alkynes with aryl halides Uses product made in 1 st lab of second semester, PEG 200 solvent Microwave 1 minute at 240 Watts Moderate success recycling catalysts and PEG solvent 11
2 nd Year: Sonogashira Coupling Reaction 20+ experiments evaluated and 7 changed Palladium (5%) and copper (8%) catalyzed coupling of terminal alkynes with aryl halides Uses product made in 1 st lab of second semester, PEG 200 solvent Microwave 1 minute at 240 Watts Moderate success recycling catalysts and PEG solvent 11
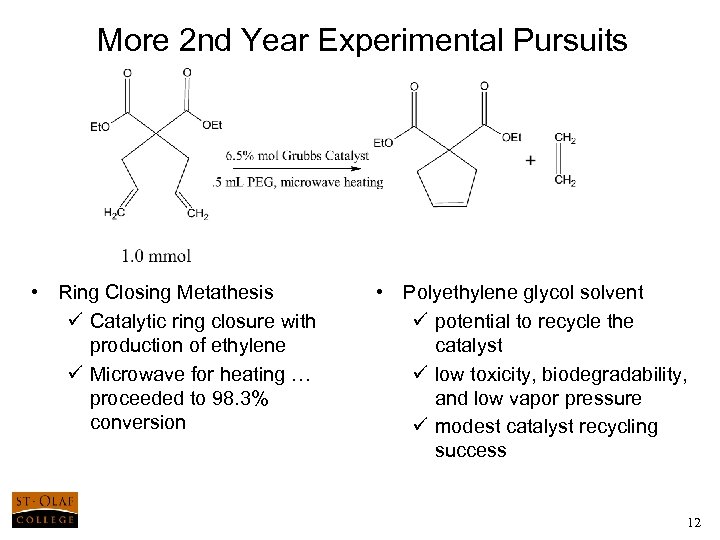 More 2 nd Year Experimental Pursuits • Ring Closing Metathesis ü Catalytic ring closure with production of ethylene ü Microwave for heating … proceeded to 98. 3% conversion • Polyethylene glycol solvent ü potential to recycle the catalyst ü low toxicity, biodegradability, and low vapor pressure ü modest catalyst recycling success 12
More 2 nd Year Experimental Pursuits • Ring Closing Metathesis ü Catalytic ring closure with production of ethylene ü Microwave for heating … proceeded to 98. 3% conversion • Polyethylene glycol solvent ü potential to recycle the catalyst ü low toxicity, biodegradability, and low vapor pressure ü modest catalyst recycling success 12
 3 rd Year: Goals & Objectives • Infuse Analytical & Physical labs with green chemistry principles • Develop appropriate metrics • Test metrics and apply to current lab experiments (benchmarking) • Determine labs with the least green characteristics ü NFPA 3 ü High material/solvent use ü High energy use ü Nonrenewable feedstocks ü Stoichiometric reactions • Reduce waste stream • Develop new or modified experiments ü Change chemistry or chemical system ü Reducing material/solvent use in currents labs ü Make volumetric reductions ü Analysis of citrus fruit essential oils by GC, GC/MS, Raman and/or IR-ATR. 13
3 rd Year: Goals & Objectives • Infuse Analytical & Physical labs with green chemistry principles • Develop appropriate metrics • Test metrics and apply to current lab experiments (benchmarking) • Determine labs with the least green characteristics ü NFPA 3 ü High material/solvent use ü High energy use ü Nonrenewable feedstocks ü Stoichiometric reactions • Reduce waste stream • Develop new or modified experiments ü Change chemistry or chemical system ü Reducing material/solvent use in currents labs ü Make volumetric reductions ü Analysis of citrus fruit essential oils by GC, GC/MS, Raman and/or IR-ATR. 13
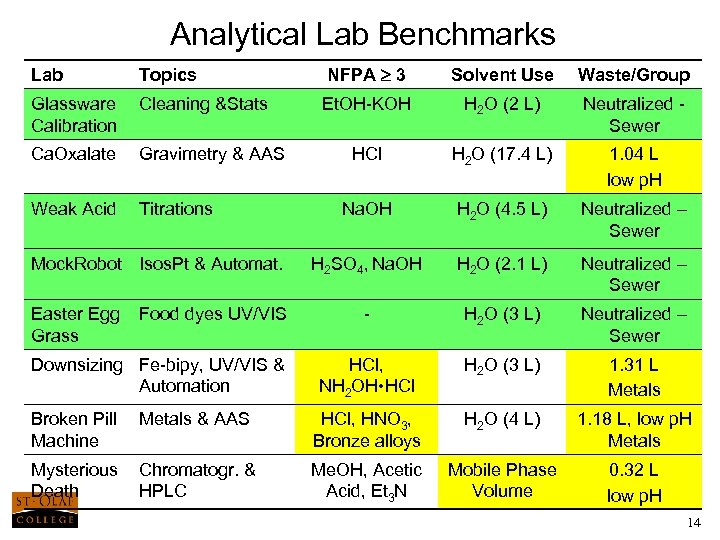 Analytical Lab Benchmarks Lab Topics NFPA 3 Solvent Use Waste/Group Glassware Calibration Cleaning &Stats Et. OH-KOH H 2 O (2 L) Neutralized - Sewer Ca. Oxalate Gravimetry & AAS HCl H 2 O (17. 4 L) 1. 04 L low p. H Weak Acid Titrations Na. OH H 2 O (4. 5 L) Neutralized – Sewer Mock. Robot Isos. Pt & Automat. H 2 SO 4, Na. OH H 2 O (2. 1 L) Neutralized – Sewer Easter Egg Food dyes UV/VIS Grass - H 2 O (3 L) Neutralized – Sewer Downsizing Fe-bipy, UV/VIS & Automation HCl, NH 2 OH • HCl H 2 O (3 L) 1. 31 L Metals Broken Pill Metals & AAS Machine HCl, HNO 3, Bronze alloys H 2 O (4 L) 1. 18 L, low p. H Metals Mysterious Chromatogr. & Death HPLC Me. OH, Acetic Acid, Et 3 N Mobile Phase Volume 0. 32 L low p. H 14
Analytical Lab Benchmarks Lab Topics NFPA 3 Solvent Use Waste/Group Glassware Calibration Cleaning &Stats Et. OH-KOH H 2 O (2 L) Neutralized - Sewer Ca. Oxalate Gravimetry & AAS HCl H 2 O (17. 4 L) 1. 04 L low p. H Weak Acid Titrations Na. OH H 2 O (4. 5 L) Neutralized – Sewer Mock. Robot Isos. Pt & Automat. H 2 SO 4, Na. OH H 2 O (2. 1 L) Neutralized – Sewer Easter Egg Food dyes UV/VIS Grass - H 2 O (3 L) Neutralized – Sewer Downsizing Fe-bipy, UV/VIS & Automation HCl, NH 2 OH • HCl H 2 O (3 L) 1. 31 L Metals Broken Pill Metals & AAS Machine HCl, HNO 3, Bronze alloys H 2 O (4 L) 1. 18 L, low p. H Metals Mysterious Chromatogr. & Death HPLC Me. OH, Acetic Acid, Et 3 N Mobile Phase Volume 0. 32 L low p. H 14
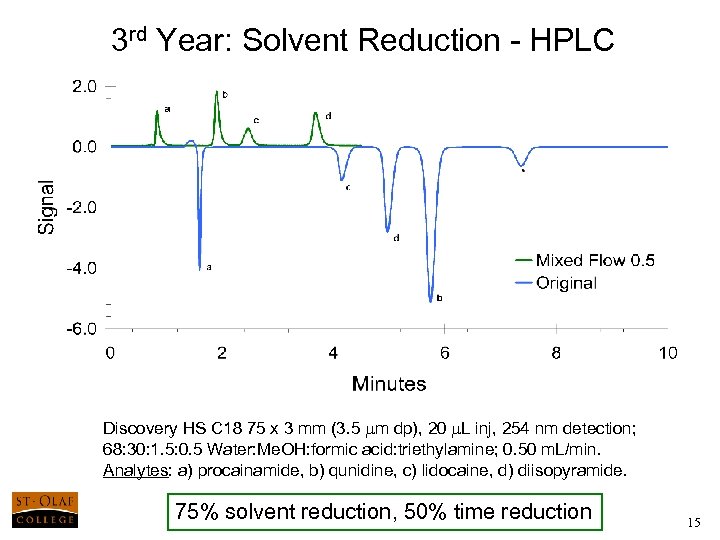 3 rd Year: Solvent Reduction - HPLC Discovery HS C 18 75 x 3 mm (3. 5 mm dp), 20 m. L inj, 254 nm detection; 68: 30: 1. 5: 0. 5 Water: Me. OH: formic acid: triethylamine; 0. 50 m. L/min. Analytes: a) procainamide, b) qunidine, c) lidocaine, d) diisopyramide. 75% solvent reduction, 50% time reduction 15
3 rd Year: Solvent Reduction - HPLC Discovery HS C 18 75 x 3 mm (3. 5 mm dp), 20 m. L inj, 254 nm detection; 68: 30: 1. 5: 0. 5 Water: Me. OH: formic acid: triethylamine; 0. 50 m. L/min. Analytes: a) procainamide, b) qunidine, c) lidocaine, d) diisopyramide. 75% solvent reduction, 50% time reduction 15
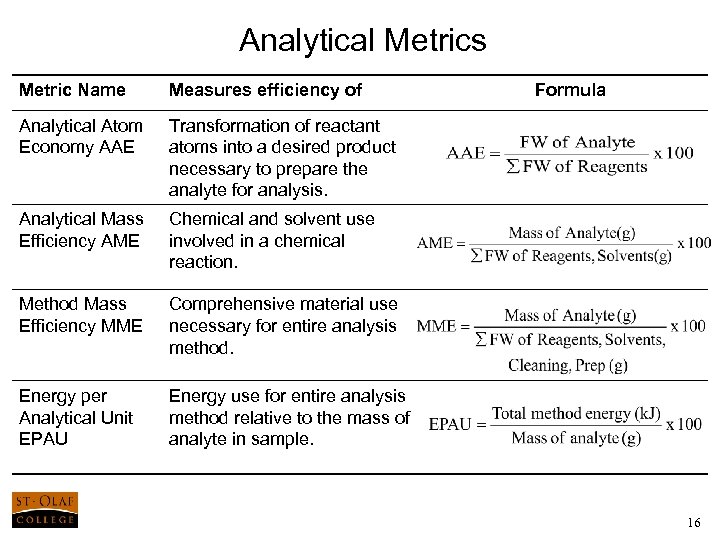 Analytical Metrics Metric Name Measures efficiency of Formula Analytical Atom Economy AAE Transformation of reactant atoms into a desired product necessary to prepare the analyte for analysis. Analytical Mass Efficiency AME Chemical and solvent use involved in a chemical reaction. Method Mass Efficiency MME Comprehensive material use necessary for entire analysis method. Energy per Analytical Unit EPAU Energy use for entire analysis method relative to the mass of analyte in sample. 16
Analytical Metrics Metric Name Measures efficiency of Formula Analytical Atom Economy AAE Transformation of reactant atoms into a desired product necessary to prepare the analyte for analysis. Analytical Mass Efficiency AME Chemical and solvent use involved in a chemical reaction. Method Mass Efficiency MME Comprehensive material use necessary for entire analysis method. Energy per Analytical Unit EPAU Energy use for entire analysis method relative to the mass of analyte in sample. 16
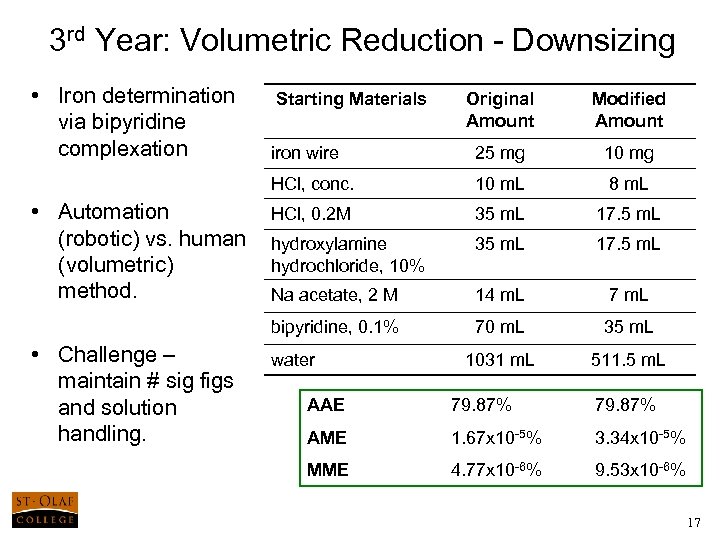 3 rd Year: Volumetric Reduction - Downsizing • Iron determination via bipyridine complexation Modified Amount iron wire 25 mg 10 m. L 8 m. L HCl, 0. 2 M 35 m. L 17. 5 m. L hydroxylamine hydrochloride, 10% 35 m. L 17. 5 m. L Na acetate, 2 M 14 m. L 7 m. L bipyridine, 0. 1% • Challenge – maintain # sig figs and solution handling. Original Amount HCl, conc. • Automation (robotic) vs. human (volumetric) method. Starting Materials 70 m. L 35 m. L 1031 m. L 511. 5 m. L water AAE 79. 87% AME 1. 67 x 10 -5% 3. 34 x 10 -5% MME 4. 77 x 10 -6% 9. 53 x 10 -6% 17
3 rd Year: Volumetric Reduction - Downsizing • Iron determination via bipyridine complexation Modified Amount iron wire 25 mg 10 m. L 8 m. L HCl, 0. 2 M 35 m. L 17. 5 m. L hydroxylamine hydrochloride, 10% 35 m. L 17. 5 m. L Na acetate, 2 M 14 m. L 7 m. L bipyridine, 0. 1% • Challenge – maintain # sig figs and solution handling. Original Amount HCl, conc. • Automation (robotic) vs. human (volumetric) method. Starting Materials 70 m. L 35 m. L 1031 m. L 511. 5 m. L water AAE 79. 87% AME 1. 67 x 10 -5% 3. 34 x 10 -5% MME 4. 77 x 10 -6% 9. 53 x 10 -6% 17
 3 rd Year: New Citrus Oil Analysis Why is lemon oil used for some consumer products and orange oil for others? How chemically similar are citrus oil extracts? How would you determine this when starting with a piece of fruit (grapefruit, lemon, lime, or orange) and doing as little sample preparation as possible? . SAMPLING STRATEGIES • Solid Phase Microextraction ü Peel / zest into vial ü PMDS-DVB fiber • Supercritical CO 2 extraction ü Peel / zest into centrifuge tube ü Dry ice & water bath 18
3 rd Year: New Citrus Oil Analysis Why is lemon oil used for some consumer products and orange oil for others? How chemically similar are citrus oil extracts? How would you determine this when starting with a piece of fruit (grapefruit, lemon, lime, or orange) and doing as little sample preparation as possible? . SAMPLING STRATEGIES • Solid Phase Microextraction ü Peel / zest into vial ü PMDS-DVB fiber • Supercritical CO 2 extraction ü Peel / zest into centrifuge tube ü Dry ice & water bath 18
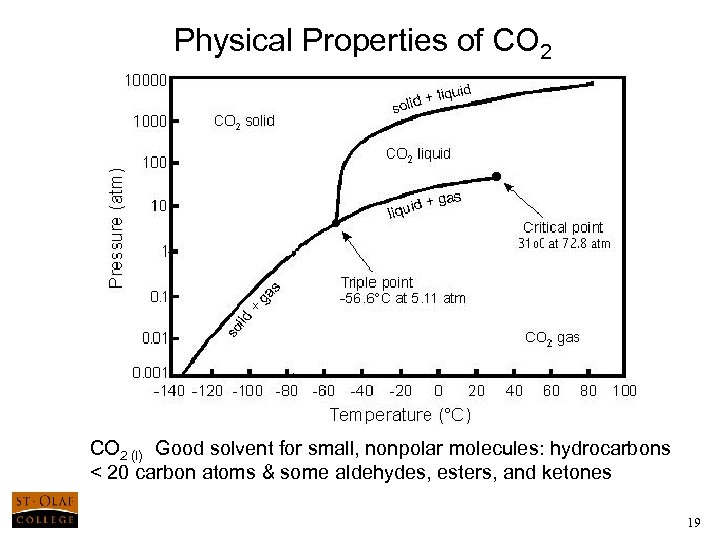 Physical Properties of CO 2 (l) Good solvent for small, nonpolar molecules: hydrocarbons < 20 carbon atoms & some aldehydes, esters, and ketones 19
Physical Properties of CO 2 (l) Good solvent for small, nonpolar molecules: hydrocarbons < 20 carbon atoms & some aldehydes, esters, and ketones 19
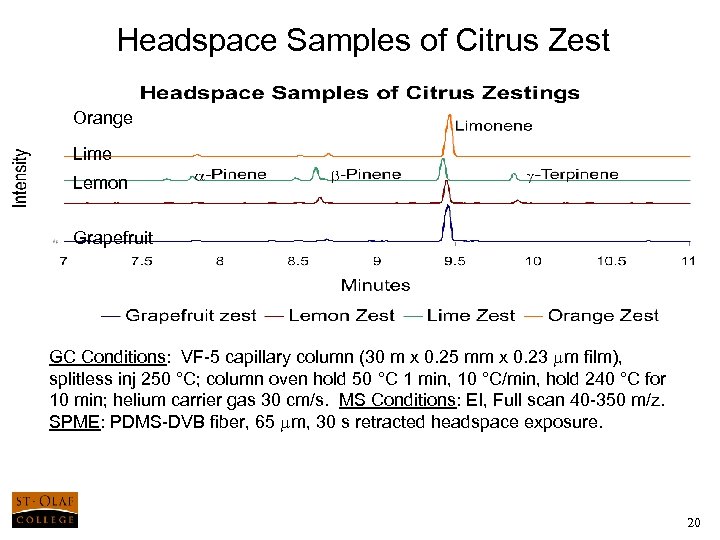 Headspace Samples of Citrus Zest Orange Lime Lemon Grapefruit GC Conditions: VF-5 capillary column (30 m x 0. 25 mm x 0. 23 mm film), splitless inj 250 °C; column oven hold 50 °C 1 min, 10 °C/min, hold 240 °C for 10 min; helium carrier gas 30 cm/s. MS Conditions: EI, Full scan 40 -350 m/z. SPME: PDMS-DVB fiber, 65 mm, 30 s retracted headspace exposure. 20
Headspace Samples of Citrus Zest Orange Lime Lemon Grapefruit GC Conditions: VF-5 capillary column (30 m x 0. 25 mm x 0. 23 mm film), splitless inj 250 °C; column oven hold 50 °C 1 min, 10 °C/min, hold 240 °C for 10 min; helium carrier gas 30 cm/s. MS Conditions: EI, Full scan 40 -350 m/z. SPME: PDMS-DVB fiber, 65 mm, 30 s retracted headspace exposure. 20
 New Science Facility Sept. 1, 2008 Opening • • Interdisciplinary Investigative Interactive Innovative Interconnected Inviting Integrity ü Green Team, Builder (Boldt), Architect (Holabird & Root) ü LEED Gold target ü Building as Teacher ü Life-cycle costs ü Chemical Fume Hood Reductions (energy, operations, first costs) • 120, 000 NASF, 26 teaching labs • Informal gathering spaces designed to extend learning beyond the classroom and laboratory. ü Green roof terrace ü Adjacent landscape ü Water management basins 65% decrease for intro/2 nd year chemistry (2. 5 linear ft/student std) 40% decrease across facility compared to initial design 21
New Science Facility Sept. 1, 2008 Opening • • Interdisciplinary Investigative Interactive Innovative Interconnected Inviting Integrity ü Green Team, Builder (Boldt), Architect (Holabird & Root) ü LEED Gold target ü Building as Teacher ü Life-cycle costs ü Chemical Fume Hood Reductions (energy, operations, first costs) • 120, 000 NASF, 26 teaching labs • Informal gathering spaces designed to extend learning beyond the classroom and laboratory. ü Green roof terrace ü Adjacent landscape ü Water management basins 65% decrease for intro/2 nd year chemistry (2. 5 linear ft/student std) 40% decrease across facility compared to initial design 21
 Future/On-going Work • Continue development and implementation in first two years of curriculum, particularly in the area of waste management and safety • Ramp up development and implementation in third year of curriculum ü Piloting upper level p-chem lab (aqueous SEC w/proteins & dextrans to calculate virial coefficients) • LEED-NC Innovation Credit – Green Chemistry & Hood Reduction • Hire another Post-Doc (Enquire here!) 22
Future/On-going Work • Continue development and implementation in first two years of curriculum, particularly in the area of waste management and safety • Ramp up development and implementation in third year of curriculum ü Piloting upper level p-chem lab (aqueous SEC w/proteins & dextrans to calculate virial coefficients) • LEED-NC Innovation Credit – Green Chemistry & Hood Reduction • Hire another Post-Doc (Enquire here!) 22


