997f275f1e3aedcb232aa914202e4643.ppt
- Количество слайдов: 30
 Grazie per aver scelto di utilizzare a scopo didattico questo materiale delle Guidelines 2011 libra. Le ricordiamo che questo materiale è di proprietà dell’autore e fornito come supporto didattico per uso personale.
Grazie per aver scelto di utilizzare a scopo didattico questo materiale delle Guidelines 2011 libra. Le ricordiamo che questo materiale è di proprietà dell’autore e fornito come supporto didattico per uso personale.
 PHARMACOLOGICAL MANAGEMENT OF COPD IN PATIENTS WITH CHRONIC CO-MORBIDITIES Professor Peter Calverley University Hospital Aintree Liverpool UK
PHARMACOLOGICAL MANAGEMENT OF COPD IN PATIENTS WITH CHRONIC CO-MORBIDITIES Professor Peter Calverley University Hospital Aintree Liverpool UK
 A RUMSFELD MOMENT! u Does having COPD influence the choice of therapy for a co-morbidity? u Does taking a treatment for a co-morbidity improve the outcome in COPD? u Does taking a treatment for COPD affect the co- morbidity?
A RUMSFELD MOMENT! u Does having COPD influence the choice of therapy for a co-morbidity? u Does taking a treatment for a co-morbidity improve the outcome in COPD? u Does taking a treatment for COPD affect the co- morbidity?
 BETA –BLOCKERS AND COPD u Good data for the benefits of selective beta- blockade in congestive heart failure, rate control of AF u Longstanding worry that beta-blockade might precipitate bronchospasm u So most people avoided beta-blockers in COPD u Now we have evidence for safety and a reason why this is the case
BETA –BLOCKERS AND COPD u Good data for the benefits of selective beta- blockade in congestive heart failure, rate control of AF u Longstanding worry that beta-blockade might precipitate bronchospasm u So most people avoided beta-blockers in COPD u Now we have evidence for safety and a reason why this is the case
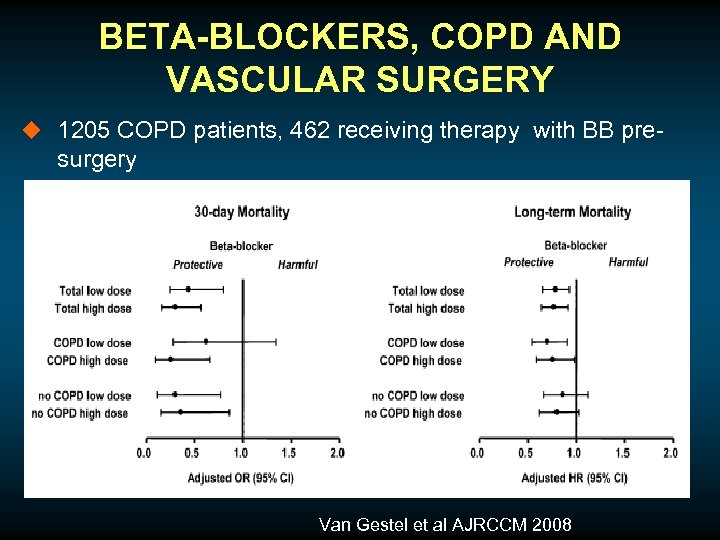 BETA-BLOCKERS, COPD AND VASCULAR SURGERY u 1205 COPD patients, 462 receiving therapy with BB pre- surgery Van Gestel et al AJRCCM 2008
BETA-BLOCKERS, COPD AND VASCULAR SURGERY u 1205 COPD patients, 462 receiving therapy with BB pre- surgery Van Gestel et al AJRCCM 2008
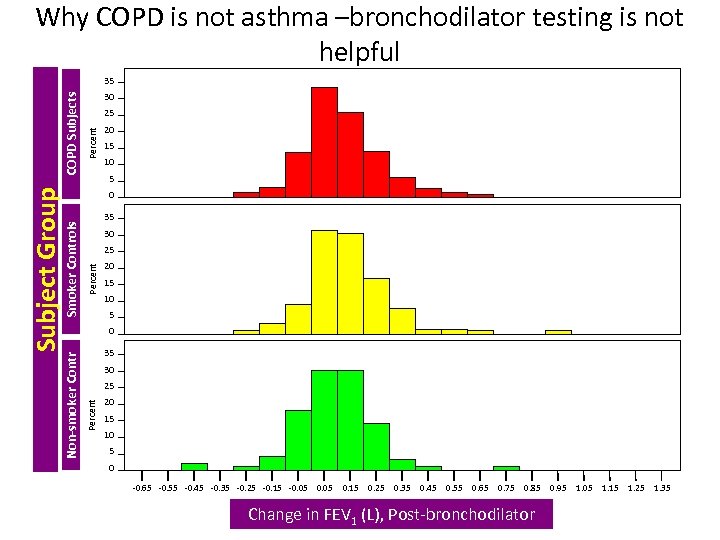 Why COPD is not asthma –bronchodilator testing is not helpful 30 Percent 25 20 15 10 5 0 35 30 25 Percent Non-smoker Contr Smoker Controls Subject Group COPD Subjects 35 20 15 10 5 0 -0. 65 -0. 55 -0. 45 -0. 35 -0. 25 -0. 15 -0. 05 0. 15 0. 25 0. 35 0. 45 0. 55 0. 65 0. 75 0. 85 Change in FEV 1 (L), Post-bronchodilator 0. 95 1. 05 1. 15 1. 25 1. 35
Why COPD is not asthma –bronchodilator testing is not helpful 30 Percent 25 20 15 10 5 0 35 30 25 Percent Non-smoker Contr Smoker Controls Subject Group COPD Subjects 35 20 15 10 5 0 -0. 65 -0. 55 -0. 45 -0. 35 -0. 25 -0. 15 -0. 05 0. 15 0. 25 0. 35 0. 45 0. 55 0. 65 0. 75 0. 85 Change in FEV 1 (L), Post-bronchodilator 0. 95 1. 05 1. 15 1. 25 1. 35
 THE STATIN STORY
THE STATIN STORY
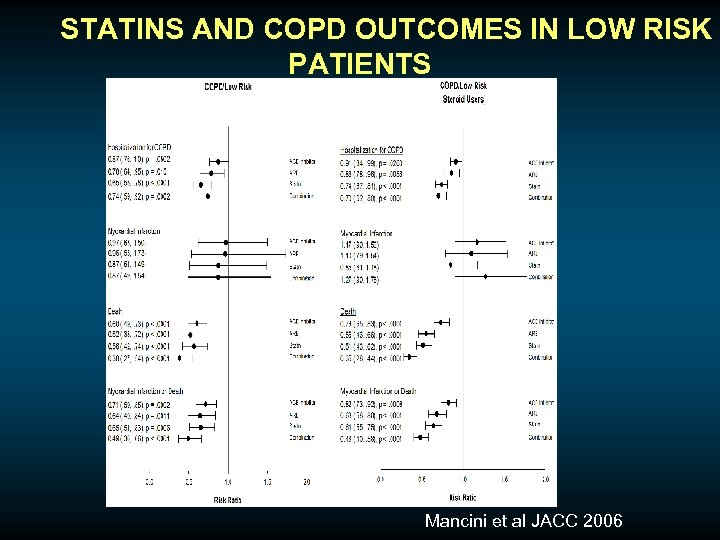 STATINS AND COPD OUTCOMES IN LOW RISK PATIENTS Mancini et al JACC 2006
STATINS AND COPD OUTCOMES IN LOW RISK PATIENTS Mancini et al JACC 2006
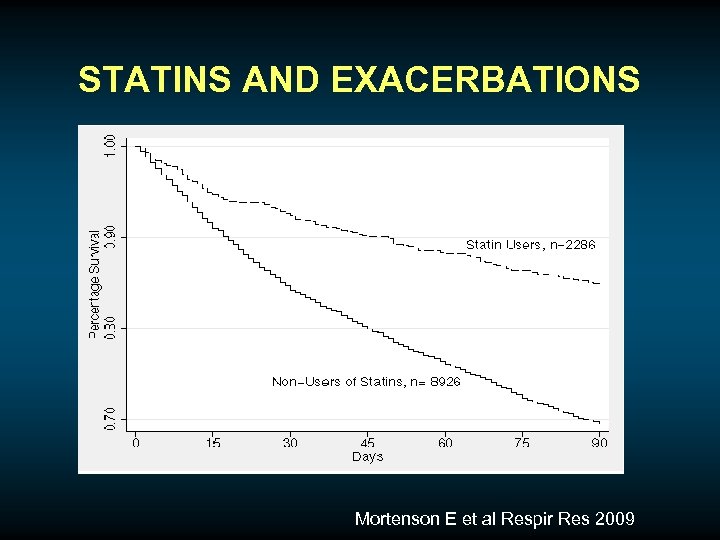 STATINS AND EXACERBATIONS Mortenson E et al Respir Res 2009
STATINS AND EXACERBATIONS Mortenson E et al Respir Res 2009
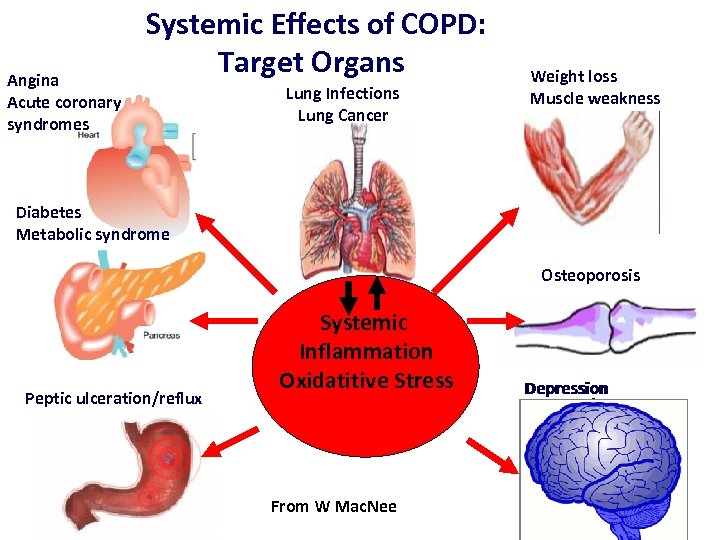 Angina Acute coronary syndromes Systemic Effects of COPD: Target Organs Lung Infections Lung Cancer Weight loss Muscle weakness Diabetes Metabolic syndrome Osteoporosis Peptic ulceration/reflux Systemic Inflammation Oxidatitive Stress From W Mac. Nee Depression
Angina Acute coronary syndromes Systemic Effects of COPD: Target Organs Lung Infections Lung Cancer Weight loss Muscle weakness Diabetes Metabolic syndrome Osteoporosis Peptic ulceration/reflux Systemic Inflammation Oxidatitive Stress From W Mac. Nee Depression
 TREATMENT AND COMPLICATIONS u Depression –common, often associated with fatigue. Interaction with therapy more likely with systemic treatment. Corticosteroids possibly – roflumilast unproven u Reflux – GI issues with theophyllines and PDEIV inhibitors u Metabolism and diabetes –ocs associated with hyperglycaemia but this is a feature of acute exacerbations. More data from roflumilast u Muscles
TREATMENT AND COMPLICATIONS u Depression –common, often associated with fatigue. Interaction with therapy more likely with systemic treatment. Corticosteroids possibly – roflumilast unproven u Reflux – GI issues with theophyllines and PDEIV inhibitors u Metabolism and diabetes –ocs associated with hyperglycaemia but this is a feature of acute exacerbations. More data from roflumilast u Muscles
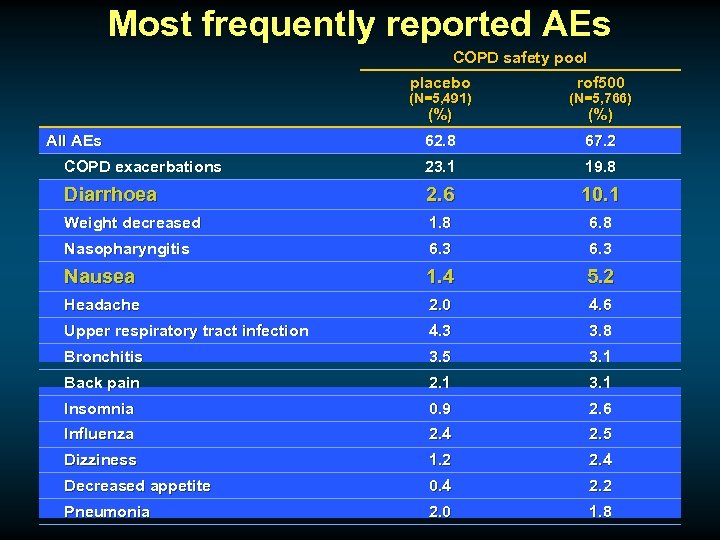 Most frequently reported AEs COPD safety pool placebo rof 500 (N=5, 491) (N=5, 766) 62. 8 67. 2 COPD exacerbations 23. 1 19. 8 Diarrhoea 2. 6 10. 1 Weight decreased 1. 8 6. 8 Nasopharyngitis 6. 3 Nausea 1. 4 5. 2 Headache 2. 0 4. 6 Upper respiratory tract infection 4. 3 3. 8 Bronchitis 3. 5 3. 1 Back pain 2. 1 3. 1 Insomnia 0. 9 2. 6 Influenza 2. 4 2. 5 Dizziness 1. 2 2. 4 Decreased appetite 0. 4 2. 2 Pneumonia 2. 0 1. 8 (%) All AEs (%)
Most frequently reported AEs COPD safety pool placebo rof 500 (N=5, 491) (N=5, 766) 62. 8 67. 2 COPD exacerbations 23. 1 19. 8 Diarrhoea 2. 6 10. 1 Weight decreased 1. 8 6. 8 Nasopharyngitis 6. 3 Nausea 1. 4 5. 2 Headache 2. 0 4. 6 Upper respiratory tract infection 4. 3 3. 8 Bronchitis 3. 5 3. 1 Back pain 2. 1 3. 1 Insomnia 0. 9 2. 6 Influenza 2. 4 2. 5 Dizziness 1. 2 2. 4 Decreased appetite 0. 4 2. 2 Pneumonia 2. 0 1. 8 (%) All AEs (%)
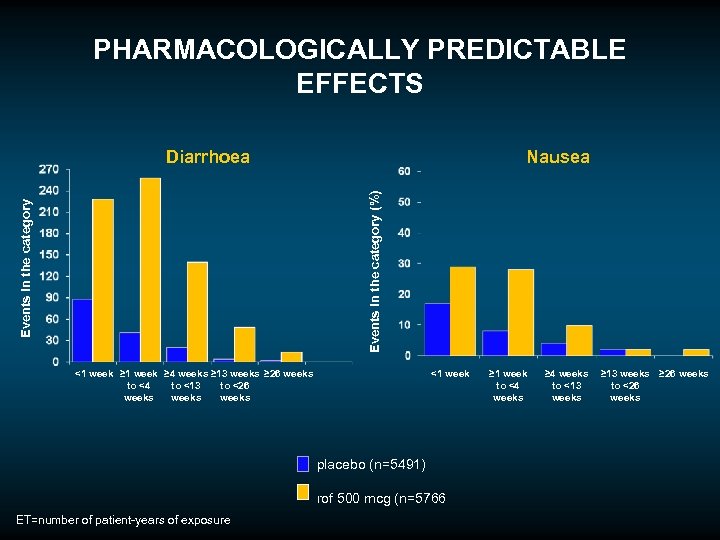 PHARMACOLOGICALLY PREDICTABLE EFFECTS Nausea Events in the category (%) Diarrhoea <1 week ≥ 4 weeks ≥ 13 weeks ≥ 26 weeks to <4 to <13 to <26 weeks <1 week placebo (n=5491) rof 500 mcg (n=5766 ET=number of patient-years of exposure ≥ 1 week to <4 weeks ≥ 4 weeks to <13 weeks ≥ 26 weeks to <26 weeks
PHARMACOLOGICALLY PREDICTABLE EFFECTS Nausea Events in the category (%) Diarrhoea <1 week ≥ 4 weeks ≥ 13 weeks ≥ 26 weeks to <4 to <13 to <26 weeks <1 week placebo (n=5491) rof 500 mcg (n=5766 ET=number of patient-years of exposure ≥ 1 week to <4 weeks ≥ 4 weeks to <13 weeks ≥ 26 weeks to <26 weeks
 Weight loss u Noted as a self-reported finding more often with roflumilast u Not just confined to patients reporting GI intolerance u Monitored with regular weight measurement in pivotal one year trials u In one 6 month study bioimpedance data were available
Weight loss u Noted as a self-reported finding more often with roflumilast u Not just confined to patients reporting GI intolerance u Monitored with regular weight measurement in pivotal one year trials u In one 6 month study bioimpedance data were available
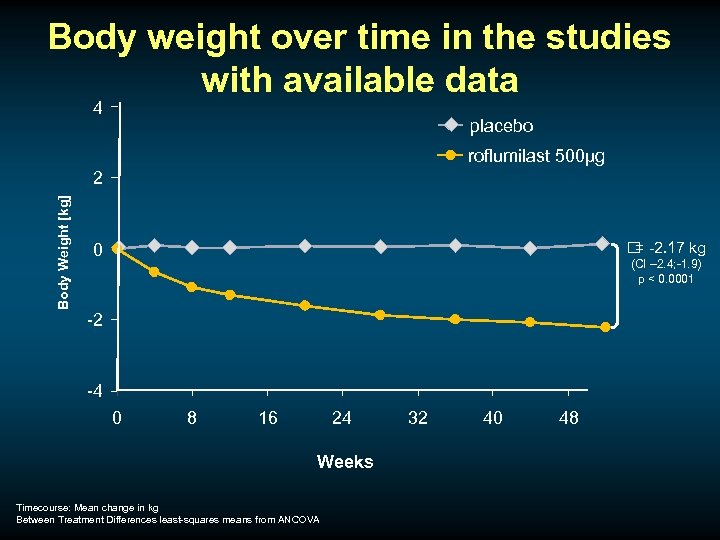 Body weight over time in the studies with available data 4 placebo roflumilast 500µg Body Weight [kg] 2 -2. 17 kg = 0 (CI – 2. 4; -1. 9) p < 0. 0001 -2 -4 0 8 16 24 Weeks Timecourse: Mean change in kg Between Treatment Differences least-squares means from ANCOVA 32 40 48
Body weight over time in the studies with available data 4 placebo roflumilast 500µg Body Weight [kg] 2 -2. 17 kg = 0 (CI – 2. 4; -1. 9) p < 0. 0001 -2 -4 0 8 16 24 Weeks Timecourse: Mean change in kg Between Treatment Differences least-squares means from ANCOVA 32 40 48
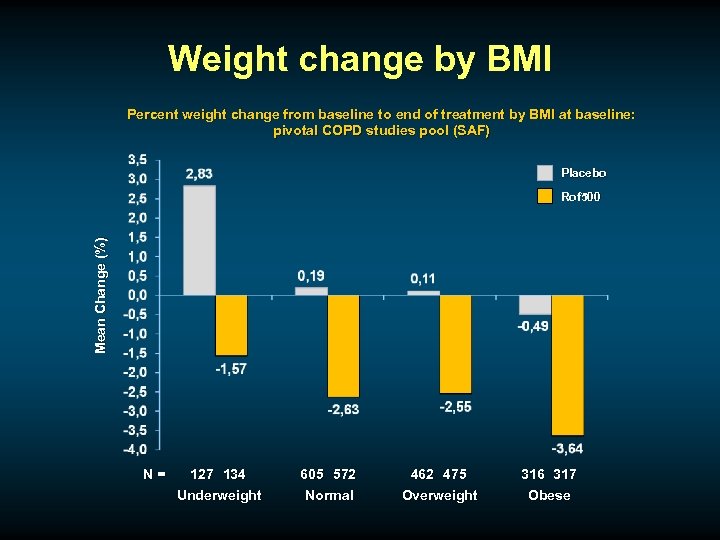 Weight change by BMI Percent weight change from baseline to end of treatment by BMI at baseline: pivotal COPD studies pool (SAF) Placebo Mean Change (%) Rof 500 N= 127 134 Underweight 605 572 Normal 462 475 Overweight 316 317 Obese
Weight change by BMI Percent weight change from baseline to end of treatment by BMI at baseline: pivotal COPD studies pool (SAF) Placebo Mean Change (%) Rof 500 N= 127 134 Underweight 605 572 Normal 462 475 Overweight 316 317 Obese
![Weight loss associated with roflumilast was primarily fat mass Mass indices [kg/m 2] Tiotropium Weight loss associated with roflumilast was primarily fat mass Mass indices [kg/m 2] Tiotropium](https://present5.com/presentation/997f275f1e3aedcb232aa914202e4643/image-17.jpg) Weight loss associated with roflumilast was primarily fat mass Mass indices [kg/m 2] Tiotropium + placebo (BMI) 0 Tiotropium + placebo (FFMI) Tiotropium + Daxas® (FFMI) -0. 5 Tiotropium + Daxas® (BMI) -1 0 4 8 12 16 20 24 Weeks Wouters EFM, Teichmann P, Brose M, et al. Am J Respir Crit Care Med 2010; 181: A 4473. FFMI: Fat Free Mass Index; BMI: Body Mass Index
Weight loss associated with roflumilast was primarily fat mass Mass indices [kg/m 2] Tiotropium + placebo (BMI) 0 Tiotropium + placebo (FFMI) Tiotropium + Daxas® (FFMI) -0. 5 Tiotropium + Daxas® (BMI) -1 0 4 8 12 16 20 24 Weeks Wouters EFM, Teichmann P, Brose M, et al. Am J Respir Crit Care Med 2010; 181: A 4473. FFMI: Fat Free Mass Index; BMI: Body Mass Index
 MUSCLES u Loss of muscle bulk vs weakness u A marker for more health care expense and mortality but the thresholds may vary u A clear relationship of weakness to ocs use long term –not seen with ics u Anabolic steroids reverse this process but only in people taking oral corticosteroids (Kreutzberg E et al)
MUSCLES u Loss of muscle bulk vs weakness u A marker for more health care expense and mortality but the thresholds may vary u A clear relationship of weakness to ocs use long term –not seen with ics u Anabolic steroids reverse this process but only in people taking oral corticosteroids (Kreutzberg E et al)
 BONES AND INHALED CORTICSTEROIDS u Database associations but confounded by disease severity
BONES AND INHALED CORTICSTEROIDS u Database associations but confounded by disease severity
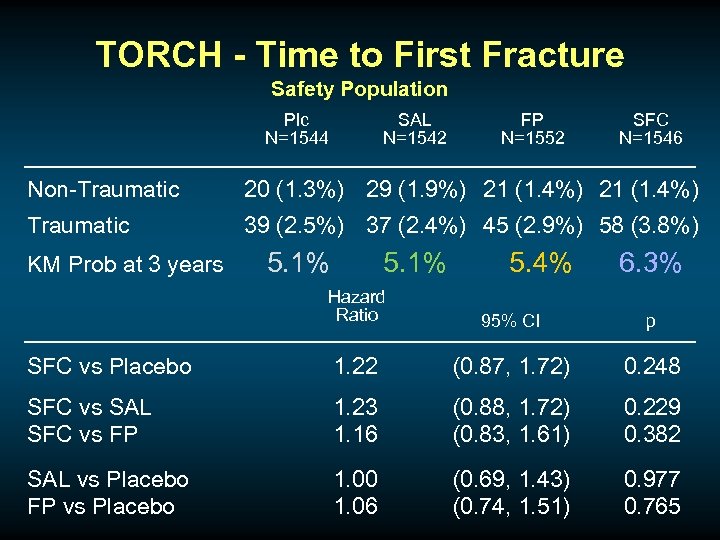 TORCH - Time to First Fracture Safety Population Plc N=1544 SAL N=1542 FP N=1552 SFC N=1546 Non-Traumatic 20 (1. 3%) 29 (1. 9%) 21 (1. 4%) Traumatic 39 (2. 5%) 37 (2. 4%) 45 (2. 9%) 58 (3. 8%) KM Prob at 3 years 5. 1% 5. 4% 6. 3% Hazard Ratio 95% CI p SFC vs Placebo 1. 22 (0. 87, 1. 72) 0. 248 SFC vs SAL SFC vs FP 1. 23 1. 16 (0. 88, 1. 72) (0. 83, 1. 61) 0. 229 0. 382 SAL vs Placebo FP vs Placebo 1. 00 1. 06 (0. 69, 1. 43) (0. 74, 1. 51) 0. 977 0. 765
TORCH - Time to First Fracture Safety Population Plc N=1544 SAL N=1542 FP N=1552 SFC N=1546 Non-Traumatic 20 (1. 3%) 29 (1. 9%) 21 (1. 4%) Traumatic 39 (2. 5%) 37 (2. 4%) 45 (2. 9%) 58 (3. 8%) KM Prob at 3 years 5. 1% 5. 4% 6. 3% Hazard Ratio 95% CI p SFC vs Placebo 1. 22 (0. 87, 1. 72) 0. 248 SFC vs SAL SFC vs FP 1. 23 1. 16 (0. 88, 1. 72) (0. 83, 1. 61) 0. 229 0. 382 SAL vs Placebo FP vs Placebo 1. 00 1. 06 (0. 69, 1. 43) (0. 74, 1. 51) 0. 977 0. 765
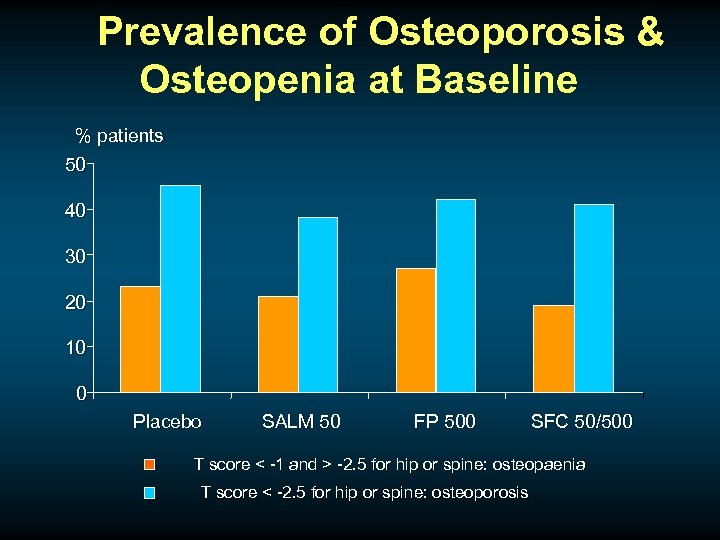 Prevalence of Osteoporosis & Osteopenia at Baseline % patients 50 40 30 20 10 0 Placebo SALM 50 FP 500 SFC 50/500 T score < -1 and > -2. 5 for hip or spine: osteopaenia T score < -2. 5 for hip or spine: osteoporosis
Prevalence of Osteoporosis & Osteopenia at Baseline % patients 50 40 30 20 10 0 Placebo SALM 50 FP 500 SFC 50/500 T score < -1 and > -2. 5 for hip or spine: osteopaenia T score < -2. 5 for hip or spine: osteoporosis
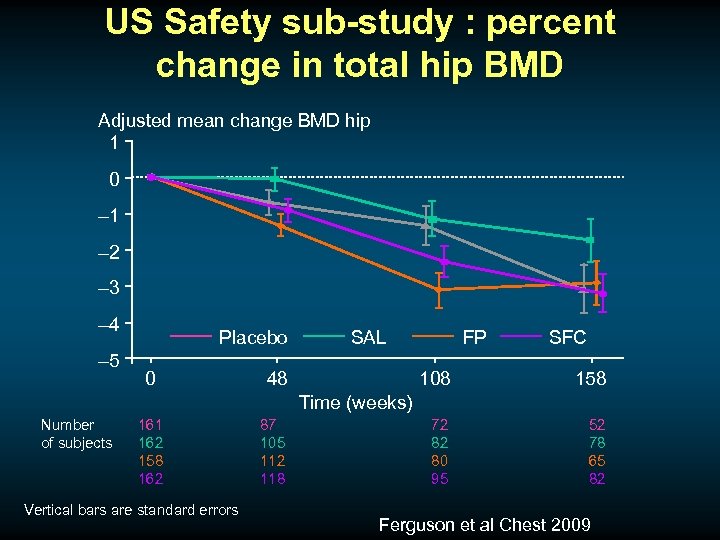 US Safety sub-study : percent change in total hip BMD Adjusted mean change BMD hip 1 0 – 1 – 2 – 3 – 4 – 5 Placebo 0 SAL 48 FP SFC 108 158 72 82 80 95 52 78 65 82 Time (weeks) Number of subjects 161 162 158 162 Vertical bars are standard errors 87 105 112 118 Ferguson et al Chest 2009
US Safety sub-study : percent change in total hip BMD Adjusted mean change BMD hip 1 0 – 1 – 2 – 3 – 4 – 5 Placebo 0 SAL 48 FP SFC 108 158 72 82 80 95 52 78 65 82 Time (weeks) Number of subjects 161 162 158 162 Vertical bars are standard errors 87 105 112 118 Ferguson et al Chest 2009
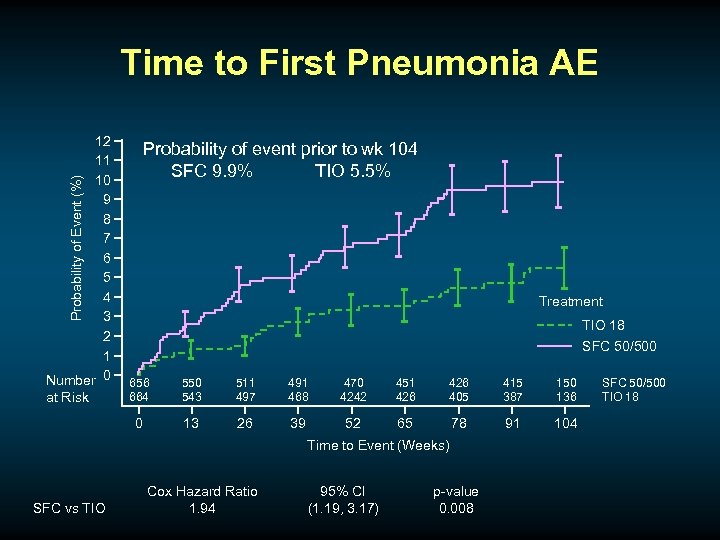 Time to First Pneumonia AE Probability of Event (%) 12 11 10 9 8 7 6 5 4 3 2 1 Number 0 Probability of event prior to wk 104 SFC 9. 9% TIO 5. 5% Treatment TIO 18 SFC 50/500 550 543 511 497 491 468 470 4242 451 426 405 415 387 150 136 0 at Risk 656 664 13 26 39 52 65 78 91 104 Time to Event (Weeks) SFC vs TIO Cox Hazard Ratio 1. 94 95% CI (1. 19, 3. 17) p-value 0. 008 SFC 50/500 TIO 18
Time to First Pneumonia AE Probability of Event (%) 12 11 10 9 8 7 6 5 4 3 2 1 Number 0 Probability of event prior to wk 104 SFC 9. 9% TIO 5. 5% Treatment TIO 18 SFC 50/500 550 543 511 497 491 468 470 4242 451 426 405 415 387 150 136 0 at Risk 656 664 13 26 39 52 65 78 91 104 Time to Event (Weeks) SFC vs TIO Cox Hazard Ratio 1. 94 95% CI (1. 19, 3. 17) p-value 0. 008 SFC 50/500 TIO 18
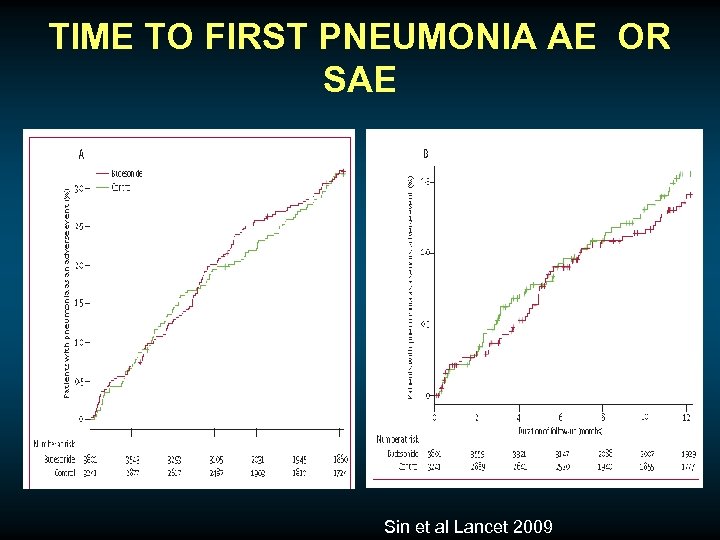 TIME TO FIRST PNEUMONIA AE OR SAE Sin et al Lancet 2009
TIME TO FIRST PNEUMONIA AE OR SAE Sin et al Lancet 2009
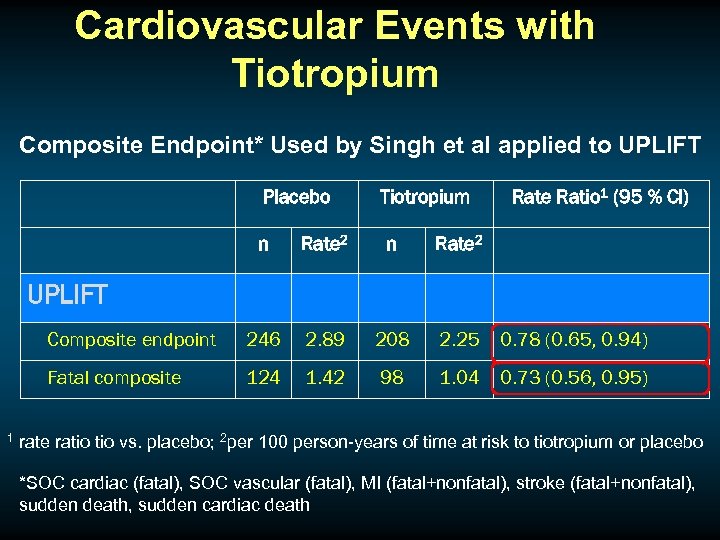 Cardiovascular Events with Tiotropium Composite Endpoint* Used by Singh et al applied to UPLIFT Placebo Tiotropium Rate Ratio 1 (95 % CI) n Rate 2 Composite endpoint 246 2. 89 208 2. 25 0. 78 (0. 65, 0. 94) Fatal composite 124 1. 42 98 1. 04 0. 73 (0. 56, 0. 95) UPLIFT 1 rate ratio vs. placebo; 2 per 100 person-years of time at risk to tiotropium or placebo *SOC cardiac (fatal), SOC vascular (fatal), MI (fatal+nonfatal), stroke (fatal+nonfatal), sudden death, sudden cardiac death
Cardiovascular Events with Tiotropium Composite Endpoint* Used by Singh et al applied to UPLIFT Placebo Tiotropium Rate Ratio 1 (95 % CI) n Rate 2 Composite endpoint 246 2. 89 208 2. 25 0. 78 (0. 65, 0. 94) Fatal composite 124 1. 42 98 1. 04 0. 73 (0. 56, 0. 95) UPLIFT 1 rate ratio vs. placebo; 2 per 100 person-years of time at risk to tiotropium or placebo *SOC cardiac (fatal), SOC vascular (fatal), MI (fatal+nonfatal), stroke (fatal+nonfatal), sudden death, sudden cardiac death
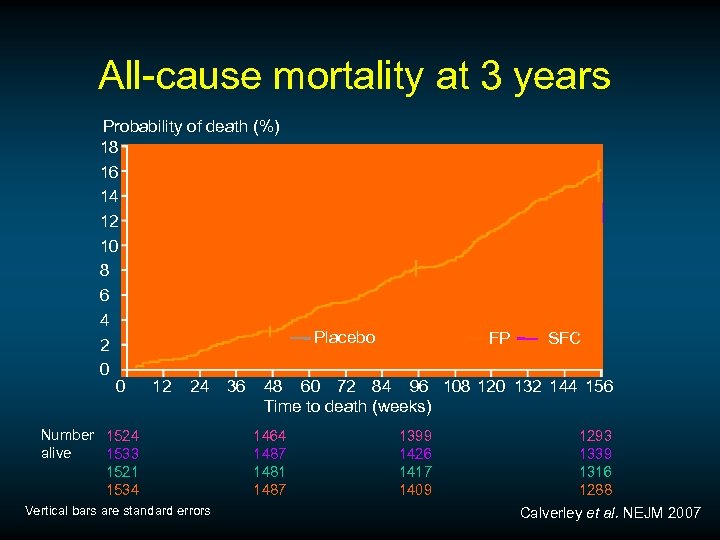 All-cause mortality at 3 years Probability of death (%) 18 16 14 12 10 8 6 4 Placebo SALM FP SFC 2 0 0 12 24 36 48 60 72 84 96 108 120 132 144 156 Time to death (weeks) Number 1524 alive 1533 1521 1534 Vertical bars are standard errors 1464 1487 1481 1487 1399 1426 1417 1409 1293 1339 1316 1288 Calverley et al. NEJM 2007
All-cause mortality at 3 years Probability of death (%) 18 16 14 12 10 8 6 4 Placebo SALM FP SFC 2 0 0 12 24 36 48 60 72 84 96 108 120 132 144 156 Time to death (weeks) Number 1524 alive 1533 1521 1534 Vertical bars are standard errors 1464 1487 1481 1487 1399 1426 1417 1409 1293 1339 1316 1288 Calverley et al. NEJM 2007
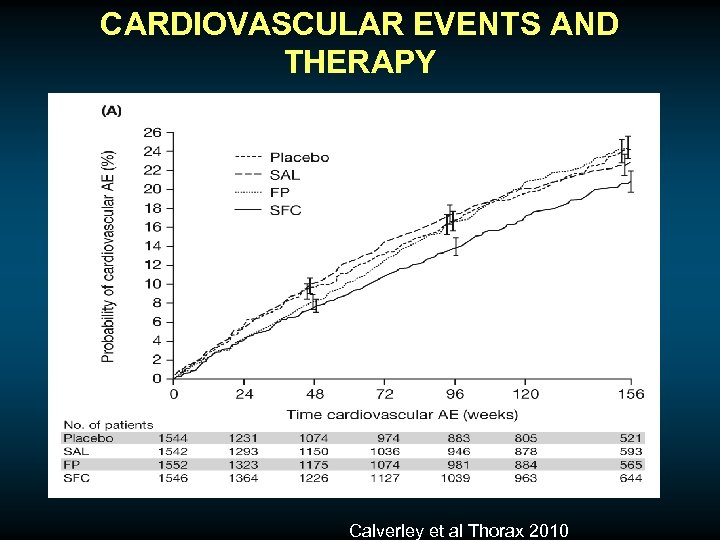 CARDIOVASCULAR EVENTS AND THERAPY Calverley et al Thorax 2010
CARDIOVASCULAR EVENTS AND THERAPY Calverley et al Thorax 2010
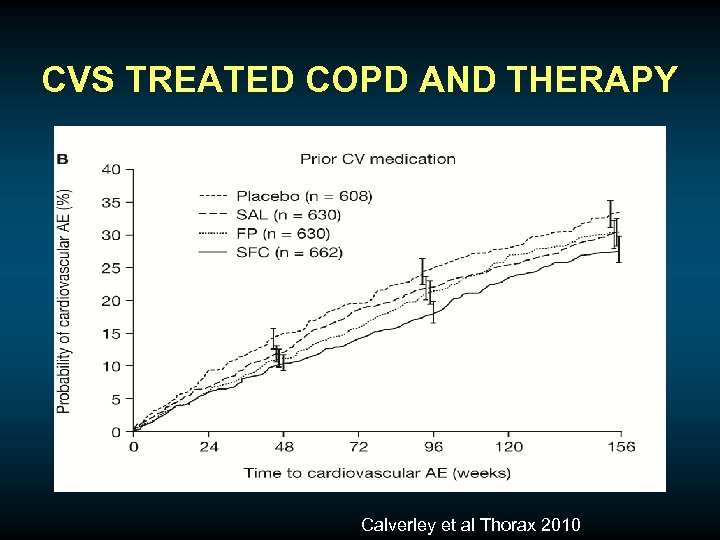 CVS TREATED COPD AND THERAPY Calverley et al Thorax 2010
CVS TREATED COPD AND THERAPY Calverley et al Thorax 2010
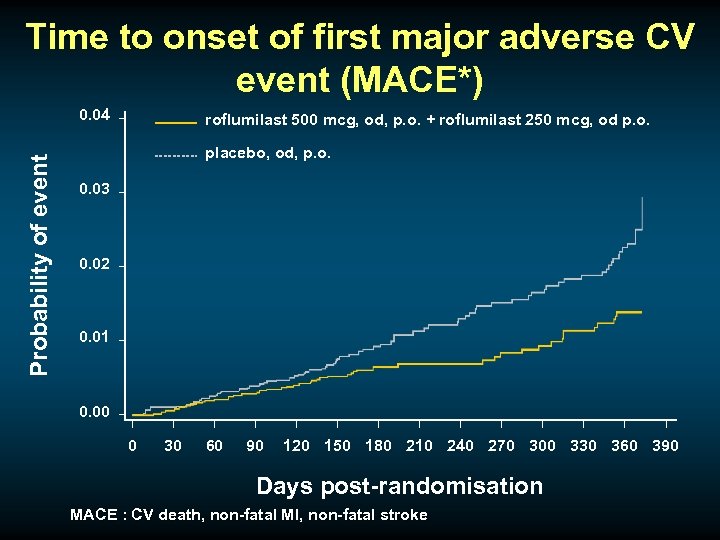 Time to onset of first major adverse CV event (MACE*) Probability of event 0. 04 roflumilast 500 mcg, od, p. o. + roflumilast 250 mcg, od p. o. placebo, od, p. o. 0. 03 0. 02 0. 01 0. 00 0 30 60 90 120 150 180 210 240 270 300 330 360 390 Days post-randomisation MACE : CV death, non-fatal MI, non-fatal stroke
Time to onset of first major adverse CV event (MACE*) Probability of event 0. 04 roflumilast 500 mcg, od, p. o. + roflumilast 250 mcg, od p. o. placebo, od, p. o. 0. 03 0. 02 0. 01 0. 00 0 30 60 90 120 150 180 210 240 270 300 330 360 390 Days post-randomisation MACE : CV death, non-fatal MI, non-fatal stroke
 CONCLUSIONS u Beta–blockers and other cardiac drugs are safe in u u u COPD Statins may improve COPD outcomes but proper trial data are needed Oral therapies produce more GI upset, oral corticosteroids long term are hazardous Inhaled corticosteroids do not seem to accelerate osteoporosis but some may induce pneumonia LAMA and LABA treatment is safe in COPD – antiinflammatory therapy may improve cardiac outcomes On balance our treatments are more friend than foe
CONCLUSIONS u Beta–blockers and other cardiac drugs are safe in u u u COPD Statins may improve COPD outcomes but proper trial data are needed Oral therapies produce more GI upset, oral corticosteroids long term are hazardous Inhaled corticosteroids do not seem to accelerate osteoporosis but some may induce pneumonia LAMA and LABA treatment is safe in COPD – antiinflammatory therapy may improve cardiac outcomes On balance our treatments are more friend than foe


