e852036a7fd647bc82e5dad84b89ba6e.ppt
- Количество слайдов: 22
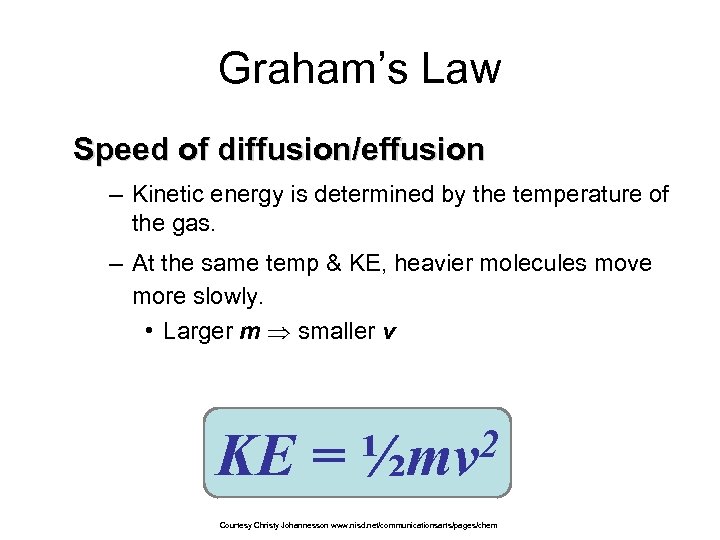 Graham’s Law Speed of diffusion/effusion – Kinetic energy is determined by the temperature of the gas. – At the same temp & KE, heavier molecules move more slowly. • Larger m smaller v KE = 2 ½mv Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem
Graham’s Law Speed of diffusion/effusion – Kinetic energy is determined by the temperature of the gas. – At the same temp & KE, heavier molecules move more slowly. • Larger m smaller v KE = 2 ½mv Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem
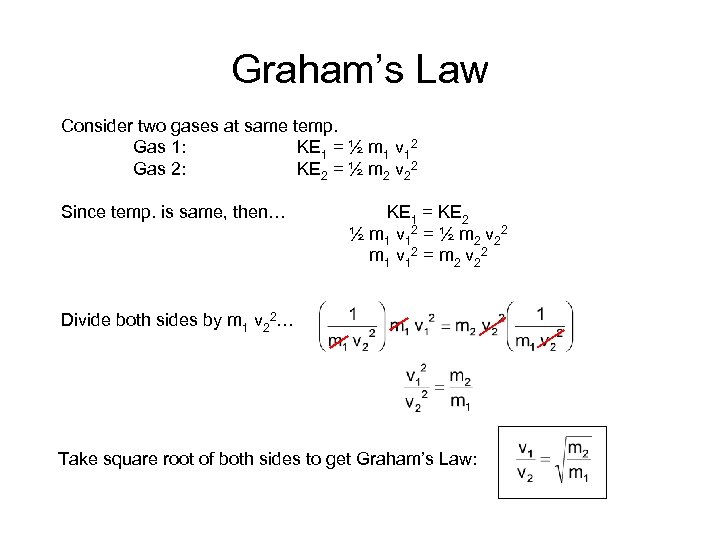 Graham’s Law Consider two gases at same temp. Gas 1: KE 1 = ½ m 1 v 12 Gas 2: KE 2 = ½ m 2 v 22 Since temp. is same, then… KE 1 = KE 2 ½ m 1 v 1 2 = ½ m 2 v 2 2 m 1 v 1 2 = m 2 v 2 2 Divide both sides by m 1 v 22… Take square root of both sides to get Graham’s Law:
Graham’s Law Consider two gases at same temp. Gas 1: KE 1 = ½ m 1 v 12 Gas 2: KE 2 = ½ m 2 v 22 Since temp. is same, then… KE 1 = KE 2 ½ m 1 v 1 2 = ½ m 2 v 2 2 m 1 v 1 2 = m 2 v 2 2 Divide both sides by m 1 v 22… Take square root of both sides to get Graham’s Law:
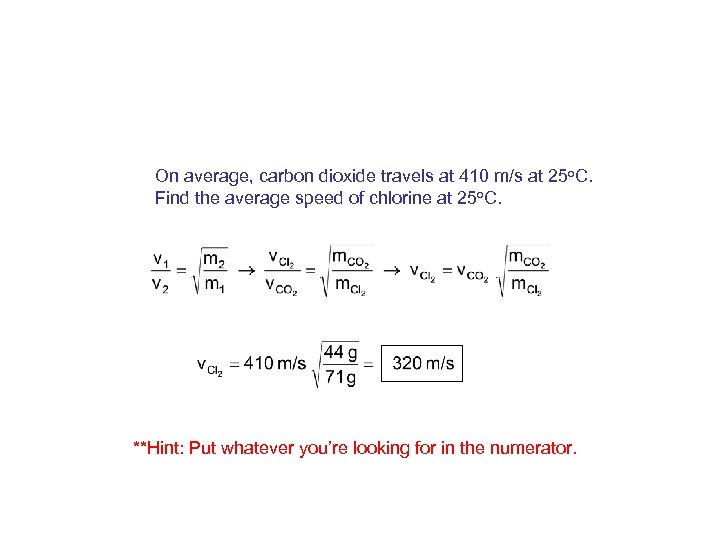 On average, carbon dioxide travels at 410 m/s at 25 o. C. Find the average speed of chlorine at 25 o. C. **Hint: Put whatever you’re looking for in the numerator.
On average, carbon dioxide travels at 410 m/s at 25 o. C. Find the average speed of chlorine at 25 o. C. **Hint: Put whatever you’re looking for in the numerator.
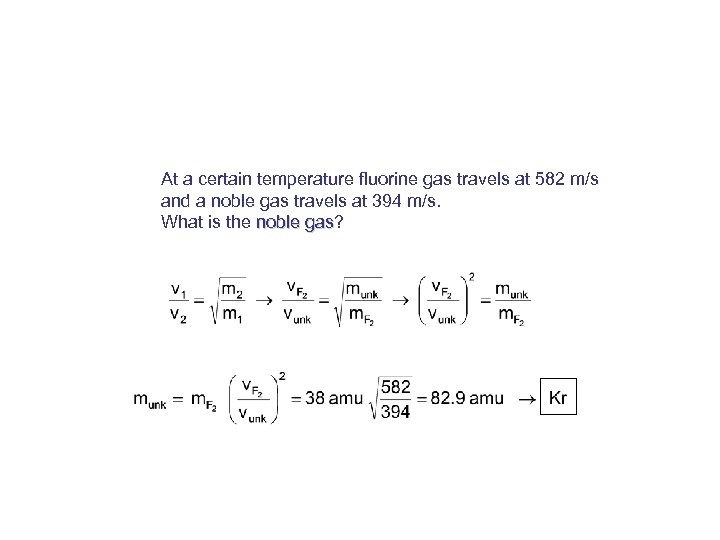 At a certain temperature fluorine gas travels at 582 m/s and a noble gas travels at 394 m/s. What is the noble gas? gas
At a certain temperature fluorine gas travels at 582 m/s and a noble gas travels at 394 m/s. What is the noble gas? gas
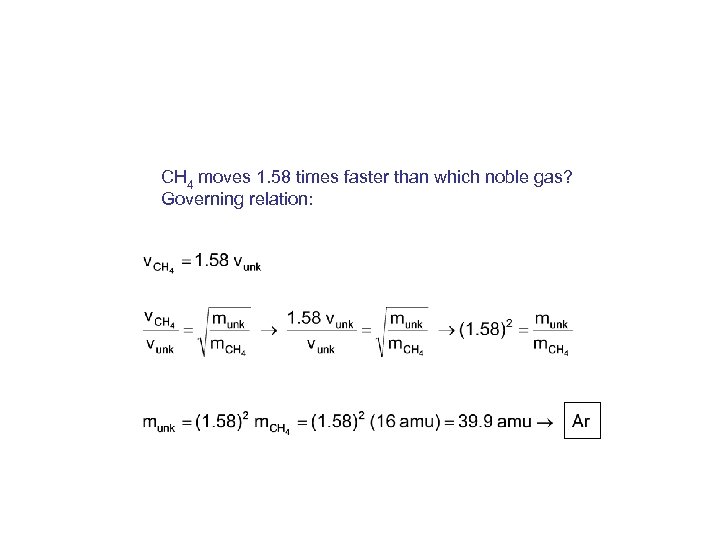 CH 4 moves 1. 58 times faster than which noble gas? Governing relation:
CH 4 moves 1. 58 times faster than which noble gas? Governing relation:
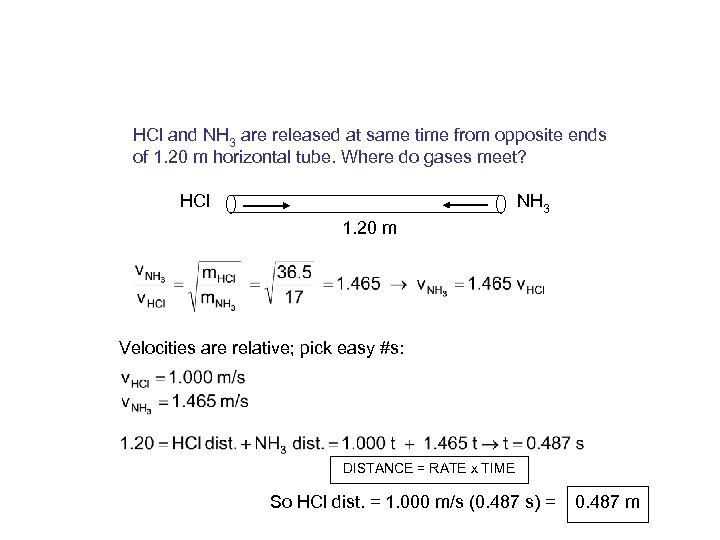 HCl and NH 3 are released at same time from opposite ends of 1. 20 m horizontal tube. Where do gases meet? HCl NH 3 1. 20 m Velocities are relative; pick easy #s: DISTANCE = RATE x TIME So HCl dist. = 1. 000 m/s (0. 487 s) = 0. 487 m
HCl and NH 3 are released at same time from opposite ends of 1. 20 m horizontal tube. Where do gases meet? HCl NH 3 1. 20 m Velocities are relative; pick easy #s: DISTANCE = RATE x TIME So HCl dist. = 1. 000 m/s (0. 487 s) = 0. 487 m
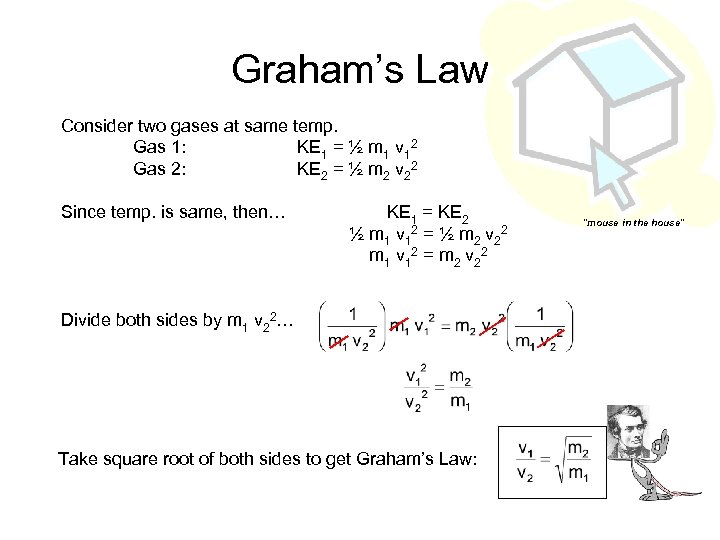 Graham’s Law Consider two gases at same temp. Gas 1: KE 1 = ½ m 1 v 12 Gas 2: KE 2 = ½ m 2 v 22 Since temp. is same, then… KE 1 = KE 2 ½ m 1 v 1 2 = ½ m 2 v 2 2 m 1 v 1 2 = m 2 v 2 2 Divide both sides by m 1 v 22… Take square root of both sides to get Graham’s Law: “mouse in the house”
Graham’s Law Consider two gases at same temp. Gas 1: KE 1 = ½ m 1 v 12 Gas 2: KE 2 = ½ m 2 v 22 Since temp. is same, then… KE 1 = KE 2 ½ m 1 v 1 2 = ½ m 2 v 2 2 m 1 v 1 2 = m 2 v 2 2 Divide both sides by m 1 v 22… Take square root of both sides to get Graham’s Law: “mouse in the house”
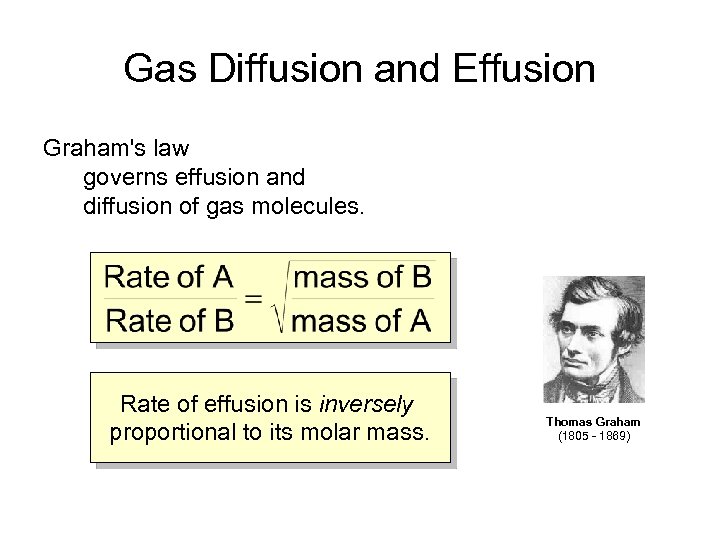 Gas Diffusion and Effusion Graham's law governs effusion and diffusion of gas molecules. Rate of effusion is inversely proportional to its molar mass. Thomas Graham (1805 - 1869)
Gas Diffusion and Effusion Graham's law governs effusion and diffusion of gas molecules. Rate of effusion is inversely proportional to its molar mass. Thomas Graham (1805 - 1869)
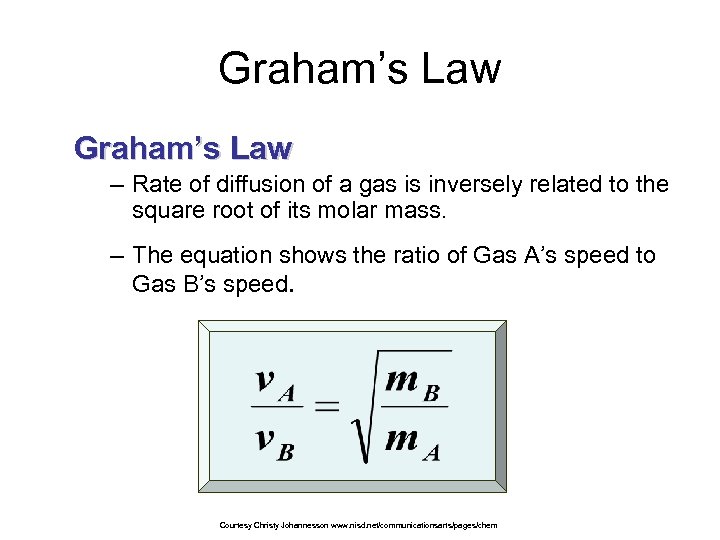 Graham’s Law – Rate of diffusion of a gas is inversely related to the square root of its molar mass. – The equation shows the ratio of Gas A’s speed to Gas B’s speed. Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem
Graham’s Law – Rate of diffusion of a gas is inversely related to the square root of its molar mass. – The equation shows the ratio of Gas A’s speed to Gas B’s speed. Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem
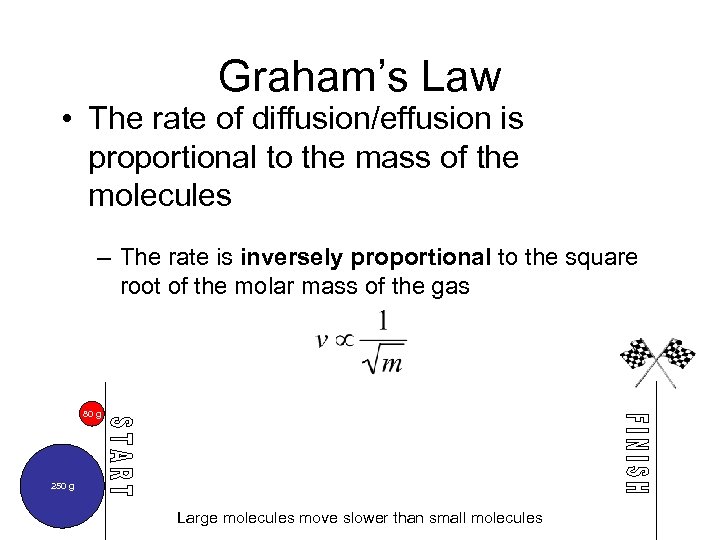 Graham’s Law • The rate of diffusion/effusion is proportional to the mass of the molecules – The rate is inversely proportional to the square root of the molar mass of the gas 80 g 250 g Large molecules move slower than small molecules
Graham’s Law • The rate of diffusion/effusion is proportional to the mass of the molecules – The rate is inversely proportional to the square root of the molar mass of the gas 80 g 250 g Large molecules move slower than small molecules
 2 17 He Cl 4. 0026 35. 453 Find the relative rate of diffusion of helium and chlorine gas Step 1) Write given information GAS 1 = helium He GAS 2 = chlorine M 1 = 4. 0 g M 2 = 71. 0 g v 1 = x Cl 2 v 2 = x Step 2) Equation Step 3) Substitute into equation and solve v 1 v 2 = 71. 0 g 4. 0 g He diffuses 4. 21 times faster than Cl 2 4. 21 1
2 17 He Cl 4. 0026 35. 453 Find the relative rate of diffusion of helium and chlorine gas Step 1) Write given information GAS 1 = helium He GAS 2 = chlorine M 1 = 4. 0 g M 2 = 71. 0 g v 1 = x Cl 2 v 2 = x Step 2) Equation Step 3) Substitute into equation and solve v 1 v 2 = 71. 0 g 4. 0 g He diffuses 4. 21 times faster than Cl 2 4. 21 1
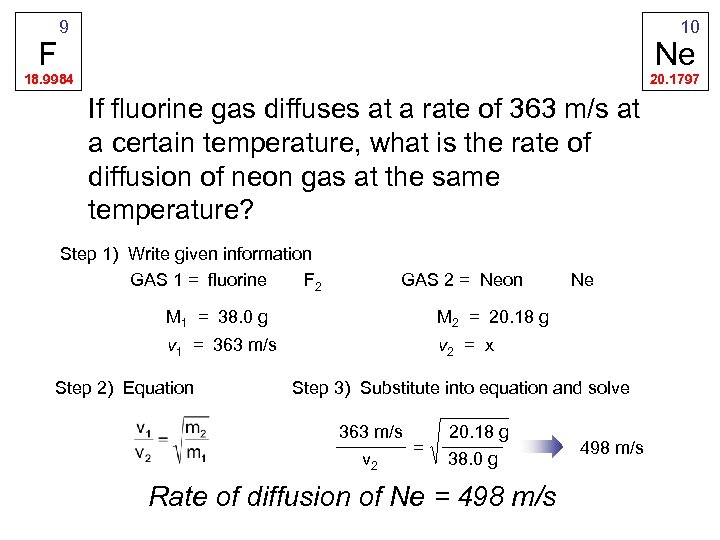 F 9 10 Ne 18. 9984 20. 1797 If fluorine gas diffuses at a rate of 363 m/s at a certain temperature, what is the rate of diffusion of neon gas at the same temperature? Step 1) Write given information GAS 1 = fluorine F 2 GAS 2 = Neon M 1 = 38. 0 g M 2 = 20. 18 g v 1 = 363 m/s Ne v 2 = x Step 2) Equation Step 3) Substitute into equation and solve 363 m/s v 2 = 20. 18 g 38. 0 g Rate of diffusion of Ne = 498 m/s
F 9 10 Ne 18. 9984 20. 1797 If fluorine gas diffuses at a rate of 363 m/s at a certain temperature, what is the rate of diffusion of neon gas at the same temperature? Step 1) Write given information GAS 1 = fluorine F 2 GAS 2 = Neon M 1 = 38. 0 g M 2 = 20. 18 g v 1 = 363 m/s Ne v 2 = x Step 2) Equation Step 3) Substitute into equation and solve 363 m/s v 2 = 20. 18 g 38. 0 g Rate of diffusion of Ne = 498 m/s
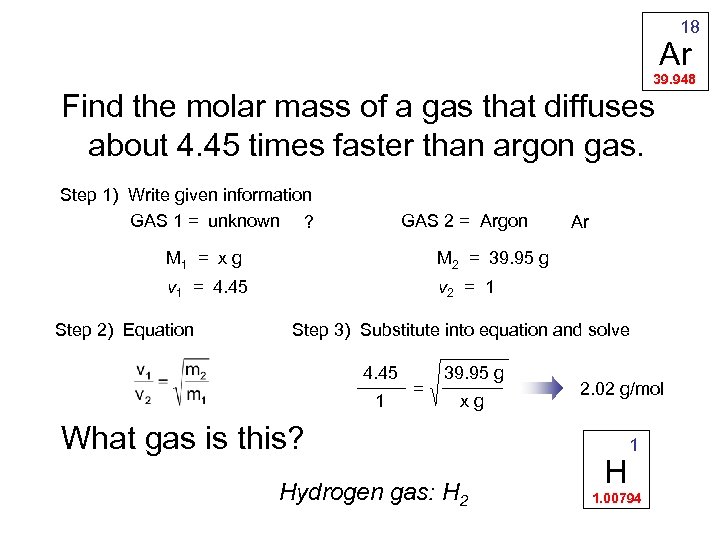 18 Ar 39. 948 Find the molar mass of a gas that diffuses about 4. 45 times faster than argon gas. Step 1) Write given information GAS 1 = unknown ? GAS 2 = Argon M 1 = x g M 2 = 39. 95 g v 1 = 4. 45 Ar v 2 = 1 Step 2) Equation Step 3) Substitute into equation and solve 4. 45 1 = 39. 95 g xg What gas is this? Hydrogen gas: H 2 2. 02 g/mol H 1 1. 00794
18 Ar 39. 948 Find the molar mass of a gas that diffuses about 4. 45 times faster than argon gas. Step 1) Write given information GAS 1 = unknown ? GAS 2 = Argon M 1 = x g M 2 = 39. 95 g v 1 = 4. 45 Ar v 2 = 1 Step 2) Equation Step 3) Substitute into equation and solve 4. 45 1 = 39. 95 g xg What gas is this? Hydrogen gas: H 2 2. 02 g/mol H 1 1. 00794
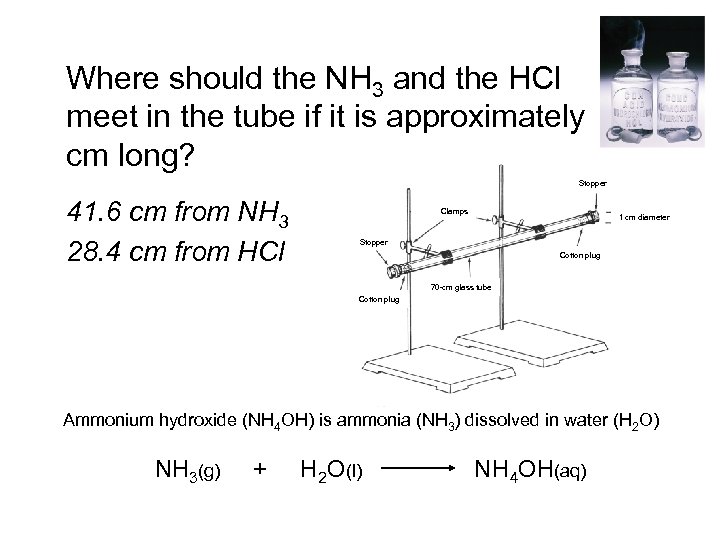 Where should the NH 3 and the HCl meet in the tube if it is approximately 70 cm long? Stopper 41. 6 cm from NH 3 28. 4 cm from HCl Clamps 1 cm diameter Stopper Cotton plug 70 -cm glass tube Cotton plug Ammonium hydroxide (NH 4 OH) is ammonia (NH 3) dissolved in water (H 2 O) NH 3(g) + H 2 O(l) NH 4 OH(aq)
Where should the NH 3 and the HCl meet in the tube if it is approximately 70 cm long? Stopper 41. 6 cm from NH 3 28. 4 cm from HCl Clamps 1 cm diameter Stopper Cotton plug 70 -cm glass tube Cotton plug Ammonium hydroxide (NH 4 OH) is ammonia (NH 3) dissolved in water (H 2 O) NH 3(g) + H 2 O(l) NH 4 OH(aq)
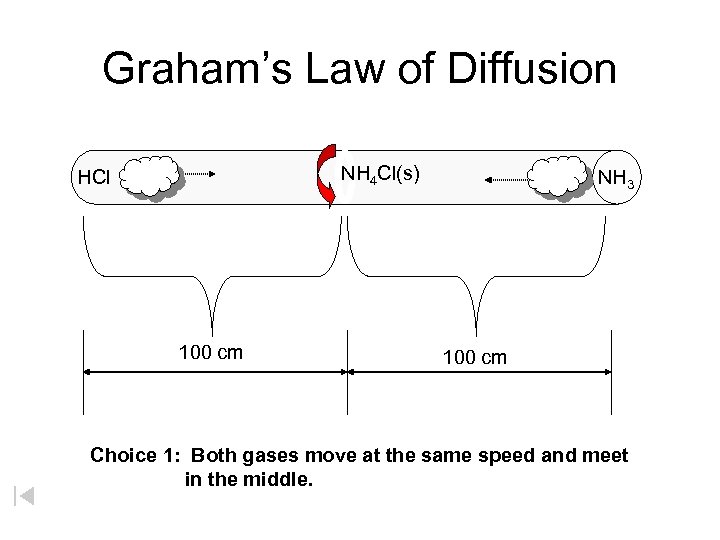 Graham’s Law of Diffusion NH 4 Cl(s) HCl 100 cm NH 3 100 cm Choice 1: Both gases move at the same speed and meet in the middle.
Graham’s Law of Diffusion NH 4 Cl(s) HCl 100 cm NH 3 100 cm Choice 1: Both gases move at the same speed and meet in the middle.
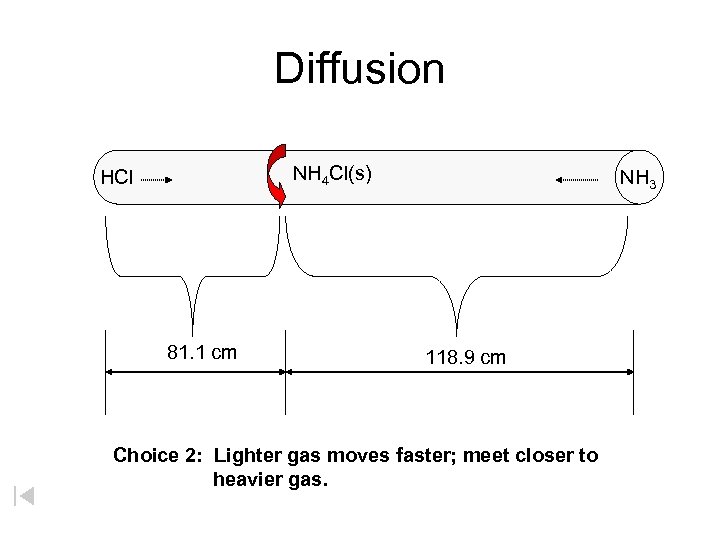 Diffusion NH 4 Cl(s) HCl 81. 1 cm NH 3 118. 9 cm Choice 2: Lighter gas moves faster; meet closer to heavier gas.
Diffusion NH 4 Cl(s) HCl 81. 1 cm NH 3 118. 9 cm Choice 2: Lighter gas moves faster; meet closer to heavier gas.
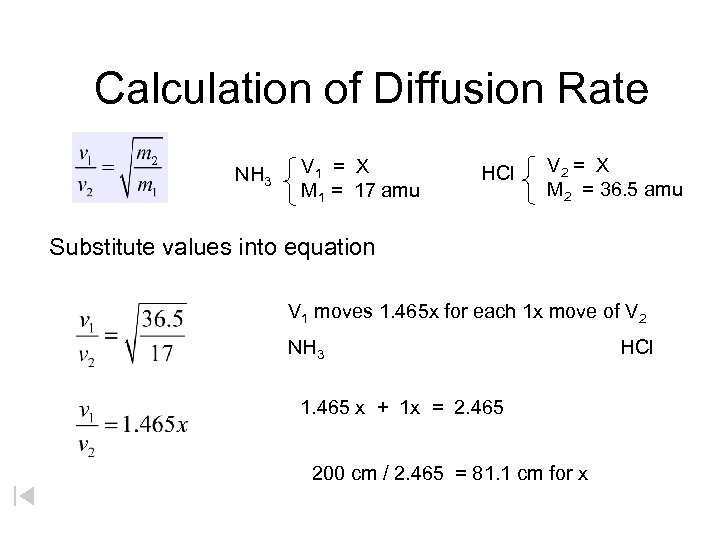 Calculation of Diffusion Rate NH 3 V 1 = X M 1 = 17 amu HCl V 2 = X M 2 = 36. 5 amu Substitute values into equation V 1 moves 1. 465 x for each 1 x move of V 2 NH 3 1. 465 x + 1 x = 2. 465 200 cm / 2. 465 = 81. 1 cm for x HCl
Calculation of Diffusion Rate NH 3 V 1 = X M 1 = 17 amu HCl V 2 = X M 2 = 36. 5 amu Substitute values into equation V 1 moves 1. 465 x for each 1 x move of V 2 NH 3 1. 465 x + 1 x = 2. 465 200 cm / 2. 465 = 81. 1 cm for x HCl
 Calculation of Diffusion Rate V 1 = V 2 m 1 NH 3 V 1 = X M 1 = 17 amu HCl V 2 = X M 2 = 36. 5 amu Substitute values into equation V 1 = V 2 36. 5 17 V 1 = V 2 1. 465 V 1 moves 1. 465 x for each 1 x move of v 2 NH 3 1. 465 x + 1 x = 2. 465 200 cm / 2. 465 = 81. 1 cm for x HCl
Calculation of Diffusion Rate V 1 = V 2 m 1 NH 3 V 1 = X M 1 = 17 amu HCl V 2 = X M 2 = 36. 5 amu Substitute values into equation V 1 = V 2 36. 5 17 V 1 = V 2 1. 465 V 1 moves 1. 465 x for each 1 x move of v 2 NH 3 1. 465 x + 1 x = 2. 465 200 cm / 2. 465 = 81. 1 cm for x HCl
 Copyright © 2007 Pearson Benjamin Cummings. All rights reserved.
Copyright © 2007 Pearson Benjamin Cummings. All rights reserved.
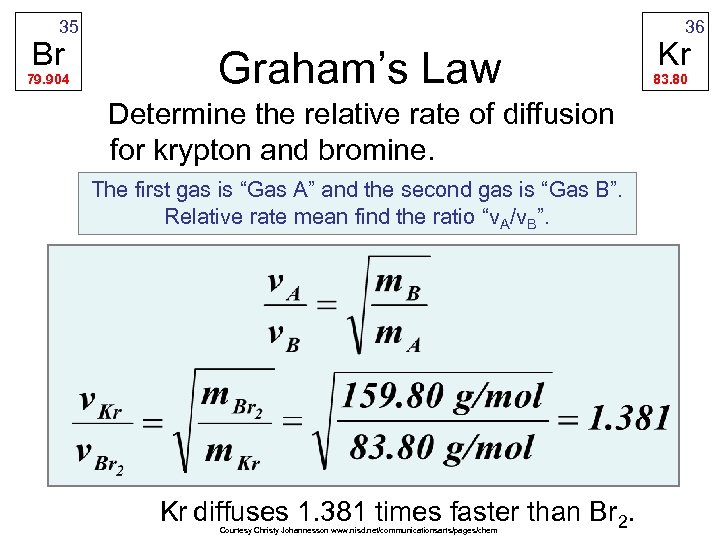 35 Br 79. 904 36 Graham’s Law Determine the relative rate of diffusion for krypton and bromine. The first gas is “Gas A” and the second gas is “Gas B”. Relative rate mean find the ratio “v. A/v. B”. Kr diffuses 1. 381 times faster than Br 2. Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem Kr 83. 80
35 Br 79. 904 36 Graham’s Law Determine the relative rate of diffusion for krypton and bromine. The first gas is “Gas A” and the second gas is “Gas B”. Relative rate mean find the ratio “v. A/v. B”. Kr diffuses 1. 381 times faster than Br 2. Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem Kr 83. 80
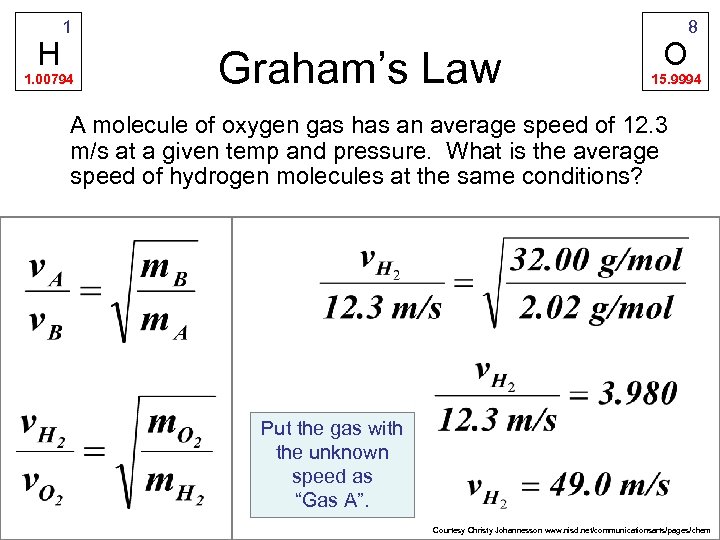 H 1 1. 00794 Graham’s Law O 8 15. 9994 A molecule of oxygen gas has an average speed of 12. 3 m/s at a given temp and pressure. What is the average speed of hydrogen molecules at the same conditions? Put the gas with the unknown speed as “Gas A”. Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem
H 1 1. 00794 Graham’s Law O 8 15. 9994 A molecule of oxygen gas has an average speed of 12. 3 m/s at a given temp and pressure. What is the average speed of hydrogen molecules at the same conditions? Put the gas with the unknown speed as “Gas A”. Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem
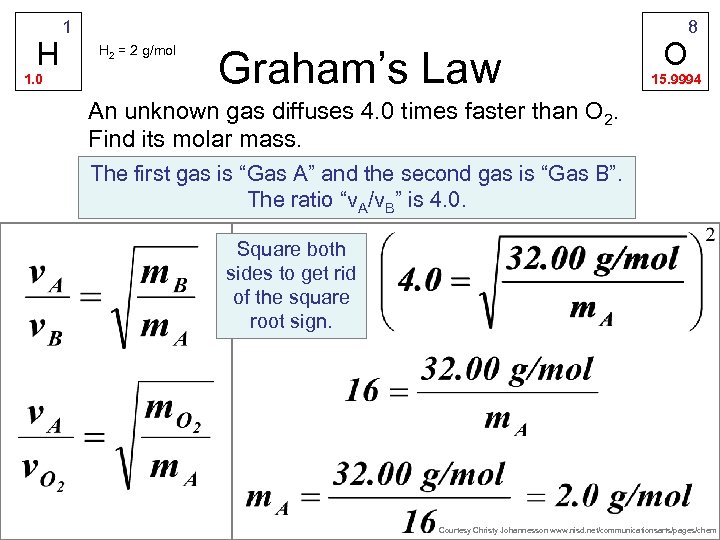 H 1. 0 1 H 2 = 2 g/mol Graham’s Law O 8 15. 9994 An unknown gas diffuses 4. 0 times faster than O 2. Find its molar mass. The first gas is “Gas A” and the second gas is “Gas B”. The ratio “v. A/v. B” is 4. 0. Square both sides to get rid of the square root sign. Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem
H 1. 0 1 H 2 = 2 g/mol Graham’s Law O 8 15. 9994 An unknown gas diffuses 4. 0 times faster than O 2. Find its molar mass. The first gas is “Gas A” and the second gas is “Gas B”. The ratio “v. A/v. B” is 4. 0. Square both sides to get rid of the square root sign. Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem


