c6c6366b4c296876307d78aa35924b03.ppt
- Количество слайдов: 25

Gideon Brückner Deputy Director General The role of good veterinary governance for harmonisation of the registration, distribution and quality control over veterinary medicinal products in Africa OIE CONFERENCE ON VETERINARY MEDICINAL PRODUCTS DAKAR, SENEGAL, 25 – 27 MARCH 2008

World Organisation for Animal Health (OIE) • Established in 1924: 172 Members • Intergovernmental organisation – predates the UN • Permanent Regional Representations: Bamako (Mali), Buenos Aires (Argentina), Tokyo (Japan), Sofia (Bulgaria) and Beirut (Lebanon) • Sub-regional Offices: Bangkok (Thailand), Gaborone (Botswana), Panama, Brussels (Belgium) • Regional Commissions: Africa, America, Asia-Pacific, Europe and Middle East 50 13 29 52 28

OIE MANDATE Historical: ‘To prevent animal diseases from spreading around the world’ The 4 th Strategic Plan 2006/2010 extends the OIE’s global mandate to: ‘The improvement of animal health all around the world’

Chapter 1. 1. 9 International standards, guidelines and recommendations • Terrestrial Animal Health Code • Aquatic Animal Health Code • Manual of Diagnostic Tests and Vaccines for Terrestrial Animals • Manual of Diagnostic Tests for Aquatic Animals Mandated in SPS Agreement of WTO

Good governance – veterinary services Ensuring Good Governance to Address Emerging and Re-emerging Animal Disease Threats Supporting the Veterinary Services of Developing Countries to meet OIE International Standards on Quality Mechanisms and strategies at Global, Regional and National levels

Use of veterinary medicinal products integral to animal disease control in Africa • • At least 90% of OIE listed diseases in Africa High circulation of illegal/counterfeit products • Outdated legislation • Insufficient standards • • Division of responsibilities between Ministries Urgent need for quality control
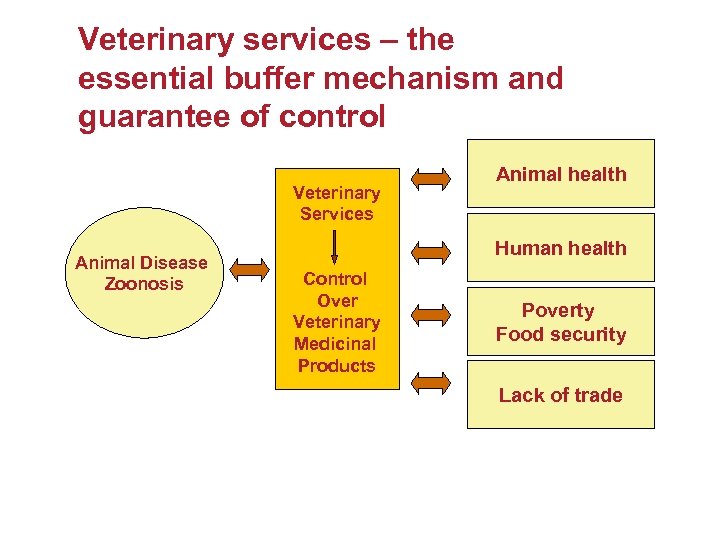
Veterinary services – the essential buffer mechanism and guarantee of control Veterinary Services Animal Disease Zoonosis Animal health Human health Control Over Veterinary Medicinal Products Poverty Food security Lack of trade

Evaluation of veterinary services OIE methodology for the evaluation of the performance of veterinary services : PVS - tool
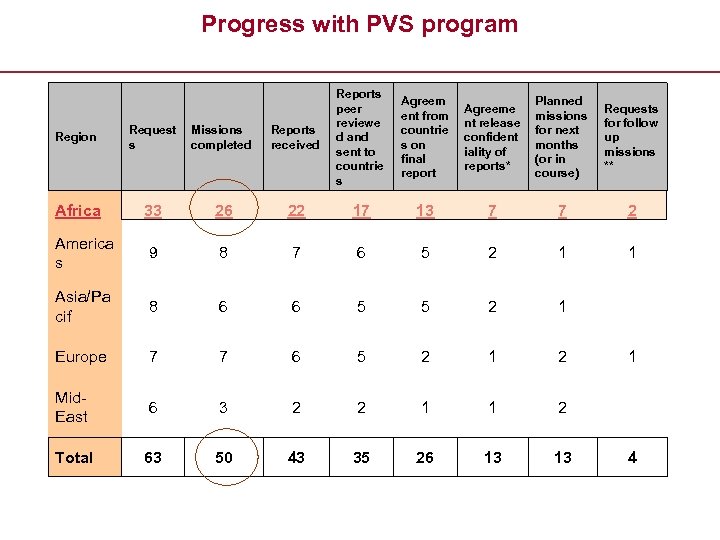
Progress with PVS program Region Request s Missions completed Reports received Reports peer reviewe d and sent to countrie s Africa 33 26 22 17 13 7 7 2 America s 9 8 7 6 5 2 1 1 Asia/Pa cif 8 6 6 5 5 2 1 Europe 7 7 6 5 2 1 2 Mid. East 6 3 2 2 1 1 2 Total 63 50 43 35 26 13 13 Agreem ent from countrie s on final report Agreeme nt release confident iality of reports* Planned missions for next months (or in course) Requests for follow up missions ** 1 4

What is the OIE-PVS Tool? • An assessment tool on level of compliance with international standards on Quality and Evaluation of Veterinary Services • Standards mandated in SPS Agreement of WTO • Chapter 1. 3. 3. : Evaluation of Veterinary Services • Chapter 1. 3. 4. : Guidelines for the Evaluation of VS

USE OF OIE-PVS • evaluation performed by internal (self evaluation) and/or OIE experts (external evaluation • assessing the performance of VS • process reviewed on a regular basis to monitor improvements

OIE PVS certified experts 4 Training Sessions (May and July ’ 06, February ’ 07 and February ’ 08) +140 OIE Certified experts (including 13 FAO Staff) List of OIE PVS certified experts sent to donors (World Bank) In Africa: requests 33 – completed evaluations 26 Gap Analysis to be developed in collaboration with FAO in several countries

OIE PVS TOOL 4 Focal components reflecting service delivery • Human, physical and financial resources • Technical authority and capability • Interaction with stakeholders • Access to markets

OIE PVS TOOL 6 - 12 critical competencies in each focal component I) Human, physical and financial resources • Professional and technical staffing • Competencies of veterinarians and veterinary para-professionals • Physical resources • Funding……
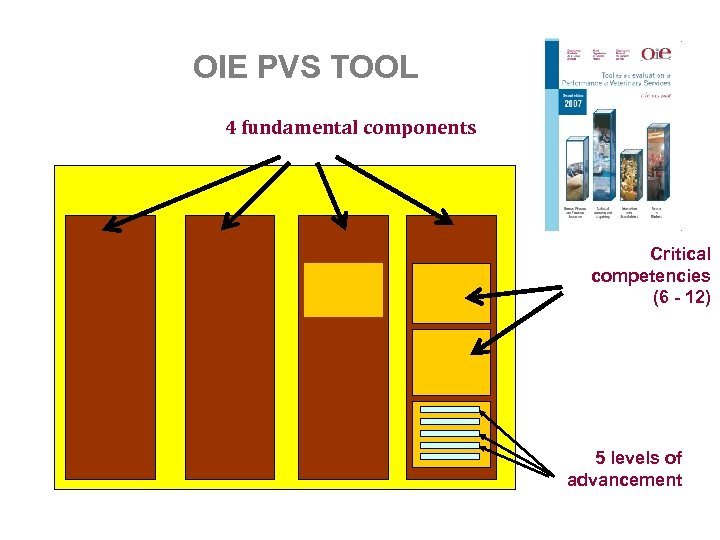
OIE PVS TOOL 4 fundamental components Critical competencies (6 - 12) 5 levels of advancement
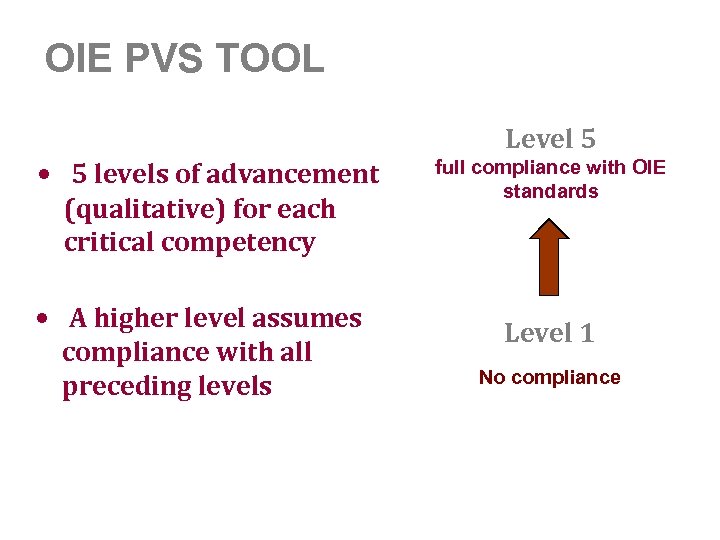
OIE PVS TOOL • 5 levels of advancement (qualitative) for each critical competency • A higher level assumes compliance with all preceding levels Level 5 full compliance with OIE standards Level 1 No compliance
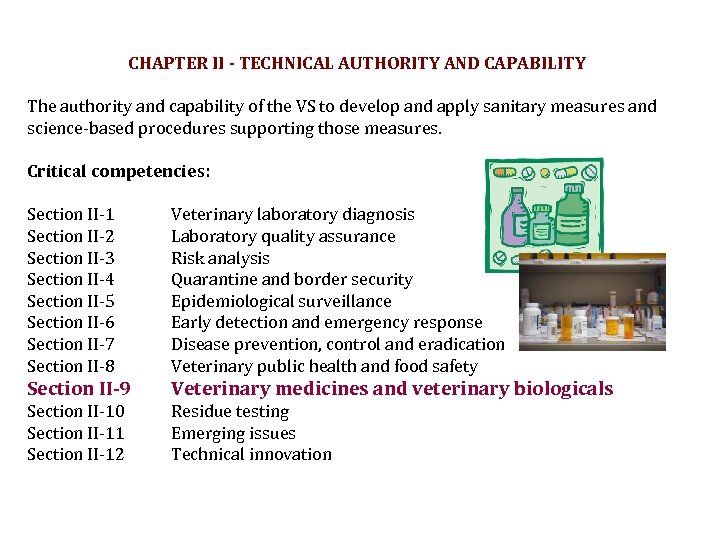
CHAPTER II - TECHNICAL AUTHORITY AND CAPABILITY The authority and capability of the VS to develop and apply sanitary measures and science-based procedures supporting those measures. Critical competencies: Section II-1 Section II-2 Section II-3 Section II-4 Section II-5 Section II-6 Section II-7 Section II-8 Veterinary laboratory diagnosis Laboratory quality assurance Risk analysis Quarantine and border security Epidemiological surveillance Early detection and emergency response Disease prevention, control and eradication Veterinary public health and food safety Section II-9 Veterinary medicines and veterinary biologicals Section II-10 Section II-11 Section II-12 Residue testing Emerging issues Technical innovation
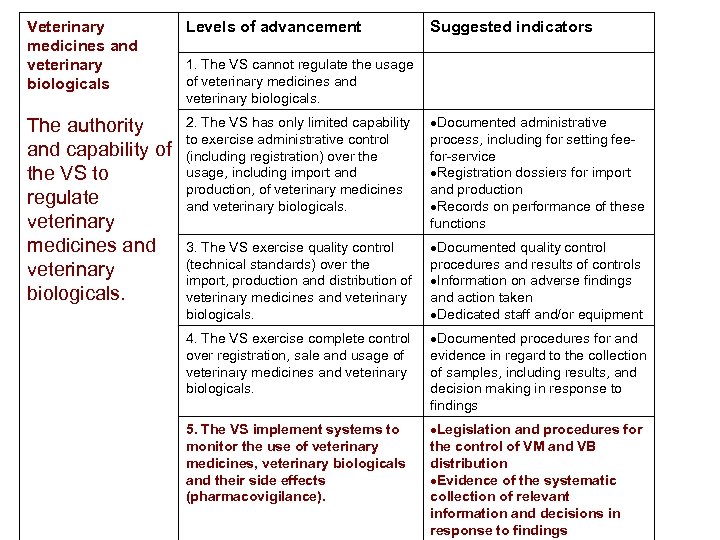
Veterinary medicines and veterinary biologicals Levels of advancement Suggested indicators The authority and capability of the VS to regulate veterinary medicines and veterinary biologicals. 2. The VS has only limited capability to exercise administrative control (including registration) over the usage, including import and production, of veterinary medicines and veterinary biologicals. Documented administrative process, including for setting feefor-service Registration dossiers for import and production Records on performance of these functions 3. The VS exercise quality control (technical standards) over the import, production and distribution of veterinary medicines and veterinary biologicals. Documented quality control procedures and results of controls Information on adverse findings and action taken Dedicated staff and/or equipment 4. The VS exercise complete control over registration, sale and usage of veterinary medicines and veterinary biologicals. Documented procedures for and evidence in regard to the collection of samples, including results, and decision making in response to findings 5. The VS implement systems to monitor the use of veterinary medicines, veterinary biologicals and their side effects (pharmacovigilance). Legislation and procedures for the control of VM and VB distribution Evidence of the systematic collection of relevant information and decisions in response to findings 1. The VS cannot regulate the usage of veterinary medicines and veterinary biologicals.

Critical issues in Africa to facilitate harmonisation of registration, distribution and quality control • Legal control over: • Registration • Importation • Standards – use/conformity/complia nce • Distribution • Sales • Quality control • • Monitoring – including residues Implementation of legislation – funds & staff

Role of OIE • Setting the standards* • Evaluate shortcomings in application of OIE standards - PVS • Provide expertise (OIE Reference Laboratories & Collaborating Centres, conferences) • Propose and adopt Resolutions by International Committee to facilitate common committment and ‘buy-in’ • Facilitate donor funding • Can not perform an inspection or policing function

OIE Standards • Not set by OIE Director General or OIE Central Bureau • OIE Standards are proposed and eventually adopted by OIE Delegates = International Committee • Adoption implies taking ownership and committment to compliance and where possible, include into national legislation

Role of OIE • Setting the standards • Evaluate shortcomings in application of OIE standards - PVS • Provide expertise (OIE Reference Laboratories & Collaborating Centres, conferences) • Propose and adopt Resolutions by International Committee to facilitate common committment and ‘buy-in’ • Facilitate donor funding • Can not perform an inspection or policing function
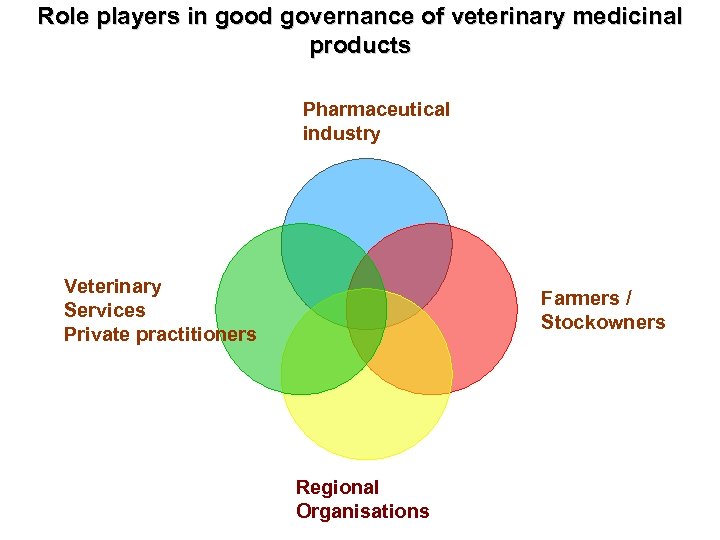
Role players in good governance of veterinary medicinal products Pharmaceutical industry Veterinary Services Private practitioners Farmers / Stockowners Regional Organisations

There already some good systems in place in Africa for veterinary medicinal products … Good veterinary governance to include the following will help us to move forward Regulation Registration Harmonisation Inspection

Thank you for your attention World Organisation for Animal Health 12 rue de Prony 75017 Paris, France Tel: 33 (0)1 44 15 18 88 Fax: 33 (0)1 42 67 09 87 Email: oie@oie. int http: //www. oie. int
c6c6366b4c296876307d78aa35924b03.ppt