ccb1b2b1f0a28a3306de129ddcdfb751.ppt
- Количество слайдов: 77
 Geri Foster, DPh Compounding & Investigational Drug Pharmacist 1
Geri Foster, DPh Compounding & Investigational Drug Pharmacist 1
 July 20, 2001, Friday Johns Hopkins Death Brings Halt To U. S. - Financed Human Studies 2
July 20, 2001, Friday Johns Hopkins Death Brings Halt To U. S. - Financed Human Studies 2
 Warning letter to Johns Hopkins No IND submission Didn’t mention all risks in consent Failed to report adverse reactions Failed to update consents following adverse reactions. 3
Warning letter to Johns Hopkins No IND submission Didn’t mention all risks in consent Failed to report adverse reactions Failed to update consents following adverse reactions. 3
 Warning letter to Johns Hopkins Failed to disclose experimental use of drug. Represented product as a medication and failed to disclose it as chemical grade intended only as lab use. Failed to submit technical information, such as, source and purity of the drug substance. 4
Warning letter to Johns Hopkins Failed to disclose experimental use of drug. Represented product as a medication and failed to disclose it as chemical grade intended only as lab use. Failed to submit technical information, such as, source and purity of the drug substance. 4
 Warning letter to Johns Hopkins Poor documentation n Undetermined amount of Sodium Bicarb added to second solution, no records Changed the dosing conditions in protocol n n n delivery system p. H, osmolarity rate of administration Changed pre-medication 5
Warning letter to Johns Hopkins Poor documentation n Undetermined amount of Sodium Bicarb added to second solution, no records Changed the dosing conditions in protocol n n n delivery system p. H, osmolarity rate of administration Changed pre-medication 5
 DEPARTMENT: INSTITUTIONAL REVIEW BOARD POLICY NUMBER: XI. A SECTION: Investigational Drugs, Biologics & Device REVIEW RESPONSIBILITIES: IRB Policy & Procedure Committee ORIGINAL CREATION DATE: February 04, 2002 REVISION DATES October 17, 2003 Subject: Storage, Handling, and Dispensing of Investigational Drugs, Agents, and/or Biologics in Clinical Trials 6
DEPARTMENT: INSTITUTIONAL REVIEW BOARD POLICY NUMBER: XI. A SECTION: Investigational Drugs, Biologics & Device REVIEW RESPONSIBILITIES: IRB Policy & Procedure Committee ORIGINAL CREATION DATE: February 04, 2002 REVISION DATES October 17, 2003 Subject: Storage, Handling, and Dispensing of Investigational Drugs, Agents, and/or Biologics in Clinical Trials 6
 Definitions: 1. Investigational Agents: A pharmaceutical form of an active ingredient or placebo being tested or used as a reference in a clinical trial. This includes products with a marketing authorization when used or assembled (formulated or packaged) in a way different from the approved form, products used for an unapproved indication, or products used to gain further information about an approved use. 7
Definitions: 1. Investigational Agents: A pharmaceutical form of an active ingredient or placebo being tested or used as a reference in a clinical trial. This includes products with a marketing authorization when used or assembled (formulated or packaged) in a way different from the approved form, products used for an unapproved indication, or products used to gain further information about an approved use. 7
 2. Definitions: Investigational Drugs/Investigational Biologics (Test Articles): A new drug/agent or biologic that is used in a clinical investigation. The term investigational biologic also includes a biological product that is used in vitro for diagnostic purposes. Investigational drugs or biologics may include: a. Products that are not generally recognized as being safe and effective for any use under the conditions prescribed, recommended, or suggested by the FDA; or b. Products already approved by the FDA as safe and effective for specific indications that are being studied for new indications (or doses, strengths, or frequency). 8
2. Definitions: Investigational Drugs/Investigational Biologics (Test Articles): A new drug/agent or biologic that is used in a clinical investigation. The term investigational biologic also includes a biological product that is used in vitro for diagnostic purposes. Investigational drugs or biologics may include: a. Products that are not generally recognized as being safe and effective for any use under the conditions prescribed, recommended, or suggested by the FDA; or b. Products already approved by the FDA as safe and effective for specific indications that are being studied for new indications (or doses, strengths, or frequency). 8
 3 Definitions: Investigational Drug Service (IDS): A division of the VUMC Pharmacy Department that provides support for clinical drug studies including Institutional Review Board consultation and dispensing services for investigational drugs, agents, or biologics. This division actively supports all Departments and Investigators involved in research. 9
3 Definitions: Investigational Drug Service (IDS): A division of the VUMC Pharmacy Department that provides support for clinical drug studies including Institutional Review Board consultation and dispensing services for investigational drugs, agents, or biologics. This division actively supports all Departments and Investigators involved in research. 9
 4. Definitions: Joint Commission on Accreditation of Healthcare Organizations (JCAHO): A national accrediting body for hospitals and other health care delivery organizations. 10
4. Definitions: Joint Commission on Accreditation of Healthcare Organizations (JCAHO): A national accrediting body for hospitals and other health care delivery organizations. 10
 Policy It is the policy of the Vanderbilt University Institutional Review Board that all investigational drugs, agents and/or biologics used in human subjects research be stored, handled, and dispensed in accordance with governing regulations and Institutional policy. 11
Policy It is the policy of the Vanderbilt University Institutional Review Board that all investigational drugs, agents and/or biologics used in human subjects research be stored, handled, and dispensed in accordance with governing regulations and Institutional policy. 11
 Policy: Storage of Investigational Drugs, Agents, or Biologics. I. A. It is the responsibility of the Investigator to comply with all Institutional, State and Federal regulations in regards to storage of investigational drugs, agents, or biologics. 12
Policy: Storage of Investigational Drugs, Agents, or Biologics. I. A. It is the responsibility of the Investigator to comply with all Institutional, State and Federal regulations in regards to storage of investigational drugs, agents, or biologics. 12
 Policy: 1. B. Investigational drugs, agents, or biologics used in the context of research, may be stored in areas other than the IDS under the direct supervision of the Investigator and is in accordance with the sponsor, if applicable. 13
Policy: 1. B. Investigational drugs, agents, or biologics used in the context of research, may be stored in areas other than the IDS under the direct supervision of the Investigator and is in accordance with the sponsor, if applicable. 13
 Policy: I. C. Controlled substances may not be stored outside of the pharmacy department. 14
Policy: I. C. Controlled substances may not be stored outside of the pharmacy department. 14
 Policy: I. D. Investigational agent storage facilities outside of the IDS must be in compliance with Institutional, State, Federal [Food and Drug Administration (FDA)], and Joint Commission on Accreditation of Hospital Organizations (JCAHO) requirements. Pharmacy monitoring may be incorporated into the IRB auditing process as needed to assure compliance. 15
Policy: I. D. Investigational agent storage facilities outside of the IDS must be in compliance with Institutional, State, Federal [Food and Drug Administration (FDA)], and Joint Commission on Accreditation of Hospital Organizations (JCAHO) requirements. Pharmacy monitoring may be incorporated into the IRB auditing process as needed to assure compliance. 15
 II. Dispensing of Investigational Drugs, Agents, or Biologics. II. A. All investigational drugs, agents, or biologics administered to inpatients should be dispensed through the IDS pharmacy. This includes all inpatient beds in the Vanderbilt University Medical Center, Clinical Research Center, Stallworth Rehabilitation Hospital, Psychiatric Hospital at Vanderbilt 16
II. Dispensing of Investigational Drugs, Agents, or Biologics. II. A. All investigational drugs, agents, or biologics administered to inpatients should be dispensed through the IDS pharmacy. This includes all inpatient beds in the Vanderbilt University Medical Center, Clinical Research Center, Stallworth Rehabilitation Hospital, Psychiatric Hospital at Vanderbilt 16
 II. B. If IDS is not utilized for the dispensing of investigational drugs, agents, or biologics, it is the responsibility of the Investigator to assure that dispensing is in accordance with all Institutional, State, Federal, and JCAHO requirements. 17
II. B. If IDS is not utilized for the dispensing of investigational drugs, agents, or biologics, it is the responsibility of the Investigator to assure that dispensing is in accordance with all Institutional, State, Federal, and JCAHO requirements. 17
 II. C. The Pharmacy must prepare and dispense controlled substances for all inpatients and outpatients. 18
II. C. The Pharmacy must prepare and dispense controlled substances for all inpatients and outpatients. 18
 II. D. Compounding of oral and intravenous drugs must be handled by the Pharmacy. The Pharmacy must prepare and dispense such medications for all inpatients and outpatients. 19
II. D. Compounding of oral and intravenous drugs must be handled by the Pharmacy. The Pharmacy must prepare and dispense such medications for all inpatients and outpatients. 19
 III. Investigations of issues related to the potential mishandling of investigational drugs, agents, or biologics will be conducted by the IDS and promptly reported to the IRB. 20
III. Investigations of issues related to the potential mishandling of investigational drugs, agents, or biologics will be conducted by the IDS and promptly reported to the IRB. 20
 Pharmacy IRB Ex-Offico Members Phil Johnston: Asst. Director of Pharmacy David Di. Persio: Clinical Pharmacist Critical Care Pharmacy Debbie Harrell: Geriatric Pharmacist Specialist 21
Pharmacy IRB Ex-Offico Members Phil Johnston: Asst. Director of Pharmacy David Di. Persio: Clinical Pharmacist Critical Care Pharmacy Debbie Harrell: Geriatric Pharmacist Specialist 21
 Vanderbilt University Institutional Review Board Pharmacy Reviewer’s Comment Form Name of Drug Is it FDA Approved? Is it being used for its approved indication? IND# for this protocol 22
Vanderbilt University Institutional Review Board Pharmacy Reviewer’s Comment Form Name of Drug Is it FDA Approved? Is it being used for its approved indication? IND# for this protocol 22
 Are the doses within acceptable dosing limits? Is the route of administration noted in the protocol? Are the side effects of all agents listed adequately in the protocol? Are other clinical considerations (pregnancy, dietary restrictions, or drug interaction potential) clearly addressed in the consent document? Are the Investigator’s descriptions of handling, storage and dispensing adequate, if not using the IDS? 23
Are the doses within acceptable dosing limits? Is the route of administration noted in the protocol? Are the side effects of all agents listed adequately in the protocol? Are other clinical considerations (pregnancy, dietary restrictions, or drug interaction potential) clearly addressed in the consent document? Are the Investigator’s descriptions of handling, storage and dispensing adequate, if not using the IDS? 23
 What regulations govern investigational drug policies? Hospital policy (IRB) ASHP (residency site) JCAHO standards (Investigational agents) Department of Health and Human Services through OHRP and FDA USP/NF 795 & 797 (FDA enforced) 24
What regulations govern investigational drug policies? Hospital policy (IRB) ASHP (residency site) JCAHO standards (Investigational agents) Department of Health and Human Services through OHRP and FDA USP/NF 795 & 797 (FDA enforced) 24
 JCAHO Standards MM. 7. 40 Investigational medications are safely controlled and administered. Specifying that when pharmacy services are provided, the pharmacy controls the storage, dispensing, labeling, and distribution of the investigational medication 25
JCAHO Standards MM. 7. 40 Investigational medications are safely controlled and administered. Specifying that when pharmacy services are provided, the pharmacy controls the storage, dispensing, labeling, and distribution of the investigational medication 25
 Why follow the USP/NF Chapter 797 standards: They may be adopted and enforced by State Boards Surveyed by accreditation organizations (JCAHO). Enforceable by the FDA 26
Why follow the USP/NF Chapter 797 standards: They may be adopted and enforced by State Boards Surveyed by accreditation organizations (JCAHO). Enforceable by the FDA 26
 USP/NF Chapter 797 Sterile Products n Training and Competence n Compounding Environment n Labeling and Expiration Dating n Aseptic Technique n Product and Environmental Testing n QC procedures n What to do after the products are made and distributed n 27
USP/NF Chapter 797 Sterile Products n Training and Competence n Compounding Environment n Labeling and Expiration Dating n Aseptic Technique n Product and Environmental Testing n QC procedures n What to do after the products are made and distributed n 27
 Who must follow 797 The standards are applicable to health care institutions, pharmacies, physician practices and other facilities in which compounded sterile products (CSP) are prepared and stored. n Not applicable to sterile products prepared for immediate use. 28
Who must follow 797 The standards are applicable to health care institutions, pharmacies, physician practices and other facilities in which compounded sterile products (CSP) are prepared and stored. n Not applicable to sterile products prepared for immediate use. 28
 Immediate Use means there is no intermediary step such as laying it down and waiting to give later- Daryl Rich Pharm. D JCAHO Surveyor n Practices that will fall under <797> using this definition: Drawing up flu vaccine or immunizations into multiple syringes for later use Anesthesiologists’ practice of drawing up all drugs into syringes at the beginning of the day 29
Immediate Use means there is no intermediary step such as laying it down and waiting to give later- Daryl Rich Pharm. D JCAHO Surveyor n Practices that will fall under <797> using this definition: Drawing up flu vaccine or immunizations into multiple syringes for later use Anesthesiologists’ practice of drawing up all drugs into syringes at the beginning of the day 29
 Is this going to be your office attire? n n n Booties or shoe covers Occlusive hair cover Mask Gown Gloves 30
Is this going to be your office attire? n n n Booties or shoe covers Occlusive hair cover Mask Gown Gloves 30
 Why use the Investigational Drug Service ? 31
Why use the Investigational Drug Service ? 31
 Investigational Drug Service Personnel Phil Johnston, Pharm. D. Assistant Director of Pharmacy Hope Campbell, Pharm. D. , BCPS, Coordinator of IDS Geri Foster, D. Ph. Compounding Coordinator Lori Choate, Certified Pharmacy Technician Deborah Allen, Certified Pharmacy Technician 32
Investigational Drug Service Personnel Phil Johnston, Pharm. D. Assistant Director of Pharmacy Hope Campbell, Pharm. D. , BCPS, Coordinator of IDS Geri Foster, D. Ph. Compounding Coordinator Lori Choate, Certified Pharmacy Technician Deborah Allen, Certified Pharmacy Technician 32
 Advantages of the IDS Investigators able to accommodate more protocols Ensure use is limited to authorized prescribers Ensures patient consent is obtained Verification of protocol compliance Available for monitoring visits and FDA audits Guaranteed compliance with all applicable state, & federal regulations 33
Advantages of the IDS Investigators able to accommodate more protocols Ensure use is limited to authorized prescribers Ensures patient consent is obtained Verification of protocol compliance Available for monitoring visits and FDA audits Guaranteed compliance with all applicable state, & federal regulations 33
 Advantages of the IDS Patient specific labeling Consistency in labeling Randomization and blinding available Patient counseling (if needed) Complete and maintain drug related case report forms Serve as the un-blinded participant Order drugs and maintain proper storage Secured drug storage/space 34
Advantages of the IDS Patient specific labeling Consistency in labeling Randomization and blinding available Patient counseling (if needed) Complete and maintain drug related case report forms Serve as the un-blinded participant Order drugs and maintain proper storage Secured drug storage/space 34
 Advantages of the IDS Placebos identical in appearance to product Approved sources of products (C of A) Formulas with sources/documentation/logs Drug accountability documentation Quality service Timely delivery 35
Advantages of the IDS Placebos identical in appearance to product Approved sources of products (C of A) Formulas with sources/documentation/logs Drug accountability documentation Quality service Timely delivery 35
 Inpatient Studies & The IDS Study medication is printed on patient MAR (medication administration record) Drug interaction check specific to protocol Drug exclusion check, specific to protocol Protocol synopsis and nursing sheets on the chart 36
Inpatient Studies & The IDS Study medication is printed on patient MAR (medication administration record) Drug interaction check specific to protocol Drug exclusion check, specific to protocol Protocol synopsis and nursing sheets on the chart 36
 Pharmacy Supported Study 37
Pharmacy Supported Study 37
 Obtain a budget 38
Obtain a budget 38
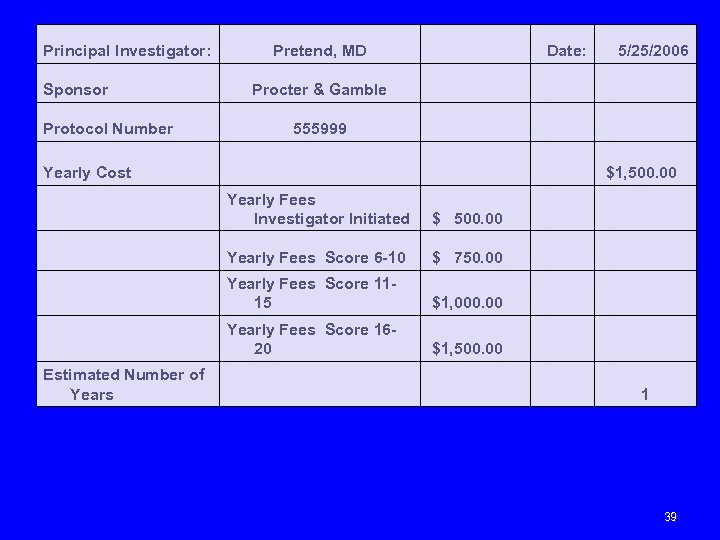 Principal Investigator: Pretend, MD Procter & Gamble Protocol Number 555999 Sponsor Date: 5/25/2006 Yearly Cost $1, 500. 00 Yearly Fees Investigator Initiated $ 500. 00 Yearly Fees Score 6 -10 $ 750. 00 Yearly Fees Score 1115 $1, 000. 00 Yearly Fees Score 1620 $1, 500. 00 Estimated Number of Years 1 39
Principal Investigator: Pretend, MD Procter & Gamble Protocol Number 555999 Sponsor Date: 5/25/2006 Yearly Cost $1, 500. 00 Yearly Fees Investigator Initiated $ 500. 00 Yearly Fees Score 6 -10 $ 750. 00 Yearly Fees Score 1115 $1, 000. 00 Yearly Fees Score 1620 $1, 500. 00 Estimated Number of Years 1 39
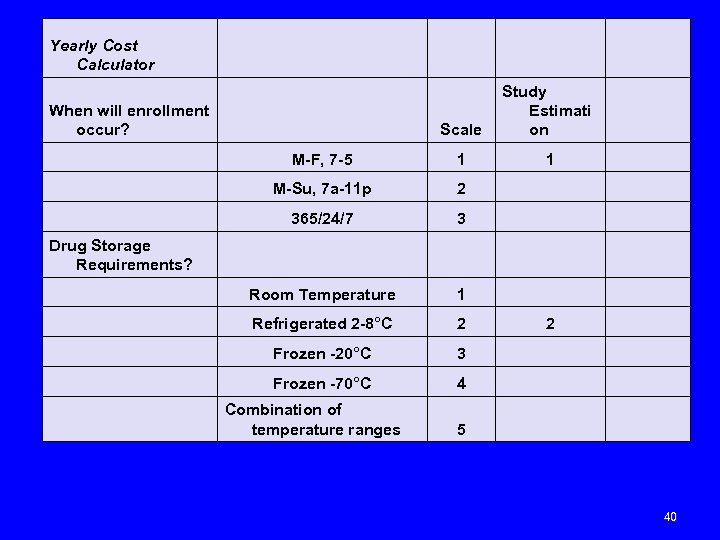 Yearly Cost Calculator Scale When will enrollment occur? Study Estimati on M-F, 7 -5 1 1 M-Su, 7 a-11 p 2 365/24/7 3 Drug Storage Requirements? Room Temperature 1 Refrigerated 2 -8°C 2 2 Frozen -20°C 3 Frozen -70°C 4 5 Combination of temperature ranges 40
Yearly Cost Calculator Scale When will enrollment occur? Study Estimati on M-F, 7 -5 1 1 M-Su, 7 a-11 p 2 365/24/7 3 Drug Storage Requirements? Room Temperature 1 Refrigerated 2 -8°C 2 2 Frozen -20°C 3 Frozen -70°C 4 5 Combination of temperature ranges 40
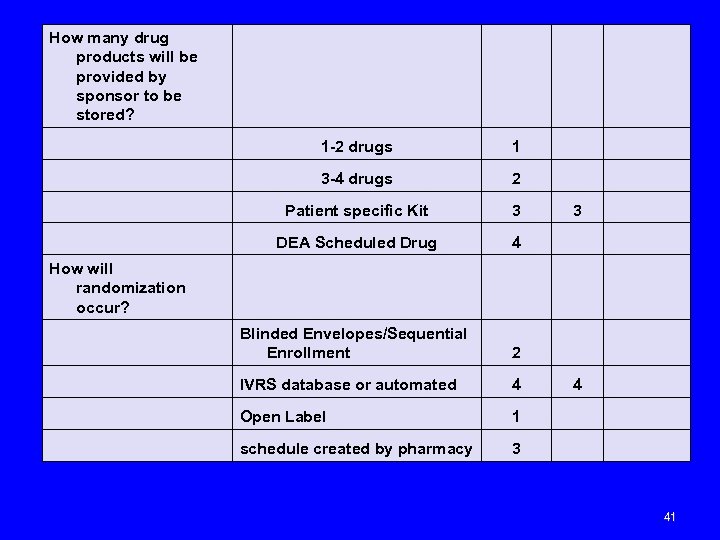 How many drug products will be provided by sponsor to be stored? 1 -2 drugs 1 3 -4 drugs 2 Patient specific Kit 3 3 DEA Scheduled Drug 4 How will randomization occur? Blinded Envelopes/Sequential Enrollment 2 IVRS database or automated 4 4 Open Label 1 schedule created by pharmacy 3 41
How many drug products will be provided by sponsor to be stored? 1 -2 drugs 1 3 -4 drugs 2 Patient specific Kit 3 3 DEA Scheduled Drug 4 How will randomization occur? Blinded Envelopes/Sequential Enrollment 2 IVRS database or automated 4 4 Open Label 1 schedule created by pharmacy 3 41
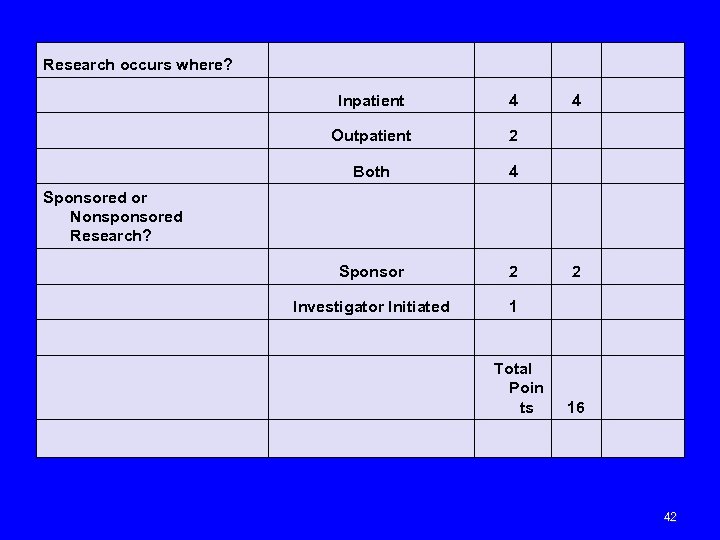 Research occurs where? Inpatient 4 4 Outpatient 2 Both 4 Sponsored or Nonsponsored Research? Sponsor 2 2 Investigator Initiated 1 Total Poin ts 16 42
Research occurs where? Inpatient 4 4 Outpatient 2 Both 4 Sponsored or Nonsponsored Research? Sponsor 2 2 Investigator Initiated 1 Total Poin ts 16 42
 Additional services provided under yearly fee cost Protocol Review Computer Files Creation Pharmacy Procedure Establishment Site Initiation Meeting Site Startup Meeting Monitoring Visits Drug Storage Continous Temperature Monitoring Drug Disposal Long Distance fax/phone charges 43
Additional services provided under yearly fee cost Protocol Review Computer Files Creation Pharmacy Procedure Establishment Site Initiation Meeting Site Startup Meeting Monitoring Visits Drug Storage Continous Temperature Monitoring Drug Disposal Long Distance fax/phone charges 43
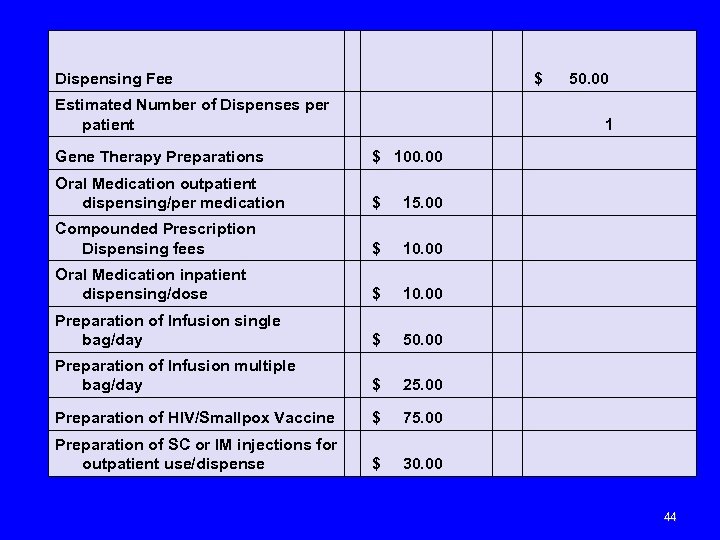 Dispensing Fee $ 50. 00 Estimated Number of Dispenses per patient 1 Gene Therapy Preparations $ 100. 00 Oral Medication outpatient dispensing/per medication $ 15. 00 Compounded Prescription Dispensing fees $ 10. 00 Oral Medication inpatient dispensing/dose $ 10. 00 Preparation of Infusion single bag/day $ 50. 00 Preparation of Infusion multiple bag/day $ 25. 00 Preparation of HIV/Smallpox Vaccine $ 75. 00 Preparation of SC or IM injections for outpatient use/dispense $ 30. 00 44
Dispensing Fee $ 50. 00 Estimated Number of Dispenses per patient 1 Gene Therapy Preparations $ 100. 00 Oral Medication outpatient dispensing/per medication $ 15. 00 Compounded Prescription Dispensing fees $ 10. 00 Oral Medication inpatient dispensing/dose $ 10. 00 Preparation of Infusion single bag/day $ 50. 00 Preparation of Infusion multiple bag/day $ 25. 00 Preparation of HIV/Smallpox Vaccine $ 75. 00 Preparation of SC or IM injections for outpatient use/dispense $ 30. 00 44
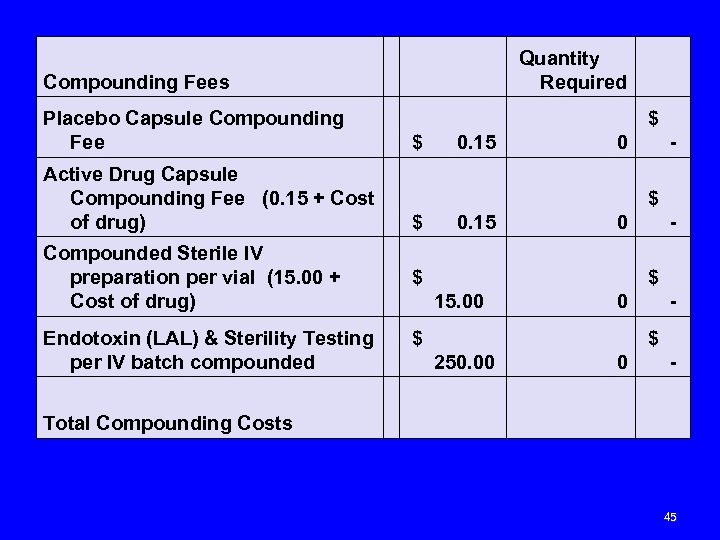 Compounding Fees Placebo Capsule Compounding Fee Quantity Required $ 0. 15 $ 0 - Active Drug Capsule Compounding Fee (0. 15 + Cost of drug) $ 0. 15 $ 0 - Compounded Sterile IV preparation per vial (15. 00 + Cost of drug) $ 0 - $ 15. 00 Endotoxin (LAL) & Sterility Testing $ per IV batch compounded 250. 00 Total Compounding Costs $ 0 - 45
Compounding Fees Placebo Capsule Compounding Fee Quantity Required $ 0. 15 $ 0 - Active Drug Capsule Compounding Fee (0. 15 + Cost of drug) $ 0. 15 $ 0 - Compounded Sterile IV preparation per vial (15. 00 + Cost of drug) $ 0 - $ 15. 00 Endotoxin (LAL) & Sterility Testing $ per IV batch compounded 250. 00 Total Compounding Costs $ 0 - 45
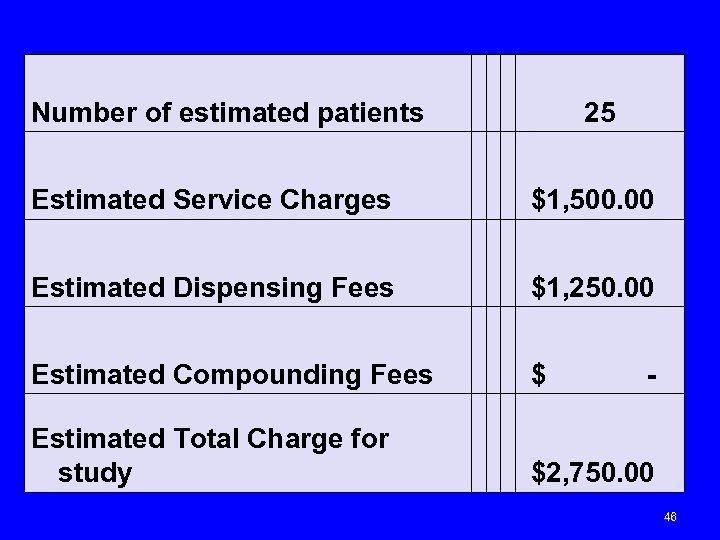 Number of estimated patients 25 Estimated Service Charges $1, 500. 00 Estimated Dispensing Fees $1, 250. 00 Estimated Compounding Fees $ - Estimated Total Charge for study $2, 750. 00 46
Number of estimated patients 25 Estimated Service Charges $1, 500. 00 Estimated Dispensing Fees $1, 250. 00 Estimated Compounding Fees $ - Estimated Total Charge for study $2, 750. 00 46
 Items needed from Principal Investigator to setup Protocol Amendments FDA 1572 IRB approval letter Consent form copy Drug Accountability forms (if sponsor provided) Drug Label Pages (if sponsor provided) Pharmacy Binder (if sponsor provided) Investigator’s brochure (if sponsor provided) Nursing Information Sheet 47
Items needed from Principal Investigator to setup Protocol Amendments FDA 1572 IRB approval letter Consent form copy Drug Accountability forms (if sponsor provided) Drug Label Pages (if sponsor provided) Pharmacy Binder (if sponsor provided) Investigator’s brochure (if sponsor provided) Nursing Information Sheet 47
 Pharmacy Commitments Budget Drug Horizon Expert Orders (Wiz) HMM (Horizon Meds Manager) Outpatient Prescription Maintain Protocol Specific Binder 48
Pharmacy Commitments Budget Drug Horizon Expert Orders (Wiz) HMM (Horizon Meds Manager) Outpatient Prescription Maintain Protocol Specific Binder 48
 Pharmacy Binder Enrollment Log/Randomization Log Drug Accountability Record Patient Worksheet RX How-to Mixing Instructions Copy of Current Consent Copy of Protocol Correspondence Current IRB Approval Letter 49
Pharmacy Binder Enrollment Log/Randomization Log Drug Accountability Record Patient Worksheet RX How-to Mixing Instructions Copy of Current Consent Copy of Protocol Correspondence Current IRB Approval Letter 49
 Documentation and Inventory Control Requirements Date of dispensing n Patients name and or study number n Manufacturer’s lot number n Balance on hand n Retest or expiration date n 50
Documentation and Inventory Control Requirements Date of dispensing n Patients name and or study number n Manufacturer’s lot number n Balance on hand n Retest or expiration date n 50
 Pharmaceutical dispensing n n n n n Dispensing Pharmacy The physician’s name, telephone, and address The words “CAUTION: New Drug Limited by Federal Law to Investigational Use” Study title Patient’s name or Study ID Date Drug name and strength & quantity dispensed Complete directions for use Storage Conditions/Expiration/Auxiliary Labels 51
Pharmaceutical dispensing n n n n n Dispensing Pharmacy The physician’s name, telephone, and address The words “CAUTION: New Drug Limited by Federal Law to Investigational Use” Study title Patient’s name or Study ID Date Drug name and strength & quantity dispensed Complete directions for use Storage Conditions/Expiration/Auxiliary Labels 51
 Storage facilities Locked Restricted access Investigational agents are kept separate from non study medications Appropriate temperature monitoring Maintain temperature logs Alarms notify of malfunctions 24/7 52
Storage facilities Locked Restricted access Investigational agents are kept separate from non study medications Appropriate temperature monitoring Maintain temperature logs Alarms notify of malfunctions 24/7 52
 Investigational Drug Service Department of Pharmaceutical Services 1161 21 st Avenue South, VUH RM B-101 Nashville, TN 37232 -7610 Phone: (615) 343 -6537 Fax: (615) 322 -6643 Investigational Drug Prescription Ketamine Nasal Spray versus placebo to decrease narcotic usage Principal Investigator: Pretend, MD Co-Investigators: Geri Foster, DPh Patient name: __________ Date: ________ Rx : Ketamine 25 mg/m. L or Placebo Treatment one: Date: ____/____ Treatment two: Date: ____/____ Deliver to GCRC S-3100 or E-507 __________________________, M. D. Patient meets study criteria and copy of informed consent signature page is attached. 53
Investigational Drug Service Department of Pharmaceutical Services 1161 21 st Avenue South, VUH RM B-101 Nashville, TN 37232 -7610 Phone: (615) 343 -6537 Fax: (615) 322 -6643 Investigational Drug Prescription Ketamine Nasal Spray versus placebo to decrease narcotic usage Principal Investigator: Pretend, MD Co-Investigators: Geri Foster, DPh Patient name: __________ Date: ________ Rx : Ketamine 25 mg/m. L or Placebo Treatment one: Date: ____/____ Treatment two: Date: ____/____ Deliver to GCRC S-3100 or E-507 __________________________, M. D. Patient meets study criteria and copy of informed consent signature page is attached. 53
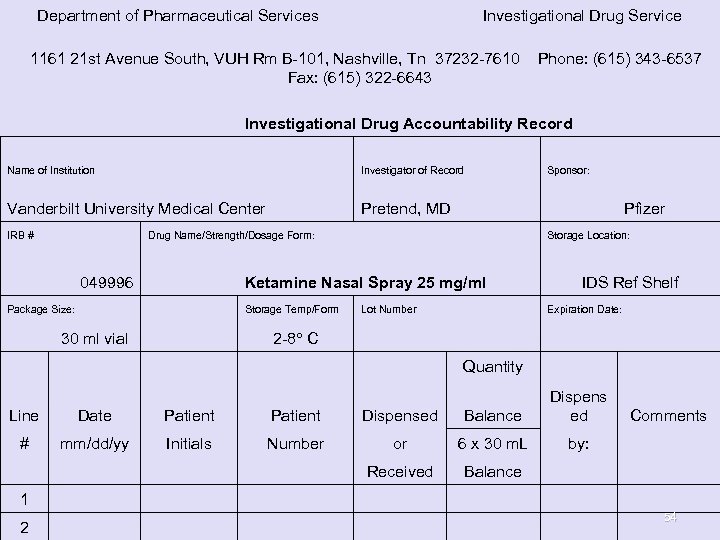 Department of Pharmaceutical Services Investigational Drug Service 1161 21 st Avenue South, VUH Rm B-101, Nashville, Tn 37232 -7610 Phone: (615) 343 -6537 Fax: (615) 322 -6643 Investigational Drug Accountability Record Investigator of Record Sponsor: Vanderbilt University Medical Center Pretend, MD Pfizer IRB # Name of Institution Drug Name/Strength/Dosage Form: 049996 Package Size: Storage Location: Ketamine Nasal Spray 25 mg/ml Storage Temp/Form 30 ml vial 2 -8° C IDS Ref Shelf Lot Number Expiration Date: Quantity Line Date Patient Dispensed Balance Dispens ed # mm/dd/yy Initials Number or 6 x 30 m. L by: Received Balance Comments 1 2 54
Department of Pharmaceutical Services Investigational Drug Service 1161 21 st Avenue South, VUH Rm B-101, Nashville, Tn 37232 -7610 Phone: (615) 343 -6537 Fax: (615) 322 -6643 Investigational Drug Accountability Record Investigator of Record Sponsor: Vanderbilt University Medical Center Pretend, MD Pfizer IRB # Name of Institution Drug Name/Strength/Dosage Form: 049996 Package Size: Storage Location: Ketamine Nasal Spray 25 mg/ml Storage Temp/Form 30 ml vial 2 -8° C IDS Ref Shelf Lot Number Expiration Date: Quantity Line Date Patient Dispensed Balance Dispens ed # mm/dd/yy Initials Number or 6 x 30 m. L by: Received Balance Comments 1 2 54
 Department of Pharmaceutical Services Investigational Drug Service 1161 21 st Avenue South, VUH Rm B-101, Nashville, Tn 37232 -7610 Phone: (615) 343 -6537 Fax: (615) 322 -6643 Pharmacy Enrollment Log Protocol # Sponsor Investigator IRB # 032229 GCRC Pretend, MD Patient Name Patient Date Number Received Enrolled Consent Order by : Ketamine Placebo 55
Department of Pharmaceutical Services Investigational Drug Service 1161 21 st Avenue South, VUH Rm B-101, Nashville, Tn 37232 -7610 Phone: (615) 343 -6537 Fax: (615) 322 -6643 Pharmacy Enrollment Log Protocol # Sponsor Investigator IRB # 032229 GCRC Pretend, MD Patient Name Patient Date Number Received Enrolled Consent Order by : Ketamine Placebo 55
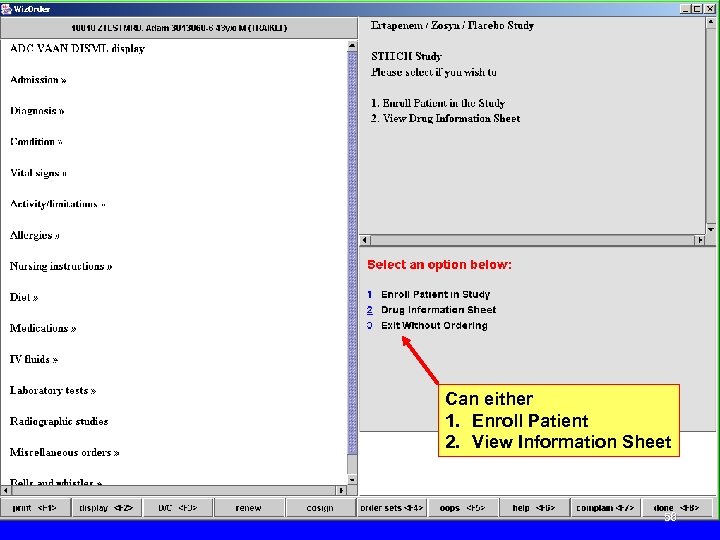 Can either 1. Enroll Patient 2. View Information Sheet 56
Can either 1. Enroll Patient 2. View Information Sheet 56
 Can Print Document 57
Can Print Document 57
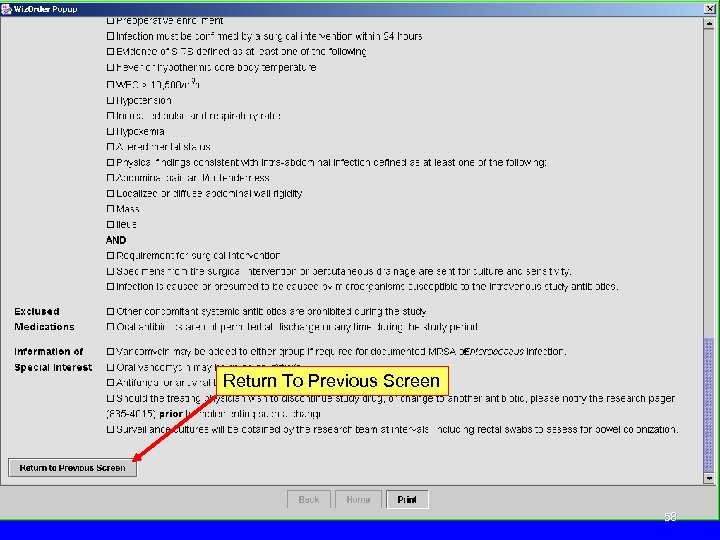 Return To Previous Screen 58
Return To Previous Screen 58
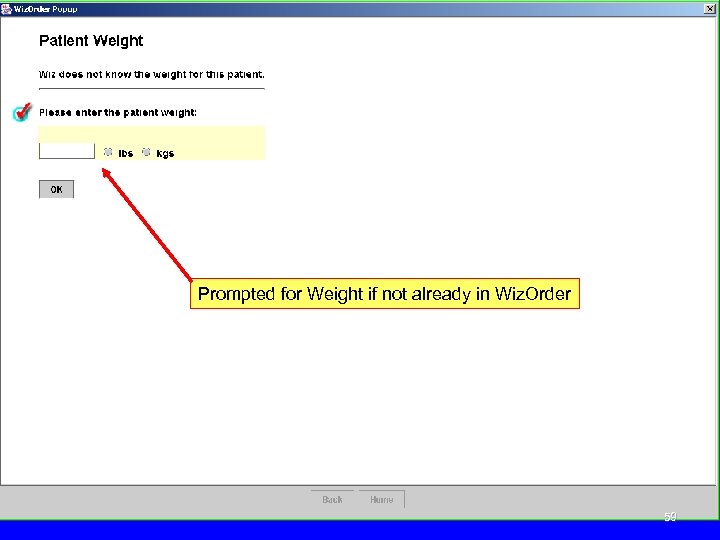 Prompted for Weight if not already in Wiz. Order 59
Prompted for Weight if not already in Wiz. Order 59
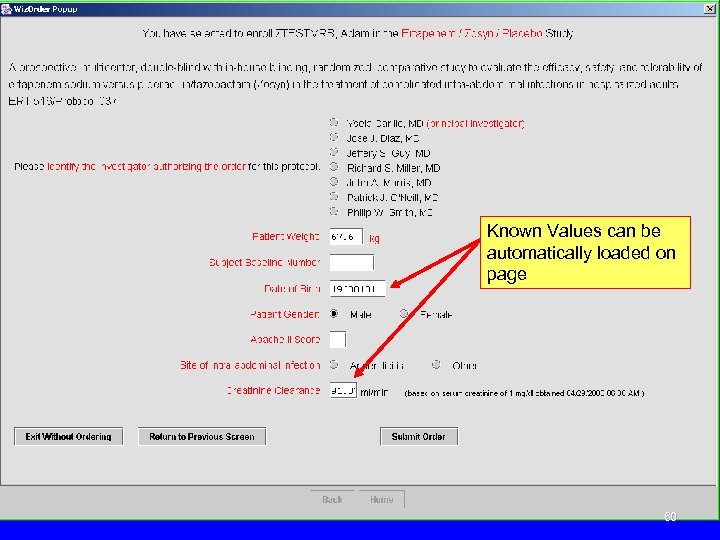 Known Values can be automatically loaded on page 60
Known Values can be automatically loaded on page 60
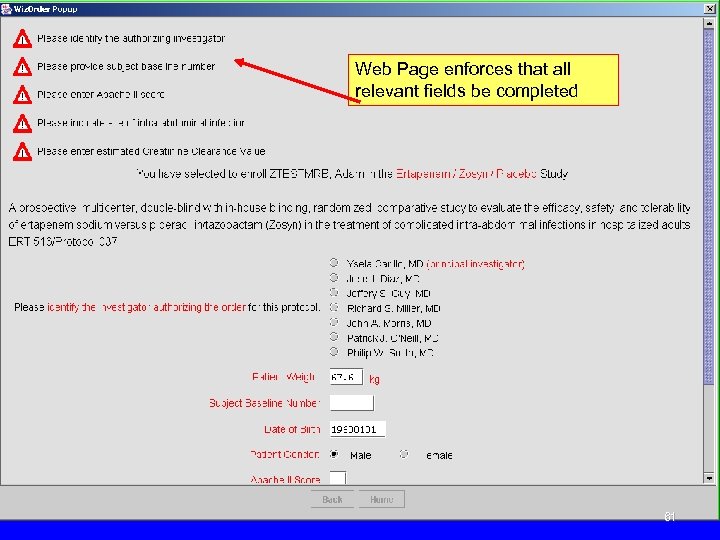 Web Page enforces that all relevant fields be completed 61
Web Page enforces that all relevant fields be completed 61
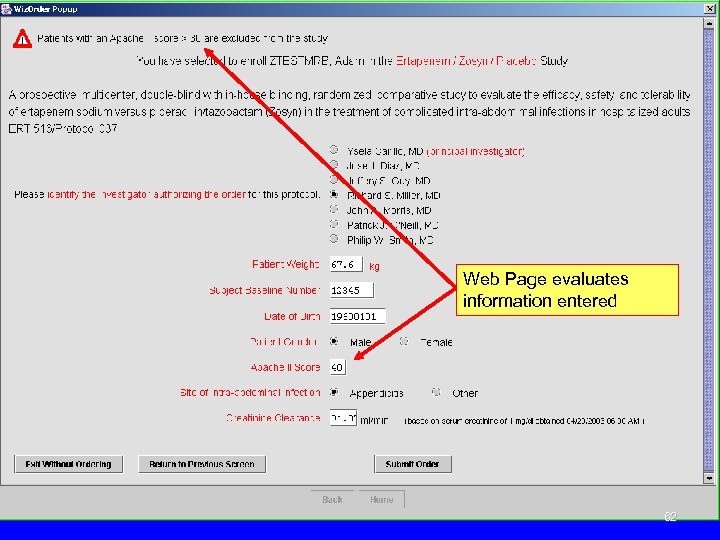 Web Page evaluates information entered 62
Web Page evaluates information entered 62
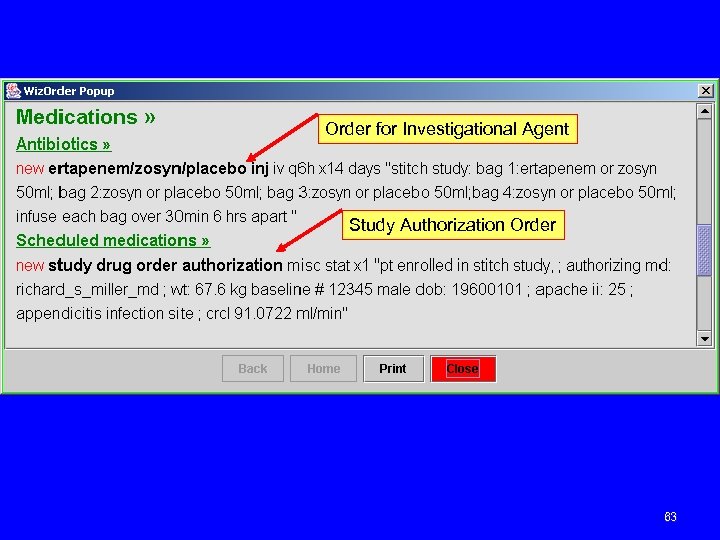 Order for Investigational Agent Study Authorization Order 63
Order for Investigational Agent Study Authorization Order 63
 Compounding Service 64
Compounding Service 64
 Why compound at VUMC? Quality patient care Serve needs of specialty patients n n Burn Pediatrics Trauma Investigational Drug Service (30%) Part of solution to healthcare problem Compliance with regulations n USP 795 & 797 65
Why compound at VUMC? Quality patient care Serve needs of specialty patients n n Burn Pediatrics Trauma Investigational Drug Service (30%) Part of solution to healthcare problem Compliance with regulations n USP 795 & 797 65
 Plan Ahead for Compounding Determine compounding costs 21 day lead time minimum Customize product for protocol Plan one dose unit per patient visit Project needs for 6 months 66
Plan Ahead for Compounding Determine compounding costs 21 day lead time minimum Customize product for protocol Plan one dose unit per patient visit Project needs for 6 months 66
 Compounding Team Geri Foster, Compounding Pharmacist Deborah Allen, Lead Compounding Technician Teresa Vo, Compounding Pharmacist Josephine Guirguis, Eileen Cooper, Van Nguyen, Linda Phaneuf, Technicians 67
Compounding Team Geri Foster, Compounding Pharmacist Deborah Allen, Lead Compounding Technician Teresa Vo, Compounding Pharmacist Josephine Guirguis, Eileen Cooper, Van Nguyen, Linda Phaneuf, Technicians 67
 “Compounding means the preparation, mixing, assembling, packaging, or labeling of a drug or device (i) as the result of a practioner’s prescription drug order or initiative based on the pharmacist/patient/prescriber relationship in the course of professional practice, or (ii) for the purpose of, as an incident to research, teaching, or chemical analysis and not for sale or dispensing. Compounding also includes the preparation of drugs and devices in anticipation of prescription drug orders based on routine, regularly observed patients. ” 68
“Compounding means the preparation, mixing, assembling, packaging, or labeling of a drug or device (i) as the result of a practioner’s prescription drug order or initiative based on the pharmacist/patient/prescriber relationship in the course of professional practice, or (ii) for the purpose of, as an incident to research, teaching, or chemical analysis and not for sale or dispensing. Compounding also includes the preparation of drugs and devices in anticipation of prescription drug orders based on routine, regularly observed patients. ” 68
 Compounding Pharmaceutical compounding is subject to standards listed in United States Pharmacopoeia (USP) or National Formulary (NF), monograph if such monograph exists If monographs do not exist then drug substance should be a component of drugs approved by the Secretary or are manufactured by an establishment registered to do so and certificates of analysis are available for each bulk component 69
Compounding Pharmaceutical compounding is subject to standards listed in United States Pharmacopoeia (USP) or National Formulary (NF), monograph if such monograph exists If monographs do not exist then drug substance should be a component of drugs approved by the Secretary or are manufactured by an establishment registered to do so and certificates of analysis are available for each bulk component 69
 Types of Compounding Oral liquids Capsules Topicals Ophthalmics Otics Inhalations Injections 70
Types of Compounding Oral liquids Capsules Topicals Ophthalmics Otics Inhalations Injections 70
 Compounding Attire Gown Gloves Quality Assurance Print Weights Log sheets Calibration Double Checks Weight Variance 71
Compounding Attire Gown Gloves Quality Assurance Print Weights Log sheets Calibration Double Checks Weight Variance 71
 72
72
 Sterile Compounding attire n n Hair cover gloves mask gown Quality assurance measures n n Endotoxin testing Sterility testing Record keeping Balance calibration 73
Sterile Compounding attire n n Hair cover gloves mask gown Quality assurance measures n n Endotoxin testing Sterility testing Record keeping Balance calibration 73
 Chemo Prep Area Morphine Bupivacaine Brilliant Green Terbutaline Cocaine Eye Drops Clonidine Hydromorphone HCl Tyramine Yohimbine Methacholine Bradykinin 74
Chemo Prep Area Morphine Bupivacaine Brilliant Green Terbutaline Cocaine Eye Drops Clonidine Hydromorphone HCl Tyramine Yohimbine Methacholine Bradykinin 74
 Sterilization Techniques Dry heat oven Filtration Gas Steam Autoclave Sterrad Future - Radiation 75
Sterilization Techniques Dry heat oven Filtration Gas Steam Autoclave Sterrad Future - Radiation 75
 Examples of Errors to Avoid Order sent before consent received Consenting volunteer to more than one study Wrong consent form signed – out of date, or wrong study Administering meds not on consent form Change practice before amendment approved Dispense without appropriate labeling/container Exceeding approved number of patients Consent obtained by non-approved personnel Inappropriate surrogate consent Inappropriate destruction of study medications 76
Examples of Errors to Avoid Order sent before consent received Consenting volunteer to more than one study Wrong consent form signed – out of date, or wrong study Administering meds not on consent form Change practice before amendment approved Dispense without appropriate labeling/container Exceeding approved number of patients Consent obtained by non-approved personnel Inappropriate surrogate consent Inappropriate destruction of study medications 76
 IDS Pharmacy VUH Room B 101 - 7610 Main Phone # 3 -6537 Hope Campbell Beeper 835 -9066 Geri Foster Beeper 835 -1797 Lori Choate Beeper 835 -9067 Deborah Allen Beeper 835 -9068 77
IDS Pharmacy VUH Room B 101 - 7610 Main Phone # 3 -6537 Hope Campbell Beeper 835 -9066 Geri Foster Beeper 835 -1797 Lori Choate Beeper 835 -9067 Deborah Allen Beeper 835 -9068 77


